Summary
Neuroimaging, both with magnetic resonance imaging (MRI) and positron emission tomography (PET), has gained a pivotal role in the diagnosis of primary neurodegenerative diseases. These two techniques are used as biomarkers of both pathology and progression of Alzheimer’s disease (AD) and to differentiate AD from other neurodegenerative diseases.
MRI is able to identify structural changes including patterns of atrophy characterizing neurodegenerative diseases, and to distinguish these from other causes of cognitive impairment, e.g. infarcts, space-occupying lesions and hydrocephalus.
PET is widely used to identify regional patterns of glucose utilization, since distinct patterns of distribution of cerebral glucose metabolism are related to different subtypes of neurodegenerative dementia.
The use of PET in mild cognitive impairment, though controversial, is deemed helpful for predicting conversion to dementia and the dementia clinical subtype. Recently, new radiopharmaceuticals for the in vivo imaging of amyloid burden have been licensed and more tracers are being developed for the assessment of tauopathies and inflammatory processes, which may underlie the onset of the amyloid cascade.
At present, the cerebral amyloid burden, imaged with PET, may help to exclude the presence of AD as well as forecast its possible onset. Finally PET imaging may be particularly useful in ongoing clinical trials for the development of dementia treatments. In the near future, the use of the above methods, in accordance with specific guidelines, along with the use of effective treatments will likely lead to more timely and successful treatment of neurodegenerative dementias.
Keywords: dementia, magnetic resonance imaging, positron emission tomography
Introduction
Dementia is a progressive deterioration of acquired cognitive abilities, a process that can result in loss of episodic memory, but may also hamper language, calculation and visuospatial skills, praxis and executive functions. It therefore drastically reduces the affected subject’s autonomy in daily activities.
Alzheimer’s disease (AD) is the most common neurodegenerative dementing disorder; it has an estimated prevalence of 20–40% in those over 85 years of age and accounts for approximately 60–80% of all dementias. Other neurodegenerative forms of dementia include frontotemporal dementia (FTD) and dementia with Lewy bodies (DLB), which together account for 14–25% of all dementias. As the population ages and the prevalence and number of individuals affected by neurodegenerative dementias continue to increase, so too does the considerable social burden they bring.
Since there exist no specific non-invasive biological markers and probes for the diagnosis of any degenerative dementia, the assessment of patients with cognitive disorders is a process of exclusion based on the use of clinical criteria as well as imaging techniques. In this regard, magnetic resonance imaging (MRI) remains the cornerstone for excluding all possible diagnoses other than probable/possible dementia in patients with cognitive impairment. In the work-up of patients showing cognitive impairment, cerebrovascular disease (with or without major stroke), head trauma and medical causes of cognitive decline must all be ruled out. If this is done, a subject with only initial symptoms may receive a diagnosis of mild cognitive impairment (MCI).
When a neurodegenerative dementia is suspected, no time must be lost in seeking to establish the correct diagnosis. There exist various types of neurodegenerative dementia and the choice and outcome of treatment will depend on timely identification of the patient’s condition: therapeutic intervention is more likely to be successful if the diagnosis is made when daily functioning is still preserved and few cognitive domains are impaired.
The procedure, in such circumstances, is usually to perform, first of all, an extensive clinical assessment with complete laboratory analysis, an evaluation for comorbid medical conditions, and various neuropsychological tests. Neuropsychological tools in particular, which are used to identify distinctive cognitive profiles associated with specific dementias, are essential in the clinical diagnosis of the different neurodegenerative dementias, while cerebrospinal fluid (CSF) analysis is performed to detect changes in amyloid β (Aβ) peptides, tau and hyperphosphorylated tau proteins, which are also elements used to classify the different forms of dementia. Genetic testing can detect mutations responsible for the rare familial forms of dementia.
In addition, neuroimaging techniques, in particular MRI and positron emission tomography (PET), may also be used, even prior to genetic testing and CSF examination, to look for specific structural changes (on MRI) and specific patterns of distribution of cerebral glucose metabolism (on PET) characterizing the various types of neurodegenerative dementia. Indeed, in the recently published IWG-2 criteria, it is suggested that both a decline in cerebral glucose metabolism (detected by PET) and MRI measurements of atrophy reflect the time course of disease progression and neurodegeneration in AD. The IWG-2 criteria also suggest that CSF biomarkers (Aβ42, tau, p-tau) and amyloid burden on PET imaging should be considered diagnostic pathological markers of AD (Dubois et al., 2014).
Because of the prolific and ongoing research in this field, diagnostic criteria for degenerative dementias, including AD and FTD, are continuously being revised, taking into account the supportive role of new biomarkers. In this paper we summarize the recent findings of imaging studies that complement clinical and laboratory data in the work-up of patients with dementia.
Imaging techniques for the assay of neuronal atrophy and dysfunction
As mentioned, the two main imaging techniques that may provide surrogate biomarkers in this field are MRI and PET imaging. In particular, certain MRI sequences make it possible to go beyond morphological imaging, while PET can now be performed not only with [18F]fluoro-deoxyglucose ([18F]FDG), a radiopharmaceutical long used in brain imaging, but also with tracers that allow imaging of the amyloid burden.
MR morphometry, diffusion tensor imaging and spectroscopy
In addition to a role in the identification of lesions that can determine secondary dementia, magnetic resonance techniques can accurately detect alterations, in cortical and subcortical gray and white matter, associated with neurodegenerative dementias, even in the prodromal stage (Pini et al., 2016). In particular, morphological MRI can visualize gray matter loss, diffusion tensor imaging can shed light on the structure of the white matter fibers, and magnetic resonance spectroscopy (MRS) can disclose metabolite changes related to the disease.
MRI morphometry
Brain atrophy in AD is strictly correlated to neurodegeneration confirmed on post-mortem histology. The presence of gray matter atrophy, visualized and measured from high quality T1-weighted sequences, can support a clinical diagnosis of AD; monitoring gray matter atrophy is also a means of tracking the progression of the disease. For this reason, MRI is considered a biomarker of neuronal injury in AD (Dubois et al., 2014) and in MCI due to AD (Albert et al., 2011).
Cortical atrophy, which is linked to the stage of neurofibrillary tangle (NFT) pathology, shows a progressive pattern that correlates with the severity of the disease. It starts from the medial temporal lobe (Fig. 1) and spreads to the temporal, parietal and frontal gray matter, with the sensorimotor and visual cortex being minimally affected only in the final stages of AD. Frisoni et al. (2009) also described a progressive pattern of atrophy, detected on MRI, that correlated with disease severity in AD: in early AD patients showing memory impairment the atrophy was limited to the hippocampus, posterior cingulate and orbitofrontal cortex. With progression of the disease and the appearance of cognitive deficits in language, visuospatial and executive functions, the atrophy was seen to spread to the neocortical temporal regions, parietal and frontal lobes; global atrophy was twofold that found in healthy elderly controls.
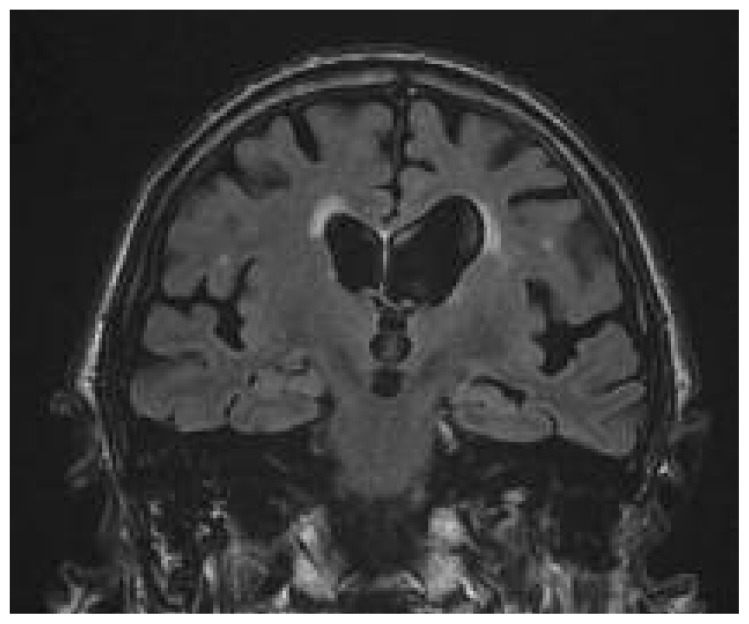
Figure 1.
Diffuse atrophy in a patient with cognitive impairment.
Coronal image from a T2-weighted FLAIR sequence. Enlarged liquoral spaces and volume loss of gyri are evident, along with bilateral periventricular hyperintentities. Note the prominent atrophy of the hippocampus (red arrow).
