Abstract
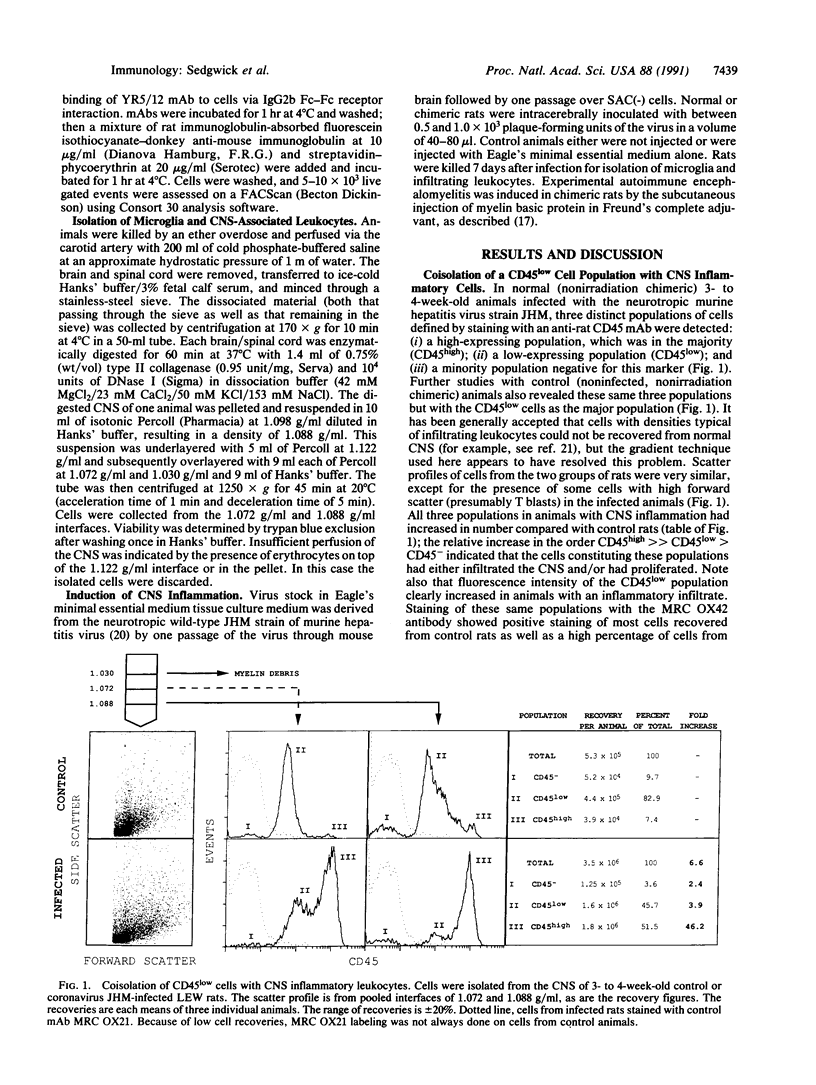
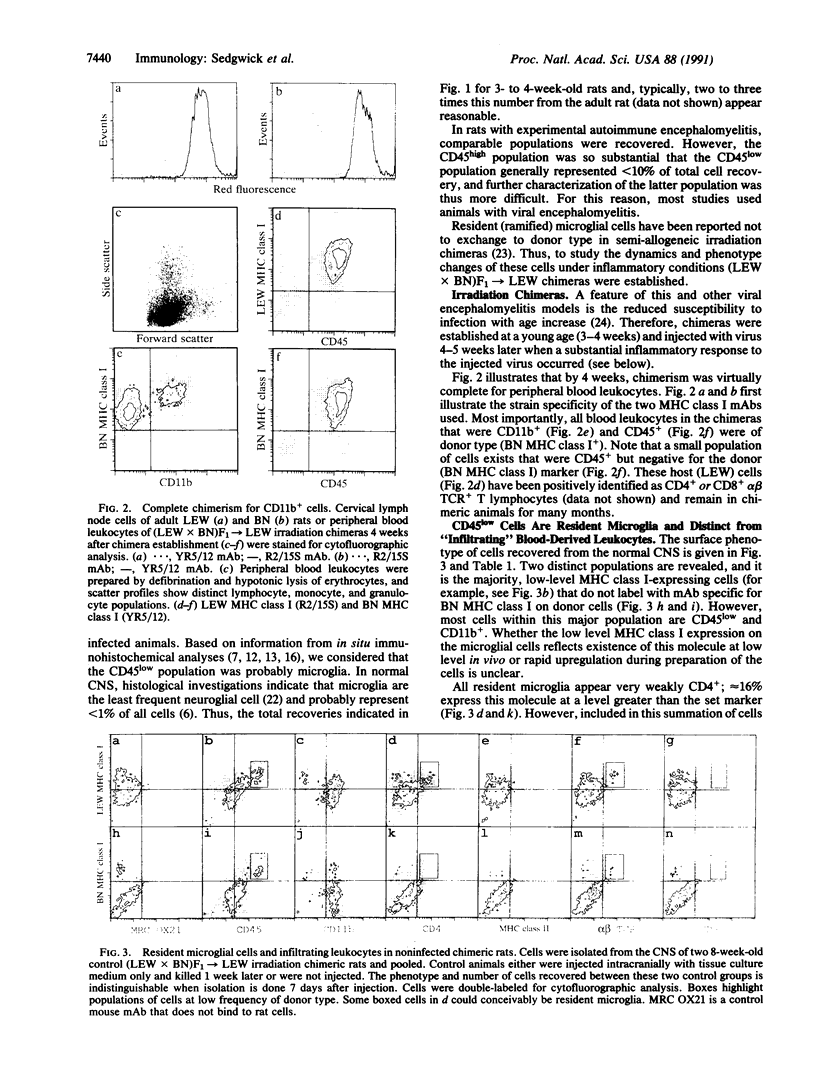
In addition to the major population of infiltrating leukocytes recovered from inflamed rat central nervous system (CNS), all of which expressed high levels of leukocyte common antigen CD45, many cells were coisolated that were MRC OX42+ (complement receptor 3/CD11b) but expressed low-to-moderate levels of CD45 and major histocompatibility complex (MHC) class I molecules. Most cells from normal CNS, in contrast, lay within this latter, CD45low population. From previous in situ immunohistochemical studies, the fortuitously isolated CD45low cells were probably resident (ramified) microglia. Using irradiation chimeras, we show that resident microglia respond to inflammation by upregulating CD45, CD4, and MHC class I molecules with a minority of these cells increasing their expression of MHC class II molecules. A 3- to 4-fold increase in the number of microglia isolated from inflamed CNS provided indirect evidence that the cells had proliferated. In normal CNS, a very small population of blood-derived CD45high-expressing cells are present; most MHC class II expression is associated with these few cells and not with the resident microglia.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butcher G. W. A list of monoclonal antibodies specific for alloantigens of the rat. J Immunogenet. 1987 Apr-Jun;14(2-3):163–176. doi: 10.1111/j.1744-313x.1987.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. A., Essayan D. M., Zweiman B., Lisak R. P. Limiting dilution analysis of the frequency of antigen-reactive lymphocytes isolated from the central nervous system of Lewis rats with experimental allergic encephalomyelitis. Cell Immunol. 1987 Aug;108(1):203–213. doi: 10.1016/0008-8749(87)90204-8. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Allan J. E., Lynch F., Ceredig R. Dissection of an inflammatory process induced by CD8+ T cells. Immunol Today. 1990 Feb;11(2):55–59. doi: 10.1016/0167-5699(90)90019-6. [DOI] [PubMed] [Google Scholar]
- Evans R. L., Faldetta T. J., Humphreys R. E., Pratt D. M., Yunis E. J., Schlossman S. F. Peripheral human T cells sensitized in mixed leukocyte culture synthesize and express Ia-like antigens. J Exp Med. 1978 Nov 1;148(5):1440–1445. doi: 10.1084/jem.148.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A., Fierz W., Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984 Jan 19;307(5948):273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Frei K., Siepl C., Groscurth P., Bodmer S., Fontana A. Immunobiology of microglial cells. Ann N Y Acad Sci. 1988;540:218–227. doi: 10.1111/j.1749-6632.1988.tb27064.x. [DOI] [PubMed] [Google Scholar]
