Abstract
P1 is a bacteriophage of Escherichia coli and other enteric bacteria. It lysogenizes its hosts as a circular, low-copy-number plasmid. We have determined the complete nucleotide sequences of two strains of a P1 thermoinducible mutant, P1 c1-100. The P1 genome (93,601 bp) contains at least 117 genes, of which almost two-thirds had not been sequenced previously and 49 have no homologs in other organisms. Protein-coding genes occupy 92% of the genome and are organized in 45 operons, of which four are decisive for the choice between lysis and lysogeny. Four others ensure plasmid maintenance. The majority of the remaining 37 operons are involved in lytic development. Seventeen operons are transcribed from σ70 promoters directly controlled by the master phage repressor C1. Late operons are transcribed from promoters recognized by the E. coli RNA polymerase holoenzyme in the presence of the Lpa protein, the product of a C1-controlled P1 gene. Three species of P1-encoded tRNAs provide differential controls of translation, and a P1-encoded DNA methyltransferase with putative bifunctionality influences transcription, replication, and DNA packaging. The genome is particularly rich in Chi recombinogenic sites. The base content and distribution in P1 DNA indicate that replication of P1 from its plasmid origin had more impact on the base compositional asymmetries of the P1 genome than replication from the lytic origin of replication.
Three bacteriophages of Escherichia coli with different life styles have been particularly instrumental in the development of the concepts and tools of molecular biology. They are the virulent phage T4 and the two temperate phages, λ and P1. The two temperate phages were first described in close succession in the middle of the last century, namely in 1951 (phage P1 [31]) and 1953 (λ [185] and informally even earlier, in the first issue of Evelyn Witkin's Microbial Genetics Bulletin, 1950 [184a]). Whereas λ prophage leads an essentially passive existence within the chromosome of its host, P1 prophage exists as an autonomous plasmid that is maintained at low copy number. The complete genome sequences of λ, T4, and Escherichia coli have been reported. We present here the complete nucleotide sequence of P1 and summarize its salient features.
P1 infects and lysogenizes E. coli and several other enteric bacteria. Its virion consists of an icosahedral head attached at one vertex to a tail that bears six kinked tail fibers (see cover figure). As in T4, the tail consists of a tail tube and a contractile sheath. A variable part of the tail fibers determines the specificity of P1 adsorption on different hosts. The variable part is encoded by an invertible segment of P1 DNA, similar to that of phage Mu (reviewed in reference 268). Infective particles of P1 contain cyclically permuted, linear, double-stranded molecules with a terminal redundancy of 10 to15 kb of DNA (293). Prior to this work, about 70 genes had been identified in P1 by genetic and physiological studies (346).
After injection into a host cell, viral DNA circularizes by recombination between redundant sequences to enter a lytic or lysogenic path. The choice between the two paths is dictated by the interplay of a number of environmental factors with the complex immunity circuitry that controls synthesis and activity of a master repressor, C1 (reviewed in reference 126). As a prophage, P1 is a stable plasmid maintained at about one copy per bacterial chromosome. Several P1 genes scattered throughout the genome are expressed in the lysogenic state. Those of primary importance are involved in plasmid maintenance functions and in inhibition of lytic development. P1 prophage replicates as a circular plasmid from an origin (oriR) resembling that of several other plasmids and, to a lesser extent, oriC of E. coli.
P1 genes expressed in the lytic pathway are those involved in the timing of phage development, in replication (from a “lytic” origin different from oriR), in the formation of phage particles, including headful packaging, and finally in cell lysis to release the phage progeny. Genes of the lytic pathway have been divided into early and late. Transcription of the late genes requires, in addition to bacterial RNA polymerase, a P1-encoded activator protein, Lpa (188), and an E. coli RNA polymerase-associated stringent starvation protein, SspA (111).
P1, like λ, made its mark early in molecular biology. The significant capacity of P1 for mediating generalized transduction (196) led promptly to P1 becoming a workhorse of genetic exchange among strains of E. coli, a role it is still playing today. Moreover, because P1 can package slightly more than twice as much DNA as can λ, and packaging can be efficiently carried out in vitro, P1-based vectors are now in common use for cloning and in vitro packaging of eukaryotic DNA (246, 293). P1 also made its mark early because of the restriction-modification genes that it carries, the analysis of which in the 1960s heralded the age of genetic engineering (16). The recognition that P1 is maintained as a plasmid prophage led to the identification of its plasmid maintenance functions. A site-specific recombinase, Cre, which appears promptly after infection, is involved in phage DNA cyclization (137), assists plasmid maintenance by resolving plasmid multimers (21), and perhaps, by a separate mechanism, may stabilize plasmid copy number (10). Ever since the introduction of the enzyme and its recombination site, lox, into Saccharomyces cerevisiae (271), the Cre-lox system has been a major tool of genetic engineering in eukaryotic cells.
The active partitioning of P1 plasmids to daughter cells requires two P1-encoded proteins and a cis-acting site (the centromere) (20). A surprising homology between the structural genes of plasmid partitioning and the soj and spoOJ sporulation genes of Bacillus subtilis provided the motivation for examining the effect of soj and spoOJ mutations on the production of anucleate progeny. Consequently, a connection was established between plasmid and nucleoid partitioning (157, 284). The finding that a gene of P1 encodes a toxin that is activated on P1 loss (192), among other findings of such “plasmid addiction” genes, helped to establish the concept that programmed cell death has its place in the life of bacterial populations, as in metazoan development (348). Finally, the analysis of P1 immunity maintenance and establishment (reviewed in reference 126) enlarges our view of controls on phage development. The master repressor, C1, unlike that of lambda, is monomeric, and the operators to which it binds are many and dispersed. It is modulated by at least two antirepressors (Ant1/2 and Coi) and a corepressor protein (Lxc), the latter capable of inducing certain C1-bound operators to loop. A trans-acting RNA (the c4 product) regulates Ant1/2 synthesis in a novel way, being excised from a transcript on which lies its site of action. Regrettably, the study of P1 immunity halted prematurely with the death of its prime mover, Heinz Schuster.
The most recent effort to compile a P1 map (made when no more than a total of 40% of the genome had been sequenced) was reported solely as a computer file (346). A recent short review of P1 biology has been prepared (186). Reviews of the older P1 literature may be found in references 29, 301, 345, and 347 and successive editions of Genetic Maps, one of which (344) was reprinted in reference 225. A thoughtful perspective on early studies of lysogeny by one of the pioneers in this field and the discoverer of P1 has recently appeared (30).
The overview of P1 genome organization and developmental regulation that we present here emerged from results of previous studies combined with the analysis of the entire genomic sequences of two strains of P1 described below.
MATERIALS AND METHODS
Sources of DNA and sequencing strategy.
DNA sequences described here were derived from two sources. One was DNA of P1 phage induced from N1706, an E. coli K-12 P1 c1-100 Tn9 lysogen preserved frozen since the early 1970s (kindly provided by J. L. Rosner). The other was DNA of P1 prophage carried by an MG1655-derived strain that was used in the E. coli genome sequencing project (35). The prophage had been acquired in the course of a P1-mediated transduction with the aid of P1c1-100 Tn9 r− m− dam (now dmt) ΔMB rev-6, a high-frequency transducing derivative of the P1 c1-100 Tn9 lysogen (303).
Prophage induction in N1706 and isolation of phage particles were performed as described previously (112). DNA was isolated from phage particles by using the QIAGEN Lambda Midi kit (QIAGEN, Inc.) according to the supplier's protocol. DNA of the prophage was isolated from a gel as described in reference 35.
The shotgun sequencing strategy was used for acquiring most of the sequencing data. Fragments of P1 phage or prophage DNA obtained by sonication were cloned in the pUC18 vector (266) or in the M13 Janus vector (45) and served as templates for sequencing. The ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction kit with AmpliTaq DNA polymerase FS (Perkin-Elmer), was used to perform sequencing reactions. Separation of products of sequencing reactions and reading of results was done with ABI 377 automated sequencers (Perkin-Elmer). After collection of initial sets of sequencing data at a four- to fivefold redundancy from readings in one direction, selected templates were resequenced in the opposite direction. The remaining gaps in the sequences were filled in by primer walking using oligonucleotides complementary to the ends of the sequenced regions and cloned P1 fragments or the entire DNA of the respective P1 strain as a template. Sequences were assembled using the program Seqman II (DNAStar Inc.).
Sequence analysis and annotation.
The majority of analyses were carried out using programs from either the GCG package version 9.0 (Genetics Computer Group, 1996) or the DNAStar package (DNAStar Inc.).
Open reading frame (ORF) identification was performed using programs based on a Markov model: the Internet version of GeneMark (http://genemark.biology.gatech.edu/GeneMark) (36, 208) and TIGR Glimmer 2.1 (75, 264). GeneMark was trained on templates for genes of E. coli, and its temperate bacteriophages and transposons, and bacteriophage T4. The model for Glimmer 2.1 was prepared with the use of Build-icm trained on P1 ORFs longer than 600 nucleotides. Additionally, the entire sequence was divided into 400-bp overlapping fragments and the predicted products of their translation were searched against the database for possible similarities with known protein sequences. Identification of a few genes, whose predicted codon usage pattern did not match that of any model organism, was based on homologies of their putative products to known proteins and on the presence in their upstream regions of sequences resembling promoters or likely to encode ribosome binding sites.
The assignment of previously identified genetic loci to newly sequenced ORFs was assisted by alignments to existing physical and genetic maps (230, 252, 280, 281, 294, 323, 346). Predicted sizes of putative P1 structural proteins were compared with sizes of head and tail components of P1 determined by polyacrylamide gel electrophoresis (PAGE) (325) to verify the identity of certain structural genes.
Searching for similarities between P1 proteins and known proteins in databases was performed using the Internet versions of programs PSI-BLAST and PHI-BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) (12, 354). Macaw (278) served to create multiple protein alignments. The putative helix-turn-helix motifs in protein sequences were identified by the HTH program at its website (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_server.html) (78).Putative signal peptides in proteins were predicted by using the SignalP program (235) at its website (http://www.cbs.dtu.dk/services/SignalP/#submission). Putative transmembrane helices in proteins were predicted by using the program TMHMM (175) at its website (http://www.cbs.dtu.dk/services/TMHMM).
Identification of putative σ70 promoters was carried out using Targsearch (118). Putative Rho-independent transcriptional terminators were identified with the GCG program Terminator (40) and TIGR program TransTerm (85). To avoid false positives, only terminators that met the following additional criteria (69, 198) were taken into consideration: a 4- to 18-bp stem and a 3- to 10 nucleotide (nt) loop of the terminator hairpin; a thymidine-rich region downstream of the terminator hairpin and separated by less than 3 nt; more than three GC/CG or GT/TG bp in the hairpin stem; at least three T residues, no more than one G, and no 5′-TVVTT stretches (V is A, C, or G) in the 5-nt-long proximal part of the thymidine-rich region; absence of four purine or cytosine residues in the 4-nt-long distal part of the thymidine-rich region and at least four T residues together in the proximal and distal parts of the thymidine-rich region. Putative integration host factor (IHF)-binding sites were identified with MacTargsearch at SEQSCAN (http://www.bmb.psu.edu/seqscan/seqform1.htm).
Oligonucleotide frequencies were determined with the program OCTAMER (L. Łobocka, unpublished). The DNA was scanned for putative tRNA genes by DNA homology searches and by using tRNAscan-SE (81, 204) at its website (http://www.genetics.wustl.edu/eddy/tRNAscan-SE/).
Nucleotide sequence accession numbers and strain availability.
The nucleotide sequences described here have been deposited in GenBank under accession numbers AF234172 (phage P1 mod749::IS5 c1-100) and AF234173 (prophage P1 mod1902::IS5 c1-100 rev-6 dmtΔMB). A lysogen (N1706) from which the first of these phages was induced has been deposited with the American Type Culture Collection (Manassas, Va.) as ATCC BAA-1001.
RESULTS AND DISCUSSION
Comparison of the sequenced P1 isolates.
Current P1 phages in circulation are derived from P1kc, a mutant selected in two steps by Lennox (196) as able to plate on E. coli K-12 with plaques of increased clarity. The induction of lysogens was facilitated by the introduction of mutations that render P1 thermoinducible (279), the most widely used being the c1-100 mutation, originally designated clr100 (261). It is present in both of the P1 isolates that we used for sequencing. For simplicity in selecting P1 lysogens as drug-resistant bacteria, Rosner (261) made use of P1 CM (171), a strain that harbors the unstable Tn9 transposon (encoding chloramphenicol acetyltransferase) acquired from an R factor. However, P1 c1-100 Tn9 has been widely used and hence was chosen for sequencing. The phage induced from the supposed P1 c1-100 Tn9 lysogen was found to have lost Tn9 and to have acquired IS5 inserted in the mod gene, which renders it r− m−. The sequence of this strain, P1 c1-100 mod749::IS5 (94,800 bp), was determined with an average 7.2-fold redundancy. The assembly was verified by comparison of the restriction map obtained by digestion with EcoRI, HindIII, HincII, BamHI, SacI, and PstI with that deduced from the entire P1 sequence (without the IS5) (346).
The other strain of P1 used here, P1 c1-100 mod1902::IS5 dmtΔMB rev-6 (94,481 bp), is a prophage carried by an MG1655-derived strain of E. coli K-12 that served in sequencing the E. coli genome (35). Its nucleotide sequence was determined with an average 7.8-fold redundancy. The prophage appears to have been acquired in the course of a P1-mediated transduction. In addition to a 319-bp deletion in dmt that impairs the function of the viral DNA methyltransferase gene, it carries a mutation, rev-6, that improves growth of the mutant in a dam mutant host and augments transduction (303). In the course of its history, the phage lost Tn9, acquired IS5, and accumulated other mutations such that, relative to the first strain, there are 13 additional mutations (four of them silent and one in a noncoding region). Five of those mutations are scattered in the variable part of the S gene (Sv), which is essential for P1 adsorption to E. coli. Which alleles are parental remains uncertain. The rev-6 mutation has not been correlated with any of the sequence differences between the two strains.
In the two sequenced strains, the IS5 insertions occurred at different TTAG sequences, known to belong to the most commonly used targets of IS5 (C/TTAA/G; reviewed in reference 212). In each case the IS5 insert, accompanied by a 4-bp duplication of its target, appears responsible for the r− m− phenotype since, in other respects, the res mod sequences are identical to those of r+ m+ P1 (145).
Genomewide features.
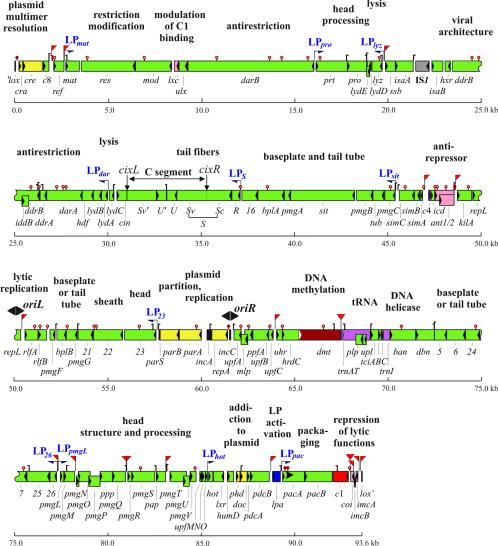
The genome of P1, without IS5 and its duplicated target, consists of 93,601 bp of double-stranded DNA, which in the prophage can be represented as a circle or line with the center of the site-specific recombination site lox assigned as the zero point such that the first nucleotide to its right on the strand written 5′ to 3′ in the direction of cre is assigned position 1 and numbering proceeds rightwards (346) (Fig. 1). The genome contains one insertion sequence, IS1, as an integral part of P1 (151). In both sequenced strains, IS1 has a base substitution mutation (IS1 G757T) in its right inverted repeat (IRR).
FIG. 1.
Genetic and physical organization of the P1 genome. Boxes with internal triangles show positions and orientations of genes, color-coded by function: yellow, plasmid maintenance; red, repression of early functions; pink, immunity control, not c1 itself; magenta, source of tRNAs; brown, DNA methylation; deep blue, transcriptional activation of late genes; grey, defective IS1; green, all other. Black boxes are intergenic regions of defined function: recombination sites, iterons to which RepA binds, plasmid centromere, and origin of DNA packaging, the direction of packaging being indicated by an arrowhead at the pac site. Bidirectional replication determined at the phage (lytic) origin, oriL, and at the plasmid origin, oriR, are indicated by black arrowheads above the genome map. C1 operator sites are marked with red flags pointing to the left or right (see also Table 6). Thin lines with terminal deep blue half arrowheads indicate the start sites and directions of the transcripts from particular late promoters. GATC sequences that overlap transcriptional promoters and clustered 5′-GATC sequences (two or more sites with pairwise separation of not more than 50 bp), substrates for Dmt or Dam methylation, are marked above the gene map by brown lollipops that are filled in the case of sites shown to alter function upon methylation. Hooks indicate Rho-independent transcriptional terminators. They face the starts of transcripts that they terminate. The map refers to the genome of P1 c1-100 mod749::IS5 without its nonintegral part, IS5.
(i) Base content and distribution.
The GC content of P1 DNA is 47.3%, slightly less than that of its E. coli host (50.8%) (35). The distribution of purines and pyrimidines between the two complementary strands is similar, with the upper strand (+; coding strand of genes transcribed clockwise) containing 49.5% (26.0% adenosine and 23.5% guanosine), and the lower strand (−) containing 50.5% (26.7% adenosine and 23.8% guanosine) purine nucleotides.
The location of extensive AT-rich regions along the genome map confirms the denaturation mapping evidence (222, 347) for the relatively recent acquisition by P1 of its restriction-modification genes (res, mod) and suggests that genes of the sim and rlf operons, as well as hot and isaB, were also incorporated in the genome of P1 late in evolution. Sharp borders of long AT-rich regions within certain genes (e.g., the 3′-moiety of parB) may be indicative of mosaic structure.
Unique restriction sites in P1 DNA are located preferentially in AT-rich regions or within the IS1 sequence (Fig. 2), suggesting their recent acquisition by P1 and selection against these sites in a P1 ancestor. A few 6-bp palindromes, the targets of known restriction enzymes, ApaI (GGGCCC), NarI (GGCGCC), NaeI (GCCGGC), SacI (GAGCTC), SalI (GTCGAC), and AvrII (CCTAGG), are absent from the P1 genome. Short DNA palindromes rare in or absent from P1 presumably identify sequences that could confer vulnerability in hosts that P1 otherwise finds particularly congenial.
FIG. 2.
Compositional organization of the P1 genome. The G+C content and (C−G)/(C+G) ratio (C-G skew) for one strand of P1 DNA are plotted as deviations from the mean. The plots were made with the GeneQuest program of DNAStar with a window size of 1,000 bp and a shift increment of 1 bp. For orientation, a simplified genome map is shown centrally. Upper-row genes are transcribed clockwise (left to right), and lower-row genes are transcribed counterclockwise (right to left). Two vertical black lines through the plots show the locations of the lytic (oriL) and plasmid (oriR) origins of replication. Two vertical grey bars show regions of polarity switch in the plot of the C-G skew. Unique recognition sites for restriction enzymes are indicated directly above the plot of G+C content; those below the line are for 8-bp cutters. See the text for absent 6-bp restriction sites. Chi sites and RAG sites (64) in the upper and lower strands are indicated by hash marks above and below the horizontal lines at the top of the figure. Where two hash marks are so close as to appear superimposed, a line at an angle to the vertical is added. The map refers to the genome of P1 c1-100 mod749::IS5 without its nonintegral parts, IS5, and the associated 4-bp duplication.
According to the pattern of C-G skew (the relative excess of cytosines versus guanosines in the top strand), the genome of P1 can be divided into two polarized arms with the average C-G skew below the mean predominating in one part and above the mean in the other part (Fig. 2). The region where the average sign of C-G skew below the mean predominates coincides with the region where transcription in the clockwise direction predominates, and vice versa. The C-G skew switches polarity in two regions: around kilobase 64 of the genome, close to the origin of plasmid replication (oriR), and around kilobase 3 of the genome. This pattern is consistent with the skew observed for bacterial and double-stranded DNA viral genomes whose replication proceeds bidirectionally from an origin (reviewed in references 101 and 216). Typically, the regions of polarity switch in the C-G skew are close to an origin and to the terminus of replication. P1 possesses two separate origins, oriR, and the origin of lytic replication, oriL, which are about 9 kb apart. Replication from both origins can be bidirectional (61, 241). As seen in Fig. 2, P1 prophage replication appears to have had more impact on the base compositional asymmetries and transcriptional organization of the genome than the theta (θ) or rolling-circle (σ) replication that occurs during lytic development. Although oriL is further away than oriR from the region of the C-G skew polarity switch at 64 kb, it is located diametrically opposite to the region of the second switch in the C-G skew polarity, which may be indicative of the terminus of P1 replication. We did not find in this region (or anywhere else in the P1 DNA) the TGTTGTAACTA sequence that in E. coli, Salmonella enterica serovar Typhimurium, and several plasmids constitutes a conserved core of Ter sites (136) at which replication terminates. However, this region is within 3 kb of the P1 site-specific recombination site lox, which is involved in resolution of plasmid multimers into monomers, playing a role analogous to that of the dif site in the E. coli chromosome. The lox site of P1 was shown to suppress the phenotype of an E. coli dif deletion strain in the presence of the lox-specific recombinase, Cre, when lox was inserted in the region of the E. coli terminus of replication (197). This suppression requires the DNA translocase activity of the essential host protein FtsK (47), as does dimer resolution at dif (292).
The major family of strongly skewed sequences in E. coli with the motif 5′-RGNAGGGS (R = purine, N = any base, S = G or C) (265) represents putative sites of binding to FtsK, a protein involved in positioning of bacterial DNA within the cell (64). The strongly biased distribution of these so-called RAG sites in P1 DNA (Fig. 2) possibly argues for a comparable role for FtsK in the life of P1 prophage, although a connection between RAG sites and FtsK is still uncertain.
The most abundant octamer in the genome of P1 corresponds to the recombinational hot spot Chi (5′-GCTGGTGG; 31 and 19 sites in the upper and lower strands [31/19]). Three other octamers in descending order of abundance (5′-TGCTGGTG, 18/14; 5′-CTGGTGGA, 14/8; and 5′-CTGGTGGC, 14/6) contain heptamers (boldface) identical to a Chi heptamer. The canonical Chi sites are two and one-half times more frequent in the P1 genome (one site per 1,872 bp) than in the genome of its E. coli host (one site per 4,598 bp) (35, 291), where they are the third most abundant octamers and sevenfold more abundant than a random octamer would be. P1 could benefit in several ways from the accumulation of Chi sites in its genome. P1 DNA encapsidated in a virion is a terminally redundant linear molecule, which upon entering cells at infection, can serve as a substrate for loading of RecBCD nuclease at its ends and for RecBCD-mediated degradation. Each encounter of RecBCD with Chi modifies the enzyme from its destructive 3′-to-5′ nucleolytic form to its recombination-promoting form (reviewed in reference 173) and thus should both protect incoming P1 DNA from further degradation and facilitate, by homologous recombination, its cyclization. The involvement of RecBCD in P1 cyclization could explain the 10-fold-reduced frequency of lysogenization by P1 of E. coli recB mutant cells observed by Rosner (261). Presumably, as in E. coli (181), Chi sites in P1, by their interaction with the RecBCD enzyme, could also facilitate the reassembly of collapsed replication forks. The burst size of P1 is reduced 20-fold in a recBC mutant of E. coli as compared to that in the recB+C+ strain (352).
The distribution of Chi sites in the P1 genome is unequal; the bias is away from the region that includes the invertible segment encoding tail fiber genes and at the right end favors the upper strand, where seven sites are within a 5-kb region around the pac site (Fig. 2). Of these, five are on the lpa side of pac. Their orientation is such that they offer protection against the nucleolytic activity of RecBCD loading onto DNA cut at the pac site and unprotected by packaging.
The two octamers that are the most frequent in the genome of E. coli (5′-CGCTGGCG and 5′-GGCGCTGG) (35) are not among the most frequent octamers in P1. However, their frequencies in both genomes are similar (approximately 1 per 6,000 nucleotides), suggesting that they have related functions. The majority of octamers that are frequent in P1 DNA, other than Chi sites and its variants, correspond to either sequences that are specific for P1 and represent fragments of 17-mer C1 binding sites or 19-mer RepA-binding iterons (Table 1) or fragments of AT-rich regions containing A or T tracts. The most infrequent tetramer in P1 DNA (5′-CTAG) is the same as the most infrequent tetramer in E. coli (35). Seven of its 27 copies are in intergenic regions. Of the remaining 20 that are within 15 protein-coding genes, 9 are within five genes of especially low GC content (three in res, two in mod, one in isaB, two in rlfA, and one in pmgU), which may represent the most recent acquisitions in the genome of P1.
TABLE 1.
Binding sites in P1 DNA of P1 proteins other than C1 and Lpa
| Namea | Coordinatesb | Strand | Sequencec | Function (protein bound) | Reference(s) |
|---|---|---|---|---|---|
| lox | 93587-93601 and 1-15 | + | AACTTCGTATAGCATACATTATACGAAGTT | Site of Cre-mediated recombination (Cre) | 138 |
| cixPp | 30379-30419 | − | TTAACAAAAACCAGTATGTGTGGAAA | cin autoregulation (Cin) | 150 |
| cixL | 31016-31035 | + | CAATCCTAAACCTTGGTTTAAGAGAA | Site of Cin-mediated C-segment inversion | 150 |
| cixR | 31016-31035 | − | TTATCCAATACCTTGGTTTAAGAGAA | Site of Cin-mediated C-segment inversion | 150 |
| parS | Plasmid centromere | 4, 92 | |||
| boxA4 | 57713-57719 | + | GTGAAAT | (ParB) | |
| boxB2 | 57722-57727 | − | TCGCCA | (ParB) | |
| boxA3 | 57722-57733 | − | GTGAAAT | (ParB) | |
| boxA2 | 57735-57741 | + | TTGAAAT | (ParB) | |
| boxA1 | 57778-57784 | + | GTGAAAT | (ParB) | |
| boxB1 | 57790-57795 | − | TCGCCA | (ParB) | |
| parO | 60027-60068 | − | TTTATGCAGCATTTTTAATTAAATTCAAAA ATACAGCATAAA | par operon autoregulation (ParA) | 73 |
| incA | Plasmid replication control | Reviewed in reference 49 | |||
| It1 | 60288-60306 | + | GATGTGTGCTGGAGGGAGA | (RepA) | |
| It2 | 60319-60337 | + | GATGTGCGATAGAGGGAAG | (RepA) | |
| It3 | 60349-60367 | + | TATGTGCTGTGGAGGGATC | (RepA) | |
| It4 | 60380-60398 | + | TATGTGTGCTGGAGGGAAA | (RepA) | |
| It5 | 60411-60419 | + | CATGTGCACTGGAGGGAAA | (RepA) | |
| It6 | 60442-60460 | + | GATGTGTGCTGGAGGGAAA | (RepA) | |
| It7 | 60482-60500 | − | GATGTGCGCTGGAGGGGGA | (RepA) | |
| It8 | 60523-60541 | − | GATGTGTGCTGCCGGGAAG | (RepA) | |
| It9 | 60554-60572 | − | AGTGTGTGCTGGAGGGAAA | (RepA) | |
| incC | Part of plasmid replication initiation region | Reviewed in reference 51 | |||
| It10 | 61489-61507 | − | GATGTGTGTTGACGGGAAA | (RepA) | |
| It11 | 61511-61529 | − | AATGTGTGCTGGCGGGATA | (RepA) | |
| It12 | 61532-61550 | − | AATGTGTGCTGACGGGTTG | (RepA) | |
| It13 | 61553-61571 | − | GATGTGTGCTGGAGGGATA | (RepA) | |
| It14 | 61574-61592 | − | GATGTGTGCTGGAGGGAAA | (RepA) | |
| pacS | Sites required to initiate packaging | 288 | |||
| 89523-89528 | + | TGATCA | (PacA) | ||
| 89530-89535 | + | TGATCA | (PacA) | ||
| 89542-89547 | + | TGATCA | (PacA) | ||
| 89557-89562 | + | TGATCA | (PacA) | ||
| 89653-89658 | + | TGATCG | (PacA) | ||
| 89665-89570 | + | TGATCA | (PacA) | ||
| 89676-89681 | + | TGATCA | (PacA) | ||
| 89704-89709 | + | TGATCG | (PacA)d |
Names of functionally linked clusters of binding sites are followed by a list of particular sites within each cluster. It, iteron.
The first number is the coordinate of the first nucleotide of upper-strand (+) binding sites or of the last nucleotide of the lower-strand (−) binding sites, based on arbitrary convention. Coordinates refer to positions of sites in the P1 c1-100 mod749::IS5 genome without its nonintegral parts, IS5, and the associated 4-bp duplication.
The 5′-GATC sequences that are targets for Dam- or Dmt-mediated N-6 adenine methylation are in boldface.
Deletion of this site reduces the efficiency of cutting.
(ii)Coding sequences.
Analysis of the complete sequence of P1 has revealed 112 protein-coding genes and five genes encoding untranslated RNA (Tables 2 and 3 see also Table S1 in the supplemental material). Of these, complete sequences of 42 protein-coding genes and two RNA genes had been determined previously (149, 187, 190, 346). The remaining 73 genes were identified as described in Materials and Methods. Of the previously published sequences of P1 genes, some differ from those presented here by single-nucleotide changes or their overall length, due either to mutations or to previous sequencing errors.
TABLE 2.
