Abstract
Chronic kidney disease (CKD) represents a major medical challenge and frequently coexists with cardiovascular disease (CVD), which can be treated by statin trerapy. However, whether statin treatment affects renal progression and outcomes in CKD patients remains unclear. We retrospectively reviewed CKD patients at Gachon University Gil Medical Center from 2003–2013. From a total of 14,497 CKD patients, 858 statin users were paired with non-users and analyze with propensity score matching was performed. The outcomes of this study were creatinine doubling, renal death, all-cause mortality, and interactive factors for composite outcomes. Statins were prescribed to 13.5% of the study subjects. Hazard ratios (HRs) [95% confidence intervals (CIs)] for statin treatment for the doubling of serum creatinine levels were significant only in CKD patients with an estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2, and were 0.744 (0.635–0.873) in the unmatched cohort and 0.767 (0.596–0.986) in the matched cohort. In analyses of secondary outcomes, the HRs (95% CIs) for all-cause mortality were 0.655 (0.502–0.855) in the unmatched cohort and 0.537 (0.297–0.973) in the matched cohort. The HRs (95% CIs) for statin therapy for composite outcomes among patients with and without an eGFR ≥30 mL/min/1.73 m2 were 0.764 (0.613–0.952) and 1.232 (0.894–1.697), respectively (P for interaction, 0.017). Thus, statin treatment may have beneficial effects on renal progression and all-cause mortality only for the patients with early- stage CKD.
Introduction
Chronic kidney disease (CKD) has been emerged as an important health concern and is a major cause of premature mortality and comorbidities [1]. Individuals with CKD have a higher risk of premature mortality related to cardiovascular disease (CVD) even during the early stages of disease; this represents a significant challenge for physicians [2]. Renal dysfunction increases inflammation, oxidative stress, platelet dysfunction, endothelial dysfunction, proteinuria, and electrolyte imbalances [3–6]. Moreover, these alterations may be related to a higher risk of CVD events [7]. Dyslipidemia and hypertension are common conditions associated with CVD [7–9]. Various toxic lipid intermediates are formed in dyslipidemic CKD patients, which may accelerate the progression of CKD [10–12]. Therefore, optimal strategies for the management of dyslipidemia are needed to prevent CVD, particularly in CKD patients.
In previous studies, statin therapy improved renal function and reduced proteinuria [13–15], possibly due to their anti-inflammatory activity and improvement of endothelial function, which reduces abnormal permeability to plasma proteins [6, 16–20]. Thus, statin administration may benefit CKD patients due to its enhancement of renal perfusion by improving endothelial and cardiac function [20, 21]. However, the indications for statin treatment are unclear, and whether it slows the progression of CKD and improves renal outcomes is controversial [22, 23]. The latest guidelines for lipid management in CKD patients from Kidney Disease: Improving Global Outcomes (KDIGO) do not suggest an appropriate point for the initiation of statin therapy; indeed, whether statin therapy has beneficial effects in all CKD patients is also uncertain [22, 23]. Therefore, we evaluated the clinical impact of statin treatment on the clinical outcomes of patients with CKD according to disease stage.
Materials and Methods
Study design and participants
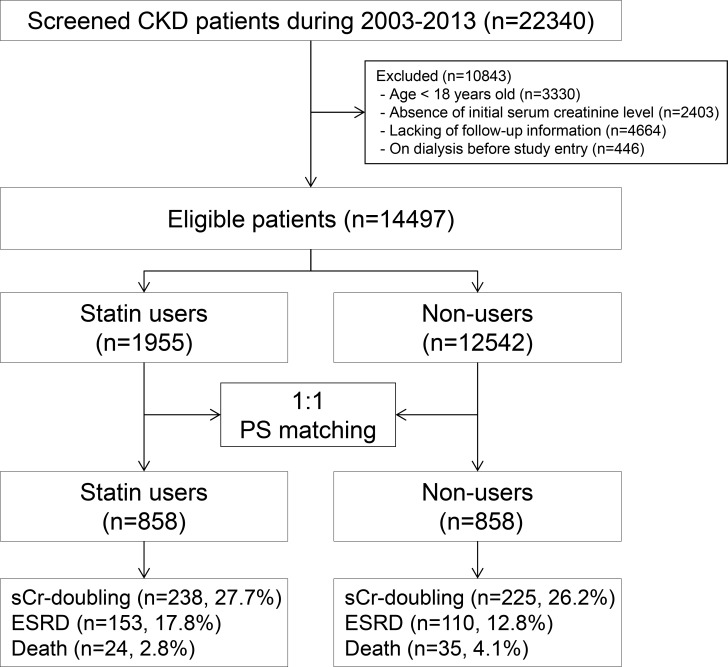
We performed a propensity score (PS)-matched analysis of a retrospective observational cohort. Data from a total of 22,340 patients with CKD between January 1, 2003, and August 31, 2013, were included. Of these patients, an analysis of 14,497 patients was performed after excluding the following groups: (i) those less than 18 years of age (n = 3,330); (ii) patients with missing information concerning comparable data (n = 2,403) or follow up (n = 4,664); and (iii) those who had previously undergone dialysis or transplantation before the index date (start point of observation, baseline creatinine measurement; n = 446; Fig 1). The patients were divided into two cohorts based on their statin prescription. Statin users (n = 1,955) were prescribed statins at least 3 months before the index date. Non-users (n = 12,542) were not prescribed statins before the index date. The research protocol and methods were approved by the Institutional Review Board (IRB) of Gachon University Gil Medical Center (GAIRB2016-085). The IRB waived the requirement for written informed consent as this was a retrospective study that did not include any interventions.
