Summary
Non-invasive mapping of brain functional connectivity (FC) has played a fundamental role in neuroscience, and numerous scientists have been fascinated by its ability to reveal the brain’s intricate morphology and functional properties. In recent years, two different techniques have been developed that are able to explore FC in pathophysiological conditions and to provide simple and non-invasive biomarkers for the detection of disease onset, severity and progression. These techniques are independent component analysis, which allows a network-based functional exploration of the brain, and graph theory, which provides a quantitative characterization of the whole-brain FC. In this paper we provide an overview of these two techniques and some examples of their clinical applications in the most common neurodegenerative disorders associated with cognitive decline, including mild cognitive impairment, Alzheimer’s disease, Parkinson’s disease, dementia with Lewy Bodies and behavioral variant frontotemporal dementia.
Keywords: brain functional connectivity, graph theoretical methods, independent component analysis, neurodegenerative disorders, resting state networks
Introduction
The human brain is probably the most complex organ to study and comprehend. Its neuronal mechanisms are able to produce movement, perception, thought, speech, learning and emotions. Non-invasive mapping of brain functional connectivity (FC) has played a fundamental role in neuroscience research, and numerous scientists have been fascinated by its ability to reveal the brain’s intricate morphology and functional properties. Furthermore, the complex brain disruption phenomena that occur in disease are the focus of a growing body of research.
Brain connectivity is a concept that embraces several different and interrelated aspects of brain organization (Horwitz, 2003) and can refer to anatomical links such as fiber pathways (anatomical connectivity) (Sporns, 2013), statistical relationships between dynamics occurring in different locations in the brain (functional connectivity, FC) (Smith, 2012), or causal interactions (effective connectivity) (Friston, 2011; Valdes-Sosa et al., 2011). It is investigated, mainly by means of in vivo and non-invasive neuroimaging techniques, at both microscopic and macroscopic levels, from that of neuronal populations to that of the anatomically segregated brain regions within the nervous system (Sporns et al., 2005).
This review focuses, in particular, on brain FC investigated by means of resting state functional MRI (rfMRI), which is currently the most widely used method for studying brain activity during rest. The rfMRI technique is based on the acquisition of T2*-sensitive MR images that record the low-frequency (<0.1 Hz) blood oxygen level-dependent (BOLD) signal fluctuations of the measured cerebral hemodynamic. The BOLD signal, recorded while subjects lie idle in the MRI scanner, is believed to characterize the neuronal baseline activity of the human brain in the absence of external stimuli. In this condition, interregional correlations of these fluctuations can be estimated, and these quantitative estimates provide measures of FC (Friston et al., 1993), defined as the temporal relationship between neuronal activation patterns of distinct and anatomically distant brain regions. FC is typically expressed through measures of correlation, covariance, or spectral coherence among gray matter voxels or regions and it reflects statistical dependencies between them (Lang et al., 2012).
The simplest method for studying FC between gray matter regions is to extract the signal from a voxel or a region of interest (ROI) and correlate it with the other gray matter voxels. The result is a correlation map spanning the whole brain (Smith et al., 2013): brain regions showing a high degree of positive correlation with the seed are assumed to play an integrative role in combining neural activity and are said to be functionally coupled with it; regions that are negatively correlated with each other are thought to belong to networks that serve opposite goals (Fox et al., 2005). FC was first investigated in rfMRI studies by means of seed-based analysis, using either a sphere centered in a ROI or an anatomical mask to extract the average BOLD signal of the ROI and correlate it with the time series of all the other voxels. Seed-based analysis has also been used to investigate multiple ROIs within spatially distributed, large-scale networks (Fox et al., 2005), called resting state networks (RSNs), which slowly activate and deactivate even in the absence of tasks or stimuli. Although this method has proven to be reliable, easily interpretable and effective in identifying the regions that are most strongly functionally connected with the seed, some weaknesses might significantly affect its results, such as its strong dependency on the seed choice (Cole et al., 2010). In fact, seeds are typically chosen based on the location of activity during a task (Biswal et al., 1995), using standardized coordinates or anatomical images as a guide (Di Martino et al., 2008). However, the anatomy of the selected regions is likely to be subject-dependent and might change depending on the presence of neurological diseases or with aging. Hence, this approach might lead to the inclusion of undesired voxels within the selected ROI, or the exclusion of functionally relevant voxels. Another limitation is the impossibility of simultaneously investigating different ROIs or networks. In order to overcome the limitations of this approach, two different directions have been pursued. The first is to use data-reduction approaches such as independent component analysis (ICA), which explores FC by decomposing the signal in each voxel into a number of components, each assigned to a specific functional network. The second is to use graph-based methods, which, by using high level graph theoretical metrics, provide a quantitative characterization of the whole-brain FC.
This review provides an overview of these two techniques, examining their advantages and disadvantages and providing some examples of their clinical applications in the most common neurodegenerative disorders associated with cognitive decline, including mild cognitive impairment, Alzheimer’s disease, Parkinson’s disease, dementia with Lewy bodies, and behavioral variant frontotemporal dementia. rfMRI is particularly attractive for this area of study, as it requires minimal patient compliance. This review is not intended to be comprehensive, but rather to illustrate some prototypical studies in which these techniques were applied.
Methods of analysis
Network-based connectivity: independent component analysis
Independent component analysis (ICA) is a data-driven approach able to decompose a signal into a number of independent sources. The method was first applied to rfMRI data by McKeown et al. (1998) to look for FC patterns across brain regions (Smith et al., 2009; Beckmann and Smith, 2004). ICA attempts to discover the underlying and maximally independent source signals only from the measured observations rather than imposing any a priori knowledge (Beckmann and Smith, 2004); in this sense it is thought of as model free. The spatial independence of the independent components obtained with the ICA approach is accepted on the basis of the assumption that brain areas that respond to a particular task are distributed independently of brain areas affected by other sources of variability (Beckmann, 2012). This independence does not require that areas belonging to different networks be completely non-overlapping, only that other sources of signal change are not distributed in the same way. This means that knowledge about the spatial distribution of a certain component does not provide any information about the spatial distribution of the others (Beckmann, 2012).
Both at single-subject level and at group level, one of the main advantages of ICA is its ability to simultaneously extract a variety of different coherent RSNs and noise sources, such as those induced by head motion, physiological confounds (e.g. cardiac pulsation or respiratory cycle) and scanner-related noise (Damoiseaux et al., 2006). For this reason, RSNs identified by ICA can be less prone to artifactual effects than those identified using other techniques due to the ability of this method to account for the existence of such structured noise effects within non-RSN ICA components (Cole et al., 2010).