Alphabetical index of P1 genes
| Gene | Location and direction or commenta | Gene | Location and direction or comment | Gene | Location and direction or commentb | ||
|---|---|---|---|---|---|---|---|
| 1 | See mat | gta | See hdf | pmgM | 77.6 → 78.2 | ||
| 2 | See lydB | icd | 47.2 → 47.4 | pmgN | 78.2 → 78.4 | ||
| 3 | See bplA | iddB | 25.3 → 25.7 | pmgO | 78.3 → 79.0 | ||
| 4 | See pro | imcA | 93.2 ← 93.3 | pmgP | 78.9 → 79.5 | ||
| 5 | 72.5 ← 73.1 | imcB | 93.1 ← 93.2 | pmgQ | 80.2 → 80.9 | ||
| 6 | 73.1 ← 74.1 | insA | 21.8 ← 22.1 | pmgR | 81.0 → 81.2 | ||
| 7 | 74.9 ← 75.6 | insB | 21.4 ← 21.9 | pmgS | 81.2 → 82.5 | ||
| 8 | See pmgR, pmgS | isaA | 20.3 → 21.3 | pmgT | 83.3 → 84.2 | ||
| 9 | See pacA | isaB | 22.3 → 22.9 | pmgU | 84.0 → 84.3 | ||
| 10 | See lpa | hdf | 28.6 ← 29.2 | pmgV | 84.3 → 84.7 | ||
| 11 | See R? | hot | 85.3 → 85.6 | ppfA | 62.3 ← 62.7 | ||
| 12 | See R? | hrdC | 64.1 → 65.2 | pro | 17.8 → 18.8 | ||
| 13 | See prt | humD | 86.3 → 86.7 | ppp | 79.5 → 80.2 | ||
| 14 | See prt, pro | hxr | 23.0 ← 23.3 | prt | 16.1 → 17.8 | ||
| 15 | See 22, 23 | kilA | 48.6 → 49.4 | R | 36.6 ← 37.0 | ||
| 16 | 37.1 ← 38.0 | lpa | 88.7 → 89.2 | rebA | See ant1 | ||
| 17 | See lyz | lxc | 8.5 ← 8.7 | rebB | See ant2 | ||
| 18 | See mat | lxr | 85.6 ← 86.2 | ref | 2.0 → 2.6 | ||
| 19 | See S | lydA | 29.6 ← 29.9 | repA | 60.0 ← 61.4 | ||
| 20 | See U | lydB | 29.2 ← 29.6 | repL | 49.5 → 50.3 | ||
| 21 | 53.3 ← 54.2 | lydC | 30.0 ← 30.3 | res | 3.5 ← 6.3 | ||
| 22 | 54.2 ← 55.8 | lydD | 18.8 ← 19.1 | rlfA | 50.5 → 51.2 | ||
| 23 | 55.8 ← 57.5 | lydE | 18.8 ← 19.0 | rlfB | 51.2 → 52.4 | ||
| 24 | 74.1 ← 74.9 | lyz | 19.1 ← 19.6 | sim | See simC | ||
| 25 | 75.6 ← 76.8 | mat | 2.7 → 3.4 | simA | 46.7 ← 46.8 | ||
| 26 | 76.8 ← 77.2 | mlp | 62.1 ← 62.8 | simB | 46.3 ← 46.6 | ||
| ant1 | 47.4 → 48.5 | mod | 6.4 ← 8.3 | simC | 45.5 ← 46.3 | ||
| ant2 | 47.7 → 48.5 | ORF0 | See cre | sit | 39.7 ← 43.2 | ||
| ban | 70.0 → 71.4 | ORF1 | See cra | ssb | 19.8 → 20.3 | ||
| bof | See lxc | ORF2 | See imcA | S (Sc + Sv) | 33.6 ← 36.6 | ||
| bplA | 38.0 ← 39.4 | ORF3 | See imcB | Sv′ | 31.0 → 32.5 | ||
| bplB | 52.2 ← 52.7 | ORF4 | See coi | tciA | 69.0 → 69.4 | ||
| c1 | 92.0 ← 92.8 | ORF50 | See simB | tciB | 69.4 → 69.6 | ||
| c2 | See mod | ORF93 | See pdcB | tciC | 69.6 → 69.7 | ||
| c3 | See mod | ORFVIII | See hxr | trnA | 68.1 → 68.2 | ||
| c4 | 47.1 → 47.2 | ORFX | See icd | trnI | 69.7 → 69.8 | ||
| c5 | See 6?, 24? | pacA | 89.3 → 90.5 | trnT | 68.2 → 68.3 | ||
| c6 | See lxc | pacB | 90.5 → 92.0 | tsu | See darA | ||
| c7 | See 22?, 23? | pacC | See pacB | tub | 44.1 ← 44.8 | ||
| c8 | 1.5 → 1.8 | pap | 82.5 → 83.0 | tsu | See darA | ||
| cin | 30.4 → 31.0 | parA | 58.8 ← 60.0 | U | 33.1 ← 33.6 | ||
| cra | 0.2 → 0.4 | parB | 57.8 ← 58.8 | U′ | 32.5 → 33.1 | ||
| cre | 0.4 → 1.4 | pdcA | 87.4 → 87.6 | uhr | 64.0 → 64.3 | ||
| coi | 92.9 ← 93.2 | pdcB | 87.6 → 88.7 | ulx | 8.7 ← 9.2 | ||
| dam | See dmt | phd | 86.8 → 87.0 | upfA | 61.7 ← 62.1 | ||
| darA | 26.7 ← 28.6 | plp | 68.2 ← 68.6 | upfB | 62.7 ← 63.6 | ||
| darB | 9.2 ← 16.0 | pmgA | 39.4 ← 39.7 | upfC | 63.5 ← 63.8 | ||
| dbn | 71.4 → 72.4 | pmgB | 43.9 ← 44.1 | upfM | 84.9 → 85.0 | ||
| ddrA | 26.3 ← 26.7 | pmgC | 44.8 ← 45.3 | upfN | 85.0 → 85.2 | ||
| ddrB | 23.3 ← 26.3 | pmgF | 51.8 ← 52.1 | upfO | 85.2 → 85.3 | ||
| dmt | 65.2 → 67.4 | pmgG | 52.7 ← 53.3 | upl | 68.5 ← 68.8 | ||
| doc | 87.0 → 87.4 | pmgL | 77.4 → 77.6 | vad | See ddrA, darA |
References to previously mapped genes whose assignment to ORFs in the P1 genome is unclear are summarized in reference 346.
Directions of the tail fiber genes are for C(+) phage.
TABLE 3.
Gene products (listed by gene position)a
| Nameb (former name[s]) | Gene coordinatesc | Coding strand | Mol mass (Da) (nucleotides in RNA) | Basis for name; known or proposed function | Reference(s)d |
|---|---|---|---|---|---|
| Cra (gpORF0) | 207-428 | + | 8,704 | Putative cre-associated function | 302S |
| Cre* | 436-1467 | + | 38,539 | Cyclization recombinase acting on P1 DNA at redundant lox sites on infection; resolves dimers, etc.; possibly modulates copy no. | 7,* 10, 21, 106, 182, 302S |
| C8 | 1518-1829 | + | 11,313 | Establishment of lysogeny? | 280, 281, twS |
| Ref | 2077-2637 | + | 21,328 | Recombination enhancement function; aids microhomology recognition, maximal function requires recBCD activity | 184, 207, 337, 338S |
| Mat (gp1; gp18?) | 2716-3468 | + | 28,933 | Particle maturation | 191, 346 |
| Res* (EcoP1; R) | 3544-6456 | − | 111,457 | Restriction component (EcoP1) of type III restriction-modification (ResMod) system | 68, 107,* 145, 152 |
| Mod* (M, C2, C3) | 6459-8399 | − | 73,485 | Modification and site recognition component of type III ResMod system; modifies pairs of 5′-AGACC sequences | 41, 42, 107,* 145,S152, 262 |
| Lxc* (Bof, C6) | 8551-8799 | − | 9,645 | Lowers expression of c1, enhances binding of C1 to its operators | 164, 272, 311, 312, 318*S |
| Ulx | 8796-9236 | − | 17,079 | Unknown; product of gene upstream of lxc | twS |
| DarB* | 9270-16037 | − | 251,542 | Defense (in cis) against a subset of type I Res enzymes, e.g. EcoB, EcoK; putative DNA methyltransferase and DNA helicase; may methylate DNA during injection | 155,* twS |
| Prt (gp13, gp14?) | 16113-17822 | + | 62,731 | Portal protein (by similarity) | 252, twS |
| Pro (gp4, gp14?) | 17815-18834 | + | 36,882 | Putative head processing protease and ? kinase; required for head morphogenesis and maturation of DarA precursor protein | 321, 324, twS |
| LydE | 18817-19029 | − | 7,914 | Lysis determinant, putative antiholin | twS |
| LydD | 18879-19133 | − | 9,936 | Lysis determinant, putative holin | twS |
| Lyz (gp17) | 19126-19683 | − | 20,255 | Lysozyme | 277S |
| Ssb | 19852-20340 | + | 18,023 | Single-stranded DNA binding protein; can suppress E. coli ssb | 187S |
| IsaA | 20370-21333 | + | 35,969 | Unknown; product of IS1 associated gene | twS |
| InsB | 21442-21945 | − | 19,742 | Transposition protein InsB of integral P1 IS1 | twS |
| InsA | 21864-22139 | − | 9,901 | Transposition protein InsA of integral P1 IS1 | twS |
| IsaB | 22328-22933 | + | 23,633 | Unknown; product of IS1-associated gene | 149S |
| Hxr (ORFVIII) | 23045-23353 | − | 11,033 | Homolog of xre; possible repressor protein | 149S |
| DdrB | 23343-26330 | − | 108,752 | Unknown; product of the second gene downstream of darA; possible protease or response regulator | 149SD |
| IddB | 25378-25776 | + | 13,540 | Unknown; product of gene internal to ddrB | tw |
| DdrA (Vad?) | 26343-26708 | − | 13,013 | Uncertain; product of gene downstream of darA; defect possibly alters P1B:P1S:P1M ratios; vad (viral architecture determinant) | 149S |
| DarA* (Vad?, Tsu?) | 26705-28624 | − | 69,479 | Defends against restriction by type I Res endonuclease and enables DarB to function; internal head protein processed by Pro | 149,S155,* 304* |
| Hdf (Gta?) | 28626-29228 | − | 22,150 | Uncertain; homolog to DarA fragment; defect possibly causes gta (generalized transduction affected) phenotype | 149 |
| LydB (gp2) | 29215-29658 | − | 17,097 | Lysis determinant; prevents premature lysis, LydA antagonist | 149, 277 |
| LydA | 29655-29984 | − | 11,433 | Lysis determinant; holin; promotes cell lysis | 149, 277 |
| LydC | 30052-30333 | − | 10,449 | Lysis determinant; putative holin (by homology) | twS |
| Cin* | 30461-31021 | + | 21,230 | C-segment inversion; cix site-specific recombinase providing for alternate fiber gene expression and hence host range enlargement | 109, 135, 142, 150* |
| gp Sv′ | 31016-32583 | + | 55,254 | Variable part of S′ tail fiber; gene expressed in (−) orientation | twS |
| gpU′ | 32586-33119 | + | 20,676 | Tail fiber assembly chaperone (by similarity to Mu homolog); variable gene of tail fiber operon, expressed in (−) orientation | 146, twS |
| gpU* (gp20) | 33148-33675 | − | 20,258 | As gpU′, but expressed in (+) orientation; both gpU′ and gpU may be virion proteins | 142,* 146, twS |
| gpS:Sc + Sv (gp19) | 33679-36642 | − | 104,837 | Tail fiber with a constant (Sc) and variable (Sv) segment as in Mu; Sv is exchanged for shorter Sv′ upon C-segment inversion | 104, 146, twS |
| gpR (gp11? gp12?) | 36654-37088 | − | 15,973 | Tail fiber structure or assembly | 104 |
| gp16 | 37167-38003 | − | 31,357 | Baseplate or tail tube | 104,190, twS |
| BplA (gp3?) | 38003-39436 | − | 53,577 | Putative baseplate structural protein (by homology), may mediate contact between hub and wedges, as does its homolog in T4 | twS |
| PmgA | 39433-39789 | − | 13,239 | Putative morphogenetic function | twS |
| Sit | 39789-43211 | − | 120,710 | Structural injection transglycosylase; putative tail tube “ruler” | 190 |
| PmgB | 43293-44174 | − | 34,138 | Putative morphogenetic function | twS |
| Tub | 44189-44800 | − | 22,316 | Major tail tube protein | twS |
| PmgC | 44811-45377 | − | 21,017 | Putative morphogenetic function | twS |
| SimC* (Sim) | 45587-46399 | − | 30,800 | Confers superimmunity when in high copy number by blocking P1 at entry; requires removal of leader sequence | 77, 170, 208*S |
| SimB (gpORF50) | 46374-46691 | − | 12,033 | Unknown; superimmunity-linked function | 213,SD twS |
| SimA | 46713-46844 | − | 4,842 | Unknown; superimmunity-linked function | twS |
| C4* | 47144-47220 | + | (77) | Antisense RNA that inhibits Icd and Ant1/Ant2 synthesis by acting on target ant RNA; processed to active form by RNase P | 26, 33, 57,* 58, 112, 115, 130 |
| Icd* (gpORFX) | 47271-47492 | + | 8,397 | Reversible inhibition of cell division, apparently required for ant expression | 123, 131, 257* |
| Ant1* (RebA) | 47489-48532 | + | 38,667 | Antagonism of C1 repression; forms complex with Ant2 | 33, 112, 122, 131, 256* |
| Ant2* (RebB) | 47738-48532 | + | 29,039 | Antagonism of C1 repression, forms complex with Ant1 | 33, 112, 131, 256* |
| KilA | 48697-49497 | + | 29,575 | Unknown, expression can kill host | 112, 295 |
| RepL | 49527-50372 | + | 30,857 | Lytic replication, initiates replication at oriL (within repL) | 112, 295 |
| RlfA | 50541-51245 | + | 27,716 | Unknown; possibly replication-linked function | twS |
| RlfB | 51245-52444 | + | 18,632 | Unknown; possibly replication-linked function | twS |
| PmgF | 51875-52171 | − | 11,423 | Putative morphogenetic function | twS |
| BplB | 52255-52764 | − | 18,804 | Baseplate | twS |
| PmgG | 52776-53357 | − | 20,696 | Putative morphogenetic function | twS |
| gp21 | 53393-54208 | − | 29,329 | Baseplate or tail tube | 323, twS |
| gp22 (gp15?, C7?) | 54218-55807 | − | 56,935 | Sheath protein | 323, 325,* twS |
| gp23 (gp15?, C7?) | 55868-57574 | − | 62,248 | Major head protein, present in P1 heads in full-length (62 kDa) and truncated (44 kDa) forms | 323, 325,* twS |
| ParB* | 57800-58801 | − | 37,440 | Plasmid partitioning; binds to parS, enhances ParA-mediated repression of par operon and ATPase of ParA; can spread over DNA flanking parS, silencing gene expression; pairs parS loci | 4, 70, 73, 83, 90,* 114, 203, 258, 343 |
| ParA* | 58818-60014 | − | 44,269 | Plasmid partitioning; weak ATPase, binds to parO repressing transcription, binds to ParB-parS partition complex | 4, 37, 70, 73,* 343 |
| RepA* | 60572-61432 | − | 32,219 | Plasmid replication initiated by chaperone-activated monomers at oriR; represses own synthesis; binds at iterated sites (incC and incA) and can handcuff them, controling plasmid copy number | 2,* 3, 6, 49, 52 |
| UpfA | 61751-62143 | − | 14,747 | Unknown protein function | twS |
| Mlp | 62155-62487 | − | 12,597 | Membrane lipoprotein precursor (by homology); ? lysis control | twS |
| PpfA | 62321-62770 | − | 15,744 | Possible periplasmic function | twS |
| UpfB | 62783-63628 | − | 29,310 | Unknown protein function | twS |
| UpfC | 63580-63864 | − | 9,478 | Unknown protein function | twS |
| Uhr | 64034-64318 | + | 10,781 | Unknown; product of gene upstream of hrdC | twS |
| HrdC | 64311-65216 | + | 33,638 | Homolog of RdgC of E. coli; possibly involved in replication, recombination, and/or Dmt function | twS |
| Dmt (Dam) | 65216-67477 | + | 83,595 | Predicted bifunctional DNA methyltransferase; methylates A's in GATC sequences, and (by homology) probably C's at unknown sites; affects replication control, late gene expression, packaging | 59, 66, twS |
| tRNA1 | 68149-68224 | + | (76) | Proposed tRNA-Asn specific for the AAC codon | twS |
| tRNA2 | 68227-68302 | + | (76) | Proposed tRNA-Thr specific for the ACA codon | twS |
| Plp | 68273-68650 | − | 13,800 | Unknown; putative lipoprotein | twS |
| Upl | 68651-68842 | − | 7,092 | Unknown; product of gene upstream of plp | twS |
| TciA | 69034-69459 | + | 15,187 | Tellurite or colicin resistance or inhibition of cell division (by homology) | twS |
| TciB | 69459-69623 | + | 5,752 | Tci accessory protein (by homology) | twS |
| TciC | 69623-69730 | + | 3,719 | Tci accessory protein | twS |
| tRNA3 | 69734-69809 | + | (76) | Proposed tRNA-Ile specific for ATA codon | twS |
| Ban* | 70096-71460 | + | 50,479 | DnaB (of E. coli) analog and homolog; replicative DNA helicase | 67, 195*S237, 254* |
| Dbn | 71460-72458 | + | 37,379 | Unknown; product of gene downstream of ban | twS |
| gp5 | 72505-73137 | − | 21,669 | Baseplate | 323, twS |
| gp6 (C5?) | 73130-74146 | − | 37,222 | Tail length determination | 323, twS |
| gp24 (C5?) | 74148-74933 | − | 28,880 | Baseplate or tail stability | 323, twS |
| gp7 | 74920-75648 | − | 27,145 | Tail stability | 323, twS |
| gp25 | 75652-76869 | − | 45,834 | Tail stability | 323, twS |
| gp26 | 76879-77256 | − | 14,456 | Baseplate | 323, twS |
| PmgL | 77436-77648 | + | 9,258 | Putative morphogenetic function | twS |
| PmgM | 77651-78229 | + | 21,888 | Putative morphogenetic function | twS |
| PmgN | 78296-78451 | + | 5,593 | Putative morphogenetic function | twS |
| PmgO | 78393-79055 | + | 24,217 | Putative morphogenetic function | twS |
| PmgP | 78953-79579 | + | 23,198 | Putative morphogenetic function | twS |
| Ppp | 79579-80253 | + | 25,568 | P1 protein phosphatase, possible role in head morphogenesis | twS |
| PmgQ | 80250-80951 | + | 26,912 | Putative morphogenetic function | twS |
| PmgR (gp8?) | 81033-81251 | + | 8,318 | Putative morphogenetic function | twS |
| PmgS (gp8?) | 81253-82515 | + | 48,345 | Putative morphogenetic function; ? prohead scaffolding protein | twS |
| Pap | 82588-83094 | + | 19,200 | P1 acid phosphatase (by homology); ? head morphogenesis | twS |
| PmgT | 83289-84218 | + | 35,155 | Putative morphogenetic function | twS |
| PmgU | 84023-84358 | + | 12,163 | Putative morphogenetic function | twS |
| PmgV | 84355-84717 | + | 13,975 | Putative morphogenetic function | twS |
| UpfM | 84900-85013 | + | 4,343 | Unknown protein function | twS |
| UpfN | 85029-85226 | + | 7,153 | Unknown protein function | twS |
| UpfO | 85204-85344 | + | 5,238 | Unknown protein function | twS |
| Hot | 85368-85631 | + | 9,694 | Homolog of theta subunit of DNA polymerase III; ? replication | twS |
| Lxr | 85638-86210 | − | 22,042 | LexA-regulated function | twS |
| HumD* | 86385-86774 | + | 14,313 | Homolog of UmuD′ subunit of E. coli repair protein DNA polymerase V (UmuD′-UmuC); complements mutant UmuD′ | 200, 217,* 276S |
| Phd* | 86847-87068 | + | 8,132 | Prevention of host death by Doc toxicity; confers, with Doc, addiction to P1; represses transcription of addiction operon | 95,* 192,S194, 210 |
| Doc* | 87068-87448 | + | 13,587 | Death on curing; toxin of P1 addiction system; reversible inhibitor of protein synthesis; corepressor of addiction operon | 95, 121, 192, 210, 211* |
| PdcA | 87432-87632 | + | 7,630 | Unknown; post-doc | 192,S tw |
| PdcB (gpORF93) | 87660-88703 | + | 39,877 | Unknown; post-doc | 192, tw |
| Lpa* (gp10) | 88792-89244 | + | 18,117 | Late promoter activator | 111,* 188S |
| PacA* (gp9) | 89330-90523 | + | 45,246 | DNA packaging; cuts at pac together with PacB | 286,S 288* |
| PacB* | 90523-92007 | + | 55,604 | DNA packaging; cuts at pac together with PacA | 286,S 287* |
| C1* | 92032-92883 | − | 32,544 | Controls plaque clarity; primary repressor of lytic functions | 79,* 84, 124, 238,S316, 319 |
| Coi* (gpORF4) | 92994-93203 | − | 7,741 | C one (reversible) inactivator; forms 1:1 complex with C1 | 25, 27, 84, 127, 130* |
| ImcB (gpORF3) | 93169-93264 | − | 3,659 | Immunity C function; may regulate c1 | 27, 84 |
| ImcA (gpORF2) | 93283-93396 | − | 4,340 | Immunity C function; may regulate c1 | 27, 84 |
A more complete table, which includes pI, number of amino acid residues (or nucleotides in RNA), and features of the amino acid sequence (closest homologs and amino acid sequence motifs) is to be found as Table S1 in the supplemental material.
Gene products marked by an asterisk were either purified or identified in PAGE gels.
Coordinates of genes refer to their positions in the P1 c1-100 mod749::IS5 genome without its nonintegral parts, IS5, and the associated 4-bp duplication. Coordinates of protein-coding genes are from the initial codon through the first stop codon.
References marked by an asterisk concern identification of a protein or RNA product of a given gene; those marked by a superscript S concern previously published sequences. Superscript SD, published sequence differs internally from sequences presented here; superscript SP, published sequence is partial; tw, this work.
Protein-coding genes account for 92% of the P1 genome. Translation of most of the ORFs is initiated independently from their own ribosome binding and translation start sites (AUG[86]≫GUG[16]>UUG[10]). Translation stops predominantly at UAA and less frequently at UGA and UAG.
Utilization of UUG as the translation initiation codon appears to be more frequent in P1 than in other coliphages (224) and in E. coli itself (35). It was confirmed experimentally that translation of the P1 lxc gene, which encodes a modulator of C1-mediated repression, initiates at UUG (272).
Whereas the translation initiation codons of P1 are generally 6 to 10 nt downstream of typical E. coli ribosome binding sites (RBS), genes plp, pmgO, and pdcA lack nearby RBSs. They are probably translationally coupled to genes immediately upstream (plp) or overlapping (pmgO and pdcA). The gene ref (recombination enhancement function) in a monocistronic operon is more unusual. The sequence that most closely resembles a canonical RBS is separated by 16 bp, including a run of eight T's, from a presumptive ref initiation codon (207, 338). What purpose the extra RNA might serve or how it is accommodated remains to be determined.
Promoter-proximal regions of four genes, pro, hrdC, pmfA, and pmgL, contain, in addition to ATG codons preceded by sequences with a good match to RBSs in mRNA, TTG codons similarly preceded by canonical RBS sequences and further upstream. Possibly, each of these genes encodes two proteins that differ in the length of their N-terminal domains. Alternative translational starts in mRNA at UUG or AUG could control expression of these genes by modulating the intracellular concentration or activity of their protein products.
Although genes of P1 are tightly packed within the genome, overlaps are rare and occur mostly at coding termini. Spaces between genes of adjacent operons are in most cases limited to short regions containing promoter and regulatory sequences. Of those that exceed 250 bp, one contains oriR and another incA, both involved in P1 prophage replication (Table 1). A 272-bp region between the first gene of the sim operon, simA, and c4, was shown to encode the 5′ region of the c4 transcript, which is cleaved to yield C4 RNA (115). Two regions within the ban operon (531 bp preceding the trnA gene and 359 bp between the trnI gene and ban, described in detail later in this paper) may also encode species of RNA that are processed.
Of previously identified or predicted products of P1 genes, 65 exhibit significant homology to known proteins of other organisms (Table 3; see also Table S1 in the supplemental material). Only 29 are homologous to proteins encoded exclusively by other phages. Although they include 11 homologs of proteins of T4 or T4-like phages, there is no clear preference in these homologies for proteins of a particular phage, suggesting that P1 has had a long, separate history. Homologs of known genes of different provenance are scattered throughout the P1 DNA. Whether their distribution results from the horizontal transfer of modules during the evolution of the P1 genome or from convergent evolution remains to be determined.
(iii)Organization of P1 genes and transcription units.
P1 genes can be grouped into 45 operons, of which 15 appear to be monocistronic. Their organization resembles that of T4 (224) in that only some genes of related function are in clusters.
Regulatory regions of 38 operons contain one or more sequences that resemble strong σ70 promoters (Table 4). Of 26 σ70 promoters identified previously by transcriptional fusion or primer extension experiments, only 9 had a match to the consensus sequence below 50% and required relaxing the stringency of the prediction program for their detection. All nine promoters are associated with genes expressed during lysogeny: three with cre, three with res or mod (including the P1lxc promoter, which can also drive transcription of lxc), one with parB, one with repA, and one with c1. A relatively high predicted strength of known and putative promoters of genes expressed during P1 lytic development implies that as soon as these promoters become accessible to the host RNA polymerase, they can effectively compete with E. coli promoters for the enzyme. Multiple promoters within regulatory regions of several operons indicate a requirement for different controls on the expression of genes at different stages of phage development or plasmid maintenance or a requirement to adapt to alternative hosts or environmental conditions.
TABLE 4.
Known and putative σ70 promoters of P1 genes
| Name (former name) | Positiona | Strand | Sequenceb
|
Basis for identificationc
|
Reference(s) | |||
|---|---|---|---|---|---|---|---|---|
| −35 | −10 | tsp | pp | cp | ||||
| P2coi | 41 | − | TGCTAAGGTA TTGAAC TGTATGGATTTACAGG | TAAATTGATCATATTC | 56.8 | |||
| P3cre | 104 | + | ACTTGATCAT TTGATC AAGGTTGCGCTACG | TAAAAT CTGTGAAAAA | √ | √ | 42.6 | 302 |
| P2cre | 283 | + | GCAAAAGGGT TTGATC GTGATAGTTGCCAACTGT | CATTAT CGCGCGGTGA | √ | √ | 45.6 | 302 |
| P1cre | 400 | + | GAGCCGATCC TGTACA CTTTACTTAAAAC | CATTAT CTGAGTGTTA | √ | √ | <40.0 | 302 |
| Pc8 | 1394 | + | AATGTAAATA TTGTCA TGAACTATATCCGTAACCTG | GATAGT GAAACAGGGG | 54.4 | |||
| P2ref (Pref-2) | 1957 | + | AACAAAATAT CTATCA TTGCTCTAATTGATTGC | TATAAT TGAGCCGCAG | 59.8 | 338 | ||
| P1ref | 2017 | + | TACGAAGACG TTGCCA TTACTTCACTCCTTGA | CATCAT TGGCGGCCAT | 52.7 | |||
| Pmat | 2666 | + | TGATAAATGA TTGACA ACTGACAAGTGACTTCAGT | CAGAAT CATCACACGC | 56.8 | |||
| P1res | 6558 | − | GATGAAGATT TTGCCC CAAACAAAGTCGTGTTT | TATGGT TGTAACTTTG | 60.4 | |||
| P2res (PR1) | 6891 | − | CAGGAGGTAT TTAAAA AAAGCACAACCACAAGT | GATAAT GCGGCTAAAA | S1 | √ | 52.1 | 285 |
| P3res | 6926 | − | AGATCTATTT TTGAAA TAACAAAAAAAAGGA | TACAGG AGGTATTTAA | S1 | √ | 47.3 | 285 |
| P1mod (PM1) | 8450 | − | CATATAGATA GTGATA GTGCCACAACTTCTGGC | TCTAAC GGGCTGGGGA | √ | 45.6 | 285 | |
| P2mod (PM2) | 8548 | − | ATCATAGATA ATGCAT TGCTTCTCAATGCGGAT | TAAACT TCCCCAAATT | √ | √ | 50.9 | 285 |
| P1lxc | 8844 | − | CTGCCAGTCC CTGAAG AAGTTGACGACCAGGAC | TATGAG TTTGAGTCTT | S1 | 46.7 | 285, 318 | |
| P2lxc | 9022 | − | AATTAAAAAA TTATTA CTGGAGCGCGGTTATGG | TAATTT CGGTCGTCTC | S1 | 51.5 | 285 | |
| P3lxc | 9167 | − | AAAACATATC TTGAGG CGGTACTGCGTACGG | CATTGT TAGGAAAGAC | S1 | 51.5 | 285 | |
| PdarB | 16093 | − | CGTGATTTTA TTGTCT GCCAAGATTGCACCTTAAT | TAGAAT AATTCACATC | 56.8 | |||
| P2ssb (Pr21) | 19787 | + | TGCAAATCAC TTGTTA GCTACGTTTCAAAGATA | TACATT ATTGCTCTAA | √ | 52.7 | 59 | |
| P1ssb | 19821 | + | ACATTATTGC TCTAAT TAATTTATTTTATTAGG | TAAGAT AAGTGGCACA | 55.6 | |||
| Plyz | 19845 | − | GATGACTTTG TTTACA CCGCGTTGTGCCACT | TATCTT ACCTAATAAA | 53.3 | |||
| PisaB | 22220 | + | CAATGATGGT TAGACA AGCAAGATAGGGCGAAT | TAAAAT TAATCAAGAG | 61.5 | |||
| PinsA | 22229 | − | AAAGTGACTC TTGATT AATTTTAATTCGCCC | TATCTT GCTTGTCTAA | 57.4 | |||
| P2cin | 30339 | + | CACATAACTA TTTCCT TAATAGTGAATTAAA | TATCAT TGGGAAACGG | 55.6 | |||
| P1cin | 30390 | + | TACTTTGTGA TTTCCA CACATACTGGTTTTTGTTAAT | TAAAAT CCGCAGCTTG | 53.3 | |||
| PlydC | 30461 | − | TGTTCATTTG TTGATA CGCGTACATAGCCTAT | TAGCAT ATTTTCTGCT | 59.2 | |||
| P1pmgC | 45555 | − | AAAATAGTGT TTGAAA GGATAGTCAATTTAAGT | TTTAAC ACAACGCCCA | 59.8 | |||
| P2pmgC | 45636 | − | CAACAATTCG TTGATG TAATGGAAGGATTTGA | TAGCAT CATGTATAAA | 59.8 | |||
| P3pmgC | 45771 | − | CAAATATATA TTCACA AATCAAGATCCTGTTCA | GATGAT GAATTTTATA | 58.0 | |||
| PsimB | 46843 | − | TGTACCCTAA ATGAGT TATAAGGCAGGTGAGGT | TATAAT GAGAAAACTA | 56.8 | |||
| PsimA | 46909 | − | GCATTATTTG TTTAAT AAATACACAGTTGGA | TCTAAT AACCTCTTTT | 51.5 | |||
| P2c4 (P51a) | 46956 | + | TCTAATAAAT TTGTAT TTTTAAGTCGCGAATGC | TATCTT TTCGCATCAT | √* | 56.8 | 131 | |
| P1c4 (P51b) | 46996 | + | TCGCATCATA TTGACC TTTTAATCGTTCAGGCT | TATAGT TCCACCGTCG | √* | 60.4 | 131 | |
| PkilA (P53) | 48593 | + | CAACCCGATCTGGATC GGGTCAGAAAAATTTGC | TCTAAT AAATTTCGTT | √ | √ | 50.3 | 295 |
| PaskilA (P53as) | 48724 | − | GGTTGAAGGA TCGACA TTTTGATGAAGGTTTGA | TATATT CATATCCGCA | √ | √ | 62.7 | 125 |
| PrepL | 49267 | + | AAACTATCTC TTCACC TGGTTGCGTGATAACGG | AATTCTGATCGCAACC | 52.1 | |||
| PrlfA | 50486 | + | CGCCCCATTA TTGCAA TTAATAAACAACTAACG | GACAAT TCTACCTAAC | 52.7 | |||
| P1rlfB | 50663 | + | AAAAAATTGC TAGAGA CATCAAGGAATTAAAAT | TAAAAC ACAATCTAAA | 55.0 | |||
| P2rlfB | 50704 | + | AATCTAAAAC CTGATT TCGAAATCAAGTGGAC | TAAAGT ATCTGCGTCC | 56.2 | |||
| P3rlfB | 50746 | + | GCGTCCAAAG TTGAAT TTTACTTAGATGTCG | TAGATT ACTTCTTCAG | 55.0 | |||
| P4rlfB | 50971 | + | ATTGAAAAAA TTGAGA AATTAAGAGGGGTTCTTCA | CAATGATCGCTATGAT | 57.4 | |||
| Pasrlf | 51784 | − | TAGCTAGCTA TTGAAA AGCTAAAGTAGATCGGT | TAGATT TGCATTACTC | 69.2 | |||
| P1pmgF | 52236 | − | TACAACTGGA TTGAGT GGGATTAAGAGTCATCCCTTG | TATTTT AAAGCTCCTT | 53.3 | |||
| P2pmgF | 52342 | − | GCTACAACAA TTGAAA TGTTGGACTGCAAAATC | TACAGT GATGCAATCG | 60.9 | |||
| P123 | 57631 | − | AAAAAATTGC TTGTCA CGAGAAAGTCAACAAGTGACT | TTCAAT AAAATCTCTT | 57.4 | |||
| P223 | 57740 | − | TATTAACTGA CTGTTT TTAAAGTAAATTACTC | TAAAAT TTCAAGGTGA | 56.2 | |||
| PparB | 59274 | − | ATTGCCGCGG AAGATT TTGCTAAAGCAGTTTTT | GACCGT ATTGAATTTA | √ | √ | <40.0 | A. Dobruk, unpublished |
| Ppar | 60069 | − | GAGAATGCTA TGTACA AGCATCTACGCATACAT | TATTAT TTTATGCAGC | √ | √ | 54.4 | 73, 120 |
| PrepA | 61505 | − | TGCTGACGGG TTGCTA ATGTGTGCTGGCGGGATA | TAGGAT GTGTGTTGAC | √ | √ | <40.0 | 52 |
| P1ppfA | 62785 | − | GATTCTGTTC TTGATG AATTAAACAAAGCGG | CACAGT AACAAAGGAC | 50.3 | |||
| P2ppfA | 63001 | − | GAACAACAAG TTGAAG CACTGAAGGAAGCTACTG | AACAAT TTGGTGCTTT | 52.7 | |||
| Pdmt | 63962 | + | CTAATAAATA TTGTTT TTTATGTCGTGTTTTCGG | TACCAT TCAGCCATCG | √ | 63.3 | 59 | |
| P2ban | 67452 | + | GGTGTAGCTC ATGACG TTTTGGGTATATTGCTCTA | TAAATT TATTAGTGTA | 52.7 | |||
| P1ban | 67469 | + | TTTGGGTATA TTGCTC TAATAAATTTATTAGTG | TAATAT CGCCTCAATG | √* | 50.3 | 209 | |
| P1upl | 69024 | − | GAATTTCTTT TTGAGA ATGCCGAGCATTTAT | TAACCT CATTACTGGT | 56.2 | |||
| P2upl | 69055 | − | TTCCATCTTC TTGACT CCGCCAGCCGCTTTGCG | GAATTT CTTTTTGAGA | 51.5 | |||
| P2dbn | 70823 | + | GTTTTTAGCC TTGAGA TGCCGAGCCACCAGCT | GATGAT GCGCTCACTG | 53.8 | |||
| P1dbn | 71207 | + | AATCGATCGC TTGAAC AGCGTGCGGACAAACGACCGG | TAAATT CAGATTTACG | 52.1 | |||
| P2pmgL | 77306 | + | TAGTCATTTG TTGAAT ATTTAACTCAATAAAA | GAAAAT TATTAGTGCA | 56.8 | |||
| P1pmgL | 77339 | + | AAAATTATTA GTGCAA TTTTGATTGTGAAATG | TATCAT TCTGCCCTTA | 56.8 | |||
| P26 | 77617 | − | AACATAATCA GTCAAA TCCTGCCGGTCGCATGC | CATATT TACGCTGTTT | 50.3 | |||
| PpmgN | 78197 | + | TGAGCATGTA TTGAAC GGCGATAAGTGGTTG | TATGCT GCTTAAAATA | 49.1 | |||
| PpmgR | 80954 | + | CAGAATAATA TTGCTC TAATAATTCCATATTTT | TAAAAC GTGATGTACA | 52.7 | |||
| P1pmgT | 82778 | + | ACTCCAGTTA TTGATA TGGTGCGCCAGTTATT | TAACGT TTACACGGTC | 56.8 | |||
| P2pmgT | 83095 | + | TCAACAATAA TTGCTC TAATAAATCTTGATTTT | TAAAAC AGAGAAAGTG | 52.7 | |||
| P3pmgT | 83110 | + | CTAATAAATC TTGATT TTTAAAACAGAGAAAGT | GAAAAT AAAAACATGC | √ | 66.3 | 59 | |
| P1upfM | 84752 | + | CAGAAAATGA TTGTTT CCACATCAAGGAGATTT | TAATGT TTCACTGAAA | 55.6 | |||
| P2upfM | 84785 | + | TAATGTTTCA CTGAAA CATTAAGTAAGCCAGTG | CATAAT TCCATTTTTT | 55.6 | |||
| PupfN | 84973 | + | CTTGCTCACG TTGACG TAGAAACCCAACCCTTA | TATAGT TGGATTCGGT | 59.8 | |||
| P1lxr | 86267 | − | TCAACAACCG CTGATG ATTTTGTGCGCTTTGC | TACTAT TCATCACCAA | 49.7 | |||
| P2lxr | 86347 | − | CCATAGCGTT TTCACC TCAATAATACTGTTCATTTA | TACAGT ACACATTAAA | 48.5 | |||
| P1humD | 86318 | + | ACGTAACGCG TTTGCA CCAAAGGTGTCTCTT | TAATGT GTACTGTATA | <40.0 | 200 | ||
| Pphd | 86787 | + | TGCAAAGTGC TGGTGC TTTATGCCTGTGAAGTT | TATAAT TGTGTACACA | √ | 52.1 | 210 | |
| Plpa (Pr94) | 88719 | + | CTCTAATGTA TTGCTA TTTCTTTAATCGAGGG | TATTAT ATTCCACGTT | √ | √ | 57.4* | 193 |
| PpacB | 90384 | + | GAAGAAATTG CCGATA TCGTCGATACAGGTGGT | TATGGT GATGTCGATG | 50.3 | |||
| Pc1 (P99a) | 92951 | − | CACTAATAAA TCTATT ATTTTCGTTGGATCCTTC | TATAAT GGTGGCCAAC | √ | √ | 46.7* | 84 |
| P1coi (P99d) | 93438 | − | TCATAGTTGT TTGACA ATTGCTCTAATAAATTAT | AGTTTT GCCGCCGTTT | √ | √ | 60.9* | 127 |
| P4cre (P99e) | 93541 | + | CTAATAATTC TTGATT TTTATGCGCAGCTGGACG | TAAACT CCTCTTCAGA | √ | √ | 63.9* | 84 |
Coordinate of the first nucleotide of the −35 sequence for promoters from which transcription proceeds on the + strand (clockwise) or of the last nucleotide of the −10 sequence for promoters from which transcription proceeds on the − strand (counterclockwise). Coordinates refer to positions of promoters in the P1 c1-100 mod749::IS5 genome without its nonintegral parts, IS5 and the associated 4-bp duplication. The locations of P3res, P1mod, P1lxc, P2lxc, and P3lxc promoters proposed in reference 285 are corrected.