Fig 1. Cohort formation.
Variables
Demographic (age, sex, and body mass index [BMI]), clinical (medication information and medical history of diabetes, hypertension and previous CVD), and laboratory (serum levels of creatinine, hemoglobin, white blood cells, platelet, albumin, calcium, phosphorus, uric acid, cholesterol, triglycerides, high-density lipoprotein [HDL]-cholesterol, and the presence of proteinuria) data of patients were obtained from electronic medical records. CVD was defined as major vascular diseases such as myocardial infarction and other ischemic heart diseases, including angina pectoris and heart failure, atrial fibrillation, and cerebrovascular disease. The four-variable Modification of Diet in Renal Disease (MDRD) Study equation was used to calculate the estimated glomerular filtration rate (eGFR) [24]. We defined and staged the CKD according to the eGFR (<60 mL/min/1.73 m2), proteinuria manifestation, or both, on two occasions at least 3 months apart [25].
Outcomes
Primary outcomes were doubling of serum creatinine and renal events reaching the end-stage renal disease (ESRD) necessitating renal replacement therapy. Secondary outcomes were all-cause mortality and composite outcomes (creatinine doubling, renal death, and all-cause mortality). Outcomes were compared on the index date until the first event.
Statistical analysis
To decrease any potential confounding bias, PS matching was adopted to adjust for significant differences in baseline covariates. As described previously [26], the probability for statin prescription was determined using a multiple logistic regression analysis model (C statistic = 0.624; Hosmer and Lemeshow goodness-of-fit test, P = 0.1494), which included demographics and clinical and laboratory values (age, sex, BMI, medical history of diabetes, hypertension, and previous CVD; baseline eGFR; presence of proteinuria, hemoglobin, albumin, calcium, phosphorus, and uric acid; cholesterol, triglycerides, and HDL-cholesterol levels; and the prescription of RAAS blockers, beta blockers, CCBs, diuretics, and aspirin). After PS matching, the balance in baseline covariates between the two groups was assessed using the standardized mean difference test, paired t-test, and McNemar’s test. PS matching was constructed using the Statistical Analysis Systems (SAS) software package version 9.3 (SAS Institute, Cary, NC).
A Cox proportional regression was performed to determine the adjusted hazard ratios (HRs) for statin prescription with 95% confidence intervals (CIs) for outcomes. Stratified subgroup analyses to test for confounding variables and interactions were also performed. All data analyses with the exception of PS matching were performed using Statistical Package for the Social Sciences (SPSS) software version 20.0 (IBM Corp., Armonk, NY). A value of P < 0.05 was considered to indicate statistical significance.
Results
Patient characteristics
A total of 14,497 CKD patients met the inclusion criteria and were followed up for a median of 30 months (interquartile range: 3–67 months). Statins were prescribed to 13.5% (1,955 of 14,497) of the patients. In particular, stain use was more common in older and male patients. Moreover, medical comorbidities and the prescription of other medications were showed more frequent in the statin user group than in non-users. After PS matching of 858 statin users with non-users, no significant differences in baseline characteristics were found between the two groups. (Table 1).
Table 1. Baseline characteristics of study participants (before after PS 1:1 matching).
