Abstract
Objective
Comprehensive studies have investigated the prognostic and clinicopathological value of tumor-associated macrophages (TAMs) in gastric cancer patients, yet results remain controversial. Therefore, we performed a meta-analysis to clarify this issue.
Methods
PubMed, Embase, and the Cochrane Library databases were searched to identify eligible studies. We extracted hazard ratios (HRs) and odds ratios (ORs) with their corresponding 95% confidence intervals (95% CIs) to estimate the effect sizes. In addition, subgroup analysis and sensitivity analysis were also conducted.
Results
A total of 19 studies involving 2242 patients were included. High generalised TAMs density was significantly associated with poor overall survival (OS) (HR 1.49, 95% CI 1.15–1.95). Subgroup analysis revealed that CD68+ TAMs had no significant effect on OS (HR 1.38, 95% CI 1.00–1.91). High M1 TAMs density was correlated with better OS (HR 0.45, 95% CI 0.32–0.65). By contrast, high density of M2 TAMs was correlated with a poor prognosis for OS (HR 1.48, 95% CI 1.25–1.75). Furthermore, high M2 TAMs density was correlated with larger tumor size, diffuse Lauren type, poor histologic differentiation, deeper tumor invasion, lymph node metastasis, and advanced TNM stage.
Conclusions
Overall, this meta-analysis reveal that although CD68+ TAMs infiltration has the neutral prognostic effects on OS, the M1/M2 polarization of TAMs are predicative factor of prognosis in gastric cancer patients.
Introduction
Gastric cancer represents the fifth most common malignancy and the third leading cause of cancer death in the world [1]. Despite recent advances in the diagnosis and medical treatment of gastric cancer, patient survival remains poor, especially for those in the advanced stages of the disease [2]. In addition, it has been reported that the current TNM classification scheme does not adequately reflect the tumor biological behavior and patient prognosis for gastric cancer [3]. Therefore, it is imperative to identify biomarkers to predict tumor progression and patient survival, as well as to provide novel therapeutic targets.
Tumor-associated macrophages (TAMs), as fundamental components of the inflammatory microenvironment of tumors, originate from circulating monocytes and are recruited to the tumor site [4, 5]. Different microenvironments can lead to two different polarizations of TAMs: the classically activated type M1 phenotype and the alternatively activated M2 phenotype. M1 macrophages are considered to be induced by Th1 cytokines (e.g., interferon-γ), microbial stimuli (e.g., lipopolysaccharide) and tumor necrosis factor α, with the function of promoting an inflammatory response and antitumor activity. M2 macrophages are mainly activated by Th2 cytokines (e.g., interleukin 4, interleukin 13), which participate in the anti-inflammatory response, tissue remodeling, angiogenesis and tumor cell activation [5–8].
The role of TAMs in the tumor microenvironment as well as their prognostic value have been widely discussed in many human cancers such as breast [9], lung [10], prostate [11], liver [12] and gastric cancer [13]. However, there exists controversy regarding the impact of TAMs on patient prognosis and clinicopathological characteristics of gastric cancer. Numerous publications have demonstrated that the TAMs density was associated with poor prognosis [13–15]; on the contrary, some studies hold different views [16–18]. Moreover, several articles reported that the polarizing subtypes of TAMs have different prognostic effects [19, 20]. To resolve these inconsistencies as well as to identify more precise prognostic biomarkers, we performed a meta-analysis to evaluate the correlation between TAMs density and its prognostic and clinicopathological significance in patients with gastric cancer.
Materials and Methods
Search strategy and selection criteria
A comprehensive literature search of PubMed, Embase, and the Cochrane Library databases was conducted from their inception through August 17, 2016. The following key words were variably combined: “stomach”, “gastric”, “neoplasm”, “cancer”, “carcinoma”, “tumor”, “macrophage”, “tumor-associated macrophage”, and “tumor-infiltrating macrophage”. Additionally, we also manually checked the reference entries of the relevant literature to minimize any omissions that may have occurred during the search process. This meta-analysis was based on previously published articles; therefore, ethical approval was not required.
To identify eligible studies, the inclusion criteria for this meta-analysis was established as follows: (1) gastric cancer as the target disease, (2) detected macrophage density in primary tumor tissues, (3) correlation of macrophage density with either prognosis (e.g., OS, DFS) or clinicopathological characteristics, (4) sufficient data to extract hazard ratios (HRs), the odds ratio (ORs), and their 95% confidence intervals (CIs), and (5) full text publications in English. Specific types of literature such as reviews, comments and conference abstracts were not included in our meta-analysis. If overlapping patients were reported with different TAMs markers or distribution among the articles, all of the reported incidents were included for different objective analysis. The process of the literature search was independently finished by two authors (Songcheng Yin and Jinyu Huang).
Quality assessment
The Newcastle-Ottawa scale (NOS) [21] was used to evaluate the quality of the original studies. This scale mainly involves three components: patient selection, study comparability and outcome assessment. Each of the included studies obtained a score between 0 and 9. Studies with an NOS score ≥6 were regarded as high quality. Two authors (Songcheng Yin and Zhan Li) independently performed this assessment, and discrepancies were resolved by discussion.
Data extraction
Two reviewers (Songcheng Yin and Jiazi Luo) independently performed the data extraction. The relevant data from the included studies comprised the first author’s name, publication year, country or area, number of patients, age, gender, makers of macrophage, detection methods of macrophage density, cut-off value, clinicopathological parameters, and survival data. Any inconsistencies were resolved through negotiation and consultation.
Statistical analysis
The hazard ratios (HRs) with 95% confidence intervals (CIs) were applied to investigate the association between the TAMs density and survival of patients with gastric cancer. For time-to-event outcomes, HRs and their 95% CIs were given directly in most of the original studies. However, several articles presented Kaplan–Meier curves rather than the HR; therefore, the HR was calculated from the survival curves using the methods reported by Parmar and Tierney [22, 23]. Odds ratios (ORs) with confidence intervals (CIs) were used to evaluate the correlation between the TAMs density and clinicopathological characteristics. A combined HR and OR >1 suggested a worse prognosis in the high TAMs density group and was regarded to be statistically significant if the 95% CI did not overlap 1.
