Summary
Aim
The aim of the present in vitro study was to evaluate the protective effects of a zinc-hydroxyapatite toothpaste against an erosive challenge produced by a soft drink (Coca-Cola) using Scanning Electron Microscopy (SEM).
Methods
Forty specimens were assigned to 4 groups of 10 specimens each (group 1: no erosive challenge, no toothpaste treatment, group 2: erosive challenge, no toothpaste treatment, group 3: erosive challenge, fluoride toothpaste treatment, group 4: erosive challenge, zinc-hydroxyapatite toothpaste treatment). The surface of each specimen was imaged by SEM. A visual rating system was used to evaluate the condition of the enamel surface; results were analyzed by nonparametric statistical methods.
Results
Statistically significant differences were found between the samples untreated and those immersed in Coca-Cola (group 1, 2); the highest grade of damage was found in group 2, while the lowest grade was recorded in the samples of group 4. Comparing the groups, the two analyzed toothpaste tended to protect in different extend.
Conclusions
In this study treatment of erosively challenged enamel with Zn-Hap toothpaste showed a clear protective effect. This was greater than the effect observed for a normal fluoride toothpaste and confirmed the potential benefit the Zn-HAP technology can provide in protecting enamel from erosive acid challenges.
Keywords: enamel erosion, fluoride, hydroxyapatite, SEM, surface analysis, toothpastes
Introduction
The prevalence of dental erosion (erosive tooth wear) is thought to be increasing, due to the wide availability, and frequent consumption of acidic drinks such as soft drinks, sports drinks and fruit juices (1). The development of erosion involves a chemical process in which the inorganic phase of the tooth is demineralized, thereby reducing the hardness of tooth substrates (2).
To prevent dental erosion many strategies have been developed (3). To improve enamel and dentin resistance, toothpastes were considered effective and affordable vehicles (4). The incorporation of protective agents in toothpastes has become increasingly common; also because dental sensitivity, a problem often related to acid erosion, is a common complaint among patients. Currently, conventional fluoride-based toothpastes do not seem to be able to effectively protect teeth against erosion (5). Recently, new toothpastes formulations have been introduced to contrast enamel and dentin erosion. Among the large amount of commercially available products, several new toothpaste technologies were subject of our previous studies (6, 7). Changes in tooth structure due to extrinsic factors, such as acidic challenges from food and drink, have been widely investigated through Scanning Electron Microscopy (SEM). The aim of the present study was to test the impact of toothpaste with Zinc-Hydroxyapatite (Zn-HAP) on preventing enamel erosion compared to toothpaste with fluoride by using Scanning Electron Microscopy (SEM).
Materials and methods
The test toothpaste was: Zn-HAP (Microrepair®) toothpaste, without fluoride (Biorepair; Coswell S.P.A., 40050 Funo, Italy). The control toothpaste was: fluoride toothpaste, 1450 ppm F- as NaF (Eufresh; CIO Farmaceutici s.r.l., 81100 Caserta, Italy).
The study involved four different treatment groups:
group 1: no erosive challenge, no toothpaste treatment;
group 2: erosive challenge, no toothpaste treatment;
group 3: erosive challenge, fluoride toothpaste treatment;
group 4: erosive challenge, zinc-hydroxyapatite toothpaste treatment.
Specimen preparation
Specimens were prepared from 40 human incisors, extracted for orthodontic and periodontal reasons. Debris and soft tissue were eliminated and teeth were inspected for cracks, hypoplasia, white spot lesions and reconstruction. Teeth were cleaned to remove soft tissue and stored in a solution of 0.1% (wt/vol) thymol. The enamel specimens were cut at the enamel-dentin junction with a high-speed diamond rotary bur with a water-air spray. The samples were placed into Teflon molds measuring 10 × 8 × 2 mm and embedded in self-curing, fast-setting acrylic resin (Rapid Repair, DeguDent GmbH, Hanau, Germany) in such a way that the exposed buccal surface was plano-parallel to the bottom of the mold.
Demineralization and remineralization
A soft drink (Coca Cola, Coca Cola Company, Milano, Italy) was chosen for the demineralization process (6). The pH at 20°C, buffering capacity, and concentration of calcium and phosphate of the beverage were measured by standard chemical methods. The pH of soft drink was measured with a pH meter (Accumet AB15, Fisher Scientific, Pittsburgh, PA). Ca2− and PO43− were determined by flame atomic absorption (Perkin Elmer 1100 B spectrophotometer). Measurements were performed in triplicate and average values calculated (Tab. 1).
