Abstract
The recently described scaffold model of murein architecture depicts the gram-negative bacterial cell wall as a gel-like matrix composed of cross-linked glycan strands oriented perpendicularly to the plasma membrane while peptide bridges adopt a parallel orientation (B. A. Dmitriev, F. V. Toukach, K. J. Schaper, O. Holst, E. T. Rietschel, and S. Ehlers, J. Bacteriol. 185:3458-3468, 2003). Based on the scaffold model, we now present computer simulation studies on the peptidoglycan arrangement of the gram-positive organism Staphylococcus aureus, which show that the orientation of peptide bridges is critical for the highly cross-linked murein architecture of this microorganism. According to the proposed refined model, staphylococcal murein is composed of glycan and oligopeptide chains, both running in a plane that is perpendicular to the plasma membrane, with oligopeptide chains adopting a zigzag conformation and zippering adjacent glycan strands along their lengths. In contrast to previous models of murein in gram-positive bacteria, this model reflects the high degree of cross-linking that is the hallmark of the staphylococcal cell wall and is compatible with distinguishing features of S. aureus cytokinesis such as the triple consecutive alteration of the division plane orientation and the strictly centripetal mode of septum closure.
Staphylococcus aureus is an important human pathogen that causes life-threatening diseases including septicemia, endocarditis, toxic shock syndrome, and abscesses in organ tissues (15, 25). The cell wall of the microorganism plays an important role in infectivity and pathogenicity (40). Over several decades of research, extensive knowledge has accumulated concerning epidemiology (9), virulence (25, 28), genetics (3), genomic evolution (14), the biochemistry of cell wall assembly (31), the crystal structures of β-lactam-resistant enzymes (24), the ultrastructure of the cell wall (4, 18), and the muropeptide composition of wild-type, methicillin-resistant (7), and vancomycin-resistant strains (34). Nonetheless, the tertiary molecular structure of the cell wall, which is central for understanding cell growth and division in staphylococci, has remained elusive. As a consequence, there is no graphic illustration of the wall architecture in the literature that adequately represents known physicochemical details of staphylococcal peptidoglycan.
Staphylococci are gram-positive bacteria, and their cell walls are composed of murein (32, 38, 41), teichoic acids (2), and wall-associated surface proteins (20, 26, 30). Stress-bearing murein represents a continuous macromolecular sacculus covering the whole cell. Murein consists of glycan strands, which are cross-linked by peptide bridges furnishing the structural integrity of the sacculus. It is a distinctive feature of staphylococci that the observed degree of murein cross-linking, which was determined as a ratio of bridged peptides to the total amount of all peptide ends in general, is extremely high, on the order of 80 to 90% (16, 35).
Glycan strands in staphylococcal murein are composed of N-acetylglucosamine (GlcpNAc) and N-acetylmuramic acid (MurpNAc) residues that furnish β-(1→4)-linked disaccharide repeating units, with MurpNAc representing the reducing terminus of the chain. The carboxyl group of each MurpNAc residue is amidated by the stem pentapeptide l-Ala-d-iso-Gln-l-Lys-d-Ala-d-Ala, and the ɛ-amino group of the lysine residue is substituted with a pentaglycine appendage (37). Thus, each peptide attached to a MurpNAc residue is a branched decapeptide with an amino group on the Gly and a carboxyl group on the d-Ala terminus, and arms of the peptide side chains interact with each other to provide a high degree of murein cross-linking.
Although the general principle of murein structural organization is simple, the muropeptide composition of the staphylococcal sacculi appears very complex, as a standard digestion of sacculi with muramidase (the enzyme that cleaves MurpNAc glycosidic bonds) releases more than 20 distinct components plus an unresolved material. The latter makes up about 50 to 60% of the muropeptide-containing oligomers, with up to 20 repeating units (7, 38). Thus, the major part of staphylococcal murein architecture could be envisaged as being constructed of interlinked glycan and oligopeptide chains, both varying in their lengths.
On electron micrographs, the cell wall of staphylococci appears as a thick (about 20- to 40-nm-thick) homogenous slab (4, 18), and it is not possible to resolve the actual orientation of the glycan strands within the cell wall. Two published models proposed that the glycan strands were arranged in shell-like parallel layers around the plasma membrane. While the first model (37, 41) accounted for a high degree of cross-linking, it was discounted because it is incompatible with the known helical conformation of glycan strands. The second model ascribed to murein an unusual “plywood”-type architecture with consecutive turns of each glycan layer by 60° (21, 22). This type of architecture is difficult to reconcile with the centripetal closure of the septum during cell division.
Based on computer simulation studies, we recently developed a new structural principle of the bacterial murein architecture. The scaffold model considers the cell wall fabrics as a solid gel-like matrix deprived of glycan layers as principal elements of the wall architecture, the glycan chains being oriented perpendicularly to the plasma membrane, and permits a graphic representation of gram-negative bacterial cell walls (11). We have now adapted this model to the specific requirements of the gram-positive cell wall of S. aureus. This tertiary model of the staphylococcal murein is compatible with a high degree of cross-linking and, at the same time, facilitates a structural understanding of essential phenomena of staphylococcal morphogenesis such as consecutive division plane alteration and centripetal septum closure.
MATERIALS AND METHODS
Definitions.
The term “muropeptide” stands for a fragment released from murein after a standard digestion with muramidase. The simplest muropeptide, or “monomer, ” is a disaccharide unit substituted with a peptide side chain; a “dimer” is a muropeptide composed of two disaccharide units cross-linked by one peptide bridge; a “tetramer” is a branched structure containing four disaccharide units cross-linked with each other by three peptide bridges; an “oligomer” is a complex branched muropeptide composed of several disaccharide units cross-linked by interconnected peptide bridges, the latter producing a long branched peptide chain.
