Abstract
Abnormalities in the signaling of the N-methyl-D-aspartate subtype of the glutamate receptor (NMDAR) within cortical and limbic brain regions are thought to underlie many of the complex cognitive and affective symptoms observed in individuals with schizophrenia. The M1 muscarinic acetylcholine receptor (mAChR) subtype is a closely coupled signaling partner of the NMDAR. Accumulating evidence suggests that development of selective positive allosteric modulators (PAMs) of the M1 receptor represent an important treatment strategy for the potential normalization of disruptions in NMDAR signaling in patients with schizophrenia. In the present studies, we evaluated the effects of the novel and highly potent M1 PAM, VU6004256, in ameliorating selective prefrontal cortical (PFC)-mediated physiologic and cognitive abnormalities in a genetic mouse model of global reduction in the NR1 subunit of the NMDAR (NR1 knockdown [KD]). Using slice-based extracellular field potential recordings, deficits in muscarinic agonist-induced long-term depression (LTD) in layer V of the PFC in the NR1 KD mice were normalized with bath application of VU6004256. Systemic administration of VU6004256 also reduced excessive pyramidal neuron firing in layer V PFC neurons in awake, freely moving NR1 KD mice. Moreover, selective potentiation of M1 by VU6004256 reversed the performance impairments of NR1 KD mice observed in two preclinical models of PFC-mediated learning, specifically the novel object recognition and cue-mediated fear conditioning tasks. VU6004256 also produced a robust, dose-dependent reduction in the hyperlocomotor activity of NR1 KD mice. Taken together, the current findings provide further support for M1 PAMs as a novel therapeutic approach for the PFC-mediated impairments in schizophrenia.
Keywords: positive allosteric modulator, antipsychotic, cognitive enhancement, M1 muscarinic, NR1 KD, VU6004256
Graphical Abstract
INTRODUCTION
Multiple lines of evidence suggest that disruptions in the normal signaling of the N-methyl-D-aspartate subtype of the glutamate receptor (NMDAR) may underlie many of the symptoms associated with schizophrenia, particularly the cognitive impairments.1–3 Previous studies have demonstrated that acute or chronic administration of noncompetitive NMDAR antagonists, including phencyclidine (PCP) and ketamine, induce psychotic-like symptoms and cognitive impairments in animals and healthy individuals and exacerbate symptoms in patients with schizophrenia (see reviews by refs 1,4). Recent copy number variation analysis studies have identified numerous de novo mutations in genes encoding the NMDAR and other proteins within the glutamatergic postsynaptic density associated with increased risk of schizophrenia.5–7 Post-mortem studies have also revealed reductions in the mRNA and protein expression of the NR1 subunit of the NMDAR in the prefrontal cortex (PFC) of individuals with schizophrenia.8 Moreover, animal studies with cell-specific deletion or global knockdown (KD) of the GluN1 subunit of the NMDAR have reported abnormalities in various physiologic measures, including changes in gamma band oscillations, and impairments in social and cognitive performance that are considered endophenotypes of schizophrenia symptoms.9–14 Taken together, targeting abnormalities in NMDAR function represents a critical strategy for the treatment of the complex symptoms associated with schizophrenia.
Development of ligands that selectively target the M1 muscarinic acetylcholine receptor (mAChR) subtype represents an important approach for modulating NMDAR function and could lead to potential therapeutic interventions for schizophrenia.15 The M1 mAChR subtype is one of the five different mAChR subtypes, termed M1–M5,16,17 which are G-protein-coupled receptors (GPCRs) activated by the neurotransmitter acetylcholine (ACh). M1 is highly expressed across many forebrain regions implicated in the pathophysiology of schizophrenia, including the striatal complex, all layers of the cortex, and postsynaptically on the dendrites and cell bodies of hippocampal granule cells and pyramidal neurons.18–22 Post-mortem studies have reported that M1 mAChR expression is reduced in the PFC, hippocampus, and other forebrain regions of a subset of patients with schizophrenia.23–25 More importantly, previous studies have shown that M1 is both physically and functionally coupled to NMDARs and activation of M1 potentiates NMDAR currents in cortical and hippocampal pyramidal cells, increases excitatory postsynaptic currents in PFC neurons, and enhances performance in several preclinical learning and memory tasks.15,26–31 Conversely, M1 knockout (KO) mice have impaired performance in medial PFC-dependent cognitive tasks and reduced hippocampal long-term potentiation (LTP).26,32–34 While clinical studies with selective M1 ligands have been limited, studies with the M1/M4-preferring mAChR agonist xanomeline produced robust efficacy in reversing psychotic symptoms and behavioral disturbances, with some modest effects on cognitive impairments, in both schizophrenia and Alzheimer’s disease patient populations.35–37 Unfortunately, xanomeline, similar to other orthosteric mAChR agonists, failed to progress in clinical development due to dose-limiting adverse effects from nonselective activation of other mAChR subtypes.38
Over the past decade, we and others have developed subtype-selective M1 mAChR ligands; these compounds do not target the orthosteric binding site of acetylcholine (ACh) that is highly conserved across all five mAChR subtypes, but rather, they act at more topographically distinct allosteric sites.15,30,31 In particular, our group and others have now reported the discovery and extensive optimization of multiple novel chemical series of highly selective M1 positive allosteric modulators (PAMs) which enhance the efficacy and/or affinity of the endogenous neurotransmitter ACh at the M1 mAChR.28,39–46 Earlier generation M1 PAMs served as important tool compounds for the in vitro characterization of the molecular and brain slice-based physiologic activity of selective M1 potentiation but provided limited utility for animal studies due to poor brain penetration.28,47–50 Using several second-generation M1 PAMs with more favorable pharmacokinetic (PK) properties for in vivo dosing, selective potentiation of M1 has been reported to reverse pharmacologically induced deficits and/or improve normal performance on many measures of learning, memory, and top-down prefrontal processing in rodents and nonhuman primates.26–28,44,46,51–56 For example, the M1 PAM BQCA ([1-(4-methoxybenzyl)-4-oxo-1,4-dihy-droquinoline-3-carboxylic acid]) robustly enhanced the synaptic excitation of pyramidal cells in the PFC and improved PFC-mediated cognitive functions, including performance in attentional set shift tasks in rodents.28 More recently, we reported the discovery of the selective M1 PAM VU0453595, which enabled further characterization of the role of M1 on PFC-mediated synaptic plasticity, cognitive function, and social interaction. Specifically, VU0453595 reversed chronic PCP-induced disruptions in muscarinic long-term depression (mLTD) in the PFC and corresponding deficits in novel object and social recognition tasks.27 However, to date the progress in understanding the role of M1 modulation in PFC-mediated physiology and behavior relevant to schizophrenia has been achieved using only acute and chronic NMDAR antagonist challenge approaches in adult animals.
Recent development of several genetic models of reduced NMDAR activity have provided important in vivo systems for studying changes in relevant cortical and limbic circuitry comparable to those observed in schizophrenia (see refs 1,9–11,13,57,58). While the null mutation of Grin1, the gene encoding the NR1 subunit of NMDARs, is lethal, a hypomorphic or partial loss-of-function mutation of Grin1 results in an NR1 subunit knockdown (NR1 KD) mouse that is still viable. These mice display only 10–15% of the wild-type levels of NMDARs and exhibits severe behavioral and physiologic impairments, many of which are consistent with those induced by acute and/or chronic NMDAR antagonist challenge.58 We and others have reported that NR1 KD mice display increased locomotor and stereotypic behaviors that can be reduced with antipsychotic drugs and a novel metabotropic glutamate receptor subtype 5 (mGlu5) PAM, as well as severe impairments in social interactions and cognition.14,58 In the present studies, we specifically evaluated PFC-mediated physiologic and cognitive functions in NR1 KD mice. First, using slice-based extracellular field potential recordings, we found deficient muscarinic agonist-induced LTD in layer V of the PFC in NR1 KD mice. Using in vivo electrophysiological recordings in awake, freely moving NR1 KD mice, we report excessive pyramidal neuron firing in layer V PFC neurons. NR1 KD mice were also impaired in their responses in two preclinical models of PFC-mediated learning and memory, the novel object recognition and cue-mediated fear conditioning tasks. Importantly, we report the ability of the novel and highly potent M1 PAM VU6004256 to reverse these selective PFC-mediated abnormalities in synaptic plasticity, in vivo physiology, and behavior in NR1 KD mice.