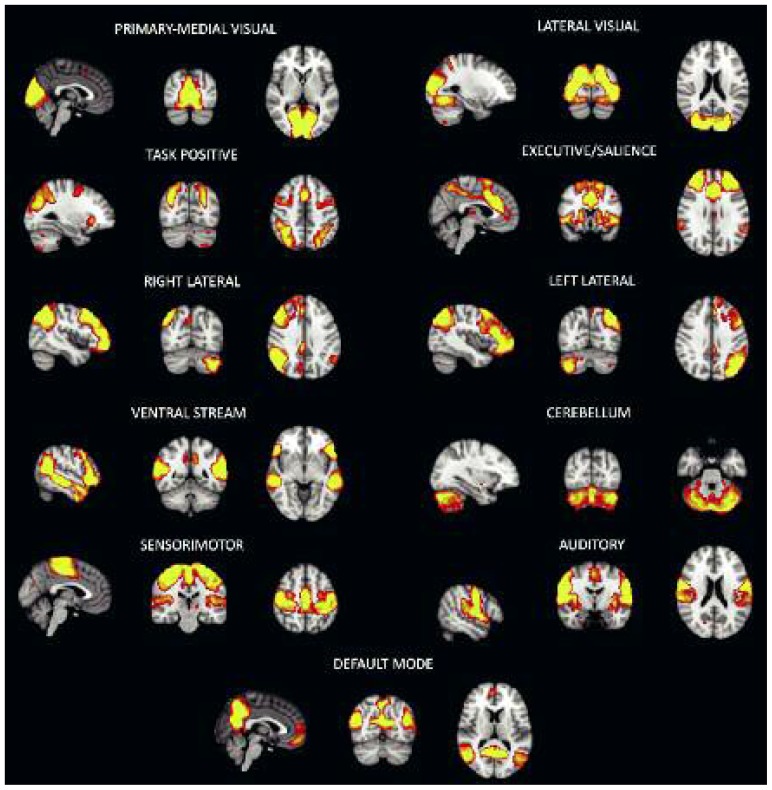
RSNs map a large variety of functional circuits, including the motor network found in the first resting-state connectivity experiment (Biswal et al., 1995), sensory systems in visual and auditory cortices, and, of particular interest for studying neurodegenerative or psychiatric disorders, networks involved in higher-level cognitive processes (Smith et al., 2009; Beckmann, 2012; Biswal et al., 1995). Figure 1 shows some of the most common and most widely investigated RSNs extracted by ICA, including the primary-medial and lateral visual networks; the executive/salience network, implicated in executive control and salience processing; the right- and left-lateralized frontoparietal networks; the ventral network; the cerebellum; the sensori-motor network (SMN); the auditory network, which involves auditory and other sensory association cortices; and the default mode network (DMN).
Figure 1.
Some of the main and more investigated resting state networks identified by independent component analysis.
This functional specificity is another advantage of ICA, especially in the clinical context, as it makes it possible to selectively focus on one or more RSNs thought to be selectively affected by a certain neurological condition or disease (Filippini et al., 2009; Greicius et al., 2004). Many studies have taken advantage of this technique to look for potential biomarkers for the diagnosis and study of disease mechanisms. For example, one of the most widely studied RSNs, due to its importance in many pathologies, is the DMN, involved in episodic memory processes and self-referential mental representations. The DMN includes the precuneus and the posterior cingulate cortex, as well as more frontal regions like the medial frontal cortex and the inferior parietal regions (Damoiseaux et al., 2006).
However, this approach also has some limitations. First, it can be difficult to determine whether a component represents a true BOLD source or noise. Second, decomposition results can vary depending on the model order choice, and reproducible patterns of RSNs are rarely found in single-subject datasets. For these reasons, it is necessary to define reference patterns at group level in the first instance, and then to extract subject-specific features by using dual regression (Filippini et al., 2009), back projection (Calhoun et al., 2001), or similar approaches (Cole et al., 2010). Moreover, there is no gold standard for model order selection at group level. In fact, the model order can be chosen manually by the operator or estimated using a sophisticated statistical criterion based on the Laplace approximation to the Bayesian evidence of the model order (Beckmann and Smith, 2004). However, a wrong model order choice can generate under-fitting, which means that the amount of explained data variance is insufficient to obtain good estimates of the signals, or over-fitting, which leads to a fragmentation of signals across multiple component maps, reducing the ability to identify the signals of interest.
An important limitation is that the ICA decomposition of a given dataset into a set of 20–30 components, of which approximately 8–13 are RSNs and the others non-recognizable networks or noise, overlooks the fact that any given brain region may share different connectivity patterns with multiple networks over time. This variability of regional co-activations between network nodes can be referred to as the “non-stationarity” of a given area in terms of its connectivity with one or more RSNs (Cole et al., 2010). Interestingly, this limit of the ICA approach can be overcome by RSN parcellation into subnetworks through high-dimensional ICA (Smith et al., 2015; Dipasquale et al., 2015). Specifically, high-dimensional ICA is performed at group level by extracting a greater number of independent components compared to the standard procedure (typically 70 or more vs 20–30 in low-dimensional ICA), in order to force the algorithm to decompose the traditional RSNs into sub-networks. This technique is particularly advantageous to investigate possible FC alterations that do not involve the whole network, e.g. in the early stages of pathology when the damage is confined to specific areas of the network. However, for a standard dataset (i.e. non-high-quality rfMRI data and a low number of subjects), high-dimensional ICA with a model order of 150–200 is not a valid option due to the intrinsic constraints of this method (Abou-Elseoud et al., 2010). Moreover, classification of the sub-networks thus obtained is more challenging than classification performed at low dimensionality as the sub-networks might not exhibit their well known spatial patterns, and for this reason it may be necessary to use specific algorithms which classify each component by comparing it in time and in space with the RSNs obtained with low-dimensional ICA performed on the same dataset (Dipasquale et al., 2015).
Whole-brain connectomics: graph theoretical methods
The subdivision of brain activity patterns into functional networks is extremely useful to investigate specific functions and study the alterations occurring in pathological conditions. Nevertheless, the brain can be described not only as a set of functional networks with specific roles, but also as one integrative circuit comprising all the brain regions and forming a complex functional system (de Reus and van den Heuvel, 2013). The ICA approach can generally overlook this functional configuration given its assumption of independence between the components (Lee et al., 2016). However, new advances in resting-state analysis techniques have opened up the possibility of examining the overall functional connectome using graph theoretical methods. Application of these methods to rfMRI data consists of modeling cortical and sub-cortical regions as a set of nodes connected to each other by edges that express the strength of the FC between node pairs. This information can easily be represented as an NxN matrix (connectivity matrix), where N is the number of nodes, and each element of the matrix corresponds to the pairwise measure of connectivity.