Simulation of the conformation and graphical representation of the major repeating unit of the S. aureus cell wall.
The computer-simulated conformation of the repeating fragment composed of the disaccharide GlcpNAc-(β1-4)-MurpNAc substituted with the branched decapeptide l-Ala-d-iso-Gln-l-Lys(Gly5)-d-Ala-d-Ala was optimized in an MM3-1996 force field by using molecular mechanics calculations (PC Model, version 7; Serena Software, Bloomington, Ind.). As the strands represent extended helices with the symmetry order N = +4 (6), they were approximated as relatively long cylinders with peptide substituents projected outward from each disaccharide unit, and with stem peptides perpendicular to each other. The bridges connecting the adjacent peptide arms were approximated as straight lines with the length of the -(Gly)5-d-Ala- cross-linking fragment, and both interacting peptides retained their antenna-like configuration. All graphical approximations presented in this paper were exclusively made to scale, thus preserving the basic atomic parameters.
Primary conformational analysis of muropeptides, the building blocks of murein.
To estimate the conformations of oligomuropeptides usually obtained from staphylococcal sacculi by a standard digestion with muramidase, it is sufficient to consider just the conformation of the tetrameric muropeptide. Since the latter could be regarded as two dimers concatenated via their short and long antenna's arms, respectively, two situations were analyzed as follows. First, each pair of disaccharide units involved in bridging was in a “vis-à-vis” position, i.e., in the vicinity of, and at the same distance from, the plasma membrane, with appropriate peptide arms pointing at each other, while both peptide antennas were in a plane perpendicular to the axis of a disaccharide cylinder. Second, the arms of the peptide antennas were oriented alongside the glycan strands to make the positions of bridged disaccharide pairs shifted in a “ladder”-type fashion along the glycan cylinders. In this type of bridged disaccharide location, the antenna's arms appear in the same plane as the axis of the glycan cylinder.
Simulation of the murein tertiary structure.
The algorithm used for murein simulation and the appropriate mathematical apparatus have been described in detail previously (11). In brief, the methodological principles are as follows:
(i) For simulation of murein assembly, chain length distributions expressed in terms of strand numbers (NL) with particular chain lengths (L) are necessary. The observed experimental data on the chain length distribution pattern in the cell wall of S. aureus were published in terms of relative amounts, expressed as percentages (5). The recalculation of the literature data on the relative mass content of the glycan strands to obtain strand numbers was achieved with equation 1:
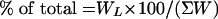 |
(1) |
In equation 1, WL is the relative mass of strands with length L and is defined as NL × NDS, where NL is the number of strands with chain length L and NDS is the number of disaccharide units in the chain.
(ii) To build up a murein matrix, a square piece of the fabric possessing 22 by 22 strand positions was virtually cut off from the sacculus. The square hole thus obtained possessed 484 positions for the glycan strands and was flanked along the perimeter by the existing fabric to allow incoming strands to cross-link with it, thus making the simulated process in agreement with murein assembly in vivo (16, 39). The maximal height of the matrix was considered to be about 45 disaccharides in order to guarantee the true thickness of the S. aureus cell walls observed in experiments omitting the procedures of fixation and staining (12). To mend the punctured fabric, peptidoglycan helices were randomly selected from the statistical pool and sequentially inserted into the square hole (MurpNAc termini pointing to the membrane) to occupy all 484 positions. Consequently, each disaccharide unit occupied a definite spatial location at a given distance from the membrane. We called these distances “levels, ” and the total number of levels within the box was 45. All the strands were inserted into the matrix vertically. Each disaccharide-peptide unit within the matrix was located at a definite height and corresponded to one particular stem peptide level. Further, each position in the matrix was occupied by at least one strand and could also be occupied by additional, shorter strands located above it. As a result, the total number of strands inserted into the matrix exceeded 484.
The distribution of strands in the matrix (probability P as a function of strand length i) was calculated by equation 2, as follows:
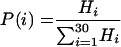 |
(2) |
In equation 2, Hi is the probability of finding a strand of length i in the experimental distribution pattern (5).
(iii) To achieve a maximal degree of cross-linking within the simulated murein, peptide side chains were oriented along the inserted glycan strands to run in parallel with their axes; the long pentaglycine arms were oriented downward, and the short alanine arms were oriented upward.
RESULTS
Conformation of the major muropeptide unit.
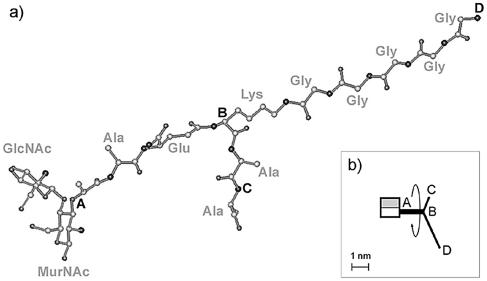
We started our simulations with the exact molecular structure of the major repeating fragment of murein, which is presented to scale in Fig. 1. The disaccharide portion of the major muropeptide unit is compact, whereas the extended peptide side chain resembles an antenna with two arms of different lengths. The stalk of the peptide antenna is almost perpendicular to the carbohydrate moiety to provide the energetic stability of the unit, while the arms can have different orientations due to the rotation around bonds inside the stalk.
FIG. 1.