Conserved −10 and −35 promoter hexamers as well as the “extended −10” motifs (T and G at positions −15 and −14, respectively) and known transcription start sites are underlined. The 5′-GATC sequences that are targets for Dam- or Dmt-mediated N-6 adenine methylation are in boldface.
Identification of promoters is based on results of transcription start point determination (tsp) or RNA polymerase binding assay (tsp*), detection with the promoter probe vector (pp), or similarity of the sequence to the consensus sequence for σ70 promoters calculated with the use of the program Targsearch (cp) (229). References that indicate existence of a promoter in a given region based on S1 nuclease mapping of 5′ ends of transcripts are marked with an S1 in the tsp column. Putative promoters which have not been verified experimentally are shown only if their homology to the consensus exceeds 50%, as calculated by the method of Hawley and McClure (118) and if their location suggests a function in initiation of transcription of genes or regulatory RNA. Exceptionally, putative promoters that have a homology score to the consensus below 50% but overlap putative regulatory sites are also shown. For comparison, homology scores are shown for both known and putative promoters, the former marked with an asterisk.
Ten promoters, including a promoter of the essential phage replication gene repL, contain the so-called “extended −10 region” characterized by the presence of T and G at positions −15 and −14, respectively. In E. coli, transcription from extended −10 promoters can initiate in the absence of typical −35 hexamers (178).
Sixteen operons end in sequences characteristic of typical Rho-independent transcriptional terminators (Fig. 1 and Table 5). Either termination of some operons of P1 occurs by a Rho-dependent mechanism or certain Rho-independent transcription termination sites are too weak to be detected. Perhaps in the latter case a terminator further downstream is alternatively used. In the case of certain P1 operons expressed during lysogeny (for instance, res and phd doc pdcA pdcB), C1 repressor-binding sites of downstream operons may possibly be used as roadblocks to transcript elongation.
TABLE 5.
Known and putative Rho-independent transcriptional terminators in P1 DNA
| Namea | Positionb | Strand | Sequencec | Relative locationd | Basis for identificatione |
|---|---|---|---|---|---|
| Tasref | 1836 | − | TTTGTGCAGCCTGGCTCCTTGCCAGGCTTTTTTTTATTTCATCATGG | 5′-c8-3′ ⌈5′-ref-3′ | TT, TR |
| Tc8 | 2046 | + | TCCTTGACATCATTGGCGGCCATTAGGCCGCCTTTTTTTTGCCATAT | 5′-c8-3′ ⌉ 5′-ref-3′ | TT, TR |
| Tref | 2788 | + | CTTCAGTTGTATTGCTAAGCCGCCGCTGGTGGCTTTTCTTTTTTGTA | 5′-mat⌉ mat-3′ | TR (338) |
| Taspro | 17761 | − | CCGATTCAAGGATTTGCGCCAGTTCCTGTGGCGGTGTTTTGATGATG | 5′-prt ⌈prt-3′ | TR |
| Tpro | 18848 | + | CCACCAAAAATAACCCCGGCAGCTGCCGGGGTTCTCGTTAACTATTA | 5′-pro-3′⌉ 3′-lyz-5′ | TR |
| Tlyz | 18848 | − | ATAGTTAACGAGAACCCCGGCAGCTGCCGGGGTTATTTTTGGTGGTT | 5′-pro-3′ ⌈3′-lyz-5′ | TT, TR |
| Taslyz | 19655 | + | GATAGCGCAAATTGCACCGCCTCCTGCGGCTGTTTTTCCCTTCATAA | 3′-lyz⌉ lyz-5′ | TR |
| TinsB | 21351 | − | TTTCGTATTATCAGGGTGCTCTCTGGCACCCCTTTTTATTCTAGTAA | 5′-isaA-3′ ⌈3′-insB-5′ | TR |
| TasddrB | 26246 | + | CACCACATACGAAACCCGCCCGAAATCGGGCAGGTTCTTTAGCAGCT | 3′-ddrB⌉ ddrB-5′ | TR |
| TddrA | 26246 | − | TGCTAAAGAACCTGCCCGATTTCGGGCGGGTTTCGTATGTGGTGACA | 3′-ddrB ⌈ddrB-5′ | TT, TR |
| TasddrA | 26508 | + | ACCAGTTTTGGCACACCATCATGTTCCGAAGGTTGTTTCTCCTCGCC | 3′-ddrA⌉ ddrA-5′ | TR |
| TplydC | 30287 | − | ACAATAATGCTCCTCTCCATCCTTGGCGGGGTGCATTCGTTTCTGAA | 3′-lydC ⌈lydC-5′ | TR |
| TU′ | 33125 | + | AACCTGAATGAGACAAGGCCCGATAGCGGGCCTTAATTTTTATTCAG | 5′-U′-3′⌉ 3′-U-5′ | TT, TR |
| T2simC | 45384 | − | ACTCGTAGAATCGGTTAACACACCAGATTCTACGAGGTTTCAATGAC | 3′-pmgC-5′ ⌈3′-simC-5′ | TT |
| T1simC | 45535 | − | GTTTTAACACAACGCCCATTAAAGGGCGTTTTATTGTTTTACTCAAA | 3′-pmgC-5′ ⌈3′-simC-5′ | TT, TR |
| TaskilA | 48546 | − | CTGCTCAGAAACCTGCCAGTTTGCTGGCAGGTTTTTTTCTTTTGTTA | 5′-ant-3′ ⌈5′-kilA-3′ | TR |
| TpmgG | 52219 | − | CATCCCTTGTATTTTAAAGCTCCTTCGGGAGCTTTTTTGTGCTTAAA | 3′-pmgG-5′ ⌈3′-pmgF-5′ | TT, TR |
| T22 | 53368 | − | GGCTAAAGGAGGAGGCCGGGGAACTCCGGCCTTTAACTTGAATGGCT | 3′-21-5′ ⌈3′-22-5′ | TT, TR |
| Tas23 | 57399 | + | CATTATCAGAGATACCCGCAAAAACCGGGTCTTTACGTGCAGCTTCA | 3′-23⌉ 23-5′ | TR |
| TparB | 57707 | − | TTTCAAGGTGAAATCGCCACGATTTCACCTTGGATTTTACCTTCCTC | 3′-23-5′ ⌈3′-parB-5′ | TT, TR |
| TrepA | 60474 | − | TTTGATGTGCGCTGGAGGGGGACGCCCCTCAGTTTGCCCAGACTTTC | 3′-parA-5′ ⌈3′-repA-5′ | TT |
| TupfA | 61718 | − | AATACGACTCCCTTCCAACCGGCTACGTTGGCCGGTTTTTCACTTAT | 3′-repA-5′ ⌈3′-upfA-5′ | TT, TR |
| ThrdC | 67115 | + | TGGAAGCCTTCCTCGGTGCGGAAGGGCGGTCTGAGTTTGTATGCGGT | 5′-dmt⌉ dmt-3′ | TT |
| Tdmt | 67659 | + | GAGCTTCCAGTTTGCCCATCTTCGGGTGGGCGTTTTTTCAGGGTTTT | 5′-dmt-3′⌉ 5′-trnA-3′ | TR |
| TastciA | 69059 | − | TCTTCTTGACTCCGCCAGCCGCTTTGCGGAATTTCTTTTTGAGAATG | 5′-tciA⌉ tciA-3′ | TR |
| Tdbn | 72485 | + | CCGTCATAACAGAAAAGCCCGAACGCCGGGCTTTTCTTAAGCCTTGT | 5′-dbn-3′⌉ 3′-5-5′ | TT, TR |
| T5 | 72485 | − | TGACAAGGCTTAAGAAAAGCCCGGCGTTCGGGCTTTTCTGTTATGAC | 5′-dbn-3′ ⌈3′-5-5′ | TT, TR |
| Tas7 | 75768 | + | ACTTTATGAGGTTGTTCGCCTGCGTTTGCAGGGCTTTTTTGAGAGCG | 3′-25⌉ 25-5′ | TR |
| TaspmgM | 77765 | − | GTGCTGCAAGTGCCTTCGCATGTGGAGAAACTTCTTTTACCGGCGCA | 5′-pmgM ⌈pmgM-3′ | TR |
| TpmgQ | 80982 | + | TTTTAAAACGTGATGTACACTCATCACGTTTTTTATTAGAGCAATCT | 5′-pmgQ-3′⌉ 5′-pmgR-3′ | TT |
| TpmgS | 82537 | + | GTCACTTTTAATGCTGGTGGAGTGCGCCCACCAGCATTTTTTTCGTC | 5′-pmgS-3′⌉ 5′-pap-3′ | TT |
| Tpap | 83146 | + | AGTGAAAATAAAAACATGCCGCAAGGCGCGGCATGTTTCCAATCAAT | 5′-pap-3′⌉ 5′-pmgT-3′ | TT, TR |
| TaspmgT | 83144 | − | GATTGGAAACATGCCGCGCCTTGCGGCATGTTTTTATTTTCACTTTC | 5′-pap-3′ ⌈5′-pmgT-3′ | TR |
| Tasdoc | 87120 | − | TCAGACATTCCCGGCAGGCCGCCGTAGCGGCTTATATTCGCATCATG | 5′-doc ⌈doc-3′ | TR |
| Tlpa | 89255 | + | ATTGATTGCTTGCCCGTTCCGGGCCTTTTGACATGTGACTTTCGTTA | 5′-lpa-3′⌉ 5′-pacA-3′ | TT |
| TpacB | 92399 | + | TTGTTTCGCTAAAGCCGTGTACGCGCATACGGAATTTTTCATCCTCC | 3′-c1⌉ c1-5′ | TR |
Names of the terminators (T) that could terminate transcription of RNA antisense to mRNA of a given gene contain “as” and the name of the gene. The terminator in lydC is marked TplydC to indicate that it could serve primarily to terminate lydC transcription prematurely.
Coordinate of the first nucleotide of the terminator hairpin for terminators that terminate transcription on the + strand (clockwise) or the last nucleotide of the terminator hairpin for terminators that terminate transcription on the − strand (counterclockwise). Coordinates refer to positions of terminators in the P1 c1-100 mod749::IS5 genome without its nonintegral parts, IS5, and the associated 4-bp duplication.
Nucleotides that form the stem of the terminator hairpin are underlined.
Terminators are represented schematically by symbols ⌉ and ⌈, which face toward the DNA region whose transcription they terminate.
Identification of terminators is based on their similarity to known transcription terminators as determined by the program TransTerm (TT) or Terminator (TR) and verified by additional criteria (see Materials and Methods).
Five predicted Rho-independent transcription terminators (Tc8, Tref, TddrA, TplydC, and Tdmt) follow closely upon apparently functional promoters. The locations of those terminators (in a leader sequence or early in a gene) suggest control by antitermination. Indeed, the Tc8 terminator was shown to participate in the regulation of transcription of a gene downstream of c8, ref, by prematurely terminating transcription from one of the ref promoters (338). Of the five terminators, four are positioned in such a way that, in addition to premature termination of ref, mat, ddrB, and dmt genes, they can function to terminate transcription of operons immediately upstream. It is likely that P1 encodes an antitermination mechanism. Whereas no P1 protein has been implicated in the process, antitermination might not require such a protein (330).
As many as 12 predicted Rho-independent terminators (Tasref, Taspro, Taslyz, TasddrB, TasddrA, TaskilA, Tas23, TastciA, Tas7, TaspmgM, TaspmgT, and Tasdoc) are at the beginning of operons but opposite in orientation to the operon. This arrangement suggests that these terminators could terminate transcription of regulatory antisense RNAs. Antisense RNA has been shown previously to regulate the expression of icd ant1/2 and kilA repL (125, 126). The location of the predicted TaskilA terminator is consistent with that expected from the length of the antisense RNA transcribed from the PaskilA promoter (125). The antisense RNA terminated at Tas23 may regulate translation of the 23 gene from an alternative, upstream start site.
Genes expressed during establishment or maintenance of P1 prophage.
The alternative life-styles of P1 require two sets of genes with little functional overlap. In general, the genes associated with lysogeny can be distinguished by the absence of regulatory regions resembling those known to control P1 lytic functions, the C1-controlled operators or the Lpa-activated promoters, which will be described later in this paper.
Of 45 P1 operons, 14 are not directly controlled by C1 or Lpa (Fig. 1). Their expression is probably not up-regulated during lytic growth, other than by gene dosage, an effect that is damped in those operons that are subject to autoregulation (ImmC and ImmI genes, repA, parAB, phd doc pdcAB, cin, and probably others).
As many as 20 genes of operons that appear to be independent of C1 and Lpa are either known or predicted to be associated with lysogeny (Fig. 1 and Table 3; see also Table S1 in the supplemental material). We are unable to predict the function of 12 other genes in this class (isaB, upfA, mlp, ppfA, upfB, upfC, plp, upl, upfM, upfN, upfO, and upfQ). However, three of them, mlp, ppfA and plp, encode products that have putative periplasmic transport or lipoprotein attachment signals likely to contribute to lysogenic conversion.
Only six structural genes are essential for stable inheritance of P1 as a prophage. Other genes associated with lysogeny may protect the lysogen from entry of foreign DNA (res and mod; reviewed in references 32 and 38), from infection by another P1 or homologous phages (sim, superinfection exclusion) (170), and from DNA damage (humD, homolog of umuD) (217). Possibly for the sim product(s) to be effective, the gene(s) must be amplified, as superimmunity was observed only with high-copy-number clones of sim. The C-segment inversion recombinase cin is expressed by the prophage, but the known benefit it confers is to produce phages that differ in host specificity from their parent.
(i) Immunity.
Whether P1 is maintained in cells as a plasmid or enters the lytic pathway is dictated by the interplay of environmental factors with the components of the immunity circuit encoded at loci designated ImmC, ImmI, and ImmT (Fig. 1 of the accompanying guest commentary [343a]; reviewed in reference 126). Genes of the immunity loci were sequenced and characterized prior to the present work. Analysis of the entire P1 genome allows us to extend this characterization.
(a) ImmC.
The key repressor of P1 lytic functions is the C1 protein, encoded by c1 within ImmC (84, 238). Inactivation of the C1 protein or a decrease in C1 synthesis triggers lytic growth of the phage. A commonly used allele of c1, c1-100, encodes a thermosensitive C1 protein (261). In both sequenced strains, c1-100 contained two mutations, T569C and G577T, as compared to what has been taken as wild-type c1 (238). The second of these mutations, which causes the amino acid substitution G193C in C1, probably confers thermosensitivity to the C1-100 protein. The thermolabile C1-100 protein of P1 and the almost identical thermostable C1 protein of P7 do not differ at the locus of the T569C substitution (238).
C1 protein exists as a monomer in solution and binds to a score of widely dispersed operators (27, 59, 84, 193). The 17-bp operator consensus sequence is asymmetric and hence has directionality. Monovalent operators have a single repressor-binding site, whereas bivalent operators consist of two overlapping repressor-binding sites oriented in opposite directions and forming an incomplete palindrome (129) (Table 6).
TABLE 6.
C1-controlled operators of P1
| Operatora (former name[s]) | Positionb | Strand | DNA sequencec | Genes controlled | Evidence for:
|
Reference(s) | |
|---|---|---|---|---|---|---|---|
| C1 binding | Regulatory function | ||||||
| Oref (Op1, Op2a) | 1962 | + | ATTGCTCTAATTGATTC | ref | √ | √ | 84, 337 |
| Omat (Op2, Op2b) | 2628 | + | CATGCACTAATAAATAT | mat | √ | √ | 84 |
| Ossb (Op21) | 19816 | + | ATTGCTCTAATTAATTT | ssb, isaA | √ | √ | 59 |
| Oc4 (Op51) | 46941 | + | AATGCTCTAATAAATTT | c4, icd, ant | √ | √ | 26, 84, 124, 316 |
| OkilA (Op53) | 48611 | + | TTTGCTCTAATAAATTT | kilA, repL | √ | 27, 84, 316 | |
| Orlf (Op55) | 50376 | + | ATTGCTCTAATTATAAC | rlfA, rlfB | √ | 112; reviewed in reference 346 | |
| Odmt (Op68) | 63946 | + | ATTGCTCTAATAAATAT | uhr, hrdC, dmt | √ | √ | 59 |
| Obanab (Op72, Op72ab) | 67468 | +/− | trnA, trnT, tciA, tciB, tciC, trnI, ban, dbn | √ | √ | 84, 129, 315, 317 | |
| a | 67468 | + | ATTGCTCTAATAAATTT | √ | 132, 316 | ||
| b | 67479 | − | ATTACACTAATAAATTT | √ | |||
| O26 (Op82) | 77329 | − | ATTGCACTAATAATTTT | 26, 25, 7, 24, 6, 5 | √ | Reviewed in 346 | |
| Oppp (Op83ab) | 78236 | − | CATGCACTAATTAATTT | pmgN, pmgO, pmgP, ppp, pmgQ | √ | Reviewed in reference 346 | |
| OpmgRa (Op86a) | 80953 | + | ATTGCTCTAATAATTCC | pmgR, pmgS | √ | 315, 346 | |
| OpmgRb (Op86, Op86b) | 81002 | − | ATTGCTCTAATAAAAAA | pmgR, pmgS | √ | 84, 316 | |
| OpmgT (Op88) | 83094 | + | ATTGCTCTAATAAATCT | pmgT, pmgU, pmgV | √ | √ | 59 |
| Olpa (Op94) | 88705 | + | ATTGCTCTAATGTATTG | lpa | √ | √ | 193 |
| OclabΛ (Op99ab, Op99a) | 92967 | −/+ | c1 | √ | √ | 27, 84, 128, 316, 317 | |
| a | 92978 | − | AATGCACTAATAAATCT | √ | 84, 316 | ||
| bd | 92967 | + | ACGAAAATAATAGATTT | √ | 317 | ||
| Oc1cΛ (Op99c) | 93150 | − | ATTGCTCTAACGCTTTA | c1 | √ | √ | 84, 128 |
| OcoiΛ (Op99d) | 93442 | − | ATTGCTCTAATAAATTA | c1, coi | √ | √ | 27, 128, 316 |
| OcreΛ (Op99e) | 93525 | + | ATTGCTCTAATAATTCT | cra, cre | √ | 27, 128, 316 | |
| Consensus | ATTGCTCTAATAAATTT | ||||||
The name of each operator refers to the name of a selected gene under its control. Bivalent operators names are underlined; constituent monovalent operators are listed below the name of each bivalent operator. Operators with a superscript Λ have been shown to engage in looping to nearby operators in vitro.
Position of the first nucleotide if the operator is assigned to the + strand or to the first nucleotide of its complement if the operator is assigned to the − strand. Coordinates refer to positions of operators in the P1 c1-100 mod749::IS5 genome without its nonintegral parts, IS5, and the associated 4-bp duplication.
The underlined nucleotides are conserved in monovalent operators and in at least one constituent operator of each bivalent operator.
Transcription of the c1 gene itself is autorepressed at operators that precede c1 and are part of ImmC (317, 128). One of these operators, Ocoi, additionally represses transcription of three small ORFs preceding c1 (27, 127). The product of the longest ORF, the 7.7-kDa Coi protein, forms a 1:1 complex with C1 and blocks its ability to bind to operators (25, 127, 130). A combination of negative effects of C1 on the synthesis of Coi and of Coi on the activity of C1 creates a sensitive regulatory system that is crucial for a choice between lysis and lysogeny. If C1 synthesis prevails in P1-infected cells, Coi synthesis is shut down, leading to the establishment of lysogeny. If Coi synthesis prevails, C1 becomes inactivated, leading to lytic growth.
The E. coli SOS response regulator LexA may be a major factor in this epigenetic switch. A LexA binding site identified previously in the P1 genome in the region upstream of the imcAB and coi genes (200) overlaps a predicted strong promoter that we designated P2coi (Tables 4 and 7; reference 343a, Fig. 1). Most likely, this promoter can drive transcription of imcAB and coi following inactivation of LexA. In support of this view, inducibility of P1 lysogens by UV light has been repeatedly reported, although not always reproduced (reviewed in reference 347).
TABLE 7.
Known and potential binding sites in P1 DNA for E. coli proteins IHF, DnaA, and LexA
| Coordinatesa | Strand | Sequence | Similarity scoreb | Known or potential function or relative location (reference[s]) |
|---|---|---|---|---|
| IHF | ||||
| 1478-1526 | − | TATGTCACCATAAATATCAAATAATTATA | 52.0 | c8 regulation |
| 6985-7132 | − | TTATATGCATACAAAAAGATGAAGTTATA | 53.9 | Internal to mod |
| 19809-19857 | − | ATCTTACCTAATAAAATAAATTAATTAGA | 55.7 | ssb regulation, lyz regulation |
| 21424-21472 | − | TGAACATAAAACACTATCAATAAGTTTGA | 50.1 | Overlaps terminal inverted repeat of defective IS1 (93, 94) |
| 30319-30367 | − | TTTAATTCACTATTAAGGAAATAGTTATG | 53.7 | cin regulation |
| 38415-38463 | − | TATAATGTTCAGAATATCAATAAGATATT | 51.8 | 16 regulation |
| 38419-38466 | + | AATATCTTATTGATATTCTGAACATTATA | 51.4 | 16 regulation |
| 46372-46419 | + | ATTAATCGGTTTAAACTCACTAATTAATT | 51.4 | sim regulation |
| 46498-46545 | − | TGGTTGATTCTTATAATCAAAAAACTATT | 50.3 | sim regulation |
| 49930-49977 | − | AAAAAATTAAATAAAGCCAATGTCTTAGC | 54.2 | Internal to repL |
| 50647-50694 | + | AAAATTGCTAGAGACATCAAGGAATTAAA | 57.0 | rlfB regulation or part of alternative replication origin |
| 50672-50719 | + | TAAAATTAAAACACAATCTAAAACCTGAT | 50.5 | rlfB regulation or part of alternative replication origin |
| 51151-51198 | + | ATAAAATCACATACAAACAGGGAGTTACT | 58.7 | rlfB regulation or part of alternative replication origin |
| 57740-57787 | + | GTAATTTACTTTAAAAACAGTCAGTTAAT | 57.2 | parS-ParB-IHF centromeric complex formation (91) |
| 61404-61451 | − | GAAAAAGTAATATGAATCAATCATTTATC | 57.3 | repA regulation (reviewed in reference 49) |
| 89562-89610 | + | CTGAAATAGCGGAAAAACAAAGAGTTAATc | 40.8 | Involved in PacA- and PacB-mediated cleavage at pac site (287) |
| DnaA | ||||
| 1076-1090 | + | TAGTTACCCCCAGGC | 54.3 | Internal to cre |
| 5894-5908 | + | GTGTTAATAACAAGA | 56.0 | Internal to res |
| 5892-5906 | − | TTGTTATTAACACGG | 55.1 | Internal to res |
| 6716-6730 | + | GTGTTGTTAACAAGC | 54.3 | Internal to mod |
| 9564-9578 | − | GAGTTATCATCAATA | 57.7 | Internal to darB |
| 41563-41577 | + | AGGTTATCGACAGTA | 55.2 | Internal to sit |
| 47322-47336 | − | GTGTTTTTTACAGGA | 54.3 | Internal to icd |
| 47945-47959 | + | GAATTTTACACAAAA | 54.3 | Internal to ant1/2 |
| 49965-49979 | + | TTTTTATCCACACCC | 56.9 | Internal to repL, presumably part of oriL |
| 50148-50162 | + | CGGTTATCCAAAAAA | 56.0 | Internal to repL, presumably part of oriL |
| 50313-50327 | + | GTTTTAGCCAAAAAT | 54.4 | Internal to repL, presumably part of oriL |
| 50986-51000 | + | GGGTTCTTCACAATG | 54.3 | Internal to rlfA, overlaps predicted promoter of rlfB, presumably regulates expression of rlfB |
| 52202-52216 | + | GATTTAAGCACAAAA | 56.9 | Internal to pmgF |
| 61448-61462 | − | AAACTATCCACACAA | 51.8 | Replication initiator complex formation at oriR (reviewed in reference 51) |
| 61457-61471 | − | CACTTATAAAAACTA | <50.0 | Replication initiator complex formation at oriR (reviewed in reference 51) |
| 61467-61481 | − | TAGTTATCACCATTA | 51.9 | Replication initiator complex formation at oriR (reviewed in reference 51) |
| 61690-61703 | − | ACATTATCCACTGGA | 51.8 | Replication initiator complex formation at oriR (reviewed in reference 51) |
| 61698-61712 | − | CACTTATCCACATTA | 56.0 | Replication initiator complex formation at oriR (reviewed in reference 51) |
| 68953-68967 | − | TGGTTATCCACCGTG | 54.3 | Between upl and its predicted promoter; regulation of upl |
| 72890-72904 | − | CGGTTATGCACAGGA | 56.9 | Internal to 5 |
| 73755-73769 | + | GGCTTATCAACAAAA | 56.9 | Internal to 6 |
| 80593-80607 | − | TTGGTATCCACAGGG | 57.7 | Internal to pmgQ |
| 85215-85229 | − | AAGTTATGCACTTGC | <50.0 | hot regulation |
| 85227-85241 | − | GATTTATCCACAAAG | 63.5 | hot regulation |
| 85851-85865 | − | GAATTTTCCTCAAGG | 55.1 | Vicinity of P2humD promoter internal to upfQ; probable humD regulation |
| 89848-89862 | − | GACTTTTCCAGAAGA | 56.0 | Vicinity of pac site internal to pacA, packaging regulation |
| LexA | ||||
| 46-65 | +/− | ACCTGTAAATCCATACAGTT | 62.7/59.8 | Regulation of ImmC genes |
| 86346-86365 | +/− | TACTGTATAAATGAACAGTA | 67.7/66.1 | humD regulation and possibly lxr regulation |
The first number is the coordinate of the first nucleotide of upper-strand (+) binding sites or of the last nucleotide of the lower-strand (−) binding sites, based on arbitrary convention. Coordinates refer to positions of sites in the P1 c1-100 mod749::IS5 genome without its nonintegral parts, IS5, and the associated 4-bp duplication.
Of predicted IHF, DnaA, or LexA binding sites, only those whose similarity score to the consensus exceeded 50.0, 54.0, or 60.0, respectively (as calculated by the GCG Fitconsensus program) are shown. Exceptions are made for tandem sites.
For PP1PAC in GenBank, boldface C is a G.
An additional component of ImmC or a separate immunity locus may be located downstream of cre. This gene, tentatively named c8, is probably the locus (c8) of a clear plaque mutation (280, 281). Temperature shift experiments with a thermosensitive allele of c8 suggested that the gene is probably not expressed in a lysogen and hence is most likely involved uniquely in the establishment of lysogeny.
(b) ImmI.
The ImmI region (antipodal to immC in the circular representation of the plasmid genome) contains a C1-controlled operon of three genes: c4, icd, and ant1/2, which confer a separate specificity to immunity. It permits P1 to plate on lysogens of the closely related phage P7, and vice versa, despite functional interchangeability of their C1 repressors (54, 131, 280, 326). The ant1/2 and icd genes determine an antirepressor protein and an inhibitor of cell division, respectively, whereas the c4 gene determines an antisense RNA that acts as a secondary repressor of Icd and antirepressor synthesis (58, 131, 257). Maintenance of lysogeny requires, in addition to the expression of c1, the expression of c4 to prevent synthesis of the antirepressor protein (reviewed in reference 126). A 77-bp antisense RNA encoded by c4 regulates expression of downstream genes in its own message (57; diagrammed in Fig. 1 of reference 343a). Two short single-stranded regions, b′ and a′, exposed in its cloverleaf-like structure, block translation of icd (and of translationally coupled ant1) by interaction with the complementary regions, a2 and b2, in the c4 icd ant1/2 mRNA. The interaction occludes the RBS in front of the icd gene (58). The resulting translational block additionally permits premature termination of ant transcription via a Rho-dependent terminator (33). Slight differences between P1 and P7 in these short regions are responsible for the heteroimmunity of the two phages. An additional specificity determinant, sas (site of Ant specificity), is involved in determining the capacity of Ant proteins to induce prophage on heteroimmune superinfection. The site resides centrally within the ant genes of P1 and P7 (J. Heinrich and H. Schuster, unpublished results). It was suggested that Ant proteins must normally load onto their phage-specific sas sites to perform their antirepressor function (294).
