Abstract
Objectives:
Cancer survivors require appropriate health care to manage their unique health needs. This study describes access to cancer care among cancer survivors in the United States and compares access to general medical care between cancer survivors and people who have no history of cancer.
Methods:
We assessed access to general medical care using the core 2011 Medical Expenditure Panel Survey (MEPS). We assessed access to cancer care using the MEPS Experiences With Cancer Survey. We used multivariable logistic regression to compare access to general medical care among 2 groups of cancer survivors (those who reported having access to all necessary cancer care [n = 1088] and those who did not [n = 70]) with self-reported access to general medical care among people who had no history of cancer (n = 22 434).
Results:
Of the 1158 cancer survivors, 70 (6.0%) reported that they did not receive all necessary cancer care. Adjusted analyses found that cancer survivors who reported not receiving all necessary cancer care were also less likely to report receiving general medical care (78.0%) than cancer survivors who reported having access to necessary cancer care (87.1%) and people who had no history of cancer (87.8%).
Conclusions:
This study provides nationally representative data on the proportion of cancer survivors who have access to necessary cancer care and yields insight into factors that impede survivors’ access to both cancer care and general medical care. This study is a reference for future work on access to care.
Keywords: cancer care, access to care, health services
Cancer and its treatment can precipitate a range of physical, functional, and psychosocial limitations that require treatment, supportive care, and self-management.1–4 As such, cancer survivors should have access to well-coordinated and appropriate health care throughout the cancer care trajectory. Studies have examined cancer survivors’ access to cancer care and general medical care services.5–8 A 2013 Institute of Medicine report found that many patients lack access to affordable high-quality cancer care.9 Other studies found that suboptimal access to cancer care is associated with adverse clinical outcomes, increased risk of recurrence, and poorer disease-free and overall survival.10–13 The cost of cancer care is a major barrier to health care access among survivors, particularly among people who are in racial/ethnic minority groups, have low socioeconomic status, or are uninsured or underinsured.14–21 Furthermore, the United States is facing projected shortages of cancer drugs and oncologists, which may negatively affect access to timely and appropriate cancer treatment.22–24
The objectives of this study were to (1) describe findings from the Medical Expenditure Panel Survey (MEPS) on the proportion of cancer survivors who reported not receiving all necessary cancer care and (2) examine whether or not access to cancer care is associated with access to general medical care. To our knowledge, no population-based studies of cancer survivors have addressed these topics. This study provides insight into whether or not barriers to cancer care influence access to health care in general and complements other studies of cancer survivors’ access to care.7
Methods
Data Source
MEPS is a nationally representative sample of the US civilian noninstitutionalized population that is administered by the Agency for Healthcare Research and Quality. MEPS collects data through an overlapping panel design. Data for each panel are collected through 5 in-person interviews conducted during a 2-year period. The interviewers collect detailed information on respondents, including data on demographic characteristics, health conditions, and access to care.25 Our analyses were based on the 2011 MEPS with data drawn from both the core survey and the Experiences With Cancer Survey.26 The MEPS Experiences With Cancer Survey was a self-administered questionnaire of adult cancer survivors comprising questions about economic burden, access to care, and other aspects of cancer survivorship. The response rate for the core MEPS in 2011 was 54.9%, and the response rate for the Experiences With Cancer Survey was 90.0%, for an overall response rate of 49.4%. Cancer survivors were identified by the question “Have you ever been told by a doctor or other health professional that you had cancer or a malignancy of any kind?” This study was based on publicly available de-identified data. As such, it was not subject to institutional review board review.
Measures
As part of a series of questions about medical care for cancer, cancer survivors were asked, “At any time since you were first diagnosed with cancer, did you get all of the medical care, tests, or treatments that you or your doctor believed were necessary?” People who responded no were asked why they did not receive all necessary care. Barriers to care were as follows: could not afford care, insurance company would not approve or pay for care, doctor did not accept insurance, had problems getting to the doctor’s office, could not get time off from work, did not know where to go to get care, could not get child care/adult care, did not have time, care took too long, and other reason. For our analysis, these responses were collapsed into 2 categories: (1) cost and insurance issues (could not afford care, insurance company would not approve or pay for care, and doctor did not accept insurance) and (2) other (had problems getting to the doctor’s office, could not get time off from work, did not know where to go to get care, could not get child care/adult care, did not have time, care took too long, and other reason).
