Abstract
The fluorophore indocyanine green accumulates in areas of ischemia-reperfusion injury due to an increase in vascular permeability and extravasation of the dye. The aim of the study was to validate an indocyanine green-based technique of in vivo visualization of myocardial infarction. A further aim was to quantify infarct size ex vivo and compare this technique with the standard triphenyltetrazolium chloride staining. Wistar rats were subjected to regional myocardial ischemia (30 minutes) followed by reperfusion (n = 7). Indocyanine green (0.25 mg/mL in 1 mL of normal saline) was infused intravenously for 10 minutes starting from the 25th minute of ischemia. Video registration in the near-infrared fluorescence was performed. Epicardial fluorescence of indocyanine green corresponded to the injured area after 30 minutes of reperfusion. Infarct size was similar when determined ex vivo using traditional triphenyltetrazolium chloride assay and indocyanine green fluorescent labeling. Intravital visualization of irreversible injury can be done directly by fluorescence on the surface of the heart. This technique may also be an alternative for ex vivo measurements of infarct size.
OCIS codes: (300.2530) Fluorescence, laser-induced; (110.3080) Infrared imaging; (170.3880) Medical and biological imaging
1. Introduction
Experimental models of myocardial infarction are widely used to study the mechanisms and pathophysiology of ischemia-reperfusion injury, post-infarction cardiac remodeling and for studies on cardioprotection [1–3]. The consequences of myocardial infarction depend on the severity of the injury and the size of the myocardial infarction. The golden standard of assessing infarct size in experimental studies is the triphenyltetrazolium chloride (TTC) assay, although functional and biochemical tests give additional information as secondary endpoints [2]. The method with TTC staining is based on histo-enzymatic reactions of dehydrogenase enzymes and cofactors in the tissue with TTC to form a formazan pigment [2]. Formazan stains viable myocardium in a red-brick color. However, irreversibly injured myocardium remains unstained, and a clear delineation of viable myocardium from necrosis is created. A limitation of this method is that the results are reliable only after ≥ 60 min of reperfusion [4].
To date, only nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FAD) autofluorescence allows in vivo visualization of the zone of ischemia on the heart surface. This method is based on monitoring of changes in autofluorescence intensity due to myocardial ischemia [5,6]. Although NADH and FAD autofluorescence allows detection of myocardial ischemia, the method’s ability to accurately measure infarct size is not known [7]. Besides, a drawback of this method is that complete darkness is necessary for good visualization. Another method of in vivo measurements is echocardiography. A correlation has been reported between infarct size measured by in vivo echocardiography and ex vivo TTC staining in both mice and rat [8,9]. Unfortunately, the individual variations between the two methods have been considerable. Expensive equipment and a very experienced examiner are also necessary.
Irreversible injury after prolonged myocardial ischemia causes severe functional and structural abnormalities to the microvasculature, with increased microvascular permeability and extravasation of proteins. In more severe cases it may lead to “no-reflow” [10]. This phenomenon has been named “vascular rhexis” [11,12]. The loss of functional integrity of the vessel wall was evident after 90 minutes of ischemia by extravasation of fluorescein isothiocyanate-conjugated albumin-fluorescein (FITC-albumin). Recently Fang et al (2016) demonstrated that ischemia-induced necrosis in skeletal muscle may be demonstrated by another fluorescent dye – indocyanine green (ICG) [13].
We hypothesize that the fluorophore ICG may accumulate in the zone of irreversible ischemia-reperfusion injury of the rat heart in vivo. ICG emits in the near-infrared (NIR) spectral range and is widely used in medicine for a variety of tasks [14]. The popularization of NIR-fluorescence imaging is largely determined by the development of appropriate systems [15–18]. Unlike TTC, ICG can be used for intravital imaging.
Furthermore, we hypothesize that ICG can be used to develop a method of intravital visualization and ex vivo quantification of myocardial irreversible ischemia-reperfusion injury. The objectives of this study were: 1) to prove the possibility of intravital visualization of irreversible ischemia-reperfusion injury using ICG; 2) to compare infarct sizes obtained using the golden standard (TTC staining) and infarct size obtained using ICG-fluorescence technique.
2. Experimental setup and method
The experiments were performed on male Wistar rats weighing 250-300g. The animals were fed standard laboratory rodent chow and given water ad libitum. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the local Ethics Committee.
2.1 In vivo rat model of myocardial ischemia-reperfusion injury
Male Wistar rats were anesthetized by a single intraperitoneal injection of sodium thiopental (60 mg/kg). Body temperature was maintained at 37,0 ± 0,5 ° (ATC1000-220, World Precision Instruments, Inc., USA). The rats were tracheostomized and mechanically ventilated (CWE-SAR-830/ AP, World Precision Instruments, Inc, USA) with 60% oxygen (respiratory rate - 60 / min, tidal volume of - 3 ml / 100 g body weight). Ventilation was adjusted by repeated arterial blood gas analyses throughout the experiment (ABL80FLEX, Radiometer, Denmark). Femoral vein catheterization was performed to maintain anesthesia and infusion of ICG solution. The right common carotid artery was cannulated with a polyethylene tube (PE-50, Intramedic, USA) for blood sample withdrawal, measurement of arterial blood pressure and heart rate. The arterial cannula was connected to a pressure transducer (Baxter, USA). During the experiments, animals had continuous monitoring of hemodynamic parameters (mean blood pressure (BP) and heart rate (HR)) using software PhysExp (LLC “Kardioprotekt”, Russia). For induction of regional ischemia-reperfusion injury, the chest was opened in the fourth intercostal space and the ribs were spread to expose the heart. The pericardium was opened and a 6.0 polypropylene non-traumatic suture was passed around the major branch of the left coronary artery, about 2 mm from its origin [19]. The ends of suture were passed through an occluder (a small polyethylene tube ~6-7 cm; PE-90, Intramedic, USA) and exteriorized. After the end of surgical procedures and 30 minutes stabilizing period, myocardial ischemia was initiated. The occluder was shifted down and locked by a surgical clamp. Registration of hemodynamic parameters was performed before occlusion, five and 15 min after the occlusion, at five minutes of reperfusion and then every 30 minutes until the experiment was finished after 120 minutes of reperfusion.