| Before matching | After PS matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Statin users (N = 1955) | Non-users (N = 12542) | P | Standardized differences | Statin users (N = 858) | Non-users (N = 858) | P | Standardized differences |
| Age, year | 55.40±13.48 | 48.92±15.91 | <0.001 | 0.603 | 53.86±13.41 | 53.37±15.31 | 0.483 | 0.059 |
| Male gender, n (%) | 1036 (53.0) | 6313 (50.3) | 0.029 | 0.054 | 457 (53.3) | 482 (56.2) | 0.233 | 0.058 |
| Diabetes, n (%) | 791 (40.5) | 1475 (11.8) | <0.001 | 0.691 | 289 (33.7) | 260 (30.3) | 0.137 | 0.073 |
| Hypertension, n (%) | 1380 (70.6) | 2946 (23.5) | <0.001 | 1.070 | 635 (74.0) | 649 (75.6) | 0.420 | 0.037 |
| BMI, kg/m2 | 25.90±5.76 | 25.49±6.45 | 0.076 | 0.075 | 26.90±6.74 | 27.69±7.51 | 0.290 | 0.053 |
| Previous CVD, n (%) | 460 (23.5) | 683 (5.4) | <0.001 | 0.533 | 141 (16.4) | 124 (14.5) | 0.271 | 0.037 |
| eGFR, ml/min/1.73m2 | 56.88±27.98 | 69.57±29.70 | <0.001 | 0.615 | 57.82±30.06 | 59.01±33.46 | 0.434 | 0.056 |
| Proteinuria, n (%) | 1172 (59.9) | 5984 (47.7) | <0.001 | 0.247 | 532 (62.0) | 547 (63.8) | 0.640 | 0.061 |
| Laboratory | ||||||||
| Hemoglobin, g/dl | 13.16±2.28 | 13.35±2.13 | 0.002 | 0.062 | 13.00±2.33 | 13.07±2.25 | 0.615 | 0.018 |
| White blood cells, x103/ml | 7.95±2.89 | 7.78±3.77 | 0.041 | 0.059 | 7.86±2.80 | 7.94±3.09 | 0.631 | 0.075 |
| Platelet, x103/ml | 266.70±78.78 | 258.90±77.59 | <0.001 | 0.137 | 265.80±78.24 | 262.80±75.98 | 0.505 | 0.035 |
| Albumin, g/dl | 4.04±0.66 | 4.25±0.51 | <0.001 | 0.131 | 4.06±0.62 | 4.05±0.64 | 0.769 | 0.005 |
| Cholesterol, mg/dl | 221.43±65.21 | 183.68±43.43 | <0.001 | 0.802 | 212.21±64.55 | 208.20±55.15 | 0.154 | 0.102 |
| Triglycerides, mg/dl | 200.93±171.02 | 140.10±105.89 | <0.001 | 0.502 | 180.84±133.26 | 186.95±172.38 | 0.419 | 0.037 |
| HDL-cholesterol, mg/dl | 48.72±15.04 | 50.54±14.63 | <0.001 | 0.155 | 48.87±15.21 | 49.55±15.34 | 0.385 | 0.049 |
| Calcium, mg/dl | 9.01±0.73 | 9.01±0.62 | 0.889 | 0.001 | 8.95±0.72 | 8.96±0.70 | 0.835 | 0.007 |
| Phosphorus, mg/dl | 3.74±0.89 | 3.65±1.86 | 0.038 | 0.063 | 3.74±0.93 | 3.69±0.89 | 0.297 | 0.028 |
| Uric acid, mg/dl | 6.27±2.14 | 5.61±2.00 | <0.001 | 0.293 | 6.37±2.17 | 6.40±2.16 | 0.813 | 0.028 |
| Statins | ||||||||
| Simvastatin | 654 (33.5) | 235 (27.4) | ||||||
| Rosuvastatin | 459 (23.5) | 217 (25.3) | ||||||
| Pravastatin | 394 (20.2) | 199 (23.2) | ||||||
| Atorvastatin | 302 (15.4) | 144 (16.8) | ||||||
| Pitavastatin | 95 (4.9) | 48 (5.6) | ||||||
| Lovastatin | 51 (2.6) | 15 (1.7) | ||||||
| Other medications | ||||||||
| RAAS blockers, n (%) | 1308 (66.9) | 2183 (17.4) | <0.001 | 1.158 | 617 (71.9) | 640 (74.6) | 0.135 | 0.061 |
| Beta-blockers, n (%) | 956 (48.9) | 1630 (13.0) | <0.001 | 0.843 | 432 (50.3) | 396 (46.2) | 0.074 | 0.082 |
| CCB, n (%) | 1085 (55.5) | 1894 (15.1) | <0.001 | 0.933 | 454 (52.9) | 475 (55.4) | 0.306 | 0.050 |
| Diuretics, n (%) | 1048 (53.6) | 1815 (14.5) | <0.001 | 0.906 | 444 (51.7) | 439 (51.2) | 0.831 | 0.010 |
| Aspirin, n (%) | 540 (27.6) | 807 (6.4) | <0.001 | 0.588 | 202 (23.5) | 175 (20.4) | 0.118 | 0.075 |
BMI: body mass index; CVD: cardiovascular disease; eGFR: estimated glomerular filtration rate; HDL: high-density lipoprotein; RAAS: renin-angiotensin-aldosterone system; CCB: calcium channel blocker. Note: Conversion factors for units were as follows: hemoglobin in g/dl to g/l, × 10; albumin in mg/dl to g/l, × 10; cholesterol mg/dl to mmol/l, × 0.02586: triglycerides mg/dl to mmol/l, × 0.01129; HDL-cholesterol mg/dl to mmol/l, × 0.02586; LDL-cholesterol mg/dl to mmol/l, × 0.0258
Primary outcome