METHODS
Chemicals
VU6004256 (4,6-difluoro-N-(1S,2S)-2-hydroxycyclo-hexyl-1-((6-(1-methyl-1H-pyrazol-4-yl)pyridine-3-yl)methyl)-1H-indole-3-carboxamide) and VU045359527,56 were synthesized in-house and dissolved in DMSO for slice physiology studies or 10% Tween 80 in sterile water for in vivo studies. Carbachol (Sigma, St. Louis, MO) stocks were prepared in water, and all other compound stocks were prepared in DMSO. For field recording experiments, all drugs were diluted in artificial cerebrospinal fluid (ACSF) (0.1% DMSO final conc.) and bath applied. All chemicals were obtained from Sigma (St. Louis, MO).
Animal Care and Housing
All in vivo studies were carried out using age-matched adult male NR1 KD mice with a C57B1/6J and 129 × 1/SvJ crossed background and wild-type littermate controls (described previously by refs 12–14). Animals were group-housed under a 12/12 h light-dark cycle (lights on at 6 AM) with food and water available ad libitum unless stated elsewhere. All animal experiments were approved by the Animal Care and Use Committees of Vanderbilt University and University of Toronto, and experimental procedures conformed to guidelines established by the National Research Council Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and the number of animals used.
Extracellular Field Potential Recordings
Eight to ten week old male wild-type or NR1 KD mice were anesthetized with isofluorane, and the brains were removed and submerged in ice-cold cutting solution (in mM: 230 sucrose, 2.5 KCl, 8 MgSO4, 0.5 CaCl2, 1.25 NaH2PO4, 10 D-glucose, 26 NaHCO3). Coronal slices containing the prelimbic prefrontal cortex were cut at 400 μm using a compresstome (Precisionary Instruments, Greenville, NC). Slices were transferred to a holding chamber containing NMDG-HEPES recovery solution (in mM: 93 NMDG, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 D-glucose, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate, 10 MgSO4, 0.5 CaCl2, 12 N-acetyl-L-cysteine, pH 7.3, < 310 mOsm) for 15 min at 32 °C. Slices were then transferred to a room-temperature holding chamber for at least 1 h containing ACSF (in mM: 126 NaCl, 1.25 NaH2PO4, 2.5 KCl, 10 D-glucose, 26 NaHCO3, 2 CaCl2, 1 MgSO4) supplemented with 600 μM sodium ascorbate for slice viability. All buffers were continuously bubbled with 95% O2/5% CO2. Subsequently, slices were transferred to a 32 °C submersion recording chamber where they were perfused with ACSF at a rate of 2 mL/min. Borosilicate glass electrodes were pulled using a Flaming/Brown micropipette puller (Sutter Instruments) and had a resistance of 2–4 MΩ when filled with ACSF. Field excitatory post synaptic potentials (fEPSP) were recorded from layer V of the prelimbic area of the mouse prefrontal cortex in response to electrical stimulation of superficial layer II/III. Input-output curves were generated to determine the stimulation intensity that produced 50–60% of the maximum response before each experiment, which was used as the baseline stimulation. A minimum of 10 min stable baseline was recorded before bath application of any drugs. 50 μM carbachol, a saturating concentration in wild-type animals, was applied for 10 min to induce a form of muscarinic LTD in the prefrontal cortex. VU6004256 was applied alone for 10 min immediately followed by coapplication with carbachol. Sampled data was analyzed offline using Clampfit 10.2 (Molecular Devices, Sunnyvale, CA). The slopes from three sequential sweeps were averaged. All slopes calculated were normalized to the average slope calculated during the predrug period (percent of baseline). Data were digitized using a Multiclamp 700B, Digidata 1322A, and pClamp 10.3 software (Molecular Devices).
In Vivo Electrophysiology
A modified version of Walker et al.59 was used for the in vivo electrophysiology studies. Under isoflurane anesthesia, mice were secured in a stereotaxic apparatus and a craniotomy was performed at the following coordinates: AP (+1.7 mm) ML (+0.5 mm).60 The 2.5 mm long electrode bundle was composed of eight 25 μm Formvar-insulated stainless-steel wires for recording single units and two 50 μm uninsulated stainless-steel ground wires, one of which served as a depth reference electrode. Mice were allowed to recover for 5 to 7 days before being introduced to the recording arena. A wire harness attached to a headstage containing unity gain amplifiers was secured to the electrode array, and mice were allowed to explore the chamber for 15 min prior to the initiation of baseline recording. Animals were placed in a chamber equipped with a Faraday cage and attached to an electric commutator which allowed for unrestricted movement during the in vivo recording. All recordings took place between 12 and 4 p.m.
Neuronal discharges were acquired by the Multichannel Acquisition Processor system following preamplification (Plexon Inc., Dallas, TX). To detect spiking activity, signals were bandpass filtered (154 Hz to 8.8 kHz) and digitized at a rate of 40 kHz. After establishing a voltage threshold ≥2.5 times background noise, waveforms were continuously collected, and principal component analysis was used to discriminate putative pyramidal neurons from fast-spiking interneurons. All recording sessions lasted for 75 min. Baseline firing rate was assessed during the final 5 min of the baseline collection period (30 min), and the effects of vehicle (10% Tween 80, i.p.) or VU6004256 (10 mg/kg, i.p.) were assessed during the final 5 min of the drug treatment period (45 min). Data are expressed as mean frequency during each period or as a percent of baseline, and significance is determined using a paired t test.
Novel Object Recognition Task
Mice were placed in a dark plexiglass arena (32 × 32 × 40 cm) and allowed to habituate for 10 min for three consecutive days. On the following day, mice were injected with either vehicle (10% Tween 80, i.p.) or VU6004256 (3 mg/kg or 10 mg/kg, i.p.) and returned to their home cages for 5 min. Mice were then placed back in the arena with 2 identical objects for 10 min. Following the initial exposure, mice were placed in their home cages for 1 h. Upon completion of the 1 h period, mice were returned to the arena and one of the familiar objects was replaced with a novel object. Mice were recorded for 10 min, and data were scored by 2 observers blinded to experimental conditions. During both the familiarization (day 1) and the test phase (day 2), location of novel versus familiar object was counterbalanced. Exploration of objects was considered sniffing or investigatory behavior; time animals spent on top of the objects was excluded.61 Recognition index scores were calculated using the following equation: (seconds spent exploring novel object – seconds spent exploring familiar object)/total time exploring either object.
Cue-Dependent Fear Conditioning
Studies were conducted using conditioning chambers in sound attenuating cubicles equipped with a stainless steel grid floor for shock deliver and a video camera for recording freezing behavior, as previously described (MedAssociates, Allentown NJ, see ref 62). Wild-type and NR1 KD mice, 8–10 weeks old, were handled for 3 days prior to conditioning and injected with saline for 1 day prior to commencement of the study. On the conditioning day, mice were habituated for 1 h in an anteroom adjacent to the test room. Mice were pretreated with vehicle (10% Tween 80, i.p.) or a dose of VU6004256 (10 mg/kg i.p.) 5 min prior to conditioning. Mice were then placed into the chamber, scented with 1.0 mL of 10% vanilla extract and illuminated by a white house light, and exposed to the following events during an 8 min session: 90 s habituation followed by four 30 s tone presentations (85 dB, 2500 Hz) coterminating with a shock (0.7 mA, 1 s) with an intertrial interval of 60 s, followed by a 90-s interval without stimuli. Approximately 24 h after conditioning, mice were returned to the anteroom where they were habituated under infrared light for 60 min. The test room and chamber were also illuminated by an infrared light only. The context of the chamber was altered with the addition of a white plexiglass floor on top of the shock grid, a black teepee to alter the shape/size of the chamber, and a 0.5 mL 10% Eucalyptus oil odor cue. Mice were exposed to the identical testing paradigm as on the conditioning day but without the shock stimuli. Freezing behavior was measured in the absence of any shock stimuli for 8 min. Data are presented as means ± SEM and analyzed by one-way ANOVA followed by Bonferroni’s test.