Different patterns of atrophy have also been described between late-onset and early-onset AD, even in the presence of similar clinical disease severity: the late-onset form usually shows atrophy of the medial temporal lobes, while early-onset AD presents with severe and widespread atrophy of the parietal and frontal cortex, along with the neocortical temporal regions, while there is relative sparing of the medial temporal lobes (Seo et al., 2011). This observation suggests that the two forms differ in aggressiveness.
Visual inspection of MR images, although easy to perform, is not adequate for the diagnosis of primary neurodegenerative diseases. To enhance the accuracy of atrophy detection and allow its use as a biomarker for the diagnosis of dementia, a standardized quantification of atrophy is required (Jack et al., 2011). Quantification of atrophy requires manual segmentation of the gray matter. This is a time-consuming process that demands specific segmentation protocols and high operator expertise, therefore it is not feasible in routine clinical practice. This limitation has led to the development of automated registration algorithms for measuring cortical volumes, and therefore tools that are independent of the operator (Fig. 2). Most of these algorithms use a reference atlas derived from several normal individuals that is aligned to the MR image of the subject under examination; applying the ideal segmentation of the atlas it is possible to identify and measure the volume of the cortical regions. Automatic segmentation tools are used to measure cortical volumes for research purposes, however they need further validation before they can be used in clinical practice or in clinical trials.
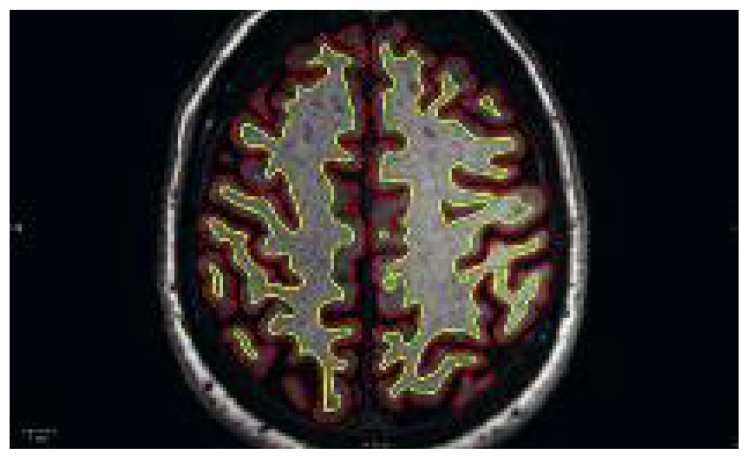
Figure 2.
Automated detection of the pial surface (red line) and interface between gray and white matter (yellow line), using Freesurfer.
Freesurfer is an open source software package for the analysis and visualization of structural neuroimaging data. It can be used to quantify gray matter volume and global atrophy from a thin-slice 3D T1-weighted sequence.
The application of automated methods of atrophy quantification has increased the research contribution that can be made by MRI, which can now be used to identify the regions that specifically differentiate patients with neurodegenerative disease from healthy elderly control subjects (Fig. 3). Among these, the mesial temporal lobe structures are the first to show signs of atrophy. In particular, hippocampal atrophy is used as a marker to stage the progression of AD pathology in the brain in research studies; in AD patients, the hippocampal volume declines significantly and progressively starting from the initial phases of the disease. Declining hippocampal volume correlates with the clinical progression of the disease; the literature reports a 10–15% reduction of hippocampal volume in the amnestic variant of MCI compared with healthy subjects (Shi et al., 2009), a 15–30% reduction in the mild dementia stage of AD (van der Flier et al., 2005), and a 15–40% reduction in the clinical stage of AD (Pantel et al., 1998). Today, technologically improved MRI scanners, such as those operating at an ultra-high magnetic field (7T), are making it feasible to measure the volume of different interconnected subregions of the hippocampus. The observation that subfields of the hippocampus may be differentially susceptible to neurodegeneration prompted further research and it is now accepted that AD patients have major atrophy in the head of the hippocampus and in the cornu ammonis sector 1 (CA1) (Boutet et al., 2014). This is in keeping with the distribution of histopathologically detected NFTs, which appear first in the CA1 subfield and later spread to other regions of the hippocampus.
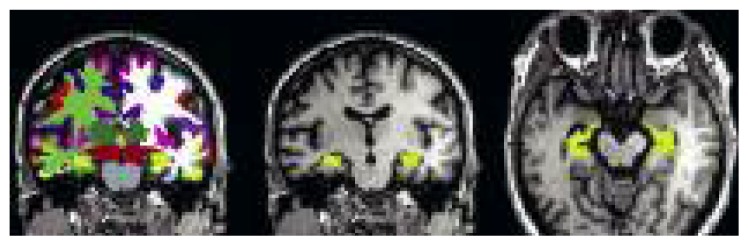
Figure 3.
Atrophy quantification using Freesurfer.
Cortical parcellation (left). Freesurfer automatically assigns brain structures a neuroanatomical label. Each color represents a unique brain anatomical structure. Parcellation of the right and left hippocampus is extracted from this dataset (coronal section in the central image and axial section on the right). Brain volumetry allows quantification of the volume of each structure. This patient with MCI shows a mild reduction of the size of the hippocampus.
CA1 atrophy has been associated with memory dysfunction in other neurodegenerative dementias, too. However, the hippocampal atrophy detected in DLB was milder than that seen in AD and this is a potential a biomarker for distinguishing between AD and DLB (Mak et al., 2016). That said, it must be borne in mind that hippocampal atrophy is not specific to AD (Boccardi et al., 2003).
Another brain region showing early atrophy is the enthorinal cortex. Located bilaterally in the medial temporal lobe this is a key region in the connection between the hippocampus and the neocortex and it plays a role in memory. It is known from post-mortem findings that this region is involved early on in the accumulation of NFTs. Accordingly, increased entorhinal cortex atrophy has been reported in AD patients (Teipel et al., 2006), with a high diagnostic accuracy. MCI subjects show an intermediate level of entorhinal cortex atrophy, between that observed in AD and the normal findings seen in healthy controls (Velayudhan et al., 2013). Morphometry of the enthorinal cortex, rather than the hippocampus, could be a better predictor of conversion to AD.
Among the subcortical structures, the amygdala is reduced in volume by 15–20% in moderate AD. Atrophy of this structure has been found to be more severe in late-onset AD than in early-onset AD. The amygdala could be an important region for the differential diagnosis with other neurodegenerative diseases as a high level of amygdala atrophy, higher than that seen in AD patients, has been described in patients with FTD (Möller et al., 2015). With advanced computational methods, it has become possible to identify a non-uniform pattern of atrophy of the amygdala, consistent with the histopathological findings. Finally, atrophy has been described in the thalamus of AD and MCI patients, and also in the caudate nucleus, putamen and nucleus accumbens.
At this time, MR morphometry has no role in the definition of preclinical AD, although it is considered a useful tool for screening subjects at risk (Dubois et al., 2016).
Diffusion tensor imaging
Diffusion-weighted MRI is a method that exploits the diffusion of water molecules to generate contrast in MR images. In the brain, diffusion of water is not free, but reflects interactions with cell membranes and myelin, resulting in water movement that is also directionally dependent. Diffusion tensor imaging (DTI), a special type of diffusion-weighted imaging, can analyze the three-dimensional shape of the diffusion and has been used extensively to map white matter tractography in the brain.
From DTI it is possible to derive the axial diffusivity, which quantifies the movement of water along the predominant direction, and its diffusivity along the orthogonal directions, which generate the radial diffusivity (RD). The average diffusivity (MD) is the mean of the three vectors that quantify the overall movement of water molecules independently of the direction, while the fractional anisotropy (FA) reflects the degree of preferential direction of water molecule diffusion.
It is generally accepted that FA reflects the structural integrity of white matter fibers with poor specificity to the type of change; axial diffusivity decreases in axonal injury and RD increases with de- or dysmyelination.
In patients with AD, myelinated fiber changes, detected by an altered MD/FA ratio, have been reported repeatedly, showing predominantly posterior involvement (Nir et al., 2015).
Several DTI studies have reported a reduction in FA with increased MD in the corpus callosum, posterior cingulate, superior longitudinal fasciculus and fornix. Fiber involvement has also been found in the fronto-occipital fasciculus and in the inferior longitudinal fasciculus. A meta-analysis of DTI studies found similar, widespread white matter abnormalities in patients with MCI (Sexton et al., 2011), with the cingulum, fornix and corpus callosum emerging as the best predictors of disease progression (Nowrangi et al., 2013), with a reported accuracy of almost 96%. Furthermore, adding FA measurement in the cingulum to the various MRI markers has been found to improve the accuracy of classification of patients with MCI from 77% to 91% (Gold et al., 2012). Nevertheless, several studies reported no changes in FA or MD in MCI patients. In addition to methodological differences, a possible explanation for these different results could be that in prodromal AD there are only minor changes in brain diffusivity. In one study, the first sign of change in MCI was an increase in DA, while increased RD better identified patients with AD (Acosta-Cabronero et al., 2012).