The following groups were included:
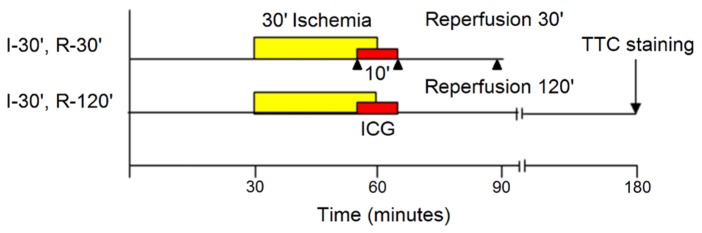
Group I: After 30 minutes of stabilization, ischemia was induced for 30 minutes followed by 30 minutes of reperfusion (Fig. 1). 1 mL of ICG solution was infused intravenously for a total of 10 minutes, beginning five minutes before end of ischemia and continuing during the first five minutes of reperfusion. Intravital video recording of distribution of ICG on the external surface of the heart was performed. At the end of 30-minute reperfusion, the area with irreversible ischemia-reperfusion injury was visualized by the fluorescence of accumulated ICG in the zone of myocardial injury (see below). Then the hearts were harvested (at 30-minute post-ischemia) for ex vivo visualization of ischemia-reperfusion injury by ICG fluorescence (n = 3).
Fig. 1.
Experimental protocol. I – ischemia, R – reperfusion, ICG - indocyanine green, TTC - triphenyltetrazolium chloride, ▲ – time points of imaging.
Group II: The protocol was identical to that of group I, except that reperfusion lasted 120 minutes. At the end of reperfusion, the hearts were harvested and processed for evaluation of infarct size by ICG and TTC staining (see below) (n = 7)
2.2 In vivo visualization of the ischemia-reperfusion injury
To prepare the fluorescence solution ICG (Pulsion medical systems, AG, Germany) was dissolved in distilled water at a final concentration of ICG 0.25 mg/ml. NaCl was added to the ICG solution to get a concentration of 0.9% NaCl.
After 20-25 minutes of ischemia, the ribs were spread to visualize the external surface of the heart for video recording. The surface of the heart was recorded by two types of videos which were saved at the same time: in visible light and in near infrared light (NIR; Fig. 2). 5W diode laser was used with a wavelength of 808 nm, which provides infrared fluorescence excitation of ICG (detailed description of the research complex is in section 2.4). ICG was infused intravenously for 10 minutes at a dose of 0.25 mg/ml as described above.
Fig. 2.
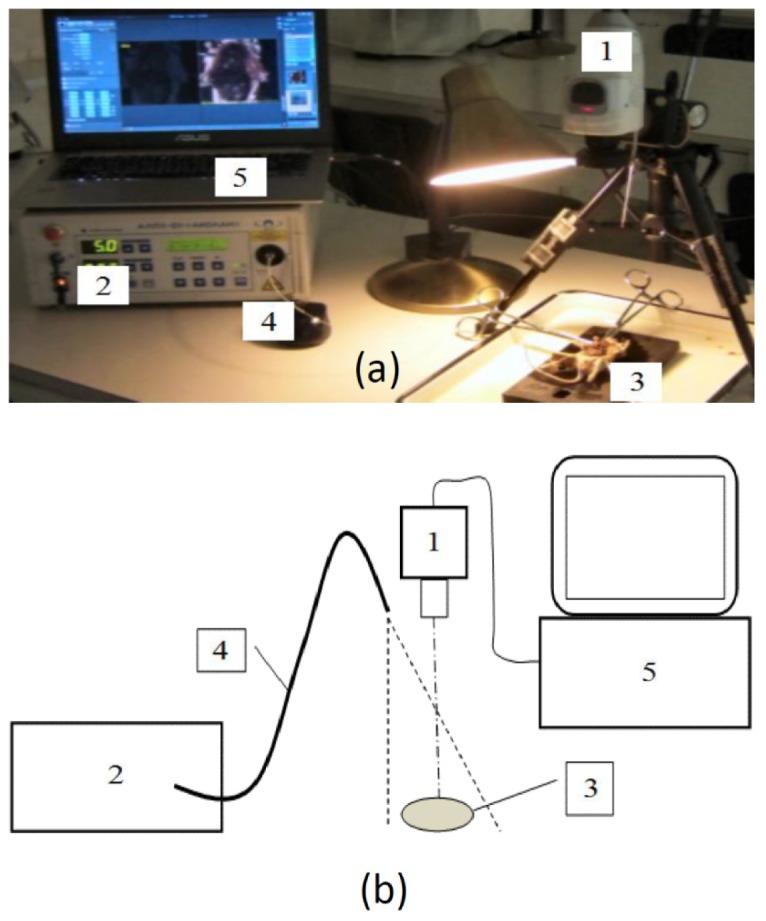
The research complex, infrared imaging system ICG-Scope, which was used for intraoperative visualization of ischemia-reperfusion myocardial injury in an experimental model in vivo. a) – Image of the setup: 1 – television camera, 2 – semiconductor laser, 3 – heart, 4 – fiber illuminator, 5 – PC., b) – Schematic image of the complex.