Locomotor Activity Studies in Mice
Spontaneous locomotor activity was assessed in 8–10 week old wild-type and NR1 KD mice. Animals were placed in an open field system (OFA-510, MedAssociates, St. Albans, VT) with three 16 × 16 arrays of infrared photobeams, as described previously.63 Mice were allowed to habituate in their home cage for 1 h prior to the start of the experiment. Following the habituation period, mice were injected with either vehicle (10% Tween 80, i.p.) or a dose of VU6004256 (1 mg/kg or 10 mg/kg, i.p.) and placed directly into the open field chambers. Spontaneous locomotor activity was recorded for 90 min. The time course of drug-induced changes in spontaneous locomotor activity is expressed as distance traveled (cm) per 5 min bin for the 90 min session. Total activity data represent the sum of all 5 min bins for the recording session. Data are represented as mean ± SEM and were analyzed using two-way ANOVA with Bonferroni’s post hoc test.
RESULTS AND DISCUSSION
VU6004256 Is a Highly Potent M1 PAM with Optimized Pharmacokinetic Properties Relative to the Previously Reported M1 PAM VU0453595
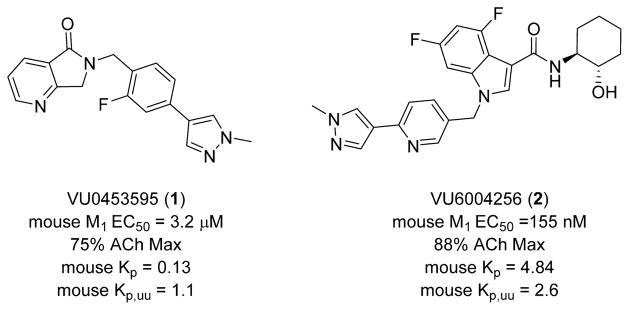
As shown in Figure 1, the structure of the novel M1 PAM 4,6-difluoro-N-(1S,2S)-2-hydroxycyclohexyl-1-((6-(1-methyl-1H-pyrazol-4-yl)-pyridine-3-yl)methyl)-1H-indole-3-carboxamide 2 (VU6004256) is compared side-by-side with previously published M1 PAM VU0453595, 1.27,64,65 VU6004256 is a potent potentiator of mouse M1 (EC50 = 155 nM, 88% ACh Max) and is ~20-fold more potent than VU0453595. In addition, VU6004256 displays a mouse brain:plasma partitioning (Kp) coefficient of 4.84 and an unbound brain:plasma coefficient (Kp,uu) of 2.6. In addition to selectivity for M1 (M2–5 EC50s > 30 μM), VU6004256 also displayed no off-target pharmacology at over 68 GPCRs, ion channels and transporters in an ancillary pharmacology radioligand binding panel (% inhibition @ 10 μM < 50%). Therefore, VU6004256 represents a more potent and centrally penetrant M1 PAM compared to VU0453595 to explore efficacy in NR1 KD mice as a genetic model of NMDAR hypofunction.
Figure 1.
Structure, potency and pharmacokinetic properties of the novel M1 PAM 4,6-difluoro-N-(1S,2S)-2-hydroxycyclohexyl-1-((6-(1-methyl-1H-pyrazol-4-yl)pyridine-3-yl)methyl)-1H-indole-3-carboxa-mide (VU6004256) (right) in comparison with the previously published M1 PAM VU0453595 (left).
Deficits in Carbachol-Induced LTD Can Be Rescued by VU6004256
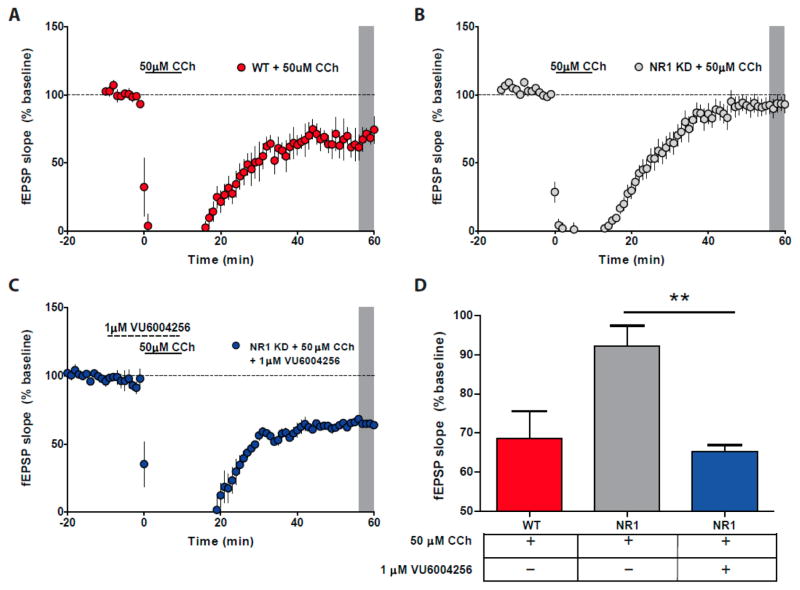
Bath application of carbachol (50 μM) induced a robust muscarinic LTD response in layer V mPFC of wild-type mouse tissue (68.5% ± 7.0 of baseline; Figure 2A). This effect was dramatically blunted in NR1 KD mouse tissue (88.3% ± 5.1 of baseline; Figure 2B), but bath application of VU6004256 (1 μM) during the carbachol application resulted in normalization of the muscarinic LTD response (Figure 2C) to levels comparable to wild-type mouse tissue (65.3% ± 1.6, H = 10.41, p < 0.01; Figure 2D).
Figure 2.
Muscarinic LTD is deficient in NR1 KD animals but can be rescued by the M1 PAM VU6004256. (A) Carbachol (CCh) application (50 μM) induced LTD in layer V PFC in wild-type mice (WT, red circles, shaded gray area). (B) Carbachol-induced LTD is absent in NR1 KD animals, but treatment with VU6004256 (1 μM) before and during carbachol application resulted in significant induction of LTD (C) (blue circles, gray shaded area). (D) Graphical representation comparing minutes 55–60 from panels A–C (** p = 0.01, ANOVA, Kruskal–Wallis followed by Dunn’s post hoc test).
VU6004256 Attenuates Excessive Pyramidal Cell Firing in the Prefrontal Cortex of NR1 KD Mice
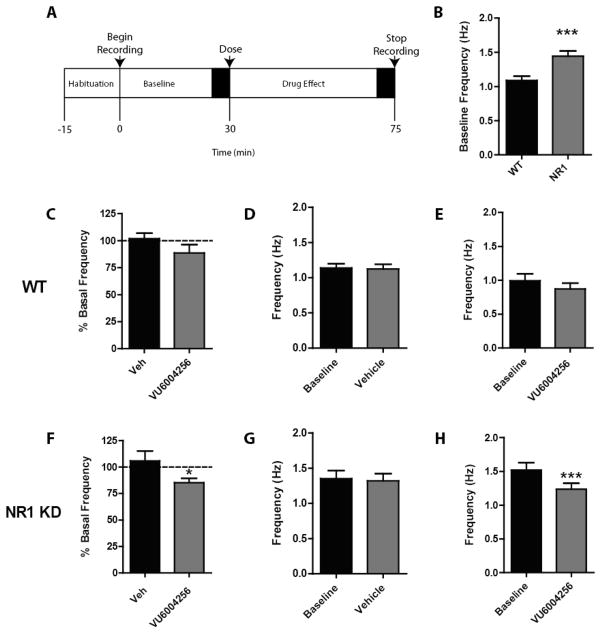
In vivo electrophysiology recordings from the prelimbic cortex of awake, behaving mice revealed a significant increase in firing rate of pyramidal neurons in NR1 KD mice (1.44 Hz ± 0.079) compared to wild-type littermate controls (1.090 Hz ± 0.0603)(t141 = 3.544, p < 0.0005; see Figure 3B). Mice received either vehicle or 10 mg/kg VU6004256 following 30 min of baseline recording (Figure 3A). Pyramidal cell firing rate was reduced in the NR1 KD animals following VU6004256 administration (85.3% ± 4.1, t69 = 2.09, df = 69, p < 0.05; Figure 3F), with no observed effect of vehicle dosing (105.8% ± 9.4; Figure 3F). Vehicle or VU6004256 administration did not affect firing rate in wild-type animals (101.8% ± 5.1 and 88.7% ± 7.7, respectively; Figure 3C). Comparison of raw firing rates using a paired t-test revealed a strong postdose decrease in NR1 KD mice following VU6004256 administration (t37= 3.76, p < 0.001; Figure 3H) but no effect in wild-type animals (Figure 3E).