Configuration and atomic parameters of the major repeating fragment of the S. aureus cell wall. Key points are marked with capital letters A, B, C, and D. Distances in a relaxed conformation are as follows: |AB| = 1.39 nm (stalk peptide), |BC| = 0.61 nm (short arm), |BD| = 2.42 nm (long arm); average disaccharide diameter, 1.1 nm; disaccharide length, 1.0 nm. (a) Tertiary structure of the disaccharide-peptide repeating unit. Bond order and hydrogen atoms are not shown. It is noteworthy that the simulation orients the arms of the peptide antenna perpendicularly to the axis of the disaccharide moiety. The atomic coordinates for this figure in PCModel format can be retrieved from the MONOUNIT.PCM file in the supplemental material. The software for interpreting this file is PCModel, version 7.00 (Serena Software). (b) Schematic representation of the disaccharide-peptide repeating unit, drawn to scale. Gray and white rectangles, GlcpNAc and MurpNAc residues, respectively. The black antenna represents the peptide side chain. The round arrow indicates that the antenna can rotate around the AB axis.
Conformation analysis of oligomeric muropeptides.
In the course of murein biosynthesis, disaccharide repeating units are attached to each other to produce glycan helices of different lengths, while the arms of the peptide side chains interact with each other to generate long oligopeptide bridges, endowing murein with a high degree of cross-linking. When performing the conformational analysis of oligomuropeptides, we made the central conceptual assumptions that within the murein tertiary structure, glycan strands have a helical conformation, which was determined by previous X-ray diffraction studies (6, 21), and that the geometry of the oligopeptide chains is undistorted and resembles that of free oligomers released from murein by muramidase digestion.
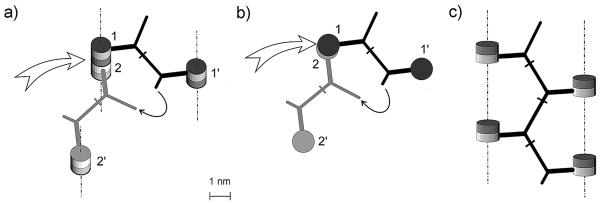
Experimentally, the muropeptide fraction represents a complex mixture of branched oligomers of different lengths with up to 20 repeating units; monomers and dimers are almost absent (7, 38). It should be noted that oligomers (at least even-numbered components) comprise chains composed of cross-linked dimers. Therefore, to achieve a general appreciation of the oligomeric muropeptide conformation, it is sufficient to carry out conformational analysis for a tetrameric muropeptide, which consists of two interconnected dimers. To guarantee the formation of a tetramer, the basic murein structure needs only to allow four monomeric muropeptides to be close enough to be cross-linked by two dimeric bridges. If cross-linked disaccharide units are located within the basic murein structure in a “vis-à-vis” manner, i.e., in agreement with the published scaffold model (11), the formation of the tetramer requires that two of four cross-linked disaccharides be arranged in one tetrasaccharide glycan block (the sequence to be split by muramidase) located within one strand while two other monomeric muropeptides belong to different strands (Fig. 2a); the minimal number of strands necessary to accomplish this is three. Thus, within the “vis-à-vis” murein structure, the two bridges are perpendicular to each other but appear in distinct planes about 1 nm (i.e., 1 disaccharide unit) apart. To make a tetrameric muropeptide, the short peptide arm of one bridge must reach the long arm of the other bridge while the two bridges are positioned in separate planes and at a rather long distance. A conventional “vis-à-vis” connection of cross-linked disaccharide pairs, therefore, seems unlikely, because it would necessitate a strong conformational deformation of the structure.
FIG. 2.
Formation of the tetrameric muropeptide from murein after virtual digestion with muramidase. Shown are considerations according to the original scaffold model (a and b) and representation of the advanced, “ladder-type” scaffold model (c). (a) Four muropeptide units belong to three strands and are located in two horizontal planes. Twin barrels correspond to the disaccharide units; those marked with a darker color (1 and 1′) are above those marked with a lighter color (2 and 2′). Vertical orientation of the strands is indicated by dashed-and-dotted lines. The white arrow points to the enzyme-cleavable bond between disaccharide units. The semicircular arrow points to the ends of short and long peptide arms which belong to two bridges that need to be connected. (b) Topside view of molecular geometry (topology) shown in panel a. Barrels filled with a darker color (1 and 1′) are located in a plane that is above the plane of a paper; lighter barrels (2 and 2′) are in the paper plane. (c) Unstressed ladder-like conformation of the tetrameric muropeptide with a zigzag geometry of the major peptide chain and four disaccharide substituents located in one plane. The atomic parameters of the muropeptides in question are identical to those presented in Fig. 1.
However, free muropeptide tetramers do exist in the muramidase digest and do possess a linkage between short and long peptide arms from two different bridges (7, 38). As depicted in Fig. 2c, the symmetrical conformation of the tetramer will be unstressed if the major peptide chain adopts a regular zigzag geometry whereas side stem peptides terminating with disaccharide residues protrude outward to avoid sterical hindrance. This deduced conformation of the tetramer is easy to insert into the murein tertiary structure under the single condition that four disaccharide residues are located within two (not three!) glycan strands, all four occupying a “ladder”-type configuration.
This requires a quarter-turn rotation of a peptide around its stem, but this conformational transition needs little energy and does not introduce any strain into the structure, as indicated by the conformational analysis of the disaccharide-peptide unit. We conclude that within the murein architecture, the major peptide chains of the oligomeric muropeptides can adopt an undistorted zigzag conformation and may run alongside the glycan strands in a plane roughly perpendicular to the plasma membrane, interconnecting them in a zipper-like manner with a high degree of cross-linking.
Graphic representation of the staphylococcal murein architecture.
