Abstract
Objective To determine whether there is an increased risk of urinary incontinence in daughters and sisters of incontinent women.
Design Population based cross sectional study.
Setting EPINCONT (the epidemiology of incontinence in the county of Nord-Trøndelag study), a substudy of HUNT 2 (the Norwegian Nord-Trøndelag health survey 2), 1995-7.
Participants 6021 mothers, 7629 daughters, 332 granddaughters, and 2104 older sisters of 2426 sisters.
Main outcome measures Adjusted relative risks for urinary incontinence.
Results The daughters of mothers with urinary incontinence had an increased risk for urinary incontinence (1.3, 95% confidence interval 1.2 to 1.4; absolute risk 23.3%), stress incontinence (1.5, 1.3 to 1.8; 14.6%), mixed incontinence (1.6, 1.2 to 2.0; 8.3%), and urge incontinence (1.8, 0.8 to 3.9; 2.6%). If mothers had severe symptoms then their daughters were likely to have such symptoms (1.9, 1.3 to 3.0; 4.0%). The younger sisters of female siblings with urinary incontinence, stress incontinence, or mixed incontinence had increased relative risks of, respectively, 1.6 (1.3 to 1.9; absolute risk 29.6%), 1.8 (1.3 to 2.3; 18.3%), and 1.7 (1.1 to 2.8; 10.8%).
Conclusion Women are more likely to develop urinary incontinence if their mother or older sisters are incontinent.
Introduction
A genetic predisposition may play a part in the development of urinary incontinence in women, a common condition which is often chronic and burdensome.1,2 We investigated the familial risk of urinary incontinence in the daughters, granddaughters, and sisters of incontinent women.
Methods
Our study, the Norwegian epidemiology of incontinence in the county of Nord-Trøndelag study (EPINCONT), is a substudy of the population based Nord-Trøndelag health survey (HUNT 2) carried out in one Norwegian county during 1995-7. We recruited women from the health survey. These women completed a questionnaire on urinary incontinence. The incontinence study is described in detail elsewhere.3
We identified female relatives from the health survey population by linking data to the kinship registry of Statistics Norway. Participants gave written consent to use their data for research. Among women with information on urinary incontinence, we identified two cohorts of mothers and older sisters and their daughters and sisters, respectively. We did not include half sisters.
When the women reported involuntary loss of urine, we inquired further about their symptoms. We classified incontinence as stress incontinence, urge incontinence, or mixed incontinence. Symptoms were defined as slight, moderate, or severe according to a validated index on the basis of the frequency of incontinence episodes and the amount of leakage at each episode.4
Statistical analysis
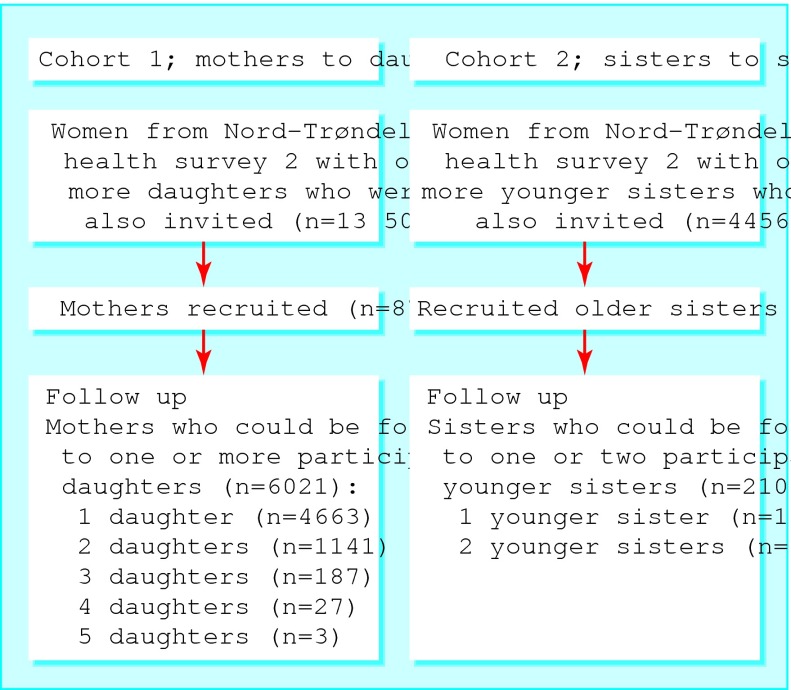
We compared the risk of urinary incontinence in the daughters of incontinent women with that in the daughters of continent women. We also compared the risk of incontinence in the sisters of incontinent older sisters with the risk in sisters of continent older sisters. We estimated relative risks with corresponding 95% confidence intervals from log binomial regression models using the general linear model program in STATA5; this was done because the odds ratios for common conditions obtained from standard regression techniques are not good approximations of relative risks. To account for correlation in data from the same family, we carried out robust estimations of variances and confidence limits with clustering of daughters who were sisters (cohort 1, figure) and clustering of sets of female siblings of older sisters (cohort 2). We adjusted for age, body mass index, and number of children of the women at risk.