Table 1.
Chemical properties of the soft drink used in the study.
| Beverage | Ph | Buffering Capacity | Po4 (Mg/L) | Ca (Mg/L) |
|---|---|---|---|---|
| Coca Cola | 2,44 | 0,0056 | 175,7 | 20,83 |
The samples were then assigned to the four treatment groups with 10 specimens per group.
The toothpastes were applied neat onto the surface of the specimens to cover the enamel surface without brushing and then wiped off with distilled water washing after every treatment to remove residual toothpaste; the control specimens (group 1) were taken on storage for the whole experimentation and they did not receive any treatment. The toothpastes were applied to the enamel surfaces for 3 min at 0, 8, 24 and 36 h; during these intervals the specimens were kept in artificial saliva. The specimens of group 2, 3 and 4 were immersed in 6 ml of the soft drink for 2 min at room temperature before rinsing with deionized water. Four consecutive intervals of the immersion procedure were carried out (8). The immersions in the soft drink were repeated as described above at 0, 8, 24 and 36 h.
Scanning Electron Microscopy (SEM)
The specimens were gently air dried, dehydrated with alcohol, sputter-coated with gold. Enamel and dentin were characterized using a field emission scanning electron microscope (FE-SEM, MIRA3, TESCAN). Serial SEM microphotographs of the surfaces of each specimen at 2.50 KX and 5.00 KX original magnifications were obtained (9). A systematic assessment method was adopted for grading the SEM images. SEM images recorded were evaluated in terms of enamel damages by three experienced assessors who randomly examined the samples twice in a blind manner. A scoring scale (Tab. 2) was adopted to describe the enamel surface (10).
Table 2.
Scoring criteria used for the evaluation of SEM images.
| Grade | Status |
|---|---|
| 0 | Enamel surface remained perfectly intact with no grooves, pits, and porosity |