Figure 3.
M1 PAM administration results in reversal of excessive pyramidal cell firing in the PFC of awake, freely moving NR1 KD mice. (A) Schematic of recording paradigm with black boxes indicating periods where firing rate averages were assessed. (B) NR1 KD mice exhibit significantly increased firing rate compared to WT littermate controls. (C–E) VU6004256 had no effect on pyramidal cell firing rate in WT littermate controls. (F–H) VU6004256 significantly reduced pyramidal cell firing rate in NR1 KD mice. Data in panels C and F represent changes in frequency compared to the baseline recording period prior to drug administration. (B, C, and F) t test, * p < 0.05. (D, E, G, and H) Paired t test, *** p < 0.001. B: n = 71–72 cells, C–H: n = 33–41 cells.
VU6004256 Administration Improves Performance in Novel Object Recognition
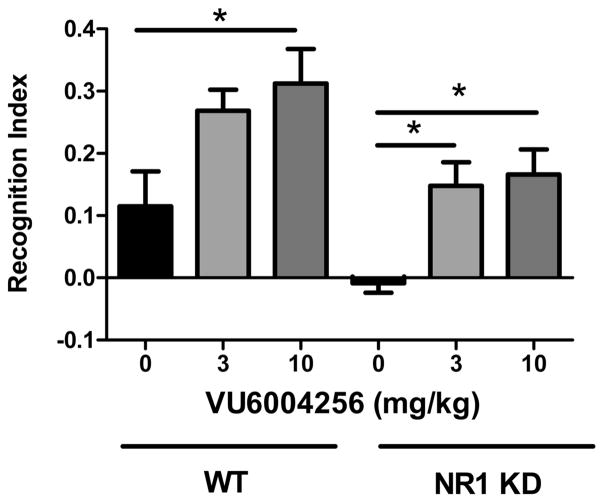
NR1 KD mice showed a marked, but statistically insignificant, reduction in recognition index compared to their wild-type littermate controls (−0.0085 ± 0.0156 vs 0.1150 ± 0.0563, respectively). Pretreatment with VU6004256 at either 3 mg/kg (0.1475 ± 0.0381) or 10 mg/kg (0.1662 ± 0.0401) significantly improved recognition index in NR1 KD mice (Fdose(1,44) = 14.64, p < 0.05; Fgenotype(2,44) = 11.43, p < 0.05; Finteraction(2,44) = 0.056, n.s.), while the top dose of 10 mg/kg (0.3125 ± 0.0552) also improved performance in wild-type mice (Figure 4).
Figure 4.
Novel object recognition deficit of NR1 KD mice is reversed by M1 PAM administration. NR1 KD animals showed no preference for the novel object when treated with vehicle. Pretreatment with VU6004256 resulted in dose-dependent increases in recognition index. Acute treatment with 10 mg/kg VU6004256 in WT animals resulted in an increase in recognition index (n = 8–10/treatment group, * p < 0.05, two-way ANOVA followed by Bonferroni’s post hoc test).
NR1 KD Mice Display Severe Deficits in Conditioned Freezing
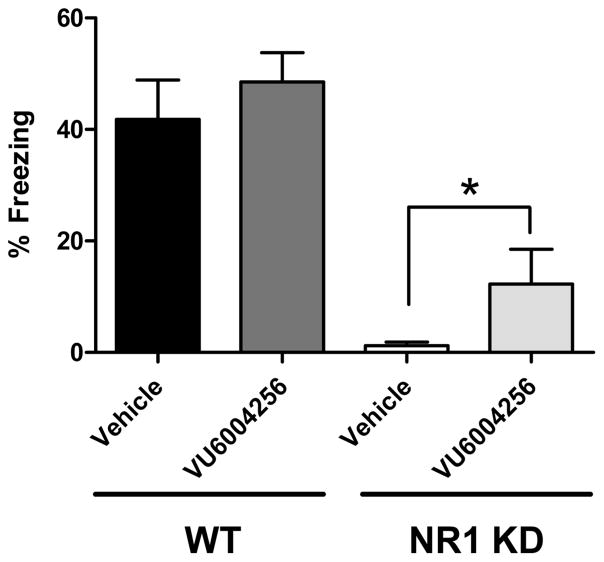
Initial studies sought to characterize the ability of wild-type and NR1 KD mice to acquire contextual- (hippocampal-dependent) and cue-mediated (PFC/amygdala-dependent) fear conditioning responses (see Supporting Information for methods and graphical representation of these data (Supplementary Figure 1A,B)). Wild-type mice were able to acquire both contextual- and cue-mediated associative learning tasks (74.9 ± 6.0 and 17.4% ± 2.9, respectively). NR1 KD mice showed a lack of freezing in both paradigms (4.6% ± 1.8 and 1.5% ± 0.3, respectively; Supplementary Figure 1A,B) that was significantly lower than that of wild-type controls (context: t24 = 9.70, p < 0.0001; cue: t23 = 4.86). To specifically determine if potentiation of M1 mAChRs would normalize PFC-mediated cognitive impairments, the effects of VU6004256 on cue-mediated fear conditioning were examined in wild-type and NR1 KD animals. NR1 KD mice displayed severe deficits in cue-mediated fear conditioning compared to their wild-type littermate controls (1.2% ± 0.7 and 41.9% ± 7.0, respectively; see Figure 5). Pretreatment with 10 mg/kg VU6004256 5 min before being placed in the chamber on the training day significantly improved performance on the test day in NR1 KD mice (12.3% ± 6.2, t14 = 1.762, p < 0.05).
Figure 5.
Pretreatment with VU6004256 on training day significantly improved performance on test day in cue-mediated conditioned freezing in NR1 KD but not WT mice (n = 8–10/group, t test, * p < 0.05).
VU6004256 Reverses Excessive Locomotor Activity in NR1 KD Mice
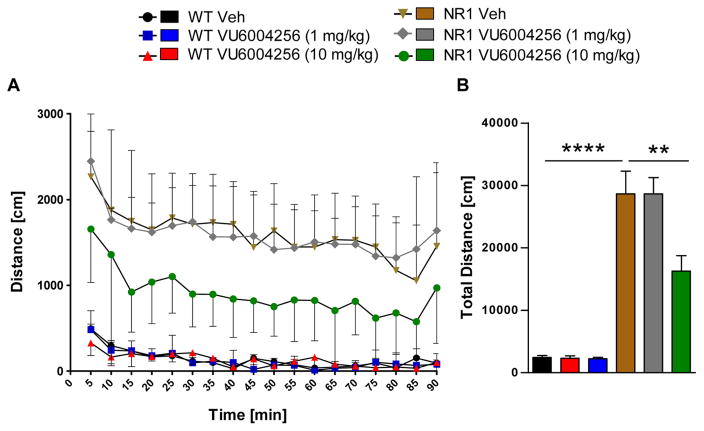
NR1 KD mice exhibit excessive spontaneous locomotor activity compared to their wild-type littermate controls (Fgenotype(5, 39) = 31.35, p < 0.0001; see Figure 6B). In NR1 KD mice, pretreatment with 10 mg/kg VU6004256 produced a significant reduction in spontaneous locomotor activity that persisted for the duration of measurement (Fdose(5,612) = 331.9, p < 0.001; Ftime(17,612) = 6.101, p < 0.001, Finteraction(85,612) = 0.46, n.s.; Figure 6A). No reduction in locomotor activity was observed at 1 mg/kg in NR1 KD mice. Additionally, pretreatment with VU6004256 at all doses did not alter spontaneous locomotor activity in wild-type mice. Spontaneous locomotor activity was also measured in response to the M1 PAM VU0453595 (Supplementary Figure 2). VU0453595 was unable to significantly alter spontaneous locomotor activity in NR1 KD or wild-type mice (Fdose(9,2400) = 246.7, p < 0.001; Ftime(23,2400) = 16.19, p < 0.001, Finteraction(207,2400) = 0.97, n.s.); though there was a strong downward trend at the 1 mg/kg dose (Supplementary Figure 2B).
Figure 6.