Magnetic resonance spectroscopy
Magnetic resonance spectroscopy (MRS) is a relatively rapid technique that, using a conventional MRI machine, provides a detailed picture of the in vivo biochemistry of the brain. Spectra can be obtained either from a single voxel of interest or from multiple areas (multivoxel spectroscopy) simultaneously, making the technique sensitive to regional changes in metabolites. It is non-invasive and free from radiation, which means that it can be used for monitoring disease progression in patients.
Due to the inverse relationship between scanner field intensity and minimum detectable concentration, only those metabolites present in high enough concentrations can be detected by scanners approved for use in humans. The ones most commonly studied are N-acetyl aspartate (NAA), choline (Cho), creatine (Cr), myoinositol (mI) and glutamate and glutamine (Glx), and they are sensitive to different pathological processes.
NAA is thought to be synthesized only within neurons and its concentration reflects neuronal density and viability. The Cho signal is mainly due to the presence of free glycerophosphocholine and phosphocholine; it increases when the cell membrane has broken down and is therefore used as a marker of membrane integrity. Cr and phosphocreatine are markers of energy metabolism. The levels of Cr are thought to be fairly constant in the brain and are therefore used as a reference value. Instead, mI is an osmolyte with a role in the second messenger system; it is a marker of glial activation. Glx, key aminoacids in the brain, appear as a single peak but can be resolved into individual wavelets in high-field MRI or less accurately using post-processing methods. Different regions of the brain have been studied using MRS, and the posterior cingulate and hippocampus show the most marked alterations. The white matter and then the gray matter of the posterior regions, the temporoparietal areas, and the prefrontal and medial temporal lobes have also been studied. A recent meta-analysis reported that NAA was significantly reduced in the posterior cingulate and bilateral hippocampus (with a major effect on the left side) in AD patients. The NAA/Cr ratio decreased markedly in the posterior cingulate. Simultaneously, an elevated mI/Cr ratio was found not only in the posterior cingulate but also in the parietal gray matter. These metabolites might therefore be potential biomarkers of brain dysfunction in AD (Wang et al., 2015).
To evaluate whether these metabolite changes are signs of early degeneration in MCI, a meta-analysis of data derived from 607 MCI patients was conducted (Tumati et al., 2013). As in AD patients, NAA measures were consistently reduced in the posterior cingulate, hippocampus and paratrigonal white matter, while Cr concentration was reduced in the hippocampus and parietal white matter. These results suggest that quantification of these metabolites with MRS is a reliable marker for differentiating MCI subjects from healthy elderly and may also contribute to the diagnosis of neurodegenerative dementia.
To define the potential of MRS in the identification of patients with MCI at risk of developing dementia, 41 subjects with amnestic MCI were studied with MRS for a year, measuring a series of metabolite ratios (NAA/Cr, Cho/Cr, ml/Cr, Glx/Cr and NAA/Cho) in the posterior cingulate gyrus, hippocampus and parietal lobe. It was found that in subjects who developed dementia, NAA/Cr in the hippocampus was significantly lower than in controls, but no differences were found between MCI patients who converted to dementia and those who remained stable (Targosz-Gajniak et al., 2013).
Technical improvement of MR technology has led to the development of scanners operating at ultra-high magnetic fields, from 7T up to 11.7T, with the potential for higher image resolution with finer definition of anatomical details; this is expected to increase the accuracy of quantification of brain atrophy, and to improve spectra definition and quantification in MRS (Balchandani and Naidich, 2015). Moreover, a potential new biomarker for AD is the increased cortical phase shift demonstrated in AD subjects on T2*-weighted images obtained with a 7T scanner, a parameter sensitive to iron content that may reflect amyloid pathology (van Rooden et al., 2014).
Positron emission tomography for the assay of neuronal dysfunction and amyloid burden
Functional brain imaging with PET is widely used to identify regions of the brain involved in cognitive and various other neurological functions, as it allows the identification of changes in regional glucose metabolism related to altered brain function.
Two major approaches are available: the assessment of neuronal function using [18F]FDG, and the assessment of amyloid deposition by means of amyloid-seeking radioactively labeled radiopharmaceuticals.
[18F]FDG is the radiopharmaceutical most commonly used in PET imaging. It was developed in the 1970s for the study of cerebral metabolism. Deoxyglucose is an analog of glucose that can be labeled with a radioactive isotope of fluorine. In this form it is phosphorylated, in neurons and glial cells, to [18F]FDG-6-phosphate.
Unlike what happens with glucose, however, [18F]FDG-6-phosphate is not further metabolized by the isomerase enzyme; instead, in relation to aerobic glycolysis, it accumulates in the cells of the nervous system. In the brain, aerobic glycolysis is the energy source that supports biosynthesis and neuroprotection; it produces about 95% of the ATP necessary for the maintenance of neuronal activity and it differs according to anatomical area and between gray and white matter (Vlassenko and Raichle, 2015); furthermore, changes in neuronal activity, both in physiological and pathological conditions, determine regional variations in glucose metabolism; for this reason, glucose metabolism is considered a biomarker of neuronal function. [18F]FDG, easily detectable in qualitative PET studies, and behaving like glucose, is considered an ideal probe for assessing neuronal degeneration, and for the diagnosis of cognitive impairment and dementia.
Although early studies were analyzed mostly through visual interpretation by expert readers, a quantitative approach by computerized image processing methods is (as with MRI) the most suitable for detecting, objectively, metabolic changes at resolutions higher than the human eye can detect. Indeed, the use of an automatic tool for the analysis of [18F]FDG imaging improves the diagnostic accuracy in dementia and pre-dementia compared to visual inspection (Perani et al., 2014).
In a large series of patients with symptoms of cognitive decline or behavioral change, cerebral uptake of [18F]FDG was found to be impaired as a consequence of neurodegeneration, and was able to predict an ensuing progressive course with a sensitivity of 91%, while a non-progressive course could be predicted with a specificity of 75% when compared with clinical follow-up. In cases with autopsy-proven AD pathology, PET correctly identified the presence or absence of AD in 88% of cases, with a sensitivity of 94% and a specificity of 73% (Silverman et al., 2001).
More important, [18F]FDG PET shows distinct patterns of cortical distribution of the radiopharmaceutical in relation to different subtypes of neurodegenerative dementia. This, allowing more accurate diagnosis, has practical consequences for patient management (Shivamurthy et al., 2015). Instead, the finding of normal distribution of [18F]FDG in the brain may suggest a different diagnosis, such as depression or other potentially reversible conditions.
In patients with AD, the typical pattern consists of reduced metabolism in the limbic system (parahippocampal gyrus and posterior cingulate), precuneus, posterior parietal cortex and temporal cortex (Fig. 4).
Figure 4.
[18F]FDG PET of a patient with dementia.
Severely reduced [18F]FDG uptake is seen in the parietal lobe (top left) and in the mesial, ventral and lateral cortex of the temporal lobes (top right). Automatic voxel-based analysis of PET images compared with a group of age-matched healthy controls showed a high level of statistical significance (blue areas) of the hypometabolism in the parietal and temporal lobes. Note the presence of small areas of hypometabolism in the left frontal lobe, too. This is the pattern of alterations typically detected in AD.
In more advanced forms of AD, the hypometabolism involves the associative prefrontal cortex and the frontal lobe, but the cortex of the anterior cingulate gyrus is typically spared, as are the primary sensory areas and primary motor cortex.
Perhaps the real clinical significance of [18F]FDG PET in the evaluation of patients with cognitive impairment is the fact that it offers the possibility of identifying different patterns in different neurodegenerative diseases. FTD is the most common form of dementia in individuals under 65 years of age. Different forms have been described: the behavioral variant (bvFTD), semantic dementia, and progressive aphasia. In bvFTD, which accounts for about 75% of cases, patients do not generally show significant memory deficits, unlike subjects with AD, but marked changes in behavior and personality, with a decline in interpersonal social conduct. Patients may become socially disinhibited and apathic and present reduced empathy. In these patients, [18F]FDG PET typically detects reduced metabolism in the frontal lobe, both in the medial and dorsolateral areas, usually related to the signs of atrophy detectable with MRI, as well as reduced metabolism in the caudate nuclei, the anterior cingulate gyrus, amygdala and hippocampus (Buhour et al., 2016). These results support the hypothesis of a deficit of a wide frontotemporal network, where apathy is related to the hypometabolism in the ventral tegmental area, disinhibition to the involvement of the anterior temporal lobes, amygdala and hippocampi, and eating disorders to alterations in the insula and in the lateral frontal cortex.