| 1 | Presence of surface irregularities on enamel surface, without demineralization of prismatic and/or |
| interprismatic enamel | |
| 2 | Presence of wrinkles and demineralization of prismatic/interprismatic enamel |
| 3 | Diffuse demineralization involved the rod core, with decomposition of morphology of prism |
Statistical Analysis
Descriptive statistics for the scores of the morphological analysis were calculated. Data were analyzed with the Kruskal-Wallis test. The Mann-Whitney U test was performed for post hoc comparisons. Significance was set at a P value <0.05. In order to check the intra - and inter-observer reliability the Intraclass Correlation Coefficient was calculated; it was greater than 0.9.
Results
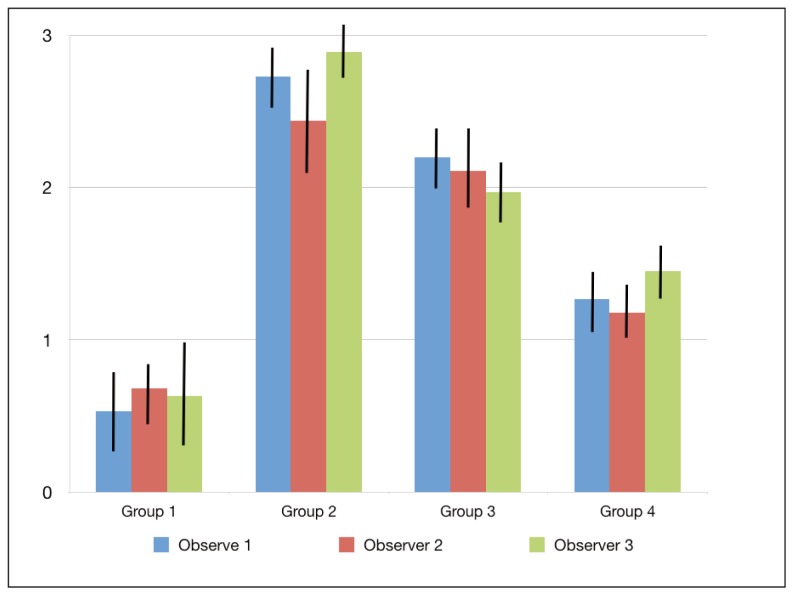
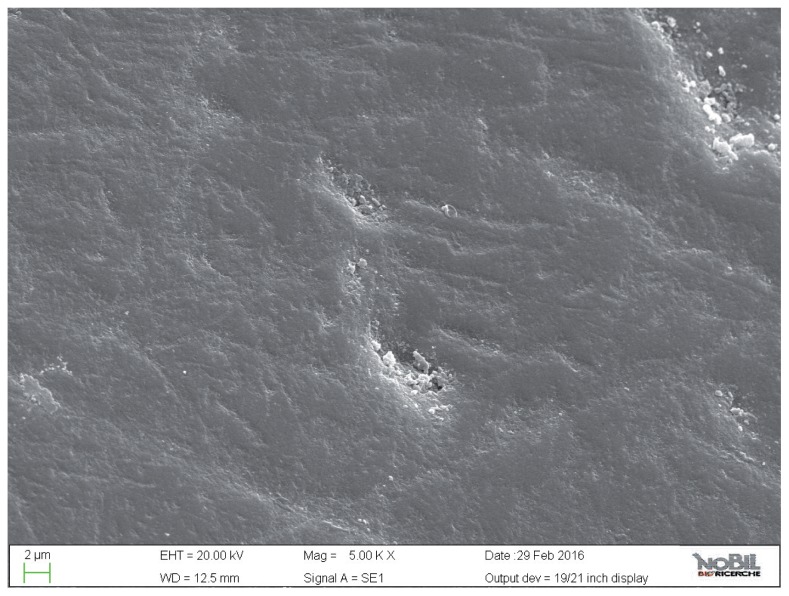
The mean amounts of scores for the morphological analysis of the images are reported in Table 3 and in Figure 1. The Kruskal-Wallis test showed the presence of significant differences among the different groups (p<0.05). On enamel surfaces not exposed to the erosive challenge by the soft drink (group 1), the typical structures of sound enamel such as grooves and perichimata lines were apparent; also small depressions or ditches or grinding marks were found indicative of the cumulative mechanical effects the teeth have experienced were observed (Figs. 2, 3).
Table 3.
Means and standard deviations of the morphological SEM scores provided by the three observers and overall. Different superscript letters indicate significant differences (p <0.05).
| Observer 1 | Observer 2 | Observer 3 | Overall | |
|---|---|---|---|---|
| Group 1 | 0.53 ± 0.52 | 0.68 ± 0.44 | 0.63 ± 0.61 | 0.61 ± 0.52 a |
| Group 2 | 2.73 ± 0.46 | 2.44 ± 0.66 | 2.89 ± 0.34 | 2.69 ± 0.49 b |
| Group 3 | 2.2 ± 0.41 | 2.11 ± 0.57 | 1.97 ± 0.44 | 2.1 ± 0.47 c |
| Group 4 | 1.27 ± 0.46 | 1.18 ± 0.35 | 1.45 ± 0.33 | 1.3 ± 0.38 d |
Figure 1.
