Abstract
Purpose
Corneal transplantation remains the last hope for vision restoration, and lymphangiogenesis (LG) is a primary mediator of transplant rejection. This study was to investigate the specific role of angiopoietin-2 (Ang-2) in transplantation-associated LG and graft rejection.
Methods
Orthotopic corneal transplantation was performed between fully mismatched C57BL/6 (donor) and BALB/c (recipient) mice to assess the effects of Ang-2 blockade via neutralizing antibody. Grafts were evaluated in vivo by ophthalmic slit-lamp biomicroscopy and anterior segment optical coherence tomography (OCT) up to 8 weeks after surgery. Additionally, whole-mount corneas were analyzed for lymphatic and blood vessels and macrophages by immunofluorescent microscopy, and draining lymph nodes were assessed for donor-derived cells by flow cytometry.
Results
Anti-Ang-2 treatment significantly suppressed LG and graft rejection. In this study, we achieved 75% suppression of LG and 80% graft survival. Our approach also inhibited donor-derived cell trafficking to draining lymph nodes and affected macrophage morphologic phenotypes in the grafted corneas. Additionally, Ang-2 blockade also reduced central corneal thickening, a parameter strongly associated with graft rejection.
Conclusions
Ang-2 is critically involved in corneal transplant rejection and anti-Ang-2 treatment significantly improves the outcomes of corneal grafts. Moreover, we have shown that anterior segment OCT offers a new tool to monitor murine corneal grafts in vivo. This study not only reveals new mechanisms for transplant rejection, but also offers a novel strategy to treat it.
Keywords: corneal transplantation, lymphangiogenesis, Ang-2
As a transparent tissue of the visual pathway, the normal adult cornea is devoid of any lymphatic vessels. However, lymphangiogenesis (LG; the formation of new lymphatic vessels) is induced into the cornea under pathological situations, such as trauma, inflammation, infection, and chemical burn. Because the lymphatic pathway constitutes the afferent arm of the immune reflect arc that mediates transplant rejection, anti-lymphangiogenic therapy is emerging as a new approach to potentially promote graft survival.1–3 Currently, there is still a lack of effective treatment for lymphatic diseases in general, it is therefore imperative to understand the underlying mechanisms of LG and to identify new molecular factors for therapeutic targeting.
Angiopoietin-2 (Ang-2) belongs to the angiopoietin-Tie family and it is involved in many pathological processes, such as hemangiogenesis (the formation of new blood vessels) and inflammation.4 Its particular functions in the lymphatic system are yet to be fully understood. Previously, we and other researchers have reported that Ang-2 deficiency leads to lymphatic defects in development and inflammation.5–7 We have also shown that Ang-2 gene knockdown suppresses lymphatic endothelial cell functions in vitro including proliferation and tube formation, and anti-Ang-2 treatment inhibits corneal inflammatory LG in a suture-induced model of 2 weeks.6 However, the potential and specific role of Ang-2 in corneal transplantation has never been investigated, which is the focus of the current study. Compared with suture-induced model of inflammation, transplantation triggers immune responses against foreign antigens and it induces a greater scale of LG as well. Results from this study are important not only for revealing new mechanisms underlying transplant rejection, but also for developing new strategies to treat it. Corticosteroids, which have been used for decades, are known to be associated with many side effects, such as cataract, glaucoma, and opportunistic infections.
In this paper, we provide the first evidence showing that Ang-2 is critically involved in corneal transplant rejection. Ang-2 blockade leads to a more significant reduction of transplantation-associated LG than hemangiogenesis. This treatment also suppresses donor-derived cell trafficking to draining lymph nodes, affects macrophage morphologic phenotype, and improves graft rejection. Additionally, in this study, we have shown, for the first time, that anterior segment optical coherence tomography (OCT) can be used to monitor murine corneal grafts in vivo, and we report that Ang-2 treatment significantly reduces central corneal thickening after transplantation, which is an important parameter strongly associated with graft rejection. Taken together, this study identifies Ang-2 as a critical mediator of transplant rejection. Given that the cornea is one of the favorite tools for lymphatic research, and results from corneal studies have been proven to be readily applied to nonocular sites, further exploration on the Ang-2 pathway may offer new therapies to manage broad lymphatic, inflammatory, or immune diseases outside the eye.