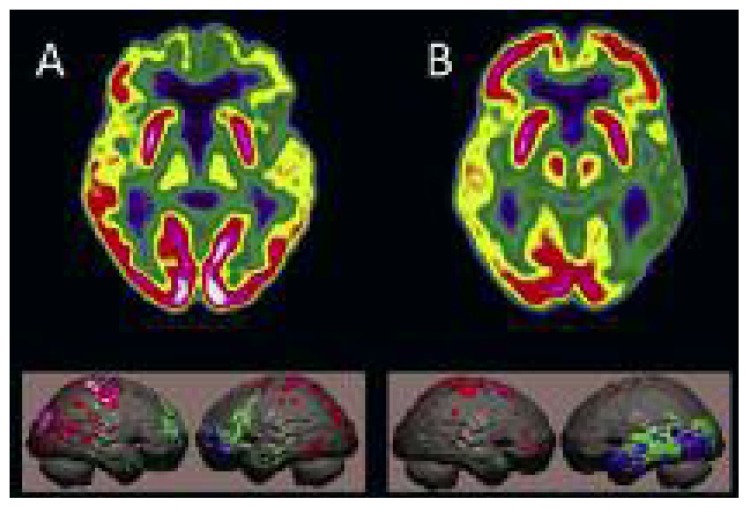
With the use of optimized voxel-based methods it has become possible to recognize, at single-patient level, differences between two variants of bvFTD, namely the “frontal” and “temporo-limbic” forms, which are correlated with differences in cognitive profiles (Cerami et al., 2016). In the primary progressive aphasia variant, patients show a progressive, insidious decline in linguistic skills during the initial phase of the disease. [18F]FDG PET detects areas of hypometabolism lateralized to the left, consistent with the aphasic disorder, in the frontal and/or in the temporal lobe, in relation to the different clinical variants (Matías-Guiu et al., 2015). The less frequent semantic variant of FTD is characterized by progressive anomia, reflecting a fundamental loss of semantic memory, with less impairment of executive functions, while memory and visuospatial functions are generally normal. In these patients, [18F]FDG PET detects marked alterations of cortical metabolism in the temporal lobe (Fig. 5), in parallel with gray matter atrophy detectable on MRI; the degree of hypometabolism in the frontal cortex is associated with the level of behavioral alterations measurable with neuropsychological tests (Iaccarino et al., 2015).
Figure 5.
Differentiation of FTD variants using [18F]FDG PET.
A) 74-year-old woman with bvFTD: marked reduction of glucose metabolism in the polar and orbital cortex of the frontal lobe, with minimal reduction in the temporal lobes. B) 80-year-old female with semantic dementia: severe reduction of metabolism in the left temporal lobe, sparing of the frontal lobe.
DLB is clinically characterized by progressive dementia that is frequently accompanied by parkinsonism and visual hallucinations. Single-photon emission computed tomography with tracers for the dopamine reuptake system can support the diagnosis as these tracers can detect early signs of neurodegeneration (Del Sole et al., 2015). [18F]FDG PET also plays a supportive role in the diagnosis of DLB, as a significant metabolic reduction in the occipital cortex can be detected, particularly in the primary visual cortex, and this allows DLB to be distinguished from AD with high sensitivity and specificity (Chiba et al., 2014). Furthermore, [18F]FDG PET studies have detected a side difference in the location of hypometabolism of the occipital cortex which may allow DLB to be differentiated from posterior cortical atrophy (Spehl et al., 2015). In patients with clinically-diagnosed DLB, different patterns of hypometabolism differentiate individuals with or without REM sleep behavior disorder, suggesting differences in the underlying pathology (Iaccarino et al., 2016). Other neurodegenerative diseases, including visuospatial deficits associated with simultanagnosia, dysgraphia, poly-mini-myoclonus and oculomotor apraxia, can also be differentiated from DLB and AD on the basis of their specific patterns of [18F]FDG distribution.
The challenge of AD imaging is not only to succeed in describing the final stages of the disease; rather, the main challenge is to recognize its signs in the earliest stages, which precede overt dementia by a decade or more. This aspect is difficult to investigate, yet there is a growing consensus that the earliest stages are the ones at which that the most effective preventive therapies will be applied. In this context, objective quantification of metabolic deficits is of the outmost relevance. MCI is a syndrome that includes a spectrum of conditions characterized by deficits in cognitive functions (memory, language, visuospatial skills, and executive functioning) that, not severe enough to be defined as dementia, constitute a transitional condition between normal cognitive functioning and dementia.
In MCI subjects with intact abilities of daily living, the detection, on [18F]FDG PET imaging, of a pattern of altered metabolism in temporoparietal areas (typical of AD) is associated with a high risk of an AD diagnosis in later years. In patients who have subjective memory complaints and isolated memory deficits on formal testing, but show normal cognitive function, a marked metabolic reduction in the posterior cingulate cortex has been found to best differentiate amnestic MCI from normal elderly subjects, while lateral parietal hypometabolism can differentiate amnestic MCI from AD (Del Sole et al., 2008; Clerici et al., 2009). Also, an AD-positive metabolic pattern shown by voxel-based methods was, compared with p-tau/Aβ42 ratio, the better predictor of conversion from MCI to AD, offering solid support for diagnosis at the individual level, showing high accuracy, and increasing the diagnostic confidence (Perani et al., 2016).
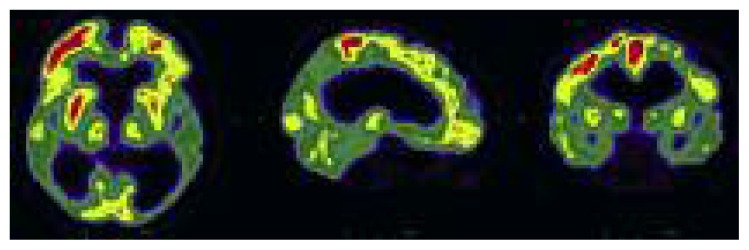
[18F]FDG PET has also been shown to be accurate in the early identification of dementia subtypes. In patients with MCI, the identification, on PET studies, of areas of hypometabolism consistent with patterns reminiscent of AD, FTD or DLB is associated with clinical progression to the different types of dementia (with a positive post-test probability of 100%) and with decline in multiple cognitive outcome measures (Cerami et al., 2015). In prodromal DLB patients, [18F]FDG PET can detect reduced metabolism in the primary visual cortex (Fig. 6); when this finding is associated with hypometabolism in the parietal and the lateral occipital cortex it may represent a greater risk of conversion to DLB (Fujishiro et al., 2013).
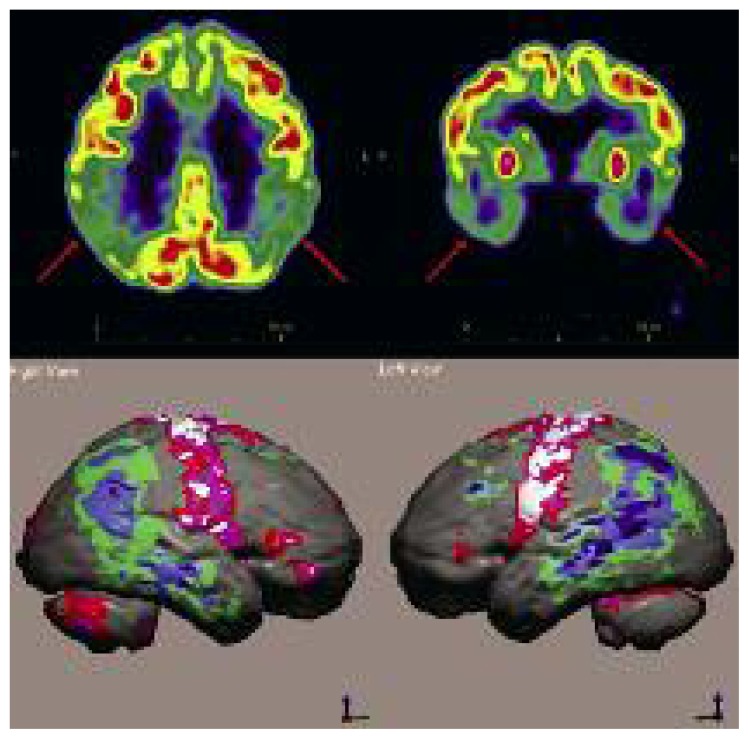
Figure 6.
Extensive areas of severely reduced [18F]FDG uptake, bilaterally, in the parietal, temporal and occipital lobes.
Note the low uptake in the primary visual cortex, which suggests a diagnosis of DLB.
In normal subjects, [18F]FDG PET is able to detect a progressive and diffuse reduction of regional consumption of glucose that parallels advancing atrophy (mainly in the frontal, temporal and parietal areas) and age. Even in the absence of cortical atrophy, a progressive reduction of metabolism in the mesial frontal region can be detected, with possible gender differences (Kakimoto et al., 2016).