Means and standard deviations of the morphological SEM scores provided by the three observers.
Figure 2.
SEM image at 5.00 KX magnification of intact enamel surface (Group 1).
Figure 3.
SEM image at 2.50 KX magnification of intact enamel surface (Group 1).
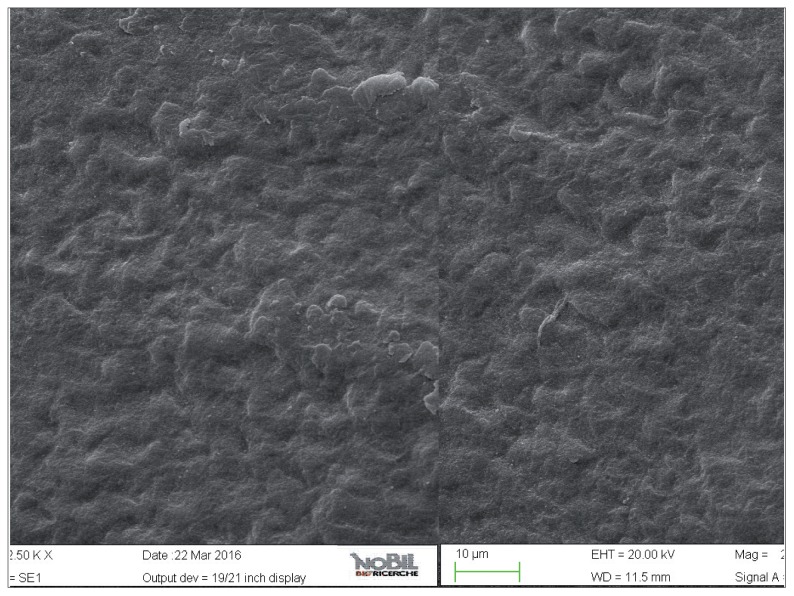
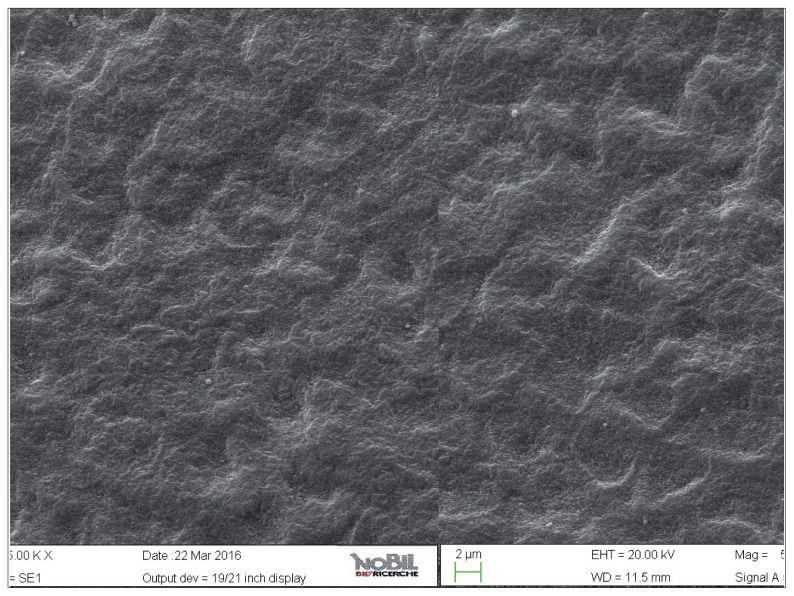
The enamel surface of teeth exposed to the acidic challenge by the soft drink clearly demonstrated deep changes in enamel structure (Figs. 4, 5). After 32 min eosure to the acidic challenge (four immersions of 8 min each) an irregular pattern of surface erosion could be observed and the presence of honeycomb structures suggests demineralization of enamel prisms. The morphological scores for the acid challenged specimens were significantly higher than the scores for the specimens not exposed to acid challenge (Mann-Whitney U test, p<0.05).
Figure 4.
SEM image at 5.00 KX magnification of enamel exposed to Coca-Cola (Group 2).
Figure 5.
SEM image at 2.50 KX magnification of enamel exposed to Coca-Cola (Group 2).
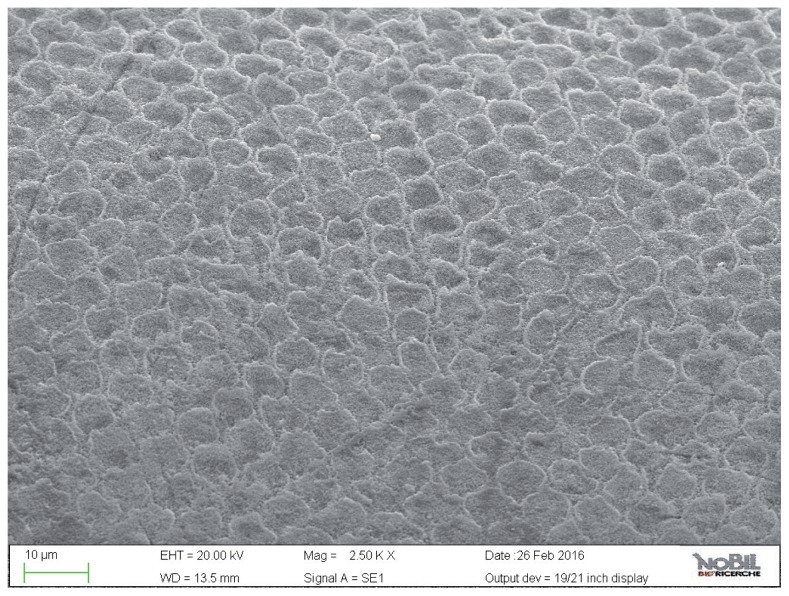
The acid-challenged specimens treated with the control and test toothpastes demonstrated a lower degree of demineralization on the enamel surface as evident in the SEM images, Figures 6–9 respectively. This is reflected in the lower morphological scores for these two groups compared to the acid-challenged samples not treated with toothpaste. In the SEM images (Figs. 6, 7) of the specimens treated with fluoride toothpaste (group 3) honeycomb structures that were typical of the demineralization enamel were still visible, and a slight irregular pattern of erosion could be observed. The average morphological score of this group was significantly lower than the scores for the acid challenged specimens (p<0.05).
Figure 6.
SEM image at 5.00 KX magnification of intact enamel surface treated with Eufresh (Group 3).