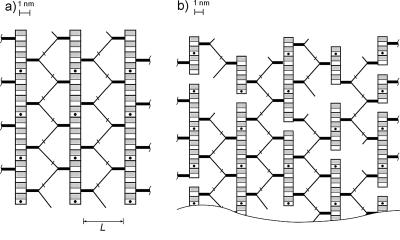
Having discovered a clue to the possible orientation of the oligopeptide bridges within the murein matrix, we applied the refined principle of the scaffold model (10, 11) to construct the S. aureus murein architecture and oriented both glycan strands and extended oligopeptide chains in a plane perpendicular to the surface of the plasma membrane. Here, the short alanine and the long pentaglycine arms went upwards and downwards, respectively, whereas the MurpNAc termini of the glycan strands pointed to the plasma membrane (Fig. 3).
FIG. 3.
Cross sections of the unstressed matrix of the hyper-cross-linked staphylococcal murein. Glycan chains run perpendicularly to the plasma membrane, and stalk peptides (thick lines) are perpendicular to the glycan chains. Long oligopeptide chains (thin lines) run in the form of zigzags between glycan chains. The linking points of the peptide arms are marked with short crossing bars. N-Acetylmuramic acid and N-acetylglucosamine residues are depicted as white and gray rectangles, respectively. The dots stand for peptides protruding above the paper plane; those protruding below are not shown. (a) Principle of staphylococcal murein organization. L stands for the distance between glycan chains. (b) Fragment of the murein matrix composed of strands with experimentally observed length distribution.
The proposed vision of the staphylococcal murein architecture is easier to explain by starting from the simplified hypothetical situation that glycan chains are long and the degree of cross-linking is about 100% (Fig. 3a). In such a case, oligopeptide chains run along the glycan chains, and the two polymers are equal in length. The oligopeptide chains adopt an unstressed zigzag conformation and actually fasten neighboring glycan strands together. Because peptide side chains project radially from glycan helices in four different directions, each peptidoglycan strand is able to interact with four others to make a dense matrix. A thin section of the murein matrix is presented in Fig. 3a, exhibiting the chains that are on the plane of the paper. However, in reality, glycan chains differ in length, and rather short oligosaccharides dominate (5). Visualization of such a situation in a model with glycan chains of actual lengths shows how the murein architecture becomes progressively irregular (Fig. 3b). Dimeric bridges are almost absent from the proposed murein structure, while bridged muropeptide units are disposed in a “ladder-like” configuration (see Fig. 2c) to allow the formation of bridges even between the ends of two separate glycan strands. The proposed scaffold model provides a rational interpretation of early X-ray diffraction data measured for the S. aureus sacculus (21), of which a weak reflection at 4.2 nm was taken to represent the distance between the glycan chains. The distance L in Fig. 3a is in accordance with this finding.
Three-dimensional model of staphylococcal murein.
To obtain an accurate three dimensional view of the proposed murein architecture, we simulated its assembly (i) by using the experimental chain length distribution (5) and (ii) by following the principle of staphylococcal murein assembly known as “restricted monomer addition” (16). To perform the simulation, three major assumptions were made as follows. First, all strands within the murein of S. aureus are oriented perpendicularly to the plasma membrane. Second, because both the biosynthesis of precursors and concomitant murein assembly proceed in association with the plasma membrane (39), the assumption was made that the MurpNAc termini of the glycan chains point to the plasma membrane while the GlcpNAc termini are oriented outward. Third, since the dynamics of murein biogenesis simultaneously comprises the assembly and turnover processes, and the latter makes the outer surface of the wall appear worn out (18), it was assumed that degraded (short) strands tend to accumulate in the outer part of the sacculus, while those that are not yet split penetrate through the wall. It bears mentioning that the experimentally determined, predominant length of the glycan chains in S. aureus is 3 to 10 disaccharide units and that an average chain length is about 6 disaccharides (5). Therefore, we did not consider chains longer than 30 disaccharide units. The program was adjusted to obtain a maximal degree of possible cross-linking, and consequently, peptide side chains were oriented along the glycan strands to run in parallel with them. Because the nascent strands emerge from the plasma membrane and then attach by their short arms (alanine donor) to the long arm (pentaglycine acceptor) of the preexisting murein (39), the long peptide arms were oriented downward.
The simulation of the murein architecture was started with the assembly of the material that had not yet undergone degradation and thus was free of very short chains. For this purpose, chains with 4 to 30 disaccharide units were selected. A certain number of strands were modeled as still attached to the membrane, while the rest were positioned as already detached. Thus, the strands could become closely interwoven to produce a maximally cross-linked material in proximity to the membrane. Evidently, a certain number of long chain ends protruded upwards from the primary matrix without being cross-linked. The remaining short strands were subsequently inserted into the box above the primary matrix so that the distance between chain ends was 1 or 2 disaccharide units. Synchronously with the strand insertion, all possible cross-links were initiated between adjacent peptides. When the arms of antenna peptides were oriented along the glycan chain, each peptide could interact with two neighbors (one from above and the other from below), thus achieving a high degree of cross-linking between the chains. During the assembly process, shorter chains tended to accumulate in the periphery, corresponding to the structure of material digested by lytic enzymes. The simulated murein matrix had 83% cross-linking, while the published degree of cross-linking for the purified cell wall preparation was 80% (16). When the distance between the glycan chain ends was programmed to be 1 disaccharide unit, the degree of murein cross-linking, as a consequence, was raised to 90%.