Heterogeneity among the studies was assessed by the Cochran’s Q statistic and I2 tests [24]. Either P<0.10 or I2 statistic >50% defined significant heterogeneity across the articles, in which case the random effects model was performed; otherwise, the fixed effects model was implemented. To find the source of heterogeneity and assess its effect on the outcome of various variables, a subgroup analysis was conducted. In addition, whether the combined results were stable, we performed a sensitivity analysis to gauge this stability. Meanwhile, Begg’s test [25] and Egger’s test [26] regression model were used to test for publication bias. All statistical analysis programs were performed using STATA version 12.0 (Stata, College Station, TX, USA), and all P values were two-sided.
Results
Search results
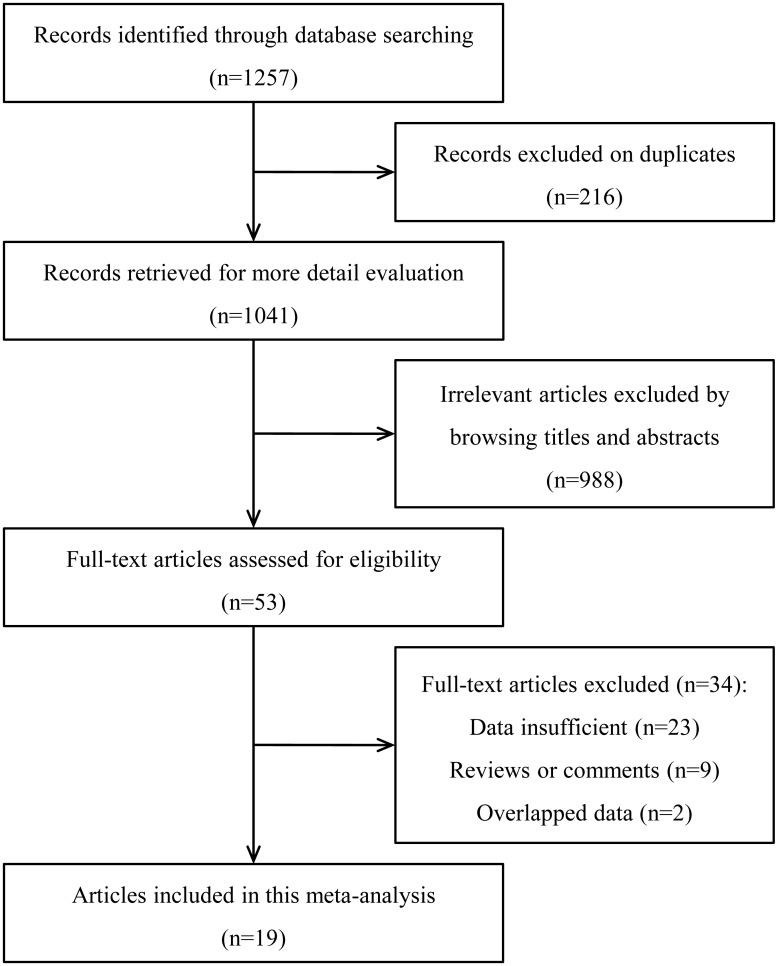
A total of 1257 articles were initially identified in our systematic literature search. Following the exclusion of duplicate publications, 1041 records remained. After screening the titles and abstracts, another 988 articles were excluded. Then, we systematically reviewed the remaining full text articles and precluded another 34 studies because of inconsistencies with the selection criteria. Finally, 19 articles [13–20, 27–37] published between 2003 and 2016 were included in this meta-analysis (Fig 1).
Fig 1. Flow chart of the study selection process.
Study characteristics
Among the included studies, different markers (CD68, CD163, CD204, etc.) were used to label the TAMs types and their localized distribution (e.g., intratumor and stroma). Some participants were enrolled twice for marker-specific or distribution-specific analyses in different articles [13, 28, 36, 37]. Therefore, 2242 gastric cancer patients were included in this meta-analysis. CD68+ was used as an ordinary maker of TAMs in 15 articles. M1 TAMs (labeled by CD11c, and NOS2) and M2 TAMs (labeled by CD163, CD204, and CD206) were reported in 2 and 9 articles, respectively. The fundamental features and study quality of the 19 eligible studies are summarized in Table 1.
Table 1. Characteristics of studies included in the meta-analysis.