The core MEPS included items about access to general medical care. Consistent with the behavioral model of health care access by Andersen and Aday as well as other research, we examined indicators of perceived and realized access to general medical care.7,27 We assessed perceived access to general medical care through two measures: (1) whether or not respondents reported having a usual source of care (yes/no) and (2) whether respondents reported a delay in obtaining or an inability to obtain medical care, dental care, or prescription medicine in the previous 12 months (yes/no). We measured realized access to general medical care as the self-reported use of selected preventive services that are recommended with an A or B rating by the US Preventive Services Task Force as of 2011. Services included cholesterol screening within the previous 2 years, dental checkup in the previous year, and influenza vaccination in the previous year. We selected these measures because they are captured in the MEPS and were answered by enough people to make meaningful inferences. We measured and analyzed perceived access to care and realized access to care independently; as such, it was possible for someone to have one type of access but not the other.
Covariates
Sociodemographic characteristics were participants’ current age, sex, education, race/ethnicity, marital status, number of children <26 years of age living in the household, current employment, and health insurance (any private, public only, or none). We defined comorbidity as the number of MEPS priority conditions that each respondent reported: angina, arthritis, asthma, coronary heart disease, diabetes, emphysema, heart attack, high cholesterol, hypertension, and stroke. We included 2 clinical characteristics of cancer survivors: cancer site (breast, prostate, other single cancer, multiple cancers) and years since last treatment.
Data Analysis
We calculated descriptive statistics to characterize the sample, and we used Wald F tests to compare differences between cancer survivors and people with no history of cancer. We used a similar approach to compare cancer survivors who reported receiving all necessary cancer care with those who did not, although we collapsed some of the categories of the variables because of concerns about small cell sizes. For example, we dichotomized education as high school or less and some college or more. We calculated population estimates of cancer survivors without access to cancer care using a special weight variable provided by the Agency for Healthcare Research and Quality that adjusts the Experiences With Cancer Survey for nonresponse and estimates the adult population self-reporting as having been diagnosed with or treated for cancer as an adult.28
We also used Wald F tests to compare differences between survivors who did and did not report receiving all necessary cancer care. We calculated the mean number of barriers to cancer care as the weighted percentage and 95% confidence interval (CI) for survivors reporting cost and insurance barriers or other barriers to cancer care. We used multivariable logistic regression analyses to examine differences in perceived and realized access to care among 3 groups: cancer survivors with (n = 1088) and without (n = 70) access to all necessary care for cancer and people with no history of cancer (n = 22 434). We adjusted regression models for current age, education, sex, health insurance, marital status, number of MEPS priority conditions, and race/ethnicity. We calculated results from the multivariable models as predicted probabilities, also known as adjusted predicted margins, which we compared using t tests.29 We considered α < .05 to be significant. Consistent with other studies, we did not count nonmelanoma skin cancer as cancer.7,19 We excluded cancer survivors from the study who were missing data for access to cancer care (n = 44). We weighted all of the estimates to account for the complex survey design of MEPS and for survey nonresponse, using SUDAAN release 11.0.1.30
Results
We based all analyses on 1158 cancer survivors and 22 434 people with no history of cancer. Compared with people with no history of cancer, cancer survivors were significantly older and more likely to be female, highly educated, non-Hispanic white, married, and insured; less likely to have children <26 years of age living in the home and to be employed for wages; and more likely to report comorbid conditions, with 43.1% of cancer survivors reporting at least 3 conditions in addition to cancer. Of the 1158 cancer survivors, 230 (18.1%) were diagnosed with breast cancer and 152 (14.2%) with prostate cancer; 693 (60.8%) reported other single cancers, and 83 (6.9%) reported multiple cancers. Eighty-five (6.6%) cancer survivors reported currently receiving treatment, and 138 (12.1%) reported being within 3 years of their last treatment (Table).
Table.