VU6004256 reduces hyperlocomotor activity in NR1 KD mice. Vehicle-treated NR1 KD mice showed excessive locomotor activity when compared to WT littermate controls. Pretreatment with VU6004256 (1–10 mg/kg) dose-dependently reduced locomotor activity in NR1 KD, but not in WT mice. (n = 7–9/treatment group, ** p < 0.01 vs NR1 KD vehicle, *** p < 0.001 vs WT vehicle, ANOVA followed by Bonferroni’s post hoc test).
In summary, potentiation of the M1 mAChR subtype represents an important indirect mechanism for the potential normalization of aberrant NMDAR signaling thought to underscore many of the symptoms observed in schizophrenia. In the present studies, we provide the first evidence of selective physiological dysfunction within the PFC and PFC-related cognitive deficits in a genetic model of global, constitutive knockdown of the NR1 subunit of the NMDAR. More importantly, selective potentiation of M1 using the optimized and highly potent M1 PAM VU6004256 resulted in a restoration of these deficits in PFC-mediated synaptic plasticity, pyramidal cell firing, and corresponding measures of learning and memory.
Discovery of highly selective and potent M1 PAMs allows for the investigation of antipsychotic-like effects that has been previously reported in preclinical and clinical studies with the M1/M4 preferring ligand, xanomeline.35–37,66 While dose-dependent reversal of psychostimulant-induced increases in locomotor activity has been observed previously with the M1 selective ligands, as demonstrated by the M1 allosteric agonist TBPB and the M1 PAM BQCA, much of the antipsychotic-like efficacy of xanomeline was attributed to M4 activation.30,44 In the current studies, we observed a significant reduction in elevated spontaneous locomotor activity of NR1 KD mice with a selective M1 PAM in a genetic model of NMDAR hypofunction that is consistent with acute psychostimulant challenge models. These data further the hypothesis that M1 modulation in cortical and limbic regions may contribute to antipsychotic-like effects observed with xanomeline. Interestingly, these effects are only observed with the more potent M1 PAM, VU6004256 (EC50 = 155 nM), and not with VU0453595 (EC50 = 3.22 μM) (Supplementary Figure 2), suggesting VU6004256 may have efficacy in other behavioral models that less potent M1 ligands do not. These findings provide valuable insight into the importance of developing more potent M1ligands to assess additional aspects of M1-related antipsychotic-like efficacy.
Traditionally, acute or repeated dosing with a use-dependent open channel blocker of NMDARs is employed to produce NMDAR hypofunction in rodents that mimics neurochemical and behavioral changes observed in patients with schizophrenia.67,68 There is a potential liability when evaluating M1 PAMs in these models because M1 is a closely associated signaling partner of NMDARs and selective activation of M1 in cortical and hippocampal regions results in the downstream potentiation of NMDAR currents, which could exacerbate the effects of the NMDAR antagonist.20 Importantly, these data provide the first evidence supporting antipsychotic-like effects with an M1 PAM in a genetic model of NMDAR hypofunction that does not rely on pharmacologic manipulation to induce behavioral disruptions. Because of the critical impact of NMDAR signaling in the modulation of normal cognition and neural circuitry hypothesized to be dysregulated in schizophrenia,20,69 ligands like the M1 PAM VU6004256 that selectively potentiate the response of ACh at M1 mAChRs may ameliorate many of the psychotic and cognitive impairments of schizophrenia through enhancement of NMDAR neurotransmission. Moreover, selective potentiation of M1 by VU6004256 provides indirect modulation of NMDAR function, which may produce therapeutic effects without the potential high risk of excitotoxicity connected with direct agonist activity at the NMDAR.
Previous reports indicate a crucial role for mAChRs in regulating synaptic plasticity in the PFC and suggest that deficits in cholinergic signaling may contribute to the electrophysiological and behavioral phenotypes associated with dysfunctional PFC plasticity. For example, nonselective activation of mAChRs is sufficient to convert transient depression into long-lasting LTD in the PFC following a subthreshold LTD protocol in rats.70 Importantly, acute coapplication of an NMDAR antagonist blocked this response, suggesting an integral role of glutamatergic activation in muscarinic-mediated cortical LTD. The effects of mAChRs on this form of plasticity in the PFC were further characterized in studies utilizing M1 knockout mice and the M1 PAM VU0453595, revealing that muscarinic LTD is dependent on M1 activation and can be potentiated by an M1 PAM in wild-type animals. Interestingly, synaptic depression was not observed following repeated NMDAR administration during early adolescent development, strengthening the importance of intact NMDA-M1 receptor signaling in cortical synaptic plasticity.27
Here we report the first electrophysiological analysis of spontaneous pyramidal cell activity in the layer V PFC of awake, free-moving NR1 KD mice in comparison with their wild-type littermate controls. Our findings revealed substantial information-processing deficits71 at the single-neuron level as denoted by a 25% increase in the firing rates in the NR1 KD mice. This disinhibition of PFC pyramidal cell firing in the NR1 KD mice is consistent with previous studies performed in rodents after acute administration of MK-801 or PCP.71 At present, the underlying mechanism(s) for this disinhibition of PFC activity in the NR1 KD mice remains unknown. However, based on NMDAR antagonist studies, one possible explanation may involve increased PFC activation resulting from dis-inhibition of hippocampal-PFC inputs,72–75 resulting in reduced LTD and enhanced LTP mechanisms.74,75 This interpretation is further supported by the observation that the novel M1 PAM VU6004256 decreased the firing rates in the NR1 KD mice. Alternatively, activation of M1 is known to increase excitability in GABAergic interneurons of the PFC76 through an NMDAR-independent mechanism. Thus, another possible explanation for the effects of VU6004256 on pyramidal cell firing rates in NR1 KD mice may by through increased activity of GABAergic interneurons in the PFC, resulting in sustained reductions in PFC excitation. In future studies, it will also be important to further understand the relative contributions of M1 modulation to both NMDAR-dependent and -independent mechanisms of PFC circuitry disrupted in the mice with global NR1 KD.
Genetic models of NMDAR hypofunction exhibit profound cognitive deficits as measured in radial arm maze performance, social and object recognition, and spatial and nonspatial memory tasks.9,77,78 Consistent with these findings, we observed robust deficits in context- and cue-mediated fear conditioning in NR1 KD mice, similar to the deficits encountered in NR2C knockout mice.79 M1 PAM administration improved deficits of NR1 KD mice in novel object recognition and cue-mediated fear conditioning, both of which are thought to heavily rely on intact PFC signaling. These data, taken together with recent reports that M1 PAMs are efficacious in similar behavioral tasks performed in a pharmacologic model of NMDAR hypofunction,27 suggest that M1 potentiation may have broad utility across several animal models of schizophrenia.
Collectively, the current studies provide important novel insights into the role of M1 modulation of PFC-mediated physiologic and cognitive functions under conditions of chronic NMDAR hypofunction. These findings also confirm and extend accumulating evidence for the broader therapeutic utility for M1 PAMs in the treatment of affective and, more importantly, cognitive impairments observed in schizophrenia.
Supplementary Material
Acknowledgments
Funding
This work was supported by grants from the National Institute of Mental Health to C.W.L. (2RO1MH082867), P.J.C. (R01MH073676), and C.K.J. (R01MH86601) and Canadian Institutes of Health Research to A.J.R. (MOP119298). C.W.L. thanks the Warren Family and the WKWF for support of our programs and for funding the William K. Warren, Jr. Chair in Medicine.
We thank Josh Luffiman and Wendy Horsfall for their support in breeding of the NR1 KD mice. The authors also thank Dr. George Rebec and Scott Barton for lending their expertise and training for the in vivo electrophysiology studies.
ABBREVIATIONS
- ACh
acetylcholine
- CCh
carbachol
- GPCR
G-protein-coupled receptor
- LTD
long-term depression
- mAChR
muscarinic acetylcholine receptor
- NMDAR
N-methyl-D-aspartate subtype of the glutamate receptor
- NR1 KD
NR1 knockdown
- PAM
positive allosteric modulator
- PFC
prefrontal cortex
Footnotes
Author Contributions
M.D.G., S.P.M., R.W.G, and C.K.J. designed the experiments. M.D.G, C.A.M., S.P.M., R.W.G, J.B., and Z.L. performed the experiments. A.J.R., M.A., H.P.C., K.D.N., A.L.B., C.M.N., P.J.C., C.W.L., and C.K.J. contributed reagents and other resources. M.D.G., C.A.M., and S.P.M. performed data analyses. M.D.G. and C.K.J. wrote the manuscript.