| Study | Year | Region | Cases | Stage | Makers | Methods | Cut-off | Outcome | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Zhang [13] | 2016 | China | 178 | I–IV | CD68 | IHC | Score ≥6 | OS | 7 |
| Kim [27] | 2016 | Korea | 396 | I–IV | CD68/CD163 | IHC | NR | OS | 6 |
| Yan [28] | 2016 | China | 178 | I–IV | CD163 | IHC | Score ≥6 | OS | 8 |
| Ichimura [29] | 2016 | Japan | 119 | I–III | CD204 | IHC | Density ≥0.22% | OS | 7 |
| Park [30] | 2016 | Korea | 113 | I–IV | CD163 | IHC | Density ≥77% | OS/DFS | 8 |
| Ding [31] | 2016 | China | 48 | I–IV | CD68 | IHC | NR | - | 6 |
| Lin [17] | 2015 | Taiwan | 170 | Early/ Advanced | CD204 | IHC | Intensity >50% | OS | 6 |
| Zhang [19] | 2015 | China | 180 | I–IV | CD68/CD11c/CD206 | IHC | Density | OS | 8 |
| Kim [32] | 2015 | Korea | 143 | I–III | CD68/CD163 | IHC | Score ≥1 | DFS | 6 |
| Wu [15] | 2015 | Taiwan | 103 | I–IV | CD68 | IHC | ≥671 cells/HPF | OS | 9 |
| Pantano [20] | 2013 | Italy | 52 | I–III | CD68+NOS2/CD68+CD163 | IF | Median score | OS | 9 |
| Peng [33] | 2012 | China | 184 | I–IV | NR | IF | Density >20% | OS | 6 |
| Osinsky [16] | 2011 | Ukraine | 105 | I–IV | CD68 | IHC | Density >23% | OS | 6 |
| Wang [18] | 2011 | China | 107 | T2–T3 | CD68 | IHC | >67.2 cells/HPF | OS | 8 |
| Kawahara [34] | 2010 | Japan | 111 | I–IV | CD68/CD163 | IHC | NR | OS | 7 |
| Haas [35] | 2009 | Germany | 52 | I–IV | CD68 | IHC | Median density | DFS | 8 |
| Ohno [36] | 2005 | Japan | 84 | T2–T3 | CD68 | IHC | Density ≥21.4% | DFS | 9 |
| Ishigami [14] | 2003 | Japan | 97 | I–IV | CD68 | IHC | ≥200 cells/HPF | OS | 7 |
| Ohno [37] | 2003 | Japan | 84 | T2–T3 | CD68 | IHC | Density ≥4.7% | DFS | 9 |
IHC, immunohistochemistry; IF, immunofluorescence; OS, overall survival; DFS, disease-free survival; NOS, Newcastle-Ottawa Scale; HPF, high-power fields; NR, not reported.
TAMs density and OS in gastric cancer patients
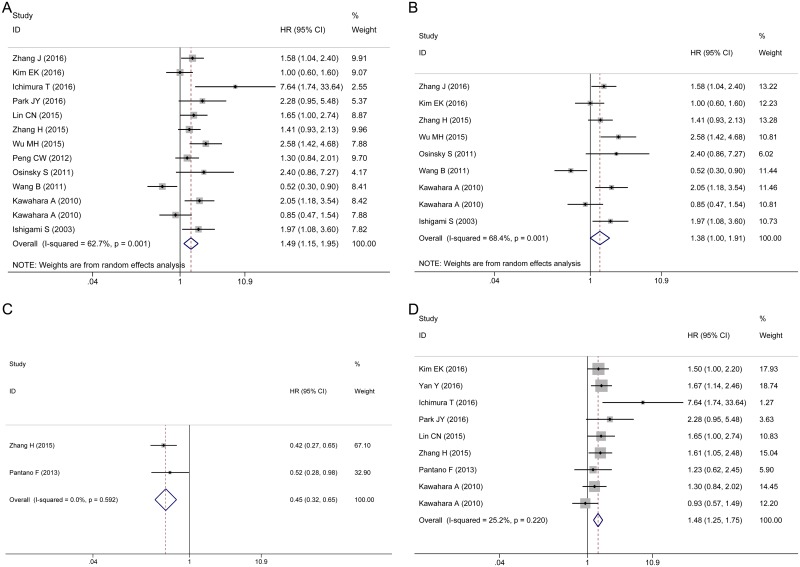
There is a phenomenon that multiple markers were used to estimate the impact of TAMs density on patient survival. When CD68 and other marker data were reported in a single study, we choose CD68, a common macrophage marker, as the indicator of TAMs detection to prevent the incorporation of duplicate samples. The pooled HR showed that a highly generalized TAMs density was significantly associated with poor OS (HR 1.49, 95% CI 1.15–1.95, Fig 2A). Due to the presence of significant heterogeneity among the studies (I2 = 62.7%, P = 0.001), the random effects model was adopted. Among the 12 studies, 11 studies estimated the TAMs density in both the intratumor and stroma regions. While the study by Park [30] respectively assessed intratumoral and stromal TAMs density, we extracted only the intratumoral data. However, the results did not change after removing this study (HR 1.46, 95% CI 1.11–1.92).
Fig 2. Forest plots of HRs for the correlation between TAMs density and OS.
(A) Generalized TAMs. (B) CD68+ TAMs. (C) M1 TAMs. (D) M2 TAMs.
In view of the variety of markers used to detect TAMs density and the existence of substantial heterogeneity, we conducted a subgroup analysis according to the different markers and polarizations. We examined the effect of CD68+, M1 and M2 TAMs on the overall survival of patients with gastric cancer. There was no significant association between the CD68+ TAMs density and OS (HR 1.38, 95% CI 1.00–1.91, P = 0.052, I2 = 68.4%, P = 0.001, random effects model, Fig 2B). A high M1 TAMs density was correlated with better OS (HR 0.45, 95% CI 0.32–0.65, P<0.001, I2 = 0%, P = 0.592, fixed effects model, Fig 2C). Nevertheless, a high density of M2 TAMs was correlated with a poor prognosis for OS (HR 1.48, 95% CI 1.25–1.75, P<0.001, I2 = 25.2%, P = 0.22, fixed effects model, Fig 2D).
TAMs density and DFS in gastric cancer patients
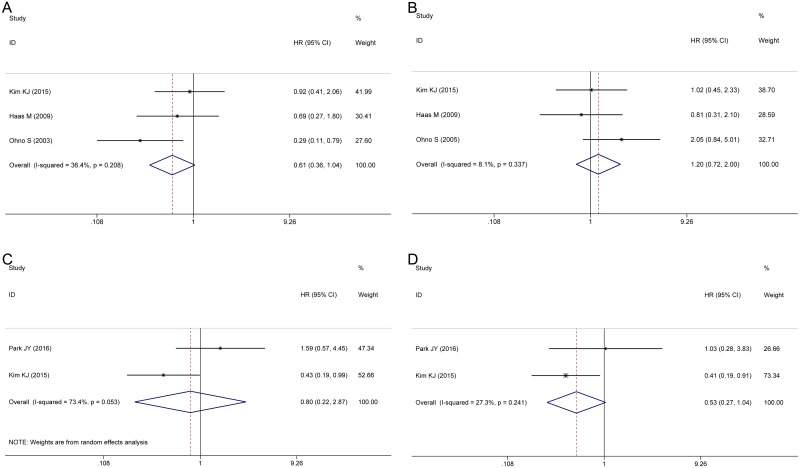
Several studies provided data concerning the association between TAMs infiltration and DFS stratified by different TAMs markers (CD68+ and M2 TAMs) and localized distribution (intratumor and stroma).