Methods
Animals
Seven- to 8-week-old male C57BL/6 and BALB/c mice (Jackson Laboratory, Sacramento, CA, USA) were used for the experiments. All mice were treated according with ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and all protocols were approved by the Animal care and Use Committee of the institute. Mice were anesthetized using a mixture of ketamine, xylazine, and acepromazine (50 mg, 10 mg, and 1 mg/kg body weight, respectively) for each surgical procedure. The experiment was performed with 10 mice in the treatment and control groups, respectively, unless otherwise indicated.
Corneal Transplantation
Orthotopic corneal transplantation was performed between fully mismatched C57BL/6 (donor) and BALB/c (recipient) mice according to the standard protocol, as described previously.2,3 Briefly, donor central cornea was marked by a 2-mm diameter microcurette (Katena Products, Inc., Denville, NJ, USA) and excised with a Vannas scissor (Storz Instruments Co., San Dimas, CA, USA). Recipient graft bed was prepared similarly with a 1.5-mm diameter microcurette where the donor button was secured by eight interrupted 11-0 nylon sutures (AROSurgical Instruments Corporation, Newport Beach, CA, USA). Antibiotic ointment was applied at the end of the surgery.
Pharmaceutical Intervention
After corneal transplantation, recipient mice were randomized to receive intraperitoneal injections of either Ang-2 neutralizing antibody (20 mg/kg, 18E5; Eli Lilly and Company, Indianapolis, IN, USA) or control PBS twice a week from Day 0 up to 8 weeks after transplantation.
Slit-Lamp Biomicroscopy
Corneal grafts were evaluated by ophthalmic slit-lamp biomicroscopy using the standard grading scheme, as reported previously.2,3 Briefly, graft opacification was scored between 0 (clear and compact graft) to 5+ (maximal opacity with total obscuration of the anterior chamber), and grafts with an opacity score of 2+ or higher after 3 weeks or an opacity score of 3+ or higher at 2 weeks were regarded as rejected.
Optical Coherence Tomography
Anterior segment OCT (Visante OCT MODEL 1000; Carl Zeiss Meditec, Dublin, CA, USA) with the resolutions of 18-μm axially and 60-μm laterally was used to evaluate central corneal thickness in vivo once a week up to 8 weeks after transplantation. High-resolution quadrant scan along four axes (0°–180°, 45°–225°, 90°–270°, and 135°–315°) was performed to ensure scanning through the central cornea and data along the 0° to 180° degree axis were used for analysis.