- Cue- and context-mediated fear conditioning performance in WT and NR1 KD mice; effects of VU0453595 on spontaneous locomotor activity in WT and NR1 KD mice (PDF)
The authors declare the following competing financial interest(s): Over the past year, C.W.L. consulted for Abbott. M.B., T.M.B., C.W.L., P.J.C., and C.K.J. received research/salary support from AstraZeneca and/or Bristol Myers Squibb. C.M.N., C.W.L., and P.J.C. are inventors on multiple composition of matter patents protecting allosteric modulators of GPCRs. The remaining authors declare no competing financial interests.
References
- 1.Balu DT. The NMDA Receptor and Schizophrenia: From Pathophysiology to Treatment. Adv Pharmacol. 2016;76:351–382. doi: 10.1016/bs.apha.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantrowitz JT, Javitt DC. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clin Schizophr Relat Psychoses. 2010;4:189–200. doi: 10.3371/CSRP.4.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010;83:108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 5.Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, Georgieva L, Rees E, Palta P, Ruderfer DM, Carrera N, Humphreys I, Johnson JS, Roussos P, Barker DD, Banks E, Milanova V, Grant SG, Hannon E, Rose SA, Chambert K, Mahajan M, Scolnick EM, Moran JL, Kirov G, Palotie A, McCarroll SA, Holmans P, Sklar P, Owen MJ, Purcell SM, O’Donovan MC. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D, Moran J, Chambert K, Toncheva D, Georgieva L, Grozeva D, Fjodorova M, Wollerton R, Rees E, Nikolov I, van de Lagemaat LN, Bayes A, Fernandez E, Olason PI, Bottcher Y, Komiyama NH, Collins MO, Choudhary J, Stefansson K, Stefansson H, Grant SG, Purcell S, Sklar P, O’Donovan MC, Owen MJ. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry. 2012;17:142–153. doi: 10.1038/mp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, O’Dushlaine C, Chambert K, Bergen SE, Kahler A, Duncan L, Stahl E, Genovese G, Fernandez E, Collins MO, Komiyama NH, Choudhary JS, Magnusson PK, Banks E, Shakir K, Garimella K, Fennell T, DePristo M, Grant SG, Haggarty SJ, Gabriel S, Scolnick EM, Lander ES, Hultman CM, Sullivan PF, McCarroll SA, Sklar P. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–190. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catts VS, Lai YL, Weickert CS, Weickert TW, Catts SV. A quantitative review of the postmortem evidence for decreased cortical N-methyl-d-aspartate receptor expression levels in schizophrenia: How can we link molecular abnormalities to mismatch negativity deficits? Biol Psychol. 2016;116:57–67. doi: 10.1016/j.biopsycho.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jadi MP, Behrens MM, Sejnowski TJ. Abnormal Gamma Oscillations in N-Methyl-D-Aspartate Receptor Hypofunction Models of Schizophrenia. Biol Psychiatry. 2016;79:716–726. doi: 10.1016/j.biopsych.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tatard-Leitman VM, Jutzeler CR, Suh J, Saunders JA, Billingslea EN, Morita S, White R, Featherstone RE, Ray R, Ortinski PI, Banerjee A, Gandal MJ, Lin R, Alexandrescu A, Liang Y, Gur RE, Borgmann-Winter KE, Carlson GC, Hahn CG, Siegel SJ. Pyramidal cell selective ablation of N-methyl-D-aspartate receptor 1 causes increase in cellular and network excitability. Biol Psychiatry. 2015;77:556–568. doi: 10.1016/j.biopsych.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 13.Milenkovic M, Mielnik CA, Ramsey AJ. NMDA receptor-deficient mice display sexual dimorphism in the onset and severity of behavioural abnormalities. Genes Brain Behav. 2014;13:850–862. doi: 10.1111/gbb.12183. [DOI] [PubMed] [Google Scholar]
- 14.Gregory KJ, Herman EJ, Ramsey AJ, Hammond AS, Byun NE, Stauffer SR, Manka JT, Jadhav S, Bridges TM, Weaver CD, Niswender CM, Steckler T, Drinkenburg WH, Ahnaou A, Lavreysen H, Macdonald GJ, Bartolome JM, Mackie C, Hrupka BJ, Caron MG, Daigle TL, Lindsley CW, Conn PJ, Jones CK. N-aryl piperazine metabotropic glutamate receptor 5 positive allosteric modulators possess efficacy in preclinical models of NMDA hypofunction and cognitive enhancement. J Pharmacol Exp Ther. 2013;347:438–457. doi: 10.1124/jpet.113.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones CK, Byun N, Bubser M. Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology. 2012;37:16–42. doi: 10.1038/npp.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonner TI, Buckley NJ, Young AC, Brann MR. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- 17.Bonner TI, Young AC, Bran MR, Buckley NJ. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron. 1988;1:403–410. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- 18.Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ. Expression of m1–m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci. 1995;15:4077–4092. doi: 10.1523/JNEUROSCI.15-05-04077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marino MJ, Rouse ST, Levey AI, Potter LT, Conn PJ. Activation of the genetically defined m1 muscarinic receptor potentiates N-methyl-D-aspartate (NMDA) receptor currents in hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 1998;95:11465–11470. doi: 10.1073/pnas.95.19.11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouse ST, Gilmor ML, Levey AI. Differential presynaptic and postsynaptic expression of m1–m4 muscarinic acetylcholine receptors at the perforant pathway/granule cell synapse. Neuroscience. 1998;86:221–232. doi: 10.1016/s0306-4522(97)00681-7. [DOI] [PubMed] [Google Scholar]
- 22.Rouse ST, Marino MJ, Potter LT, Conn PJ, Levey AI. Muscarinic receptor subtypes involved in hippocampal circuits. Life Sci. 1999;64:501–509. doi: 10.1016/s0024-3205(98)00594-3. [DOI] [PubMed] [Google Scholar]
- 23.Dean B, McLeod M, Keriakous D, McKenzie J, Scarr E. Decreased muscarinic1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2002;7:1083–1091. doi: 10.1038/sj.mp.4001199. [DOI] [PubMed] [Google Scholar]
- 24.Gibbons AS, Scarr E, Boer S, Money T, Jeon WJ, Felder C, Dean B. Widespread decreases in cortical muscarinic receptors in a subset of people with schizophrenia. Int J Neuro-psychopharmacol. 2013;16:37–46. doi: 10.1017/S1461145712000028. [DOI] [PubMed] [Google Scholar]
- 25.Scarr E, Craig JM, Cairns MJ, Seo MS, Galati JC, Beveridge NJ, Gibbons A, Juzva S, Weinrich B, Parkinson-Bates M, Carroll AP, Saffery R, Dean B. Decreased cortical muscarinic M1 receptors in schizophrenia are associated with changes in gene promoter methylation, mRNA and gene targeting microRNA. Transl Psychiatry. 2013;3:e230. doi: 10.1038/tp.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gould RW, Dencker D, Grannan M, Bubser M, Zhan X, Wess J, Xiang Z, Locuson C, Lindsley CW, Conn PJ, Jones CK. Role for the M1Muscarinic Acetylcholine Receptor in Top-Down Cognitive Processing Using a Touchscreen Visual Discrimination Task in Mice. ACS Chem Neurosci. 2015;6:1683–1695. doi: 10.1021/acschemneuro.5b00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghoshal A, Rook JM, Dickerson JW, Roop GN, Morrison RD, Jalan-Sakrikar N, Lamsal A, Noetzel MJ, Poslusney MS, Wood MR, Melancon BJ, Stauffer SR, Xiang Z, Daniels JS, Niswender CM, Jones CK, Lindsley CW, Conn PJ. Potentiation of M1Muscarinic Receptor Reverses Plasticity Deficits and Negative and Cognitive Symptoms in a Schizophrenia Mouse Model. Neuropsychopharmacology. 2016;41:598–610. doi: 10.1038/npp.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shirey JK, Brady AE, Jones PJ, Davis AA, Bridges TM, Kennedy JP, Jadhav SB, Menon UN, Xiang Z, Watson ML, Christian EP, Doherty JJ, Quirk MC, Snyder DH, Lah JJ, Levey AI, Nicolle MM, Lindsley CW, Conn PJ. