Public health surveillance is focused on the detection of acute, chronic, and emerging threats to the health of the population to direct disease control and prevention efforts.1 Public health surveillance relies on health care providers to report to public health agencies conditions or outbreaks that may impact the broader population. This case reporting is mandated through laws and regulations at the state and local levels. Notification of cases to the Centers for Disease Control and Prevention (CDC) is facilitated by agreements between states and the federal government.2 Historically, case reporting has been based on paper reports or Internet-based entry of reports to state health department systems, but these reports are often slow or incomplete and place a substantial burden of work on health care providers and public health agencies.3 The future of surveillance is electronic case reporting (eCR), by which cases of reportable conditions are automatically generated from electronic health record (EHR) systems and transmitted to public health agencies for review and action.
eCR holds promise for enhancing the quality and effectiveness of public health surveillance.4 Greater use of eCR could result in (1) more complete and accurate case data in near real time for public health action; (2) earlier detection of cases, permitting earlier intervention and lowered transmission of disease; (3) improved detection of outbreaks to allow earlier investigation and, potentially, earlier identification of risk factors for the spread of disease; and (4) creation of a new infrastructure to support rapid reporting of newly recognized and emerging conditions. In this commentary, we review the promise of eCR and present our vision for a nationally interoperable eCR system that allows for timely reporting to public health and information sharing among jurisdictions.
Coordination between health care providers and public health agencies is essential for the monitoring, control, and prevention of disease and is best carried out through a bidirectional exchange of information. In 2009, the US Congress passed the Health Information Technology for Economic and Clinical Health Act to promote and expand the use of interoperable health information technology (IT) to improve the quality of health care.5,6 The act—funded with $19.2 billion from the American Recovery and Reinvestment Act—provided financial incentives to eligible health care providers and hospitals to convert from paper records to EHR systems. The act empowered the Centers for Medicare & Medicaid Services and the Office of the National Coordinator for Health Information Technology to provide incentives for meaningful use of EHRs for population and public health. The Centers for Medicare & Medicaid Services and the Office of the National Coordinator for Health Information Technology added eCR as an option that eligible health care providers and hospitals could choose to implement starting in 2018 to receive these financial incentives.7
Challenges to National Implementation
In the past, agencies trying to establish local eCR systems faced several challenges. One of the reasons to pursue eCR is efficiency, and health care providers and IT developers have sought efficient solutions for reporting cases to most jurisdictions nationwide. However, reaching such efficiencies has been frustrated when individual public health jurisdictions requested different eCR data elements, reporting formats, and structures and used their own criteria for reporting rather than a standardized approach. Efficiency has also been frustrated by heterogeneity in the diagnostic and order codes used by providers and laboratories in medical records.
Many public health jurisdictions are unprepared to receive and process eCR without additional investment, guidance, and assistance to enhance their IT infrastructure and workforce. In addition, some jurisdictions have expressed legal and privacy concerns that the personally identifiable information of their constituents may be vulnerable if the data are available for more than a minimal amount of time on intermediary platforms outside their control or jurisdiction.
These challenges apply to any efforts at national implementation of eCR. Implementation of national eCR requires collaboration among 3 key partners: health care delivery organizations, commercial EHR developers, and public health. To successfully implement national eCR, each partner must help prioritize and define what is best for the nation in the long run, as opposed to what is immediately most advantageous for each health care system, software product strategy, or public health agency. Until recently, these partners differed on how to plan and implement national eCR. Insufficient discussion among the 3 partners made it difficult to reach consensus. Successful implementation of eCR among these partners requires good relationships built on trust and understanding (eg, a recognition of the continuous evolution of technology and standards and the uses of data). We also have learned that eCR implementation is an evolving enterprise and that, to be successful, we must achieve incremental progress. We must avoid a one-and-done mentality and acknowledge that we cannot build a perfect system immediately.
Our Proposed Technical Framework
We propose the adoption of a technical framework for eCR that has the overall goals of fostering interoperability, reducing the work of case reporting for EHR developers and health care providers, addressing the jurisdiction-specific reporting requirements of state and local health agencies, and establishing a governance structure to support the evolution and improvement of eCR. To accomplish this goal for eCR, we propose initially using standards that are already available and widely adopted by health care providers in their existing EHR systems.