2.3 Ex vivo visualization of the ischemia-reperfusion injury
At the end of the experiments (after 30 or 120 minutes of reperfusion) the left coronary artery was reoccluded, followed by intravenous administration of 2.5 mL of 2.5% Evans Blue (MP Biomedicals, Santa Ana, CA) for identification of the area at risk (perfusion deficit zone following a coronary artery occlusion). The hearts were excised for photographing of the outer surface followed by the registration of the fluorescence area and intensity of ICG in areas of irreversible ischemia-reperfusion injury and the surrounding undamaged myocardium. Then hearts were cut into five 2 mm thick slices parallel to the atrioventricular groove. The basal surface of each slice was photographed, using a digital camera. To compare results of ICG-fluoroscopy (potential infarct indicator) with TTC assay (infarct indicator after >90 min reperfusion), the slices were immersed in a 1% solution of 2,3,5-triphenyltetrazolium chloride (TTC; TTH, ICN Pharmaceuticals, USA) at 37°C (pH 7.4) for 15 minutes and photographed again for identification of infarct area (TTC-negative sites) and registration of fluorescence area of ICG. The images were analyzed using software ImageJ (https://imagej.nih.gov/ij/). The area at risk was expressed as a percentage of the whole slice, and the infarct area (TTC-negative sites or ICG fluorescence area) was expressed as a percentage of the area at risk. We introduced an arbitrary threshold for measurement of the size of myocardial infarction to delineate nonviable myocardium in the area at risk. The area at risk and area of necrosis of each slice were calculated by an investigator blinded to the results of staining with another dye. Values of the area at risk and infarct size for each heart were obtained by summarizing data for the slices and calculating the percentage.
2.4 Near-infrared fluorescence imaging system
Video registration of the NIR fluorescence was performed by using custom built NIR fluorescence optical imaging system FLUM-808 (Fig. 2) [18]. It includes 5W diode laser with a wavelength of 808 nm, which provides infrared fluorescence excitation, and a multispectral video system ICG-scope, created by the Russian Research Centre in Seoul [20]. The laser beam is directed at the surface of the heart through the optical fiber. The excitation power density at the site was 44mW / cm2. The measurements were made using a power meter QB230 (Advantest Corp, Japan). The camera has two CCD image sensors – black-and-white and color. TV sensors installed after the dichroic prism which separates the light entering the camera in two different directions - infrared and visible (VIS). The color sensor features long-pass edge filter which, basing on the spectral sensitivity of the TV sensor, enables reception of the NIR light in the range 820-950 nm. The color sensor features Bayer filter mosaic RGBG, which allowing the formation of three spectral channels: B (410-500 nm), G (490-585 nm) and R (575-730 nm). NIR and VIS digital images (1280x960) are simultaneously transmitting to a computer via the same USB-3 cable with frequency 25 frames-per-seconds. Image analysis was performed by using a computer program (RSScam, South Korea) that in dual-mode allows simultaneous viewing on the screen of one object in two adjacent picture frames – black-and-white and color. A black-and-white image of the object is formed in NIR fluorescence using laser illumination, while a color picture is representing a normal view of the object when illuminated by a white light source. Color imaging is used for anatomical identification of different areas of the object with infrared fluorescence. Analysis of the obtained image in interactive mode of the program allows measuring NIR fluorescence signal in areas of ICG accumulation caused by irreversible myocardial ischemia-reperfusion injury and comparing with the signal in undamaged areas.
2.5 Statistics
Statistical analyses were performed by GraphPad Prism 5.0 software (GraphPad Software, Inc., California, USA). The Wilcoxon matched pairs test was used to compare medians of two repeated measurements of infarct sizes (TTC and ICG). Data was presented in median with range (min-max). Linear regression was used to test for significant correlation of infarct sizes measured by two methods. p< 0.05 is considered to be statistically significant.
3. Results
No differences in hemodynamic parameters (HR and BP) were observed between the two groups. Neither were any important changes in hemodynamics observed (data not shown).