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci. 2009;29:14271–14286. doi: 10.1523/JNEUROSCI.3930-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sur C, Mallorga PJ, Wittmann M, Jacobson MA, Pascarella D, Williams JB, Brandish PE, Pettibone DJ, Scolnick EM, Conn PJ. N-desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-D-aspartate receptor activity. Proc Natl Acad Sci U S A. 2003;100:13674–13679. doi: 10.1073/pnas.1835612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones CK, Brady AE, Davis AA, Xiang Z, Bubser M, Tantawy MN, Kane AS, Bridges TM, Kennedy JP, Bradley SR, Peterson TE, Ansari MS, Baldwin RM, Kessler RM, Deutch AY, Lah JJ, Levey AI, Lindsley CW, Conn PJ. Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci. 2008;28:10422–10433. doi: 10.1523/JNEUROSCI.1850-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Digby GJ, Noetzel MJ, Bubser M, Utley TJ, Walker AG, Byun NE, Lebois EP, Xiang Z, Sheffler DJ, Cho HP, Davis AA, Nemirovsky NE, Mennenga SE, Camp BW, Bimonte-Nelson HA, Bode J, Italiano K, Morrison R, Daniels JS, Niswender CM, Olive MF, Lindsley CW, Jones CK, Conn PJ. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J Neurosci. 2012;32:8532–8544. doi: 10.1523/JNEUROSCI.0337-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2002;6:51–58. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- 33.Bartko SJ, Romberg C, White B, Wess J, Bussey TJ, Saksida LM. Intact attentional processing but abnormal responding in M1 muscarinic receptor-deficient mice using an automated touchscreen method. Neuropharmacology. 2011;61:1366–1378. doi: 10.1016/j.neuropharm.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyakawa T, Yamada M, Duttaroy A, Wess J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci. 2001;21:5239–5250. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, Shannon HE, Tollefson GD, Rasmussen K, Bymaster FP, Hurley DJ, Potter WZ, Paul SM. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54:465–473. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- 36.Bodick NC, Offen WW, Shannon HE, Satterwhite J, Lucas R, van Lier R, Paul SM. The selective muscarinic agonist xanomeline improves both the cognitive deficits and behavioral symptoms of Alzheimer disease. Alzheimer Dis Assoc Disord. 1997;11(Suppl4):S16–S22. [PubMed] [Google Scholar]
- 37.Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dube S, Mallinckrodt C, Bymaster FP, McKinzie DL, Felder CC. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165:1033–1039. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- 38.Bymaster FP, Felder C, Ahmed S, McKinzie D. Muscarinic receptors as a target for drugs treating schizophrenia. Curr Drug Targets: CNS Neurol Disord. 2002;1:163–181. doi: 10.2174/1568007024606249. [DOI] [PubMed] [Google Scholar]
- 39.Bridges TM, Lewis LM, Dawson ES, Weaver CD, Lindsley CW. Discovery and development of the a highly selective M1 Positive Allosteric Modulator (PAM). Probe Reports from the NIH Molecular Libraries Program; Bethesda, MD. 2010. [PubMed] [Google Scholar]
- 40.Bridges TM, Kennedy JP, Noetzel MJ, Breininger ML, Gentry PR, Conn PJ, Lindsley CW. Chemical lead optimization of a pan Gq mAChR M1, M3, M5 positive allosteric modulator (PAM) lead. Part II: development of a potent and highly selective M1 PAM. Bioorg Med Chem Lett. 2010;20:1972–1975. doi: 10.1016/j.bmcl.2010.01.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bridges TM, Reid PR, Lewis LM, Dawson ES, Weaver CD, Wood MR, Lindsley CW. Discovery and development of a second highly selective M1 Positive Allosteric Modulator (PAM). Probe Reports from the NIH Molecular Libraries Program; Bethesda, MD. 2010. [PubMed] [Google Scholar]
- 42.Marlo JE, Niswender CM, Days EL, Bridges TM, Xiang Y, Rodriguez AL, Shirey JK, Brady AE, Nalywajko T, Luo Q, Austin CA, Williams MB, Kim K, Williams R, Orton D, Brown HA, Lindsley CW, Weaver CD, Conn PJ. Discovery and characterization of novel allosteric potentiators of M1 muscarinic receptors reveals multiple modes of activity. Mol Pharmacol. 2009;75:577–588. doi: 10.1124/mol.108.052886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melancon BJ, Poslusney MS, Gentry PR, Tarr JC, Sheffler DJ, Mattmann ME, Bridges TM, Utley TJ, Daniels JS, Niswender CM, Conn PJ, Lindsley CW, Wood MR. Isatin replacements applied to the highly selective, muscarinic M1 PAM ML137: continued optimization of an MLPCN probe molecule. Bioorg Med Chem Lett. 2013;23:412–416. doi: 10.1016/j.bmcl.2012.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma L, Seager MA, Wittmann M, Jacobson M, Bickel D, Burno M, Jones K, Graufelds VK, Xu G, Pearson M, McCampbell A, Gaspar R, Shughrue P, Danziger A, Regan C, Flick R, Pascarella D, Garson S, Doran S, Kreatsoulas C, Veng L, Lindsley CW, Shipe W, Kuduk S, Sur C, Kinney G, Seabrook GR, Ray WJ. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc Natl Acad Sci U S A. 2009;106:15950–15955. doi: 10.1073/pnas.0900903106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mistry SN, Valant C, Sexton PM, Capuano B, Christopoulos A, Scammells PJ. Synthesis and pharmacological profiling of analogues of benzyl quinolone carboxylic acid (BQCA) as allosteric modulators of the M1 muscarinic receptor. J Med Chem. 2013;56:5151–5172. doi: 10.1021/jm400540b. [DOI] [PubMed] [Google Scholar]
- 46.Uslaner JM, Eddins D, Puri V, Cannon CE, Sutcliffe J, Chew CS, Pearson M, Vivian JA, Chang RK, Ray WJ, Kuduk SD, Wittmann M. The muscarinic M1 receptor positive allosteric modulator PQCA improves cognitive measures in rat, cynomolgus macaque, and rhesus macaque. Psychopharmacology (Berl) 2013;225:21–30. doi: 10.1007/s00213-012-2788-8. [DOI] [PubMed] [Google Scholar]
- 47.Kuduk SD, Chang RK, Di Marco CN, Ray WJ, Ma L, Wittmann M, Seager MA, Koeplinger KA, Thompson CD, Hartman GD, Bilodeau MT. Quinolizidinone carboxylic acids as CNS penetrant, selective m1 allosteric muscarinic receptor modulators. ACS Med Chem Lett. 2010;1:263–267. doi: 10.1021/ml100095k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuduk SD, Di Marco CN, Cofre V, Pitts DR, Ray WJ, Ma L, Wittmann M, Veng L, Seager MA, Koeplinger K, Thompson CD, Hartman GD, Bilodeau MT. N-heterocyclic derived M1 positive allosteric modulators. Bioorg Med Chem Lett. 2010;20:1334–1337. doi: 10.1016/j.bmcl.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 49.Kuduk SD, Di Marco CN, Cofre V, Ray WJ, Ma L, Wittmann M, Seager MA, Koeplinger KA, Thompson CD, Hartman GD, Bilodeau MT. Fused heterocyclic M1 positive allosteric modulators. Bioorg Med Chem Lett. 2011;21:2769–2772. doi: 10.1016/j.bmcl.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 50.Thomsen M, Lindsley CW, Conn PJ, Wessell JE, Fulton BS, Wess J, Caine SB. Contribution of both M1 and M4 receptors to muscarinic agonist-mediated attenuation of the cocaine discriminative stimulus in mice. Psychopharmacology (Berl) 2012;220:673–685. doi: 10.1007/s00213-011-2516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chambon C, Jatzke C, Wegener N, Gravius A, Danysz W. Using cholinergic M1 receptor positive allosteric modulators to improve memory via enhancement of brain cholinergic communication. Eur J Pharmacol. 2012;697:73–80. doi: 10.1016/j.ejphar.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Chambon C, Wegener N, Gravius A, Danysz W. A new automated method to assess the rat recognition memory: validation of the method. Behav Brain Res. 2011;222:151–157. doi: 10.1016/j.bbr.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 53.Galloway CR, Lebois EP, Shagarabi SL, Hernandez NA, Manns JR. Effects of selective activation of M1 and M4 muscarinic receptors on object recognition memory performance in rats. Pharmacology. 2014;93:57–64. doi: 10.1159/000357682. [DOI] [PubMed] [Google Scholar]