Immunofluorescent Microscopic Assay and Quantification
The experiments were performed as described previously.3,6,8 For vascular analysis, whole-mount corneas were harvested at the end of the 8-week study, and fixed in acetone for immunostaining with primary antibodies against LYVE-1 (rabbit-anti-mouse; Abcam, Inc., Cambridge, MA, USA) and CD31 (rat-anti-mouse; Santa Cruz Biotechnology, Santa Cruz, CA, USA), which were visualized by FITC conjugated donkey-anti-rabbit and Cy3 conjugated donkey-anti-rat secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA), respectively. For macrophage analysis, whole-mount corneas were harvested at 2 weeks post transplantation and stained with rat-anti-mouse F4/80 antibody (Abcam, Inc.), which was recognized by a FITC conjugated donkey-anti-rat secondary antibody (Jackson ImmunoResearch Laboratories). Samples were mounted by Vector Shield mounting medium (Vector Laboratories, Burlingame, CA, USA) and photographed using an AxioImager M1 epifluorescence deconvolution microscope with AxioVision 4.8 software (Carl Zeiss AG, Göttingen, Germany). Quantification analysis was performed as reported previously.3,6,8 Vascular structures stained as CD31+LYVE-1+ were defined as lymphatic vessels and stained as CD31+LYVE-1− were defined as blood vessels. Vessel coverage area was quantified using ImageJ software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA) and normalized to total corneal area to obtain a percentage coverage score for each sample. Macrophages were quantified in central grafted corneas (520 × 520 μm) for each sample (experiments repeated twice with 7 mice in the treatment and control groups, respectively). The percentage scores were obtained by normalizing to the control condition defined as being 100%.6,9
Flow Cytometry
The experiment was performed as reported previously.2,9 Briefly, ipsilateral submandibular lymph nodes were harvested at 8 weeks after corneal transplantation. Cell suspension was blocked with Fc-blocking antibody (CD16/32; BD Bioscience, Franklin Lakes, NJ, USA) and stained for donor type major histocompatibility complex (MHC) class II I-A [b] alloantigen (BD Bioscience). Data were acquired and analyzed by a Guava easyCyte HT cytometer and InCyte 2.6 software (Millipore, Billerica, MA, USA).
Statistical Analysis
Data were reported as mean ± SEM. Corneal graft survival was assessed using Kaplan-Meier survival curves. The statistical difference between two groups was assessed by Mann-Whitney U test except for where Student's t-test was used (see Fig. 4B). Data from multiple time-point studies were analyzed by ANOVA. The correlation analysis between central corneal thickening and graft rejection was performed in RStudio using linear mixed model (RStudio, Inc., Boston, MA, USA). All other statistical analysis was performed with GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA). The differences were considered statistically significant when P less than 0.05.
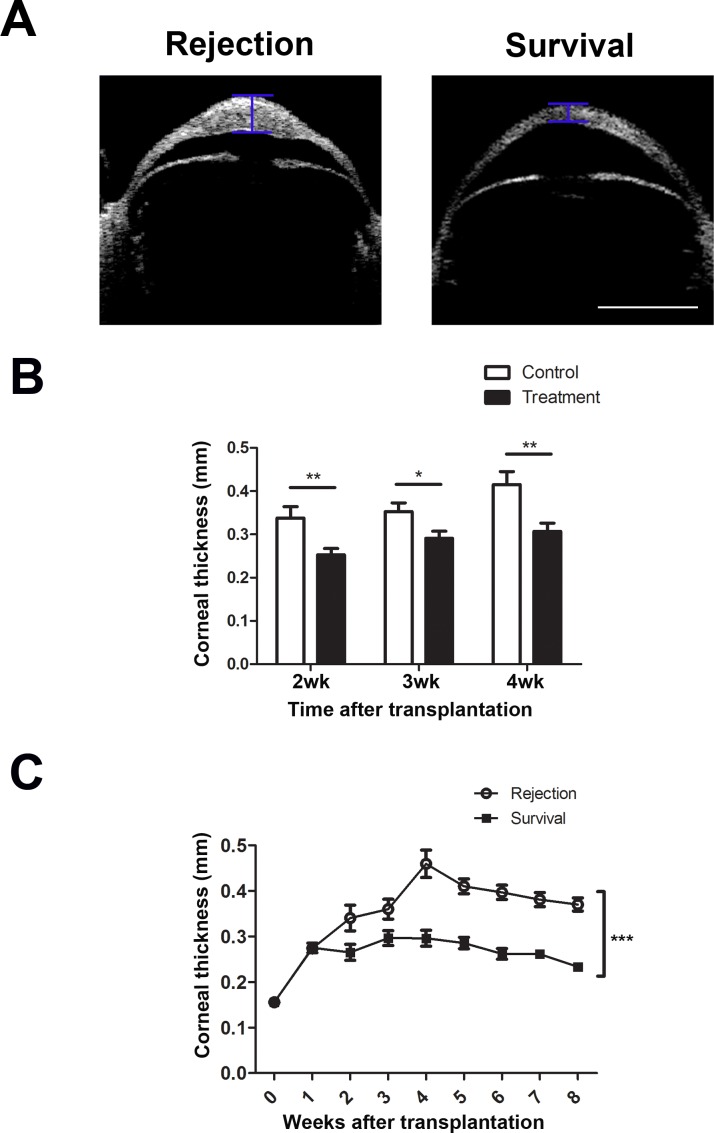
Figure 4.