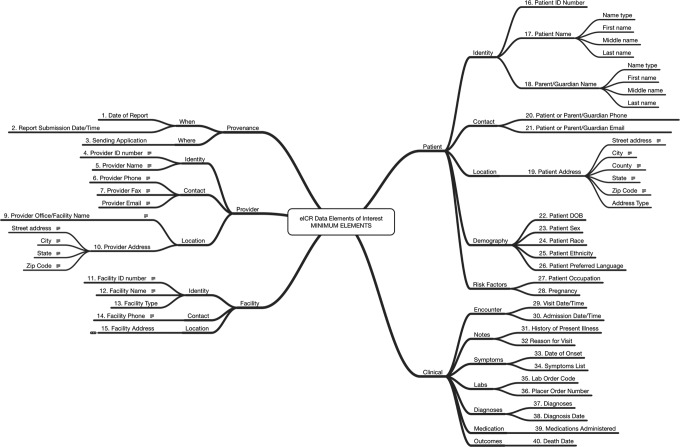
Under our proposed framework, the generation of an electronic initial case report (eICR) would take place in the EHR (Figure 1). The eICR for all reported conditions would consist of a standard set of data elements, vocabularies, and value sets (ie, a group of acceptable responses for a data element or question). The list of data elements has been drafted by the Council of State and Territorial Epidemiologists (CSTE) based on elements already used for meaningful use and will not require new functions for the EHR. The data elements will include such items as patient name and demographic characteristics, location, condition or disease, provider, and facility (Figure 2). Put together, these elements will include enough information to allow public health personnel to decide whether to initiate a public health investigation of a case, thus substantially decreasing the burden of work from follow-up telephone calls, facsimiles, and e-mails sent to gather initial case-related information from the health care providers.
Figure 1.
Overview of a proposed model of components and data flow for electronic case reporting in the United States. Abbreviation: CDC, Centers for Disease Control and Prevention. Adapted from: Association of State and Territorial Health Officials. Public Health Community Platform: condition referral from EHR to public health use case. http://www.thephcp.org/committees/case-reporting. Accessed August 25, 2016.
Figure 2.
Draft data elements for inclusion in electronic initial case reports (eICRs) from electronic health records to intermediary platforms in the United States. Abbreviations: DOB, date of birth; ID, identification. Source: Council of State and Territorial Epidemiologists’ Initial Case Report Task Force.
We believe that, in the future, there may be opportunity to further decrease the follow-up burden of work on health care providers by expanding the data elements in the initial case message or by electronically requesting and receiving supplementary EHR information not included in the eICR. One way of obtaining supplementary data would be to use electronic forms to capture data through a structured data capture standard. Any expansion of the data received electronically by public health would require stakeholder agreement.
The eICR would be identified in the EHR through a standard set of trigger codes that flag when a provider diagnoses, or considers diagnosing, a reportable condition based on International Classification of Diseases, Tenth Revision codes for diagnoses, LOINC (Logical Observation Identifiers Names and Codes) for laboratory testing orders, or SNOMED CT (Systematized Nomenclature of Medicine–Clinical Terms) for clinical information and laboratory results. The Association of Public Health Laboratories (APHL), CSTE, and CDC have already vetted the reportable trigger codes for 5 conditions (ie, gonorrhea, chlamydia, salmonella, pertussis, and Zika virus infections) and are developing trigger codes for all reportable conditions. We have learned that trigger codes will not remain static; they must evolve as diagnostic tests and case definitions change. The list of standard trigger codes will reside in EHRs but can be updated from a CDC subscription service that curates and allows access to trigger codes to EHR developers as needed. Additionally, new and updated trigger codes can rapidly be made available in response to new or evolving conditions, such as severe acute respiratory syndrome, Middle East respiratory syndrome coronavirus, enterovirus D68, and Zika virus.
After potential cases are identified through trigger codes, the eICR will automatically be generated with case information and transmitted from the EHR to an intermediary platform (eg, a health information exchange or shared public health platform) via secure, broadly used data transport mechanisms (Figure 1). On these platforms, a software application will serve health care providers by assessing the reportability of the disease or condition via a logic model based on the relevant jurisdiction’s mandated case reporting requirements and then will route adjudicated cases to the appropriate agency or agencies.