Demographic characteristics of cancer survivors and people with no history of cancer, Medical Expenditure Panel Survey, 2011a
| Cancer Survivors (n = 1158) | People With no History of Cancer (n = 22 434) | ||||
|---|---|---|---|---|---|
| Characteristics | No. | Weighted %b (95% CI) | No. | Weighted %b (95% CI) | P Value |
| Current age, y | |||||
| 18-39 | 59 | 4.7 (3.5-6.3) | 9816 | 41.5 (40.4-42.6) | <.001 |
| 40-49 | 113 | 8.8 (7.1-11.0) | 4144 | 17.9 (17.2-18.7) | |
| 50-59 | 215 | 18.7 (15.7-22.1) | 3908 | 18.5 (17.7-19.4) | |
| 60-69 | 337 | 28.1 (25.1-31.4) | 2527 | 12.2 (11.5-12.9) | |
| 70-79 | 243 | 22.2 (19.3-25.3) | 1299 | 6.1 (5.6-6.6) | |
| ≥80 | 191 | 17.5 (14.4-21.2) | 740 | 3.7 (3.3-4.2) | |
| Sex | |||||
| Male | 451 | 41.8 (38.5-45.2) | 10 499 | 48.7 (48.0-49.3) | <.001 |
| Female | 707 | 58.2 (54.8-61.5) | 11 935 | 51.3 (50.7-52.0) | |
| Education when first entered MEPS | |||||
| <High school | 172 | 10.7 (8.8-12.9) | 4805 | 14.3 (13.5-15.2) | <.001 |
| High school graduate | 401 | 31.2 (27.9-34.6) | 7209 | 29.4 (28.3-30.5) | |
| ≥Some college | 583 | 58.1 (54.5-61.6) | 10 287 | 55.9 (54.5-57.2) | |
| Race/ethnicity | |||||
| Non-Hispanic white | 877 | 87.1 (84.9-89.1) | 9703 | 64.9 (62.7-67.1) | <.001 |
| Otherc | 281 | 12.9 (10.9-15.1) | 12 731 | 35.1 (32.9-37.3) | |
| Marital status | |||||
| Married | 620 | 56.9 (52.9-60.7) | 10 964 | 52.0 (50.8-53.2) | .02 |
| Not married | 538 | 43.1 (39.3-47.1) | 11 470 | 48.0 (46.8-49.2) | |
| No. of children aged <26 y living in household | |||||
| 0 | 944 | 83.3 (80.4-85.8) | 13 266 | 63.4 (62.3-64.5) | <.001 |
| 1 | 104 | 8.7 (6.9-10.8) | 3404 | 14.7 (13.9-15.4) | |
| ≥2 | 110 | 8.0 (6.3-10.1) | 5764 | 21.9 (20.9-23.0) | |
| Current employment | |||||
| Full-time | 294 | 27.5 (24.0-31.3) | 11 104 | 52.5 (51.5-53.5) | <.001 |
| Part-time | 104 | 9.3 (7.4-11.7) | 2286 | 10.8 (10.2-11.4) | |
| Unemployed | 748 | 61.8 (57.5-66.0) | 8604 | 34.9 (33.7-36.0) | |
| Health insurance | |||||
| Any private | 721 | 69.2 (66.0-72.2) | 12 738 | 67.7 (66.3-69.1) | <.001 |
| Public only | 374 | 26.6 (23.8-29.7) | 4784 | 16.7 (15.8-17.8) | |
| None | 63 | 4.2 (3.0-5.9) | 4912 | 15.5 (14.6-16.5) | |
| No. of MEPS priority conditionsd | |||||
| 0 | 721 | 69.2 (66.0-72.2) | 12 738 | 67.7 (66.3-69.1) | <.001 |
| 1 | 374 | 26.6 (23.8-29.7) | 4784 | 16.7 (15.8-17.8) | |
| 2 | 63 | 4.2 (3.0-5.9) | 4912 | 15.5 (14.6-16.5) | |
| ≥3 | 527 | 43.1 (39.2-47.1) | 3630 | 16.5 (15.8-17.3) | |
| Cancer site | |||||
| Breast | 230 | 18.1 (16.0-20.6) | NA | ||
| Prostate | 152 | 14.2 (11.6-17.1) | NA | ||
| Other single cancers | 693 | 60.8 (57.5-63.9) | NA | ||
| Multiple cancers | 83 | 6.9 (5.5-8.7) | NA | ||
| Years since last treatment | |||||
| <1 | 40 | 3.0 (1.9-4.5) | NA | ||
| 1 to <3 | 98 | 9.1 (7.2-11.4) | NA | ||
| 3 to <5 | 132 | 11.2 (9.2-13.7) | NA | ||
| 5 to <10 | 121 | 10.5 (8.6-12.8) | NA | ||
| ≥10 | 200 | 16.4 (14.2-18.9) | NA | ||
| Never received treatment | 308 | 27.6 (25.1-30.3) | NA | ||
| Currently receiving treatment | 85 | 6.6 (5.2-8.4) | NA | ||
Abbreviations: CI, confidence interval; MEPS, Medical Expenditure Panel Survey; NA, not applicable.
aAnalyses were based on the 2011 MEPS with data drawn from both the core survey and the Experiences With Cancer Survey.25,26 Estimates were weighted to account for the complex survey design of the MEPS. Wald F tests were used to compare differences between cancer survivors and people without a history of cancer.
bWeighted percentages may not total to 100 because of missing data.
cOther races/ethnicities are non-Hispanic black, American Indian/Alaska Native, Asian, Native Hawaiian/Pacific Islander, multiple races, and Hispanic.
dMEPS priority conditions are angina, arthritis, asthma, coronary heart disease, diabetes, emphysema, heart attack, high cholesterol, hypertension, and stroke.