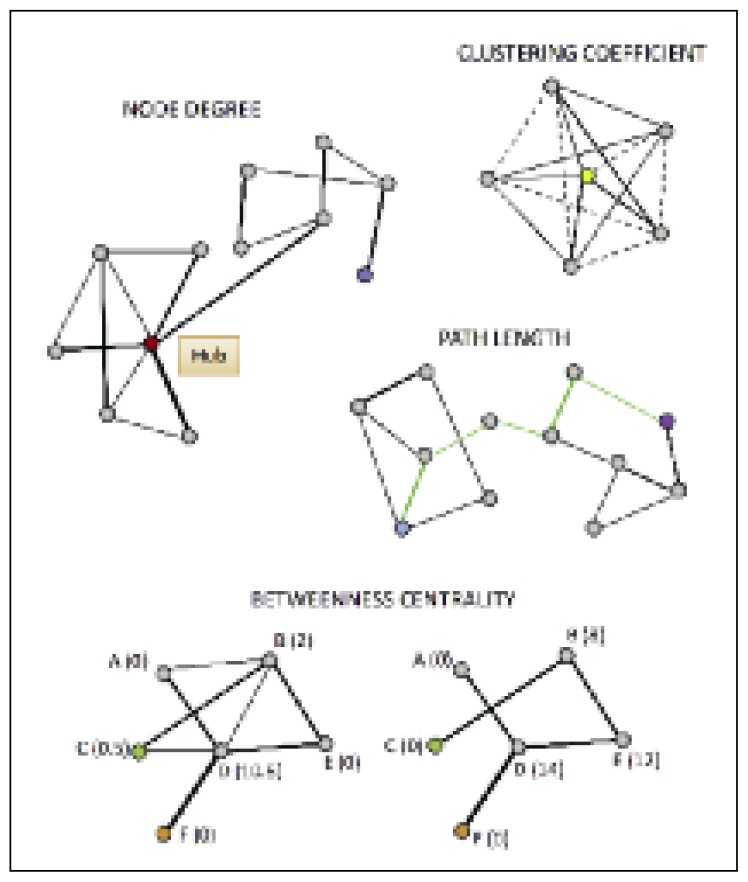
The increasing use of graph theory approaches to study brain connectivity has led neuroscientists to hypothesize that the brain shows an efficient “small-world” functional architecture (Achard et al., 2006); i.e. nodes have greater local interconnections (edges) than would be expected for a random network, and smaller minimum path lengths between node pairs than regular or lattice type networks have (Watts and Strogatz, 1998). This functional architecture affords a number of substantial benefits: it reduces wiring cost and ensures a high degree of robustness, i.e. preservation of network integrity following random damage to a node or a connection. Such networks are also characterized by a smaller number of highly connected nodes, called hubs, and “high centrality” nodes that provide the shortest connection paths between many other node pairs. These nodes are crucial to efficient communication (van den Heuvel et al., 2008) but also vulnerable to targeted insults that can result in a rapid reduction in the network efficiency and whole-brain connectivity. In order to study these connectional characteristics, so-called graph metrics are typically used. These are higher-level indices that make it possible to investigate the topology of the whole-brain network (Bullmore and Sporns, 2009; Rubinov and Sporns, 2010; van den Heuvel and Hulshoff Pol, 2010; van den Heuvel et al., 2008). Examples of measures of interest include the node degree, a measure of the number of connections (edges) of each node with the other network nodes; the average path length, a measure of global connectedness which indicates the average shortest connection length (i.e. the mean of the shortest path length values recorded for all the single node pairs); the clustering coefficient, which measures the rate of existing edges between the nearest neighbors versus possible connections and reflects the presence of smaller subgraphs; the betweenness centrality, which measures the number of shortest paths that pass through a node and indicates the importance of a node for efficient communication and integration across a network; efficiency, which indicates the information transfer between nodes; in particular, the local efficiency of an individual node is the inverse of the shortest path length connecting all the neighbors of that node, while global efficiency is the average of the local efficiency and includes all the nodes of the graph (Fig. 2).
Figure 2.
Graphical representation of some graph indices.
Node degree: the red dot (node) represents a high degree node and it is directly connected with six out of 11 nodes; the blue dot represents a low degree node and it is directly connected with only one node. Clustering coefficient: quantification of the number of connections that exist between the nearest neighbors of the yellow node (continuous lines) as a proportion of the maximum number of its possible connections (dotted lines). Path length: the shortest connection between the light-blue and the purple nodes corresponds to the path length and is highlighted by green edges. Betweenness centrality: on the left, nodes A, B, C, D, E and F are well connected and maintain efficient network communication. Numbers in parentheses refer to each node’s betweenness centrality, which indicates how many of the shortest paths between all other node pairs in the network pass through it. To reach node C (green dot) from node F (orange dot), information flow is efficient and passes only through D. In the graph on the right, some connections have been lost. To reach C from F, information now has to go through more nodes (D, E and B).
Contrary to traditional low-dimensional ICA (20–30 components extracted), by studying the brain as a whole it is possible to take into account the fact that each area can be functionally connected not only with the regions within the same RSN, but also with other areas. However, a challenge in this approach is the requirement for a parcellation of the gray matter into a set of ROIs (corresponding to the nodes), which must be performed a priori. While a number of methods for data-driven parcellation based on either anatomical or functional criteria have been proposed (Glasser et al., 2016), the most common approaches are based either on the use of a priori ROIs from predefined anatomical templates (e.g. the Harvard-Oxford atlas and the Automated Anatomical Labeling atlas), or on randomly generated templates and voxel-based divisions. The choice of the nodes has a non-negligible impact on the estimation of the topological and spatial features of the brain network (de Reus and van den Heuvel, 2013; Wang et al., 2009; Minati et al., 2013). It is worth noting that even if the different parcellation schemes cover the same gray matter structures, differences between them could exist, such as the number of extracted brain regions or the fact that some areas might belong to the same ROI in a certain atlas but to different ROIs in another (de Reus and van den Heuvel, 2013). Hence, these differences inevitably affect the comparability of published studies based on different parcellation schemes.
Another problem with graph theory approaches is the choice of the threshold for FC matrices, which tends to be quite arbitrary. In order to minimize the inter-subject variability, typically, FC matrices are not thresholded using a specific FC value, but using the wiring cost (K) (also known as “density”), which corresponds to the number of existing edges over the maximum possible number of edges (Achard and Bullmore, 2007). However, the choice of K can be equally arbitrary and bias the results, thus different K values are typically used to reduce this bias and boost the robustness of the analysis (Liu et al., 2012).
Clinical applications
The studies included in this section are also listed in table I, where they are classified by type of pathology. For each study, we have specified the number of subjects included, the method used to perform the FC analysis and the main findings.
Table 1.
Studies examining functional connectivity alterations in neurodegenerative conditions by means of network-based or graph theoretical approaches and summary of the main results.