The pooled HRs from three studies indicated that there was no association between the CD68+ TAMs density and DFS in either the intratumor (HR 0.61, 95% CI 0.36–1.04, P = 0.068, I2 = 36.4%, P = 0.208, Fig 3A) or stroma (HR 1.20, 95% CI 0.72–2.00, P = 0.458, I2 = 8.1%, P = 0.337, Fig 3B). Two studies estimated the correlation between M2 TAMs and DFS in the intratumor and stroma (Fig 3C and 3D). The pooled HRs showed that the density of M2 TAMs was not correlated with DFS in either the intratumor (HR 0.80, 95% CI 0.22–2.87, P = 0.728, I2 = 73.4%, P = 0.053) or stroma (HR 0.53, 95% CI 0.27–1.04, P = 0.063, I2 = 27.3%, P = 0.241). Due to the limited number of articles, one should be cautious regarding the interpretation of these results.
Fig 3. Forest plots of HRs for the correlation between TAMs density and DFS.
(A) Intratumor CD68+ TAMs. (B) Stroma CD68+ TAMs. (C) Intratumor M2 TAMs. (D) Stroma M2 TAMs.
TAMs density and clinicopathological features
To further elucidate the role of TAMs infiltration on tumor progression, we investigated the correlation between TAMs density and the clinicopathological features of gastric cancer according to different markers and polarizations. Because only one study reported M1 TAMs data, we focused on CD68+ TAMs and M2 TAMs. All of the studies describing the patients’ clinicopathological characteristics detected TAMs in both the intratumor and stroma except the study by Park [30], in which the intratumoral data were used.
As shown in Table 2, the CD68+ TAMs density was not associated with age (older vs. younger: OR 0.83, 95% CI 0.56–1.24, P = 0.365), gender (male vs. female: OR 0.73, 95% CI 0.53–1.00, P = 0.051), tumor size (large vs. small: OR 1.27, 95% CI 0.89–1.80, P = 0.185), depth of invasion (T3–T4 vs. T1–T2: OR 1.54, 95% CI 0.90–2.63, P = 0.112), lymph node metastasis (present vs. absent: OR 1.67, 95% CI 0.92–3.03, P = 0.093), or Lauren type (diffuse vs. intestinal: OR 1.49, 95% CI 0.77–2.88, P = 0.232). However, a high density of CD68+ TAMs was significantly correlated with advanced TNM stage (III–IV vs. I–II: OR 2.61, 95% CI 1.82–2.73, P<0.001) and poor histological differentiation (poorly vs. well to moderately: OR 1.73, 95% CI 1.00–2.98, P = 0.048).
Table 2. The relationship between TAMs and clinicopathological characteristics.
| Clinicopathological features | No. of studies | Pooled OR | P value | Heterogeneity | Effect model | Publication bias | ||
|---|---|---|---|---|---|---|---|---|
| (95% CI) | I2 (%) | P value | PEgger | PBegg | ||||
| CD68+ TAMs | ||||||||
| Age | 4 | 0.83 (0.56–1.24) | 0.365 | 6.4 | 0.361 | Fixed | 0.590 | 0.734 |
| Gender | 6 | 0.73 (0.53–1.00) | 0.051 | 0.0 | 0.791 | Fixed | 0.564 | 0.452 |
| Tumor size | 4 | 1.27 (0.89–1.80) | 0.185 | 45.1 | 0.141 | Fixed | 0.740 | 1.000 |
| Lauren classifcation | 4 | 1.49 (0.77–2.88) | 0.232 | 63.9 | 0.040 | Random | 0.489 | 0.308 |
| Grade of differentiation | 5 | 1.73 (1.00–2.98) | 0.048 | 53.9 | 0.069 | Random | 0.903 | 1.000 |
| Depth of invasion | 6 | 1.54 (0.90–2.63) | 0.112 | 50.2 | 0.074 | Random | 0.090 | 0.707 |
| Lymph node metastasis | 7 | 1.67 (0.92–3.03) | 0.093 | 69.0 | 0.004 | Random | 0.735 | 1.000 |
| TNM stage | 5 | 2.61 (1.82–3.73) | <0.001 | 31.4 | 0.212 | Fixed | 0.333 | 0.462 |
| M2 TAMs | ||||||||
| Age | 5 | 1.09 (0.60–1.97) | 0.780 | 66.1 | 0.019 | Random | 0.821 | 0.806 |
| Gender | 6 | 0.79 (0.59–1.06) | 0.117 | 0.0 | 0.453 | Fixed | 0.232 | 0.707 |
| Tumor size | 4 | 1.61 (1.17–2.23) | 0.004 | 46.2 | 0.134 | Fixed | 0.530 | 0.734 |
| Lauren classifcation | 5 | 1.52 (1.10–2.11) | 0.012 | 23.7 | 0.264 | Fixed | 0.315 | 0.462 |
| Grade of differentiation | 4 | 2.78 (1.94–3.97) | <0.001 | 16.9 | 0.307 | Fixed | 0.457 | 0.734 |
| Depth of invasion | 5 | 2.56 (1.24–5.28) | 0.011 | 75.2 | 0.003 | Random | 0.453 | 1.000 |
| Lymph node metastasis | 6 | 2.17 (1.40–3.38) | 0.001 | 52.9 | 0.059 | Random | 0.788 | 1.000 |
| TNM stage | 6 | 2.26 (1.32–3.87) | 0.003 | 67.5 | 0.009 | Random | 0.828 | 1.000 |
In addition, the pooled analysis revealed no significant association between M2 TAMs and age (older vs. younger: OR 1.09, 95% CI 0.60–1.97, P = 0.780) or gender (male vs. female: OR 0.79, 95% CI 0.59–1.06, P = 0.117). Nevertheless, a high M2 TAMs density was correlated with several clinical parameters, including tumor size (large vs. small: OR 1.61, 95% CI 1.17–2.23, P = 0.004), depth of invasion (T3–T4 vs. T1–T2: OR 2.56, 95% CI 1.24–5.28, P = 0.011), lymph node metastasis (present vs. absent: OR 2.17, 95% CI 1.40–3.38, P = 0.001), TNM stage (III–IV vs. I–II: OR 2.26, 95% CI 1.32–3.87, P = 0.003), Lauren type (diffuse vs. intestinal: OR 1.52, 95% CI 1.10–2.11, P = 0.012), and histological differentiation (poorly vs. well to moderately: OR 2.78, 95% CI 1.94–3.97, P<0.001) (Table 2).