Access to Cancer Care
Of the 1158 cancer survivors, 1088 (94.0%) reported receiving all necessary cancer care. The 70 (6.0%) cancer survivors who reported that they did not receive all necessary cancer care corresponded to an estimated 901 470 (95% CI, 700 372-1 155 918) adults with cancer in the US population. Compared with cancer survivors who reported receiving all necessary cancer care, a smaller proportion of those who reported not receiving all necessary cancer care had some college or more (557 of 1088 [59%] vs 26 of 70 [42.3%], P = .034). Likewise, compared with cancer survivors who reported receiving all necessary cancer care, a greater proportion of those who reported not receiving all necessary cancer care was publicly insured or uninsured (395 of 1088 [29.3%] vs 42 of 70 [55.7%], P < .001). We found no significant differences between survivors who reported receiving all necessary cancer care and those who did not, in terms of age, cancer site, employment status, marital status, number of children <26 years of age living in the household, number of MEPS priority conditions, race/ethnicity, sex, or years from last treatment.
Of the 70 cancer survivors who reported not receiving all necessary cancer care, 24 (32%) reported 1 barrier; 11 (17%), 2 barriers; 14 (18%), at least 3 barriers; and 21 (34%), no barriers or missing data. Of 70 cancer survivors who reported not receiving all necessary cancer care, 29 cited cost and insurance issues as barriers to care (weighted percentage = 40.4%; 95% CI, 27.6%-54.5%). More than half of cancer survivors (39 of 70) who reported not receiving all necessary cancer care cited other barriers (weighted percentage = 54.4%; 95% CI, 40.7%-67.5%).
Perceived and Realized Access to Care
Perceived Access to General Medical Care
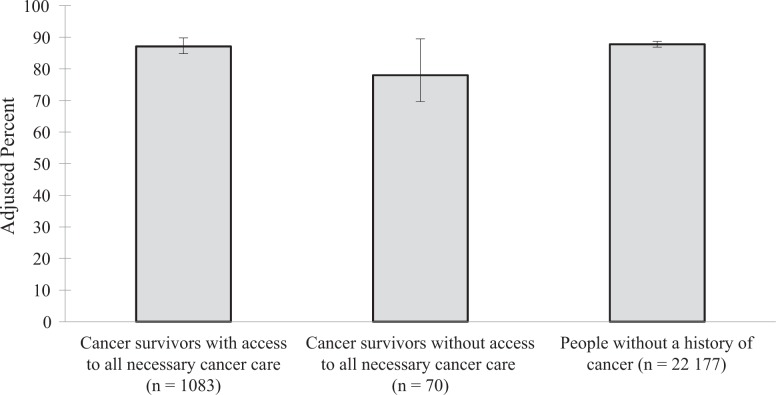
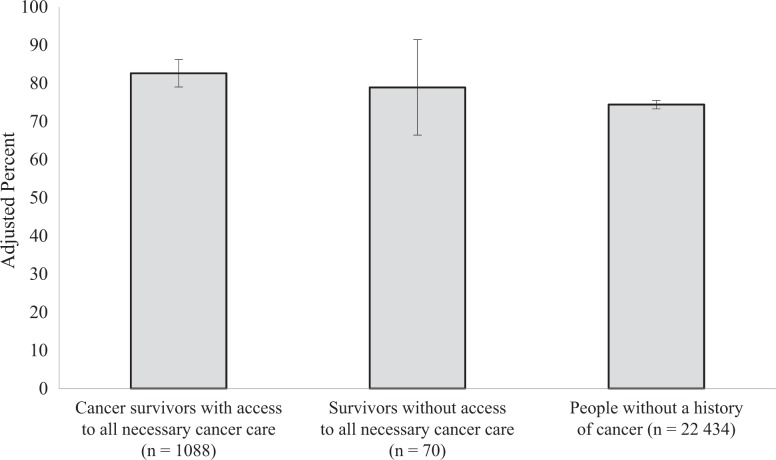
The predicted probability of having access to general medical care was 78.0% among cancer survivors who reported not receiving all necessary cancer care, 87.1% among cancer survivors who reported having access to all necessary cancer care, and 87.8% among adults with no history of cancer (F 2,21 = 2.84, P = .06; Figure 1). Group differences were not significant between cancer survivors who did and did not report receiving all necessary cancer care (P = .08). Likewise, group differences were not significant between cancer survivors who reported not receiving all necessary cancer care and those with no history of cancer (P = .06). The predicted probability of having a usual source of care was 78.9% for cancer survivors who reported not receiving all necessary cancer care, 82.6% for cancer survivors who reported receiving all necessary cancer care, and 74.4% for people with no history of cancer (F 2,21 = 9.76, P < .001). The predicted probability of having a usual source of care differed significantly between cancer survivors who reported receiving all necessary cancer care and people with no history of cancer (P < .001; Figure 2).