In cognitively normal subjects with risk factors for AD, PET can detect changes in cerebral metabolism in the very cortical areas involved in AD. For example, elderly patients with type 2 diabetes and peripheral insulin resistance show extensive reduction of cerebral glucose metabolism, particularly significant in the hippocampus, mesial temporal lobe, posterior and rostral cingulate, precuneus and cuneus, consistent with that which precedes the development of clinical AD.
Unlike the familial forms of AD, the risk of developing the more common late-onset form appears to be influenced only in part by genetic factors; the gene encoding apolipoprotein E (ApoE), located on chromosome 19q13, is the one most strongly correlated with the risk of developing late-onset AD. In subjects who are cognitively normal but with one or both parents affected by AD, FDG PET studies have shown alterations similar to those found in patients with AD pathology (Mosconi et al., 2014). Like MRI, [18F]FDG PET is not presently considered suitable for defining preclinical AD (Dubois et al., 2016).
Amyloid imaging
There is increasing evidence supporting the “amyloid cascade” hypothesis, in other words the hypothesis that abnormal processing and clearance of Aβ proteins, eventually leading to an accumulation of plaques of longer amyloid-β species (particularly Aβ40 and Aβ42), is the start of a series of events that eventually leads to AD neurodegeneration. The accumulation of Aβ in the brain may occur as a result of overproduction of this protein or its reduced clearance from the brain. It is now becoming clear that in sporadic late-onset AD there is an age-related failure of pathways involved in the elimination of Aβ from the brain (Duara et al. 2015).
The “amyloid cascade hypothesis” has prompted the development of radiopharmaceuticals that, able to bind to amyloid plaques, allow imaging of the amyloid load in the brain.
The first amyloid-seeking radiopharmaceutical used for cerebral amyloid burden imaging was 2-(1-(6-[(2-[18F] fluoroethyl)(methyl)amino]-2-naphthyl)ethylidene) ma lo nonitrile ([18F]FDDNP) followed by the currently termed [11C]Pittsburgh Compound-B or [11C]PiB, which was developed by investigators at the University of Pittsburgh, by labeling a derivative of thioflavin-S with carbon-11. To overcome the disadvantages of the short half-life of carbon-11, several research teams have sought to develop fluorine-18-labeled amyloid-seeking tracers. To date, three commercial radiopharmaceuticals have been approved by the European Medicines Agency and by the Food and Drug Administration in the USA: [18F]florbetapir (Amyvid®, Eli Lilly, Utrecht, The Netherlands) (Lin et al., 2016), [18F]flutemetamol (Vizamyl®, GE Healthcare Ltd, Little Chalfont, United Kingdom) (Heurling et al., 2016), and [18F]florbetaben (Neuraceq®, Piramal Imaging Ltd, Havant, United Kingdom) (Sabri et al., 2015). It has been demonstrated that all these substances bind to amyloid fibrils consistently with pathology findings. This fact has changed the view of AD as a clinical-biological entity and raises the prospect of amyloid imaging being introduced into the core diagnostic pathway. For example, whereas MCI patients with a positive amyloid PET scan have a high risk of converting to AD in comparison with MCI patients with a negative amyloid PET scan (Nordberg et al., 2013), a negative amyloid PET scan in patients with dementia excludes AD with high probability, given that neuritic amyloid plaques are a constant finding in patients with AD. A recent meta-analysis of the diagnostic accuracy of fluorinated amyloid tracers found a high sensitivity (95%, with confidence interval 89–97%) while the specificity values ranged more widely, from 63% to 93%, without noticeable differences between the different agents (Morris et al., 2016). The negative likelihood ratios were all smaller than 0.2 and some were <0.1 indicating almost no likelihood of AD when amyloid PET imaging is negative, in accordance with the current label indication. Results obtained by PET with amyloid radiopharmaceuticals should not be considered as diagnostic for AD, but rather to reflect of the presence, or absence, of cerebral amyloid deposition (Frey and Perani, 2015). In fact, demonstration of elevated cerebral Aβ does not conclusively establish a diagnosis of AD, but it does confirm beta-amyloidosis of the brain, and this condition has been hypothesized to lead eventually to AD or other neurodegenerative entities. Aβ deposition may, in fact, occur in disorders other than AD, such as DLB and amyloid angiopathy, but is not found in certain other neurodegenerative disorders, such as those of the FTD spectrum, Creutzfeld-Jacob disease, and others. It was recently reported that amyloid PET imaging was positive in all patients in a series of 25 subjects with a clinical diagnosis of posterior cortical atrophy (Singh et al., 2015). Also in primary progressive aphasia, significant, although asymmetric, deposition of amyloid has been demonstrated with PET (Martersteck et al., 2016). On the other hand, amyloid imaging was reported as negative in patients with other frontotemporal dementias (Drzezga et al., 2008; Kobylecki et al., 2015).
Amyloid imaging reveals the accumulation of neuritic plaques in 60% of MCI patients, which is consistent with the incidence of AD pathology in autopsy studies (Jack et al., 2013). The probability of a positive scan is higher in amnestic MCI (68%) than in non-amnestic MCI (19%) and when other biomarkers of neurodegeneration are detected (Banzo et al., 2016). In a series of 344 MCI patients, the effect of ApoE ɛ4 on amyloid uptake was highly significant, with ApoE ɛ4-positive MCI patients showing a greater mean plaque density than ɛ4-negative AD patients (Murphy et al., 2013). On the other hand, patients with major depressive disorders presented elevated retention only when MCI was associated, whereas in depressed patients without MCI tracer retention was low (Wu et al., 2016).
The use of amyloid imaging extends beyond AD. Comprehensive reviews of its use in Lewy body diseases, cerebral amyloid angiopathy, brain trauma and Down’s syndrome have recently been published (Catafau and Bullich, 2015; Frey and Petrou, 2015); further research will clarify these issues, possibly increasing the spectrum of applications for amyloid PET imaging (Frey and Perani, 2015).
From a clinical point of view, it has to be underlined that the systematic use of [18F]FDG PET and amyloid PET scans in MCI in clinical practice is still not supported by evidence-based analysis of clinical effectiveness, given the considerable variability of specificity values and the limited effects of the currently available treatments for AD (Smailagic et al., 2015). The Alzheimer Association and the Society of Nuclear Medicine and Molecular Imaging have proposed some recommendations on the appropriate use of amyloid imaging in patients with persistent or progressive unexplained persistent MCI, in AD patients with an atypical course or an etiologically mixed presentation, and in patients with progressive dementia and atypically early age at onset (Johnson et al., 2013). The use of amyloid PET scanning in clinical practice provides unique information on the amyloid burden that may change a diagnosis of neurodegenerative dementia in a varying proportion of patients and therefore influence treatment strategies. Moreover, a positive amyloid PET scan makes the neurologist more confident in the diagnosis of AD, with practical implications for therapeutic management and information to patients and caregivers (Nordberg, 2015). From a practical point of view, there are still other limitations to the extensive use of amyloid PET. Even though the use of these radiopharmaceuticals has been authorized by the monitoring agencies for several years, in many cases, national health systems and insurance companies have not yet defined the criteria of eligibility for PET examinations, and this limits patients’ access to them (Herscovitch, 2015).
When [18F]FDG and amyloid imaging are obtained in the same subject, the pattern of distribution of these two radiopharmaceuticals differs considerably (Fig. 7): although in parietal associative areas both a high load of amyloid deposition and severe hypometabolism are detected, in the frontal lobe, where amyloid deposition is usually high, metabolic impairment is usually low. At present the significance of these radiopharmaceutical distribution differences is not known, but it is interesting to note that unlike amyloid imaging, [18F]FDG PET can better identify the changes in brain metabolism that mark the transition to AD dementia (Roy et al., 2014).
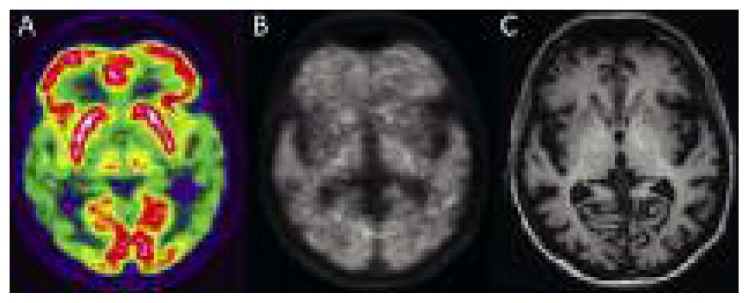
Figure 7.
Images of a patient with mild dementia.