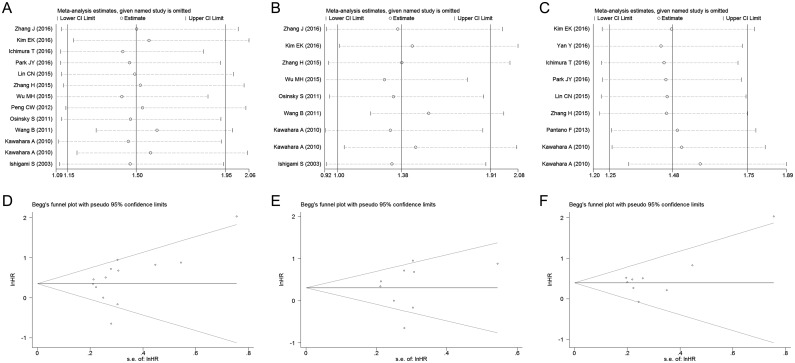
Sensitivity analysis
We conducted a sensitivity analysis by removing each individual study to evaluate the effect of individual datasets on the pooled HRs and ORs. The results shown in Fig 4A–4C indicated that the pooled HRs for OS was not substantially changed. Similarly, our findings of the pooled ORs for the clinicopathological characteristics were also robust (S1 and S2 Figs).
Fig 4. Sensitivity analysis of TAMs on OS and funnel plots of publication bias in analysis of OS.
(A) Sensitivity analysis of generalised TAMs. (B) Sensitivity analysis of CD68+ TAMs. (C) Sensitivity analysis of M2 TAMs. (D) Funnel plot for generalised TAMs on OS. (E) Funnel plot for CD68+ TAMs. (F) Funnel plot for M2 TAMs.
Publication bias
Both Begg’s test and Egger’s test were applied to estimate the publication bias. The P values from these two tests suggested no evidence of publication bias on TAMs and OS (generalized TAMs: PBegg = 0.127, PEgger = 0.152, Fig 4D; CD68+ TAMs: PBegg = 0.602, PEgger = 0.686, Fig 4E; M2 TAMs: PBegg = 0.466, PEgger = 0.145, Fig 4F). The publication bias of M1 TAMs on OS and TAMs on DFS were not performed because of the small number of available articles. Moreover, all of the instances of P>0.05 (Begg’s test and Egger’s test) indicated that the assessment of publication bias was not significant in analysis of the clinicopathological features (Table 2).
Discussion
It is well known that Hanahan and Weinberg emphasized the central role of tumor cells in tumor progression based on changes in the intracellular signaling of the tumor [38]. After a decade, they published another important review to further propose the key role of the tumor microenvironment in the occurrence and development of tumors [39]. The tumor microenvironment is mainly composed of tumor cells, immune cells, the extracellular matrix, and cytokines [40]. Recently, immunotherapy targeting immunosuppressive proteins such as cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed cell death 1 (PD-1) has provided more treatment options for cancer patients [41, 42]. Because the composition of the tumor microenvironment and the interactions among the molecules within this setting are complex, exploring novel targets for combination therapy is still crucial. TAMs, as important members of the tumor microenvironment, have two polarization subtypes: M1 and M2. M1 TAMs function by combating pathogens and tumor cells [7, 43]. In contrast, M2 TAMs are involved in promoting tumor progression. M2 TAMs not only inhibit immune response by producing TGF-β and IL-10 but also produce a variety of enzymes to degrade the matrix, which promotes the dissolution of the matrix membrane, interstitial digestion and remodeling. Moreover, they produce cytokines (e.g., VEGF, PDGF) that participate in angiogenesis and lymphatic vessel formation. Therefore, TAMs themselves and their polarization mechanism are regarded as novel therapeutic targets for cancer patients [4, 7, 44].
Indeed, the density of TAMs was found to be involved in the prognosis of various cancers. Nonomura suggested that a higher TAMs density correlated with poor recurrence-free survival in patients with prostate cancer [11]. Mei reported that high levels of M1 or M2 were associated with good or poor survival, respectively, in patients with non-small cell lung cancer [45]. However, there are still inconsistent prognostic data of TAMs and their polarization subtypes in gastric cancer. Therefore, we performed this meta-analysis to evaluate the prognostic impact and clinicopathological significance of TAMs in patients with gastric cancer.