Figure 1.
Perceived access to general medical care among cancer survivors and people with no history of cancer, Medical Expenditure Panel Survey (MEPS), 2011. Analyses were based on the 2011 MEPS, with data drawn from both the core survey and the Experiences With Cancer Survey.25,26 Predicted probabilities were derived from multivariate logistic regression analyses adjusting for current age, education, health insurance, marital status, number of MEPS priority conditions, race/ethnicity, and sex. Estimates were weighted to account for the complex survey design of MEPS. The numbers in parentheses are the sample size of each group. Perceived access to general medical care was defined as reporting no delay or inability to obtain medical care, dental care, or prescription medicine in the previous 12 months. The 70 cancer survivors without access to necessary cancer care represent 901 470 adults with cancer in the US population. Error bars indicate 95% confidence intervals.
Figure 2.
Perceived access to a usual source of care among cancer survivors and people without a history of cancer, Medical Expenditure Panel Survey (MEPS), 2011. Analyses were based on the 2011 MEPS, with data drawn from both the core survey and the Experiences With Cancer Survey.25,26 Predicted probabilities were derived from multivariate logistic regression analyses adjusting for current age, education, health insurance, marital status, number of MEPS priority conditions, race/ethnicity, and sex. Estimates were weighted to account for the complex survey design of MEPS. The numbers in parentheses are the sample size of each group. The 70 cancer survivors without access to necessary cancer care represent 901 470 adults with cancer in the US population. Error bars indicate 95% confidence intervals.
Realized Access to General Medical Care
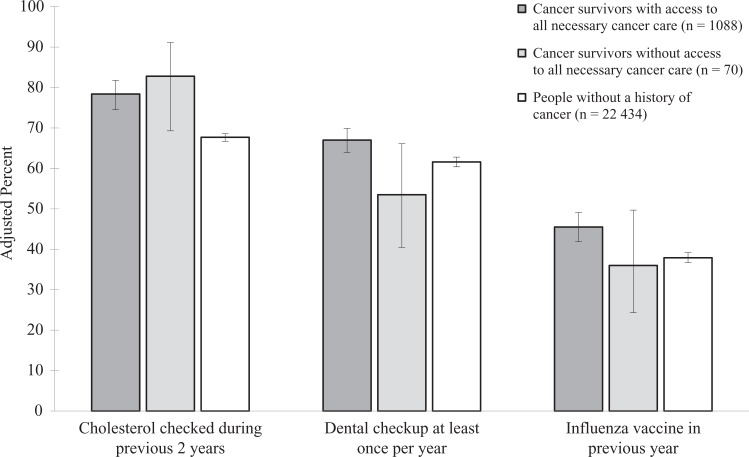
The predicted probability of a cholesterol check in the previous 2 years was 82.8% for cancer survivors who reported not receiving all necessary cancer care, 78.4% for cancer survivors who reported receiving all necessary cancer care, and 67.7% for people with no history of cancer (F 2,21 = 15.83, P < .001). Both cancer survivors who reported having and not having access to necessary cancer care had a higher probability of a cholesterol check in the previous 2 years than people with no history of cancer (P < .001 and P = .007, respectively). The predicted probability of having at least 1 dental checkup per year was 53.5% for cancer survivors who reported not having access to necessary cancer care, 67.0% for cancer survivors who reported having access to necessary cancer care, and 61.6% for people with no history of cancer (F 2,21 = 5.48, P = .005).
Cancer survivors who reported not receiving all necessary cancer care had a lower probability of reporting at least 1 dental checkup in the previous year than cancer survivors who reported receiving all necessary cancer care (P = .05); however, cancer survivors who reported receiving all necessary cancer care had a higher probability of reporting at least 1 dental checkup per year than people with no history of cancer (P < .01). The predicted probability of reporting having an influenza vaccine in the previous year was 36.0% for cancer survivors who reported not having access to necessary cancer care, 45.5% for cancer survivors who reporting having access to necessary cancer care, and 37.9% for people with no history of cancer (F 2,21 = 8.61, P < .001). Cancer survivors who reported receiving all necessary cancer care had a higher probability of reporting having an influenza vaccine in the previous year than people with no history of cancer (P < .001; Figure 3).
Figure 3.