Multivariable Cox proportional regression analyses were performed for doubling of serum creatinine or renal events (long-term dialysis and kidney transplantation) according to the stages of CKD (eGFR ≥30 mL/min/1.73 m2 or <30 mL/min/1.73 m2). Statins delayed the doubling of serum creatinine without considering eGFR in the unmatched cohort (HR, 0.824; 95% CI, 0.722–0.939; P = 0.004; Table 2), but this significant association was not seen in the matched cohort (HR, 0.871; 95% CI, 0.711–1.066; P = 0.181; Table 2). For the eGFR ≥30 mL/min/1.73 m2 group, statins significantly reduced the incidence of creatinine doubling in both the unmatched (HR, 0.744; 95% CI, 0.635–0.873; P < 0.001; Table 2) and matched (HR, 0.767; 95% CI, 0.596–0.986; P = 0.039; Table 2) cohorts. There was no significant relationship between statin treatment and creatinine doubling in the eGFR <30 mL/min/1.73 m2 group. In addition, the risk of renal events was not related to statin use in the unmatched (HR, 1.041; 95% CI, 0.866–1.250; P = 0.671) and matched (HR, 1.095; 95% CI, 0.833–1.440; P = 0.515; Table 2) cohorts.
Table 2. Association between statin and risk of CKD progression and risk of ESRD.
| Unmatched cohort | Matched cohort | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| SCr x 2a | ||||
| Non-users | 1 (reference) | 1 (reference) | ||
| Statin users | 0.824 (0.722–0.939) | 0.004 | 0.871 (0.711–1.066) | 0.181 |
| eGFR≥30ml/min/1.73m2 | 0.744 (0.635–0.873) | <0.001 | 0.767 (0.596–0.986) | 0.039 |
| eGFR<30ml/min/1.73m2 | 0.992 (0.786–1.253) | 0.948 | 1.101 (0.773–1.569) | 0.595 |
| Simvastatin | 0.742 (0.618–0.891) | 0.001 | 0.689 (0.518–0.918) | 0.011 |
| Rosuvastatin | 1.009 (0.909–1.120) | 0.868 | 1.076 (0.923–1.254) | 0.349 |
| Pravastatin | 1.017 (0.944–1.096) | 0.656 | 1.034 (0.931–1.150) | 0.532 |
| Atorvastatin | 0.999 (0.940–1.062) | 0.999 | 1.004 (0.922–1.093) | 0.936 |
| Pitavastatin | 0.772 (0.648–0.921) | 0.004 | 0.824 (0.655–1.036) | 0.098 |
| Lovastatin | 0.991 (0.840–1.169) | 0.916 | 1.043 (0.749–1.452) | 0.804 |
| ESRD | ||||
| Non-users | 1 (reference) | 1 (reference) | ||
| Statin users | 1.040 (0.866–1.249) | 0.675 | 1.095 (0.833–1.440) | 0.515 |
| eGFR≥30ml/min/1.73m2 | 0.884 (0.673–1.161) | 0.374 | 1.142 (0.753–1.733) | 0.532 |
| eGFR<30ml/min/1.73m2 | 1.220 (0.953–1.563) | 0.115 | 1.136 (0.784–1.646) | 0.500 |
| Simvastatin | 0.970 (0.769–1.225) | 0.800 | 0.875 (0.619–1.238) | 0.451 |
| Rosuvastatin | 1.093 (0.948–1.260) | 0.220 | 1.130 (0.924–1.383) | 0.233 |
| Pravastatin | 1.081 (0.977–1.197) | 0.131 | 1.136 (0.999–1.293) | 0.053 |
| Atorvastatin | 0.956 (0.873–1.048) | 0.341 | 0.940 (0.829–1.065) | 0.330 |
| Pitavastatin | 0.831 (0.662–1.044) | 0.111 | 0.946 (0.749–1.195) | 0.641 |
| Lovastatin | 0.991 (0.840–1.169) | 0.916 | 1.043 (0.749–1.452) | 0.804 |
Abbreviations: HR, hazards ratio; CI, confidence interval; SCr, serum creatinine; ESRD, end-stage renal disease. HRs were obtained from Cox models adjusted for age (years), diabetes, hypertension, previous CVD, proteinuria, baseline eGFR, hemoglobin level (< 10 g/dl), albumin level (< 3.5 g/dl), and cholesterol level and use of medications (RAAS blockers, aspirin, beta-blockers, CCB, and diuretics)
aSCr x 2: a doubling of the baseline serum creatinine concentration
All-cause mortality
Statin users showed a lower mortality rate than those of non-users before (P = 0.002) and after PS matching (P = 0.04; Table 3). In a multivariable Cox proportional analysis, statin users had a lower incidence of mortality than non-users in both the unmatched cohort (HR, 0.655; 95% CI, 0.502–0.855; P = 0.002; Table 3) and in the matched cohort (HR, 0.537; 95% CI, 0.297–0.973; P = 0.040; Table 3). In the eGFR ≥30 mL/min/1.73 m2 group, statin users showed significantly less mortality risk in both the unmatched (HR, 0.518; 95% CI, 0.365–0.734; P < 0.001; Table 3) and matched (HR, 0.457; 95% CI, 0.214–0.979; P = 0.044; Table 3) cohorts. However, statin treatment did not significantly affect all-cause mortality in the patients with an eGFR <30 mL/min/1.73 m2.