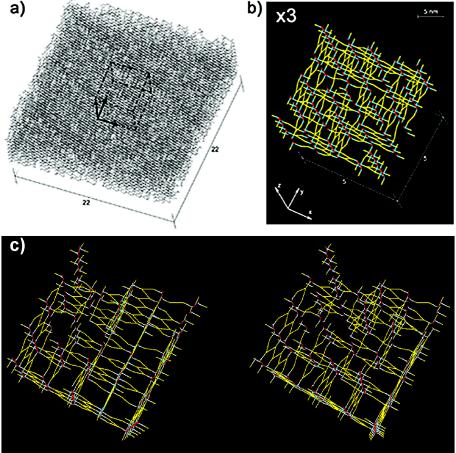
The overall visualization of the simulated murein architecture is presented in Fig. 4. The thick fragment of the murein matrix (Fig. 4a) is almost impermeable and produces the impression of a tightly interwoven thicket, which very much resembles the ultrastructure observed on electron micrographs (4, 18). Evidently, there is an immense inconvenience in any two-dimensional representation of the cross-linked murein architecture due to two intrinsic features of the staphylococcal cell wall: its considerable thickness and the high degree of cross-linking. Therefore, to demonstrate the details of the architecture, we selected a rather thin and small fragment from the upper part of the simulated murein matrix for enlargement (Fig. 4b). The porous structure, oligopeptide zigzags, and distinct glycan chains of different lengths can be seen clearly. A stereo view of the selected murein fragment is presented in Fig. 4c.
FIG. 4.
Simulated tertiary structure of the staphylococcal murein. The chain length distribution corresponds to the high-performance liquid chromatography data observed for S. aureus (5). The normalized cross-linking degree is 83%. All glycan strands are oriented perpendicularly to the plasma membrane, with GlcpNAc termini pointing upward. (a) Panorama of a simulated murein matrix occupying 22 by 22 positions for the strands and 45 layers thick. Dashed lines outline a cube corresponding to a smaller murein fragment that is selected for enlargement in panel b. Boldfaced arrows correspond to three coordinate axes. (b) Top view of the threefold-enlarged fragment of the murein, composed of 5 by 5 glycan strand positions and 20 levels thick, extracted from the matrix as outlined by dashed lines in panel a. The z axis is perpendicular to the plasma membrane, which is represented by a black background. The residues of GlcpNAc are presented as red dots, while those of MurpNAc are presented as cyan blue dots. Cross-linked oligopeptide chains are clearly visible as yellow zigzags. (c) Stereo view of the simulated murein matrix shown in panel b.
DISCUSSION
At present, a reliable evaluation of the actual murein tertiary structure by direct experimental approaches is impossible, because bacterial sacculi undergo continuous synthesis and concomitant turnover, making them heterogeneous, irregular, and distorted by tensile forces. Therefore, it is not surprising that different models for the S. aureus cell wall have been proposed.
The refined scaffold model of peptidoglycan architecture, when applied to the gram-positive wall of S. aureus, has three distinct advantages over previously discussed concepts: (i) it can account for the extremely high degree of cross-linking (80 to 90%), (ii) it is compatible with the phenomenon of consecutive division plane alternation, and (iii) it can explain appositional growth during centripetal septum closure. No other published structural views of the cell wall architecture can simultaneously accommodate all of these experimentally confirmed biological facts about S. aureus (for examples, see the most recently published figures in references 13 and 38).
Four decades ago it was presumed that glycan chains possess a chitin-like conformation and run parallel to the plasma membrane as well as to each other, thus producing a set of glycan planes. Beneath each plane and at right angles to the glycan chains, there was a plane of peptide chains that were cross-linked at considerable distances; the whole structure made a covalently closed sphere joined by glycan chains in one direction and by oligopeptide chains at 90o to the glycan chains (29, 32, 36, 37, 41). This model was discounted by X-ray diffraction data (6, 21), which proved that glycan strands do not possess a straight chitin-like conformation but represent right-handed helices with 4 disaccharides units per turn. Although there are no conditions under which helices can be introduced into the proposed type of architecture, the “chitin” model is surprisingly present even in today's microbiology textbooks.
In an alternative model, peptidoglycan strands were approximated as long cylinders (21, 22) or stretched ropes (23) around which a continuous and narrow spiral, imitating the discrete peptide substituents, was wound. The approximated strands were arranged parallel to the plasma membrane and to each other to simulate a peptidoglycan layer. To achieve a maximal degree of cross-linking, the layers were proposed to pile one over the other with a 60° turn of each consecutive layer relative to its neighbor. In this way, the murein architecture resembled a plywood-layered structure (21). For its assembly, this model apparently demands strong conformational distortions of the oligopeptide chains to allow consecutive turns of the layers and to accomplish a high degree of cross-linking.
The scaffold model, in contrast, readily guarantees 80 to 90% cross-linking in accordance with published experimental data (16, 35) and implies that all the structural elements of murein architecture are unstressed and adopt energetically minimized conformations of helices and zigzags.
Staphylococci divide in three successively different planes. Each new plane changes its direction at an angle of 90o to the preceding division plane (18, 27, 38), but conserved patches of old wall sectors still exist during division, as visualized recently in spherical cells of Escherichia coli (8). By the principle of glycan strand arrangement proposed by the “chitin” model, the consequences of this phenomenon for the cell wall would be those presented in Fig. 5.
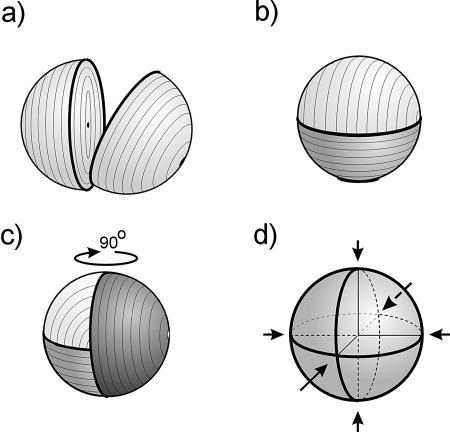
FIG. 5.