Figure 7.
SEM image at 2.50 KX magnification of intact enamel surface treated with Eufresh (Group 3).
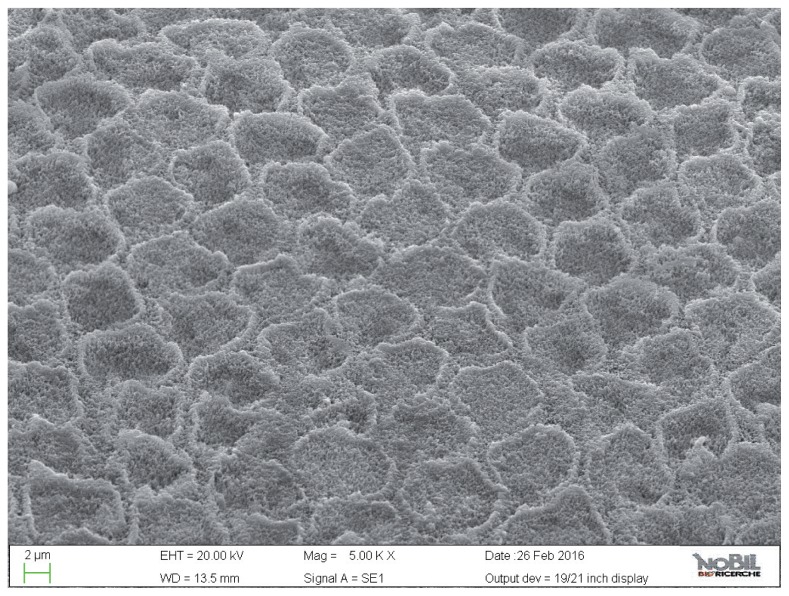
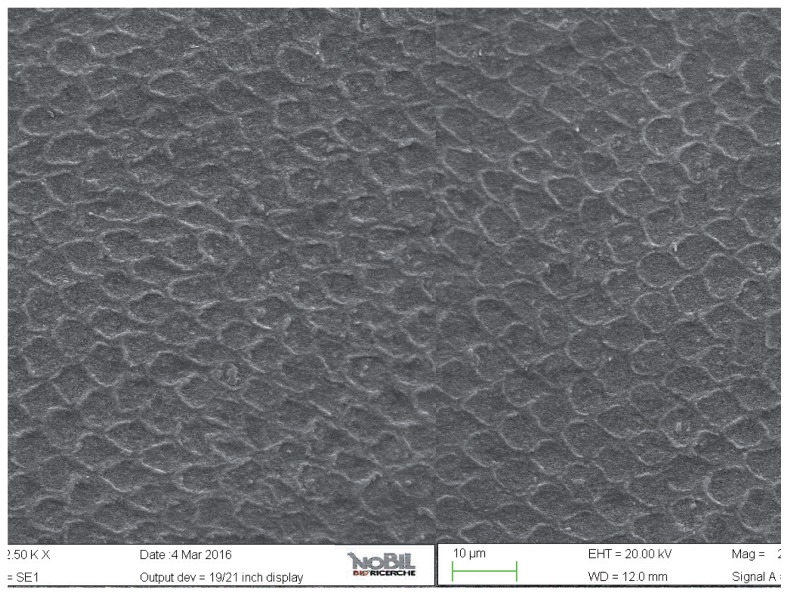
Figure 8.
SEM image at 5.00 KX magnification of intact enamel surface treated with Biorepair (Group 4).
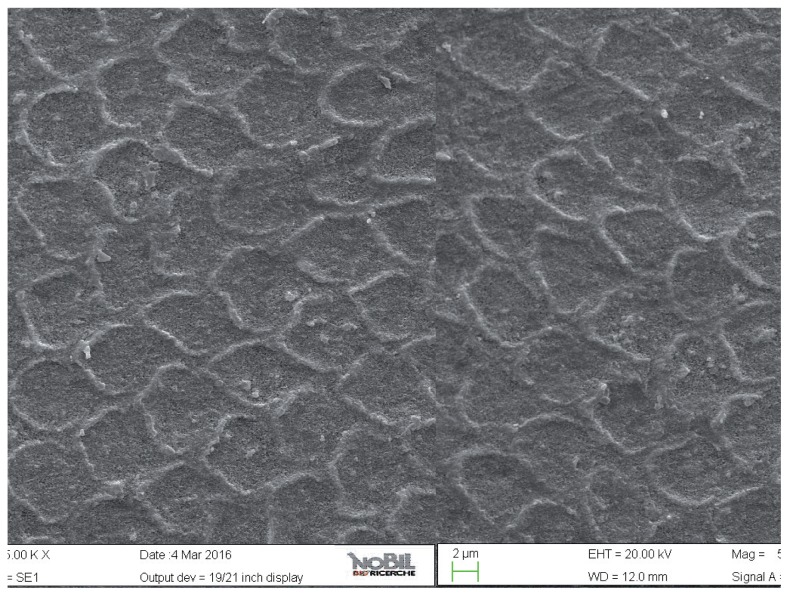
Figure 9.