Table 3. Association between statin and risk for all-cause mortality.
| Unmatched cohort | Matched cohort | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| All-cause mortality | ||||
| Non-users | 1 (reference) | 1 (reference) | ||
| Statin users | 0.655 (0.502–0.855) | 0.002 | 0.537 (0.297–0.973) | 0.040 |
| eGFR≥30ml/min/1.73m2 | 0.518 (0.365–0.734) | <0.001 | 0.457 (0.214–0.979) | 0.044 |
| eGFR<30ml/min/1.73m2 | 0.946 (0.619–1.446) | 0.798 | 0.752 (0.248–2.279) | 0.752 |
| Simvastatin | 0.640 (0.435–0.942) | 0.024 | 0.450 (0.172–1.178) | 0.104 |
| Rosuvastatin | 1.021 (0.827–1.260) | 0.845 | 0.917 (0.570–1.475) | 0.721 |
| Pravastatin | 0.892 (0.741–1.075) | 0.231 | 0.850 (0.571–1.264) | 0.421 |
| Atorvastatin | 0.906 (0.784–1.048) | 0.185 | 1.002 (0.791–1.269) | 0.987 |
| Pitavastatin | 0.923 (0.734–1.159) | 0.489 | 0.985 (0.656–1.479) | 0.940 |
| Lovastatin | 0.969 (0.768–1.223) | 0.790 | — | — |
Abbreviations: HR, hazards ratio; CI, confidence interval; SCr, serum creatinine. HRs were obtained from Cox models adjusted for age (years), diabetes, hypertension, previous CVD, proteinuria, baseline eGFR, hemoglobin level (< 10 g/dl), albumin level (< 3.5 g/dl), and cholesterol level and use of medications (RAAS blockers, aspirin, beta-blockers, CCB, and diuretics)
Other secondary endpoints; associations of statin use with composite outcomes according to eGFR
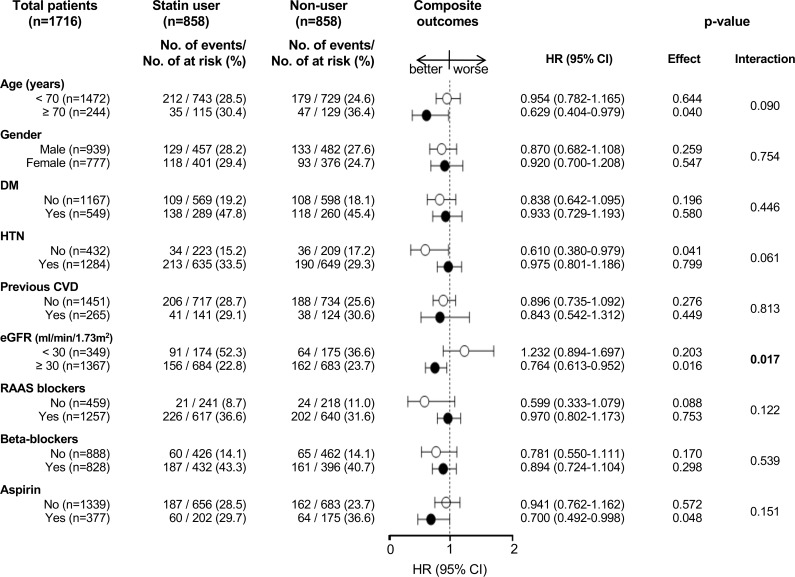
In the matched cohort, 79.6% (1,367 of 1,716) of the patients had early-stage CKD (stages 1 to 3, eGFR ≥30 mL/min/1.73 m2). Among statin users in the PS-matched cohort, 22.8% (156 of 684) of patients with early-stage CKD were statin users, while 52.3% (91 of 174) of late-stage CKD patients (stages 4 and 5; eGFR <30 mL/min/1.73 m2) were statin users. The HRs (95% CIs) for composite outcomes among early- and late-stage CKD patients were 0.764 (0.613–0.952, P = 0.016) and 1.232 (0.894–1.697, P = 0.203), respectively (P interaction, 0.017; Fig 2). The influence of statin treatment on outcome was consistent across the stratified patient subgroups (Fig 2).