Relationship between the division plane direction and the orientation of glycan chains. Equatorial rings are shown as boldfaced arcs. (a) The staphylococcal cell after the first division. The division plane goes through the equatorial ring, and glycan strands run concentrically to the latter in both hemispheres. (b) The cell after the second division with subsequent alternation of the division plane. (c) The cell after the third division and subsequent alternation of the division plane. The round arrow indicates that to see a new hemisphere, the image should be turned by 90°. (d) Three equatorial rings produced by alternating division planes of the staphylococcal cell. Arrows mark the vertical orientation of the glycan chains in the scaffold model, which is in agreement with any of the division planes.
If the glycan chains run in the described way, the first division plane goes through the equatorial ring oriented parallel to these chains, leading to the formation of two identical daughter cells (Fig. 5a). In the second division, glycan chains in both hemispheres of four daughter cells take up two different orientation patterns (Fig. 5b). The third division leads to a murein structure with three different glycan chain orientations within one sacculus (Fig. 5c), resulting in eight chimerical cells altogether. The situation presented in Fig. 5b and c seems to be impossible because glycan chains in all members of the cell clone display a uniform orientation, and it is obvious that glycan chains within such patched sacculi cannot be arranged at 90° angles. Theoretically, the “plywood” model is compatible with the phenomenon of division plane alternation, although it is difficult to envisage a biosynthetic apparatus that would ensure consecutive regular turns—at an angle of exactly 60°—of the strands layer after layer. In contrast, the simplest way for glycan strands to fit the phenomenon of division plane alternation is to be arranged perpendicularly to the plasma membrane (Fig. 5d), as implied by the scaffold model.
How do the different models of the staphylococcal murein architecture account for the process of cell wall morphogenesis, as it has been revealed both by advanced electron microscopy (1, 4, 18) and by recent biochemical studies (38, 39)? The spherical walls of staphylococci seem to grow mostly via a zonal mechanism of cross-wall assembly. During cell division there is a clearly exposed leading edge, and septum growth proceeds strictly centripetally, like the iris diaphragm of a camera, from the peripheral edges to the center (1). The septum consists of two newly born walls with a “splitting system” sandwiched in between; each newly born wall represents the primary wall of the daughter cell in its pure form. The ongoing secondary process known as wall “thickening” accompanies the primary wall assembly, and when the septum is completed, the maternal wall becomes cleaved and skinned off along the “stripping” layer to allow daughter cells to be separated (18). The process is repeated in the next cell cycle, with the only distinction that the next division plane is turned by 90°.
The proposed scaffold model is in agreement with the cell wall morphogenesis described above because it implies the appositional attachment of the nascent peptidoglycan strands to the centripetally moving leading edge of the closing septum. Enzymatic cutting of the inner maternal wall into two hemispheres produces two bare leading edges, and their contact with the plasma membrane is initiated by invagination of the cell membrane, thus allowing the nascent strands to interact with the exposed edges of the growing walls. This is possible only if the glycan strands are oriented perpendicularly to the plasma membrane, because any horizontal attachment of nascent strands would be in conflict with the phenomenon of division plane alternation.
As mentioned above, staphylococci possess on their surfaces type-specific teichoic acid (2) and a set of proteins (20), all of which are covalently attached to murein. Teichoic acid (19) and surface-associated proteins (26, 30) are first synthesized in vivo in the form of the corresponding biosynthetic precursors, which are closely related to the precursors of murein biosynthesis (39). These precursors could concomitantly participate in cell wall assembly, i.e., teichoic acid and surface-associated proteins could become attached to murein in the course of its assembly. The scaffold model is in accord with such molecular mechanisms of cell wall assembly because both the teichoic acid and surface protein intermediates could be readily inserted into the nascent peptidoglycan chain during its attachment to the edge of the growing septum. The involvement of all precursors in a zonal process of murein assembly provides teichoic acid and surface proteins with a unique opportunity to reach the bacterial surface directly.
Although the “chitin” model may theoretically account for the centripetal mechanism of staphylococcal cell wall growth, other shortcomings mentioned above have rendered this model obsolete. The “plywood” model seems to contradict the centripetal leading-edge growth of the closing septum. The growing septum is a round plate with a hole in the center, and the movement of the edge of a constricting hole (zone of growth) has to be coherent with that of the constricting membrane, both being strictly centripetal (1, 18). This implies that the orientation of all glycan strands in all layers (if those exist in bacterial murein) of the septum has to correlate unvaryingly with the centripetal edge movement; otherwise the septum will never be closed. In contrast, the plywood model states that glycan strands diverge their orientation in consecutive layers and therefore, by definition, concurrently run both centripetally and centrifugally. In conclusion, only the advanced model described here adequately reflects the biological and physicochemical requirements for the assembly of highly cross-linked murein within the cell wall of S. aureus.
Finally, it is pertinent to consider the relationship between the scaffold-like model originally proposed by us for gram-negative cell walls (11) and that described here for the highly cross-linked cell wall of S. aureus. It is evident that the previous model does not account for a high degree of murein cross-linking, a characteristic feature of gram-positive walls, and therefore cannot serve as a general model. The question arises whether the advanced scaffold model is universally applicable to all types of bacterial walls or whether it applies only in the special situation of gram-positive walls.
With the Gram staining procedure, all bacteria described so far can be divided into three large classes, i.e., positive, negative and indeterminate, while according to the chemical classification (33) of murein structures described thus far, there are about 20 types of murein. Actually, the number of murein types is larger, because particular chemotypes were not included in the list and an additional type of architecture comprising the subsequently discovered form of cross-linking via Dap-Dap bridges was not considered. Despite this complicated situation, it can be stated that the walls of all gram-negative bacteria belong to type A1 and possess the simplest murein structures (33). Here, glycan strands are loosely cross-linked by ordinary bridges, and oligomeric peptide fragments are practically absent. The tertiary structures are therefore similar and can be adequately presented in their entirety by the previously published scaffold model (11). Indeed, even if the advanced scaffold model with a shifted “ladder-type” location of the cross-linked disaccharide units is applied to gram-negative walls, the tensile forces that act to expand the wall material will inevitably revert the construction to the “vis-à-vis” tertiary structure with ordinary peptide bridges oriented parallel to the plasma membrane.