SEM image at 2.50 KX magnification of intact enamel surface treated with Biorepair (Group 4).
The specimens treated with the Zn-HAP toothpaste (group 4) showed evidence of deposited material in the SEM images (Figs. 8, 9), with little evidence of erosive damage to the tooth surface.
Specimens of group 4 showed the lowest morphological SEM scores of even if not as similar as intact enamel (P<0.05). Overall mean and standard deviations of the morphological SEM scores confirmed that Zn-HAP toothpaste provided the lowest evidence of erosive damage to the tooth surface (p < 0.001).
Thus, if comparing the action of Zn-HAP (group 4) and fluoride (group 3) toothpastes against an eroded enamel surface (group 2), it resulted that enamel specimens of group 4 tended to be significantly more protected after the treatment.
Discussion and conclusions
In this study the morphological analysis of enamel surfaces after an erosive acid challenge from a soft drink followed by treatment with Zn-Hap toothpaste showed a clear protective effect. This was greater than the effect observed for a normal fluoride toothpaste and confirmed the potential benefit the Zn-HAP technology can provide in protecting enamel from erosive acid challenges.
The morphological analysis of enamel was based on images taken by scanning electron microscopy (SEM), a technique that is suitable for use with native unpolished surface samples and enamel having been exposed to acidic challenge or toothpaste treatment. In the present in vitro study, SEM was used to verify the protective effect of the two toothpastes on enamel exposed to erosive action of a soft drink. The SEM study allowed to understand qualitatively the processes of demineralization of the enamel surface through the observation of specific morphological and structural features that characterize the enamel itself.
A classification scale was used in order to help quantifying and describe the damage grade on enamel. Scoring criteria modification of demineralization evaluation (11) was followed, as reported in Table 2: a score of zero was assigned to enamel surface perfectly intact with no grooves, pits and porosity, while a score of three to those where diffuse demineralization involved the rod core, resulting in a lesion forming the “keyhole” like structure.
The experimental protocol of the present study was conducted in attempt to better simulate the daily habits of soft drink consumption. To predict the erosive potential of a soft drink, the method used should simulate what happens in vivo when the drink enters the mouth. For this reason, the method used in the present study (four consecutive intervals of 2 minutes for four times, at 0, 8, 24 and 36 hours) was considered to mimic, as closely as possible, the natural consumption of cola drink during the main daily meals. During the entire experimental protocol, the specimens were maintained in fresh artificial saliva until the next time of application of pastes. This means that the specimens were in contact with the bioactive agent for 12 min without suffering a demineralizing acid attack and then stayed in remineralizing solution.
In the oral environment, host factors (such as the mineral concentration of the tooth, and the pellicle and plaque formation) can influence the progression of demineralization (12–14).
Salivary factors, such as the salivary flow rate, composition and buffering capacity, might exert protective action on dental surface (12, 15, 16). For this reason, a further step was taken in this study to enhance the relevance of the model by storing specimens in artificial saliva between the experimental procedures.
Among soft drinks, Cola drink has the highest erosive potential (17, 18) and this was the rationale for using it in the present study.
The effect of demineralizing, acidic drinks such as Coca Cola was assessed in this study by comparing the SEM images of enamel treated with the drink with those of the unchallenged samples, shown in Figure 2 and 3. As expected, the surface of enamel treated with an acidic drink shows the presence of honeycomb structures, which suggests demineralization of enamel prisms. Diffuse demineralization involved the rod core, with decomposition of morphology of prims: they were severely affected and a greater prism-core dissolution compared with that in the interprismatic areas gave the enamel a “keyhole pattern” or “honeycomb pattern” of demineralization (Figs. 4, 5).
The main aim of the present study was to evaluate, by SEM analysis, the protective efficacy of a Zn-HaP toothpaste on enamel after acidic challenge, compared to a standard fluoride toothpaste and untreated controls. The results presented in Table 3, supported by the images in Figures 2–9 clearly demonstrate that the Zn-HAP technology was superior to standard fluoride toothpaste in protecting the enamel surface.