Realized access to general medical care among cancer survivors and people without a history of cancer, Medical Expenditure Panel Survey (MEPS), 2011. Analyses were based on the 2011 MEPS, with data drawn from both the core survey and the Experiences With Cancer Survey.25,26 The figure shows predicted margins derived from multivariate logistic regression analyses adjusting for current age, education, health insurance, marital status, number of MEPS priority conditions, race/ethnicity, and sex. The numbers in parentheses are the sample size of each group. Realized access to general medical care was defined as the self-reported use of preventive services that were recommended with an A or B rating by the US Preventive Services Task Force as of 2011. Error bars indicate 95% confidence intervals.
Discussion
Ninety-four percent of cancer survivors reported receiving the medical care for cancer that they or their doctors considered necessary, suggesting high levels of access to care across that population. However, 6.0% of cancer survivors reported that they did not receive all necessary cancer care, corresponding to an estimated 901 470 adult cancer survivors in the US population. We based access to necessary cancer care on a measure of cancer survivors’ perceived access to care from the point of diagnosis forward. Thus, these data provide a global indicator of cancer care access from the patient’s perspective, which complements other literature in this area.31–34
Cancer survivors who reported not receiving all necessary cancer care had lower levels of education and were more likely to have public insurance or no insurance than cancer survivors who reported receiving all necessary cancer care. Thus, it is not surprising that cost and insurance issues were reported by more than 40% of survivors who did not receive all necessary cancer care. This finding is consistent with other research, suggesting that cancer survivors were more likely than people with no history of cancer to delay or forgo medical services because of cost15,19 and that cancer survivors who reported financial problems stemming from their cancer were more likely to delay or forgo medical care than people with a history of cancer.16 The cost of cancer is considerable and persists beyond the period of acute treatment, leading to medical debt and, in some cases, bankruptcy.35–38 Having health insurance is tied to one’s ability to access affordable health care. In other studies, uninsured cancer survivors were more likely to delay or forgo care and prescription medication because of cost18 and were less likely to have cancer-related medical care in the previous year than cancer survivors with insurance.20 Although insurance is an important determinant of health care access, having health insurance does not always translate to affordable care. Cost sharing in the form of copayments, coinsurance, and deductibles can lead to large out-of-pocket costs, which may limit access to appropriate treatment.17
The Patient Protection and Affordable Care Act addressed some economic barriers to care through provisions aimed at expanding access to health insurance, prohibiting insurers from denying coverage on the basis of a preexisting condition, eliminating lifetime limits on coverage for essential health benefits, capping in-network out-of-pocket costs, and covering standard costs for clinical trials.39 Many provisions of the Affordable Care Act that are relevant to cancer survivors’ access to care were implemented in part or in full after 2011 (the survey year on which this study was based). Thus, this largely pre–Affordable Care Act implementation study is a reference for future work on access to care. Future research should include larger samples to explore whether or not the proportion of cancer survivors who have access to necessary cancer care and general medical care increases from the levels observed in our study. An important part of this research will be to assess whether or not fewer survivors report cost and insurance as barriers to care.
After cancer diagnosis and treatment, cancer survivors need access to ongoing clinical surveillance, symptom management, and preventive services. A usual source of care is important for ensuring that all people receive timely and appropriate medical care. In this study, a higher proportion of cancer survivors than the general population reported having a usual source of care, although differences were significant only between cancer survivors who reported having access to all necessary cancer care and people with no history of cancer. Likewise, cancer survivors who reported receiving all necessary cancer care had equivalent or better realized access to general medical care than people with no history of cancer, possibly because of better insurance coverage and a usual source of care. Thus, factors contributing to suboptimal access to necessary cancer care may also be related to receipt of suboptimal general medical care services.
Cancer survivors with and without access to necessary cancer care were more likely than people with no history of cancer to have their cholesterol checked in the previous 2 years. Cancer survivors had more comorbid conditions (in addition to cancer) than people with no history of cancer; thus, cholesterol may be screened more frequently among cancer survivors than among people with no history of cancer simply because they have more contact with the health care system. Additionally, cancer survivors were more likely than people with no history of cancer to report having a usual source of care, which may also explain these findings.40
Research generally finds that cancer survivors have poorer access to care than people with no history of cancer.15,19,41 However, research suggests that among cancer survivors <65 years of age, those who are uninsured or publicly insured are more likely than the general population to have poorer access to preventive care, whereas privately insured cancer survivors generally have equivalent or better access to care than the general population.7 Furthermore, access to care for cancer survivors aged ≥65 years is better than or equal to access to care for the general population. Although we did not stratify results by age or insurance, our pattern of results is consistent with this research.