Fig 2. Association of statin treatment and composite outcomes in subgroups of matched cohort.
Discussion
The effect of statin prescription on clinical outcomes in patients with CKD according to stage was evaluated using PS matching. We found that statin treatment delayed the deterioration of kidney function and reduced all-cause mortality only in patients with early-stage CKD (eGFR ≥30 mL/min/1.73 m2). Thus, we may consider statin treatment in such patients.
Several studies have evaluated the mechanism by which statins affect CKD [3, 12–15, 21, 27, 28]. Statins not only lower lipid profiles, they also exert anti-proteinuric effects [13]. However, the beneficial effects of statins had not previously been assessed according stage of CKD, dosage, and treatment duration. A previous study compared the renoprotective effects of atorvastatin and rosuvastatin in progressive renal disease patients with or without diabetes (PLANET I and PLANET II) [27]. The results suggested rosuvastatin to have a greater lipid-lowering effect. In contrast, atorvastatin exerts a greater renoprotective effect in CKD patients. Therefore, the renoprotective effect varies according to the type of statin; moreover, there was no correlation between the reduction in lipid levels and renoprotection.
Statins’ renoprotective effects may be dosage-dependent. High-dose statin therapy ameliorated the decline in eGFR in CKD patients (eGFR <60 mL/min/1.73 m2), while statin therapy had no effect on renal progression in patietns with low-dose prescriptions [3]. Hence, the renoprotective effect of statins may vary according to the dose and type of statin, as well as CKD stage. Although the effects of statins in patients with CKD of various stages are uncertatin, a favorable effect of statins has empirically been adopted in these populations; indeed, statins are frequently used in CKD patients. In our study, simvastatin exerted beneficial effects on renal progression and all-cause mortality only for patients with early-stage CKD. However, there were no significant differences on outcomes regarding outcomes when different doses of simvastatin were compared (data not shown).
The first guidelines for lipid therapy in CKD patients were published in 2003. According to those guidelines, treatment should be considered for CKD patients with a low-density lipoprotein (LDL) level >100 mg/dL to reduce the LDL level to <100 mg/dL [29]. As indicated above, statins are recommended in patients with all stages of CKD. However, two large randomized trials failed to show a beneficial effect of statin treatment in patients undergoing hemodialysis [30, 31]. The Die Deutsche Diabetes Dialysis (4D) trial [30] and The Assessment of Survival and Cardiovascular Events (AURORA) trial [31] demonstrated that statin therapy in dialysis patients lowers the LDL level but does not affect the incidence of CVD or all-cause mortality. Therefore, the revised guidelines suggested that statin treatment should not be initiated in patients on maintenance hemodialysis [32]. Subsequently, the authors reported a clear benefit of statins, even in patients undergoing hemodialysis in the Study of Heart and Renal Protection (SHARP) [33]. However, the efficacy of statins in CKD patients undergoing hemolysis have remains uncertain. The 2014 KDIGO guidelines recommend not initiating statin therapy in adults with dialysis-dependent CKD and continuation in adults taking statins at the time of dialysis initiation [22]. KDIGO guidelines on lipid management adopt a risk-based approach for statin therapy in accordance with ACC-AHA guidelines [23]. However, the guidelines did not suggest the time at which statin treatment should be commenced. Our findings suggest that statin therapy in early-stage CKD patients may increase the time until serum creatinine doubles and decrease all-cause mortality.
In the subgroup analysis, only those with early-stage CKD had better outcomes when taking statins. We evaluated the association between statin use and eGFR. The use of statins by patients whose disease has advanced to ESRD requiring renal replacement therapy is controversial, and results regarding statin use in dialysis patients are inconsistent [30, 31, 33]. It would be worth performing randomized controlled interventional studies to confirm the clinical usefulness of statins in terms of clinical outcome in dialysis patients. However, statins may benefit patients not undergoing dialysis. In agreement with these observations, our findings suggest that statins may exert an eGFR-dependent renoprotective effect.
Although our findings show that statin use is associated with delayed progression of CKD after adjustment for underlying CKD, this study had several limitations. First, we could not conclude whether efficacy depends on statin type or dosage. To address this, we need to determine whether high-dose statin treatment lengthens the doubling time for serum creatinine and whether it decreases the risk of ESRD and mortality in a future study considering the dosage and type of statin therapy. Another limitation is the involvement of a single center and an ethnically homogenous population. An additional, multiple-center study of statin efficacy in CKD patients of other ethnicities is thus warranted. Also, this was a retrospective observational study, therefore is not a perfect substitute for a randomized trial. Even though PS matched analyses were performed, out study may contain biased results due to differences in unmeasured characteristics. Despite these limitations, this was a large-scale observational cohort study that used PS matching to enhance the statistical significance of the findings.