(A) [18F]FDG, (B) [18F]florbetaben, and (C) T1-weighted MPRAGE MRI images show severe bilateral reduction of glucose metabolism in both temporoparietal regions, associated with diffuse deposition of amyloid-seeking tracer in the gray matter. MRI shows mild enlargement of cortical sulci. (Image courtesy of Prof. Diego Cecchin, Department of Medicine (DIMED), Nuclear Medicine, University of Padua, Italy).
The accumulation of amyloid in cognitively normal subjects has been described ever since the validation studies of amyloid-imaging radiopharmaceuticals. The likelihood of having a positive amyloid PET increases with patient age, as does the detection of amyloid on autopsy. Also the ApoE ɛ4 genotype, which represents a risk factor for the development of AD in the elderly, is positively correlated with the accumulation of amyloid. Other risk factors, such as high blood pressure and, in particular, inadequately treated/controlled hypertension, are associated with a high probability of Aβ accumulation (Rodrigue et al., 2013). The evolution of amyloid imaging has also raised new hypotheses on the complex interrelations between patterns of neuronal degeneration and amyloid deposition, imaging findings with both [18F]FDG and amyloid-seeking tracers, other biomarkers, particularly CSF assay, and the different clinical syndromes.
Recently, a meta-analysis that measured the prevalence of amyloid in more than 2,900 cognitively normal subjects aged between 18 and 100 years found that age is a major risk factor that predicts the positivity of PET but, above all, that amyloid detection precedes dementia onset by 20–30 years (Jansen et al., 2015).
The importance of a positive amyloid PET study must not be underestimated; in fact, one study showed the existence of a correlation between a positive PET scan and reduced thickness of the cortical gray matter in the precuneus and hippocampi and disorders of episodic memory in cognitively normal subjects (Doré et al., 2013). The same study also found that gray matter atrophy in the temporal lobes progressed faster in subjects with a positive PET than in those who have a negative PET.
The use of amyloid PET imaging is considered appropriate in patients with persistent and progressive unexplained MCI, in patients with established dementia with an atypical clinical course or etiology, and in young patients with atypical-onset dementia. It has also been shown that when amyloid imaging is performed in memory clinic settings the diagnostic confidence may change in 20 to 50% of patient series (Nordberg, 2015).
PET imaging of amyloid burden is becoming an established procedure and will likely complement the use of 18F-FDG PET in MCI while new PET tracers for imaging of tau deposition and the assessment of inflammatory processes (Varrone and Nordberg, 2015) in the brain will further increase understanding of the time course AD disease pathology and clinical progression.
As with other neuroimaging techniques, quantification of amyloid burden in the brain plays a crucially important role. Furthermore, given that positive scans increase in proportion with the increasing age of the subjects examined, quantification of the amyloid burden could, by differentiating between low and high amyloid load, provide a means of differentiating the normal aging process associated with amyloid deposits from AD (Herholz, 2014).
Hybrid PET/MR imaging
Over the past 25 years, the understanding and use of MRI and PET in dementia have advanced in parallel and independently and it has become the clinician’s task to integrate the results of each technique in a single patient in order to refine the diagnosis. Indeed multimodal imaging and the combined evaluation of PET and MRI data increase diagnostic accuracy substantially (Dukart et al., 2011).
Recently, hybrid PET/MRI scanners have become available and they seem to be the most promising instruments from the perspective of the search for sensitive biomarkers of dementia (Garibotto et al., 2013). The use of this hybrid technology has several advantages. First, all the important information is captured in a single session. The examination therefore takes less time than the sum of separate examinations — PET or computed tomography (CT) and MRI —, which are performed at different times and, occasionally, in different locations. It may therefore be more convenient for patients with dementia and for their caregivers and increase compliance. The simultaneous acquisition of PET and MRI also allows the introduction of patient motion correction methods, thereby limiting a common cause of artifacts in PET imaging.
A further advantage of simultaneous acquisition is that it allows simultaneous comparison of different biomarkers. This is particularly important in research settings, as it increases the homogeneity of the data collected, such as disease stage and drug effectiveness. In addition, hybrid technology is also associated with lower radiation exposure, and CT scanning could in some cases be avoided. From a technical point of view, hybrid imaging also has other advantages: PET imaging, due to its limited spatial resolution, is affected by the underlying cortical atrophy, with possible overestimation of metabolic deficits and underestimation of the amount of amyloid in the case of cortical atrophy. The application of corrections derived from the MRI data is expected to allow more precise quantification of the parameters derived from PET (Drzezga et al., 2014).
Experience with hybrid PET/MRI in dementia is still partial due to the limited number of available scanners. Some technical issues still exist: considerable region-dependent differences have been observed between [18F]FDG brain imaging data acquired on PET/MR compared with corresponding PET/CT images (Hitz et al., 2014), with quantitative errors of up to 20%. Accordingly, there is a need for further improvement of the technique (Cabello et al., 2016).
It was recently demonstrated that amyloid PET/MRI hybrid imaging is well tolerated by patients and allows accurate visual identification and quantification of the amyloid burden and of medial temporal lobe atrophy, allowing rapid classification of patients according to the IWG-2 criteria (Schütz et al., 2016).
It is possible to envisage that, before too long, hybrid PET/MRI may be a “one-stop shop” able to provide information on molecular neuropathology (from amyloid PET) and structural atrophy and neuronal deafferentation (from MRI) in a single visit.
Concluding remarks
Primary neurodegenerative diseases require extensive clinical evaluation associated with neuropsychological tests and assay of the Aβ42, tau and p-tau protein levels in the CSF, but these examinations can only suggest the probability of neurodegenerative dementias, and the specificity of the diagnosis remains limited.
Over the past decade, neuroimaging features detected on MRI and PET have emerged as reliable surrogate biomarkers. Volumetric MRI is widely used to stage AD patients. The identification of atrophy alone lacks specificity for AD, but it is a valuable tool for evaluating disease progression. Quantification of local atrophy with automatic morphometry software provides a topographical marker of neurodegeneration, with the hippocampus and enthorinal cortex being the most affected regions and predictive of a further worsening of cognitive functions in the future. MR tractography with DTI detects demyelination of the white matter in vivo. Many studies have reported widespread changes in the cingulum, fornix and corpus callosum, both in AD and in MCI. The MRS technique identifies changes in brain metabolites; in dementia and MCI, the reduction of NAA in the posterior cingulate, hippocampus and the paratrigonal white matter is a sign of reduced neuronal viability.
PET imaging with [18F]FDG can identify specific patterns of reduced glucose metabolism that are associated with different neurodegenerative diseases, and constitute surrogate markers of the ongoing pathological process. In particular, bilateral temporoparietal hypometabolism is a signature of AD, diffuse hypometabolism in the frontal cortex is commonly found in FTD, while asymmetric temporal hypometabolism is usually reported in FTD variants. Specific patterns of hypometabolism have also been described in DLB and other neurodegenerative dementias. Alterations in glucose metabolism have been consistently described in MCI patients who convert to AD. More recently, the availability of radiopharmaceuticals that bind to amyloid has extended the role of PET, which can now be used to quantify the amyloid load in the brain as a surrogate marker of pathology. Amyloid PET shows a high specificity for Aβ plaques, and a positive scan is an expression of neurodegenerative disease, although not specific for AD. A positive amyloid PET scan in a healthy subject is an early sign of preclinical dementia. On the other hand, a negative scan excludes AD pathology.
The recent availability of hybrid PET/MRI scanners has encouraged the synergistic use of the information derived from each technique performed separately. Although some technical issues still need to be resolved so that the techniques achieve an adequate clinical quality level, early clinical experience has demonstrated the advantages of hybrid imaging. This sophisticated technique can probably be used most efficiently for the concurrent detection of diagnostic markers of neurodegenerative pathology (with amyloid PET) and monitoring of the progression of markers (with volumetric MRI) in order to arrive, rapidly, at reliable diagnosis of neurodegenerative disease.