Ang-2 blockade significantly reduced central corneal thickness in grafted corneas. (A) Representative in vivo OCT images showing rejected and survived grafts in the control and treatment groups at 8 weeks after transplantation, respectively. The survived graft demonstrated reduced thickness, as indicated by the blue brackets. Scale bar: 1 mm. (B) Summarized data showing reduced central cornea thickness in the treatment group at 2, 3, and 4 weeks (wk) after transplantation. *P < 0.05; **P < 0.01. (C) Summarized data showing increased central corneal thickness in the rejected than in the survived grafts. ***P < 0.001.
Results
Ang-2 Blockade Improved Graft Transparency and Survival
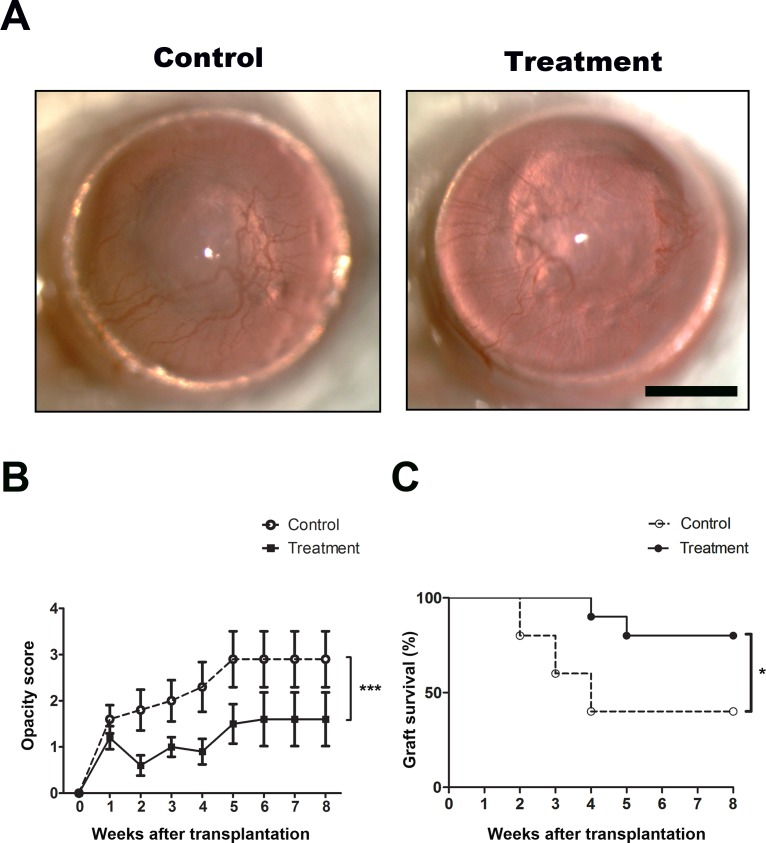
We first performed corneal transplantation between C57BL/6 (donor) and BALB/c (recipient) mice, which were fully mismatched in both major and minor histocompatibility antigens, and assessed the effect of anti-Ang-2 treatment on graft opacity for 8 weeks after the surgery. As presented in Figures 1A and 1B, our data from in vivo ophthalmic slit-lamp biomicroscopy showed that anti-Ang-2–treated corneas enjoyed greater clarity with lower opacity scores at all time-points studied (***P < 0.001). Moreover, the treated corneas demonstrated delayed onset and reduced degree of rejection (Fig. 1C). Within 4 weeks after transplantation, 60% of the grafts were already rejected in the control condition while as high as 90% of the treated corneas survived. By the end of the 8-week study when the peak of transplant rejection already passed in this setting of allogeneic combination, the significant difference between the treatment and control groups still remained obvious with a survival rate of 80% in contrast to 40% (*P < 0.05).