3.1 ICG fluorescence for in vivo and ex vivo visualization of irreversible ischemia-reperfusion injury
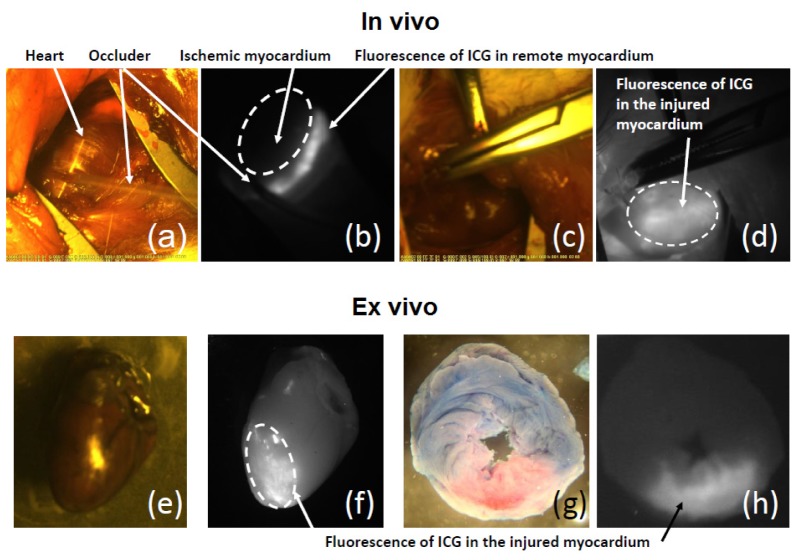
Figure 3 shows example images of anterior and lateral walls of the left ventricle through the fourth intercostal space by fluoroscopic angiography at the time of formation of local ischemia-reperfusion injury (Fig. 3(a)-3(d)). Panels 3(a) and 3(b) shows ICG distribution in the myocardium during ischemia (last five minutes of occlusion). The occluder obstructed blood flow and passage of fluorophore distal to the coronary occlusion. Panel 3(b) shows fluorescent dye-filling defect: no fluorescent emission of ICG in the ischemic zone, while fluorescence was observed in the neighboring or remote myocardium.
Fig. 3.
– Intravital and post-mortem visualization of the ischemic-reperfused area of the heart with ICG. (a), (b) – Perfusion defect immediately after ICG administration; (c), (d) – Intravital epicardial ICG fluorescence; (e), (f) - Epicardial ICG fluorescence in the excised heart; (g), (h) – Transverse heart slices stained with Evans Blue and ICG obtained after 30 min of reperfusion.
At the onset of reperfusion and the remaining five minutes of infusion of ICG, its fluorescence was observed on the entire surface of the heart. clear demarcation from normal surrounding myocardium was found at 20 minutes after the completion of infusion of the fluorescent dye, which was due to the accumulation of the ICG in the zone of irreversible injury (Fig. 3(c), 3(d)). Panel 3(d) shows intense fluorescence distal to the site of coronary occlusion and low fluorescence intensity in the surrounding tissue.
At the end of 30 minutes of reperfusion, the heart was excised and viewed in NIR light in whole and sliced. Plots 3(f) and 3(h) show fluorescence of accumulated ICG in the area at risk with clear demarcation from normal surrounding myocardium at the 30th minute of reperfusion. Within the area at risk (Evans-negative, pink region, Fig. 3(g) accumulation of ICG, was seen (Fig. 3(h)).
3.2 Comparing infarct size evaluated by ICG fluorescence and TTC staining
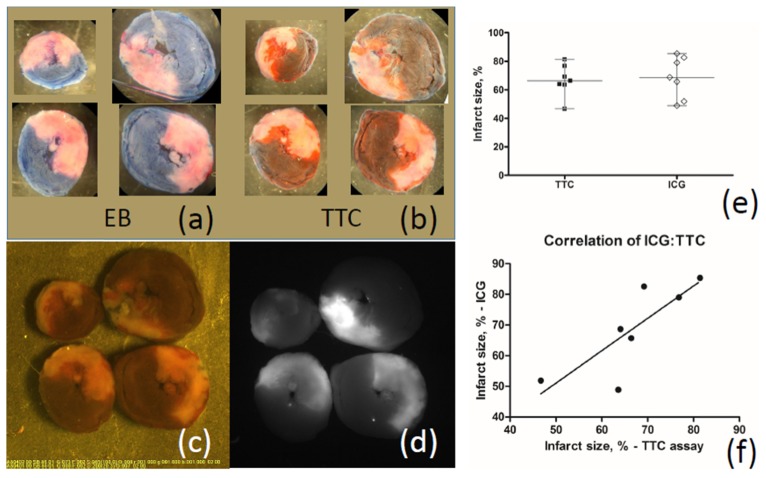
To compare infarct size obtained by TTC staining and ICG-fluorescence technique ex vivo, the length of reperfusion was 120 minutes which is essential for TTC staining. Figure 4 shows increased uptake of ICG (Fig. 4(d)) in areas of irreversible injury within area at risk 43 ± 8% of ventricular mass (Fig. 4(a)) with a matching TTC-negative staining (ischemia-reperfusion injury (IRI)) in the same sections of the rat heart (Fig. 4(b) and 4(c)). Infarct size was similar when measured by TTC assay and ICG fluorescent labeling (medians 66% and 69%, respectively, n = 7, Fig. 4(e)).
Fig. 4.
Comparison of images of infarction areas obtained by the new method of visualization using ICG and using the traditional method of staining with Evans blue and TTC. There was no difference in infarct size determined by two methods of staining. (a), (b) - Transverse heart slices stained with Evans blue and TTC after 120 min of reperfusion; (c), (d) – The images of the same slices obtained using ICG-scope television camera: (c) - in the visible light, (d) - in the NIR light; (e) – Comparing infarct size evaluated by ICG fluorescence and TTC staining. Medians are not different; (f) – Correlation of infarct sizes obtained by ICG fluorescence and TTC staining is significant.
4. Discussion
The present study showed that ICG accumulates in the zone of irreversible IRI of the heart already after 30 minutes, demonstrating real-time, myocardial injury in vivo. Furthermore, ICG accumulated solely in the TTC-negative area of myocardium, and infarct size measured by ICG was identical to infarct size measured by TTC staining.