As to the cell walls of gram-positive bacteria, they exhibit a wide diversity from simple to very complex structures with variably high degrees of cross-linking. Staphylococcal cell walls have a rather extraordinary type of architecture, belonging to the most highly cross-linked type; only the advanced scaffold model adequately describes this specific feature. The walls of other gram-positive bacteria exhibit a much lower degree of cross-linking, and the muropeptide fraction of these walls does not contain long oligomeric chains. For example, the walls of bacilli exhibit about 50 to 55% cross-linking, and the major component of the oligopeptide fraction is trimeric and tetrameric muropeptides (17). Furthermore, the arm lengths of the peptide antennas in the walls of other gram-positive bacteria are not as long as those in staphylococci. However, it is likely that the same major principle, i.e., that both glycan and oligopeptide chains tend to run in a plane perpendicular to the plasma membrane, holds for the architecture of all gram-positive walls. As these walls contain sufficient amounts of unbridged disaccharide units along with dimeric and trimeric bridges, their architecture might be of a mixed type, exhibiting the tertiary structural elements of both the basic and advanced scaffold models.
Supplementary Material
Acknowledgments
B.A.D. and F.V.T. gratefully acknowledge the Research Center Borstel for providing research fellowships. Work in S.E.'s laboratory is funded in part by the Deutsche Forschungsgemeinschaft, SFB 470-C9.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Amako, K., and A. Umeda. 1984. Cross wall synthesis and the arrangement of the wall polymers in the cell wall of Staphylococcus spp. Microbiol. Immunol. 28:1293-1301. [DOI] [PubMed] [Google Scholar]
- 2.Baddiley, J. 1989. Bacterial cell walls and membranes. Discovery of the teichoic acids. Bioassays 10:207-210. [DOI] [PubMed] [Google Scholar]
- 3.Berger-Bachi, B., and S. Rohrer. 2002. Factors influencing methicillin resistance in staphylococci. Arch. Microbiol. 178:165-171. [DOI] [PubMed] [Google Scholar]
- 4.Beveridge, J. 2000. Ultrastructure of gram-positive cell walls, p. 3-10. In V. A. Fishetti, R. P. Novick, J. J. Feretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 5.Boneca, I. G., Z. H. Huang, D. A. Gage, and A. Tomasz. 2000. Characterization of Staphylococcus aureus cell wall glycan strands: evidence for a new beta-N-acetylglucosaminidase activity. J. Biol. Chem. 275:9910-9918. [DOI] [PubMed] [Google Scholar]
- 6.Burge, R. E., A. G. Fowler, and D. A. Reaveley. 1977. Structure of the peptidoglycan of bacterial cell walls. J. Mol. Biol. 117:927-953. [DOI] [PubMed] [Google Scholar]
- 7.de Jonge, B. L., Y. S. Chang, D. Gage, and A. Tomasz. 1992. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2A. J. Biol. Chem. 267:11248-11254. [PubMed] [Google Scholar]
- 8.de Pedro, M. A., W. D. Donachie, J. V. Holtje, and H. Schwarz. 2001. Constitutive septal murein synthesis in Escherichia coli with impaired activity of the morphogenetic proteins RodA and penicillin-binding protein 2. J. Bacteriol. 183:4115-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diekema, D. J., M. A. Pfaller, F. J. Schmitz, J. Smayevsky, J. Bell, R. N. Jones, and M. Beach. 2001. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S114-S132. [DOI] [PubMed] [Google Scholar]
- 10.Dmitriev, B. A., S. Ehlers, and E. T. Rietschel. 1999. Layered murein revisited: a fundamentally new concept of bacterial cell wall structure, biogenesis and function. Med. Microbiol. Immunol. (Berlin) 187:173-181. [DOI] [PubMed] [Google Scholar]
- 11.Dmitriev, B. A., F. V. Toukach, K. J. Schaper, O. Holst, E. T. Rietschel, and S. Ehlers. 2003. Tertiary structure of bacterial murein: the scaffold model. J. Bacteriol. 185:3458-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubochet, J., A. W. McDowall, B. Menge, E. N. Schmid, and K. G. Lickfeld. 1983. Electron microscopy of frozen-hydrated bacteria. J. Bacteriol. 155:381-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischetti, V.A. 2000. Surface proteins on gram-positive bacteria, p. 11-23. In V. A. Fishetti, R. P. Novick, J. J. Feretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 14.Fitzgerald, J. R., D. E. Sturdevant, S. M. Mackie, S. R. Gill, and J. M. Musser. 2001. Evolutionary genomics of Staphylococcus aureus: insights into the origin of methicillin-resistant strains and the toxic shock syndrome epidemic. Proc. Natl. Acad. Sci. USA 98:8821-8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flock, J. I. 1999. Extracellular-matrix-binding proteins as targets for the prevention of Staphylococcus aureus infections. Mol. Med. Today 5:532-537. [DOI] [PubMed] [Google Scholar]
- 16.Gally, D., and A. R. Archibald. 1993. Cell wall assembly in Staphylococcus aureus: proposed absence of secondary crosslinking reactions. J. Gen. Microbiol. 139:1907-1913. [DOI] [PubMed] [Google Scholar]