| Authors | Subjects | Method | Main findings |
|---|---|---|---|
| Mild cognitive impairment and Alzheimer’s disease | |||
| Greicius et al. 2004 | 13 mild AD, 13 HCs | ICA | Decreased activity in the posterior cingulate and hippocampus in AD |
| Sorg et al. 2007 | 24 aMCI and 16 HCs | ICA | Only selected areas of the DMN and the executive attention network demonstrated reduced network-related activity in aMCI. FC between both hippocampi and the posterior cingulate cortex of the DMN was present in healthy controls but absent in patients. |
| Sanz-Arigita et al. 2010 | 18 mild AD and 21 HCs | Graph theory | Path length, used as global network measures, showed significant changes in global brain FC in AD specifically affecting long-distance connectivity. These results reflect the randomization of the brain functional networks in AD, suggesting a loss of global information integration in disease. |
| Gili et al. 2011 | 10 aMCI, 11 AD and 10 HCs | ICA | AD and aMCI patients showed a similar brain disconnection between the posterior cingulate cortex and the medial prefrontal cortex and the rest of the brain; aMCI patients also showed a reduced connectivity in the posterior cingulate cortex in the absence of GM atrophy, which was, by contrast, detectable at the stage of fully developed AD. |
| Damoiseaux et al. 2012 | 21 AD and 18 HCs at baseline; 9 AD and 10 HCs at follow-up | ICA | Decreased connectivity in AD in the posterior DMN and increased connectivity in the anterior and ventral DMNs. At follow-up, FC decreased across all DMNs in AD patients. |
| Dipasquale et al. 2015 | 21 AD and 20 HCs | ICA | Within-network FC alteration (AD<HCs) in the anterior and posterior DMNs and in the sensorimotor network. |
| Bozzali et al. 2015 | 11 AD, 18 MCI and 16 HCs | ICA | A strong association was detected between patients’ education level and FC within the PCC: the effect was highly significant in AD patients, less significant in patients with MCI. No correlation was found in HCs. |
| Serra et al. 2016 | 31 aMCI (14 AD converters and 17 non-converters after 2 years) and 26 HCs | ICA | Discriminant analysis revealed that FC of the precuneus within the DMN at baseline is the parameter able to correctly classify patients as converters and non-converters. |
| Minati et al. 2014 | 49 aMCI and 32 HCs | ICA and graph theory | ICA: Decreased connectivity only for the DMN component in the medial parietal region, precuneus and posterior cingulate cortex. Graph theory: aMCI patients were consistently characterized by decreased network completeness and clustering coefficient, reduced global network efficiency and node degree. This widespread disconnection was observed primarily in some cortical hubs, i.e. precuneus, parietal lobules, supramarginal and angular gyri, and cuneus, with additional involvement of subcortical regions, sensorimotor cortex and insula. |
| Zhao et al. 2012 | 33 moderate AD and 20 HCs | Graph theory | Increased local efficiency and decreased global efficiency were found in AD, mainly located in the DMN, temporal lobe and certain subcortical regions associated with the neuropathological changes in AD; the authors also showed that the ApoE genotype modulates brain network properties, especially in AD patients. |
| Challis et al. 2015 | 27 AD, 50 aMCI and 39 HCs | Graph theory | The single elements of the connectivity metrics were used as features for a machine-learning algorithm to perform patient stratification between HCs, aMCI and AD. The model achieves 75% accuracy disambiguating HC from aMCI and 97% accuracy disambiguating aMCI from AD. |
| Parkinson’s disease | |||
| Szewczyk-Krolikowski et al. 2014 | 32 PD on and off medication and 19 HCs | ICA | PD showed reduced FC within the basal ganglia network in a wide range of areas. Medication significantly improved connectivity. |
| Putcha et al. 2015 | 20 PD and 20 HCs | ICA | PD showed significantly less coupling between salience network and executive network and greater coupling between DMN and executive network. Disease severity was also related to reduced functional coupling between the striatum and salience network. |
| Karunanayaka et al. 2016 | 17 PD with primary akinetic/rigidity (PDAR), 15 PD with tremor-predominant symptoms (PDT) and 24 HCs | ICA and the left posterior cingulate cortex within the DMN between PDT and HCs; resting state activity in the inferior | Decreased activity was found in the left inferior parietal cortex between PDAR and both HCs and PDT subjects, but not parietal cortex and posterior cingulate cortex were correlated with some measures of cognitive performance in PD but not in HCs. |
| Wei et al. 2014 | 37 PD and 34 HCs | Graph theory | Remarkable decreased efficiency in PD was observed in the corticobasal ganglia motor network, with the most pronounced changes in the rostral SMA, caudal SMA, primary motor cortex, primary somatosensory cortex, thalamus, globus pallidus and putamen. Reduced efficiency in SMA, primary motor cortex, thalamus and globus pallidus was significantly correlated with UPDRS motor scores in PD patients. |
| Zhang et al. 2015 | 16 PD and 20 HCs | Graph theory | Increased information transformation efficiency was found in PD. The identified network that encompassed cortical and subcortical regions and the cerebellum and brainstem correlated with clinical manifestations in PD and could distinguish PD from HCs. |
| Berman et al. 2016 | 19 PD on and off medication and 16 HCs | Graph theory | PD patients off medication showed no significant changes in global efficiency and overall local efficiency, but they showed increased local efficiency in executive and salience networks. Levodopa significantly decreased local efficiency in PD except within the subcortical network, in which it significantly increased local efficiency. |
| Dementia with Lewy bodies | |||
| Lowther et al. 2014 | 15 DLB, 13 AD and 40 HCs | ICA | DMN, salience and executive networks showed reduced FC in DLB subjects compared with AD and HCs and increased FC in the basal ganglia network. |
| Peraza et al. 2014 | 16 DLB and 17 HCs | ICA | Significant differences between DLB and HCs were found in the left frontoparietal, temporal, and sensorimotor networks. Desynchronization of a number of cortical and subcortical areas related to the left frontoparietal network was also associated with the severity and frequency of cognitive fluctuations. |
| Peraza et al. 2015 | 22 DLB, 24 AD and 17 HCs | Graph theory | DLB group showed a lower synchronization compared with AD and HCs. DLB also showed higher small-worldness and global efficiency (DLB > controls > AD) and lower clustering coefficient (DLB < controls < AD). Significant associations between network performance measures and global cognitive impairment and severity of cognitive fluctuations were also found in DLB. |
| Behavioral variant frontotemporal dementia | |||
| Zhou et al. 2010 | 12 bvFTD, 12 AD and 12 HCs | ICA | bvFTD attenuated salience network connectivity, in frontoinsular, cingulate, striatal, thalamic and brainstem nodes, but enhanced connectivity within the DMN. AD, by contrast, reduced DMN connectivity to the posterior hippocampus, medial cingulo-parieto-occipital regions and dorsal raphe nucleus, but intensified salience network connectivity. Clinical severity in bvFTD correlated with loss of right frontoinsular salience network connectivity and with bi-parietal DMN connectivity enhancement. A combined index of salience network and DMN connectivity achieved 92% accuracy in discriminating between the three groups. |
| Borroni et al. 2012 | 11 bvFTD carriers of GRN mutation, 16 bvFTD non-carriers, 11 HCs, 9 siblings carriers of GRN mutation and 13 non-carrier siblings | ICA | FC within the salience network was reduced in all FTD patients (more markedly in the mutation carriers), while it was enhanced in the DMN. Conversely, pre-symptomatic carriers showed increased connectivity in the salience network, with no changes in the DMN. |
| Hafkemeijer et al. 2016 | 12 bvFTD, 20 AD and 22 HCs at baseline and 1.8-year follow-up | ICA and graph theory | “At follow-up, connectivity between angular gyrus and right frontoparietal network, and between paracingulate gyrus and DMN was lower in bvFTD compared with controls, and lower compared with AD between anterior cingulate gyrus and executive control network, and between lateral occipital cortex and medial visual network. Over time, connectivity decreased in AD between precuneus and right frontoparietal network and in bvFTD between inferior frontal gyrus and left frontoparietal network. Longitudinal changes in connectivity between supramarginal gyrus and right frontoparietal network differ between both patient groups and controls.” |
| Sedeño et al. 2016 | 14 bvFTD and 12 HCs | Graph theory | Average betweenness centrality across regions was examined within different networks. The authors found a significantly de creased betweenness centrality in the bilateral frontotemporoinsular network in bvFTD patients versus HCs. |
Abbreviations: AD=Alzheimer’s disease; PD=Parkinson’s disease; DLB=dementia with Lewy bodies; bvFTD=behvioral variant frontotemporal dementia; HCs=healthy controls; ICA=independent component analysis; DMN=default mode network; FC=functional connectivity; aMCI=amnestic mild cognitive impairment; MCI=mild cognitive impairment; SMA=supplementary motor area; UPDRS=Unified Parkinson’s disease Rating Scale; GRN=granulin
Mild cognitive impairment and Alzheimer’s disease
Alzheimer’s disease (AD) is currently the most common cause of neurodegenerative dementia. The typical clinical presentation of AD is characterized by an early and prominent impairment of memory, followed by a progressive decline of all cognitive functions, eventually resulting in dementia (Sunderland et al., 2006). In the early stage, memory impairment is the prominent feature because the pathology initiates near the medial temporal cortex. In the moderate stage, language problems or visuospatial dysfunctions become conspicuous as the pathology propagates to the other temporal and parietal cortices (Förstl and Kurz, 1999). In the late stage of the illness, most cognitive functions are severely impaired, including frontal executive functions such as judgment, abstract or logical reasoning, and planning (Braak and Braak, 1991).