As expected, the highest degree of damage was found in the samples challenged by the acidic drink and without toothpaste treatment (group 2), as the enamel prism pattern showed a predominant dissolution of rods exposing interprismatic enamel (Figs. 4, 5). The lowest score of damage was recordered in the samples treated with Zn-HAP containing toothpaste (Figs. 8, 9) after acidic challenge.
In the case of the Zn-HAP technology, this indicates that supplying calcium-phoshate minerals is a suitable and effective route to counteract the effect of an erosive challenge. The mode of action is a combination of reducing the demineralization effect of the acidic challenge and a remineralization/repair effect brought about by the extra provision of calcium and phosphates. The grade of damage observed in enamel surfaces after treatment with Zn-HAP containing dentifrice (group 4) highlighted the persistence of rod integrity resembling a less advanced demineralization level if compared with samples treated with fluoride containing toothpaste (group 3).
Toothpastes have been considered effective and accessible vehicles to provide enamel resistance and to improve enamel resistance to further erosive attacks (19).
According to Rao et al. (20) dentifrices would be the preferable mode of delivering topical protective agents since they are used routinely as an oral hygiene measure. Jager et al. (21) showed that different exposure times to acid beverages also result in very different estimates of erosive potential, and that effect of the choice of study methodology may affect the results of the study.
For this reason, the effects of dentifrices on dental erosion have been exhaustively studied (12, 22–24). While some studies have shown that products with bioactive agents such as fluoride, CPP-ACP, and calcium sodium phosphosilicate have the potential to prevent enamel demineralization (20, 23, 24), other studies have shown no favorable effects of these agents (12, 22).
Acknowledgements
We are grateful to Clara Cassinelli (Nobil Bio Ricerche S.r.l., Portacomaro, Asti, Italy) for providing the SEM images and technical assistance.
Footnotes
Conflict of interest statement
The Authors of this study have no conflict of interest to disclose.
References
- 1.Lussi A, Hellwig E, Zero D, Jaeggi T. Erosive tooth wear: diagnosis, risk factors and prevention. American Journal of Dentistry. 2006;19:319–25. [PubMed] [Google Scholar]
- 2.Lussi A, Schlueter N, Rakhmatullina E, Ganss C. Dental Erosion - an overview with emphasis on chemical and histopathological aspects. Caries Research. 2011;45:2–12. doi: 10.1159/000325915. [DOI] [PubMed] [Google Scholar]
- 3.Magalhaes AC, Wiegand A, Rios D, Honorio HM, Buzalaf MA. Insights into preventive measures for dental erosion. Journal of Applied Oral Sciences. 2009;17:75–86. doi: 10.1590/S1678-77572009000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato MT, Lancia M, Sales-Peres SH, Buzalaf MA. Preventive effect of commercial desensitizing toothpastes on bovine enamel erosion in vitro. Caries Research. 2010;44:85–9. doi: 10.1159/000282668. [DOI] [PubMed] [Google Scholar]
- 5.Moron BM, Miyazaki SS, Ito N, Wiegand A, Vilhena F, Buzalaf MA, Magalhaes AC. Impact of different fluoride concentrations and pH of dentifrices on tooth erosion/abrasion in vitro. Australian Dental Journal. 2013;58:106–11. doi: 10.1111/adj.12016. [DOI] [PubMed] [Google Scholar]
- 6.Poggio C, Lombardini M, Vigorelli P, Ceci M. Analysis of Dentin/Enamel Remineralization by a CPP-ACP Paste: AFM and SEM Study. Scanning. 2013;35:366–74. doi: 10.1002/sca.21077. [DOI] [PubMed] [Google Scholar]
- 7.Lombardini M, Ceci M, Colombo M, Bianchi S, Poggio C. Preventive effect of different toothpaste on enamel erosion: AFM and SEM studies. Scanning. 2014;36:401–10. doi: 10.1002/sca.21132. [DOI] [PubMed] [Google Scholar]