The injured area was visible by ICG fluoroscopy with a clear delineation between injured and viable myocardium already at 20-30 minutes of reperfusion. Because ICG fluoroscopy allows earlier estimates of infarct size than the traditional TTC method, the ICG method may possibly be used as an alternative method for measurement of the infarct size. The present study is also the first report on ICG fluoroscopy for in vivo evaluation of irreversible ischemia-reperfusion injury in the heart.
ICG accumulation at the site of injury has been described for different injuries such as ischemic tissue damage, inflammation, and tumors [13–16] and observed in various organs, but the accumulation of ICG in the myocardium to date has not yet been described. ICG choroidal angiography allows the observation of injury caused by ischemia or inflammatory processes that are detected by leakage of ICG [16,21]. Irreversible ischemia-reperfusion injury in the heart leads to extravasation of fluorescein isothiocyanate-tagged albumin [10]. The authors named this phenomenon “vascular rhexis” [10–12] and explained it as the loss of integrity of the coronary microcirculation caused by the death of vascular cells. The studies by French et al (2010) [11] and Zaman et al (2011, 2013) [10,12] described that one-hour ischemia or longer initiated vascular rhexis, whereas shorter ischemic periods did not. The present study showed that shorter ischemic insults followed by reperfusion caused increased permeability of the coronary vasculature and extravasation of ICG. Some explanation for these discrepancies may lie in the different experimental protocol, fluorophore structure, and method of detection. Zaman et al (2013) injected FITC–albumin 24 hours after the induction of nonsustained ischemia for subsequent measurement of fluorescent activity in the entire left ventricular homogenate [10]. Despite these discrepancies, there is now evidence that increased permeability of coronary vessels may allow detection of irreversible injury during extended periods of time, beginning within 30 minutes after start of reperfusion till 24 hours. Along with the increased vascular permeability, decrease of lymph drainage is another possible explanation of accumulation of ICG in the area of myocardial injury. Intravenous injection of ICG delivers the dye to the blood circulatory system. Return of the ~10% of fluid/ICG that will not enter the venous capillaries, but instead enters the lymphatic capillaries may be deferred, because the inflammation caused by ischemia could induce production of inflammatory cytokines and other molecular mediators of lymphatic flow arrest [22,23]. Fang et al (2016) detected necrosis in the rat skeletal muscle using fluorescence molecular imaging with ICG [13]. The authors suggest that increased vascular permeability is an insufficient explanation for indocyanine staining of necrosis, and proposed a mechanism by which ICG selectively binds to necrotic tissue. The mechanism is based on the interaction between the ICG-lipoprotein complex circulating in the blood stream and hydrophobic tails of phospholipids of injured cell membranes. However, in our view, increased vascular permeability may be the sole reason for indocyanine accumulation in necrotic tissue.
Fluorescence in the NIR spectrum provides important additional benefits for intraoperative visualization due to the greater depth of penetration of the radiation into the tissue as compared with the radiation in the UV and visible region of the spectrum, as well as the ability to work in well-lit rooms. Also, fluorescence in the NIR region provides a high contrast due to lower background fluorescence and lack of autofluorescence. The amphiphilic properties of ICG allow these molecules easily bind to plasma proteins such as albumin, globulins, and lipoproteins. Because of this, ICG has extended time of circulation with decay kinetics consisting of two phases: the initial phase with a half-life of four minutes and a second slow phase lasting about one hour. After intravenous injection of ICG solution, the dye is retained for 3-4 minutes in the capillaries and then rapidly cleared from the bloodstream by the liver (less than 4% remaining in the plasma 20 min after administration of the initial bolus) [24]. Fluorescence emission of ICG is concentration and solvent dependent [25]. In aqueous solution, ICG in the chosen concentration has very low fluorescence because of the tendency of ICG molecules to aggregate. It fluoresces in the bloodstream due to its binding to plasma proteins, leading to disaggregation of dimers and formation of ICG monomers having a bright fluorescence [26]. One-two seconds are required for almost complete plasma protein binding [27]. Only 2% of ICG remains not bound to plasma proteins [28]. Consequently, the feasibility of pre-binding of ICG to albumin in experimental studies to enhance the brightness of fluorescence at the infarct site awaits further exploration. Binding of ICG with albumin was used by Chen et al (2015) prior to intravenous injection to mice to avoid self-aggregation of ICG in physiological solution, nonspecific binding to plasma proteins, and rapid renal elimination from the body [29].
Indocyanine green fluorescence angiography is used in cardiac surgery as an additional instrumental method to control grafts function during coronary artery bypass graft surgery [30]. The interpretation of ICG angiography is based on analysis of three main phases of ICG distribution in the coronary vasculature, each phase provides useful information: 1) arterial phase - distribution of ICG in large coronary vessels 2) capillary (microcirculatory) phase - distribution of ICG in small vessels, 3) and venous phase – clearance of ICG.
Arterial phase begins immediately after administration of ICG. With a delay of about one minute, depending on the site of injection and the observed organ, fluorescence is observed. During this time ICG binds to plasma proteins, and the pericardial coronary arteries brightly fluoresce. An occlusion in the coronary circulation is seen as a perfusion defect with no fluorescence in the vessel [14,31].
In the capillary phase, dye fills small vessels, which leads to a uniform fluorescence of the entire surface of the organ. If there is perfusion defect, the intact (remote) myocardium will begin to fluoresce, while ischemic myocardium will remain unstained. The duration of the second phase is about 10 minutes.