AD pathology (accumulation of beta-amyloid plaques and neurofibrillary tangles) is believed to begin decades before the clinical manifestations of the disease appear. As a consequence, the diagnosis of AD is typically made when it is too late to reverse, or even stop, the disease process. To allow the early identification of subjects at risk of developing AD, the concept of mild cognitive impairment (MCI) was introduced (Petersen et al., 1999). MCI is considered an intermediate stage between normal aging and dementia (Boyle et al., 2006), and it is characterized by mild memory deterioration. Progression from MCI is hard to predict, as people diagnosed with MCI can develop forms of dementia other than AD, or even revert to normal cognition (Larrieu et al., 2002). Different subtypes of MCI exist and amnestic MCI (aMCI) is considered a prodromal stage of AD, carrying a high risk of progression to AD.
AD and MCI have been extensively studied using rfMRI, particularly after the first reports that highlighted a prominent vulnerability of the DMN (Greicius et al., 2004). In fact, many studies (mainly based on ICA) have shown that DMN structures, involved in memory processes, are particularly affected by atrophy and by deposition of the amyloid protein, and generally show reduced glucose metabolism (Buckner et al., 2005). Several studies reported DMN FC alterations in patients with MCI (Esposito et al., 2013; Gili et al., 2011) and in healthy subjects at high risk of developing dementia (Filippini et al., 2009; Hafkemeijer et al., 2012). Stronger evidence can be found in AD studies, where decreased FC of the DMN in AD subjects compared with healthy elderly has been shown both in posterior areas, i.e. the precuneus (Damoiseaux et al., 2012) and the posterior cingulate cortex (Dipasquale et al., 2015; Griffanti et al., 2015), and in the anterior cingulate and medial prefrontal cortex (Hafkemeijer et al., 2012; Gili et al., 2011). Interestingly, one study reported a counter trend with higher FC in the precuneus and in the frontal pole found in early AD versus healthy subjects (Damoiseaux et al., 2012). This was explained by the authors as a compensatory mechanism that disappears with disease progression. However, other factors might be responsible for these findings. In particular, the cognitive reserve hypothesis (Stern, 2006) postulates the existence of functional brain mechanisms, developed as a result of lifestyle and cognitive stimuli, that enable certain individuals to cope with cerebral damage better than others. Within the DMN, it was shown that education (a proxy of cognitive reserve) can modulate FC in the posterior cingulate cortex (Bozzali et al., 2015). Overall, the existing literature suggests that changes in FC of the precuneus within the DMN might be a key feature in AD evolution. A recent longitudinal study showed that this parameter measured at baseline is able to classify patients as converters and con-converters (after 2 years) with higher sensitivity, specificity and accuracy than measures of atrophy (Serra et al., 2016).
Other networks have been investigated, and found to show alterations from the early stages of the disease. The SMN, which did not show altered signs in the study performed by Damoiseaux and colleagues (2012), exhibited a within-network FC loss (Dipasquale et al., 2015) when a high-dimensional approach was used to decompose the SMN into sub-networks. The executive control network, which is typically anti-correlated with the DMN, showed reduced FC in the superior frontal lobule and in the prefrontal cortex in MCI compared with healthy subjects (Sorg et al., 2007). In a longitudinal study, Hafkemeijer and colleagues (2016) reported lower FC in the right and left frontoparietal networks in AD versus healthy controls, involving the parietal lobule, paracingulate and postcingulate gyrus, and frontal pole (only the results in the right frontoparietal network survived after correction for gray matter volume).
Fewer graph analysis studies have been published. Some of the first reports provided inconsistent results on the altered brain network pattern in AD patients (Supekar et al., 2008; Stam et al., 2007). Sanz-Arigita and colleagues (2010) used graph analysis to study AD alterations at whole-brain level by comparing the “small-world” structure in a group of AD patients compared with a group of healthy subjects. They found a relative randomization of AD architecture driven by a different connectivity pattern compared with healthy group. Specifically, in the AD subjects they found higher FC within in the frontal cortices and between these and the corpus striatum and thalamus, as well as decreased connectivity between the temporal lobe and parietal and occipital cortices.
Zhao et al. (2012) showed that the topological properties of the brain networks were disrupted in moderate-stage AD compared with an elderly healthy population. In particular, they found a higher clustering coefficient in AD, higher average shortest path length values and a lower global efficiency. These findings suggested that long distance information integration and information transfer ability are compromised in AD patients. However, they showed stronger local information processing capacity in the moderate stage of the disease. More recently, reduced clustering coefficient and reduced global efficiency were found in MCI patients compared with healthy controls using fine cortical parcellation (mean ROI volume = 1.55 ± 0.33 ml) (Minati et al., 2014). The same study demonstrated a widespread reduction in node degree (a measure of the local density of connections), which significantly exceeded the changes detected using ICA, both in amplitude and topographical extent. For this reason, the authors underlined that graph-based analysis showed a superior ability to detect disease-related disconnection in MCI, suggesting that this technique might potentially be used in the determination of biomarkers of early dementia.
Challis et al. (2015) did not evaluate specific graph metrics, but used the single elements of the connectivity metrics as features for a machine-learning algorithm to perform patient stratification between healthy control subjects and either aMCI or AD subjects. The model achieved 75% accuracy disambiguating healthy participants from patients with aMCI and 97% accuracy disambiguating aMCI subjects from those with AD, and confirming the importance of functional disconnection in the evolution of AD.
Parkinson’s disease
Parkinson’s disease (PD) is a progressive neurodegenerative disease characterized by a specific range of motor symptoms, including slowness of movement, rigidity, tremor at rest and postural instability (Jankovic, 2008). Its core pathophysiological mechanism is degeneration of nigrostriatal dopaminergic neurons, which leads to a deficiency of dopamine in the striatum and the early motor features of the disease (Greffard et al., 2006). Moreover, about 40% of patients with PD are also affected by dementia (Emre, 2003).
The considerable importance of rfMRI in PD studies derives from the fact that, unlike task-based fMRI, it allows the FC disruption mechanisms that occur in the motor areas to be investigated without the patients having to perform any motor tasks (PD patients are typically affected by motor impairments). This neurodegenerative disease has been investigated using ICA and focusing on specific networks, and with graph theory analysis, the latter allowing a whole-brain exploration using specific graph indices.