The action of C4 appears limited to the mRNA of its own operon, as we did not find any pair of sequences complementary to b′ and a′ in the P1 genome, other than those designated a1 and b1, located upstream of c4, and presumed to participate in unmasking of a2 and b2 by competitive binding of b′ and a′.
(c) ImmT.
The ImmT region, distant from ImmC and ImmI, contains a small gene, lxc, whose product modulates the function of C1 by formation of a ternary complex with C1 and operator DNA (272, 317, 318). Participation of Lxc in the C1-operator complexes that control c1 expression increases the affinity of the repressor for the operator and down-regulates the synthesis of C1 itself (318). Lxc can thus relieve repression at weak operators by decreasing the intracellular concentration of C1 below that critical for their binding and simultaneously enhance repression at strong operators by increasing their affinity for C1. Consequently, lxc mutations can have divergent effects on transcription from different C1-controlled promoters (272, 273, 311). Part of the modulating effect of Lxc may be mediated by looping between nearby operators complexed with C1, as looping is dramatically increased by Lxc (128, 343a). As Lxc has been reported to strongly inhibit the ability of Coi to dissociate operator-C1 complexes (317), it may assist in establishing P1 as a prophage.
The initiation codon of lxc appears to overlap with the termination codon of the preceding ulx gene of unknown function, indicating transcriptional and translational coupling of the two genes (Table 3). The coupling of lxc and ulx implies that lxc, in addition to being transcribed from its own promoters, may be transcribed from PdarB, which presumably drives transcription of ulx and the preceding gene, darB (Table 4). None of the promoters that might drive transcription of lxc is under the control of C1, suggesting that Lxc-mediated modulation of C1 function is independent of ImmC and ImmI.
(ii) Plasmid maintenance.
Despite its low copy number per bacterial origin (0.7 to 1.4) (248), the P1 plasmid is lost with a frequency of only about 10−5 per cell per generation (261). Like other plasmids that have accepted the tradeoff of large size for low copy number, P1 must counteract the increased risk of loss at cell division. Four functions have been identified that allow it to accomplish this task: a regulated plasmid replicon, a partitioning mechanism, a site-specific recombination system, and a plasmid addiction (postsegregational killing or growth inhibition) mechanism. These are discussed below. Analysis of the genome of P1 indicates that whereas the lytic origin may contribute to plasmid replication (349), there is no second addiction or partition module in P1 as there are in certain other plasmids (80, 140).
(a) Plasmid replication and partition.
The P1 plasmid replication and partition genes are encoded within one region of P1 DNA, where they form two operons transcribed in the same direction. The P1 replicon has been intensively studied (reviewed in references 49 and 51). It belongs to the large family of plasmid replicons that encode an initiator protein, called RepA in P1, and contain multiple binding sites for that initiator (19-bp iterons in P1). One set of these sites, incC, precedes the repA gene and is part of the replication origin (oriR). A second set, incA, follows repA and is a regulatory locus (Table 1). Replication from the P1 plasmid origin, oriR, proceeds in both directions (241). P1 plasmid and E. coli chromosome replication have much in common, including sequestration of the origin when its 5′-GATC sequences are hemimethylated (1) and a requirement for DnaA (Table 7) (113). One difference is that the ADP form of DnaA suffices for P1 replication, whereas the ATP form is essential for bacterial replication (333).
The P1 repA promoter is nested among the incC iterons and consequently is subject to autorepression by RepA (5). Replication control is exerted by incC and the additional iterons of incA in more than one way. By sequestering RepA, the incA iterons can prevent the concentration of RepA at the origin from attaining the threshold value required for firing (reviewed in reference 49). By becoming “handcuffed” to the iterons of incC via bound RepA, the additional iterons can interfere with both replisome assembly (239) and the release of autorepression that would otherwise replenish sequestered RepA (50). The availability of RepA for replication is limited not only by sequestration and handcuffing but also by the formation of inactive RepA dimers which require bacterial chaperones for conversion to active protein monomers (334). We suggest an additional role of incA in the control of repA expression. The putative transcriptional terminator of repA is located within the incA region (Table 5), and thus occupation of incA iterons by RepA may act as a roadblock to the completion of repA transcripts. Abbreviated repA transcripts might undergo more rapid degradation than intact transcripts.
The partition module of P1, which is situated downstream of the repA gene and the incA iterons, is a major contributor to stability of the plasmid. It ensures partition of plasmid molecules to daughter cells and has been, with its homologs, the subject of numerous detailed studies and reviews (reviewed most recently in references 97 and 306). The module consists of an operon of two genes, parA and parB, which ends at a site, parS, a centromere analog (4). ParA is an ATPase whose activity is stimulated by ParB (73). Both ATP and ADP forms of ParA act as autorepressors, the ADP form being the more effective (70, 71). A stimulation of repression by ParB (87) is assisted by parS (114). The parS site contains two kinds of recognition sequences for ParB, four heptameric and two hexameric boxes, and a binding site for the host protein IHF (Tables 1 and 7) (249). ParB and IHF form a complex at parS (72, 89) to which ParA, in the ATP form, can bind via ParB (37, 90). Binding of ParB to parS can permit parS sites to pair (83) and can nucleate spreading of ParB to the flanking DNA and silence transcription of genes as far as several kilobases away (258). Whether ParA and ParB translocate plasmid molecules to their target positions within a cell during partitioning by themselves or attach them to unknown host components of partition machinery is unclear.
We did not find in P1 DNA any additional sequences similar to parS that could serve as potential binding sites for ParB. This suggests that the function of ParB is limited to partitioning in P1, whereas in certain other plasmids, e.g., RK2 and N15, the homologous proteins appear to have additional functions (102, 251, 335). Two putative σ70 promoters of P1 gene 23, immediately downstream of parB, of which one overlaps the IHF binding region of parS, appeared weak when tested in vivo in the absence of ParB (259; A. Dobruk and M. Łobocka, unpublished results). The possibility that conditions might exist leading to their significant expression and regulation by ParB has not been studied.
(b) DNA cyclization and multimer resolution.
Faithful partitioning of newly replicated plasmid molecules is assisted by prior resolution to monomers of plasmid multimers that are inevitably formed in recA+ bacteria. The resolution is usually accomplished by a site-specific recombination. In P1 it is ensured by the recombinase Cre, encoded near its site of action, lox (8, 299, 300), far from the genes of replication and partition (Fig. 1). The resolution of plasmid multimers into monomers increases the number of partitionable P1 molecules and hence plasmid stability (21). The same accessory proteins that are required for the resolution of ColE1 dimers by the bacterial recombinase XerCD are also required for the stable maintenance of P1 prophage (244). These proteins (ArgR, for which a putative binding site to the left of lox has been located, and PepA) are proposed to constrain the directionality of the recombination in vivo. The possibility that the lox-cre module might assist plasmid stability in another way has also been suggested (10). Interplasmid recombination involving a replicating plasmid could generate a concatemeric replication substrate that, following amplification as a rolling circle, could be resolved into plasmid monomers by Cre-mediated recombination. The plasmid yield per replication initiation event would be increased, providing protection against RepA insufficiency.
The lox site is the unique target for Cre action in P1 DNA, as we did not find any other similar sites. In the P1 genome, the cre gene is downstream of cra, a gene of unknown function. In the prophage, cre is weakly expressed, either from promoters P1cre and P2cre, which lie within cra, or together with cra from a third promoter, P3cre (302). The −35 regions of P3cre and P2cre promoters overlap GATC sequences (Table 4), and P3cre was shown to be down-regulated in E. coli by Dam methylation, as P2cre probably also is. Whether methylation of one strand is sufficient for the repression is unknown. If this was not so, the expression of cre in a prophage would increase immediately following each round of replication and be attenuated later as a result of methylation of the newly replicated strand in the region of P3cre and P2cre promoters.
A putative fourth cre promoter, P4cre, predicted to be the strongest, lies almost 500 bp upstream of cre and on the opposite side of lox (Table 4). Its −35 region is partially overlapped by the C1 operator, Ocre (Table 6), indicating that the promoter is inactive in the P1 prophage. Apparently, the cre gene is expressed most efficiently at the time of infection and during P1 lytic development. Following infection, the Cre recombinase can cyclize the DNA of phages that carry a lox site on each redundant end (302). The first phages to be packaged belong to this privileged minority and hence are to be found in every productive burst. The other phage DNAs must rely on homologous recombination to cyclize.
(c) Plasmid addiction.
A fail-safe P1 stabilization mechanism is encoded by the addiction operon, phd doc, located in a region far from other plasmid maintenance genes (Fig. 1). The phd doc operon programs the inhibition of growth (and the eventual death) of any daughter cells emerging plasmid free. Two small genes of this operon encode a stable protein toxin, Doc, and an unstable cognate antidote, Phd (95, 96, 192, 211). Doc appears to block protein synthesis reversibly (R. Magnuson and M. Yarmolinsky, unpublished results). Phd and Doc can form a complex. This interaction prevents Doc from killing plasmid-containing cells and enhances the Phd-mediated repression of the autoregulated phd doc promoter (210, 211). Phd is slowly degraded by the host protease ClpXP (194), allowing for the release of the toxin protein upon plasmid loss. Recently documented connections among prokaryotic toxin-antitoxin systems, Phd-Doc among them, and their relationship to the eukaryotic nonsense-mediated RNA decay system places Phd-Doc in a grander evolutionary context than anticipated (13).
The nucleotide sequence downstream of doc contains two ORFs that could encode proteins of 66 and 347 residues, designated here pdcA and pdcB (post-doc). The 5′ end of pdcA overlaps 17 terminal nucleotides of the doc gene. The phd-doc transcript appears to include the transcripts of pdcA and pdcB, as suggested by the lack of predicted Rho-independent terminators of the phd doc operon within pdcA or pdcB, and the lack of a separate recognizable σ70 promoter sequence that could drive their transcription. Whether the functions of pdcA and pdcB are accessory or unrelated to the function of phd doc remains to be seen.
(iii) Restriction-modification.
P1 mod and res are tandem genes that encode the subunits of a type III restriction-modification enzyme, EcoPI. This bifunctional enzyme has been the subject of extensive studies (reviewed in references 32 and 38; see also reference 161).
The P1 Mod subunit recognizes the DNA sequence 5′-AGACC and catalyzes methylation of the central adenine residue at the N-6 position (23), using adenosyl methionine as the methyl donor. The Res subunit catalyzes the double-strand cleavage of DNA about 25 bp to the 3′ side of a recognition sequence, but it does so only when bound to the Mod subunit in a Res2Mod2 complex (161).
Whereas methylation by Mod can occur at any recognition sequence, each double-strand scission by Res-Mod requires a pair of unmethylated recognition sequences, in head-to-head configuration (32, 219). The cut appears to be triggered by collision between two converging Res-Mod enzymes that, powered by ATP hydrolysis, have been translocated along the DNA (220). The requirement for collision, in combination with the asymmetry of enzyme recognition sites, provides an efficient protection from cleavage of newly replicated DNA in which only one strand is unmethylated, because the unmodified sites in one orientation are always paired with modified sites in the opposite orientation.
Host killing by restriction is avoided during the process of establishing a P1 prophage in a bacterial cell with an unmodified chromosome. This is achieved by complex regulatory mechanisms that delay restriction long enough to allow complete methylation of host DNA (253). In P1 lysogens, transcripts containing either mod or res messages are detected. This indicates that mod and res form separate operons, although the beginning of res immediately follows the end of mod (285). The σ70 promoters for res, separate from those for mod, have been identified previously or are predicted here (Table 4). Additional regulation occurs at the translational and posttranslational levels (253).
(iv) Superinfection exclusion.
During attempts to clone the c1 gene of P1, Devlin et al. isolated a fragment of DNA that, although not carrying c1, protected cells from superinfection by wild-type P1, its c1 and virs mutants, and the heteroimmune P7 phage (77). This extended immunity phenotype, designated superimmunity and subsequently attributed to superinfection exclusion, was associated with a gene upstream of the c4 icd ant operon and transcribed in the opposite direction (170, 213). We find that this gene, designated previously sim and here simC, is the last of three genes of the simABC operon. The sim genes appear to encode precursors of periplasmic proteins, suggesting that their functions are related. The location of putative promoter sequences in the region preceding simC indicates that simC is cotranscribed with simB, or with simA and simB (Table 4). A plasmid carrying the sim operon was found by minicell analysis to specify a processed, as well as full-length, form of the SimC protein (213). Two additional proteins apparently expressed from this clone were seen following sodium dodecyl sulfate (SDS)-PAGE (170). One is probably SimB (12.0 kDa). The other migrated as a very small protein and, although not mentioned by the authors, is probably SimA (predicted molecular weight, 4.8 kDa).
The superimmunity (or superinfection exclusion) phenotype was observed in the presence of SimC alone (213). The SimC protein appears to be an analog of superinfection exclusion proteins that act to prevent injection of superinfecting phage DNA into the cytoplasm of the infected cell. Many phages, both temperate and virulent, encode such proteins. SimC appears to act in the periplasmic space, where the processed form is found, or in the cytoplasmic membrane, similarly to the SieA protein of P22 (139) and the Imm protein of T4 (205), either by helping to destroy the injected DNA or by preventing its entry into the cytoplasm (139, 170, 206, 213). The processing of SimC requires SecA (213), as probably does the processing of SimA and SimB, which, like SimC, have putative signal peptides.
Products of the sim operon, like the product of sieA of P22 (139, 307), exclude both phage and transducing particle DNA. However, exclusion of the latter by Sim is much less efficient, indicating that the Sim system can discriminate between P1 and foreign DNA. Conceivably, SimC or another Sim protein can interact with a P1 protein that is bound specifically with phage DNA as it enters a cell during infection.
Cells carrying low-copy-number sim+ plasmids display a Sim− phenotype (77, 170), which appears inconsistent with a role of the sim genes in lysogeny. It is possible that in P1 lysogens, the sim functions are induced only under certain circumstances or that the main role of Sim proteins is to protect from superinfection those cells in which lytic development has been initiated and the sim gene dosage is high. The physiological role of sim functions could be analogous to that of superinfection exclusion functions encoded by the immT and sp genes of the lytic phage T4. It has been proposed that superinfection exclusion mechanisms may preserve genetic diversity among phages, protecting phage populations from dominance by a phage that would otherwise outcompete any related phage when given a possibility to propagate in a superinfected cell (205).
(v) HumD and Lxr.
The monocistronic operon immediately upstream of the P1 addiction module encodes HumD protein, a homolog of E. coli UmuD′ (200). The P1 humD gene, like E. coli umuD, is transcribed from a LexA-regulated promoter (Tables 4 and 7). P1 lacks a homolog of umuC, which in E. coli is functionally associated and cotranscribed with umuD.
Aside from recA, umuD and umuC of E. coli are the only LexA-regulated genes of the SOS response that are required for DNA damage-induced mutagenesis (290). They encode a low-fidelity and low-processivity DNA polymerase, PolV, whose main function is to bypass DNA lesions (309). A RecA-mediated autocleavage of the umuD gene product (215) is required to form active PolV, which is a complex of UmuD′ and UmuC in a 2:1 ratio (43). P1 HumD corresponds to the processed form of UmuD, UmuD′, and can functionally replace it (217).
The regulatory region of humD overlaps the regulatory region of a divergently transcribed gene of unknown function. This gene could encode a 190-residue, slightly acidic protein that has no homologs in databases. We surmise that its transcription, like that of humD, is negatively regulated by LexA. One of two putative σ70 promoters of this gene overlaps the LexA binding site that controls humD (Tables 4 and 7), and thus we name the gene lxr (LexA regulated).
Genes expressed in lytic development.
Expression of the majority of P1 genes during lytic growth follows a strict temporal pattern that can lead to the production of mature phage particles within less than 1 h. For many bacteriophages such as T4 (180), Mu (18, 55), P2 (339), and P4 (98), the regulatory cascade has been subdivided into early, middle, and late stages. In P1, the cascade appears to be simpler; early transcription switches directly to late transcription, without a well-defined intermediate stage (188, 193). C1 operator sequences, which bind the primary phage repressor C1, act as a switch of early genes. Lpa (late promoter activator) binding sequences, which are in the −22 region of late promoters and enable RNA polymerase to initiate transcription upon binding of Lpa, act as a switch of late genes.
(i) C1-controlled operons.
Analysis of the entire P1 genome has revealed 17 operons that have C1 operators in the region of their σ70 promoter sequences or, in some cases, between promoters and proximal genes (Table 6). One of these operons (pmgR) is preceded by two nonoverlapping operators, and two others (ban and c1) are preceded by operators that are bivalent, bringing the total number of monovalent C1-binding sites to 20, all of which were identified previously. Although we did not find any additional sequences with strong similarity to known C1-binding sites, sequences that resemble the C1-binding sites but are missing the highly conserved C at position 7, are present in the parS site and in the promoter region close to the darB gene. The sequence in parS did not interact with C1 in vitro (N. Sternberg, personal communication). Whether these sequences can act cooperatively with other C1 operator sequences to bind C1 in vivo, as does the Oc1b operator, which also has the C at position 7 replaced by another base, remains to be seen. Looping between C1-bound operators located close to each other has been demonstrated (128), but looping between well-separated operator sequences has not.
The 17 C1-controlled operons, transcribed from σ70 promoters, contain 49 genes that are derepressed early in lytic development. Transcription does not start simultaneously, since the operators exhibit different affinities for C1 and are differently influenced by the Lxc protein, which modulates the C1-operator interaction. Lytic replication genes start to be transcribed within 5 min of infection, allowing a prompt initiation of phage DNA replication. Increased amounts of P1 DNA can be detected about 15 min after infection, at about the same time as cleavage of the packaging site, pac, in P1 DNA (297). Detection of DNA synthesis within 5 min has also been reported (282). Expression of other early functions was reported to start 10 to 15 min after induction of P1 lysogens (187). The intense transcription of P1 early functions is attenuated later in lytic development, prior to transcriptional activation of late genes (111). The attenuation depends on the E. coli RNA polymerase-associated protein SspA. Whether SspA acts directly on complexes of RNA polymerase with P1 σ70 promoter sequences or requires the action of a P1 protein is unknown. We did not find any conserved sequences, other than promoter sequences and C1-binding sites, in the regulatory regions of P1 early genes.
(a) P1-encoded tRNAs.
Several phages encode their own tRNAs (e.g., see references 88, 177, and 247); the T4-like vibriophage KVP40 encodes at least 25 tRNAs at a single locus (223). Some of these tRNAs supplement the host pool of rare tRNAs to facilitate efficient expression of selected phage genes during lysogeny or at certain stages of lytic development (179, 247). Additionally, it has been proposed that phage tRNAs can provide selective pressure to keep base composition and codon usage of phage DNA significantly different from those of its host (summarized in reference 227). We find that the P1 genome contains three sequences characteristic of tRNA genes. All three are located between the ban gene and its promoter, in the apparently untranslated regions of the ban operon. Two are adjacent; one is further downstream, separated from the two by three protein-coding genes. Control of the P1 tRNA genes from a promoter repressed by C1 implies that they are not expressed during lysogeny.
The proposed cloverleaf structures of all predicted P1 tRNAs are shown in Fig. 3. The first tRNA, tRNA1, has the anticodon GUU, characteristic of E. coli tRNAAsn. It is 90% identical to the predicted tRNAAsn of the Salmonella enterica serovar Typhi plasmid pHCM2 (242) (GenBank accession no. AL513384) and contains all bases essential for the aminoacylation of tRNAAsn in E. coli: the anticodon bases G34, U35, and U36 and the discriminator base G73 (201).
FIG. 3.
P1-encoded tRNAs and codons presumably recognized by them. The identity determinants of tRNAs are marked by asterisks. The putative modification of tRNA3 by lysinylation at the 2 position of cytosine alters the recognition specificity of the anticodon. The bar graphs compare the usage frequencies of codons recognized by P1 tRNAs in protein-coding genes of P1 (black bars) and E. coli K-12 (grey bars) per 1,000 codons. The usage frequency of a particular codon in P1 is its relative abundance among codons in genes that encode proteins. The usage frequency of a particular codon in E. coli K-12 is according to the March 2004 edition of the Codon Usage Database found at its website (http://www.kazusa.or.jp/codon/).
The second tRNA, tRNA2, has the anticodon UGU, characteristic of E. coli tRNAThr. Although it is highly homologous to alanine tRNA-UGC of a cyanobacterium, Synechocystis sp. (SYCSLRB; 83% identity), and to tRNAAla of numerous other organisms, it contains neither the G3-U70 wobble pair, which is the primary determinant of the acceptor identity of the E. coli tRNAAla, nor the discriminator base G20, which is also an important characteristic of this tRNA (250, 308). Instead, in addition to the anticodon bases G35 and U36, which are identity determinants of all E. coli tRNAThrs, it contains base pairs G1-C72 and C2-G71, which in E. coli tRNAThr, are crucial for threonine charging activity (116).
The third tRNA, tRNA3, has the anticodon CAU, which should correspond to the Met codon AUG. However, in its sequence this tRNA appears to be 89% identical to the E. coli tRNAIle-2, encoded by the ileX gene. None of the differences (outside the anticodon) between tRNA3 and tRNAIle-2 affect residues known to be essential for the function of E. coli tRNAIle-2. In the tRNAIle-2 of E. coli the wobble position C34 is modified by lysinylation to lysidine (i.e., 2-lysylcytidine). The modified anticodon acquires the AUA (Ile)-decoding capacity and is required for the recognition of this tRNA by isoleucyl-tRNA synthetase (231, 232, 283). The other identifying characteristics of E. coli tRNAIle are the anticodon loop bases A37 and A38, the discriminator base A73, and the base pairs C4-G69, U12-A23, and C29-G41 (236, 240). All of them are present in P1 tRNA3. Certain other phages, including 933W (247), T4 (224), and the T4-like phages RB69 (see http://phage.bioc.tulane.edu/) and KVP40 (223) also encode homologs of E. coli tRNAIle-2, predicted to contain the lysylcytidine modification at C34. The T4 homolog of E. coli tRNAIle-2 was confirmed to have the capacity to decode ATA (274).
Of the codons that are apparently recognized by P1 tRNAs, two, ACA and ATA, are rare in E. coli genes but overrepresented in certain P1 genes (Fig. 3). The ACA codon is the rarest threonine codon in E. coli, but its effect on translation has not been studied. The ATA codon, which is the fifth rarest codon in E. coli, is known to dramatically decrease translation of those E. coli mRNAs that contain it, especially when present in multiple copies, or in tandem, or in a single copy in the anterior part of a gene (99, 166, 353). The ATA codons are overrepresented more than twofold in 59 of 113 protein-coding genes of P1 as compared to their representation in E. coli genes. Four of these 59 genes (isaB, rlfA, 7, and pacB) contain both single ATAs and tandem ATAs. In eight, ATAs are among the first five codons of the proximal region (at position 5 of lydC, 4 of 21, 2 of ppfA, 3 of tciB, 2 of ppp, 4 of doc, 4 of pdcB, and 2 of c1). At least two of these genes, doc and c1, are known to be expressed, albeit not efficiently, during lysogeny, indicating that the low levels of ileX tRNA that are normally present in E. coli cells are sufficient for their translation, since tRNA3 is not expressed. However, the known translation-limiting role of ATA codons in E. coli implies that the efficiency of translation of doc, c1, and other genes rich in the ATA codons increases under conditions of abundance of P1 tRNA3. In the case of doc and c1, the physiological significance of such an increase, if it does occur, is unclear. In the case of certain other genes, the requirement for abundant tRNA3 to permit their efficient expression may provide an additional control on the timing of P1 lytic development at the translational level. All P1 tRNA genes appear to be transcribed from the C1-controlled promoter of the ban operon and so become available early during lytic development.
It is noteworthy that lpa, whose product activates transcription of P1 late genes, has in its proximal region three rare E. coli codons, recognized by P1 tRNAs, namely ATA at position 13 and an ATA ACA pair at positions 18 and 19. Whether the abundance of P1 tRNA3, and perhaps tRNA2, early in P1 lytic development normally contributes to efficient translation of lpa mRNA, which in turn ensures efficient transcription of late genes, remains to be tested.
Insertions of numerous phages into bacterial chromosomes occur at or near tRNA genes (reviewed in reference 46). Conceivably, tRNA genes could have been acquired by a P1 ancestor either through its integration and then imperfect excision from the DNA of its host or via recombination from other phages whose prophages can integrate into a bacterial chromosome.
(b) C1-controlled replication functions.
P1 DNA synthesis has been reported to start as early as 5 min after infection of E. coli (282). Lytic replication initiates bidirectionally in the theta mode from an origin, oriL, distinct from the plasmid origin and located within the essential replication gene, repL (61, 112, 295). Later, lytic replication switches into the rolling-circle mode (60). The shift is accompanied by a gradual decrease in accumulation of θ-shaped replication intermediates as σ-shaped intermediates accumulate.
Most likely oriL is within the second half of the repL gene, where we find putative binding sites for DnaA and IHF proteins and two 5′-GATC sequences (Table 7). P1 phage replication in the rolling-circle (σ) mode, predominant in the later stages of phage development, might initiate from an origin other than oriL. One possible location for an additional P1 origin of lytic replication is within the rlf operon, which directly follows the repL gene. It contains multiple sets of GATC sequences and several IHF binding sites, which are internal to its two protein-coding regions (Table 7). An alternative proposal is that rolling-circle replication starts at pac from a nick introduced in DNA by pacase (296).
In addition to the essential replication protein, RepL, P1 encodes homologs of certain E. coli replication-associated proteins: DnaB helicase (67, 237), single-stranded DNA-binding protein, Ssb (164, 187), Dam methyltransferase (66), and the theta subunit of DNA polymerase III. Genes encoding these homologs (ban, ssb, dmt, and hot, respectively), are in separate operons and, with the exception of the last mentioned, are controlled by C1. Four host proteins have been found to be essential for P1 lytic replication: DNA primase (DnaG), the host helicase-loading factor (DnaC), and the DnaE and DnaX subunits of the DNA polymerase III holoenzyme (119, 234). This list of essential host proteins is probably incomplete.
The repL gene is in an operon with the preceding gene, kilA, whose function is unclear (112, 295). When expressed, kilA is lethal to E. coli. Its product is dispensable for phage replication. The 180-nt antisense RNA transcribed from the constitutive PaskilA promoter internal to kilA interferes with transcription of the kilA repL operon initiated from PkilA (125). It may counteract any leakiness of the C1-mediated repression of PkilA in P1 lysogens. The activity of PaskilA is much stronger on linear than on circular templates. Thus, it was proposed that transcription from PaskilA during lytic development serves to down-regulate (the initiation of) P1 DNA replication at two stages: immediately after infection before the P1 DNA is circularized and late after infection when rolling-circle replication predominates and linear P1 DNA concatemers accumulate (125).
Presumably the origin region is transcriptionally activated (61, 295). A candidate promoter providing the activation, as well as the initiator message, is the putative PrepL promoter, which we find directly upstream of repL (Table 4).
The ssb gene of P1 is in an operon with a downstream gene of unknown function, isaA, which does not have a separate promoter sequence (Fig. 1). The isaA gene probably has no essential function as it is absent from the ssb operon of the closely related P7 phage (M. Łobocka, unpublished results). The cloned P1 ssb gene can substitute for E. coli ssb (28).
A large P1 gene able to complement the defect in dam mutants of E. coli (originally dam, renamed here dmt) was shown to be directly under C1 control (59). We find it to be the last gene in a three-gene operon. The first gene of this operon (uhr) encodes a small protein without homology to proteins of known function. The second gene encodes a 34-kDa protein that appears to be a homolog of the E. coli nucleoid-associated protein RdgC (233), and thus we designate it hrdC. Proteins of the RdgC family are conserved in several species of bacteria. RdgC was proposed to be an exonuclease that is involved in the removal of stalled replication forks, based on its being required for proper replication in cells deficient in the recombination enzymes RecABC and SbcCD (263). The gonococcal homolog of RdgC plays a role in pilin antigenic variation, suggesting that it may have a role in site-specific DNA recombination (218). The amino acid sequence of the P1 RdgC homolog contains a motif characteristic of the active site of E. coli DNA polymerase I and related polymerases (see Table S1 in the supplemental material) (39, 74, 160; reviewed in reference 243). The function of HrdC, which is one-third the size of DNA polymerase I (PolI), remains to be elucidated.
The P1 homolog of E. coli Dam methyltransferase is a 754-residue protein. Its homologies to Dam are contained solely within the C-terminal region of 266 residues. The major part of the protein is highly homologous to C-5 cytosine methyltransferases of various bacteria and bacteriophages. Most likely the protein is able to methylate DNA at both the N-6 positions of adenines and the C-5 positions of cytosines. Accordingly, we designate it Dmt (DNA methyltransferase).
In E. coli, the Dam methyltransferase has multiple functions. It is involved in postreplicative methyl-directed mismatch repair, contributes to the control of initiation of DNA replication, and regulates the transcription of certain genes (reviewed in reference 255). Functions of the adenine methyltransferase activity of the P1-encoded Dmt protein are apparent only in a dam host. In such a host, P1 dmt mutants that are defective in adenine methylation of 5′-GATC sequences show a diminished burst size of 5 to 10 viable phage per cell (303). One of the two sequenced P1 strains contains a 319-bp deletion (formerly designated damΔMB) in the region of dmt that codes for the N-6 methyltransferase domain. Decreased specificity of the packaging endonuclease, resulting in impaired cleavage of P1 DNA at the pac site and increased cleavage of host DNA, could account for the low phage yield as well as the increased yield of transducing particles that has led to widespread use of the mutant phage.
In order to ascertain possible targets of Dmt-mediated adenine methylation, we searched for 5′-GATC sequences in the entire sequence of P1. About 20 clusters of two or three 5′-GATC sites were found, including the few that had been described previously (Fig. 1). One cluster is in the region of the lytic origin (30 nt from a predicted DnaA box). The location of another suggests that it regulates the kilA repL genes and controls transcriptional activation of the origin region. Two other clusters are internal to genes of the rlf operon, downstream of repL, where an additional origin of P1 lytic replication may be located. One that is internal to rlfA is flanked by putative IHF binding sites. The predicted strongest promoter in the genome of P1 (Pasrlf), which is located immediately downstream of the rlfAB genes and could drive transcription of RNA antisense to the mRNA of the rlfAB operon, overlaps another cluster of 5′-GATC sites and thus also appears to be controlled by DNA methylation. Several additional clusters within coding sequences, such as the 5′-GATC sequences that are internal to pacA, may be associated with particular stages of phage development. Of single 5′-GATC sites, 10 overlap known or putative promoters of P1 genes (Table 4) and presumably affect their function, as was shown for the 5′-GATC sites that overlap certain promoters of cre (302). The list of genes that are most likely controlled by methylation of 5′-GATC sites may thus be extended to include c1, coi, rlfB, and genes of the sim and sit operons.
The ban gene (67, 237), which can complement the replicative helicase defect of E. coli dnaB mutants, was mapped to a 1.6-kb region located 2.5 kb downstream of the bivalent C1 operator Obanab (formerly Op72ab; Table 6) (132, 316). It encodes a 455-residue protein (195) identical in 78% of its amino acid sequence to E. coli DnaB. The Ban protein cross-reacts with anti-DnaB serum (129) and forms active thermally stabilized heteromultimers with the DnaB protein of E. coli dnaB(Ts) mutants (82, 183, 310). Although ban suppresses a number of conditionally lethal dnaB mutations in E. coli (67), the functional replacement of DnaB by Ban is somewhat incomplete, as indicated by the thermosensitivity of a dnaB deletion strain that carries the ban gene (195). Mutations exist which permit growth of this strain at the nonpermissive temperature (E. Lanka, personal communication). Whether they reside in the chromosome or in the ban gene remains to be determined. No phenotype was associated with a ban mutation of P1 in a dnaB+ E. coli strain. Presumably ban can provide a selective advantage to P1 in other hosts or under other conditions.
The ban gene is the penultimate gene in a 5-kb operon of unusual structure (Fig. 1). Immediately downstream of the promoter (designated P1ban) is a 1.5-kb region that apparently specifies untranslated RNA, including tRNA1 and tRNA2. This region is followed by two clusters of ORFs separated by another region (0.3 kb) that specifies untranslated RNA, including tRNA3. The ban gene is the first of two ORFs in the second ORF cluster.