Figure 1.
Recruitment and follow up of two familial cohorts from Nord-Trøndelag health survey 2, 1995-7
Results
We recruited 8771 of 13 501 (65.0%) mothers and 2866 of 4456 (64.3%) older sisters who were surveyed for the Nord-Trøndelag health survey during 1995-7 (figure). We were able to follow up 68.6% (6021 of 8771) of the mothers (cohort 1, mothers to daughters) and 73.4% (2104 of 2866) of the sisters (cohort 2, sisters to sisters). Table 1 lists the characteristics of the two cohorts.
Table 1.
Characteristics of two cohorts of women. Values are numbers (percentages) of women unless stated otherwise
|
Cohort 1 |
Cohort 2 |
|||
|---|---|---|---|---|
| Characteristic | Mothers | Daughters | Oldest sisters | Younger sisters |
| Median (interquartile range) age (years) |
60 (52-69) |
33 (26-40) |
39 (33-45) |
34 (28-40) |
| Median (interquartile range) parity |
3 (2-4) |
2 (0-3) |
2 (2-3) |
2 (1-3) |
| Median (interquartile range) body mass index |
27.0 (24.3-30.1) |
24.2 (22.1-27.1) |
24.7 (22.6-27.8) |
24.2 (22.1-27.1) |
| Type of incontinence: |
||||
| Any* |
2257 (29.6) |
1427 (18.7) |
548 (22.6) |
471 (19.4) |
| Severe* |
678 (8.9) |
152 (2.0) |
72 (3.0) |
57 (2.3) |
| Stress† |
979 (43.4) |
823 (57.7) |
323 (58.9) |
268 (56.9) |
| Urge† |
268 (11.9) |
127 (8.9) |
36 (6.5) |
39 (8.3) |
| Mixed† | 932 (41.3) | 415 (29.1) | 160 (29.1) | 142 (30.1) |
Estimates of prevalence.
Distribution among incontinent women. Values do not add up to 100% because women with unclassifiable incontinence due to lack of information were excluded.
Daughters of mothers with any type of urinary incontinence had a 1.3-fold risk of being incontinent (table 2). The risk for having the same type of incontinence as the mother was slightly higher (urge incontinence was not statistically significant). The relative risk of severe urinary incontinence in the daughters of mothers with severe incontinence was 1.9. The risks were highest for severe stress incontinence and severe mixed incontinence. We could not calculate risk estimates for severe urge incontinence because of small population numbers.
Table 2.
Observed risks of incontinence in daughters of mothers with incontinence. Risks relate to daughters whose mothers were continent
|
Slight, moderate, or severe incontinence |
Severe incontinence |
|||||||
|---|---|---|---|---|---|---|---|---|
| Condition in mother | No of daughters at risk | No (%) incontinent | Crude relative risk (95% CI) | Adjusted relative risk (95% CI)* | No of daughters at risk | No (%) incontinent | Crude relative risk (95% CI) | Adjusted relative risk (95% CI)* |
| Type of incontinence: |
||||||||
| Any |
2257 |
525 (23.3) |
1.39 (1.25 to 1.53) |
1.31 (1.19 to 1.44) |
678 |
27 (4.0) |
2.37 (1.56 to 3.59) |
1.94 (1.26 to 3.00) |
| Stress |
979 |
143 (14.6) |
1.50 (1.26 to 1.79) |
1.52 (1.28 to 1.81) |
201 |
5 (2.5) |
3.58 (1.42 to 8.98) |
2.98 (1.11 to 8.03) |
| Urge |
268 |
7 (2.6) |
1.79 (0.84 to 3.85) |
1.80 (0.83 to 3.92) |
87 |
— |
— |
— |
| Mixed | 932 | 77 (8.3) | 1.74 (1.35 to 2.23) | 1.55 (1.21 to 1.99) | 379 | 8 (2.1) | 2.96 (1.39 to 6.30) | 2.07 (0.92 to 4.64) |
Values for three types do not add up to number at risk for any incontinence as some women had unclassifiable incontinence due to lack of information.