One of the most affected RSNs in rigidity-predominant PD is the basal ganglia network. Szewczyk-Krolikowski and colleagues (2014) found reduced basal ganglia motor network connectivity in non-tremor patients with early PD and without cognitive deficits; this reduced connectivity involved the putamen and caudate bilaterally, the midbrain, the superior temporal gyrus bilaterally, the dorsolateral prefrontal cortex bilaterally, the medial prefrontal cortex, and the precuneus. Topological efficiency was also evaluated in a graph-based study to estimate the impairment of the basal ganglia motor circuit in non-tremor patients with mild to moderate PD (Wei et al., 2014). In this study, the PD patients showed decreased efficiency in the basal ganglia motor pathway, especially in the right rostral supplementary motor area, the left caudal supplementary motor area, and the bilateral primary motor cortex, primary somatosensory cortex, thalamus, globus pallidus and putamen.
However, PD patients with tremor and without cognitive impairment have been reported to show alteration of different circuits involving different areas. Zhang et al. (2015) reported a widespread increase of FC in such PD patients compared with healthy subjects, increased centrality in the frontal, parietal, and occipital regions, and decreased centrality in the cerebellum anterior lobe and thalamus. Increased efficiency in information transfer was also found in a distributed network encompassing different regions, including the nodes of the DMN, the sensorimotor cortex, prefrontal and occipital areas, the thalamus, basal ganglia, cerebellum and brainstem.
Beyond its motor symptoms, PD also affects the cognitive domain in a high percentage of patients. However, the risk of developing cognitive impairment is associated with different factors. Using ICA, Karunanayaka and colleagues (2016) tested the hypothesis that PD patients with primary rigidity are more likely to develop cognitive deficits than those with tremor-predominant symptoms. They focused on the DMN as it is supposed to be involved in cognitive processing, and found a decrease in FC in rigidity-predominant PD patients compared with healthy controls and tremor-predominant PD patients. This result led them to hypothesize that the discrepancies in the literature (Krajcovicova et al., 2012; Tessitore et al., 2012) might be related, in part, to the fact that other studies did not take PD subtypes into account.
In order to evaluate possible alterations of the cognitive domain, together with the DMN, other RSNs have recently been investigated both with ICA and using graph theory analysis (Berman et al., 2016; Putcha et al., 2015). The graph-based study led by Berman and colleagues reported significantly reduced FC in the auditory and visual network areas and an increased integrated local efficiency in the DMN, executive regions and salience networks, which are typically referred to as the “core” neurocognitive networks responsible for maintaining effective neural communication. This result is in line with the ICA-based study performed by Putcha et al. (2015), in which aberrant coupling was found between the DMN and executive network, together with reduced coupling between the salience network and the executive one.
Dementia with Lewy bodies
Dementia with Lewy bodies (DLB), which is the one of the most frequent types of neurodegenerative dementia, is associated with greater deficits on attentional and visuoperceptual tasks (Calderon et al., 2001; Collerton et al., 2003). The clinical symptoms of DLB can overlap with those of AD and PD, making this disorder difficult to differentiate from these conditions. Compared with the more extensive literature on AD and PD, fewer neuroimaging studies have investigated DLB, and the neural changes responsible for its characteristic distressing symptoms — attentional deficits, motor features of parkinsonism, and depression — are still not well understood. Most studies of DLB used seed-based analysis to focus on certain core regions, including the posterior cingulate cortex, precuneus, primary visual cortex, hippocampus, putamen, caudate and thalamus (Kenny et al., 2012, 2013; Galvin et al., 2011). However, as highlighted in the methods of analysis section, the limitation of this approach is that it only tests the FC of specific brain regions and the choice is therefore linked to a priori hypotheses or previous studies. A more extensive study was performed by Lowther and colleagues (2014), who, by means of ICA, analyzed different RSNs and compared FC in DLB patients versus healthy controls and AD patients, finding significant alterations in the default mode, salience and executive control networks. Their results also showed greater FC in the basal ganglia and limbic networks in DLB subjects compared with healthy controls, a finding that may be related to the parkinsonian symptoms and mood disturbances that are typical in DLB. In another study, an ICA-based approach was used to assess whether cognitive fluctuations, which are a core symptom in DLB, are related to pathological alterations in distributed brain networks (Peraza et al., 2014). No DMN FC alterations were found in DLB compared with controls, but significant cluster differences between DLB and controls were found in the left frontoparietal, temporal and sensorimotor networks (DLB < healthy subjects).
A desynchronization of a number of cortical and subcortical areas related to the left frontoparietal network was also shown to be associated with the severity and frequency of cognitive fluctuations.
Graph theory studies of network organization in DLB are scarce. A graph-based study was performed to describe possible alterations in DLB brain complexity at global and local levels and to compare these alterations with those caused by AD (Peraza et al., 2015).
The authors found significant differences in the functional brain network measures of DLB patients (higher global efficiency and clustering coefficient, lower average path length) compared with healthy controls and broader network alterations compared with AD. Of note, global efficiency was significantly correlated with cognitive and fluctuating attention scores in DLB. Moreover, compared with the healthy subjects, the DLB patients showed higher small-worldness, while the AD patients showed lower small-worldness. The authors also investigated regional network differences between the groups, finding lower node degree and nodal clustering in parietal, occipital and frontal cortices and higher node degree in both thalamic nodes in DLB patients compared with controls.
Behavioral variant frontotemporal dementia
Frontotemporal dementia (FTD) is another kind of dementia that groups various types of neurodegenerative disorders associated with atrophy of the frontal and temporal lobes, and is clinically characterized by language or behavioral impairments (Cardarelli et al., 2010). The behavioral variant of FTD (bvFTD) is one of the subtypes most studied so far and is mainly characterized by changes in behavior, personality and motivation (Rascovsky et al., 2011). However, bvFTD symptoms may vary considerably and sometimes symptoms such as memory disturbances and behavioral abnormalities can be misinterpreted as AD symptoms and lead to misdiagnosis. Clinical differentiation between these types of dementia may be challenging, particularly in their early stages. Therefore, it has been suggested that analysis of brain FC could be used to find early markers of brain changes associated with the two types of dementia, so as to differentiate one disease from the other. Zhou and colleagues (2010) studied alterations of the DMN and salience network in bvFTD and AD patients, demonstrating a divergent effect of the two diseases on core neural network dynamics. Their results showed that, in comparison with a control group, bvFTD attenuates salience network connectivity, mainly in frontoinsular, cingulate, striatal, thalamic and brainstem nodes, and enhances DMN FC, while connectivity in AD is reduced in the posterior hippocampus, medial cingulo-parieto-occipital regions and the dorsal raphe nucleus, and increased in the salience network. Consistent with those findings, a selective disruption of the salience network in the presence of bvFTD was reported by Borroni et al. (2012). In the same study, genetic data were used to investigate the effect of granulin mutation, which has been identified as a major cause of FTD, on brain FC. Healthy subjects, bvFTD patients, carriers and non-carriers of granulin mutation, and pre-symptomatic carriers were recruited. The results showed a more marked involvement of the salience network in the bvFTD patients who carried the granulin mutation than in the mutation non-carriers, and an increase in FC in the asymptomatic granulin mutation carriers compared with the healthy controls, while no changes were found in the DMN. These results suggest that changes in FC might not be monotonic throughout the course of the disease, but instead manifest as an initial increase — a coping strategy — followed by a decrease caused by the accrual of pathology.