The first ORF cluster upstream of ban, named by us tciABC, and the ORF downstream of ban, named by us dbn, encode proteins that have no homologies with known replication-associated proteins. The predicted products of tciA and tciB have homologs of unknown function in various prophages (see Table S1 in the supplemental material). P1 TciA has significant similarities to the TerB proteins of R478 and other plasmids of the IncHI2 incompatibility group. The terB genes in those plasmids are internal genes of multigenic operons that confer upon E. coli resistance to tellurite and to channel-forming colicins and cause inhibition of cell division and of the propagation of certain phages (163, 260, 331, 332).
(c) Recombination enhancement.
The product of the P1 gene ref (recombination enhancement function) can stimulate RecA-dependent recombination between regions of DNA homology shorter than the minimum needed for recombination mediated by RecA alone (207, 337, 338). The proximity of ref to cre might suggest that Ref plays a role in the cyclization of P1 DNA molecules that, lacking two copies of lox, are not substrates of the cyclization recombinase, Cre. However, within the limits of the assays, no enhancement of P1 cre− lysogenization by Ref could be detected. The substrates of Ref appear confined to regions of microhomology (337, 338).
Regulation of ref is complex (338). One presumptive promoter, 91 bp upstream of the first ATG of ref, is overlapped by a C1-recognition sequence. A second presumptive promoter (which we locate closer to ref than originally proposed) lies within a region that could fold into a long stem-loop and prevent formation of a terminator stem-loop further downstream. It is likely that transcripts from the C1-controlled promoter form the antiterminator structure, whereas transcripts from the C1-independent promoter would be blocked by the terminator (338). In addition, translation of ref message can be from either of two AUG codons 46 amino acid residues apart, although the shorter product has low activity. The benefit to P1 that ref confers remains to be determined.
(d) Late transcription activation.
With a few exceptions, genes involved in late functions are preceded by late promoter sequences (Table 8) and are partly or entirely dependent on the product of lpa (late promoter activator) for their transcription (188, 193). The lpa gene is under the control of a C1-controlled promoter, Plpa. Early in lytic development, it is cotranscribed with two genes, pacA and pacB, that directly follow lpa and that are additionally transcribed from the late promoter LPpac (111). The sites recognized by the Lpa protein and requirements for its function are considered in the section “Lpa-controlled operons.”
TABLE 8.
Known and putative late promoters of P1
| Name | Positiona | Strand | Sequence
|
Identificationb
|
Reference | |||
|---|---|---|---|---|---|---|---|---|
| −22 | −10 | tsp | pp | cmp | ||||
| LPmat | 2678 | + | TTGACAACTGAC AAGTGACTT CAGT | CAGAAT CATCACACGC | √ | 189 | ||
| LPpro | 16052 | + | GTAATCCTTAAC AAGTGACTA GTGT | TAAATT CCGTTCAAAC | √ | This work | ||
| LPlyz | 19770 | − | AACGTAGCTAAC AAGTGATTT GCGT | TATCCT GTGTCTTCTA | √ | √ | 277 | |
| LPdarA | 30013 | − | TGACCGGTTCAA AAGTTACTT TGCA | TACCAT TACCTCCTGA | √ | √ | 105 | |
| LPS | 37119 | − | GGGCGGGGTGAC AAGTTACTT ATCT | TACAAT GAGGCTTCAC | √ | √ | 105 | |
| LPsit | 45409 | − | TACTCAATGAAC AAATGACTA CTCG | TAGAAT CGGTTAACAC | √ | H. Lehnherr, unpublished | ||
| LP23 | 57626 | − | GAGAAAGTCAAC AAGTGACTT TCAA | TAAAAT CTCTTCCGAA | √ | This work | ||
| LP26 | 77286 | − | TAAATATTCAAC AAATGACTA GCGG | TAGAAT CACCATCATC | √ | 275 | ||
| LPpmgL | 77296 | + | TCATTCTACCGC TAGTCATTT GTTG | AATATT TAACTCAATA | √ | H. Lehnherr, unpublished | ||
| LPhot | 85322 | + | AAAAAAAATTAA AAGTTACTT TGCTGGTTA | AATAAT AGTCGTTACT | √ | √ | 275 | |
| LPpac | 89276 | + | GGGCCTTTTGAC ATGTGACTT TCGT | TACCCT CGCGTCAAAA | √ | H. Lehnherr, unpublished | ||
The coordinate is that of the first nucleotide of the promoter, that is, the first nucleotide of the −22 sequence for promoters read from the + strand (clockwise) or the last nucleotide of the −10 sequence for promoters read from the − strand (counterclockwise). Coordinates refer to positions of promoters in the P1 c1-100 mod749::IS5 genome without its nonintegral parts, IS5, and the associated 4-bp duplication. Note that LPmat shares its −10 sequence with Pmat.
Promoter identification is based on a transcription start point determination (tsp), detection with the promoter probe vector (pp), or computer analysis of the sequence (cmp). The promoters distinguished by √ in the cmp column were identified on the basis of the similarity of their sequences to the sequences of the other nine late promoters, using the GCG Fitconsensus program and the consensus table of −22 sequences of the known late promoters made by the GCG program Consensus. Only the sequences with homology scores similar to the known promoters and located 4 to 14 nt (189) upstream from sequences similar to -10 hexamers of RNA polymerase promoters of E. coli (as estimated by the program Targsearch [229]) were considered.
(e) Morphogenetic functions that start to be expressed early in lytic development.
Among 17 P1 operons controlled by C1, five (mat, 26, ppp, pap, and pmgT) encode morphogenetic functions (Fig. 1 and Table 3; see also Table S1 in the supplemental material). Although early expression of these operons may seem surprising, it is noteworthy that, in addition to being controlled by C1, they all appear to be activated by Lpa. Two of them, mat and 26, contain in their immediate upstream regulatory regions, in addition to a C1-controlled σ70 promoter, an Lpa-activated late promoter. Three others, pmgN, pmgR, and pmgT, are likely to be transcribed from an Lpa-activated promoter of the upstream pmgN gene by read-through transcription.
An Ssp-dependent mechanism was found to turn down transcription from C1-controlled promoters before the Lpa-controlled promoters are activated (28). This finding indicates that, for those genes transcribed from a C1-controlled promoter, additional control from a late promoter is required to ensure the continuity of their expression throughout lytic development and to satisfy the requirement late in development for large amounts of certain proteins. Dual regulatory signals in control regions of certain operons are common in T4 and other phages, where they can serve to extend expression of a given gene to more than one developmental stage and to modulate it differently at different stages (224, 228).
P1 mat mutants produce viral particles with empty heads and unstable tails with tail sheaths in various stages of contraction (323). The pattern of mat expression following induction of a P1 lysogen is consistent with its being subject to dual control (191). Expression was seen to start within 10 to15 min of inception of lytic development, to continue at a low level for about 10 min, and then to increase sharply, exactly when the expression of other genes activated by Lpa begins. Expression profiles of the remaining genes that are controlled directly by both C1 and Lpa may be similar. As some products of the remaining C1- and Lpa-regulated operons are structural virion proteins, they are described later. Mat was proposed to have a regulatory role in morphogenesis (323), although with scant justification.
The pacAB operon, which encodes two subunits of the P1 DNA packaging enzyme, is also subject to dual control. Although it is directly preceded only by the late promoter LPpac (Table 8), it is expressed early from the C1-controlled promoter of the upstream lpa gene (105). Moreover, some PacAB-mediated cleavage of pac sites occurred in the absence of Lpa, indicating that Lpa is not essential for pacAB operon expression during P1 lytic development (297).
(ii) Lpa-controlled operons.
P1 late promoters resemble typical E. coli σ70 promoters at their −10 regions but lack the −35 hexamer (188). Instead, they have a conserved 9-bp inverted repeat (previously termed late operator) that is centered about position −22 of the transcription start site and interacts with Lpa (111, 189). Results of in vivo footprinting experiments confirm that RNA polymerase binds to late promoter sequences in the presence, but not in the absence, of Lpa (111). In addition to Lpa, the E. coli RNA polymerase-associated protein, SspA (158), is required both in vivo and in vitro for late promoter activation (111, 336). The activation requires the σ70 RNA polymerase holoenzyme and does not occur when σ70 is replaced by σS. How Lpa and SspA cooperate in redirecting the host RNA polymerase towards P1 late promoter sequences is unknown.
Nine P1 late promoters were identified previously by empirical studies (Table 8). The optimal spacing between −10 and −22 regions of late promoters is 4 bp, and it is conserved in all but one of these promoters, LPhot. Although in the LPhot promoter the spacing (9 bp) is suboptimal, a promoter probe assay confirmed in vivo LPhot transcriptional activity to be dependent upon Lpa (275).
Here we identified two additional putative late promoters, based on the strong similarity of their predicted −22 regions to the −22 regions of known late promoters and on the presence of appropriately positioned putative −10 hexamers (Table 8). Both promoters have the optimal spacing between their predicted −10 and −22 regions, and both are located in the regulatory regions of genes known to encode phage morphogenetic functions. The 11 late promoters control at least 12 and as many as 14 P1 operons. We suspect that transcription from LPpmgL, before encountering the closest terminator, TpmgQ (Table 5), can read through two genes that are immediately downstream, pmgL and pmgM, and a C1-controlled operon further downstream that encodes morphogenetic functions (Fig. 1). It is likely that this transcript includes an additional two operons downstream from TpmgQ that also encode morphogenetic functions. Expression of these operons late during lytic development implies that P1 uses an efficient antitermination mechanism to attenuate the function of TpmgQ, TpmgS, and Tpap terminators. Attenuation of terminator functions may also occur at T22, TpmgG, and TddrA terminators, since these would otherwise prevent late expression of genes 22, pmgG, pmgF, ddrB, and hxr, encoding known or predicted late functions. Alternatively, these terminators may be inefficient and serve to decrease the expression of certain late genes distant from the LPpmgL, LP23, and LPdar late promoters. One other late promoter, LPsit, could drive transcription of both the sit operon and the following R S U operon. However, the R S U operon is also transcribed from its own late promoter, LPS. The remaining nine late promoters drive transcription of single operons that adjoin operons transcribed in the opposite direction. In total, 52 genes of P1 can be transcribed from late promoter sequences.
In E. coli at 37°C, late gene products start to be synthesized 20 to 30 min after either transient thermal induction of a P1c1ts lysogen or infection of sensitive cells by P1. The completion of phage morphogenesis at about min 40 is accompanied by the onset of cell lysis. The late genes of P1 are involved in DNA replication, phage tail and head morphogenesis, packaging of DNA, synthesis and incorporation of injectable proteins, and cell lysis. Some of them, as explained above, are expressed both early and late in lytic development. Some that are apparently expressed in late developmental stages cannot be associated with any late promoter sequences. The darB gene, encoding an antirestriction protein and a P1 head component, is an example. Transcription of these genes may be controlled either indirectly by Lpa through another regulatory protein or by an as-yet-unidentified mechanism.
(a) Head morphogenesis.
The morphogenesis of P1 resembles that of other tailed phages in that heads and tails have independent assembly pathways (323). At least some of the head proteins appear to be proteolytically processed (304). DNA is packaged into proheads from concatemers (60) by a headful mechanism, as in T4, but the packaging of each concatemer, unlike that in T4, starts from an initial cut at a specific pac site (286, 297, 298). Infective particles of P1 contain cyclically permuted, linear, double-stranded molecules with a terminal redundancy of about 10 kb of DNA (296). P1 tail fibers, unlike those of T4, can attach to the tail before head-tail joining occurs (323).
P1 virions resemble in many respects those of T4, although they are less complex, especially in the structures of the head and the baseplate (323). Mature P1 virions contain at least 28 proteins (325), as compared to at least 49 proteins in mature virions of T4 (summarized in reference 224). In T4, a further five proteins assist in morphogenesis and are removed during virion maturation. Proteins of similar function in P1 await identification.
Analysis of P1 virion proteins by SDS-PAGE indicates that P1 heads are composed of 15 to 19 protein components, possibly an underestimate as small proteins may have escaped detection (325). Although little is known about the assembly pathway of the P1 head, it is likely that, as in many other tailed phages, a scaffold of the head is assembled on an initiator complex, which is then degraded after being surrounded by structural head proteins, allowing the resultant prohead to expand to a mature head in a process coupled to entry of DNA (34).
Mutations affecting head morphogenesis were mapped to five unlinked regions of P1 DNA (323; reviewed in references 346 and 347). Each of these regions contains an operon that is under the control of a late promoter, LPpro, LPdar, LP23, and LPpmgL, or LPpac (Fig. 1). Together these operons contain 21 genes. Two genes of the pac operon encode subunits of the P1 DNA packaging enzyme and are described here separately.
The assignment of the majority of head components to genes that encode them cannot be made based on simple size correlations. Certain P1 virion polypeptides are truncated by proteolytic processing; one or more are increased in size by the addition of multimers of an amino acid chain of 1 to 2 kDa, most likely through disulfide bonds (325). With two exceptions, the lack of significant homologies of P1 head proteins to characterized head proteins of other phages excludes sequence-based predictions of their function.
At least four head proteins (with molecular masses of 220, 76, 47.5, and 10 kDa) are dispensable for morphogenesis, as they were absent from heads of phages missing the 9-kb DNA segment containing the darA operon (325). The missing 220-kDa protein must correspond to DarB, the only P1-encoded protein larger than 120 kDa (predicted molecular mass, 251 kDa). Although the darB gene itself is outside the missing segment, it may require a protein encoded by this segment for its expression or the incorporation of its product into phage heads.
The other three dispensable proteins are probably products of the darA operon. The 47.5-kDa protein may represent DarA, which is proteolytically cleaved from a 69.6-kDa precursor. The 76-kDa polypeptide may be a processed form of the 108-kDa product of the second large gene of the darA operon, ddrB. The 10-kDa polypeptide could represent the product of hdf, a protein that is homologous to a fragment of DarA and may be a head component, as is DarA itself.
An unusual feature of P1 is the variety of head sizes. In addition to normal, infectious particles with big heads (P1B), P1 also produces small (P1S) and, more infrequently, minute (P1M) particles. S and M particles lack the capacity to hold the complete P1 genome and are nonproductive in single infections (14, 156, 322). A gene that regulates the proportion of phages of different head sizes in the progeny, designated vad (viral architecture determinant) was mapped to the late operon, under LPdar, but it is unclear whether it corresponds to the dar gene of this operon or to a gene close to dar (147, 149, 155).
The major head component is a 44-kDa protein that was present in different stoichiometric amounts in heads of big-headed (P1B) and small-headed (P1S) phages (325). It appears to represent a truncated product of gene 23 (H. Lehnherr, unpublished results). The full-length product, a 62.2-kDa protein, is also one of the major bands of P1 head proteins separated by SDS-PAGE. A mutation in gene 23 resulted in the production of complete tails and only a few heads following induction of the mutant lysogen (323). Whereas the neck and head connector are normally associated with the head, the tails of the mutant contained structures resembling head-neck connectors or aberrant tail extensions. The product of gene 23 has no homologies to known capsid proteins of other phages. Either its evolutionary distance from these proteins is so great that only similarities at the structural level have been retained or gp23 is representative of a separate class of phage capsid proteins characteristic of P1.
Gene 23 is the first of six genes transcribed from the LP23 late promoter and perhaps the only head morphogenetic gene of the 23 operon, since other mutations mapped to this operon cause phenotypes not associated with head morphogenesis (281, 323). Homologies of the predicted gene products of the genes downstream of 23 to known phage proteins suggest that they have roles in sheath, tail tube, and baseplate morphogenesis.
The major set of head structure and assembly genes must be contained within the array of 13 genes transcribed from the LPpmgL promoter, as other late operons of P1 could not encode all the unassigned head proteins. An amber mutation in a gene of this cluster, previously designated 8, conferred a phenotype similar to that of a 23 mutation, except that there were half as many empty heads as tails (323). Apparently, gene 8 encodes an essential head structure or assembly protein. Which of the 13 LPpmgL-controlled genes corresponds to 8 is unclear. One candidate is the largest gene of this cluster, pmgS, whose predicted product is a 48-kDa protein of almost entirely alpha-helical structure, characteristic of prohead scaffolding proteins of other tailed phages (221, 314). The gene that directly precedes pmgS, pmgR, is another candidate, as its predicted product contains a putative transmembrane domain. In T4, the assembly of the major head protein into proheads is initiated on membrane-bound connectors built of other T4-encoded proteins (34).
Only one protein encoded by genes transcribed from LPpmgL, the product of the fourth gene in this array, pmgO, appears to have homologies to putative phage head morphogenesis proteins, namely to the products of two directly linked ORFs of similar sequence (ORF7 and ORF8) of the P. aeruginosa D3 phage, which in the D3 genome directly follow a gene for the major capsid protein (176).
The predicted product of the sixth gene of this array, designated by us ppp (P1 protein phosphatase) appears to be 64% identical to phage λ serine/threonine protein phosphatase (lambdaPP), which is a representative of a group of phage-encoded protein phosphatases and has structural similarity to mammalian Ser/Thr phosphoprotein phosphatases, including calcineurin (62, 172, 320). LambdaPP is a product of the 221-codon ORF (ORF221) in the region of eight nonessential delayed-early genes (called the ninR region) of the λ genome. An ORF221 mutation, known as byp, which abolishes the phosphatase activity of lambdaPP, caused a defect in the establishment of lysogeny, unlike a nin deletion (62, 65). The defect was proposed to depend either on the inability of altered lambdaPP to dephosphorylate a host protein that normally participates in termination of transcription of λ early genes or on constitutive transcription, from a promoter created by the mutation, of the λ Q gene, encoding the antiterminator of late gene transcription (24, 53). Whether the product of P1 ppp serves P1 by dephosphorylating P1-encoded proteins involved in virion morphogenesis or a host protein or both remains to be determined.
The tenth gene in the array, pap, like ppp, can be transcribed both early and late in P1 lytic development. The gene encodes a protein that is homologous to the C-terminal moiety of the polynucleotide kinase (Pnk) of the T4 bacteriophage. By itself, this acid phosphatase domain of Pnk can remove 3′-PO4 termini from DNA or RNA polynucleotides (328), hence our assignment of the name pap (P1 acid phosphatase). The aspartate residue at position 167 of T4 Pnk, which is an essential and presumably catalytic constituent of the Pnk 3′-phosphatase domain, is conserved in P1 Pap, as are the surrounding amino acid residues. A gene whose product would be a homolog of the N-terminal kinase domain of T4 Pnk could not be found in the genome of P1. Thus P1 Pap appears to have a different role from that of Pnk in the lytic development of T4; determination of that role will require further study.
As noted above, some structural head proteins of P1 are synthesized as precursors that are proteolytically cleaved to the form found in mature phage particles (304). One such cleavage, of the product of late gene darA to 9-kDa and 68-kDa proteins, was associated with a locus initially designated 4 and later pro (summarized in reference 346). The pro function is essential for P1 development as indicated by the conditionally nonproductive phenotype of all the mutants isolated. It was proposed that the product of pro must be involved in processing other structural proteins in addition to DarA, as darA is not an essential gene (304).
The pro gene belongs to an operon of two genes of previously unknown sequence which appear to be controlled by the putative late promoter, LPpro. They were originally defined as two complementation groups to which mutations with similar pleiotropic phenotypes could be assigned (230, 252, 294, 321, 324). In addition to being defective in the proteolytic processing of DarA, amber mutations in both complementation groups were found to cause production of complete phage tails and unattached empty heads, suggesting a defect in a protein involved in head-to-tail connection (304, 323). In support of this conclusion, the predicted product of the first gene of the pro operon is a protein that has similar length and some sequence similarities to the portal protein of bacteriophage T4, gp20, and its homologs in several T4-like cyanophages (110). We conclude that this gene encodes the P1 portal protein and designate it prt.
The predicted product of the pro gene is a protein of complex structure. Its N-terminal region is homologous to the N-terminal moiety of the ATP-dependent Hpr kinase/phosphatase of Enterococcus faecalis, HprK (174). Its central part has homologies to the C-terminal region of the ClpX protein of E. coli, which is an alternative ATP-binding subunit of the Clp protease (100, 350). Although it seems likely that Pro activates, and acts in a complex with a host protease, ClpP is excluded from that role since P1 is able to plate on a clpP null mutant of E. coli and transduce the mutation.
The Prt protein may be a substrate for Pro-mediated proteolytic processing. Thus, a likely explanation for similar pleiotropic phenotypes of amber mutations in either of the genes of the prt pro operon, each of which encodes a different function, is that mutations in prt have a polar effect on pro expression, whereas mutations in pro may lead to accumulation of an unprocessed product of prt that can not be incorporated into P1 virions.
(b) DNA packaging into proheads.
The processive headful packaging from a specific cut in a concatemer that produces cyclically permuted, terminally redundant DNA in T4 phage populations was originally proposed to start from a specific cut in a concatemeric molecule. T4 was later shown not to be specifically cut for packaging, but certain other phages were, including P1 (22).
The site, pac, at which each concatemeric P1 DNA is cut to be packaged into a prohead lies just within the pacA gene, encoding one of the two subunits of the pacase enzyme (286). Packaging into the first prohead proceeds from pac towards the site-specific recombination site lox (22), 4 kb away (Fig. 1). It continues through the next pac and lox and roughly 4 kb further until the prohead is filled and packaging into a second prohead can begin. Consequently, the first DNA that is packaged from each concatemer contains two lox sites, one at the beginning and the second at its redundant end. Upon entering a cell, this DNA has the advantage of being able to cyclize either by homologous or site-specific recombination. The processivity of packaging appears limited to three or four headfuls, as determined by the proportion of nonstoichiometric fragments (generated by cuts at pac) in restriction digests of DNA from phage particles (22). The limiting factor may not be the processivity of pacase itself but the extent to which the substrate of packaging is subject to RecBCD-mediated degradation from the end unprotected by bound pacase (297).
Cleavages within the pac site are distributed over 13 bp, which are flanked on each side by four similarly oriented hexanucleotide elements (5′-TGATCA/G) (298). These sites are substrates for adenine methylation by the bacterial and viral methyltransferases Dam and Dmt, respectively. If the sites are unmethylated or hemimethylated, cleavage does not occur, although hemimethylated (as well as fully methylated) DNA forms complexes with the pacase (296).
These observations have suggested that pacase complexed with hemimethylated pac sites prevents their full methylation and hence their cleavage. The late expression of dmt could switch pacase function from nonselective sequestration of pac sites to selective cutting of those pac sites that are newly generated and able to become fully methylated. Presumably only late in lytic development, when Lpa has activated the transcription of dmt and accelerated the transcription of pacA and pacB, does cleavage at pac sites occur and then only at some nascent sites as they are generated by rolling-circle replication. This proposal implies that DNA packaging proceeds from the rolling circle in the direction of the first DNA to roll out. As suggested earlier, the nick responsible for initiating rolling-circle replication may be made by the same enzyme that cleaves both DNA strands to initiate packaging (296). The proposed roles of pacase in successively preventing and promoting pac site cleavage could explain the finding that pacA and pacB start to be transcribed from an early promoter well before packaging begins.
Cleavage of the P1 packaging site requires the product of both proteins PacA and PacB, presumably as heterooligomers. An additional protein (PacC) encoded within the distal third of pacB and translated in the same reading frame as PacB has been identified, but its role in packaging, if any, remains undetermined (286). The PacA subunit recognizes and binds to methylated pac sites independently of PacB but requires a host factor to do so (287). The requirement can be satisfied by IHF, for which a binding site lies immediately adjacent to the cleavage region. HU can also satisfy this requirement, and these two host proteins work in synergy, apparently cooperating in bending the DNA at pac to bring together the two sets of hexameric repeats (287).
PacA is likely to interact with the P1 portal protein, since an analogous interaction of a DNA pacase complex with a portal protein in T4 has been demonstrated (34, 202). Homologies between P1 and T4 portal proteins strongly support this hypothesis. P1 PacB bears an ATP binding motif (see Table S1 in the supplemental material) and presumably transduces the energy of ATP hydrolysis into the energy of DNA translocation into the capsid, as does the analogous T4 gp17 protein.
(c) Tail and tail fiber morphogenesis.
The P1 tail consists of a long cylindrical tube surrounded by a contractile sheath and attached at one end to a baseplate containing six tail fibers. Nine virion proteins (with apparent molecular masses of 117, 105, 72, 33.5, 32.7, 27.2, 24.0, 21.4, and 15.4 kDa) were identified as P1 tail structural components on the basis of their absence in preparations of P1 heads (325). The assignment of four additional proteins to a tail structure was uncertain. Small proteins that could not be detected may extend this list.
Mutations affecting tail assembly were assigned to regions where four P1 late operons, transcribed from the LPS, LPsit, LP23, and LP26 promoters, are located. Head and tail assembly pathways appear independent, as each tail-defective mutant produced complete heads (323). Homologies of putative products of certain P1 tail genes to tail proteins of T4 or T4-like phages indicate a common ancestry of P1 and T4 tail morphogenetic functions. The clearest difference is in the appearance of the baseplate, which in P1 is thinner and smaller than in T4 (323).
A major tube component is a product of the second gene in the sit operon (designated here tub). Its C-terminal moiety is homologous to C-terminal moieties of tail tube proteins, products of gene 19 of T4-related phages RB49 and nt-1, and, to a lesser extent, to gp19 of T4 itself. In addition, the predicted molecular mass of the P1 Tub protein (22.3 kDa) roughly corresponds to the estimated molecular mass of one of the two most abundant tail components (21.4 kDa) (325), which by analogy to other phages, must correspond to either the tail or sheath subunits. In T4, the tail tube consists of gp19 subunits, arranged in stacked hexameric rings, which form a tunnel through which the phage DNA can pass (reviewed in reference 63). The organization of Tub protein molecules in the tail tube of P1 seems likely to be similar.
In the genomes of T4 and related phages, a gene encoding the tube protein is typically located between a gene encoding the sheath protein and a gene encoding a portal vertex (110). In the genome of P1, this organization is not conserved. The tub gene is flanked by genes whose products resemble an essential baseplate hub subunit of T4-related phage RB49 and a protein of unknown function of another T4-related phage, Aeh1.
A single P1 amber mutation that caused production of complete heads and tail tubes without sheaths was mapped to a gene designated 22 (323). This gene is contained within the late operon 23 and distant from the sit operon, in which the tub gene is located. Gene 22 was proposed to encode the sheath protein. This assignment is consistent with the presence in gp22 of a motif characteristic of conserved domains of bacteriophage tail sheath proteins (see Table S1 in the supplemental material) and regions similar to a nucleotide-binding motif (data not shown) that is present in the sheath protein of T4 (63). Although the calculated molecular mass of gp22 is only 57 kDa, smaller by 15 kDa than that of the P1 sheath protein estimated by SDS-PAGE, there is no other unassigned P1 ORF transcribed from a late promoter that could encode an essential protein of about 72 kDa. It is possible that the mature sheath protein of 72 kDa found in virions of P1 is formed by cross-linking of gp22 with another protein. Although translational frameshifting was found to occur during synthesis of λ and P2 phage tail components (56, 199), it is unlikely to be responsible for the extension of P1 gp22. No single frameshift within the 22 sequence could generate a significantly longer protein.
All four operons that encode P1 tail proteins contain genes for baseplate components. Products of five of these genes have homologies to baseplate proteins of T4 or T4-related phages. In T4, the baseplate consists of a hub surrounded by six wedges. Baseplate morphogenesis begins with assembly of wedges that polymerize around the independently assembled hub (reviewed in references 63 and 86).
Two P1 genes, belonging to well-separated operons, encode proteins that are likely to participate early in the assembly of baseplate wedges. One of them, which we name bplA, is identified by the extensive homology of its product to gp6, the largest baseplate protein and major wedge component of T4 (329). The bplA gene is located downstream of the tubA gene in the sit operon. The other gene, 26, in the 26 operon, encodes a protein with homology to gp53 of T4 and related phages Aeh1 and RB49. The products of both P1 genes are somewhat smaller than their T4 homologs. In T4, the order of addition is gp6 before gp53 and the two proteins may interact (reviewed in reference 224). T4 phages mutant in either gp6 or gp53 produce polysheaths in addition to complete heads, but no visible tail structures, consistent with the requirement of gp6 and gp53 for the assembly of the T4 tail. P1 proteins BplA and gp26 may play analogous roles in baseplate and tail assembly. In support of this view, gene 26 mutant phages produced complete heads and no tail structures other than polysheaths (323). A similar phenotype was attributed to P1 mutants in a gene previously named 3, which was mapped to the pmgC tub pmgB sit pmgA bplA 16 operon region. Whether bplA and 3 are identical remains to be seen. Mutations in three other P1 genes, R, 16, and 21, cause phenotypes identical to those of gene 26 mutants (323). The products of these genes lack homology to proteins of other phages, indicating that they form those parts of the P1 baseplate whose evolutionary history is different from baseplate components of T4, and they are perhaps unique to P1.
The largest tail component is the product of the sit gene (121 kDa). The Sit protein has a mosaic structure. Between amino acid residues 757 and 885, close to the C-terminal end of this 1,140-amino-acid protein, are motifs characteristic of soluble lytic transglycosylases (190). Sit was proposed to contact the outer surface of the bacterial cell wall at infection and facilitate passage of the viral genome through the cell wall by introducing small gaps into its peptidoglycan layer, as does the soluble lytic transglycosylase of E. coli. The structure of Sit has similarities to that of T4 gp5, a 575-residue protein that contains a separable domain with lysozyme activity (17, 165). Posttranslational cleavage of gp5 allows this domain to join in the assembly of the baseplate and form a cell-puncturing device that penetrates the outer membrane and locally dissolves the cell wall at infection. Sit does not undergo major proteolytic processing; a protein of Sit's length can be isolated from virions of P1 (323). The maintenance of the integrity of Sit protein may be necessary to fulfill a putative second Sit function, that of a “ruler. ” Sit is the only protein long enough to be a candidate for the P1 tail length determinant. Moreover, its central region (residues 450 to 691) has homologies to the C-terminal moiety of gp29 protein of T4 and the T4-related phage RB69. In T4, gp29 is the largest baseplate hub protein (63). Additionally, gp29 serves as a tail tube ruler which fills the core of the tube during assembly and determines the tail length (9). The modular organization of P1 Sit is not the same as T4 gp29 but resembles that of tail tube rulers of some mycobacteriophages, which have additional domains embedded near their C-terminal ends (245).
Genes of P1 that determine tail fibers and the specificity of P1 adsorption to different hosts were previously localized to two operons. One of them, the tail fiber operon, consists of three genes, R, S, and U, transcribed from the late promoter LPS (Tables 3 and 8) (104, 105). S specifies a structural protein that extends along the tail fiber with its constant N-terminal part at the baseplate, while its variable C-terminal part is available to bind a specific receptor on the host cell surface (reviewed in reference 347). The host range specificity of P1 is subject to alteration by inversion of the C segment of P1 DNA (Fig. 1). The C segment adjoins the anterior part of the S gene (Sc) and contains two alternative variants of the posterior part of the S gene (Sv and Sv′) and two alternative variants of the entire U gene (U and U′). The alternative variants are organized in oppositely oriented Sv′U′ and SvU segments so that the inversion connects either of the segments, in frame, to the anterior part of the S gene (135, 146, 153). In the so-called C+ phage, expression of Sv and U enables infection of E. coli strains K-12 and C (153).
Analysis of the predicted amino acid sequence of S and U gene products and their alternative forms in the C− phage (Sc+Sv′ and U′) confirms extensive homologies of these proteins to the products of the corresponding Mu genes (see Table S1 in the supplemental material), consistent with the results of early complementation and DNA hybridization experiments.
The gpS protein of P1 appears to be extended at its N terminus by 200 residues relative to its Mu-encoded counterpart. This extra region is partly identical to the N-terminal region of the tail fiber structural protein encoded by plasmid p15B, which has extensive regions of homology to P1 (268, 269). The tail fiber genes of P1, p15B, and Mu appear to have evolved by exchange of segments within a common pool of tail fiber genes.