Figure 1.
Ang-2 blockade significantly improved corneal graft survival. (A) Representative slit-lamp micrographs demonstrating rejected and survived corneal grafts in the control and treatment groups, respectively, at 8 weeks after transplantation. Scale bar: 1 mm. (B) Summarized data showing anti-Ang-2–treated corneas had lower opacity scores at all time-points studied. ***P < 0.001. (C) Kaplan-Meier survival curves showing anti-Ang-2–treated corneas enjoyed higher rate of survival. *P < 0.05.
Ang-2 Blockade Suppressed Transplantation-Induced Lymphangiogenesis
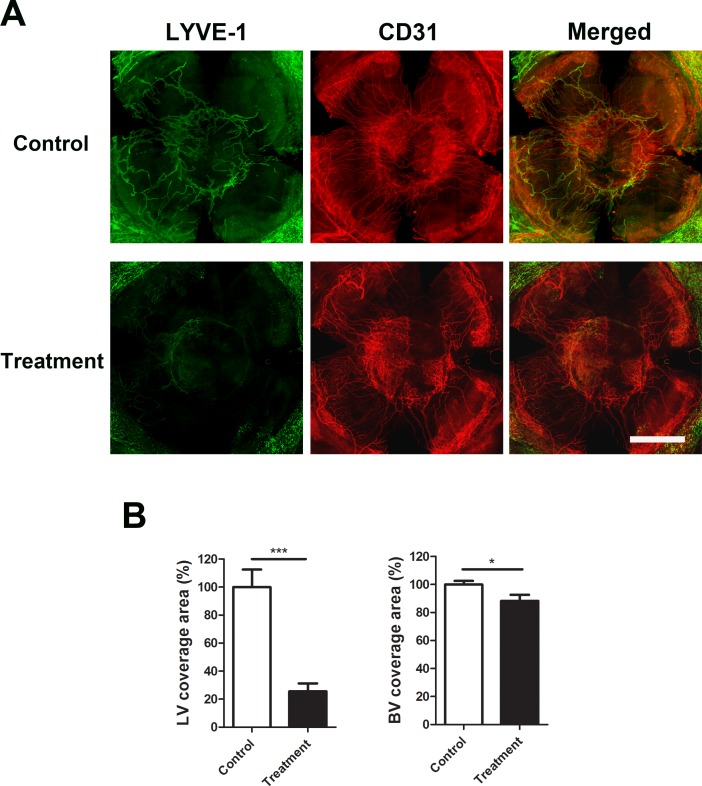
To further evaluate the effect of Ang-2 blockade on the formation of lymphatic and blood vessels after corneal transplantation, we harvested whole-mount corneas at the end of the 8-week transplantation study, and immunostained the samples for LYVE-1 and CD31. As demonstrated in Figure 2, Ang-2 blockade led to a dramatic decrease in lymphatic coverage area (75% reduction, ***P < 0.001), and the inhibitory effect on blood vessels was much less significant (10% reduction, *P < 0.05).
Figure 2.
Ang-2 blockade markedly inhibited lymphatic formation in grafted corneas. (A) Representative whole-mount images showing significant reduced lymphatic vessels in anti-Ang-2–treated corneas 8 weeks after transplantation. Fewer blood vessels were observed in the treated corneas but to a less degree. Green: LYVE-1; Red: CD31. Scale bar: 1 mm. (B) Summarized data in vascular coverage area. ***P < 0.001; *P < 0.05. LV, lymphatic vessel; BV, blood vessel.