We primarily assessed the association between generalized TAMs density and overall survival. The overall analysis showed that high density of generalized TAMs predicts a poor OS. Subgroup analysis indicated that the CD68+ TAMs density had no significant association with OS. However, a high M1 TAMs density was significantly correlated with better OS. In contrast, a high density of M2 TAMs was significantly correlated with a poor OS. It is well known that TAMs are a heterogeneous group of immune cells. The M1 and M2 phenotypes are more accurate descriptors of TAMs after polarization. A subgroup analysis revealed completely different significantly prognostic effects of both phenotypes, which was consistent with the function of M1 and M2 TAMs regarding anti- and pro-tumor progression, respectively. However, CD68 is a common marker that identifies both M1 and M2 TAMs and cannot reflect the TAMs polarization subtypes. The reason that CD68+ TAMs are not reliable as a prognostic marker in our analysis might be because of the neutralization of the M1 and M2 prognostic effects. Taking this into account, the relationship between CD68 and OS was not critically significant (P = 0.052). CD68 as a common marker of TAMs, whose relationship with OS was inconsistent with the result of generalized TAMs. Therefore, the result that high generalized TAMs density was associated with OS might be not robust. However, the M1 or M2 TAMs are predicative factor of prognosis in gastric cancer patients.
Moreover, neither CD68+ TAMs nor M2 TAMs was associated with DFS. However, these results were merely derived from three studies and two studies, respectively. We anticipate further research in this area to evaluate the relationship between TAMs and DFS. In the aspect of the analysis of clinicopathological features, a high CD68+ TAMs density was associated with an advanced TNM stage and poor histological differentiation. A high M2 TAMs density was correlated with larger tumor size, deeper tumor invasion, lymph node metastasis, advanced TNM stage, diffuse Lauren type, and poor histological differentiation. In general, a high density of M2 TAMs can signify poor clinicopathological characteristics in patients with gastric cancer.
The tumor promoting activity observed by TAMs can be attributed to the function of M2 TAMs [46–48]. Many researchers have reported the characteristics of M2 TAMs in the tumor progression of different malignant tumors. Intraperitoneal TAMs that polarized towards the M2 phenotype facilitated peritoneal dissemination in gastric cancer by introducing peritoneal mesothelial cell injury and promoting tumor cell proliferation [49, 50]. Zhang et al. reported that M2 TAMs displayed the ability to induce the expression of VEGF-C in Lewis lung carcinoma cells and to increase lymphangiogenesis [51]. In human basal cell carcinoma (BCC), M2 TAMs enhanced the potential of invasion and angiogenesis through a COX-2-dependent pathway, resulting in the elevated release of VEGF-A, bFGF and MMP-9 from BCC cells [52]. In our meta-analysis, we also observed the poor prognostic impact of M2 TAMs in gastric cancer. In addition, corosolic acid, a triterpenoid compound, has been shown to significantly inhibit macrophage polarization into the M2 phenotype and suppress subcutaneous tumor development and lung metastasis in a murine cancer model [7]. As a result, targeted therapy based on this mechanism has the potential to be applied clinically, and accordingly, gastric cancer patients with a high M2 TAMs density may obtain a survival benefit from this approach.
Previously, there were two meta-analysis involving the density of TAMs and the prognosis of gastric cancer. Zhang et al. reported that TAMs had a negative effect on OS in gastric cancer, but only 5 studies were included in this analysis [53]. The other publication by Liu et al. summarized from 11 studies and showed no association between TAMs and OS in gastric cancer [54]. There was a clear inconsistency between the results of these two publications. To clarify this confusion, we conducted this meta-analysis. Comparing with the previous meta-analyses, we used a broad search strategy to systematically search electronic databases and manually scanned reference entries of relevant literature. As a result, the 19 eligible studies included in our meta-analysis. Our original goal was to assess the prognostic value of generalized TAMs density in gastric cancer patients. Nevertheless, taking into account the complexity of TAMs, it might be not reliable to evaluate the relationship between generalized TAMs and OS simply. Thus, we performed stratified analysis according to the polarization of TAMs, which was not conducted in previous articles. And the results showed that completely different significantly prognostic effects of M1 and M2 TAMs, respectively. The inconsistence of previous meta-analysis might be due to huge heterogeneity of TAMs. We performed specifically concentrate on the prognostic value of the TAMs subtypes, which has made our meta-analysis more constructive and advisable.
However, some limitations exist in our study. First, there was significant heterogeneity among the analysis of CD68+ TAMs on OS and TAMs on clinicopathological features. Nevertheless, it is well known that heterogeneity among the studies exists when conducting meta-analysis of observational studies [55, 56]. In our meta-analysis it might be derived from the differences in sample size, demographic data, tumor location, EBV status and experimental technique. We adopted a more conservative approach and used the random effects model if there was significant heterogeneity. Second, the included studies used different types of antibodies or dilution ratios even if detecting the same TAMs marker. In addition, there was no international unification cutoff value to identify the density of the TAMs. Third, the number of the included studies was not enough to analyze the prognostic role of M1 TAMs, which was in turn weakened the power of the results.
In summary, our findings reveal that although CD68+ TAMs infiltration has the neutral prognostic effects on OS, the M1/M2 polarization of TAMs are predicative factor of prognosis in gastric cancer patients. Additional well-designed studies, especially multicenter and randomized controlled trials, are warranted to confirm our results and would provide more valuable prognostic information for gastric cancer patients.
Supporting Information
(DOC)
(A) Patient's age. (B) Gender. (C) Tumor size. (D) Lauren classification. (E) Grade of differentiation. (F) Depth of invasion. (G) Lymph node metastasis. (H) TNM stage.
(TIF)
(A) Patient's age. (B) Gender. (C) Tumor size. (D) Lauren classification. (E) Grade of differentiation. (F) Depth of invasion. (G) Lymph node metastasis. (H) TNM stage.