- Graeber M. B., Streit W. J., Kiefer R., Schoen S. W., Kreutzberg G. W. New expression of myelomonocytic antigens by microglia and perivascular cells following lethal motor neuron injury. J Neuroimmunol. 1990 May;27(2-3):121–132. doi: 10.1016/0165-5728(90)90061-q. [DOI] [PubMed] [Google Scholar]
- Hart D. N., Fabre J. W. Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. J Exp Med. 1981 Aug 1;154(2):347–361. doi: 10.1084/jem.154.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes G. M., Woodroofe M. N., Cuzner M. L. Characterisation of microglia isolated from adult human and rat brain. J Neuroimmunol. 1988 Sep;19(3):177–189. doi: 10.1016/0165-5728(88)90001-x. [DOI] [PubMed] [Google Scholar]
- Hayes G. M., Woodroofe M. N., Cuzner M. L. Microglia are the major cell type expressing MHC class II in human white matter. J Neurol Sci. 1987 Aug;80(1):25–37. doi: 10.1016/0022-510x(87)90218-8. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988 Jan 15;239(4837):290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Hinrichs D. J., Wegmann K. W., Dietsch G. N. Transfer of experimental allergic encephalomyelitis to bone marrow chimeras. Endothelial cells are not a restricting element. J Exp Med. 1987 Dec 1;166(6):1906–1911. doi: 10.1084/jem.166.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hünig T., Wallny H. J., Hartley J. K., Lawetzky A., Tiefenthaler G. A monoclonal antibody to a constant determinant of the rat T cell antigen receptor that induces T cell activation. Differential reactivity with subsets of immature and mature T lymphocytes. J Exp Med. 1989 Jan 1;169(1):73–86. doi: 10.1084/jem.169.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawetzky A., Tiefenthaler G., Kubo R., Hünig T. Identification and characterization of rat T cell subpopulations expressing T cell receptors alpha/beta and gamma/delta. Eur J Immunol. 1990 Feb;20(2):343–349. doi: 10.1002/eji.1830200217. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Leblond C. P. Investigation of glial cells in semithin sections. II. Variation with age in the numbers of the various glial cell types in rat cortex and corpus callosum. J Comp Neurol. 1973 May 1;149(1):73–81. doi: 10.1002/cne.901490105. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Fujiwara M. Absence of donor-type major histocompatibility complex class I antigen-bearing microglia in the rat central nervous system of radiation bone marrow chimeras. J Neuroimmunol. 1987 Dec;17(1):71–82. doi: 10.1016/0165-5728(87)90032-4. [DOI] [PubMed] [Google Scholar]