Ang-2 Blockade Reduced Donor-Derived Cell Trafficking to Draining Lymph Nodes
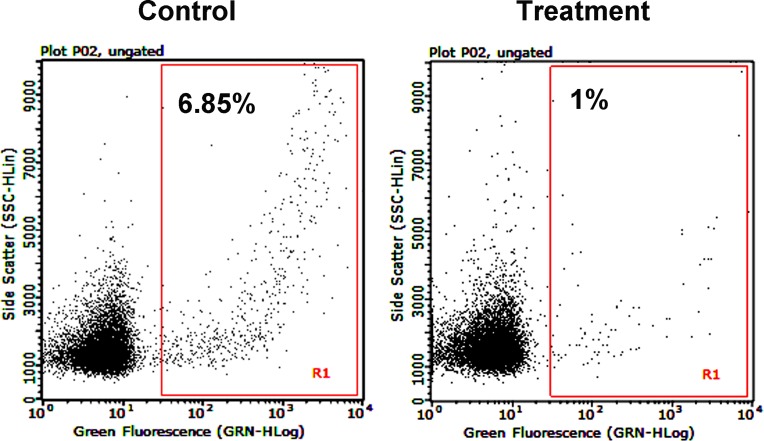
We next assessed the effect of Ang-2 blockade on donor-derived cell trafficking to recipient draining lymph nodes, which is an important parameter for assessing the afferent pathway of the immune reflex arc for graft rejection.2 We performed flow cytometric analysis and analyzed donor type MHC class II alloantigen in the ipsilateral submandibular lymph nodes after corneal transplantation. Our results showed that anti-Ang-2 treatment led to a significant reduction of donor-derived cells in the draining lymph nodes, as demonstrated in Figure 3.
Figure 3.
Ang-2 blockade significantly reduced donor-derived cell trafficking to draining lymph nodes. Flow cytometric analysis of the ipsilateral draining lymph nodes at 8 weeks after corneal transplantation showing reduced donor-derived I-A [b]–positive cells in the treatment condition (R1 gate).
Ang-2 Blockade Reduced Central Corneal Thickness in Grafted Corneas
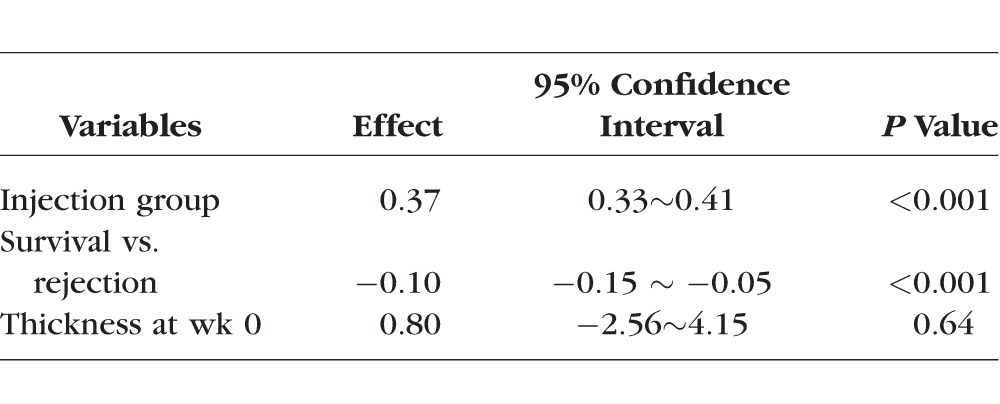
Corneal transplantation surgery is associated with corneal thickening and edema. OCT is an advanced technology that can be used to obtain accurate measurement of corneal thickness but has yet to be applied to murine corneal transplantation. To explore this possibility and to investigate whether corneal thickness can be used as a parameter for murine graft rejection, we assessed the central corneal thickness of the grafts with an anterior segment OCT and compared the results between anti-Ang-2 treatment and control conditions in the 8-week study (Fig. 4). Our results showed that anti-Ang-2–treated corneas had reduced central thickness at 2, 3, and 4 weeks post transplantation (Fig. 4B, *P < 0.05; **P < 0.01). Further, central corneal thickness was significantly higher in the rejected than the survived corneas in the 8-week study (Fig. 4C, ***P < 0.001). As presented in the Table, further evaluation using linear mixed model showed that central corneal thickening was significantly correlated with graft rejection. While there was no difference in central corneal thickness between the survived and rejected groups at the baseline level (week 0), the group difference changed significantly over time. On average, thickness in the rejection group was 0.37 mm (95% CI: 0.33∼0.41). Compared with the rejection group, thickness in the survival group was 0.10 mm lower (95% CI: −0.15 ∼ −0.05) across the 8 weeks. These results support central corneal thickening as a valuable parameter to assess murine graft rejection, and this study has validated anterior segment OCT as a new tool to monitor this parameter in vivo.