Other studies have been conducted to assess whether the FC changes in bvFTD involve further large-scale distributed networks and to look for other alterations able to discriminate between bvFTD and AD. Hafkemeijer and colleagues (2016) used both ICA and the graph theory approach to compare the effects of these two types of dementia on brain connectivity. Network analysis performed by ICA highlighted FC differences in the lateral visual cortical network (AD > bvFTD), dorsal visual stream network (AD < bvFTD) and auditory system network, where a decreased negative FC between this network and the angular gyrus was found in patients with bvFTD compared to AD. On comparing the bvFTD patients with a group of healthy controls, altered FC was found in the auditory network. The graph theory approach provided additional information about the right superior temporal gyrus, by detecting decreased FC with the cuneal cortex, supracalcarine cortex, intracal-carine cortex and lingual gyrus in bvFTD versus AD.
A recent graph-based study was performed by Sedeño and colleagues (2016). They used a specific graph index, i.e. the average betweenness centrality across regions within different networks, to characterize the central role of each network in the dynamics of the overall system in bvFTD. Their main finding was a significantly decreased betweenness centrality in the bilateral frontotemporoinsular network in bvFTD patients compared with the healthy group.
Concluding remarks
Brain FC is likely to have a fundamental role in the study and understanding of several diseases, as it has proven to have great potential as a biomarker for the characterization or detection of their onset, severity and progression.
In this scenario, both ICA and graph theory approaches have become very powerful tools for studying different pathological conditions and highlight — at network and at whole-brain level — the cerebral functional reorganization occurring in neurodegenerative diseases in association with various kinds of deficits (e.g. cognitive impairment, motor deficits, etc.).
The continuous improvement of these advanced analysis techniques aims at extracting, from data, useful information for understanding the complex mechanisms that regulate brain connectivity in pathophysiological conditions and provide simple and non-invasive biomarkers for data-driven patient stratification, differential diagnosis and disease monitoring.
In conclusion, ICA and graph theoretical methods can be considered complementary tools for exploring brain FC, as ICA studies the RSN connectivity, while graph theoretical methods go further, analyzing different aspects of FC architecture. Hence, a further step towards a more detailed FC analysis of the brain might be a combination of the two, e.g. using high-dimensional ICA to obtain a data-driven functional atlas of the gray matter regions and graph theoretic indices on the nodes thus obtained to investigate the small-world functional architecture of the brain and its alterations in pathology.
References
- Abou-Elseoud A, Starck T, Remes J, et al. The effect of model order selection in group PICA. Hum Brain Mapp. 2010;31:1207–1216. doi: 10.1002/hbm.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard S, Salvador R, Whitcher B, et al. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF. Modelling with independent components. Neuroimage. 2012;62:891–901. doi: 10.1016/j.neuroimage.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Berman BD, Smucny J, Wylie KP, et al. Levodopa modulates small-world architecture of functional brain networks in Parkinson’s disease. Mov Disord. 2016;31:1676–1684. doi: 10.1002/mds.26713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Borroni B, Alberici A, Cercignani M, et al. Granulin mutation drives brain damage and reorganization from preclinical to symptomatic FTLD. Neurobiol Aging. 2012;33:2506–2520. doi: 10.1016/j.neurobiolaging.2011.10.031. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Aggarwal NT, et al. Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- Bozzali M, Dowling C, Serra L, et al. The impact of cognitive reserve on brain functional connectivity in Alzheimer’s disease. J Alzheimers Dis. 2015;44:243–250. doi: 10.3233/JAD-141824. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Calderon J, Perry RJ, Erzinclioglu SW, et al. Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;70:157–164. doi: 10.1136/jnnp.70.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, et al. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardarelli R, Kertesz A, Knebl JA. Frontotemporal dementia: a review for primary care physicians. Am Fam Physician. 2010;82:1372–1377. [PubMed] [Google Scholar]
- Challis E, Hurley P, Serra L, et al. Gaussian process classification of Alzheimer’s disease and mild cognitive impairment from resting-state fMRI. Neuroimage. 2015;112:232–43. doi: 10.1016/j.neuroimage.2015.02.037. [DOI] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collerton D, Burn D, McKeith I, et al. Systematic review and meta-analysis show that dementia with Lewy bodies is a visual-perceptual and attentional-executive dementia. Dement Geriatr Cogn Disord. 2003;16:229–237. doi: 10.1159/000072807. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Prater KE, Miller BL, et al. Functional connectivity tracks clinical deterioration in Alzheimer’s disease. Neurobiol Aging. 2012;33:828.e19–30. doi: 10.1016/j.neurobiolaging.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Reus MA, van den Heuvel MP. The parcellation-based connectome: limitations and extensions. Neuroimage. 2013;80:397–404. doi: 10.1016/j.neuroimage.2013.03.053. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dipasquale O, Griffanti L, Clerici M, et al. High-dimensional ICA analysis detects wthin-network functional connectivity damage of default-mode and sensory-motor networks in Alzheimer’s disease. Front Hum Neurosci. 2015;9:43. doi: 10.3389/fnhum.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M. Dementia associated with Parkinson’s disease. Lancet Neurol. 2003;2:229–237. doi: 10.1016/s1474-4422(03)00351-x. [DOI] [PubMed] [Google Scholar]
- Esposito R, Mosca A, Pieramico V, et al. Characterization of resting state activity in MCI individuals. PeerJ. 2013;1(1):e135. doi: 10.7717/peerj.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förstl H, Kurz A. Clinical features of Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 1999;249:288–290. doi: 10.1007/s004060050101. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1:13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, et al. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Price JL, Yan Z, et al. Resting bold fMRI differentiates dementia with Lewy bodies vs Alzheimer disease. Neurology. 2011;76:1797–1803. doi: 10.1212/WNL.0b013e31821ccc83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gili T, Cercignani M, Serra L, et al. Regional brain atrophy and functional disconnection across Alzheimer’s disease evolution. J Neurol Neurosurg Psychiatry. 2011;82:58–66. doi: 10.1136/jnnp.2009.199935. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greffard S, Verny M, Bonnet AM, et al. Motor score of the unified Parkinson disease rating scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch Neurol. 2006;63:584–588. doi: 10.1001/archneur.63.4.584. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, et al. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L, Dipasquale O, Laganà MM, et al. Effective artifact removal in resting state fMRI data improves detection of DMN functional connectivity alteration in Alzheimer’s disease. Front Hum Neurosci. 2015;9:449. doi: 10.3389/fnhum.2015.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafkemeijer A, Möller C, Dopper EG, et al. A longitudinal study on resting state functional connectivity in behavioral variant frontotemporal dementia and Alzheimer’s disease. J Alzheimers Dis. 2016 doi: 10.3233/JAD-150695. [DOI] [PubMed] [Google Scholar]