References
- Acosta-Cabronero J, Alley S, Williams GB, et al. Diffusion tensor metrics as biomarkers in Alzheimer’s disease. PLoS One. 2012;7:e49072. doi: 10.1371/journal.pone.0049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balchandani P, Naidich TP. Ultra-high-field MR neuroimaging. AJNR Am J Neuroradiol. 2015;36:1204–1215. doi: 10.3174/ajnr.A4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banzo I, Jiménez-Bonilla JF, Martínez-Rodríguez I, et al. Patterns of 11C-PIB cerebral retention in mild cognitive impairment patients. Rev Esp Med Nucle imagen Mol. 2016;35:171–174. doi: 10.1016/j.remn.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Laakso MP, Bresciani L, et al. The MRI pattern of frontal and temporal brain atrophy in fronto-temporal dementia. Neurobiol Aging. 2003;24:95–103. doi: 10.1016/s0197-4580(02)00045-3. [DOI] [PubMed] [Google Scholar]
- Boutet C, Chupin M, Lehéricy S, et al. Detection of volume loss in hippocampal layers in Alzheimer’s disease using 7 T MRI: a feasibility study. Neuroimage Clin. 2014;5:341–348. doi: 10.1016/j.nicl.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhour MS, Doidy F, Laisney M, et al. Pathophysiology of the behavioral variant of frontotemporal lobar degeneration: A study combining MRI and FDG-PET. Brain Imaging Behav. 2016 doi: 10.1007/s11682-016-9521-x. (In press) [DOI] [PubMed] [Google Scholar]
- Cabello J, Lukas M, Rota Kops E, et al. Comparison between MRI-based attenuation correction methods for brain PET in dementia patients. Eur J Nucl Med Mol Imaging. 2016;43:2190–2200. doi: 10.1007/s00259-016-3394-5. [DOI] [PubMed] [Google Scholar]
- Catafau AM, Bullich S. Amyloid PET imaging: applications beyond Alzheimer’s disease. Clin Transl Imaging. 2015;3:39–55. doi: 10.1007/s40336-014-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami C, Dodich A, Lettieri G, et al. Different FDG-PET metabolic patterns at single-subject level in the behavioral variant of fronto-temporal dementia. Cortex. 2016;83:101–112. doi: 10.1016/j.cortex.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Cerami C, Della Rosa PA, Magnani G, et al. Brain metabolic maps in mild cognitive impairment predict heterogeneity of progression to dementia. Neuroimage Clin. 2015;7:187–194. doi: 10.1016/j.nicl.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y, Iseki E, Fujishiro H, et al. Primary visual cortical metabolism and rapid eye movement sleep behavior disorder in dementia with Lewy bodies. Psychiatry Clin Neurosci. 2014;68:137–144. doi: 10.1111/pcn.12101. [DOI] [PubMed] [Google Scholar]
- Clerici F, Del Sole A, Chiti A, et al. Differences in hippocampal metabolism between amnestic and non-amnestic MCI subjects: automated FDG-PET image analysis. Q J Nucl Med Mol Imaging. 2009;53:646–557. [PubMed] [Google Scholar]
- Del Sole A, Perini G, Lecchi M, et al. Correlation between 123I-FP-CIT brain SPECT and parkinsonism in dementia with Lewy bodies: caveat for clinical use. Clin Nucl Med. 2015;40:32–35. doi: 10.1097/RLU.0000000000000602. [DOI] [PubMed] [Google Scholar]
- Del Sole A, Clerici F, Chiti A, et al. Individual cerebral metabolic deficits in Alzheimer’s disease and amnestic mild cognitive impairment: an FDG PET study. Eur J Nucl Med Mol Imaging. 2008;35:1357–1366. doi: 10.1007/s00259-008-0773-6. [DOI] [PubMed] [Google Scholar]
- Doré V, Villemagne VL, Bourgeat P, et al. Cross-sectional and longitudinal analysis of the relationship between Aβ deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer disease. JAMA Neurol. 2013;70:903–911. doi: 10.1001/jamaneurol.2013.1062. [DOI] [PubMed] [Google Scholar]
- Drzezga A, Barthel H, Minoshima S, Sabri O. Potential Clinical Applications of PET/MR Imaging in Neurodegenerative Diseases. J Nucl Med. 2014;55:47–55. doi: 10.2967/jnumed.113.129254. [DOI] [PubMed] [Google Scholar]
- Drzezga A, Grimmer T, Henriksen G, et al. Imaging of amyloid plaques and cerebral glucose metabolism in semantic dementia and Alzheimer’s disease. Neuroimage. 2008;39:619–633. doi: 10.1016/j.neuroimage.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Duara R, Barker W, Loewenstein D, et al. Insights into cognitive aging and Alzheimer’s disease using amyloid PET and structural MRI scans. Clin Transl Imaging. 2015;3:65–74. [Google Scholar]
- Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- Dukart J, Mueller K, Horstmann A, et al. Combined evaluation of FDG-PET and MRI improves detection and differentiation of dementia. PLoS One. 2011;6:e18111. doi: 10.1371/journal.pone.0018111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey KA, Petrou M. Imaging amyloidopathy in Parkinson disease and parkinsonian dementia syndromes. Clin Transl Imaging. 2015;3:57–64. doi: 10.1007/s40336-015-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey KA, Perani D. Amyloid PET imaging: a challenge for research in clinical neuroimaging. Clin Transl Imaging. 2015;3:3–5. [Google Scholar]
- Frisoni GB, Prestia A, Rasser PE, et al. In vivo mapping of incremental cortical atrophy from incipient to overt Alzheimer’s disease. J Neurol. 2009;256:916–24. doi: 10.1007/s00415-009-5040-7. [DOI] [PubMed] [Google Scholar]
- Fujishiro H, Iseki E, Kasanuki K, et al. A follow up study of non-demented patients with primary visual cortical hypometabolism: prodromal dementia with Lewy bodies. J Neurol Sci. 2013;334:48–54. doi: 10.1016/j.jns.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Garibotto V, Förster S, Haller S, et al. Molecular neuroimaging with PET/MRI. Clin Transl Imaging. 2013;1:53–63. [Google Scholar]
- Gold BT, Jiang Y, Powell DK, et al. Multimodal imaging evidence for axonal and myelin deterioration in amnestic mild cognitive impairment. J Alzheimers Dis. 2012;31(Suppl 3):S19–31. doi: 10.3233/JAD-2012-112165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herholz K. The role of PET quantification in neurological imaging: FDG and amyloid imaging in dementia. Clin Transl Imaging. 2014;2:320–330. [Google Scholar]
- Herscovitch P. Regulatory approval and insurance reimbursement: the final steps in clinical translation of amyloid brain imaging. Clin Transl Imaging. 2015;3:75–77. [Google Scholar]
- Heurling K, Leuzy A, Zimmer ER, et al. Imaging β-amyloid using [(18)F]flutemetamol positron emission tomography: from dosimetry to clinical diagnosis. Eur J Nucl Med Mol Imaging. 2016;43:362–373. doi: 10.1007/s00259-015-3208-1. [DOI] [PubMed] [Google Scholar]
- Hitz S, Habekost C, Fürst S, et al. Systematic comparison of the performance of integrated whole-body PET/MR imaging to conventional PET/CT for 18F-FDG brain imaging in patients examined for suspected dementia. J Nucl Med. 2014;55:923–931. doi: 10.2967/jnumed.113.126813. [DOI] [PubMed] [Google Scholar]
- Iaccarino L, Marelli S, Iannaccone S, et al. Severe brain metabolic decreases associated with REM sleep behavior disorder in dementia with Lewy bodies. J Alzheimers Dis. 2016;52:989–997. doi: 10.3233/JAD-151000. [DOI] [PubMed] [Google Scholar]
- Iaccarino L, Crespi C, Della Rosa PA, et al. The semantic variant of primary progressive aphasia: Clinical and neuroimaging evidence in single subjects. PLoS One. 2015;10:e0120197. doi: 10.1371/journal.pone.0120197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Barrio JR, Kepe V. Cerebral amyloid PET imaging in Alzheimer’s disease. Acta Neuropathol. 2013;126:643–657. doi: 10.1007/s00401-013-1185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Barkhof F, Bernstein MA, et al. Steps to standardization and validation of hippocampal volumetry as a biomarker in clinical trials and diagnostic criterion for Alzheimer’s disease. Alzheimers Dement. 2011;7:474–485. doi: 10.1016/j.jalz.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement. 2013;9:e-1–16. doi: 10.1016/j.jalz.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimoto A, Ito S, Okada H, et al. Age-related sex-specific changes in brain metabolism and morphology. J Nucl Med. 2016;57:221–225. doi: 10.2967/jnumed.115.166439. [DOI] [PubMed] [Google Scholar]
- Kobylecki C, Langheinrich T, Hinz R, et al. 18F-florbetapir PET in patients with frontotemporal dementia and Alzheimer disease. J Nucl Med. 2015;56:386–391. doi: 10.2967/jnumed.114.147454. [DOI] [PubMed] [Google Scholar]
- Lin KJ, Hsiao IT, Hsu JL, et al. Imaging characteristic of dual-phase (18)F-florbetapir (AV-45/Amyvid) PET for the concomitant detection of perfusion deficits and beta-amyloid deposition in Alzheimer’s disease and mild cognitive impairment. Eur J Nucl Med Mol Imaging. 2016;43:1304–1314. doi: 10.1007/s00259-016-3359-8. [DOI] [PubMed] [Google Scholar]