P1 gpU and gpU′ appear to be 95% identical to their Mu-encoded counterparts and have informative homologies to certain tail fiber assembly proteins of other phages, including gp38 of T4, λ Tfa, and the Tfa protein of a wild strain of λ with tail fibers (Ur-λ) (133). In T4, gp38 acts as a chaperone essential for the assembly of the distal part of long tail fibers and is absent from virions (48; reviewed in reference 134). In contrast to T4 gp38, the Tfa protein of Ur-λ is a virion component (133). Although there is little homology between the two, each can replace the chaperone function of the other (117, 226). This function of Tfa does not require virion attachment. Whereas the C-terminal region of λ Tfa is not homologous to that of T4 gp38, it is homologous to those of P1 gpU and P1 gpU′. It is thus likely that the gpU and gpU′ proteins of P1, like λ Tfa, can function as both tail fiber assembly chaperones and virion components.
Comparison of variable parts of alternative P1 gpS proteins and variable P1 gpU and gpU′ proteins reveals that the main sequence differences between them reside within the C-terminal regions (Fig. 4). Further analysis of these differences may help identify subregions of gpS and gpU proteins essential for adsorption of P1 on its particular hosts.
FIG. 4.
Alignment of alternative tail fiber proteins and protein segments.
Inversion of the C segment is accomplished by the Cin site-specific recombinase, the only product of an operon that adjoins the C segment on the opposite side from Sc (Fig. 1). Unlike the tail fiber operon, which is expressed from the late promoter, the cin operon is expressed from a σ70 promoter, allowing inversions of the C segment to occur in the prophage (Table 4).
Cin acts on a pair of inverted 26-bp crossover sites, cixL and cixR, at the C-segment borders (Table 1). The cixL site overlaps with the end of the cin gene. Inversions within the analog of the C segment in phages with similar invertible sequences have been reported and may well occur in P1 as well (267). In the p15B plasmid, whose S gene is homologous to that of P1 in its posterior region, inversions can occur at six crossover sites within the corresponding invertible segment and may result in 240 isomeric configurations of this region (269, 270). The inversions depend on the p15B invertase Min, which can functionally replace the Cin invertase of P1 (154).
A pseudo-cix site, cixPp, overlaps one of the two predicted cin promoters, possibly allowing autoregulation of Cin synthesis (Tables 1 and 4) (135, 150). The cin segment, flanked by cixPp and cixL, does not invert at a noticeable frequency, perhaps because the cixPp site does not contain the conserved core sequence, TT, important for efficient Cin-mediated recombination (148).
Inversion is an infrequent process and, as a result, the progeny of an infecting phage and the infecting phage itself are generally of the same host range, whereas an induced lysogen is likely to produce a burst of phages of mixed genotypes, which are able to plate either on E. coli strains K-12 and C or on other P1 hosts. Control of inversion appears to be mediated at a palindromic recombinational enhancer located 18 bp downstream of the translational start signal of the cin gene (141). The host architectural protein Fis (factor for inversion stimulation) binds at the enhancer, stimulates (by more than 500-fold) recombination in cis to the recombination sites (109, 142-144), and determines the topological specificity of DNA inversion (108). An additional factor that influences the rate of C-segment inversion is probably IHF protein. We find a putative IHF binding site overlapping one of the two σ70 promoters that can drive cin transcription (Tables 4 and 7). IHF protein is three times more abundant in early stationary phase and two times more abundant in late stationary phase than in exponential phase (11). Fis levels in turn are drastically reduced in stationary phase. These considerations suggest that C-segment inversion will be especially infrequent during the stationary phase due to the predicted IHF-mediated down-regulation of cin expression and the lack of Fis-mediated enhancer function.
Arber has suggested that the ability of Cin to mediate occasional deletions and intermolecular reactions (e.g., under conditions of relaxed topological specificity) could lead to exchange of tail fiber segments and could have been selected for during evolution (15). Tail fiber genes from unrelated phages of enteric bacteria exhibit a mosaic of homologous segments suggestive of extensive horizontal transfer (267). On the other hand, conservation of the order of the various motifs has been taken to imply that independent divergence from a common ancestor also played a major role in tail fiber evolution (342).
(d) Antirestriction.
Two P1 head components, DarA and DarB, protect infecting P1 DNA against degradation by different subsets of enterobacterial type I restriction enzymes (155, 304, 325). Both Dar (defense against restriction) proteins are synthesized late during lytic development. The corresponding dar genes were mapped to two unlinked operons (Fig. 1).
P1 darA is the fourth gene in a multigene operon, whose nucleotide sequence was determined previously (149) and corrected in this study. The operon is transcribed from the late promoter LPdar (Table 8) (105) and, in addition to darA, contains two lysis-determining genes that will be described separately and four other genes (Fig. 1). None of these genes are essential for P1 development, as cells infected with a P1 deletion mutant lacking the entire darA operon can produce infective phages.
The 5′ region of darA and the entire preceding gene appear to have evolved through internal duplication, as suggested by the corresponding homology of the predicted products of both genes (see Table S1 in the supplemental material). To reflect this property, we give the name hdf (homolog of darA fragment) to the gene that is directly upstream of darA. It is unclear whether the Hdf protein contributes to P1 antirestriction or has evolved to serve a different function.
In addition to antirestriction and cell lysis, the darA operon determines three previously identified functions designated Vad, Gta, and Tsu (reviewed in reference 347, see also reference 149). The corresponding mutants have been identified on the basis of their variable proportions of heads of different sizes, altered generalized transduction, and transduction stimulation by UV, respectively. Assigning these functions to particular genes of the darA operon will require further study.
The DarA protein has no homologies to known proteins, other than Hdf. It is synthesized as a precursor and packed into phage particles following proteolytic processing. DarA and DarB are injected into the host cell with the phage DNA, where they act exclusively in cis. The Dar proteins do not directly inactivate type I restriction-modification enzymes (155). Moreover P1 DNA isolated from phage particles was not protected against type I restriction in vitro, suggesting that the protection requires entry of phage DNA into cells at infection.
DarB appears to be the largest P1-encoded protein (252 kDa). Analysis of the predicted amino acid sequence of DarB (see Table S1 in the supplemental material) reveals that this gigantic protein consists of at least two domains. The N-terminal domain contains nine appropriately ordered amino acid sequence motifs characteristic of DNA N6-adenine methyltransferases of type “gamma” (Fig. 5) (reviewed in references 44 and 214) and like the majority of them, may methylate adenine residues in a TNNA motif. DarB contains within motif IV the sequence NPPY/F (with one permitted mismatch) known to be essential for adenine DNA methyltransferase catalytic activity. The C-terminal moiety of DarB contains sequence motifs characteristic of DNA or RNA helicases (see Table S1 in the supplemental material). Most likely DarB methylates DNA on infection. The DNA methylase function of DarB is not specific for P1 DNA. Any DNA packed into P1 heads is protected from a DarB-counteracted host restriction system following its injection (155).
FIG. 5.
Conserved sequence motifs in the predicted amino acid sequences of the N-terminal domain of DarB and of selected γ-type DNA N6-adenine methyltransferases. The motifs are indicated by Roman numerals according to reference 214. Methylated nucleotides in target sequences of DarB homologs are underlined (32, 159, 162, 167, 340).
Although expressed late in P1 lytic development, darB is not preceded by any sequence resembling a late promoter. As the function of darB depends on functional darA, it is possible that the product of darA (or a downstream gene in the same operon) is required for transcriptional activation of darB.
(e) Replicative functions expressed late in lytic development.
A late promoter that we designate LPhot can drive transcription of a gene whose predicted product, an 83-residue, basic polypeptide, appears to be a homolog of the θ subunit of the major replicative enzyme of E. coli, DNA polymerase III, whence our gene designation, hot (homolog of theta). In the regions of homology, which include 65 amino acid residues of both proteins, 62% of the amino acid residues are identical.
E. coli DNA polymerase III is a complex of at least 10 different protein subunits, which cooperate to ensure the rapid synthesis of DNA with high fidelity (reviewed in reference 168). The θ subunit binds tightly to another subunit, ε (proofreading 3′-5′exonuclease), which, in turn, binds to α (5′-3′ polymerase) in a linear array that forms the catalytic core of PolIII (76, 305). A loop region in θ was identified that directly interacts with the ε subunit (169). It consists of nine amino acid residues (AAAGVAFKE) at position 29 of ε. In the Hot protein of P1 this region is highly conserved (seven identical and two similar residues), suggesting that Hot can replace E. coli θ in the assembly of the E. coli core of PolIII, a conclusion recently confirmed (R. Schaaper, personal communication).
Whereas ε is known to play several roles, affecting the processivity, thermal stability, and fidelity of PolIII, the function of θ is elusive. There is some evidence to suggest a role in maintaining fidelity of the enzyme via stabilization of the ε subunit (76, 305). No mutant phenotype has been associated with a null mutation in holE, the structural gene for θ (289).
Upstream of the hot gene, in addition to the late promoter LPhot, there is a 69-bp region that is 91 to 96% identical to the complement of the regulatory region of the ribonucleotide diphosphate reductase encoded in the nrd operon of certain E. coli and Shigella flexneri strains. The homologous regions include two sequence motifs characteristic of E. coli DnaA protein recognition sites (Table 7). Transcription of the nrd operon was shown to be regulated by the cooperative binding of DnaA to the upstream sites (19, 313). Whether hot may be subjected to regulation by DnaA bound to oppositely oriented DnaA boxes is unknown. Expression of an LPhotΦ (hot′-′lacZ) in-frame fusion from an insert that, in addition to LPhot, contained about 200 bp of upstream P1 DNA, was initiated coordinately with expression of other Lpa-controlled operons upon induction of a P1 lysogen (275). Apparently, Hot synthesis starts late in P1 lytic development, much later than the synthesis of other known P1-encoded replication proteins, which are expressed from genes controlled directly by C1. Hot may possibly compensate for a limitation that appears only well after phage replication has begun or, as a virion protein that is injected into host cells together with P1 DNA, may facilitate early stages of P1 replication.
(f) Lysis.
Lysis of the host cell to release phage progeny is the last stage of P1 lytic development. Lysis starts 60 to 70 min after infection under normal laboratory conditions and releases a burst of 100 to 200 infective phage particles. Studies with lysis-defective mutants of P1 have revealed three lytic functions typical of tailed phages: a primary cell-wall-degrading enzyme (endolysin), a helper protein (holin), and its antagonist (antiholin). The function of holins is to form hole-like lesions in the cytoplasmic membrane that provide endolysins access to the peptidoglycan layer of the cell wall (327, 351). Antiholins regulate the timing of lysis. They block function of their cognate holins until a complete set of mature phage particles is ready for release.
Genes for the P1 lytic functions were located previously in two unlinked operons (277, 324) (Fig. 1), both transcriptionally activated by Lpa. Mutations in only one of these genes, lyz (formerly 17), located separately from the two others, prevent lysis. The lytic development of lyz mutant phages is normal until the expected lysis time. Then the production of phages ceases and even chloroform treatment of the phage-laden cells cannot induce lysis (324). This phenotype is characteristic of phage mutants with mutations in genes for primary cell-wall-degrading enzymes (reviewed in reference 351). The product of the lyz gene was found to be a 185-residue protein homologous to several proteins of the T4 lysozyme family (277), indicating that it is a “true” lysozyme capable of hydrolyzing peptidoglycan N-acetylmuramyl-β(1,4) linkages. However, unlike the lysozyme of T4, P1 Lyz does not require holin for its passage to the periplasm, at least in E. coli (341). Its N-terminal domain, which resembles a signal sequence, can mediate export of Lyz to the membrane and its release into the periplasm in a sec-dependent manner that is not associated with cleavage of Lyz by SecA.
Two other lysis-associated genes, lydA and lydB (formerly gene 2), encode a holin and antiholin, respectively (277, 324). They are the first genes in the darA operon (Fig. 1), which also encodes functions not associated with cell lysis. LydA possesses two putative transmembrane domains characteristic of class II holins (327). LydB has no homologs among known proteins. Phages carrying an amber mutation in lydB lyse their host cells prematurely, indicating that LydB antagonizes a P1 holin (324).
Analysis of the entire genome sequence of P1 reveals two additional putative holin genes (Fig. 6). One of them, designated by us lydC, immediately precedes the promoter-operator region of the dar operon (Fig. 1). It can encode a 93-residue protein whose predicted amino acid sequence is 30% identical to that of LydA and features two putative transmembrane helices, a hallmark of class II holins (327). Recent studies confirm the holin function of LydC (R. Young, personal communication). It is likely that either lydC evolved from a duplication of the neighboring lydA or vice versa.
FIG. 6.
Known and putative holins encoded by P1. (Top) Alignment of the amino acid sequences of the LydA holin and the putative holin, LydC. Two putative transmembrane helices (TMH) are highlighted in grey. (Bottom) The amino acid sequence of the predicted holin LydD. Putative transmembrane helices are highlighted.
Although the end of lydC is separated from the beginning of lydA by a 67-nt noncoding region that contains the late promoter of lydA and lydB (LPdar), there is no predicted Rho-independent terminator in this interval. Conceivably lydA and lydB and perhaps other genes of the darA operon, in addition to being transcribed from the late promoter in front of lydA, can also be transcribed from a putative strong promoter that drives transcription of lydC.
The regulatory region of lydC overlaps the regulatory region of the cin gene, which is transcribed in the opposite direction. Some coregulation of the two genes is expected. The −35 and −10 regions of a putative lydC promoter (Table 4) overlap the sequence that acts as an enhancer of Cin-mediated C-segment inversion and bind the Fis protein. The region between the predicted lydC promoter and the beginning of lydC also contains a binding site for the Cin protein (cixPp; Table 1) and a putative binding site for the host protein IHF (Table 7). Occupation of these sites by Cin and IHF, respectively, could possibly repress the expression of lydC (as well as cin). Another factor that could limit the expression of lydC is a putative strong Rho-independent terminator at the beginning of the lydC coding sequence (Table 5). It is likely that transcription of lydC terminates prematurely in the absence of an efficient antitermination mechanism. Since lydC appears subject to multiple levels of repression, it is probably expressed at low levels. Expression of lydA and lydB from the late promoter LPdar, downstream of lydC, is expected to make LydA predominant over LydC late in lytic development. It is likely that LydB antiholin can control the time of lysis mediated by the related LydA and LydC proteins.
A second predicted holin gene, which we designate lydD, is apparently transcribed from the same promoter, Plyz, as the upstream gene lyz, which partially overlaps it. It can encode an 84-residue protein, which contains two putative transmembrane helices, like LydA and LydC. LydD contains a cluster of five highly basic (arginine) residues at its C-terminal end, another hallmark of phage holins (327). LydD may be the primary holin involved in the P1-mediated lysis of E. coli cells, as suggested by the inability of a lyz gene clone to complement fully a lyz amber mutant, unless lydD was part of the clone (59). Polarity of the amber lyz mutation, expected from an overlap of lyz and lydD, could explain this result. It is likely that, in addition to regulation by Lpa, lyz and lydD expression depends on RNA antisense to the proximal region of the lyz gene and is terminated in that region by a predicted Rho-independent terminator (Table 5).
In the central region of lydD starts yet another gene that is oriented in the same direction and is read in a different frame (Fig. 1). It can encode a 71-residue protein, which is likely to be an antiholin antagonistic to LydD and which we designate lydE. As a general rule, a phage holin gene is associated with a gene encoding its specific antagonist (327). The most frequent organization involves a “dual start motif”; the holin genes encode two polypeptides, read in the same frame, that differ in length and have antagonistic functions (holin and antiholin) (103). In P1, genes for known and predicted holins and their specific antiholins are either closely linked (lydA and lydB) or overlapping (lydD and lydE) but their products differ significantly. Thus, the P1 lysis machinery appears atypical in its complexity and organization.
Supplementary Material
Acknowledgments
We are deeply indebted to the many members of phage community for their helpful comments on a rough draft of the manuscript and for their encouragement. We owe particular thanks to colleagues who have reviewed the initial draft in its entirety— Hans W. Ackermann, Richard D'Ari, David Lane, and Erich Miller—and to Sherwood Casjens and Roger Hendrix for suggestions concerning phage morphogenesis. We thank Judah L. Rosner for providing the N1706 strain from which P1 was induced, Włodzimierz Zagórski-Ostoja for crucial support of the project at the IBB PAS, and Sankar Adhya and Wacław Szybalski for their help in initiating this collaborative effort.
The main part of this work, including sequencing of P1 c1-100 mod749::IS5, was supported by grant Nr6P04B01116 from the Polish State Committee for Scientific Research (KBN). Sequencing of the second P1 strain, P1 c1-100 mod1902::IS5 dmtΔMB rev-6, was supported by NIH grants P01HG01428 and S10RR10379.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/
REFERENCES
- 1.Abeles, A., T. Brendler, and S. Austin. 1993. Evidence of two levels of control of P1 oriR and host oriC replication origins by DNA adenine methylation. J. Bacteriol. 175:7801-7807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abeles, A. L. 1986. P1 plasmid replication. Purification and DNA-binding activity of the replication protein RepA. J. Biol. Chem. 261:3548-3555. [PubMed] [Google Scholar]
- 3.Abeles, A. L., and S. J. Austin. 1991. Antiparallel plasmid-plasmid pairing may control P1 plasmid replication. Proc. Natl. Acad. Sci. USA 88:9011-9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abeles, A. L., S. A. Friedman, and S. J. Austin. 1985. Partition of unit-copy miniplasmids to daughter cells. III. The DNA sequence and functional organization of the P1 partition region. J. Mol. Biol. 185:261-272. [DOI] [PubMed] [Google Scholar]
- 5.Abeles, A. L., L. D. Reaves, and S. J. Austin. 1989. Protein-DNA interactions in regulation of P1 plasmid replication. J. Bacteriol. 171:43-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abeles, A. L., K. M. Snyder, and D. K. Chattoraj. 1984. P1 plasmid replication: replicon structure. J. Mol. Biol. 173:307-324. [DOI] [PubMed] [Google Scholar]
- 7.Abremski, K., and R. Hoess. 1984. Bacteriophage P1 site-specific recombination. Purification and properties of the Cre recombinase protein. J. Biol. Chem. 259:1509-1514. [PubMed] [Google Scholar]
- 8.Abremski, K., R. Hoess, and N. Sternberg. 1983. Studies on the properties of P1 site-specific recombination: evidence for topologically unlinked products following recombination. Cell 32:1301-1311. [DOI] [PubMed] [Google Scholar]
- 9.Abuladze, N. K., M. Gingery, J. Tsai, and F. A. Eiserling. 1994. Tail length determination in bacteriophage T4. Virology 199:301-310. [DOI] [PubMed] [Google Scholar]
- 10.Adams, D. E., J. B. Bliska, and N. Cozzarelli. 1992. Cre-lox recombination in Escherichia coli cells: mechanistic differences from the in vitro reaction. J. Mol. Biol. 226:661-673. [DOI] [PubMed] [Google Scholar]
- 11.Ali Azam, T., A. Iwata, A. Nishimura, S. Ueda, and A. Ishihama. 1999. Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J. Bacteriol. 181:6361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anantharaman, V., and L. Aravind. 2003. New connections in the prokaryotic toxin-antitoxin network: relationship with the eukaryotic nonsense-mediated RNA decay system. Genome Biol. 4:R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson, T. F., and D. H. J. Walker. 1960. Morphological variants of the bacteriophage P1. Science 132:1488. [Google Scholar]
- 15.Arber, W. 1991. Elements in microbial evolution. J. Mol. Evol. 33:4-12. [DOI] [PubMed] [Google Scholar]
- 16.Arber, W., and D. Dussoix. 1962. Host specificity of DNA produced by Escherichia coli. I. Host controlled modification of bacteriophage λ. J. Mol. Biol. 5:18-36. [DOI] [PubMed] [Google Scholar]
- 17.Arisaka, F., S. Kanamaru, P. Leiman, and M. G. Rossmann. 2003. The tail lysozyme complex of bacteriophage T4. Int. J. Biochem. Cell Biol. 35:16-21. [DOI] [PubMed] [Google Scholar]
- 18.Artsimovitch, I., K. Murakami, A. Ishihama, and M. M. Howe. 1996. Transcription activation by the bacteriophage Mu Mor protein requires the C-terminal regions of both alpha and sigma70 subunits of Escherichia coli RNA polymerase. J. Biol. Chem. 271:32343-32348. [DOI] [PubMed] [Google Scholar]
- 19.Augustin, L. B., B. A. Jacobson, and J. A. Fuchs. 1994. Escherichia coli Fis and DnaA proteins bind specifically to the nrd promoter region and affect expression of an nrd-lac fusion. J. Bacteriol. 176:378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin, S., and A. Abeles. 1983. Partition of unit-copy miniplasmids to daughter cells. II. The partition region of miniplasmid P1 encodes an essential protein and a centromere-like site at which it acts. J. Mol. Biol. 169:373-387. [DOI] [PubMed] [Google Scholar]
- 21.Austin, S., M. Ziese, and N. Sternberg. 1981. A novel role for site-specific recombination in maintenance of bacterial replicons. Cell 25:729-736. [DOI] [PubMed] [Google Scholar]
- 22.Bächi, B., and W. Arber. 1977. Physical mapping of BglII, BamHI, EcoRI, HindIII and PstI restriction fragments of bacteriophage P1 DNA. Mol. Gen. Genet. 153:311-324. [DOI] [PubMed] [Google Scholar]
- 23.Bächi, B., J. Reiser, and V. Pirrotta. 1979. Methylation and cleavage sequences of the EcoP1 restriction-modification enzyme. J. Mol. Biol. 128:143-163. [DOI] [PubMed] [Google Scholar]
- 24.Barik, S. 1993. Expression and biochemical properties of a protein serine/threonine phosphatase encoded by bacteriophage lambda. Proc. Natl. Acad. Sci. USA 90:10633-10637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumstark, B. R., S. R. Stovall, and P. Bralley. 1990. The ImmC region of phage Pl codes for a gene whose product promotes lytic growth. Virology 179:217-227. [DOI] [PubMed] [Google Scholar]
- 26.Baumstark, B. R., and J. R. Scott. 1987. The c4 gene of phage P1. Virology 156:197-203. [DOI] [PubMed] [Google Scholar]
- 27.Baumstark, B. R., S. R. Stovall, and S. Ashkar. 1987. Interaction of the Plcl repressor with Pl DNA. Localization of repressor binding sites near the cl gene. Virology 156:404-413. [DOI] [PubMed] [Google Scholar]
- 28.Bendtsen, J. D., A. S. Nilsson, and H. Lehnherr. 2002. Phylogenetic and functional analysis of the bacteriophage P1 single-stranded DNA-binding protein. J. Virol. 76:9695-9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertani, G. 1958. Lysogeny. Adv. Virus Res. 5:151-193. [DOI] [PubMed] [Google Scholar]
- 30.Bertani, G. 2004. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J. Bacteriol. 186:595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertani, G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bickle, T. A., and D. H. Krüger. 1993. Biology of DNA restriction. Microbiol. Rev. 57:434-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biere, A. L., M. Citron, and H. Schuster. 1992. Transcriptional control via translational repression by c4 antisense RNA of bacteriophages P1 and P7. Genes Dev. 6:2409-2416. [DOI] [PubMed] [Google Scholar]
- 34.Black, L., M. Showe, and A. Steven. 1994. Morphogenesis of the T4 head., p. 218-258. In J. D. Karam (ed.), Molecular biology of bacteriophage T4. ASM Press, Washington, D.C.
- 35.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 36.Borodovsky, M., and J. McIninch. 1993. Recognition of genes in DNA sequence with ambiguities. Biosystems 30:161-171. [DOI] [PubMed] [Google Scholar]
- 37.Bouet, J. Y., and B. E. Funnell. 1999. P1 ParA interacts with the P1 partition complex at parS and an ATP-ADP switch controls ParA activities. EMBO J. 18:1415-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourniquel, A. A., and T. A. Bickle. 2002. Complex restriction enzymes: NTP-driven molecular motors. Biochimie 84:1047-1059. [DOI] [PubMed] [Google Scholar]
- 39.Braithwaite, D. K., and J. Ito. 1993. Compilation, alignment, and phylogenetic relationships of DNA polymerases. Nucleic Acids Res. 21:787-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brendel, V., and E. N. Trifonov. 1984. A computer algorithm testing potential prokaryotic terminators. Nucleic Acids Res. 12:4411-4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brockes, J. P., P. R. Brown, and K. Murray. 1972. The deoxyribonucleic acid modification enzyme of bacteriophage P1. Biochem. J. 127:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brockes, J. P., P. R. Brown, and K. Murray. 1974. Nucleotide sequences at the sites of action of the deoxyribonucleic acid modification enzyme of bacteriophage P1. J. Mol. Biol. 88:437-443. [DOI] [PubMed] [Google Scholar]
- 43.Bruck, I., R. Woodgate, K. McEntee, and M. F. Goodman. 1996. Purification of a soluble UmuD′C complex from Escherichia coli. Cooperative binding of UmuD′C to single-stranded DNA. J. Biol. Chem. 271:10767-10774. [DOI] [PubMed] [Google Scholar]
- 44.Bujnicki, J. M. 2001. Understanding the evolution of restriction-modification systems: clues from sequence and structure comparisons. Acta Biochim. Pol. 48:935-967. [PubMed] [Google Scholar]
- 45.Burland, V., D. L. Daniels, G. Plunkett III, and F. R. Blattner. 1993. Genome sequencing on both strands: the Janus strategy. Nucleic Acids Res. 21:3385-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell, A. 2003. Prophage insertion sites. Res. Microbiol. 154:277-282. [DOI] [PubMed] [Google Scholar]
- 47.Capiaux, H., C. Lesterlin, K. Perals, J. M. Louarn, and F. Cornet. 2002. A dual role for the FtsK protein in Escherichia coli chromosome segregation. EMBO Rep. 3:532-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cerritelli, M. E., J. S. Wall, M. N. Simon, J. F. Conway, and A. C. Steven. 1996. Stoichiometry and domainal organization of the long tail-fiber of bacteriophage T4: a hinged viral adhesin. J. Mol. Biol. 260:767-780. [DOI] [PubMed] [Google Scholar]
- 49.Chattoraj, D. K. 2000. Control of plasmid DNA replication by iterons: no longer paradoxical. Mol. Microbiol. 37:467-476. [DOI] [PubMed] [Google Scholar]
- 50.Chattoraj, D. K., R. J. Mason, and S. H. Wickner. 1988. Mini-P1 plasmid replication: the autoregulation-sequestration paradox. Cell 52:551-557. [DOI] [PubMed] [Google Scholar]
- 51.Chattoraj, D. K., and T. D. Schneider. 1997. Replication control of plasmid P1 and its host chromosome: the common ground. Prog. Nucleic Acid Res. Mol. Biol. 57:145-186. [DOI] [PubMed] [Google Scholar]
- 52.Chattoraj, D. K., K. M. Snyder, and A. L. Abeles. 1985. P1 plasmid replication: multiple functions of RepA protein at the origin. Proc. Natl. Acad. Sci. USA 82:2588-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng, S. W., D. L. Court, and D. I. Friedman. 1995. Transcription termination signals in the nin region of bacteriophage lambda: identification of Rho-dependent termination regions. Genetics 140:875-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chesney, R. H., and J. R. Scott. 1975. Superinfection immunity and prophage repression in phage P1. II. Mapping of the immunity-difference and ampicillin-resistance loci of P1 and phi amp. Virology 67:375-384. [DOI] [PubMed] [Google Scholar]
- 55.Chiang, L. W., and M. M. Howe. 1993. Mutational analysis of a C-dependent late promoter of bacteriophage Mu. Genetics 135:619-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Christie, G. E., L. M. Temple, B. A. Bartlett, and T. S. Goodwin. 2002. Programmed translational frameshift in the bacteriophage P2 FETUD tail gene operon. J. Bacteriol. 184:6522-6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Citron, M., and H. Schuster. 1992. The c4 repressor of bacteriophage P1 is a processed 77 base antisense RNA. Nucleic Acids Res. 20:3085-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Citron, M., and H. Schuster. 1990. The c4 repressors of bacteriophages Pl and P7 are antisense RNAs. Cell 62:591-598. [DOI] [PubMed] [Google Scholar]
- 59.Citron, M., M. Velleman, and H. Schuster. 1989. Three additional operators, Op21, Op68, and Op88, of bacteriophage P1. Evidence for the control of the P1 dam methylase by Op68. J. Biol. Chem. 264:3611-3617. [PubMed] [Google Scholar]
- 60.Cohen, G. 1983. Electron microscopy study of early lytic replication forms of bacteriophage P1 DNA. Virology 131:159-170. [DOI] [PubMed] [Google Scholar]
- 61.Cohen, G., E. Or, W. Minas, and N. L. Sternberg. 1996. The bacteriophage P1 lytic replicon: directionality of replication and cis-acting elements. Gene 175:151-155. [DOI] [PubMed] [Google Scholar]
- 62.Cohen, P. T., and P. Cohen. 1989. Discovery of a protein phosphatase activity encoded in the genome of bacteriophage lambda. Probable identity with open reading frame 221. Biochem. J. 260:931-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coombs, D. H., and F. Arisaka. 1994. T4 tail structure and function., p. 259-281. In J. D. Karam (ed.), Molecular biology of bacteriophage T4. ASM Press, Washington, D.C.