The venous phase is characterized by a gradual decline in the concentration of the ICG in the bloodstream. Therefore, the brightness of the intact tissue is gradually reduced. This phase lasts more than two hours.
Figure 5 shows changes in the distribution of ICG in the case of restoration of blood flow in the ischemic myocardium. During the arterial phase (Fig. 5(b), ICG will fill coronary arteries of the entire heart, including arteries with restored blood flow. In the next capillary phase (Fig. 5(c)) delayed fluorescence occurs in the region of myocardial injury with altered coronary microcirculation. The blood vessels in an area of irreversible IRI have excessive leakage of plasma containing ICG. This causes retention of ICG in the injured tissues and increased the brightness of fluorescence in the venous phase (Fig. 5(d)) as compared to remote, unharmed myocardium. It allows identification of the areas of irreversibly damaged myocardium as early as 15-20 minutes after administration of ICG.
Fig. 5.
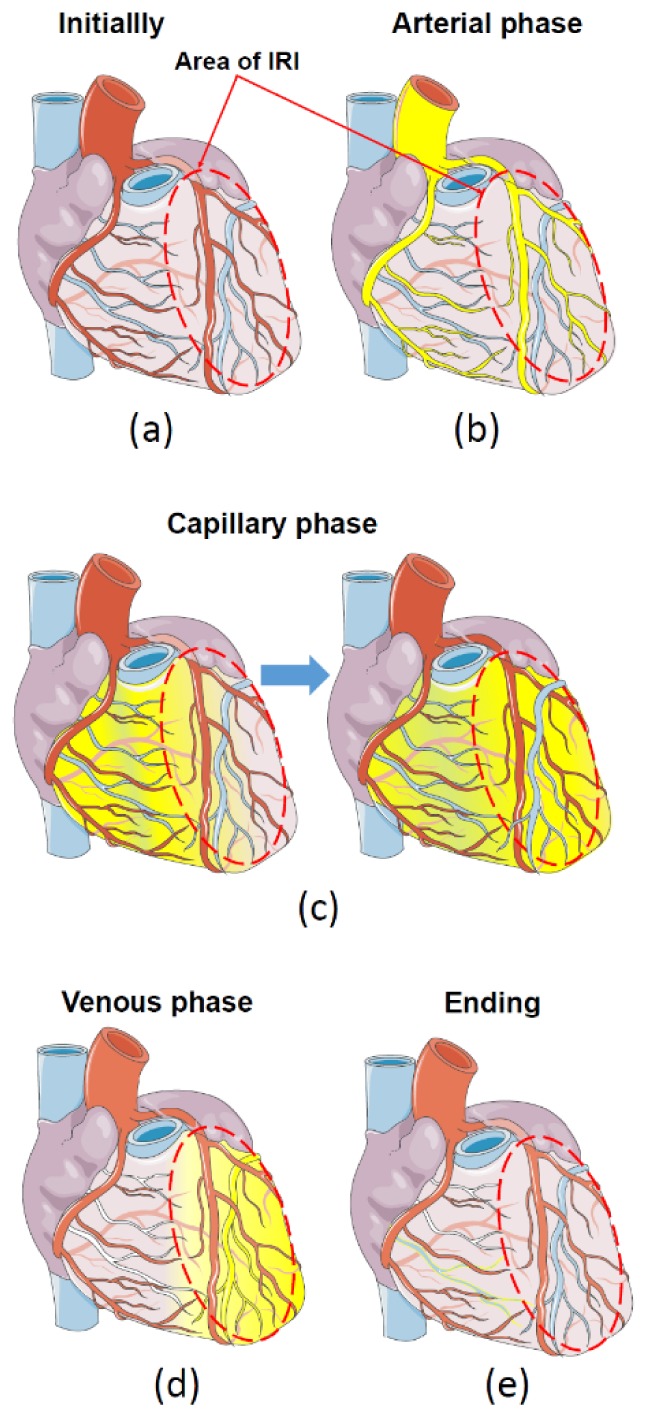
Phases of distribution of fluorescent dye (ICG) in the heart with regional IRI. (a) - initially, before administration of ICG, red oval denotes the area of IRI. (b) - the first, arterial phase - distribution of ICG on large coronary vessels. (c) - Capillary phase - distribution of ICG on small vessels. (d) – the venous phase of ICG with delay of ICG in the area of IRI and complete clearance from undamaged myocardium, (e) - the final washout of ICG.
The present ICG method may provide additional armamentarium to study IRI of the heart as well as cardioprotection. It provides an alternative to the standard TTC staining to quantify infarct size ex vivo. It also allows measurement of infarct size in an early phase of reperfusion. The detection sensitivity of this approach is high and comparable with TTC assay. The smallest necrosis that is successfully detected is 0.6 mm [13]. However, maybe most important is the ability to visualize the infarcted area in vivo. Future studies are necessary to decide if surface fluorescence in vivo may provide a reliable measurement of infarct size as well as to investigate whether the early infarct size after 30 minutes of reperfusion is similar to the more delayed infarct size.