In summary, the lipid-lowering effect of statins prevents cardiovascular events and may exert similar effects in CKD patients. Little is known about the effect of statins in ESRD patients or the point at which statin treatment should be initiated in CKD patients. This study demonstrates that statins improved the prognosis of early-stage CKD patients with an eGFR ≥ 30 mL/min/1.73 m2, as evidenced by a reduced time to serum creatinine doubling and decreased all-cause mortality.
In conclusion, although the effect of statins on renal progression in CKD patients is unclear, our results suggest statins to have beneficial effects in patients with an eGFR ≥30 mL/min/1.73 m2. Therefore, the commencement of statin treatment during the early stages of CKD may be beneficial. Future randomized controlled studies are warranted to confirm the efficacy of statin therapy in early-stage CKD patients.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction, when the researchers used it in this form. All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.James MT, Hemmelgarn BR, Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet (London, England). 2010;375(9722):1296–309. Epub 2010/04/13. [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–47. Epub 2007/11/08. 10.1001/jama.298.17.2038 [DOI] [PubMed] [Google Scholar]
- 3.Sanguankeo A, Upala S, Cheungpasitporn W, Ungprasert P, Knight EL. Effects of Statins on Renal Outcome in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis. PLoS One. 2015;10(7):e0132970 Epub 2015/07/08. PubMed Central PMCID: PMCPmc4495033. 10.1371/journal.pone.0132970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoja C, Corna D, Rottoli D, Cattaneo D, Zanchi C, Tomasoni S, et al. Effect of combining ACE inhibitor and statin in severe experimental nephropathy. Kidney international. 2002;61(5):1635–45. Epub 2002/04/23. 10.1046/j.1523-1755.2002.00332.x [DOI] [PubMed] [Google Scholar]
- 5.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, et al. Adiponectin regulates albuminuria and podocyte function in mice. The Journal of clinical investigation. 2008;118(5):1645–56. Epub 2008/04/24. PubMed Central PMCID: PMCPmc2323186. 10.1172/JCI32691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikolic D, Banach M, Nikfar S, Salari P, Mikhailidis DP, Toth PP, et al. A meta-analysis of the role of statins on renal outcomes in patients with chronic kidney disease. Is the duration of therapy important? International journal of cardiology. 2013;168(6):5437–47. Epub 2013/09/11. 10.1016/j.ijcard.2013.08.060 [DOI] [PubMed] [Google Scholar]
- 7.Uhlig K, Levey AS, Sarnak MJ. Traditional cardiac risk factors in individuals with chronic kidney disease. Seminars in dialysis. 2003;16(2):118–27. Epub 2003/03/19. [DOI] [PubMed] [Google Scholar]
- 8.Jungers P, Massy ZA, Nguyen Khoa T, Fumeron C, Labrunie M, Lacour B, et al. Incidence and risk factors of atherosclerotic cardiovascular accidents in predialysis chronic renal failure patients: a prospective study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 1997;12(12):2597–602. Epub 1998/02/12. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez CJ, Swett K, Agarwal SK, Folsom AR, Fox ER, Loehr LR, et al. Systolic blood pressure levels among adults with hypertension and incident cardiovascular events: the atherosclerosis risk in communities study. JAMA internal medicine. 2014;174(8):1252–61. Epub 2014/06/18. PubMed Central PMCID: PMCPmc4573449. 10.1001/jamainternmed.2014.2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallamaci F, Zoccali C, Tripepi G, Fermo I, Benedetto FA, Cataliotti A, et al. Hyperhomocysteinemia predicts cardiovascular outcomes in hemodialysis patients. Kidney international. 2002;61(2):609–14. Epub 2002/02/19. 10.1046/j.1523-1755.2002.00144.x [DOI] [PubMed] [Google Scholar]
- 11.Schaeffner ES, Kurth T, Curhan GC, Glynn RJ, Rexrode KM, Baigent C, et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. Journal of the American Society of Nephrology: JASN. 2003;14(8):2084–91. Epub 2003/07/23. [DOI] [PubMed] [Google Scholar]
- 12.Gluba A, Rysz J, Banach M. Statins in patients with chronic kidney disease: why, who and when? Expert opinion on pharmacotherapy. 2010;11(16):2665–74. Epub 2010/10/28. 10.1517/14656566.2010.512419 [DOI] [PubMed] [Google Scholar]
- 13.Rysz J, Gluba-Brzozka A, Banach M, Wiecek A. Should we use statins in all patients with chronic kidney disease without dialysis therapy? The current state of knowledge. International urology and nephrology. 2015;47(5):805–13. Epub 2015/03/12. 10.1007/s11255-015-0937-9 [DOI] [PubMed] [Google Scholar]