The CSTE has been working with CDC to build the Reportable Conditions Knowledge Management System (RCKMS), the software application that will unpack, transform, and adjudicate the eICR automatically in a secure environment to determine whether the potential case meets minimal criteria consistent with mandated reporting based on a standard logic specific to jurisdictional requirements.8 The RCKMS will additionally transmit reportable cases to state and local jurisdictions for final classification and action and will inform the health care provider when cases have been reported. The CSTE has completed an initial pilot of the RCKMS. It includes logic for adjudicating potential cases of pertussis, hepatitis, elevated blood lead levels, and tuberculosis for 10 jurisdictions. The process for determining the logic for adjudicating the eICR for other reportable conditions will engage local, state, and CDC programs in that process. We envision the RCKMS as a service that can be deployed on intermediary platforms.
On most intermediary platforms, data containing personally identifiable information will be resident very briefly, and only limited data that contain no personally identifiable information will be saved for auditing. For efficiency, some jurisdictions may choose to allow these data to be available for a longer period on a secure cloud-based platform.
CDC has supported the Health Level 7 Consolidated Clinical Document Architecture as the initial structure for transmitting the eICR, based on standards that are already in use. A Health Level 7 Consolidated Clinical Document Architecture implementation guide based on the data elements shown in Figure 2 has been published.9 We expect a phased transition to new and improved structures (eg, Health Level 7 Fast Healthcare Interoperability Resources) as the standard matures and the business case becomes apparent to stakeholders.
Working Toward National eCR Implementation
CDC, CSTE, Association of State and Territorial Health Officials, APHL, National Association of County and City Health Officials, and Public Health Informatics Institute have worked together to explore and begin eCR implementation. Together, these organizations have identified the important technical elements needed for the first phase of eCR implementation, including initial standards, platform structure, tools, and guides. This work began in 2013 with a 3-year, $3-million investment through a cooperative agreement with the Association of State and Territorial Health Officials, in which these organizations explored the concept of a Public Health Community Platform (PHCP): an intermediary platform that will serve for many, if not all, implementations of eCR during its first phase of implementation. Currently, the PHCP is leveraging the platform infrastructure provided by the APHL Informatics Messaging Service, a secure, auditable cloud-based platform that can receive, hold, and transmit electronic messages.10 The PHCP is envisioned to support information sharing (eg, immunization registries, case reporting) between public health and health care providers and among jurisdictions and to provide shared services (eg, messaging, visualization, analysis) to public health partners.11
On June 14-15, 2016, the Robert Wood Johnson Foundation convened a meeting of stakeholders to discuss vision, governance, and initial steps toward eCR implementation. We agreed that, to be effective, we need a governance structure for eCR that includes 3 principal partners: health care delivery organizations, EHR developers, and public health. Participating stakeholders agreed that we must focus on the secure sharing of reports of potential cases, the periodic evaluation and evolution of standards, and the tools and processes for continual improvement. Any governance structure for eCR should support the iterative construction of systems, routine communication and alignment to regulatory timelines, and realistic software release cycles.
Participating stakeholders also agreed that successful eCR implementation will require a commitment by state and local public health agencies and public health associations (eg, APHL, Association of State and Territorial Health Officials, CSTE, National Association of County and City Health Officials) to a clear, shared vision of an interoperable system for eCR. One recent sign of progress is that on June 23, 2016, the CSTE adopted a position statement supporting changes in practice, shared governance, and the collaborative efforts needed for eCR implementation.12 Another positive recent sign is that the Public Health Informatics Institute has begun to outline a robust communications plan on eCR with public health partners that will engage their leadership and members. CDC is expanding the potential for the use of funds from the Public Health Emergency Preparedness and the Epidemiology and Laboratory Capacity cooperative agreements to support eCR implementation and strengthen public health informatics capacities at state and local health departments.
With this progress, there is now a need for effective project management, better technical expertise, and additional experienced partners to develop eCR on a large scale. The next steps are to (1) conduct proof-of-concept demonstration projects for eCR to provide initial incremental success, (2) ensure communication and coordination of partners, (3) provide clear timelines to partners for broader implementation of major eCR components, and (4) hold partners accountable for timelines and deliverables. CDC has engaged the MITRE Corporation—a federally funded research and development corporation that has experience in the health care domain—to assist in guiding this eCR effort.
Conclusion
Coordination and bidirectional information exchange between health care providers and public health agencies are essential for the prevention and control of disease. eCR offers the promise of improving public health by enhancing the speed and accuracy of this crucial exchange and by transforming how we approach population health and disease prevention.