5. Conclusion
The data presented in this study indicate that ICG accumulates in the area of IRI, which become visible in the NIR light 20-30 minutes after ICG administration. Visualization of irreversible ischemia-reperfusion injury of the heart can be done directly on the surface of the myocardium in animal models with the chest opened. Infarct size in rats obtained by the ICG assay was similar to the golden standard of infarct size measurements, TTC. There are two possible mechanisms of accumulation of indocyanine after injury: 1) extravasation followed by slow elimination of the dye because of the low rate of lymph flow, 2) extravasation followed by interacting of indocyanine with phospholipid membranes of injured cells. The present ICG technique is a new, alternative method to measure infarct size and may in the future be used to measure infarct size in animal studies in vivo.
Funding
This study was supported by Government of Russian Federation (Grant 074-U01) and by the Russian Science Foundation (Project 14-15-00473).
References and links
- 1.Goldman S., Raya T. E., “Rat infarct model of myocardial infarction and heart failure,” J. Card. Fail. 1(2), 169–177 (1995). 10.1016/1071-9164(95)90019-5 [DOI] [PubMed] [Google Scholar]
- 2.Ito W. D., Schaarschmidt S., Klask R., Hansen S., Schäfer H. J., Mathey D., Bhakdi S., “Infarct size measurement by triphenyltetrazolium chloride staining versus in vivo injection of propidium iodide,” J. Mol. Cell. Cardiol. 29(8), 2169–2175 (1997). 10.1006/jmcc.1997.0456 [DOI] [PubMed] [Google Scholar]
- 3.Grieve S. M., Mazhar J., Callaghan F., Kok C. Y., Tandy S., Bhindi R., Figtree G. A., “Automated quantification of myocardial salvage in a rat model of ischemia-reperfusion injury using 3D high-resolution magnetic resonance imaging (MRI),” J. Am. Heart Assoc. 3(4), e000956 (2014). 10.1161/JAHA.114.000956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwarz E. R., Somoano Y., Hale S. L., Kloner R. A., “What is the required reperfusion period for assessment of myocardial infarct size using triphenyltetrazolium chloride staining in the rat?” J. Thromb. Thrombolysis 10(2), 181–187 (2000). 10.1023/A:1018770711705 [DOI] [PubMed] [Google Scholar]
- 5.Mayevsky A., Rogatsky G. G., “Mitochondrial Function In vivo Evaluated by NADH Fluorescence: From Animal Models to Human Studies,” Am. J. Physiol. Cell Physiol. 292(2), C615–C640 (2006). 10.1152/ajpcell.00249.2006 [DOI] [PubMed] [Google Scholar]
- 6.Papayan G., Petrishchev N., Galagudza M., “Autofluorescence Spectroscopy for NADH and Flavoproteins Redox State Monitoring in the Isolated Rat Heart Subjected to Ischemia-Reperfusion,” Photodiagn. Photodyn. Ther. 11(3), 400–408 (2014). 10.1016/j.pdpdt.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 7.Ranji M., Motlagh M. M., Salehpour F., Sepehr R., Heisner J. S., Dash R. K., Camara A. K. S., “Optical Cryoimaging Reveals a Heterogeneous Distribution of Mitochondrial Redox State in ex vivo Guinea Pig Hearts and Its Alteration During Ischemia and Reperfusion,” IEEE J. Transl. Eng. Health Med. 4, 1800210 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X., Cui K., Xiu J., Lin H., Lao Y., Zhou B., Liang F., Zha D., Bin J., Liu Y., “Evaluation and simplified measurement of infarct size by myocardial contrast echocardiography in a rat model of myocardial infarction,” Int. J. Cardiovasc. Imaging 25(7), 713–716 (2009). 10.1007/s10554-009-9474-x [DOI] [PubMed] [Google Scholar]
- 9.Yuan L. J., Wang T., Kahn M. L., Ferrari V. A., “High-resolution echocardiographic assessment of infarct size and cardiac function in mice with myocardial infarction,” J. Am. Soc. Echocardiogr. 24(2), 219–226 (2011). 10.1016/j.echo.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 10.Tarikuz Zaman A. K., Spees J. L., Sobel B. E., “Attenuation of cardiac vascular rhexis: a promising therapeutic target,” Coron. Artery Dis. 24(3), 245–252 (2013). 10.1097/MCA.0b013e32835d6688 [DOI] [PubMed] [Google Scholar]
- 11.French C. J., Zaman A. K. M. T., Kelm R. J., Jr, Spees J. L., Sobel B. E., “Vascular Rhexis: Loss of Integrity of Coronary Vasculature in Mice Subjected to Myocardial Infarction,” Exp. Biol. Med. (Maywood) 235(8), 966–973 (2010). 10.1258/ebm.2010.010108 [DOI] [PubMed] [Google Scholar]
- 12.Zaman A. K. M. T., French C. J., Spees J. L., Binbrek A. S., Sobel B. E., “Vascular Rhexis in Mice Subjected to Non-Sustained Myocardial Ischemia and Its Therapeutic Implications,” Exp. Biol. Med. (Maywood) 236(5), 598–603 (2011). 10.1258/ebm.2011.011026 [DOI] [PubMed] [Google Scholar]