Table.
Statistical Inference for Central Corneal Thickness Between Survived and Rejected Corneas Over 8 Weeks
Ang-2 Blockade Affected Macrophage Morphologic Phenotype in Grafted Corneas
Previously, we showed that Ang-2 is expressed on corneal macrophages.6 More recently, it was reported that anti-Ang-2 treatment reduced the number of macrophages in the corneas 10 days after suture placement.10 Because macrophage infiltration peaks around 2 weeks after corneal transplanation,11 we next evaluated the effect of Ang-2 blockade on corneal macrophages at 2 weeks after transplantation. As shown in Supplementary Figure S1, our results showed that Ang-2 blockade tended to reduce the number of macrophages inside the grafted corneas though the data were not statistically significant (P = 0.9). Interestingly, it was found that this treatment affected the morphologic phenotype of macrophages. While macrophages in Ang-2–treated corneas showed a rounded inactive shape, those in the control corneas had a spindle-shaped appearance of activation.12
Discussion
In summary, we present in this study a critical role of Ang-2 in mediating corneal transplant rejection. Our data have revealed Ang-2 as a new therapeutic target to interfere with transplantation-induced LG, to monitor donor-derived cell trafficking to draining lymph nodes, to affect macrophage activation, and to ultimately promote graft survival. Additionally, we have demonstrated that anterior segment OCT is a valuable tool in monitoring murine corneal transplants in vivo and have shown that anti-Ang-2 treatment reduces central corneal thickening, which is an important parameter strongly associated with graft rejection.
Previously, we reported that suture-induced corneal LG is abolished in Ang-2 knockout mice where blood vessels still develop but to a less degree.6 In the current study with transplantation, a much more complicated procedure than suture placement, we observed similar preferential effect on LG in anti-Ang-2–treated wild-type mice. Taken together, our studies have demonstrated that Ang-2 is differentially involved in lymphatic versus blood vessel formation, and this differential role of Ang-2 indeed renders it as an ideal candidate to manage graft rejection where blood vessels are desired for wound healing and lymphatic vessels need to be inhibited for immune rejection, which demands further investigation. This study also supports that the lymphatic pathway is a primary mediator of transplant rejection, as reported by us and other researchers in the field.2,3,13
In the immune reflex arc, donor-derived cell trafficking to draining lymph nodes is mediated by lymphatic vessels. It was reported before that surgical removal of the draining lymph nodes can promote corneal graft survival.14 However, this strategy is not practical in clinic. It is therefore necessary to explore the underlying molecular mechanisms in order to identify new therapeutic targets. In this study, using a preclinical model of corneal transplantation, we show that by antagonizing Ang-2, we can achieve this goal and to ultimately promote graft survival. Our data show that at the end of the 8-week study, our anti-Ang-2 strategy has led to a 75% reduction of corneal LG in wild-type mice. This is a dramatic effect that cannot be easily achieved. It may be partially due to a role of Ang-2 in lymphatic maturation and stabilization, as reported in development.5,15 In this study, we show that even at 8 weeks after corneal transplantation, donor-derived cells can be detected in draining lymph nodes, and this can be suppressed by anti-Ang-2 treatment. Because corneal results have been approved to be readily applicable to nonocular sites, and the lymphatic pathway has been identified in major organ transplant rejection outside the eye,1,16–18 it is plausible that similar therapeutic results can be achieved at other sites of the body. It would be interesting to explore whether Ang-2 blockade affects other indices of transplant immunity, such as delayed type hypersensitivity (DTH). Because it is known that intervention of the lymphatic pathway can suppress this T cell–mediated activity, it is highly likely that anti-Ang-2 treatment can inhibit DTH as well.