- 54.Reid PR, Bridges TM, Sheffler DJ, Cho HP, Lewis LM, Days E, Daniels JS, Jones CK, Niswender CM, Weaver CD, Conn PJ, Lindsley CW, Wood MR. Discovery and optimization of a novel, selective and brain penetrant M1 positive allosteric modulator (PAM): the development of ML169, an MLPCN probe. Bioorg Med Chem Lett. 2011;21:2697–2701. doi: 10.1016/j.bmcl.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poslusney MS, Melancon BJ, Gentry PR, Sheffler DJ, Bridges TM, Utley TJ, Daniels JS, Niswender CM, Conn PJ, Lindsley CW, Wood MR. Spirocyclic replacements for the isatin in the highly selective, muscarinic M1 PAM ML137: the continued optimization of an MLPCN probe molecule. Bioorg Med Chem Lett. 2013;23:1860–1864. doi: 10.1016/j.bmcl.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panarese JD, Cho HP, Adams JJ, Nance KD, Garcia-Barrantes PM, Chang S, Morrison RD, Blobaum AL, Niswender CM, Stauffer SR, Conn PJ, Lindsley CW. Further optimization of the M1 PAM VU0453595: Discovery of novel heterobicyclic core motifs with improved CNS penetration. Bioorg Med Chem Lett. 2016;26:3822–3825. doi: 10.1016/j.bmcl.2016.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferris MJ, Milenkovic M, Liu S, Mielnik CA, Beerepoot P, John CE, Espana RA, Sotnikova TD, Gainetdinov RR, Borgland SL, Jones SR, Ramsey AJ. Sustained N-methyl-d-aspartate receptor hypofunction remodels the dopamine system and impairs phasic signaling. Eur J Neurosci. 2014;40:2255–2263. doi: 10.1111/ejn.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramsey AJ. NR1 knockdown mice as a representative model of the glutamate hypothesis of schizophrenia. Prog Brain Res. 2009;179:51–58. doi: 10.1016/S0079-6123(09)17906-2. [DOI] [PubMed] [Google Scholar]
- 59.Walker AG, Miller BR, Fritsch JN, Barton SJ, Rebec GV. Altered information processing in the prefrontal cortex of Huntington’s disease mouse models. J Neurosci. 2008;28:8973–8982. doi: 10.1523/JNEUROSCI.2804-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2. Academic Press; San Diego: 2001. [Google Scholar]
- 61.Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cognitive Processing. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bubser M, Bridges TM, Dencker D, Gould RW, Grannan M, Noetzel MJ, Lamsal A, Niswender CM, Daniels JS, Poslusney MS, Melancon BJ, Tarr JC, Byers FW, Wess J, Duggan ME, Dunlop J, Wood MW, Brandon NJ, Wood MR, Lindsley CW, Conn PJ, Jones CK. Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents. ACS Chem Neurosci. 2014;5:920–942. doi: 10.1021/cn500128b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Byun NE, Grannan M, Bubser M, Barry RL, Thompson A, Rosanelli J, Gowrishankar R, Kelm ND, Damon S, Bridges TM, Melancon BJ, Tarr JC, Brogan JT, Avison MJ, Deutch AY, Wess J, Wood MR, Lindsley CW, Gore JC, Conn PJ, Jones CK. Antipsychotic drug-like effects of the selective M4 muscarinic acetylcholine receptor positive allosteric modulator VU0152100. Neuropsychopharmacology. 2014;39:1578–1593. doi: 10.1038/npp.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lindsley C, Conn PJ, Wood MR, Tarr JC, Bridges TM. WO 2011163280 A1 Indole compounds as positive allosteric modulators of the muscarinic receptor. 2014
- 65.Tarr JC, Turlington ML, Reid PR, Utley TJ, Sheffler DJ, Cho HP, Klar R, Pancani T, Klein MT, Bridges TM, Morrison RD, Blobaum AL, Xiang Z, Daniels JS, Niswender CM, Conn PJ, Wood MR, Lindsley CW. Targeting selective activation of M(1) for the treatment of Alzheimer’s disease: further chemical optimization and pharmacological characterization of the M(1) positive allosteric modulator ML169. ACS Chem Neurosci. 2012;3:884–895. doi: 10.1021/cn300068s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stanhope KJ, Mirza NR, Bickerdike MJ, Bright JL, Harrington NR, Hesselink MB, Kennett GA, Lightowler S, Sheardown MJ, Syed R, Upton RL, Wadsworth G, Weiss SM, Wyatt A. The muscarinic receptor agonist xanomeline has an antipsychotic-like profile in the rat. J Pharmacol Exp Ther. 2001;299:782–792. [PubMed] [Google Scholar]
- 67.Bubenikova-Valesova V, Horacek J, Vrajova M, Hoschl C. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci Biobehav Rev. 2008;32:1014–1023. doi: 10.1016/j.neubiorev.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 68.Jentsch JD, Roth RH. The neuro-psychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuro-psychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 69.Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- 70.Lopes-Aguiar C, Bueno-Junior LS, Ruggiero RN, Romcy-Pereira RN, Leite JP. NMDA receptor blockade impairs the muscarinic conversion of sub-threshold transient depression into long-lasting LTD in the hippocampus-prefrontal cortex pathway in vivo: correlation with gamma oscillations. Neuropharmacology. 2013;65:143–155. doi: 10.1016/j.neuropharm.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 71.Jackson ME, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci U S A. 2004;101:8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jodo E. The role of the hippocampo-prefrontal cortex system in phencyclidine-induced psychosis: a model for schizophrenia. J Physiol. 2013;107:434–440. doi: 10.1016/j.jphysparis.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 73.Jodo E, Suzuki Y, Katayama T, Hoshino KY, Takeuchi S, Niwa S, Kayama Y. Activation of medial prefrontal cortex by phencyclidine is mediated via a hippocampo-prefrontal pathway. Cereb Cortex. 2005;15:663–669. doi: 10.1093/cercor/bhh168. [DOI] [PubMed] [Google Scholar]
- 74.Kamiyama H, Matsumoto M, Otani S, Kimura SI, Shimamura KI, Ishikawa S, Yanagawa Y, Togashi H. Mechanisms underlying ketamine-induced synaptic depression in rat hippocampus-medial prefrontal cortex pathway. Neuroscience. 2011;177:159–169. doi: 10.1016/j.neuroscience.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 75.Thomases DR, Cass DK, Meyer JD, Caballero A, Tseng KY. Early adolescent MK-801 exposure impairs the maturation of ventral hippocampal control of basolateral amygdala drive in the adult prefrontal cortex. J Neurosci. 2014;34:9059–9066. doi: 10.1523/JNEUROSCI.1395-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yi F, Ball J, Stoll KE, Satpute VC, Mitchell SM, Pauli JL, Holloway BB, Johnston AD, Nathanson NM, Deisseroth K, Gerber DJ, Tonegawa S, Lawrence JJ. Direct excitation of parvalbumin-positive interneurons by M1 muscarinic acetylcholine receptors: roles in cellular excitability, inhibitory transmission and cognition. J Physiol. 2014;592:3463–3494. doi: 10.1113/jphysiol.2014.275453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dzirasa K, Ramsey AJ, Takahashi DY, Stapleton J, Potes JM, Williams JK, Gainetdinov RR, Sameshima K, Caron MG, Nicolelis MA. Hyperdopaminergia and NMDA receptor hypofunction disrupt neural phase signaling. J Neurosci. 2009;29:8215–8224. doi: 10.1523/JNEUROSCI.1773-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- 79.Hillman BG, Gupta SC, Stairs DJ, Buonanno A, Dravid SM. Behavioral analysis of NR2C knockout mouse reveals deficit in acquisition of conditioned fear and working memory. Neurobiol Learn Mem. 2011;95:404–414. doi: 10.1016/j.nlm.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.