Limitations
This study had several limitations. First, access to cancer care was identified by self-report, and information was not available about types of care or delivery setting. Recall bias could have affected the accuracy of the information reported. Second, the number of cancer survivors who did not receive all necessary cancer care was small, although the Agency for Healthcare Research and Quality considers a cell size of 70 adequate for stable estimates.42 Nevertheless, we could not evaluate other preventive services because of power limitations. Likewise, comparisons of the subgroup differences between cancer survivors who did and did not receive all necessary cancer care were underpowered; as such, we may have failed to identify meaningful predictors of access to cancer care.
Third, most participants with a history of cancer in MEPS were long-term cancer survivors, so it was not possible to explore issues in newly diagnosed cancers or cancers with a poor prognosis. Similarly, survivors who died as a result of poor access to health care were not represented in our sample. Finally, the reference period differed among the access-to-care questions. The question about access to cancer care referred to any time after cancer diagnosis, and the questions about access to general medical care referred to the previous 1 to 2 years. The reference period also varied among cancer survivors by time since diagnosis, which may have led us to underestimate the association between access to cancer care and general medical care.
Conclusion
This study provides nationally representative data on the proportion of cancer survivors who had access to necessary cancer care and yields insight into factors that impede survivors’ access to cancer care and general medical care. Although the proportion of cancer survivors not receiving all necessary care was relatively low, the population affected was substantial, and economic factors were the most common barriers reported. To optimize cancer survivors’ health and quality of life, it is important to address factors that impede access to appropriate health care. Future research should include larger samples to explore whether or not the proportion of cancer survivors who have access to necessary cancer care and general medical care increased from the levels observed in this study. An important part of this work will be to assess whether or not fewer cancer survivors report cost and insurance as barriers to care.
Acknowledgments
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the National Cancer Institute, the Centers for Disease Control and Prevention, the American Cancer Society, or the Department of Health and Human Services.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Alfano CM, Rowland JH. Recovery issues in cancer survivorship: a new challenge for supportive care. Cancer J. 2006;12(5):432–443. [DOI] [PubMed] [Google Scholar]
- 2. Keating NL, Nørredam M, Landrum MB, Huskamp HA, Meara E. Physical and mental health status of older long-term cancer survivors. J Am Geriatr Soc. 2005;53(12):2145–2152. [DOI] [PubMed] [Google Scholar]
- 3. Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11)(suppl):2577–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weaver KE, Forsythe LP, Reeve BB, et al. Mental and physical health-related quality of life among US cancer survivors: population estimates from the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21(11):2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Treanor C, Donnelly M. An international review of the patterns and determinants of health service utilisation by adult cancer survivors. BMC Health Serv Res. 2012;12:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Snyder CF, Frick KD, Peairs KS, et al. Comparing care for breast cancer survivors to non-cancer controls: a five-year longitudinal study. J Gen Intern Med. 2009;24(4):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yabroff KR, Short PF, Machlin S, et al. Access to preventive health care for cancer survivors. Am J Prev Med. 2013;45(3):304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101(8):1712–1719. [DOI] [PubMed] [Google Scholar]
- 9. Levit LA, Balogh EP, Nass SJ, Ganz PA, eds. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- 10. Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013;148(6):5165–5173. [DOI] [PubMed] [Google Scholar]
- 11. Badakhshi H, Gruen A, Sehouli J, Budach V, Boehmer D. The impact of patient compliance with adjuvant radiotherapy: a comprehensive cohort study. Cancer Med. 2013;2(5):712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barron TI, Cahir C, Sharp L, Bennett K. A nested case-control study of adjuvant hormonal therapy persistence and compliance, and early breast cancer recurrence in women with stage I-III breast cancer. Br J Cancer. 2013;109(6):1513–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erickson BK, Martin JY, Shah MM, Straughn JM, Jr, Leath CA. 3 rd. Reasons for failure to deliver National Comprehensive Cancer Network (NCCN)–adherent care in the treatment of epithelial ovarian cancer at an NCCN cancer center. Gynecol Oncol. 2014;133(2):142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palmer NR, Geiger AM, Felder TM, Lu L, Case LD, Weaver KE. Racial/ethnic disparities in health care receipt among male cancer survivors. Am J Public Health. 2013;103(7):1306–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kirchhoff AC, Lyles CR, Fluchel M, Wright J, Leisenring W. Limitations in health care access and utilization among long-term survivors of adolescent and young adult cancer. Cancer. 2012;118(23):5964–5972. [DOI] [PubMed] [Google Scholar]