- 13.Fang C., Wang K., Zeng C., Chi C., Shang W., Ye J., Mao Y., Fan Y., Yang J., Xiang N., Zeng N., Zhu W., Fang C., Tian J., “Illuminating necrosis: From mechanistic exploration to preclinical application using fluorescence molecular imaging with indocyanine green,” Sci. Rep. 6(6), 21013 (2016). 10.1038/srep21013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alander J. T., Kaartinen I., Laakso A., Pätilä T., Spillmann T., Tuchin V. V., Venermo M., Välisuo P., “A Review of Indocyanine Green Fluorescent Imaging in Surgery,” Int. J. Biomed. Imaging 2012, 940585 (2012). 10.1155/2012/940585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray D. C., Kim E. M., Cotero V. E., Bajaj A., Staudinger V. P., Hehir C. A., Yazdanfar S., “Dual-mode laparoscopic fluorescence image-guided surgery using a single camera,” Biomed. Opt. Express 3(8), 1880–1890 (2012). 10.1364/BOE.3.001880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z., Zhu N., Pacheco S., Wang X., Liang R., “Single camera imaging system for color and near-infrared fluorescence image guided surgery,” Biomed. Opt. Express 5(8), 2791–2797 (2014). 10.1364/BOE.5.002791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akopov A. L., Papayan G. V., Chistyakov I. V., Karlson A., Gerasin A. V., Agishev A. S., “Intraoperative detection of sentinel lymph nodes using infrared imaging system in local non-small cell lung carcinoma of the lung,” Vestn. Khir. Im. I I Grek. 174(4), 13–17 (2015). [PubMed] [Google Scholar]
- 18.Papayan G. V., Akopov A. L., “Near-infrared fluorescence diagnostics: Devices, Applications,” J. Opt. Technol. 83(9), 586–596 (2016). [Google Scholar]
- 19.Himori N., Matsuura A., “A simple technique for occlusion and reperfusion of coronary artery in conscious rats,” Am. J. Physiol. 256(6 Pt 2), H1719–H1725 (1989). [DOI] [PubMed] [Google Scholar]
- 20.Uk. Kang and G. V. Papayan, “Fluorescent endoscope system having improved image detection module,” US Patent No US7635330. 22.12.2009.
- 21.Jung J. W., Lee D. Y., Nam D. H., “Choroidal ischemia and serous macular detachment associated with severe postoperative pain,” Korean J. Ophthalmol. 22(2), 133–136 (2008). 10.3341/kjo.2008.22.2.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aldrich M. B., Sevick-Muraca E. M., “Cytokines are systemic effectors of lymphatic function in acute inflammation,” Cytokine 64(1), 362–369 (2013). 10.1016/j.cyto.2013.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakraborty S., Zawieja S. D., Wang W., Lee Y., Wang Y. J., von der Weid P. Y., Zawieja D. C., Muthuchamy M., “Lipopolysaccharide modulates neutrophil recruitment and macrophage polarization on lymphatic vessels and impairs lymphatic function in rat mesentery,” Am. J. Physiol. Heart Circ. Physiol. 309(12), H2042–H2057 (2015). 10.1152/ajpheart.00467.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malbrain M. L., Viaene D., Kortgen A., De Laet I., Dits H., Van Regenmortel N., Schoonheydt K., Bauer M., “Relationship between intra-abdominal pressure and indocyanine green plasma disappearance rate: hepatic perfusion may be impaired in critically ill patients with intra-abdominal hypertension,” Ann. Intensive Care 2(2 Suppl 1), S19 (2012). 10.1186/2110-5820-2-S1-S19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Philip R., Penzkofer A., Biiumler W., Szeimies R. M., Abels C., “Absorption and Fluorescence Spectroscopic Investigation of Indocyanine Green,” J. Photochem. Photobiol. Chem. 96(1-3), 137–148 (1996). 10.1016/1010-6030(95)04292-X [DOI] [Google Scholar]
- 26.Jung B., Vullev V. I., Anvari B., “Revisiting indocyanine green: effects of serum and physiological temperature on absorption and fluorescence characteristics,” IEEE J. Sel. Top. Quantum Electron. 20(2), 149–157 (2014). 10.1109/JSTQE.2013.2278674 [DOI] [Google Scholar]
- 27.Levesque E., Martin E., Dudau D., Lim C., Dhonneur G., Azoulay D., “Current use and perspective of indocyanine green clearance in liver diseases,” Anaesth Crit Care Pain Med 35(1), 49–57 (2016). 10.1016/j.accpm.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 28.Reinhart M. B., Huntington C. R., Blair L. J., Heniford B. T., Augenstein V. A., “Indocyanine Green: Historical Context, Current Applications, and Future Considerations,” Surg. Innov. 23(2), 166–175 (2016). 10.1177/1553350615604053 [DOI] [PubMed] [Google Scholar]
- 29.Chen Q., Liang C., Wang C., Liu Z., “An imagable and photothermal “Abraxane-like” nanodrug for combination cancer therapy to treat subcutaneous and metastatic breast tumors,” Adv. Mater. 27(5), 903–910 (2015). 10.1002/adma.201404308 [DOI] [PubMed] [Google Scholar]
- 30.Ferguson T. B., Jr, Chen C., Babb J. D., Efird J. T., Daggubati R., Cahill J. M., “Fractional Flow Reserve-Guided Coronary Artery Bypass Grafting: Can Intraoperative Physiologic Imaging Guide Decision Making?” J. Thorac. Cardiovasc. Surg. 146(4), 824–835 (2013). 10.1016/j.jtcvs.2013.06.026 [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto M., Sasaguri S., Sato T., “Assessing intraoperative blood flow in cardiovascular surgery,” Surg. Today 41(11), 1467–1474 (2011). 10.1007/s00595-010-4553-0 [DOI] [PubMed] [Google Scholar]