- 64.Corre, J., and J. M. Louarn. 2002. Evidence from terminal recombination gradients that FtsK uses replichore polarity to control chromosome terminus positioning at division in Escherichia coli. J. Bacteriol. 184:3801-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Costantino, N., M. Zuber, and D. Court. 1990. Analysis of mutations in the ninR region of bacteriophage lambda that bypass a requirement for lambda N antitermination. J. Bacteriol. 172:4610-4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coulby, J. N., and N. L. Sternberg. 1988. Characterization of the phage P1 dam gene. Gene 74:191. [DOI] [PubMed] [Google Scholar]
- 67.D'Ari, R., A. Jaffé-Brachet, D. Touati-Schwartz, and M. B. Yarmolinsky. 1975. A dnaB analog specified by bacteriophage P1. J. Mol. Biol. 94:341-366. [DOI] [PubMed] [Google Scholar]
- 68.Dartois, V., O. De Backer, and C. Colson. 1993. Sequence of the Salmonella typhimurium StyLT1 restriction-modification genes: homologies with EcoP1 and EcoP15 type-III R-M systems and presence of helicase domains. Gene 127:105-110. [DOI] [PubMed] [Google Scholar]
- 69.d'Aubenton Carafa, Y., E. Brody, and C. Thermes. 1990. Prediction of rho-independent Escherichia coli transcription terminators. A statistical analysis of their RNA stem-loop structures. J. Mol. Biol. 216:835-858. [DOI] [PubMed] [Google Scholar]
- 70.Davey, M. J., and B. E. Funnell. 1997. Modulation of the P1 plasmid partition protein ParA by ATP, ADP, and P1 ParB. J. Biol. Chem. 272:15286-15292. [DOI] [PubMed] [Google Scholar]
- 71.Davey, M. J., and B. E. Funnell. 1994. The P1 plasmid partition protein ParA. A role for ATP in site-specific DNA binding. J. Biol. Chem. 269:29908-29913. [PubMed] [Google Scholar]
- 72.Davis, M. A., and S. J. Austin. 1988. Recognition of the P1 plasmid centromere analog involves binding of the ParB protein and is modified by a specific host factor. EMBO J. 7:1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davis, M. A., K. A. Martin, and S. J. Austin. 1992. Biochemical activities of the ParA partition protein of the P1 plasmid. Mol. Microbiol. 6:1141-1147. [DOI] [PubMed] [Google Scholar]
- 74.Delarue, M., O. Poch, N. Tordo, D. Moras, and P. Argos. 1990. An attempt to unify the structure of polymerases. Protein Eng. 3:461-467. [DOI] [PubMed] [Google Scholar]
- 75.Delcher, A. L., D. Harmon, S. Kasif, O. White, and S. L. Salzberg. 1999. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 27:4636-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeRose, E. F., T. Darden, S. Harvey, S. Gabel, F. W. Perrino, R. M. Schaaper, and R. E. London. 2003. Elucidation of the epsilon-theta subunit interface of Escherichia coli DNA polymerase III by NMR spectroscopy. Biochemistry 42:3635-3644. [DOI] [PubMed] [Google Scholar]
- 77.Devlin, B. H., B. R. Baumstark, and J. R. Scott. 1982. Superimmunity: characterization of a new gene in the immunity region of P1. Virology 120:360-375. [DOI] [PubMed] [Google Scholar]
- 78.Dodd, I. B., and J. B. Egan. 1990. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 18:5019-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dreiseikelmann, B., M. Velleman, and H. Schuster. 1988. The c1 repressor of bacteriophage P1. Isolation and characterization of the repressor protein. J. Biol. Chem. 263:1391-1397. [PubMed] [Google Scholar]
- 80.Ebersbach, G., and K. Gerdes. 2001. The double par locus of virulence factor pB171: DNA segregation is correlated with oscillation of ParA. Proc. Natl. Acad. Sci. USA 98:15078-15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eddy, S. R., and R. Durbin. 1994. RNA sequence analysis using covariance models. Nucleic Acids Res. 22:2079-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Edelbluth, C., E. Lanka, W. von der Hude, M. Mikolajczyk, and H. Schuster. 1979. Association of the prophage P1ban protein with the dnaB protein of Escherichia coli. Overproduction of ban protein by a P1bac crr mutant. Eur. J. Biochem. 94:427-435. [DOI] [PubMed] [Google Scholar]
- 83.Edgar, R., D. K. Chattoraj, and M. Yarmolinsky. 2001. Pairing of P1 plasmid partition sites by ParB. Mol. Microbiol. 42:1363-1370. [DOI] [PubMed] [Google Scholar]
- 84.Eliason, J. L., and N. Sternberg. 1987. Characterization of the binding sites of cl repressor of bacteriophage Pl: evidence for multiple asymmetric sites. J. Mol. Biol. 198:281-293. [DOI] [PubMed] [Google Scholar]
- 85.Ermolaeva, M. D., H. G. Khalak, O. White, H. O. Smith, and S. L. Salzberg. 2000. Prediction of transcription terminators in bacterial genomes. J. Mol. Biol. 301:27-33. [DOI] [PubMed] [Google Scholar]
- 86.Ferguson, P. L., and D. H. Coombs. 2000. Pulse-chase analysis of the in vivo assembly of the bacteriophage T4 tail. J. Mol. Biol. 297:99-117. [DOI] [PubMed] [Google Scholar]
- 87.Friedman, S. A., and S. J. Austin. 1988. The P1 plasmid-partition system synthesizes two essential proteins from an autoregulated operon. Plasmid 19:103-112. [DOI] [PubMed] [Google Scholar]
- 88.Fukada, K., and J. Abelson. 1980. DNA sequence of a T4 transfer RNA gene cluster. J. Mol. Biol. 139:377-391. [DOI] [PubMed] [Google Scholar]
- 89.Funnell, B. E. 1988. Mini-P1 plasmid partitioning: excess ParB protein destabilizes plasmids containing the centromere parS. J. Bacteriol. 170:954-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Funnell, B. E. 1991. The P1 plasmid partition complex at parS. The influence of Escherichia coli integration host factor and of substrate topology. J. Biol. Chem. 266:14328-14337. [PubMed] [Google Scholar]
- 91.Funnell, B. E. 1988. Participation of Escherichia coli integration host factor in the P1 plasmid partition system. Proc. Natl. Acad. Sci. USA 85:6657-6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Funnell, B. E., and L. Gagnier. 1993. The P1 plasmid partition complex at parS. II. Analysis of ParB protein binding activity and specificity. J. Biol. Chem. 268:3616-3624. [PubMed] [Google Scholar]
- 93.Gamas, P., M. G. Chandler, P. Prentki, and D. J. Galas. 1987. Escherichia coli integration host factor binds specifically to the ends of the insertion sequence IS1 and to its major insertion hot-spot in pBR322. J. Mol. Biol. 195:261-272. [DOI] [PubMed] [Google Scholar]
- 94.Gamas, P., D. Galas, and M. Chandler. 1985. DNA sequence at the end of IS1 required for transposition. Nature 317:458-460. [DOI] [PubMed] [Google Scholar]
- 95.Gazit, E., and R. T. Sauer. 1999. The Doc toxin and Phd antidote proteins of the bacteriophage P1 plasmid addiction system form a heterotrimeric complex. J. Biol. Chem. 274:16813-16818. [DOI] [PubMed] [Google Scholar]
- 96.Gazit, E., and R. T. Sauer. 1999. Stability and DNA binding of the phd protein of the phage P1 plasmid addiction system. J. Biol. Chem. 274:2652-2657. [DOI] [PubMed] [Google Scholar]
- 97.Gerdes, K., J. Møller-Jensen, G. Ebersbach, T. Kruse, and K. Nordström. 2004. Bacterial mitotic machineries. Cell 116:359-366. [DOI] [PubMed] [Google Scholar]
- 98.Ghisotti, D., F. Briani, F. Forti, F. Piazza, S. Polo, P. Sabbattini, T. Sturniolo, S. Terzano, S. Zangrossi, M. Zappone, G. Sironi, and G. Dehò. 1995. Multiple regulatory mechanisms controlling phage-plasmid P4 propagation. FEMS Microbiol. Rev. 17:127-134. [DOI] [PubMed] [Google Scholar]
- 99.Goldman, E., A. H. Rosenberg, G. Zubay, and F. W. Studier. 1995. Consecutive low-usage leucine codons block translation only when near the 5′ end of a message in Escherichia coli. J. Mol. Biol. 245:467-473. [DOI] [PubMed] [Google Scholar]
- 100.Gottesman, S., W. P. Clark, V. de Crecy-Lagard, and M. R. Maurizi. 1993. ClpX, an alternative subunit for the ATP-dependent Clp protease of Escherichia coli. Sequence and in vivo activities. J. Biol. Chem. 268:22618-22626. [PubMed] [Google Scholar]
- 101.Grigoriev, A. 1999. Strand-specific compositional asymmetries in double-stranded DNA viruses. Virus Res. 60:1-19. [DOI] [PubMed] [Google Scholar]
- 102.Grigoriev, P., and M. B. Łobocka. 2001. Determinants of segregational stability of the linear plasmid prophage N15 of Escherichia coli. Mol. Microbiol. 42:355-368. [DOI] [PubMed] [Google Scholar]
- 103.Gründling, A., M. D. Manson, and R. Young. 2001. Holins kill without warning. Proc. Natl. Acad. Sci. USA 98:9348-9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guidolin, A., J. M. Zingg, and W. Arber. 1989. Organization of the bacteriophage P1 tail-fibre operon. Gene 76:239-243. [DOI] [PubMed] [Google Scholar]
- 105.Guidolin, A., J. M. Zingg, H. Lehnherr, and W. Arber. 1989. Bacteriophage P1 tail-fibre and dar operons are expressed from homologous phage-specific late promoter sequences. J. Mol. Biol. 208:615-622. [DOI] [PubMed] [Google Scholar]
- 106.Guo, F., D. N. Gopaul, and G. D. van Duyne. 1997. Structure of Cre recombinase complexed with DNA in a site-specific recombination synapse. Nature 389:40-46. [DOI] [PubMed] [Google Scholar]
- 107.Hadi, S. M., B. Bächi, S. Iida, and T. A. Bickle. 1983. DNA restriction-modification enzymes of phage P1 and plasmid p15B. Subunit functions and structural homologies. J. Mol. Biol. 165:19-34. [DOI] [PubMed] [Google Scholar]
- 108.Haffter, P., and T. A. Bickle. 1988. Enhancer-independent mutants of the Cin recombinase have a relaxed topological specificity. EMBO J. 7:3991-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haffter, P., and T. A. Bickle. 1987. Purification and DNA-binding properties of FIS and Cin, two proteins required for the bacteriophage P1 site-specific recombination system, cin. J. Mol. Biol. 198:579-587. [DOI] [PubMed] [Google Scholar]
- 110.Hambly, E., F. Tetart, C. Desplats, W. H. Wilson, H. M. Krisch, and N. H. Mann. 2001. A conserved genetic module that encodes the major virion components in both the coliphage T4 and the marine cyanophage S-PM2. Proc. Natl. Acad. Sci. USA 98:11411-11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hansen, A.-M., H. Lehnherr, X. Wang, V. Mobley, and D. J. Jin. 2003. Escherichia coli SspA is a transcription activator for bacteriophage P1 late genes. Mol. Microbiol. 48:1621-1631. [DOI] [PubMed] [Google Scholar]
- 112.Hansen, E. B. 1989. Structure and regulation of the lytic replicon of phage Pl. J. Mol. Biol. 207:135-149. [DOI] [PubMed] [Google Scholar]
- 113.Hansen, E. B., and M. B. Yarmolinsky. 1986. Host participation in plasmid maintenance: dependence upon DnaA of replicons derived from P1 and F. Proc. Natl. Acad. Sci. USA 83:4423-4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hao, J. J., and M. Yarmolinsky. 2002. Effects of the P1 plasmid centromere on expression of P1 partition genes. J. Bacteriol. 184:4857-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hartmann, R. K., J. Heinrich, J. Schlegl, and H. Schuster. 1995. Precursor of C4 antisense RNA of bacteriophages P1 and P7 is a substrate for RNase P of Escherichia coli. Proc. Natl. Acad. Sci. USA 92:5822-5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hasegawa, T., M. Miyano, H. Himeno, Y. Sano, K. Kimura, and M. Shimizu. 1992. Identity determinants of E. coli threonine tRNA. Biochem. Biophys. Res. Commun. 184:478-484. [DOI] [PubMed] [Google Scholar]
- 117.Hashemolhosseini, S., Y. D. Stierhof, I. Hindennach, and U. Henning. 1996. Characterization of the helper proteins for the assembly of tail fibers of coliphages T4 and lambda. J. Bacteriol. 178:6258-6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hawley, D. K., and W. R. McClure. 1983. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 11:2237-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hay, N., and G. Cohen. 1983. Requirement of E. coli DNA synthesis functions for the lytic replication of bacteriophage P1. Virology 131:193-206. [DOI] [PubMed] [Google Scholar]
- 120.Hayes, F., L. Radnedge, M. A. Davis, and S. J. Austin. 1994. The homologous operons for P1 and P7 plasmid partition are autoregulated from dissimilar operator sites. Mol. Microbiol. 11:249-260. [DOI] [PubMed] [Google Scholar]
- 121.Hazan, R., B. Sat, M. Reches, and H. Engelberg-Kulka. 2001. Postsegregational killing mediated by the P1 phage “addiction module” phd-doc requires the Escherichia coli programmed cell death system mazEF. J. Bacteriol. 183:2046-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Heilmann, H., J. N. Reeve, and A. Pühler. 1980. Identification of the repressor and repressor bypass (antirepressor) polypeptides of bacteriophage P1 synthesized in infected minicells. Mol. Gen. Genet. 178:149-154. [DOI] [PubMed] [Google Scholar]
- 123.Heinrich, J., M. Citron, A.Günther, and H. Schuster. 1994. Second-site suppressors of the bacteriophage P1 virs mutant reveal the interdependence of the c4, icd, and ant genes in the P1 immI operon. J. Bacteriol. 176:4931-4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heinrich, J., H. D. Riedel, B. R. Baumstark, M. Kimura, and H. Schuster. 1989. The c1 repressor of bacteriophage P1 operator-repressor interaction of wild-type and mutant repressor proteins. Nucleic Acids Res. 17:7681-7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heinrich, J., H. D. Riedel, B. Rückert, R. Lurz, and H. Schuster. 1995. The lytic replicon of bacteriophage P1 is controlled by an antisense RNA. Nucleic Acids Res. 23:1468-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Heinrich, J., M. Velleman, and H. Schuster. 1995. The tripartite immunity system of phages P1 and P7. FEMS Microbiol. Rev. 17:121-126. [DOI] [PubMed] [Google Scholar]
- 127.Heinzel, T., M. Velleman, and H. Schuster. 1990. The cl repressor inactivator protein coi of bacteriophage Pl. Cloning and expression of coi and its interference with cl repressor function. J. Biol. Chem. 265:17928-17934. [PubMed] [Google Scholar]
- 128.Heinzel, T., R. Lurz, B. Dobrinsky, M. Velleman, and H. Schuster. 1994. C1 repressor-mediated looping is involved in C1 autoregulation of bacteriophage P1. J. Biol. Chem. 269:31885-31890. [PubMed] [Google Scholar]
- 129.Heinzel, T., M. Velleman, and H. Schuster. 1989. ban operon of bacteriophage P1. Mutational analysis of the c1 repressor-controlled operator. J. Mol. Biol. 205:127-135. [DOI] [PubMed] [Google Scholar]
- 130.Heinzel, T., M. Velleman, and H. Schuster. 1992. Cl repressor of phage Pl is inactivated by non-covalent binding of Pl Coi protein. J. Biol. Chem. 267:4183-4188. [PubMed] [Google Scholar]
- 131.Heisig, A., H.-D. Riedel, B. Dobrinski, R. Lurz, and H. Schuster. 1989. Organization of the immunity region immI of bacteriophage Pl and synthesis of the Pl antirepressor. J. Mol. Biol. 209:525-538. [DOI] [PubMed] [Google Scholar]
- 132.Heisig, A., I. Severin, A. K. Seefluth, and H. Schuster. 1987. Regulation of the ban gene containing operon of prophage P1. Mol. Gen. Genet. 206:368-376. [DOI] [PubMed] [Google Scholar]
- 133.Hendrix, R. W., and R. L. Duda. 1992. Bacteriophage lambda PaPa: not the mother of all lambda phages. Science 258:1145-1148. [DOI] [PubMed] [Google Scholar]
- 134.Henning, U., and S. Hashemolhosseini. 1994. Receptor recognition by T-even-type coliphages, p. 182-185. In J. D. Karam (ed.), Molecular biology of bacteriophage T4. ASM Press, Washington, D.C.
- 135.Hiestand-Nauer, R., and S. Iida. 1983. Sequence of the site-specific recombinase gene cin and of its substrates serving in the inversion of the C segment of bacteriophage P1. EMBO J. 2:1733-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hill, T. M. 1996. Features of the chromosomal terminus region., p. 1602-1614. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 2. ASM Press, Washington, D.C.
- 137.Hochman, L., N. Segev, N. Sternberg, and G. Cohen. 1983. Site-specific recombinational circularization of bacteriophage P1 DNA. Virology 131:11-17. [DOI] [PubMed] [Google Scholar]
- 138.Hoess, R. H., M. Ziese, and N. Sternberg. 1982. P1 site-specific recombination: nucleotide sequence of the recombining sites. Proc. Natl. Acad. Sci. USA 79:3398-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hofer, B., M. Ruge, and B. Dreiseikelmann. 1995. The superinfection exclusion gene (sieA) of bacteriophage P22: identification and overexpression of the gene and localization of the gene product. J. Bacteriol. 177:3080-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Holcik, M., and V. N. Iyer. 1997. Conditionally lethal genes associated with bacterial plasmids. Microbiology 143:3403-3416. [DOI] [PubMed] [Google Scholar]
- 141.Huber, H. E., S. Iida, W. Arber, and T. A. Bickle. 1985. Site specific DNA inversion is enhanced by a specific DNA element in cis. Proc. Natl. Acad. Sci. USA. 82:3776-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huber, H. E., S. Iida, and T. A. Bickle. 1985. Expression of the bacteriophage P1 cin recombinase gene from its own and heterologous promoters. Gene 34:63-72. [DOI] [PubMed] [Google Scholar]
- 143.Hübner, P., and W. Arber. 1989. Mutational analysis of a prokaryotic recombinational enhancer element with two functions. EMBO J. 8:577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hübner, P., P. Haffter, S. Iida, and W. Arber. 1989. Bent DNA is needed for recombinational enhancer activity in the site-specific recombination system Cin of bacteriophage P1. The role of FIS protein. J. Mol. Biol. 205:493-500. [DOI] [PubMed] [Google Scholar]
- 145.Hümbelin, M., B. Suri, D. N. Rao, D. P. Hornby, H. Eberle, T. Pripfl, S. Kenel, and T. A. Bickle. 1988. Type III DNA restriction and modification systems EcoP1 and EcoP15. Nucleotide sequence of the EcoP1 operon, the EcoP15 mod gene and some EcoP1 mod mutants. J. Mol. Biol. 200:23-29. [DOI] [PubMed] [Google Scholar]
- 146.Iida, S. 1984. Bacteriophage P1 carries two related sets of genes determining its host range in the invertible C segment of its genome. Virology 134:421-434. [DOI] [PubMed] [Google Scholar]
- 147.Iida, S., and W. Arber. 1977. Plaque forming specialized transducing phage P1: isolation of P1CmSmSu, a precursor of P1Cm. Mol. Gen. Genet. 153:259-269. [DOI] [PubMed] [Google Scholar]
- 148.Iida, S., and R. Hiestand-Nauer. 1987. Role of the central dinucleotide at the crossover sites for the selection of quasi sites in DNA inversion mediated by the site-specific Cin recombinase of phage P1. Mol. Gen. Genet. 208:464-468. [DOI] [PubMed] [Google Scholar]
- 149.Iida, S., R. Hiestand-Nauer, H. Sandmeier, H. Lehnherr, and W. Arber. 1998. Accessory genes in the darA operon of bacteriophage P1 affect antirestriction function, generalized transduction, head morphogenesis, and host cell lysis. Virology 251:49-58. [DOI] [PubMed] [Google Scholar]
- 150.Iida, S., H. Huber, R. Hiestand-Nauer, J. Meyer, T. A. Bickle, and W. Arber. 1984. The bacteriophage P1 site-specific recombinase cin: recombination events and DNA recognition sequences. Cold Spring Harbor Symp. Quant. Biol. 49:769-777. [DOI] [PubMed] [Google Scholar]
- 151.Iida, S., J. Meyer, and W. Arber. 1978. The insertion element IS1 is a natural constituent of coliphage P1 DNA. Plasmid 1:357-365. [DOI] [PubMed] [Google Scholar]
- 152.Iida, S., J. Meyer, B. Bächi, M. Stålhammar-Carlemalm, S. Schrickel, T. A. Bickle, and W. Arber. 1983. DNA restriction-modification genes of phage P1 and plasmid p15B. Structure and in vitro transcription. J. Mol. Biol. 165:1-18. [DOI] [PubMed] [Google Scholar]
- 153.Iida, S., J. Meyer, K. E. Kennedy, and W. Arber. 1982. A site-specific, conservative recombination system carried by bacteriophage P1. Mapping the recombinase gene cin and the cross-over sites cix for the inversion of the C segment. EMBO J. 1:1445-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Iida, S., H. Sandmeier, P. Hübner, R. Hiestand-Nauer, K. Schneitz, and W. Arber. 1990. The Min DNA inversion enzyme of plasmid p15B of Escherichia coli 15T-: a new member of the Din family of site-specific recombinases. Mol. Microbiol. 4:991-997. [DOI] [PubMed] [Google Scholar]
- 155.Iida, S., M. B. Streiff, T. A. Bickle, and W. Arber. 1987. Two DNA antirestriction systems of bacteriophage P1, darA and darB: characterization of darA− phages. Virology 157:156-166. [DOI] [PubMed] [Google Scholar]
- 156.Ikeda, H., and J.-I. Tomizawa. 1965. Transducing fragments in generalized transduction by phage P1. III. Studies with small phage particles. J. Mol. Biol. 14:120-129. [DOI] [PubMed] [Google Scholar]
- 157.Ireton, K., N. W. Günther, and A. D. Grossman. 1994. Spo0J is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J. Bacteriol. 176:5320-5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ishihama, A., and T. Saitoh. 1979. Subunits of RNA polymerase in function and structure. IX. Regulation of RNA polymerase activity by stringent starvation protein (SSP). J. Mol. Biol. 129:517-530. [DOI] [PubMed] [Google Scholar]
- 159.Ito, H., A. Sadaoka, H. Kotani, N. Hiraoka, and T. Nakamura. 1990. Cloning, nucleotide sequence, and expression of the HincII restriction-modification system. Nucleic Acids Res. 18:3903-3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ito, J., and D. K. Braithwaite. 1991. Compilation and alignment of DNA polymerase sequences. Nucleic Acids Res. 19:4045-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Janscak, P., U. Sandmeier, M. D. Szczelkun, and T. A. Bickle. 2001. Subunit assembly and mode of DNA cleavage of the type III restriction endonucleases EcoP1I and EcoP15I. J. Mol. Biol. 306:417-431. [DOI] [PubMed] [Google Scholar]
- 162.Janulaitis, A., M. Petrusyte, Z. Maneliene, S. Klimasauskas, and V. Butkus. 1992. Purification and properties of the Eco57I restriction endonuclease and methylase-prototypes of a new class (type IV). Nucleic Acids Res. 20:6043-6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jobling, M. G., and D. A. Ritchie. 1988. The nucleotide sequence of a plasmid determinant for resistance to tellurium anions. Gene 66:245-258. [DOI] [PubMed] [Google Scholar]
- 164.Johnson, B. F. 1982. Suppression of the lexC (ssbA) mutation of Escherichia coli by a mutant of bacteriophage P1. Mol. Gen. Genet. 186:122-126. [DOI] [PubMed] [Google Scholar]
- 165.Kanamaru, S., P. G. Leiman, V. A. Kostyuchenko, P. R. Chipman, V. V. Mesyanzhinov, F. Arisaka, and M. G. Rossmann. 2002. Structure of the cell-puncturing device of bacteriophage T4. Nature 415:553-557. [DOI] [PubMed] [Google Scholar]
- 166.Kane, J. F. 1995. Effects of rare codon clusters on high-level expression of heterologous proteins in Escherichia coli. Curr. Opin. Biotechnol. 6:494-500. [DOI] [PubMed] [Google Scholar]
- 167.Kawakami, B., H. Christophe, M. Nagatomo, and M. Oka. 1991. Cloning and nucleotide sequences of the AccI restriction-modification genes in Acinetobacter calcoaceticus. Agric. Biol. Chem. 55:1553-1559. [PubMed] [Google Scholar]
- 168.Kelman, Z., and M. O'Donnell. 1995. DNA polymerase III holoenzyme: structure and function of a chromosomal replicating machine. Annu. Rev. Biochem. 64:171-200. [DOI] [PubMed] [Google Scholar]
- 169.Keniry, M. A., H. A. Berthon, J. Y. Yang, C. S. Miles, and N. E. Dixon. 2000. NMR solution structure of the theta subunit of DNA polymerase III from Escherichia coli. Protein Sci. 9:721-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kliem, M., and B. Dreiseikelmann. 1989. The superimmunity gene sim of bacteriophage P1 causes superinfection exclusion. Virology 171:350-355. [DOI] [PubMed] [Google Scholar]
- 171.Kondo, E., and S. Mitsuhashi. 1964. Drug resistance of enteric bacteria. IV. Active transducing bacteriophage P1CM produced by the combination of R-factor with bacteriophage P1. J. Bacteriol. 88:1266-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Koonin, E. V. 1993. Bacterial and bacteriophage protein phosphatases. Mol. Microbiol. 8:785-786. [DOI] [PubMed] [Google Scholar]
- 173.Kowalczykowski, S. C. 2000. Initiation of genetic recombination and recombination-dependent replication. Trends Biochem. Sci. 25:156-165. [DOI] [PubMed] [Google Scholar]
- 174.Kravanja, M., R. Engelmann, V. Dossonnet, M. Blüggel, H. E. Meyer, R. Frank, A. Galinier, J. Deutscher, N. Schnell, and W. Hengstenberg. 1999. The hprK gene of Enterococcus faecalis encodes a novel bifunctional enzyme: the HPr kinase/phosphatase. Mol. Microbiol. 31:59-66. [DOI] [PubMed] [Google Scholar]
- 175.Krogh, A., B. Larsson, G. von Heijne, and E. L. Sonnhammer. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567-580. [DOI] [PubMed] [Google Scholar]
- 176.Kropinski, A. M. 2000. Sequence of the genome of the temperate, serotype-converting, Pseudomonas aeruginosa bacteriophage D3. J. Bacteriol. 182:6066-6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kropinski, A. M., and M. J. Sibbald. 1999. Transfer RNA genes and their significance to codon usage in the Pseudomonas aeruginosa lamboid bacteriophage D3. Can J. Microbiol. 45:791-796. [PubMed] [Google Scholar]
- 178.Kumar, A., R. A. Malloch, N. Fujita, D. A. Smillie, A. Ishihama, and R. S. Hayward. 1993. The minus 35-recognition region of Escherichia coli sigma 70 is inessential for initiation of transcription at an “extended minus 10” promoter. J. Mol. Biol. 232:406-418. [DOI] [PubMed] [Google Scholar]
- 179.Kunisawa, T. 1992. Synonymous codon preferences in bacteriophage T4: a distinctive use of transfer RNAs from T4 and from its host Escherichia coli. J. Theor. Biol. 159:287-298. [DOI] [PubMed] [Google Scholar]
- 180.Kutter, E., K. Gachechiladze, A. Poglazov, E. Marusich, M. Shneider, P. Aronsson, A. Napuli, D. Porter, and V. Mesyanzhinov. 1995. Evolution of T4-related phages. Virus Genes 11:285-297. [DOI] [PubMed] [Google Scholar]
- 181.Kuzminov, A. 1995. Collapse and repair of replication forks in Escherichia coli. Mol. Microbiol. 16:373-384. [DOI] [PubMed] [Google Scholar]
- 182.Kwon, H. J., R. Tirumalai, A. Landy, and T. Ellenberger. 1997. Flexibility in DNA recombination: structure of the lambda integrase catalytic core. Science 276:126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lanka, E., B. Geschke, and H. Schuster. 1978. Escherichia coli dnaB mutant defective in DNA initiation: isolation and properties of the dnaB protein. Proc. Natl. Acad. Sci. USA 75:799-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Laufer, C. S., J. B. Hays, B. E. Windle, T. S. Schaefer, E. H. Lee, S. L. Hays, and M. R. McClure. 1989. Enhancement of Escherichia coli plasmid and chromosomal recombination by the Ref function of bacteriophage P1. Genetics 123:465-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184a.Lederberg, E. M. 1950. Lysogenicity of Escherichia coli strain K-12. Microb. Genet. Bull. 1:5-7. [Google Scholar]
- 185.Lederberg, E. M., and J. Lederberg. 1953. Genetic studies of lysogenicity in E. coli. Genetics 38:51-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lehnherr, H. Bacteriophage P1. In R. Calendar (ed.), The bacteriophages, in press. Oxford University Press, New York, N.Y.
- 187.Lehnherr, H., J. D. Bendtsen, F. Preuss, and T. V. Ilyina. 1999. Identification and characterization of the single-stranded DNA-binding protein of bacteriophage P1. J. Bacteriol. 181:6463-6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lehnherr, H., A. Guidolin, and W. Arber. 1991. Bacteriophage P1 gene 10 encodes a trans-activating factor required for late gene expression. J. Bacteriol. 173:6438-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lehnherr, H., A. Guidolin, and W. Arber. 1992. Mutational analysis of the bacteriophage P1 late promoter sequence PS. J. Mol. Biol. 228:101-107. [DOI] [PubMed] [Google Scholar]
- 190.Lehnherr, H., A.-M. Hansen, and T. V. Ilyina. 1998. Penetration of the bacterial cell wall: a family of lytic transglycosylases in bacteriophages and conjugative plasmids. Mol. Microbiol. 30:454-457. [DOI] [PubMed] [Google Scholar]
- 191.Lehnherr, H., C. D. Jensen, A. R. Stenholm, and A. Dueholm. 2001. Dual regulatory control of a particle maturation function of bacteriophage P1. J. Bacteriol. 183:4105-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lehnherr, H., E. Maguin, S. Jafri, and M. Yarmolinsky. 1993. Plasmid addiction genes of bacteriophage P1: doc, which causes cell death on curing of prophage, and phd, which prevents host death when prophage is retained. J. Mol. Biol. 233:414-428. [DOI] [PubMed] [Google Scholar]
- 193.Lehnherr, H., M. Velleman, A. Guidolin, and W. Arber. 1992. Bacteriophage P1 gene 10 is expressed from a promoter-operator sequence controlled by C1 and Bof proteins. J. Bacteriol. 174:6138-6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lehnherr, H., and M. B. Yarmolinsky. 1995. Addiction protein Phd of plasmid prophage P1 is a substrate of the ClpXP serine protease of Escherichia coli. Proc. Natl. Acad. Sci. USA 92:3274-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lemonnier, M., G. Ziegelin, T. Reick, A. M. Gomez, R. Diaz-Orejas, and E. Lanka. 2003. Bacteriophage P1 Ban protein is a hexameric DNA helicase that interacts with and substitutes for Escherichia coli DnaB. Nucleic Acids Res. 31:3918-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lennox, E. S. 1955. Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1:190-206. [DOI] [PubMed] [Google Scholar]
- 197.Leslie, N. R., and D. J. Sherratt. 1995. Site-specific recombination in the replication terminus region of Escherichia coli: functional replacement of dif. EMBO J. 14:1561-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lesnik, E. A., R. Sampath, H. B. Levene, T. J. Henderson, J. A. McNeil, and D. J. Ecker. 2001. Prediction of rho-independent transcriptional terminators in Escherichia coli. Nucleic Acids Res. 29:3583-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Levin, M. E., R. W. Hendrix, and S. R. Casjens. 1993. A programmed translational frameshift is required for the synthesis of a bacteriophage lambda tail assembly protein. J. Mol. Biol. 234:124-139. [DOI] [PubMed] [Google Scholar]
- 200.Lewis, L. K., G. R. Harlow, L. A. Gregg-Jolly, and D. W. Mount. 1994. Identification of high affinity binding sites for LexA which define new DNA damage-inducible genes in Escherichia coli. J. Mol. Biol. 241:507-523. [DOI] [PubMed] [Google Scholar]
- 201.Li, S., H. Pelka, and L. H. Schulman. 1993. The anticodon and discriminator base are important for aminoacylation of Escherichia coli tRNA(Asn). J. Biol. Chem. 268:18335-18339. [PubMed] [Google Scholar]
- 202.Lin, H., V. B. Rao, and L. W. Black. 1999. Analysis of capsid portal protein and terminase functional domains: interaction sites required for DNA packaging in bacteriophage T4. J. Mol. Biol. 289:249-260. [DOI] [PubMed] [Google Scholar]
- 203.Łobocka, M., and M. Yarmolinsky. 1996. P1 plasmid partition: a mutational analysis of ParB. J. Mol. Biol. 259:366-382. [DOI] [PubMed] [Google Scholar]
- 204.Lowe, T. M., and S. R. Eddy. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25:955-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lu, M. J., and U. Henning. 1994. Superinfection exclusion by T-even-type coliphages. Trends Microbiol. 2:137-139. [DOI] [PubMed] [Google Scholar]
- 206.Lu, M. J., Y. D. Stierhof, and U. Henning. 1993. Location and unusual membrane topology of the immunity protein of the Escherichia coli phage T4. J. Virol. 67:4905-4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lu, S. D., D. Lu, and M. Gottesman. 1989. Stimulation of IS1 excision by bacteriophage P1 ref function. J. Bacteriol. 171:3427-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lukashin, A. V., and M. Borodovsky. 1998. GeneMark.hmm: new solutions for gene finding. Nucleic Acids Res. 26:1107-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lurz, R., A. Heisig, M. Velleman, B. Dobrinski, and H. Schuster. 1987. The ban operon of bacteriophage P1. Localization of the promoter controlled by P1 repressor. J. Biol. Chem. 262:16575-16579. [PubMed] [Google Scholar]
- 210.Magnuson, R., H. Lehnherr, G. Mukhopadhyay, and M. B. Yarmolinsky. 1996. Autoregulation of the plasmid addiction operon of bacteriophage P1. J. Biol. Chem. 271:18705-18710. [DOI] [PubMed] [Google Scholar]
- 211.Magnuson, R., and M. B. Yarmolinsky. 1998. Corepression of the P1 addiction operon by Phd and Doc. J. Bacteriol. 180:6342-6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mahillon, J., and M. Chandler. 1998. Insertion sequences. Microbiol. Mol. Biol. Rev. 62:725-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Maillou, J., and B. Dreiseikelmann. 1990. The sim gene of Escherichia coli phage P1: nucleotide sequence and purification of the processed protein. Virology 175:500-507. [DOI] [PubMed] [Google Scholar]
- 214.Malone, T., R. M. Blumenthal, and X. Cheng. 1995. Structure-guided analysis reveals nine sequence motifs conserved among DNA amino-methyltransferases, and suggests a catalytic mechanism for these enzymes. J. Mol. Biol. 253:618-632. [DOI] [PubMed] [Google Scholar]
- 215.McDonald, J. P., T. S. Peat, A. S. Levine, and R. Woodgate. 1999. Intermolecular cleavage by UmuD-like enzymes: identification of residues required for cleavage and substrate specificity. J. Mol. Biol. 285:2199-2209. [DOI] [PubMed] [Google Scholar]
- 216.McLean, M. J., K. H. Wolfe, and K. M. Devine. 1998. Base composition skews, replication orientation, and gene orientation in 12 prokaryote genomes. J. Mol. Evol. 47:691-696. [DOI] [PubMed] [Google Scholar]
- 217.McLenigan, M. P., O. I. Kulaeva, D. G. Ennis, A. S. Levine, and R. Woodgate. 1999. The bacteriophage P1 HumD protein is a functional homolog of the prokaryotic UmuD′-like proteins and facilitates SOS mutagenesis in Escherichia coli. J. Bacteriol. 181:7005-7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mehr, I. J., C. D. Long, C. D. Serkin, and H. S. Seifert. 2000. A homologue of the recombination-dependent growth gene, rdgC, is involved in gonococcal pilin antigenic variation. Genetics 154:523-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Meisel, A., T. A. Bickle, D. H. Krüger, and C. Schroeder. 1992. Type III restriction enzymes need two inversely oriented recognition sites for DNA cleavage. Nature 355:467-469. [DOI] [PubMed] [Google Scholar]
- 220.Meisel, A., P. Mackeldanz, T. A. Bickle, D. H. Krüger, and C. Schroeder. 1995. Type III restriction endonucleases translocate DNA in a reaction driven by recognition site-specific ATP hydrolysis. EMBO J. 14:2958-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mesyanzhinov, V. V., B. N. Sobolev, E. I. Marusich, A. G. Prilipov, and V. P. Efimov. 1990. A proposed structure of bacteriophage T4 gene product 22, a major prohead scaffolding core protein. J. Struct. Biol. 104:24-31. [DOI] [PubMed] [Google Scholar]
- 222.Meyer, J., M. Stalhammar-Carlemalm, and S. Iida. 1981. Denaturation map of bacteriophage P1 DNA. Virology 110:167-175. [DOI] [PubMed] [Google Scholar]
- 223.Miller, E. S., J. F. Heidelberg, J. A. Eisen, W. C. Nelson, A. S. Durkin, A. Ciecko, T. V. Feldblyum, O. White, I. T. Paulsen, W. C. Nierman, J. Lee, B. Szczypinski, and C. M. Fraser. 2003. Complete genome sequence of the broad-host-range vibriophage KVP40: comparative genomics of a T4-related bacteriophage. J. Bacteriol. 185:5220-5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Miller, E. S., E. Kutter, G. Mosig, F. Arisaka, T. Kunisawa, and W. Rüger. 2003. Bacteriophage T4 genome. Microbiol. Mol. Biol. Rev. 67:86-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Miller, J. H. 1992. A short course in bacterial genetics. A laboratory handbook for Escherichia coli and related bacteria, p. 15.3-15.10. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 226.Montag, D., and U. Henning. 1987. An open reading frame in the Escherichia coli bacteriophage lambda genome encodes a protein that functions in assembly of the long tail fibers of bacteriophage T4. J. Bacteriol. 169:5884-5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mosig, G. 1994. Synthesis and maturation of T4-encoded tRNas, p. 182-185. In J. D. Karam (ed.), Molecular biology of bacteriophage T4. ASM Press, Washington, D.C.