- Mak E, Su L, Williams GB, et al. Differential atrophy of hippocampal subfields: a comparative study of dementia with Lewy bodies and Alzheimer disease. Am J Geriatr Psychiatry. 2016;24:136–143. doi: 10.1016/j.jagp.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Martersteck A, Murphy C, Rademaker A, et al. Is in vivo amyloid distribution asymmetric in primary progressive aphasia? Ann Neurol. 2016;79:496–501. doi: 10.1002/ana.24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matías-Guiu JA, Cabrera-Martín MN, Pérez-Castejón MJ, et al. Visual and statistical analysis of 18F-FDG PET in primary progressive aphasia. Eur J Nucl Med Mol Imaging. 2015;42:916–927. doi: 10.1007/s00259-015-2994-9. [DOI] [PubMed] [Google Scholar]
- Möller C, Dieleman N, van der Flier WM, et al. More atrophy of deep gray matter structures in frontotemporal dementia compared to Alzheimer’s disease. J Alzheimers Dis. 2015;44:635–647. doi: 10.3233/JAD-141230. [DOI] [PubMed] [Google Scholar]
- Morris E, Chalkidou A, Hammers A, et al. Diagnostic accuracy of (18)F amyloid PET tracers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2016;43:374–385. doi: 10.1007/s00259-015-3228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Murray J, Tsui WH, et al. Brain imaging of cognitively normal individuals with 2 parents affected by late-onset AD. Neurology. 2014;82:752–760. doi: 10.1212/WNL.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KR, Landau SM, Choudhury KR, et al. Mapping the effects of ApoE4, age and cognitive status on 18F-florbetapir PET measured regional cortical patterns of beta-amyloid density and growth. Neuroimage. 2013;78:474–780. doi: 10.1016/j.neuroimage.2013.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir TM, Villalon-Reina JE, Prasad G, et al. Diffusion weighted imaging-based maximum density path analysis and classification of Alzheimer’s disease. Neurobiol Aging. 2015;36(Suppl 1):S132–140. doi: 10.1016/j.neurobiolaging.2014.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg A. The use of amyloid imaging in clinical praxis: a critical review. Clin Transl Imaging. 2015;3:7–11. [Google Scholar]
- Nordberg A, Carter SF, Rinne J, et al. A European multi-centre PET study of fibrillar amyloid in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2013;40:104–114. doi: 10.1007/s00259-012-2237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowrangi MA, Lyketsos CG, Leoutsakos JM, et al. Longitudinal, region-specific course of diffusion tensor imaging measures in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 2013;9:519–528. doi: 10.1016/j.jalz.2012.05.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantel J, Schröder J, Essig M, et al. In vivo quantification of brain volumes in subcortical vascular dementia and Alzheimer’s disease. An MRI-based study. Dement Geriatr Cogn Disord. 1998;9:309–316. doi: 10.1159/000017082. [DOI] [PubMed] [Google Scholar]
- Perani D, Cerami C, Caminiti SP, et al. Cross-validation of biomarkers for the early differential diagnosis and prognosis of dementia in a clinical setting. Eur J Nucl Med Mol Imaging. 2016;43:499–508. doi: 10.1007/s00259-015-3170-y. [DOI] [PubMed] [Google Scholar]
- Perani D, Della Rosa PA, Cerami C, et al. Validation of an optimized SPM procedure for FDG-PET in dementia diagnosis in a clinical setting. Neuroimage Clin. 2014;6:445–454. doi: 10.1016/j.nicl.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini L, Pievani M, Bocchetta M, et al. Brain atrophy in Alzheimer’s Disease and aging. Ageing Res Rev. 2016;30:25–48. doi: 10.1016/j.arr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Rieck JR, Kennedy KM, et al. Risk factors for β-amyloid deposition in healthy aging: vascular and genetic effects. JAMA Neurol. 2013;70:600–606. doi: 10.1001/jamaneurol.2013.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K, Pepin LC, Philiossaint M, et al. Regional fluorodeoxyglucose metabolism and instrumental activities of daily living across the Alzheimer’s disease spectrum. J Alzheimers Dis. 2014;42:291–300. doi: 10.3233/JAD-131796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri O, Seibyl J, Rowe C, et al. Beta-amyloid imaging with florbetaben. Clin Transl Imaging. 2015;3:13–26. doi: 10.1007/s40336-015-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz L, Lobsien D, Fritzsch D, et al. Feasibility and acceptance of simultaneous amyloid PET/MRI. Eur J Nucl Med Mol Imaging. 2016;43:2236–2243. doi: 10.1007/s00259-016-3462-x. [DOI] [PubMed] [Google Scholar]
- Seo SW, Im K, Lee JM, et al. Effects of demographic factors on cortical thickness in Alzheimer’s disease. Neurobiol Aging. 2011;32:200–209. doi: 10.1016/j.neurobiolaging.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Sexton CE, Kalu UG, Filippini N, et al. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2011;32:2322.e5–e18. doi: 10.1016/j.neurobiolaging.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Shi F, Liu B, Zhou Y, et al. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer’s disease: Meta-analyses of MRI studies. Hippocampus. 2009;19:1055–1064. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- Shivamurthy VK, Tahari AK, Marcus C, et al. Brain FDG PET and the diagnosis of dementia. AJR Am J Roentgenol. 2015;204:W76–85. doi: 10.2214/AJR.13.12363. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. JAMA. 2001;286:2120–2127. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- Singh TD, Josephs KA, Machulda MM, et al. Clinical, FDG and amyloid PET imaging in posterior cortical atrophy. J Neurol. 2015;262:1483–1492. doi: 10.1007/s00415-015-7732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smailagic N, Vacante M, Hyde C, et al. 18F-FDG PET for the early diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI) Cochrane database Syst Rev. 2015;1:CD010632. doi: 10.1002/14651858.CD010632.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spehl TS, Hellwig S, Amtage F, et al. Syndrome-specific patterns of regional cerebral glucose metabolism in posterior cortical atrophy in comparison to dementia with Lewy bodies and Alzheimer’s disease – a [F-18]-FDG PET study. J Neuroimaging. 2015;25:281–288. doi: 10.1111/jon.12104. [DOI] [PubMed] [Google Scholar]
- Targosz-Gajniak MG, Siuda JS, Wicher MM, et al. Magnetic resonance spectroscopy as a predictor of conversion of mild cognitive impairment to dementia. J Neurol Sci. 2013;335:58–63. doi: 10.1016/j.jns.2013.08.023. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Pruessner JC, Faltraco F, et al. Comprehensive dissection of the medial temporal lobe in AD: measurement of hippocampus, amygdala, entorhinal, perirhinal and parahippocampal cortices using MRI. J Neurol. 2006;253:794–800. doi: 10.1007/s00415-006-0120-4. [DOI] [PubMed] [Google Scholar]
- Tumati S, Martens S, Aleman A. Magnetic resonance spectroscopy in mild cognitive impairment: systematic review and meta-analysis. Neurosci Biobehav Rev. 2013;37:2571–2586. doi: 10.1016/j.neubiorev.2013.08.004. [DOI] [PubMed] [Google Scholar]
- van der Flier WM, van Straaten EC, Barkhof F, et al. Medial temporal lobe atrophy and white matter hyperintensities are associated with mild cognitive deficits in non-disabled elderly people: the LADIS study. J Neurol Neurosurg Psychiatry. 2005;76:1497–1500. doi: 10.1136/jnnp.2005.064998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooden S, Versluis MJ, Liem MK, et al. Cortical phase changes in Alzheimer’s disease at 7T MRI: a novel imaging marker. Alzheimers Dement. 2014;10:e19–26. doi: 10.1016/j.jalz.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Varrone A, Nordberg A. Molecular imaging of neuroinflammation in Alzheimer’s disease. Clin Transl Imaging. 2015;3:437–447. [Google Scholar]
- Velayudhan L, Proitsi P, Westman E, et al. Entorhinal cortex thickness predicts cognitive decline in Alzheimer’s disease. J Alzheimers Dis. 2013;33:755–766. doi: 10.3233/JAD-2012-121408. [DOI] [PubMed] [Google Scholar]
- Vlassenko AG, Raichle ME. Brain aerobic glycolysis functions and Alzheimer’s disease. Clin Transl Imaging. 2015;3:27–37. doi: 10.1007/s40336-014-0094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tan L, Wang HF, et al. Magnetic resonance spectroscopy in Alzheimer’s disease: systematic review and meta-analysis. J Alzheimers Dis. 2015;46:1049–1070. doi: 10.3233/JAD-143225. [DOI] [PubMed] [Google Scholar]
- Wu KY, Liu CY, Chen CS, et al. Beta-amyloid deposition and cognitive function in patients with major depressive disorder with different subtypes of mild cognitive impairment: (18)F-florbetapir (AV-45/Amyvid) PET study. Eur J Nucl Med Mol Imaging. 2016;43:1067–1076. doi: 10.1007/s00259-015-3291-3. [DOI] [PubMed] [Google Scholar]