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by National Natural Science Foundation of China (No. 81372550); and Key Laboratory Programme of Liaoning Province (LZ2015080). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136(5):E359–86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA: a cancer journal for clinicians. 2014;64(4):252–71. [DOI] [PubMed] [Google Scholar]
- 3.Sawada T, Yashiro M, Sentani K, Oue N, Yasui W, Miyazaki K, et al. New molecular staging with G-factor supplements TNM classification in gastric cancer: a multicenter collaborative research by the Japan Society for Gastroenterological Carcinogenesis G-Project committee. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2015;18(1):119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. 10.1016/j.immuni.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Seminars in immunopathology. 2013;35(5):585–600. 10.1007/s00281-013-0367-7 [DOI] [PubMed] [Google Scholar]
- 6.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, et al. Macrophage polarization in tumour progression. Seminars in cancer biology. 2008;18(5):349–55. 10.1016/j.semcancer.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 7.Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Advanced drug delivery reviews. 2016;99(Pt B):180–5. 10.1016/j.addr.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Locati M. Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: lessons and open questions. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(7):1478–83. Epub 2013/06/15. 10.1161/ATVBAHA.113.300168 [DOI] [PubMed] [Google Scholar]
- 9.Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. Tumour-infiltrating macrophages and clinical outcome in breast cancer. Journal of clinical pathology. 2012;65(2):159–63. 10.1136/jclinpath-2011-200355 [DOI] [PubMed] [Google Scholar]
- 10.Zhang BC, Gao J, Wang J, Rao ZG, Wang BC, Gao JF. Tumor-associated macrophages infiltration is associated with peritumoral lymphangiogenesis and poor prognosis in lung adenocarcinoma. Medical oncology (Northwood, London, England). 2011;28(4):1447–52. Epub 2010/08/03. [DOI] [PubMed] [Google Scholar]
- 11.Nonomura N, Takayama H, Nakayama M, Nakai Y, Kawashima A, Mukai M, et al. Infiltration of tumour-associated macrophages in prostate biopsy specimens is predictive of disease progression after hormonal therapy for prostate cancer. BJU international. 2011;107(12):1918–22. Epub 2010/11/04. 10.1111/j.1464-410X.2010.09804.x [DOI] [PubMed] [Google Scholar]
- 12.Li YW, Qiu SJ, Fan J, Gao Q, Zhou J, Xiao YS, et al. Tumor-infiltrating macrophages can predict favorable prognosis in hepatocellular carcinoma after resection. Journal of cancer research and clinical oncology. 2009;135(3):439–49. Epub 2008/09/11. 10.1007/s00432-008-0469-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Yan Y, Yang Y, Wang L, Li M, Wang J, et al. High Infiltration of Tumor-Associated Macrophages Influences Poor Prognosis in Human Gastric Cancer Patients, Associates With the Phenomenon of EMT. Medicine. 2016;95(6):e2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Okumura H, Matsumoto M, et al. Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer research. 2003;23(5A):4079–83. [PubMed] [Google Scholar]
- 15.Wu MH, Lee WJ, Hua KT, Kuo ML, Lin MT. Macrophage Infiltration Induces Gastric Cancer Invasiveness by Activating the beta-Catenin Pathway. PloS one. 2015;10(7):e0134122 10.1371/journal.pone.0134122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osinsky S, Bubnovskaya L, Ganusevich I, Kovelskaya A, Gumenyuk L, Olijnichenko G, et al. Hypoxia, tumour-associated macrophages, microvessel density, VEGF and matrix metalloproteinases in human gastric cancer: interaction and impact on survival. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2011;13(2):133–8. Epub 2011/02/18. [DOI] [PubMed] [Google Scholar]
- 17.Lin CN, Wang CJ, Chao YJ, Lai MD, Shan YS. The significance of the co-existence of osteopontin and tumor-associated macrophages in gastric cancer progression. BMC cancer. 2015;15:128 10.1186/s12885-015-1114-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B, Xu D, Yu X, Ding T, Rao H, Zhan Y, et al. Association of intra-tumoral infiltrating macrophages and regulatory T cells is an independent prognostic factor in gastric cancer after radical resection. Annals of surgical oncology. 2011;18(9):2585–93. Epub 2011/02/25. 10.1245/s10434-011-1609-3 [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Wang X, Shen Z, Xu J, Qin J, Sun Y. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2015;18(4):740–50. Epub 2014/09/19. [DOI] [PubMed] [Google Scholar]
- 20.Pantano F, Berti P, Guida FM, Perrone G, Vincenzi B, Amato MM, et al. The role of macrophages polarization in predicting prognosis of radically resected gastric cancer patients. Journal of cellular and molecular medicine. 2013;17(11):1415–21. 10.1111/jcmm.12109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 22.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in medicine. 1998;17(24):2815–34. [DOI] [PubMed] [Google Scholar]
- 23.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16 Epub 2007/06/09. 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics (Oxford, England). 2005;21(18):3672–3. Epub 2005/06/16. [DOI] [PubMed] [Google Scholar]
- 25.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim EK, Yoon SO, Jung WY, Lee H, Kang Y, Jang YJ, et al. Implications of NOVA1 suppression within the microenvironment of gastric cancer: association with immune cell dysregulation. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2016. Epub 2016/06/20. [DOI] [PubMed] [Google Scholar]
- 28.Yan Y, Zhang J, Li JH, Liu X, Wang JZ, Qu HY, et al. High tumor-associated macrophages infiltration is associated with poor prognosis and may contribute to the phenomenon of epithelial-mesenchymal transition in gastric cancer. OncoTargets and therapy. 2016;9:3975–83. 10.2147/OTT.S103112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichimura T, Abe H, Morikawa T, Yamashita H, Ishikawa S, Ushiku T, et al. Low density of CD204-positive M2-type tumor-associated macrophages in Epstein-Barr virus-associated gastric cancer: a clinicopathologic study with digital image analysis. Human pathology. 2016;56:74–80. 10.1016/j.humpath.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 30.Park JY, Sung JY, Lee J, Park YK, Kim YW, Kim GY, et al. Polarized CD163+ tumor-associated macrophages are associated with increased angiogenesis and CXCL12 expression in gastric cancer. Clinics and research in hepatology and gastroenterology. 2016;40(3):357–65. 10.1016/j.clinre.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 31.Ding H, Zhao L, Dai S, Li L, Wang F, Shan B. CCL5 secreted by tumor associated macrophages may be a new target in treatment of gastric cancer. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2016;77:142–9. [DOI] [PubMed] [Google Scholar]