- Paterson D. J., Jefferies W. A., Green J. R., Brandon M. R., Corthesy P., Puklavec M., Williams A. F. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987 Dec;24(12):1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Gordon S. Macrophages and microglia in the nervous system. Trends Neurosci. 1988 Jun;11(6):273–277. doi: 10.1016/0166-2236(88)90110-5. [DOI] [PubMed] [Google Scholar]
- Pryce G., Male D., Sedgwick J. Antigen presentation in brain: brain endothelial cells are poor stimulators of T-cell proliferation. Immunology. 1989 Feb;66(2):207–212. [PMC free article] [PubMed] [Google Scholar]
- Robinson A. P., White T. M., Mason D. W. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986 Feb;57(2):239–247. [PMC free article] [PubMed] [Google Scholar]
- Sedgwick J. D., Hughes C. C., Male D. K., MacPhee I. A., ter Meulen V. Antigen-specific damage to brain vascular endothelial cells mediated by encephalitogenic and nonencephalitogenic CD4+ T cell lines in vitro. J Immunol. 1990 Oct 15;145(8):2474–2481. [PubMed] [Google Scholar]
- Sedgwick J., Brostoff S., Mason D. Experimental allergic encephalomyelitis in the absence of a classical delayed-type hypersensitivity reaction. Severe paralytic disease correlates with the presence of interleukin 2 receptor-positive cells infiltrating the central nervous system. J Exp Med. 1987 Apr 1;165(4):1058–1075. doi: 10.1084/jem.165.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen O., Perry D., Dales S. In vivo and in vitro models of demyelinating diseases. III. JHM virus infection of rats. Arch Neurol. 1980 Aug;37(8):478–484. doi: 10.1001/archneur.1980.00500570026003. [DOI] [PubMed] [Google Scholar]
- Spencer S. C., Fabre J. W. Characterization of the tissue macrophage and the interstitial dendritic cell as distinct leukocytes normally resident in the connective tissue of rat heart. J Exp Med. 1990 Jun 1;171(6):1841–1851. doi: 10.1084/jem.171.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit W. J., Graeber M. B., Kreutzberg G. W. Functional plasticity of microglia: a review. Glia. 1988;1(5):301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979 Feb;9(2):155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Traugott U., Scheinberg L. C., Raine C. S. On the presence of Ia-positive endothelial cells and astrocytes in multiple sclerosis lesions and its relevance to antigen presentation. J Neuroimmunol. 1985 Apr;8(1):1–14. doi: 10.1016/s0165-5728(85)80043-6. [DOI] [PubMed] [Google Scholar]
- Watkins B. A., Dorn H. H., Kelly W. B., Armstrong R. C., Potts B. J., Michaels F., Kufta C. V., Dubois-Dalcq M. Specific tropism of HIV-1 for microglial cells in primary human brain cultures. Science. 1990 Aug 3;249(4968):549–553. doi: 10.1126/science.2200125. [DOI] [PubMed] [Google Scholar]