- 16. Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119(20):3710–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dusetzina SB, Winn AN, Abel GA, Huskamp HA, Keating NL. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32(4):306–311. [DOI] [PubMed] [Google Scholar]
- 18. Sabatino SA, Coates RJ, Uhler RJ, Alley LG, Pollack LA. Health insurance coverage and cost barriers to needed medical care among US adult cancer survivors age <65 years. Cancer. 2006;106(11):2466–2475. [DOI] [PubMed] [Google Scholar]
- 19. Weaver KE, Rowland JH, Bellizzi KM, Aziz NM. Forgoing medical care because of cost: assessing disparities in healthcare access among cancer survivors living in the United States. Cancer. 2010;116(14):3493–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keegan TH, Tao L, DeRouen MC, et al. Medical care in adolescents and young adult cancer survivors: what are the biggest access-related barriers? J Cancer Surviv. 2014;8(2):282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. King CJ, Chen J, Dagher RK, Holt CL, Thomas SB. Decomposing differences in medical care access among cancer survivors by race and ethnicity. Am J Med Qual. 2015;30(5):459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists: challenges to assuring access to oncology services. J Oncol Pract. 2007;3(2):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chabner BA. Drug shortages—a critical challenge for the generic-drug market. N Engl J Med. 2011;365(23):2147–2149. [DOI] [PubMed] [Google Scholar]
- 24. Gatesman ML, Smith TJ. The shortage of essential chemotherapy drugs in the United States [published erratum appears in N Engl J Med. 2011;365(25):2441]. N Engl J Med. 2011;365(18):1653–1655. [DOI] [PubMed] [Google Scholar]
- 25. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Medical Expenditure Panel Survey. https://meps.ahrq.gov/mepsweb. Accessed August 9, 2016.
- 26. Yabroff KR, Dowling E, Rodriguez J, et al. The Medical Expenditure Panel Survey (MEPS) experiences with cancer survivorship supplement. J Cancer Surviv. 2012;6(4):407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andersen R, Aday LA. Access to medical care in the US: realized and potential. Med Care. 1978;16(7):533–546. [DOI] [PubMed] [Google Scholar]
- 28. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Medical Expenditure Panel Survey: MEPS HC-147 2011—full year consolidated data file. https://meps.ahrq.gov/mepsweb/data_stats/download_data_files_detail.jsp?cboPufNumber=HC-147. Published September 2013. Accessed August 9, 2016.
- 29. Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55(2):652–659. [DOI] [PubMed] [Google Scholar]
- 30. RTI International. SUDAAN: Release 11.0.1. Research Triangle Park, NC: RTI International; 2016. [Google Scholar]
- 31. Weaver KE, Aziz NM, Arora NK, et al. Follow-up care experiences and perceived quality of care among long-term survivors of breast, prostate, colorectal, and gynecologic cancers. J Oncol Pract. 2014;10(4):e231–e239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schroeck FR, Kaufman SR, Jacobs BL, Hollenbeck BK. Receipt of best care according to current quality of care measures and outcomes in men with prostate cancer. J Urol. 2015;193(2):500–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anderson RT, Morris CR, Kimmick G, et al. Patterns of locoregional treatment for nonmetastatic breast cancer by patient and health system factors. Cancer. 2015;121(5):790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ryoo JJ, Ordin DL, Antonio AL, et al. Patient preference and contraindications in measuring quality of care: what do administrative data miss? J Clin Oncol. 2013;31(21):2716–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pisu M, Azuero A, McNees P, Burkhardt J, Benz R, Meneses K. The out of pocket cost of breast cancer survivors: a review. J Cancer Surviv. 2010;4(3):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jagsi R, Pottow JA, Griffith KA, et al. Long-term financial burden of breast cancer: experiences of a diverse cohort of survivors identified through population-based registries. J Clin Oncol. 2014;32(12):1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood). 2013;32(6):1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guy GP, Jr, Ekwueme DU, Yabroff KR, et al. Economic burden of cancer survivorship among adults in the United States. J Clin Oncol. 2013;31(30):3749–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Virgo KS, Bromberek JL, Glaser A, Horgan D, Maher J, Brawley OW. Health care policy and cancer survivorship. Cancer. 2013;119(suppl 11):2187–2199. [DOI] [PubMed] [Google Scholar]
- 40. VanGompel EC, Jerant AF, Franks PM. Primary care attributes associated with receipt of preventive care services: a national study. J Am Board Fam Med. 2015;28(6):733–741. [DOI] [PubMed] [Google Scholar]
- 41. Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101(8):1712–1719. [DOI] [PubMed] [Google Scholar]
- 42. Machlin SR, Chowdhury SR, Ezzati-Rice T, et al. Estimation Procedures for the 2007 Medical Expenditure Panel Survey Household Component. Rockville, MD: Agency for Healthcare Research and Quality; 2010. [Google Scholar]