- 228.Mosig, G., and D. H. Hall. 1994. Gene expression: a paradigm of integrated circuits., p. 127-131. In J. D. Karam (ed.), Molecular biology of bacteriophage T4. ASM Press, Washington, D.C.
- 229.Mulligan, M. E., D. K. Hawley, E. Entriken, and W. R. McClure. 1984. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 12:789-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mural, R. J., R. H. Chesney, D. Vapnek, M. M. Kropf, and J. R. Scott. 1979. Isolation and characterization of cloned fragments of bacteriophage P1 DNA. Virology 93:387-397. [DOI] [PubMed] [Google Scholar]
- 231.Muramatsu, T., K. Nishikawa, F. Nemoto, Y. Kuchino, S. Nishimura, T. Miyazawa, and S. Yokoyama. 1988. Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature 336:179-181. [DOI] [PubMed] [Google Scholar]
- 232.Muramatsu, T., S. Yokoyama, N. Horie, A. Matsuda, T. Ueda, Z. Yamaizumi, Y. Kuchino, S. Nishimura, and T. Miyazawa. 1988. A novel lysine-substituted nucleoside in the first position of the anticodon of minor isoleucine tRNA from Escherichia coli. J. Biol. Chem. 263:9261-9267. [DOI] [PubMed] [Google Scholar]
- 233.Murphy, L. D., J. L. Rosner, S. B. Zimmerman, and D. Esposito. 1999. Identification of two new proteins in spermidine nucleoids isolated from Escherichia coli. J. Bacteriol. 181:3842-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Nainen, O. 1975. Ph.D. thesis. University of Georgia, Athens.
- 235.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 236.Nureki, O., T. Niimi, T. Muramatsu, H. Kanno, T. Kohno, C. Florentz, R. Giege, and S. Yokoyama. 1994. Molecular recognition of the identity-determinant set of isoleucine transfer RNA from Escherichia coli. J. Mol. Biol. 236:710-724. [DOI] [PubMed] [Google Scholar]
- 237.Ogawa, T. 1975. Analysis of dnaB function of Escherichia coli K12 and the dnaB-like function of P1 prophage. J. Mol. Biol. 94:327-340. [DOI] [PubMed] [Google Scholar]
- 238.Osborne, F. A., S. R. Stovall, and B. R. Baumstark. 1989. The c1 genes of P1 and P7. Nucleic Acids Res. 17:7671-7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pal, S. K., and D. K. Chattoraj. 1988. P1 plasmid replication: initiator sequestration is inadequate to explain control by initiator-binding sites. J. Bacteriol. 170:3554-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Pallanck, L., M. Pak, and H. Schulman. 1995. tRNA discrimination in aminoacylation, p. 371-394. In D. Soll and U. I. RajBhandary (ed.), tRNA: structure, biosynthesis, and function. ASM Press, Washington, D.C.
- 241.Park, K., and D. K. Chattoraj. 2001. DnaA boxes in the P1 plasmid origin: the effect of their position on the directionality of replication and plasmid copy number. J. Mol. Biol. 310:69-81. [DOI] [PubMed] [Google Scholar]
- 242.Parkhill, J., G. Dougan, K. D. James, N. R. Thomson, D. Pickard, J. Wain, C. Churcher, K. L. Mungall, S. D. Bentley, M. T. Holden, M. Sebaihia, S. Baker, D. Basham, K. Brooks, T. Chillingworth, P. Connerton, A. Cronin, P. Davis, R. M. Davies, L. Dowd, N. White, J. Farrar, T. Feltwell, N. Hamlin, A. Haque, T. T. Hien, S. Holroyd, K. Jagels, A. Krogh, T. S. Larsen, S. Leather, S. Moule, P. O'Gaora, C. Parry, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848-852. [DOI] [PubMed] [Google Scholar]
- 243.Patel, P. H., M. Suzuki, E. Adman, A. Shinkai, and L. A. Loeb. 2001. Prokaryotic DNA polymerase I: evolution, structure, and “base flipping” mechanism for nucleotide selection. J. Mol. Biol. 308:823-837. [DOI] [PubMed] [Google Scholar]
- 244.Paul, S., and D. Summers. 2004. ArgR and PepA, accessory proteins for XerCD-mediated resolution of ColE1 dimers, are also required for stable maintenance of the P1 prophage. Plasmid 52:63-68. [DOI] [PubMed] [Google Scholar]
- 245.Pedulla, M. L., M. E. Ford, J. M. Houtz, T. Karthikeyan, C. Wadsworth, J. A. Lewis, D. Jacobs-Sera, J. Falbo, J. Gross, N. R. Pannunzio, W. Brucker, V. Kumar, J. Kandasamy, L. Keenan, S. Bardarov, J. Kriakov, J. G. Lawrence, W. R. Jacobs, Jr., R. W. Hendrix, and G. F. Hatfull. 2003. Origins of highly mosaic mycobacteriophage genomes. Cell 113:171-182. [DOI] [PubMed] [Google Scholar]
- 246.Pierce, J. C., and N. L. Sternberg. 1992. Using bacteriophage P1 system to clone high molecular weight genomic DNA. Methods Enzymol. 216:549-574. [DOI] [PubMed] [Google Scholar]
- 247.Plunkett, G., III, D. J. Rose, T. J. Durfee, and F. R. Blattner. 1999. Sequence of Shiga toxin 2 phage 933W from Escherichia coli O157:H7: Shiga toxin as a phage late-gene product. J. Bacteriol. 181:1767-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Prentki, P., M. Chandler, and L. Caro. 1977. Replication of prophage P1 during the cell cycle of Escherichia coli. Mol. Gen. Genet. 152:71-76. [DOI] [PubMed] [Google Scholar]
- 249.Radnedge, L., M. A. Davis, and S. J. Austin. 1996. P1 and P7 plasmid partition: ParB protein bound to its partition site makes a separate discriminator contact with the DNA that determines species specificity. EMBO J. 15:1155-1162. [PMC free article] [PubMed] [Google Scholar]
- 250.Ramos, A., and G. Varani. 1997. Structure of the acceptor stem of Escherichia coli tRNA Ala: role of the G3.U70 base pair in synthetase recognition. Nucleic Acids Res. 25:2083-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ravin, N. V., J. Rech, and D. Lane. 2003. Mapping of functional domains in F plasmid partition proteins reveals a bipartite SopB-recognition domain in SopA. J. Mol. Biol. 329:875-889. [DOI] [PubMed] [Google Scholar]
- 252.Razza, J. B., C. A. Watkins, and J. R. Scott. 1980. Phage P1 temperature-sensitive mutants with defects in the lytic pathway. Virology 105:52-59. [DOI] [PubMed] [Google Scholar]
- 253.Redaschi, N., and T. A. Bickle. 1996. Posttranscriptional regulation of EcoP1I and EcoP15I restriction activity. J. Mol. Biol. 257:790-803. [DOI] [PubMed] [Google Scholar]
- 254.Reeve, J. N., E. Lanka, and H. Schuster. 1980. Synthesis of P1 ban protein in minicells infected by P1 mutants. Mol. Gen. Genet. 177:193-197. [DOI] [PubMed] [Google Scholar]
- 255.Reisenauer, A., L. S. Kahng, S. McCollum, and L. Shapiro. 1999. Bacterial DNA methylation: a cell cycle regulator? J. Bacteriol. 181:5135-5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Riedel, H. D., J. Heinrich, A. Heisig, T. Choli, and H. Schuster. 1993. The antirepressor of phage P1. Isolation and interaction with the C1 repressor of P1 and P7. FEBS Lett. 334:165-169. [DOI] [PubMed] [Google Scholar]
- 257.Riedel, H. D., J. Heinrich, and H. Schuster. 1993. Cloning, expression, and characterization of the icd gene in the immI operon of bacteriophage P1. J. Bacteriol. 175:2833-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Rodionov, O., M. Łobocka, and M. Yarmolinsky. 1999. Silencing of genes flanking the P1 plasmid centromere. Science 283:546-549. [DOI] [PubMed] [Google Scholar]
- 259.Rodionov, O., and M. Yarmolinsky. 2004. Plasmid partitioning and the spreading of P1 partition protein ParB. Mol. Microbiol. 52:1215-1223. [DOI] [PubMed] [Google Scholar]
- 260.Rodriguez-Lemoine, V. 1992. PacB, a plasmid-encoded property which confers to E. coli K12 resistance to channel-forming colicins. Zentralbl. Bakteriol. 276:374-379. [DOI] [PubMed] [Google Scholar]
- 261.Rosner, J. L. 1972. Formation, induction and curing of bacteriophage Pl lysogens. Virology 48:679-689. [DOI] [PubMed] [Google Scholar]
- 262.Rosner, J. L. 1973. Modification-deficient mutants of bacteriophage P1. I. Restriction by P1 cryptic lysogens. Virology 52:213-222. [DOI] [PubMed] [Google Scholar]
- 263.Ryder, L., G. J. Sharples, and R. G. Lloyd. 1996. Recombination-dependent growth in exonuclease-depleted recBC sbcBC strains of Escherichia coli K-12. Genetics 143:1101-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Salzberg, S. L., A. L. Delcher, S. Kasif, and O. White. 1998. Microbial gene identification using interpolated Markov models. Nucleic Acids Res. 26:544-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Salzberg, S. L., A. J. Salzberg, A. R. Kerlavage, and J. F. Tomb. 1998. Skewed oligomers and origins of replication. Gene 217:57-67. [DOI] [PubMed] [Google Scholar]
- 266.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning. a laboratory manual. Cold Spring Harber Laboratory Press, Cold Spring Harbor, N.Y.
- 267.Sandmeier, H. 1994. Acquisition and rearrangement of sequence motifs in the evolution of bacteriophage tail fibres. Mol. Microbiol. 12:343-350. [DOI] [PubMed] [Google Scholar]
- 268.Sandmeier, H., S. Iida, and W. Arber. 1992. DNA inversion regions Min of plasmid pl5B and Cin of bacteriophage Pl: evolution of bacteriophage tail fiber genes. J. Bacteriol. 174:3936-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sandmeier, H., S. Iida, P. Hübner, R. Hiestand-Nauer, and W. Arber. 1991. Gene organization in the multiple DNA inversion region min of plasmid p15B of E.coli 15T-: assemblage of a variable gene. Nucleic Acids Res. 19:5831-5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Sandmeier, H., S. Iida, J. Meyer, R. Hiestand-Nauer, and W. Arber. 1990. Site-specific DNA recombination system Min of plasmid p15B: a cluster of overlapping invertible DNA segments. Proc. Natl. Acad. Sci. USA 87:1109-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Sauer, B. 1987. Functional expression of the cre-lox site-specific recombination system in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 7:2087-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Schaefer, T., and J. Hays. 1990. The bof gene of bacteriophage P1: DNA sequence and evidence for roles in regulation of phage c1 and ref genes. J. Bacteriol. 172:3269-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Schaefer, T. S., and J. B. Hays. 1991. Bacteriophage Pl Bof protein is an indirect positive effector of transcription of the Pl bac-1 ban gene in some circumstances and a direct negative effector in other circumstances. J. Bacteriol. 173:6469-6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Scherberg, N. H., and S. B. Weiss. 1972. T4 transfer RNAs: codon recognition and translational properties. Proc. Natl. Acad. Sci. USA 69:1114-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Schmidt, C. 1991. Ph.D thesis. University of Basel, Basel, Switzerland.
- 276.Schmidt, C., H. Lehnherr, A. Guidolin, and W. Arber. 1994. GenBank accession no. M95666.
- 277.Schmidt, C., M. Velleman, and W. Arber. 1996. Three functions of bacteriophage P1 involved in cell lysis. J. Bacteriol. 178:1099-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Schuler, G. D., S. F. Altschul, and D. J. Lipman. 1991. A workbench for multiple alignment construction and analysis. Proteins 9:180-190. [DOI] [PubMed] [Google Scholar]
- 279.Scott, J. R. 1970. Clear plaque mutants of phage Pl. Virology 41:66-71. [DOI] [PubMed] [Google Scholar]
- 280.Scott, J. R., M. Kropf, and L. Mendelson. 1977. Clear plaque mutants of phage P7. Virology 76:39-46. [DOI] [PubMed] [Google Scholar]
- 281.Scott, J. R., and M. M. Kropf. 1977. Location of new clear plaque genes on the P1 map. Virology 82:362-368. [DOI] [PubMed] [Google Scholar]
- 282.Segev, N., A. Laub, and G. Cohen. 1980. A circular form of bacteriophage P1 DNA made in lytically infected cells of Escherichia coli. Characterization and kinetics of formation. Virology 101:261-271. [DOI] [PubMed] [Google Scholar]
- 283.Senger, B., S. Auxilien, U. Englisch, F. Cramer, and F. Fasiolo. 1997. The modified wobble base inosine in yeast tRNA-Ile is a positive determinant for aminoacylation by isoleucyl-tRNA synthetase. Biohemistry 36:8269-8275. [DOI] [PubMed] [Google Scholar]
- 284.Sharpe, M. E., and J. Errington. 1996. The Bacillus subtilis soj-spo0J locus is required for a centromere-like function involved in prespore chromosome partitioning. Mol. Microbiol. 21:501-509. [DOI] [PubMed] [Google Scholar]
- 285.Sharrocks, A. D., and D. P. Hornby. 1991. Transcriptional analysis of the restriction and modification genes of bacteriophage P1. Mol. Microbiol. 5:685-694. [DOI] [PubMed] [Google Scholar]
- 286.Skorupski, K., J. C. Pierce, B. Sauer, and N. Sternberg. 1992. Bacteriophage Pl genes involved in the recognition and cleavage of the phage packaging site (pac). J. Mol. Biol. 223:977-989. [DOI] [PubMed] [Google Scholar]
- 287.Skorupski, K., B. Sauer, and N. Sternberg. 1994. Faithful cleavage of the P1 packaging site (pac) requires two phage proteins, PacA and PacB, and two Escherichia coli proteins, IHF and HU. J. Mol. Biol. 243:268-282. [DOI] [PubMed] [Google Scholar]
- 288.Skorupski, K., N. Sternberg, and B. Sauer. 1994. Purification and DNA-binding activity of the PacA subunit of the bacteriophage P1 pacase enzyme. J. Mol. Biol. 243:258-267. [DOI] [PubMed] [Google Scholar]
- 289.Slater, S. C., M. R. Lifsics, M. O'Donnell, and R. Maurer. 1994. holE, the gene coding for the theta subunit of DNA polymerase III of Escherichia coli: characterization of a holE mutant and comparison with a dnaQ (epsilon-subunit) mutant. J. Bacteriol. 176:815-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Sommer, S., J. Knezevic, A. Bailone, and R. Devoret. 1993. Induction of only one SOS operon, umuDC, is required for SOS mutagenesis in Escherichia coli. Mol. Gen. Genet. 239:137-144. [DOI] [PubMed] [Google Scholar]
- 291.Sourice, S., V. Biaudet, M. El Karoui, S. D. Ehrlich, and A. Gruss. 1998. Identification of the Chi site of Haemophilus influenzae as several sequences related to the Escherichia coli Chi site. Mol. Microbiol. 27:1021-1029. [DOI] [PubMed] [Google Scholar]
- 292.Steiner, W., G. Liu, W. D. Donachie, and P. Kuempel. 1999. The cytoplasmic domain of FtsK protein is required for resolution of chromosome dimers. Mol. Microbiol. 31:579-583. [DOI] [PubMed] [Google Scholar]
- 293.Sternberg, N. 1990. Bacteriophage P1 cloning system for the isolation, amplification, and recovery of DNA fragments as large as 100 kilobase pairs. Proc. Natl. Acad. Sci. USA 87:103-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sternberg, N. 1979. A characterization of bacteriophage P1 DNA fragments cloned in a lambda vector. Virology 96:129-142. [DOI] [PubMed] [Google Scholar]
- 295.Sternberg, N., and G. Cohen. 1989. Genetic analysis of the lytic replicon of bacteriophage Pl. II. Organization of replicon elements. J. Mol. Biol. 207:111-133. [DOI] [PubMed] [Google Scholar]
- 296.Sternberg, N., and J. Coulby. 1990. Cleavage of the bacteriophage P1 packaging site (pac) is regulated by adenine methylation. Proc. Natl. Acad. Sci. USA 87:8070-8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Sternberg, N., and J. Coulby. 1987. Recognition and cleavage of the bacteriophage Pl packaging site (pac) I. Differential processing of the cleaved ends in vivo. J. Mol. Biol. 194:453-468. [DOI] [PubMed] [Google Scholar]
- 298.Sternberg, N., and J. Coulby. 1987. Recognition and cleavage of the bacteriophage Pl packaging site (pac) II. Functional limits of pac and location of pac cleavage termini. J. Mol. Biol. 194:469-480. [DOI] [PubMed] [Google Scholar]
- 299.Sternberg, N., D. Hamilton, S. Austin, M. Yarmolinsky, and R. Hoess. 1981. Site-specific recombination and its role in the life cycle of bacteriophage P1. Cold Spring Harbor Symp. Quant. Biol. 45:297-309. [DOI] [PubMed] [Google Scholar]
- 300.Sternberg, N., D. Hamilton, and R. Hoess. 1981. Bacteriophage P1 site-specific recombination. II. Recombination between loxP and the bacterial chromosome. J. Mol. Biol. 150:487-507. [DOI] [PubMed] [Google Scholar]
- 301.Sternberg, N., and R. Hoess. 1983. The molecular genetics of bacteriophage P1. Annu. Rev. Genet. 17:123-154. [DOI] [PubMed] [Google Scholar]
- 302.Sternberg, N., B. Sauer, R. Hoess, and K. Abremski. 1986. Bacteriophage Pl cre structural gene and its regulatory region. Evidence for multiple promoters and for regulation by DNA methylation. J. Mol. Biol. 187:197-212. [DOI] [PubMed] [Google Scholar]
- 303.Sternberg, N. L., and R. Maurer. 1991. Bacteriophage-mediated generalized transduction in Escherichia coli and Salmonella typhimurium. Methods Enzymol. 204:18-43. [DOI] [PubMed] [Google Scholar]
- 304.Streiff, M. B., S. Iida, and T. A. Bickle. 1987. Expression and proteolytic processing of the darA anti-restriction gene product of bacteriophage P1. Virology 157:167-171. [DOI] [PubMed] [Google Scholar]
- 305.Studwell-Vaughan, P. S., and M. O'Donnell. 1993. DNA polymerase III accessory proteins. V. Theta encoded by holE. J. Biol. Chem. 268:11785-11791. [PubMed] [Google Scholar]
- 306.Surtees, J. A., and B. E. Funnell. 2003. Plasmid and chromosome traffic control: how ParA and ParB drive partition. Curr. Top. Dev. Biol. 56:145-180. [DOI] [PubMed] [Google Scholar]
- 307.Susskind, M. M., A. Wright, and D. Botstein. 1971. Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. II. Genetic evidence for two exclusion systems. Virology 45:638-652. [DOI] [PubMed] [Google Scholar]
- 308.Tamura, K., H. Asahara, H. Himeno, T. Hasegawa, and M. Shimizu. 1991. Identity elements of Escherichia coli tRNA(Ala). J. Mol. Recognit. 4:129-132. [DOI] [PubMed] [Google Scholar]
- 309.Tang, M., X. Shen, E. G. Frank, M. O'Donnell, R. Woodgate, and M. F. Goodman. 1999. UmuD′(2)C is an error-prone DNA polymerase, Escherichia coli pol V. Proc. Natl. Acad. Sci. USA 96:8919-8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Touati-Schwartz, D. 1979. A dnaB analog ban, specified by bacteriophage P1: genetic and physiological evidence for functional analogy and interactions between the two products. Mol. Gen. Genet. 174:173-188. [DOI] [PubMed] [Google Scholar]
- 311.Touati-Schwartz, D. 1979. A new pleiotropic bacteriophage P1 mutation, bof, affecting c1 repression activity, the expression of plasmid incompatibility and the expression of certain constitutive prophage genes. Mol. Gen. Genet. 174:189-202. [DOI] [PubMed] [Google Scholar]
- 312.Touati-Schwartz, D. 1978. Two replication functions in phage P1: ban, an analog of dnaB, and bof, involved in the control of replication., p. 683-691. In I. Molineaux, and M. Kohiyama (ed.), DNA synthesis: present and future, vol. 17. Plenum Press, New York, N.Y. [Google Scholar]
- 313.Tuggle, C. K., and J. A. Fuchs. 1986. Regulation of the synthesis of ribonucleotide reductase in Escherichia coli., p. 227-232. In C.-I. Brandon, A. Holmgren, J.-O. Hoag, H. Jornvall, and B.-M. Sjoberg (ed.), Thioredoxin and glutaredoxin systems: structure and function. Raven Press, New York, N.Y.
- 314.Tuma, R., and G. J. Thomas, Jr. 1997. Mechanisms of virus assembly probed by Raman spectroscopy: the icosahedral bacteriophage P22. Biophys. Chem. 68:17-31. [DOI] [PubMed] [Google Scholar]
- 315.Velleman, M. 1989. PhD thesis. Freie Universitat Berlin, Berlin, Germany.
- 316.Velleman, M., B. Dreisekelmann, and H. Schuster. 1987. Multiple repressor binding sites in the genome of bacteriophage Pl. Proc. Natl. Acad. Sci. USA 84:5570-5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Velleman, M., T. Heinzel, and H. Schuster. 1992. The Bof protein of bacteriophage Pl exerts its modulating function by formation of a ternary complex with operator DNA and Cl repressor. J. Biol. Chem. 267:12174-12181. [PubMed] [Google Scholar]
- 318.Velleman, M., M. Heirich, A. Günther, and H. Schuster. 1990. A bacteriophage P1-encoded modulator protein affects the P1 c1 repression system. J. Mol. Biol. 265:18511-18517. [PubMed] [Google Scholar]
- 319.Velleman, M., and S. Parbus. 1992. Purification of the C1 repressor of bacteriophage P1 by fast protein liquid chromatography. J. Chromatogr. 625:41-46. [DOI] [PubMed] [Google Scholar]
- 320.Voegtli, W. C., D. J. White, N. J. Reiter, F. Rusnak, and A. C. Rosenzweig. 2000. Structure of the bacteriophage lambda Ser/Thr protein phosphatase with sulfate ion bound in two coordination modes. Biochemistry 39:15365-15374. [DOI] [PubMed] [Google Scholar]
- 321.Walker, D. H., Jr., and J. T. Walker. 1976. Genetic studies of coliphage P1. III. Extended genetic map. J. Virol. 20:177-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Walker, D. H. J., and T. F. Anderson. 1970. Morphological variants of coliphage P1. J. Virol. 5:765-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Walker, J. T., and D. H. J. Walker. 1983. Coliphage P1 morphogenesis: analysis of mutants by electron microscopy. J. Bacteriol. 45:1118-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Walker, J. T., and D. H. J. Walker. 1980. Mutations in coliphage P1 affecting host cell lysis. J. Virol. 35:519-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Walker, J. T., and D. H. J. Walker. 1981. Structural proteins of coliphage P1. Prog. Clin. Biol. Res. 64:69-77. [PubMed] [Google Scholar]
- 326.Wandersman, C., and M. Yarmolinsky. 1977. Bipartite control of immunity conferred by the related heteroimmune plasmid prophages, P1 and P7 (formerly phi Amp). Virology 77:386-400. [DOI] [PubMed] [Google Scholar]
- 327.Wang, I. N., D. L. Smith, and R. Young. 2000. Holins: the protein clocks of bacteriophage infections. Annu. Rev. Microbiol. 54:799-825. [DOI] [PubMed] [Google Scholar]
- 328.Wang, L. K., and S. Shuman. 2001. Domain structure and mutational analysis of T4 polynucleotide kinase. J. Biol. Chem. 276:26868-26874. [DOI] [PubMed] [Google Scholar]
- 329.Watts, N. R., and D. H. Coombs. 1990. Structure of the bacteriophage T4 baseplate as determined by chemical cross-linking. J. Virol. 64:143-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Weisberg, R. A., and M. E. Gottesman. 1999. Processive antitermination. J. Bacteriol. 181:359-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Whelan, K. F., E. Colleran, and D. E. Taylor. 1995. Phage inhibition, colicin resistance, and tellurite resistance are encoded by a single cluster of genes on the IncHI2 plasmid R478. J. Bacteriol. 177:5016-5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Whelan, K. F., R. K. Sherburne, and D. E. Taylor. 1997. Characterization of a region of the IncHI2 plasmid R478 which protects Escherichia coli from toxic effects specified by components of the tellurite, phage, and colicin resistance cluster. J. Bacteriol. 179:63-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Wickner, S., J. Hoskins, D. Chattoraj, and K. McKenney. 1990. Deletion analysis of the mini-P1 plasmid origin of replication and the role of Escherichia coli DnaA protein. J. Biol. Chem. 265:11622-11627. [PubMed] [Google Scholar]
- 334.Wickner, S., J. Hoskins, and K. McKenney. 1991. Monomerization of RepA dimers by heat shock proteins activates binding to DNA replication origin. Proc Natl Acad Sci USA 88:7903-7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Williams, D. R., D. P. Macartney, and C. M. Thomas. 1998. The partitioning activity of the RK2 central control region requires only incC, korB and KorB-binding site O(B)3 but other KorB-binding sites form destabilizing complexes in the absence of O(B)3. Microbiology 144:3369-3378. [DOI] [PubMed] [Google Scholar]
- 336.Williams, M. D., J. A. Fuchs, and M. C. Flickinger. 1991. Null mutation in the stringent starvation protein of Escherichia coli disrupts lytic development of bacteriophage P1. Gene 109:21-30. [DOI] [PubMed] [Google Scholar]
- 337.Windle, B. E., and J. B. Hays. 1986. A phage P1 function that stimulates homologous recombination of the Escherichia coli chromosome. Proc. Natl. Acad. Sci. USA 83:3885-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Windle, B. E., C. S. Laufer, and J. B. Hays. 1988. Sequence and deletion analysis of the recombination enhancement gene (ref) of bacteriophage P1: evidence for promoter-operator and attenuator-antiterminator control. J. Bacteriol. 170:4881-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Wood, L. F., N. Y. Tszine, and G. E. Christie. 1997. Activation of P2 late transcription by P2 Ogr protein requires a discrete contact site on the C terminus of the alpha subunit of Escherichia coli RNA polymerase. J. Mol. Biol. 274:1-7. [DOI] [PubMed] [Google Scholar]
- 340.Xu, G. L., W. Kapfer, J. Walter, and T. A. Trautner. 1992. BsuBI-an isospecific restriction and modification system of PstI: characterization of the BsuBI genes and enzymes. Nucleic Acids Res. 20:6517-6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Xu, M., D. K. Struck, J. Deaton, I. N. Wang, and R. Young. 2004. A signal-arrest-release sequence mediates export and control of the phage P1 endolysin. Proc. Natl. Acad. Sci. USA 101:6415-6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Xue, Q., and J. B. Egan. 1995. DNA sequence of tail fiber genes of coliphage 186 and evidence for a common ancestor shared by dsDNA phage fiber genes. Virology 212:128-133. [DOI] [PubMed] [Google Scholar]
- 343.Yamaichi, Y., and H. Niki. 2000. Active segregation by the Bacillus subtilis partitioning system in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:14656-14661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343a.Yarmolinsky, M. B. 2004. Bacteriophage P1 in retrospect and in prospect. J. Bacteriol. 186:7032-7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Yarmolinsky, M. 1990. Bacteriophage P1, p. 1.52-1.58. In S. J. O'Brien (ed.), Genetic maps. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 345.Yarmolinsky, M. 1977. Genetic and physical structure of bacteriophage P1 DNA, p. 721-732. In I. A. Bukhari, J. A. Shapiro, and S. L. Adhya (ed.), DNA insertion elements, plasmids, and episomes. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 346.Yarmolinsky, M., and M. £obocka. 1993. Bacteriophage Pl, p. 42-54. In S. J. O'Brien (ed.), Locus maps of complex genomes., vol. 3. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y. [Google Scholar]
- 347.Yarmolinsky, M., and N. Sternberg. 1988. Bacteriophage Pl, p. 291-438. In R. Calendar (ed.), The Bacteriophages, vol. 1. Plenum Press, New York, N.Y. [Google Scholar]
- 348.Yarmolinsky, M. B. 1995. Programmed cell death in bacterial populations. Science 267:836-837. [DOI] [PubMed] [Google Scholar]
- 349.Yarmolinsky, M. B., E. B. Hansen, S. Jafri, and D. K. Chattoraj. 1989. Participation of the lytic replicon in bacteriophage P1 plasmid maintenance. J. Bacteriol. 171:4785-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Yoo, S. J., J. H. Seol, M. S. Kang, D. B. Ha, and C. H. Chung. 1994. clpX encoding an alternative ATP-binding subunit of protease Ti (Clp) can be expressed independently from clpP in Escherichia coli. Biochem. Biophys. Res. Commun. 203:798-804. [DOI] [PubMed] [Google Scholar]
- 351.Young, R. 1992. Bacteriophage lysis: mechanism and regulation. Microbiol. Rev. 56:430-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Zabrovitz, S., N. Segev, and G. Cohen. 1977. Growth of bacteriophage P1 in recombination-deficient hosts of Escherichia coli. Virology 80:233-248. [DOI] [PubMed] [Google Scholar]
- 353.Zdanovsky, A. G., and M. V. Zdanovskaia. 2000. Simple and efficient method for heterologous expression of clostridial proteins. Appl. Environ. Microbiol. 66:3166-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Zhang, Z., A. A. Schaffer, W. Miller, T. L. Madden, D. J. Lipman, E. V. Koonin, and S. F. Altschul. 1998. Protein sequence similarity searches using patterns as seeds. Nucleic Acids Res. 26:3986-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.