Adjusted for daughters' age, body mass index, and parity.
When we could investigate urinary incontinence in both the daughters and the granddaughters of mothers (322 families), we found no increased risk in the granddaughters if only the mothers were incontinent. When both mothers and daughters had urinary incontinence, however, the risk for incontinence in granddaughters was 2.4 (95% confidence interval 1.1 to 4.3). We were unable to estimate risks according to type of incontinence because too few women could be followed through the three generations.
Female siblings had a 1.6-fold increased risk of urinary incontinence if their older sisters were incontinent (table 3). This risk was not significantly different from that between mothers and daughters (1.3-fold risk; P = 0.07). The risks for stress and mixed incontinence in female siblings of older sisters with similar symptoms were 1.8-fold and 1.7-fold, respectively. Too few sisters had severe or urge incontinence to estimate the risk of these symptoms.
Table 3.
Observed risks of incontinence in younger sisters of older sisters with incontinence (risks relate to younger sisters whose older sisters were continent)
| Type of incontinence in oldest sister | Younger sisters at risk | No (%) of cases | Crude relative risk (95% CI) | Adjusted relative risk (95% CI)* |
|---|---|---|---|---|
| Any |
548 |
161 (29.4) |
1.78 (1.50 to 2.11) |
1.59 (1.34 to 1.89) |
| Stress |
323 |
59 (18.3) |
1.99 (1.51 to 2.62) |
1.77 (1.34 to 2.33) |
| Mixed | 160 | 17 (10.6) | 2.10 (1.33 to 3.33) | 1.74 (1.08 to 2.82) |
[YSH1][E2]* Adjusted for younger sisters' age, body mass index, and parity.
Discussion
Women are at an increased risk of urinary incontinence if their mothers or older sisters are incontinent. In contrast to previous studies,6,7 our study investigated the familial risk of urinary incontinence based on independently self reported information from individual women. Our results are consistent as we found an increased risk both from mothers to daughters and from sisters to younger sisters. The quality of our familial data was ensured because we were able to link the records of the women through an official registry using their unique personal identification numbers.
The prevalence of urinary incontinence among the mothers whose daughters were lost to follow up and among available daughters whose mothers were not recruited was similar to that of women of the same age in previous analyses of the study population. This reduces the likelihood of bias from non-recruitment or loss to follow up.
Reporting bias was possible in our study as women with incontinent relatives may be more knowledgeable about symptoms and therefore more likely to report having the condition than women with continent relatives. Previous research, however, indicates that such bias should have only a marginal effect on the observed risks.8-10
What is already known on this topic
Older age, parity, and high body mass index are established risk factors for urinary incontinence in women
A genetic predisposition may also play a part in the development of this condition
What this study adds
Women are at an increased risk of urinary incontinence if their mothers or older sisters are incontinent
This association is present for both stress and mixed incontinence
Our estimation of relative risks with confidence limits using log binomial models was validated against alternatives, including Poisson regression with robust estimation of confidence limits and transformation from odds ratios using the methods of Zhang and Yu, which provided similar results.5,11
Age and parity are important factors in the development of urinary incontinence. The women in our study who had a familial predisposition for urinary incontinence but were young and nulliparous, may develop incontinence when they have had children and are getting older.1-3 We found, however, no significant differences across different age strata for the development of urinary incontinence.
One strength of our study is that we investigated several types of urinary incontinence, although there may be some discrepancy between symptom scores and urodynamic investigations.12-14 We found that daughters were more likely to develop stress or mixed incontinence if their mother had these conditions as were the sisters of older siblings with these types of urinary incontinence. Urge incontinence is the least common type of incontinence and its prevalence is particularly low among young women.1,3 For this reason we were unable to obtain precise values for urge incontinence in the daughters and sisters in our cohorts.
The symptoms of urinary incontinence are likely to have a complex cause, and known risk factors such as increasing age, pregnancy and childbirth, and high body mass index may further increase the risk among women with a familial predisposition.2,3,15-19
The Nord-Trøndelag health study (HUNT) is a collaboration between the HUNT Research Centre, Faculty of Medicine, Norwegian University of Science and Technology, Verdal; the National Institute of Public Health; the national health screening service of Norway, and Nord-Trøndelag county council.
Contributors: All authors participated in the design of the study, the analyses of the material, the interpretation of the data, and the writing of the paper. YSH is guarantor.