In this commentary, we outline some of the complexities and challenges of implementing eCR nationwide. We know that questions and issues will arise during implementation. For example, as is true for most surveillance enhancements, it is likely that eCR will increase the reporting of cases of disease. We anticipate that eCR will ultimately decrease the amount of work needed by local and state public health agencies to obtain case information from health care providers. In the short term, however, the workload for public health agencies may increase. But we believe that the benefits from eCR will greatly outweigh the costs in time and money. For example, eCR can give us a truer sense of the magnitude and distribution of reportable diseases and conditions. It can foster a reprioritization of activities and resources at state and local health departments that can, in turn, lead to enhanced tools and methods to prevent disease and improve outcomes. Ultimately, the success of a standards-based framework for eCR implementation can be a building block for broader data sharing about noncommunicable conditions.4 Broad data sharing can support health care reform by providing more timely, complete, and accurate population health data.5
The technical problems of implementing eCR can be solved. What we need now is a collective commitment among public health, health care, and health IT organizations to achieve our vision of interoperable, secure, and nationwide eCR in the United States.
Acknowledgments
We acknowledge the following people for their roles in advancing electronic case reporting thus far and contributing to the knowledge that led to the technical framework: Oscar Alleyne, Rita Altamore, Edward Baker, Bill Brand, Jim Collins, Jim Daniel, David Friedman, Kate Goodin, Janet Hamilton, Gillian Haney, Erin Holt, Monica Huang, Janet Hui, Charlie Ishikawa, Paul Jarris, Bryant Karras, Wes Kennemore, J. T. Lane, Marty LaVenture, Brian Lee, Meredith Lichtenstein, John Loonsk, John Lumpkin, Shu McGarvey, Joe McLaughlin, Michelle Meigs, Riki Merrick, Robert Pinner, Marcus Rennick, David Ross, Paula Soper, Catherine Staes, Sanjeev Tandon, Jon White, and Paula Yoon.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Lee LM, Thacker SB. The cornerstone of public health practice: public health surveillance, 1961-2011. MMWR Morb Mortal Wkly Rep. 2011;60(4):15–21. [PubMed] [Google Scholar]
- 2. Case definitions for infectious conditions under public health surveillance. MMWR Recomm Rep. 1997;46(RR-10):1–55. [PubMed] [Google Scholar]
- 3. Lee LM, Teutsch SM, Thacker SB, St Louis ME, eds. Principles and Practice of Public Health Surveillance. New York, NY: Oxford University Press; 2010. [Google Scholar]
- 4. Birkhead GS, Klompas M, Shah NR. Uses of electronic health records for public health surveillance to advance public health. Annu Rev Public Health. 2015;36:345–359. [DOI] [PubMed] [Google Scholar]
- 5. Buntin MB, Jain SH, Blumenthal D. Health information technology: laying the infrastructure for national health reform. Health Aff (Millwood). 2010;29(6):1214–1219. [DOI] [PubMed] [Google Scholar]
- 6. Blumenthal D. Launching HITECH. N Engl J Med. 2010;362(5):382–385. [DOI] [PubMed] [Google Scholar]
- 7. US Government Printing Office. Medicare and Medicaid programs: electronic health record incentive program—stage 3 and modifications to meaningful use in 2015 through 2017. https://www.federalregister.gov/articles/2015/10/16/2015-25595/medicare-and-medicaid-programs-electronic-health-record-incentive-program-stage-3-and-modifications#h-113. Accessed August 9, 2016. [PubMed]
- 8. Council of State and Territorial Epidemiologists. Surveillance/informatics: reportable condition knowledge management system. http://www.cste.org/group/RCKMS. Accessed August 9, 2016.
- 9. Health Level Seven International. Product brief: section 3: clinical and administration domains; section 5: implementation guides. http://www.h17.org/implement/standards/product_brief.cfm?product_id=34. Accessed August 9, 2016.
- 10. Association of Public Health Laboratories. AIMS platform. http://www.aphl.org/programs/informatics/pages/aims_platform.aspx. Accessed August 9, 2016.
- 11. Association of State and Territorial Health Officials. Public Health Community Platform. http://www.thephcp.org. Accessed August 9, 2016.
- 12. Council of State and Territorial Epidemiologists. Position statement 16-SI-02 on surveillance: electronic case reporting (eCR). http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/2016PS/16_SI_02.pdf. Accessed August 9, 2016.