- 17.Gally, D. L., I. C. Hancock, C. R. Harwood, and A. R. Archibald. 1991. Cell wall assembly in Bacillus megaterium: incorporation of new peptidoglycan by a monomer addition process. J. Bacteriol. 173:2548-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giesbrecht, P., T. Kersten, H. Maidhof, and J. Wecke. 1998. Staphylococcal cell wall: morphogenesis and fatal variations in the presence of penicillin. Microbiol. Mol. Biol. Rev. 62:1371-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrington, C. R., and J. Baddiley. 1985. Biosynthesis of wall teichoic acids in Staphylococcus aureus H, Micrococcus varians and Bacillus subtilis W23. Involvement of lipid intermediates containing the disaccharide N-acetylmannosaminyl N-acetylglucosamine. Eur. J. Biochem. 153:639-645. [DOI] [PubMed] [Google Scholar]
- 20.Höök, M., and T. J. Foster. 2000. Staphylococcal surface proteins, p. 386-391. In V. A. Fishetti, R. P. Novick, J. J. Feretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 21.Labischinski, H., G. Barnickel, H. Bradaczek, and P. Giesbrecht. 1979. On the secondary and tertiary structure of murein. Low and medium-angle X-ray evidence against chitin-based conformations of bacterial peptidoglycan. Eur. J. Biochem. 95:147-155. [DOI] [PubMed] [Google Scholar]
- 22.Labischinski, H., G. Barnickel, D. Naumann, and P. Keller. 1985. Conformational and topological aspects of the three-dimensional architecture of bacterial peptidoglycan. Ann. Inst. Pasteur Microbiol. 136A:45-50. [DOI] [PubMed] [Google Scholar]
- 23.Labischinski, H., and H. Maidhof. 1994. Bacterial peptidoglycan: overview and evolving concepts, p. 23-38. In J. M. Ghuysen and R. Hackenbeck (ed.), Bacterial cell wall. Elsevier Science B.V., Amsterdam, The Netherlands.
- 24.Lim, D., and N. C. Strynadka. 2002. Structural basis for the beta lactam resistance of PBP2a from methicillin-resistant Staphylococcus aureus. Nat. Struct. Biol. 9:870-876. [DOI] [PubMed] [Google Scholar]
- 25.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 26.Mazmanian, S. K., H. Ton-That, and O. Schneewind. 2001. Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus. Mol. Microbiol. 40:1049-1057. [DOI] [PubMed] [Google Scholar]
- 27.Novick, R. P. 1990. Staphylococci, p. 539-551. In B. D. Davis, R. Dulbecco, H. N. Eisen, and H. S. Ginsberg (ed.), Microbiology, 4th ed. Lippincott Co., Philadelphia, Pa.
- 28.Novick, R. P. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 48:1429-1449. [DOI] [PubMed] [Google Scholar]
- 29.Oldmixon, E. H., S. Glauser, and M. L. Higgins. 1974. Two proposed general configurations for bacterial cell wall peptidoglycans shown by space-filling molecular models. Biopolymers 13:2037-2060. [DOI] [PubMed] [Google Scholar]
- 30.Perry, A. M., H. Ton-That, S. K. Mazmanian, and O. Schneewind. 2002. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. III. Lipid II is an in vivo peptidoglycan substrate for sortase-catalyzed surface protein anchoring. J. Biol. Chem. 277:16241-16248. [DOI] [PubMed] [Google Scholar]
- 31.Pinho, M. G., S. R. Filipe, H. de Lencastre, and A. Tomasz. 2001. Complementation of the essential peptidoglycan transpeptidase function of penicillin-binding protein 2 (PBP2) by the drug resistance protein PBP2A in Staphylococcus aureus. J. Bacteriol. 183:6525-6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers, H. J, H. R. Perkins, and J. B. Ward. 1980. Microbial cell walls and membranes, p. 190-214. Chapman and Hall, London, United Kingdom.
- 33.Schleifer, K. H., and O. Kandler. 1972. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 36:407-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sieradzki, K., and A. Tomasz. 1997. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J. Bacteriol. 179:2557-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snowden, M. A., H. R. Perkins, A. W. Wyke, M. V. Hayes, and J. B. Ward. 1989. Cross-linking and O-acetylation of newly synthesized peptidoglycan in Staphylococcus aureus H. J. Gen. Microbiol. 135:3015-3022. [DOI] [PubMed] [Google Scholar]
- 36.Strominger, J. L. 1970. Penicillin-sensitive enzymatic reactions in bacterial cell wall synthesis. Harvey Lect. 64:179-213. [PubMed] [Google Scholar]
- 37.Strominger, J. L., and J. M. Ghuysen. 1967. Mechanisms of enzymatic bacteriolysis. Cell walls of bacteria are solubilized by action of either specific carbohydrases or specific peptidases. Science 156:213-221. [DOI] [PubMed] [Google Scholar]
- 38.Tomasz, A. 2000. The staphylococcal cell wall, p. 351-360. In V. A. Fishetti, R. P. Novick, J. J. Feretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 39.van Heijenoort, J. 2001. Formation of the glycan chains in the synthesis of bacterial peptidoglycan. Glycobiology 11:25R-36R. [DOI] [PubMed] [Google Scholar]
- 40.van Heijenoort, J., and L. Gutmann. 2000. Correlation between the structure of the bacterial peptidoglycan monomer unit, the specificity of transpeptidation, and susceptibility to beta-lactams. Proc. Natl. Acad. Sci. USA 97:5028-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weidel, W., and H. Pelzer. 1964. Bagshaped macromolecules—a new outlook on bacterial cell walls. Adv. Enzymol. Relat. Areas Mol. Biol. 26:193-232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.