- 8.Barbour ME, Finke M, Parker DM, Hughes JA, Allen GC, Addy M. The relationship between enamel softening and erosion caused by soft drinks at a range of temperatures. Journal of Dentistry. 2007;34:207–13. doi: 10.1016/j.jdent.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Bertassoni LE, Habelitz S, Pugach M, et al. Evaluation of surface structural and mechanical changes following remineralization of dentin. Scanning. 2010;32:312–9. doi: 10.1002/sca.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nucci C, Marchionni S, Piana G, Mazzoni A, Prati C. Morphological evaluation of enamel surface after application of the two “home” whitening products. Oral Health & Preventive Dentistry. 2004;2:221–9. [PubMed] [Google Scholar]
- 11.Alessandri Bonetti G, Pazzi E, Zanarini M, Marchionni S, Checchi L. The effect of zinc-carbonate hydroxyapatite versus fluoride on enamel surfaces after interproximal reduction. Scanning. 2014;36:356–61. doi: 10.1002/sca.21125. [DOI] [PubMed] [Google Scholar]
- 12.Lussi A, Megert B, Eggenberger D, Jaeggi T. Impact of different toothpastes on the prevention of erosion. Caries Research. 2008;42:62–7. doi: 10.1159/000112517. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira MC, Ramos-Jorge ML, Delbem AC, de Vieirac RS. Effect of Toothpastes with different abrasives on eroded human enamel: An in situ/ex vivo Study. The Open Dentistry Journal. 2013;7:132–9. doi: 10.2174/1874210601307010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Silva E, de Sa Rodrigues C, Dias D, da Silva S, Amaral C, Guimaraes J. Effect of toothbrushing-mouthrinse-cycling on surface roughness and topography of nanofilled, micro-filled, and microhybrid resin composites. Operative Dentistry. 2014;39:521–9. doi: 10.2341/13-199-L. [DOI] [PubMed] [Google Scholar]
- 15.Manton DJ, Cai F, Yuan Y, Walker GD, Cochrane NJ, Reynolds C, et al. Effect of casein phosphopeptide-amorphous calcium phosphate added to acidic beverages on enamel erosion in vitro. Australian Dental Journal. 2010;55:275–9. doi: 10.1111/j.1834-7819.2010.01234.x. [DOI] [PubMed] [Google Scholar]
- 16.Wongkhantee S, Patanapiradej V, Maneenut C, Tantbirojn D. Effect of acidic food and drinks on surface hardness of enamel, dentine, and tooth-coloured filling materials. Journal of Dentistry. 2006;34:214–20. doi: 10.1016/j.jdent.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Jensdottir T, Holbrook P, Nauntofte B, Buchwald C, Bardow A. Immediate erosive potential of cola drinks and orange juices. Journal of Dental Research. 2006;85:226–30. doi: 10.1177/154405910608500304. [DOI] [PubMed] [Google Scholar]
- 18.Torres CP, Chinelatti MA, Gomes-Silva JM, Rizoli FA, Oliveira MA, Palma-Dibb RG, et al. Surface and subsurface erosion of primary enamel by acid beverages over time. Brazilian Dental Journal. 2010;21:337–45. doi: 10.1590/s0103-64402010000400009. [DOI] [PubMed] [Google Scholar]
- 19.Lussi A, Jaeggi T, Zero The role of diet in the aetiology of dental erosion. Caries Research. 2004;38:34–44. doi: 10.1159/000074360. [DOI] [PubMed] [Google Scholar]
- 20.Rao SK, Bhat GS, Aradhya S, Devi A, Bhat M. Study of the efficacy of toothpaste containing casein phosphopeptide in the prevention of dental caries: A randomized controlled trial in 12- to 15-year-old high caries risk children in Bangalore, India. Caries Research. 2009;43:430–5. doi: 10.1159/000252976. [DOI] [PubMed] [Google Scholar]
- 21.Jager DH, Vieira AM, Ruben JL, Huysmans MC. Estimated erosive potential depends on exposure time. Journal of Dentistry. 2012;40:1103–8. doi: 10.1016/j.jdent.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Lennon AM, Pfeffer M, Buchalla W, Becker K, Lennon S, Attin T. Effect of a casein/calcium phosphate containing tooth cream and fluoride on enamel erosion in vitro. Caries Research. 2006;40:154–157. doi: 10.1159/000091063. [DOI] [PubMed] [Google Scholar]
- 23.Oshiro M, Yamaguchi K, Takamizawa T, Inage H, et al. Effect of CPP-ACP paste on tooth mineralization: an FE-SEM study. Journal of Oral Sciences. 2007;49:115–20. doi: 10.2334/josnusd.49.115. [DOI] [PubMed] [Google Scholar]
- 24.Tantbirojin D, Huang A, Ericson MD, Poolthong S. Change in surface hardness of enamel by a cola drink and a CPP-ACP paste. Journal of Dentistry. 2008;36:74–9. doi: 10.1016/j.jdent.2007.10.008. [DOI] [PubMed] [Google Scholar]