Funding: The epidemiology of incontinence in the county of Nord-Trøndelag study (EPINCONT) was funded by the Research Council of Norway and the University of Bergen. Additional support came from an unrestricted grant from the Lilly Centre for Women's Health, Eli Lilly.
Competing interests: None declared.
Ethical approval: This study was approved by the data inspectorate of Norway and by the regional committee for medical research ethics.
References
- 1.Hunskaar S, Arnold EP, Burgio K, Diokno AC, Herzog AR, Mallett VT. Epidemiology and natural history of urinary incontinence. Int Urogynecol J 2000;11: 301-19. [DOI] [PubMed] [Google Scholar]
- 2.Thom D. Variation in estimates of urinary incontinence prevalence in the community: effects of differences in definition, population characteristics, and study type. J Am Geriatr Soc 1998;46: 473-80. [DOI] [PubMed] [Google Scholar]
- 3.Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. J Clin Epidemiol 2000;53: 1150-7. [DOI] [PubMed] [Google Scholar]
- 4.Sandvik H, Seim A, Vanvik A, Hunskaar S. A severity index for epidemiological surveys of female urinary incontinence: comparison with 48-hour pad-weighing tests. Neurourol Urodyn 2000;19: 137-45. [DOI] [PubMed] [Google Scholar]
- 5.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol 2003;157: 940-3. [DOI] [PubMed] [Google Scholar]
- 6.Elia G, Bergman J, Dye TD. Familial incidence of urinary incontinence. Am J Obstet Gynecol 2002;187: 53-5. [DOI] [PubMed] [Google Scholar]
- 7.Mushkat Y, Bukovsky I, Langer R. Female urinary stress incontinence—does it have familial prevalence? Am J Obstet Gynecol 1996;174: 617-9. [DOI] [PubMed] [Google Scholar]
- 8.Rekers H, Drogendijk AC, Valkenburg H, Riphagen F. Urinary incontinence in women from 35 to 79 years of age: prevalence and consequences. Eur J Obstet Gynecol Reprod Biol 1992;43: 229-34. [DOI] [PubMed] [Google Scholar]
- 9.Hannestad YS, Rortveit G, Hunskaar S. Help-seeking and associated factors in female urinary incontinence. The Norwegian EPINCONT Study. Scand J Prim Health Care 2002;20: 102-7. [PubMed] [Google Scholar]
- 10.Schulman C, Claes H, Matthijs J. Urinary incontinence in Belgium: a population-based epidemiological survey. Eur Urol 1997;32: 315-20. [PubMed] [Google Scholar]
- 11.Zhang J, Yu KF. What's the relative risk?: a method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280: 1690-1. [DOI] [PubMed] [Google Scholar]
- 12.Sandvik H, Hunskaar S, Vanvik A, Bratt H, Seim A, Hermstad R. Diagnostic classification of female urinary incontinence: an epidemiological survey corrected for validity. J Clin Epidemiol 1995;48: 339-43. [DOI] [PubMed] [Google Scholar]
- 13.Jensen JK, Nielsen FJ, Ostergard DR. The role of patient history in the diagnosis of urinary incontinence. Obstet Gynecol 1994;83: 904-10. [PubMed] [Google Scholar]
- 14.FitzGerald MP, Brubaker L. Urinary incontinence symptom scores and urodynamic diagnoses. Neurourol Urodyn 2002;21: 30-5. [DOI] [PubMed] [Google Scholar]
- 15.Thom DH, van den Eeden SK, Brown JS. Evaluation of parturition and other reproductive variables as risk factors for urinary incontinence in later life. Obstet Gynecol 1997;90: 983-9. [DOI] [PubMed] [Google Scholar]
- 16.Rortveit G, Hannestad YS, Daltveit AK, Hunskaar S. Age- and type-dependent effects of parity on urinary incontinence: the Norwegian EPINCONT study. Obstet Gynecol 2001;98: 1004-10. [DOI] [PubMed] [Google Scholar]
- 17.Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S. Urinary incontinence after vaginal delivery or cesarean section. N Engl J Med 2003;348: 900-7. [DOI] [PubMed] [Google Scholar]
- 18.Mommsen S, Foldspang A. Body mass index and adult female urinary incontinence. World J Urol 1994;12: 319-22. [DOI] [PubMed] [Google Scholar]
- 19.Hannestad YS, Rortveit G, Daltveit AK, Hunskaar S. Are smoking and other lifestyle factors associated with female urinary incontinence? The Norwegian EPINCONT Study. BJOG 2003;110: 247-54. [PubMed] [Google Scholar]