- 32.Kim KJ, Wen XY, Yang HK, Kim WH, Kang GH. Prognostic Implication of M2 Macrophages Are Determined by the Proportional Balance of Tumor Associated Macrophages and Tumor Infiltrating Lymphocytes in Microsatellite-Unstable Gastric Carcinoma. PloS one. 2015;10(12):e0144192 10.1371/journal.pone.0144192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng CW, Tian Q, Yang GF, Fang M, Zhang ZL, Peng J, et al. Quantum-dots based simultaneous detection of multiple biomarkers of tumor stromal features to predict clinical outcomes in gastric cancer. Biomaterials. 2012;33(23):5742–52. 10.1016/j.biomaterials.2012.04.034 [DOI] [PubMed] [Google Scholar]
- 34.Kawahara A, Hattori S, Akiba J, Nakashima K, Taira T, Watari K, et al. Infiltration of thymidine phosphorylase-positive macrophages is closely associated with tumor angiogenesis and survival in intestinal type gastric cancer. Oncology reports. 2010;24(2):405–15. [DOI] [PubMed] [Google Scholar]
- 35.Haas M, Dimmler A, Hohenberger W, Grabenbauer GG, Niedobitek G, Distel LV. Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC gastroenterology. 2009;9:65 10.1186/1471-230X-9-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohno S, Inagawa H, Dhar DK, Fujii T, Ueda S, Tachibana M, et al. Role of tumor-associated macrophages (TAM) in advanced gastric carcinoma: the impact on FasL-mediated counterattack. Anticancer research. 2005;25(1B):463–70. [PubMed] [Google Scholar]
- 37.Ohno S, Inagawa H, Dhar DK, Fujii T, Ueda S, Tachibana M, et al. The degree of macrophage infiltration into the cancer cell nest is a significant predictor of survival in gastric cancer patients. Anticancer research. 2003;23(6d):5015–22. Epub 2004/02/26. [PubMed] [Google Scholar]
- 38.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. [DOI] [PubMed] [Google Scholar]
- 39.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 40.Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. Journal of cellular biochemistry. 2007;101(4):805–15. 10.1002/jcb.21159 [DOI] [PubMed] [Google Scholar]
- 41.Merelli B, Massi D, Cattaneo L, Mandala M. Targeting the PD1/PD-L1 axis in melanoma: biological rationale, clinical challenges and opportunities. Critical reviews in oncology/hematology. 2014;89(1):140–65. 10.1016/j.critrevonc.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 42.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12(4):252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. The Journal of experimental medicine. 2015;212(4):435–45. 10.1084/jem.20150295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer cell. 2015;27(4):462–72. 10.1016/j.ccell.2015.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mei J, Xiao Z, Guo C, Pu Q, Ma L, Liu C, et al. Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: A systemic review and meta-analysis. Oncotarget. 2016;7(23):34217–28. 10.18632/oncotarget.9079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. The Journal of pathology. 2002;196(3):254–65. 10.1002/path.1027 [DOI] [PubMed] [Google Scholar]
- 47.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nature reviews Cancer. 2004;4(1):71–8. 10.1038/nrc1256 [DOI] [PubMed] [Google Scholar]
- 48.Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer science. 2014;105(1):1–8. 10.1111/cas.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaguchi T, Fushida S, Yamamoto Y, Tsukada T, Kinoshita J, Oyama K, et al. Tumor-associated macrophages of the M2 phenotype contribute to progression in gastric cancer with peritoneal dissemination. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2016;19(4):1052–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu XY, Miao ZF, Zhao TT, Wang ZN, Xu YY, Gao J, et al. Milky spot macrophages remodeled by gastric cancer cells promote peritoneal mesothelial cell injury. Biochemical and biophysical research communications. 2013;439(3):378–83. 10.1016/j.bbrc.2013.08.073 [DOI] [PubMed] [Google Scholar]
- 51.Zhang B, Zhang Y, Yao G, Gao J, Yang B, Zhao Y, et al. M2-polarized macrophages promote metastatic behavior of Lewis lung carcinoma cells by inducing vascular endothelial growth factor-C expression. Clinics. 2012;67(8):901–6. 10.6061/clinics/2012(08)08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao YH, Chu CY, et al. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. The Journal of investigative dermatology. 2009;129(4):1016–25. 10.1038/jid.2008.310 [DOI] [PubMed] [Google Scholar]
- 53.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PloS one. 2012;7(12):e50946 10.1371/journal.pone.0050946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu JY, Yang XJ, Geng XF, Huang CQ, Yu Y, Li Y. Prognostic significance of tumor-associated macrophages density in gastric cancer: a systemic review and meta-analysis. Minerva medica. 2016;107(5):314–21. [PubMed] [Google Scholar]
- 55.Rahbari NN, Aigner M, Thorlund K, Mollberg N, Motschall E, Jensen K, et al. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010;138(5):1714–26. Epub 2010/01/27. 10.1053/j.gastro.2010.01.008 [DOI] [PubMed] [Google Scholar]
- 56.Rahbari NN, Bork U, Motschall E, Thorlund K, Buchler MW, Koch M, et al. Molecular detection of tumor cells in regional lymph nodes is associated with disease recurrence and poor survival in node-negative colorectal cancer: a systematic review and meta-analysis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(1):60–70. Epub 2011/11/30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(A) Patient's age. (B) Gender. (C) Tumor size. (D) Lauren classification. (E) Grade of differentiation. (F) Depth of invasion. (G) Lymph node metastasis. (H) TNM stage.
(TIF)
(A) Patient's age. (B) Gender. (C) Tumor size. (D) Lauren classification. (E) Grade of differentiation. (F) Depth of invasion. (G) Lymph node metastasis. (H) TNM stage.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.