- Hafkemeijer A, van der Grond J, Rombouts SA. Imaging the default mode network in aging and dementia. Biochim Biophys Acta. 20121822:431–441. doi: 10.1016/j.bbadis.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Horwitz B. The elusive concept of brain connectivity. Neuroimage. 2003;19:466–470. doi: 10.1016/s1053-8119(03)00112-5. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Karunanayaka PR, Lee EY, Lewis MM, et al. Default mode network differences between rigidity-and tremor-predominant Parkinson’s disease. Cortex. 2016;81:239–250. doi: 10.1016/j.cortex.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny ER, O’Brien JT, Firbank MJ, et al. Subcortical connectivity in dementia with Lewy bodies and Alzheimer’s disease. Br J Psychiatry. 2013;203:209–214. doi: 10.1192/bjp.bp.112.108464. [DOI] [PubMed] [Google Scholar]
- Kenny ER, Blamire AM, Firbank MJ, et al. Functional connectivity in cortical regions in dementia with Lewy bodies and Alzheimer’s disease. Brain. 2012;135:569–581. doi: 10.1093/brain/awr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajcovicova L, Mikl M, Marecek R, et al. The default mode network integrity in patients with Parkinson’s disease is levodopa equivalent dose-dependent. J Neural Transm (Vienna) 2012;119:443–454. doi: 10.1007/s00702-011-0723-5. [DOI] [PubMed] [Google Scholar]
- Lang EW, Tomé AM, Keck IR, et al. Brain connectivity analysis: a short survey. Comput Intell Neurosci. 20122012:412512. doi: 10.1155/2012/412512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- Lee YB, Lee J, Tak S, et al. Sparse SPM: Group Sparse-dictionary learning in SPM framework for resting-state functional connectivity MRI analysis. Neuroimage. 2016;125:1032–1045. doi: 10.1016/j.neuroimage.2015.10.081. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang Y, Yan H, et al. Altered topological patterns of brain networks in mild cognitive impairment and Alzheimer’s disease: a resting-state fMRI study. Psychiatry Res. 2012;202:118–125. doi: 10.1016/j.pscychresns.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Lowther ER, O’Brien JT, Firbank MJ, et al. Lewy body compared with Alzheimer dementia is associated with decreased functional connectivity in resting state networks. Psychiatry Res. 2014;223:192–201. doi: 10.1016/j.pscychresns.2014.06.004. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, et al. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp. 1998;6:160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minati L, Chan D, Mastropasqua C, et al. Widespread alterations in functional brain network architecture in amnestic mild cognitive impairment. J Alzheimers Dis. 2014;40:213–220. doi: 10.3233/JAD-131766. [DOI] [PubMed] [Google Scholar]
- Minati L, Nigri A, Cercignani M, et al. Detection of scale-freeness in brain connectivity by functional MRI: signal processing aspects and implementation of an open hardware co-processor. Med Eng Phys. 2013;35:1525–1531. doi: 10.1016/j.medengphy.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Peraza LR, Taylor JP, Kaiser M. Divergent brain functional network alterations in dementia with Lewy bodies and Alzheimer’s disease. Neurobiol Aging. 2015;36:2458–2467. doi: 10.1016/j.neurobiolaging.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraza LR, Kaiser M, Firbank M, et al. fMRI resting state networks and their association with cognitive fluctuations in dementia with Lewy bodies. Neuroimage Clin. 2014;4:558–565. doi: 10.1016/j.nicl.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Putcha D, Ross RS, Cronin-Golomb A, et al. Altered intrinsic functional coupling between core neurocognitive networks in Parkinson’s disease. Neuroimage Clin. 2015;7:449–455. doi: 10.1016/j.nicl.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sanz-Arigita EJ, Schoonheim MM, Damoiseaux JS, et al. Loss of ‘small-world’ networks in Alzheimer’s disease: graph analysis of FMRI resting-state functional connectivity. PLoS One. 2010;5:e13788. doi: 10.1371/journal.pone.0013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedeño L, Couto B, García-Cordero I, et al. Brain network organization and social executive performance in frontotemporal dementia. J Int Neuropsychol Soc. 2016;22:250–262. doi: 10.1017/S1355617715000703. [DOI] [PubMed] [Google Scholar]
- Serra L, Cercignani M, Mastropasqua C, et al. Longitudinal changes in functional brain connectivity predicts conversion to Alzheimer’s disease. J Alzheimers Dis. 2016;51:377–389. doi: 10.3233/JAD-150961. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE, Vidaurre D, et al. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci. 2015;18:1565–1567. doi: 10.1038/nn.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Vidaurre D, Beckmann CF, et al. Functional connectomics from resting-state fMRI. Trends Cogn Sci. 2013;17:666–682. doi: 10.1016/j.tics.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. The future of FMRI connectivity. Neuroimage. 2012;62:1257–1266. doi: 10.1016/j.neuroimage.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Structure and function of complex brain networks. Dialogues Clin Neurosci. 2013;15:247–262. doi: 10.31887/DCNS.2013.15.3/osporns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Kötter R. The human connectome: a structural description of the human brain. PLoS Comput Biol. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, Jones BF, Nolte G, et al. Small-world networks and functional connectivity in Alzheimer’s disease. Cereb Cortex. 2007;17:92–99. doi: 10.1093/cercor/bhj127. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(3 Suppl 2):S69–74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- Sunderland T, Hampel H, Takeda M, et al. Biomarkers in the diagnosis of Alzheimer’s disease: are we ready? J Geriatr Psychiatry Neurol. 2006;19:172–179. doi: 10.1177/0891988706291088. [DOI] [PubMed] [Google Scholar]
- Supekar K, Menon V, Rubin D, et al. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput Biol. 2008;4:e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk-Krolikowski K, Menke RA, Rolinski M, et al. Functional connectivity in the basal ganglia network differentiates PD patients from controls. Neurology. 2014;83:208–214. doi: 10.1212/WNL.0000000000000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, Esposito F, Vitale C, et al. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology. 2012;79:2226–2232. doi: 10.1212/WNL.0b013e31827689d6. [DOI] [PubMed] [Google Scholar]
- Valdes-Sosa PA, Roebroeck A, Daunizeau J, et al. Effective connectivity: influence, causality and biophysical modeling. Neuroimage. 2011;58:339–361. doi: 10.1016/j.neuroimage.2011.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Stam CJ, Boersma M, et al. Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. Neuroimage. 2008;43:528–539. doi: 10.1016/j.neuroimage.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang L, Zang Y, et al. Parcellation-dependent small-world brain functional networks: a resting-state fMRI study. Hum Brain Mapp. 2009;30:1511–1523. doi: 10.1002/hbm.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Wei L, Zhang J, Long Z, Wu GR, et al. Reduced topological efficiency in cortical-basal ganglia motor network of Parkinson’s disease: a resting state fMRI study. PLoS One. 2014;9:e108124. doi: 10.1371/journal.pone.0108124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Liu X, Chen J, et al. Widespread increase of functional connectivity in Parkinson’s disease with tremor: a resting-state fMRI study. Front Aging Neurosci. 2015;7:6. doi: 10.3389/fnagi.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Liu Y, Wang X, et al. Disrupted small-world brain networks in moderate Alzheimer’s disease: a resting-state fMRI study. PLoS One. 2012;7:e33540. doi: 10.1371/journal.pone.0033540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain. 2010;133:1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]