- 14.Athyros VG, Katsiki N, Karagiannis A, Mikhailidis DP. Statins can improve proteinuria and glomerular filtration rate loss in chronic kidney disease patients, further reducing cardiovascular risk. Fact or fiction? Expert opinion on pharmacotherapy. 2015;16(10):1449–61. Epub 2015/06/04. 10.1517/14656566.2015.1053464 [DOI] [PubMed] [Google Scholar]
- 15.Tonelli M. Do statins protect the kidney by reducing proteinuria? Annals of internal medicine. 2006;145(2):147–9. Epub 2006/07/19. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. The New England journal of medicine. 2005;352(1):20–8. Epub 2005/01/07. 10.1056/NEJMoa042378 [DOI] [PubMed] [Google Scholar]
- 17.Tonelli M, Isles C, Curhan GC, Tonkin A, Pfeffer MA, Shepherd J, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation. 2004;110(12):1557–63. Epub 2004/09/15. 10.1161/01.CIR.0000143892.84582.60 [DOI] [PubMed] [Google Scholar]
- 18.Epstein M, Campese VM. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors on renal function. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2005;45(1):2–14. Epub 2005/02/08. [DOI] [PubMed] [Google Scholar]
- 19.Tonelli M, Moye L, Sacks FM, Cole T, Curhan GC. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. Journal of the American Society of Nephrology: JASN. 2003;14(6):1605–13. Epub 2003/05/23. [DOI] [PubMed] [Google Scholar]
- 20.O'Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95(5):1126–31. Epub 1997/03/04. [DOI] [PubMed] [Google Scholar]
- 21.Barylski M, Nikfar S, Mikhailidis DP, Toth PP, Salari P, Ray KK, et al. Statins decrease all-cause mortality only in CKD patients not requiring dialysis therapy—a meta-analysis of 11 randomized controlled trials involving 21,295 participants. Pharmacological research. 2013;72:35–44. Epub 2013/04/02. 10.1016/j.phrs.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 22.Wanner C, Tonelli M. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney international. 2014;85(6):1303–9. Epub 2014/02/21. 10.1038/ki.2014.31 [DOI] [PubMed] [Google Scholar]
- 23.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–45. Epub 2013/11/14. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of internal medicine. 1999;130(6):461–70. Epub 1999/03/13. [DOI] [PubMed] [Google Scholar]
- 25.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2002;39(2 Suppl 1):S1–266. Epub 2002/03/21. [PubMed] [Google Scholar]
- 26.Kim AJ, Lim HJ, Ro H, Ko KP, Han SY, Chang JH, et al. Low-dose aspirin for prevention of cardiovascular disease in patients with chronic kidney disease. PLoS One. 2014;9(8):e104179 PubMed Central PMCID: PMCPMC4122498. 10.1371/journal.pone.0104179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Zeeuw D, Anzalone DA, Cain VA, Cressman MD, Heerspink HJ, Molitoris BA, et al. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): a randomised clinical trial. The lancet Diabetes & endocrinology. 2015;3(3):181–90. Epub 2015/02/11. [DOI] [PubMed] [Google Scholar]
- 28.Deedwania PC, Stone PH, Fayyad RS, Laskey RE, Wilson DJ. Improvement in Renal Function and Reduction in Serum Uric Acid with Intensive Statin Therapy in Older Patients: A Post Hoc Analysis of the SAGE Trial. Drugs & aging. 2015;32(12):1055–65. Epub 2015/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.K/DOQI clinical practice guidelines for management of dyslipidemias in patients with kidney disease. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2003;41(4 Suppl 3):I–iv, s1-91. Epub 2003/04/03. [PubMed] [Google Scholar]
- 30.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. The New England journal of medicine. 2005;353(3):238–48. Epub 2005/07/22. 10.1056/NEJMoa043545 [DOI] [PubMed] [Google Scholar]
- 31.Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. The New England journal of medicine. 2009;360(14):1395–407. Epub 2009/04/01. 10.1056/NEJMoa0810177 [DOI] [PubMed] [Google Scholar]
- 32.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2007;49(2 Suppl 2):S12–154. Epub 2007/02/06. [DOI] [PubMed] [Google Scholar]
- 33.Sharp Collaborative G. Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. American heart journal. 2010;160(5):785–94.e10. Epub 2010/11/26. 10.1016/j.ahj.2010.08.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction, when the researchers used it in this form. All relevant data are within the paper.