Corneal opacity has been used as a single parameter to assess mouse graft rejection and it has been assessed by conventional ophthalmic slit-lamp microscopy for decades. In this study, we present a new method to monitor murine corneal grafts in vivo using the advanced technology of anterior segment OCT. Using this technology, we have demonstrated that central corneal thickening is strongly associated with graft rejection in mice. Our data align well with a recent rat study by Liu et al.19 In the current study, we have also shown that central corneal thickening can be interfered with a molecular intervention, such as anti-Ang-2 treatment. These results together support OCT as a new and reliable technology to monitor murine corneal grafts in vivo. Because murine transplantation model is the most widely employed animal model to study corneal transplantation immunity, it is our hope that future utilization of this new technology will facilitate corneal transplantation research in the field.
Supplementary Material
Acknowledgments
The authors thank the Core Translational Module at Program in Vision Science and School of Optometry, University of California at Berkeley, for statistical assistance.
Supported by grants from National Institutes of Health (Bethesda, MD, USA) and the University of California at Berkeley (LC; Berkeley, CA, USA).
Disclosure: L. Zhang, None; G. Li, None; R. Sessa, None; G.J. Kang, None; M. Shi, None; S. Ge, None; A.J. Gong, None; Y. Wen, None; S. Chintharlapalli, None; L. Chen, None
References
- 1. Chen L. Ocular lymphatics: state-of-the-art review. Lymphology. 2009; 42: 66–76. [PMC free article] [PubMed] [Google Scholar]
- 2. Chen L,, Hamrah P,, Cursiefen C,, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004; 10: 813–815. [DOI] [PubMed] [Google Scholar]
- 3. Zhang H,, Grimaldo S,, Yuen D,, Chen L. Combined blockade of VEGFR-3 and VLA-1 markedly promotes high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2011; 52: 6529–6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scholz A,, Plate KH,, Reiss Y. Angiopoietin-2: a multifaceted cytokine that functions in both angiogenesis and inflammation. Ann N Y Acad Sci. 2015; 1347: 45–51. [DOI] [PubMed] [Google Scholar]
- 5. Dellinger M,, Hunter R,, Bernas M,, et al. Defective remodeling and maturation of the lymphatic vasculature in angiopoietin-2 deficient mice. Dev Biol. 2008; 319: 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yuen D,, Grimaldo S,, Sessa R,, et al. Role of angiopoietin-2 in corneal lymphangiogenesis. Invest Ophthalmol Vis Sci. 2014; 55: 3320–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zheng W,, Nurmi H,, Appak S,, et al. Angiopoietin 2 regulates the transformation and integrity of lymphatic endothelial cell junctions. Genes Dev. 2014; 28: 1592–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sessa R,, Yuen D,, Wan S,, et al. Monocyte-derived Wnt5a regulates inflammatory lymphangiogenesis. Cell Res. 2016; 26: 262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Altiok E,, Ecoiffier T,, Sessa R,, et al. Integrin alpha-9 mediates lymphatic valve formation in corneal lymphangiogenesis. Invest Ophthalmol Vis Sci. 2015; 56: 6313–6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrari G,, Giacomini C,, Bignami F,, et al. Angiopoietin 2 expression in the cornea and its control of corneal neovascularization [published online ahead of print May 4, 2016]. Br J Ophthalmol. doi:10.1136/bjophthalmol-2015-307901. [DOI] [PubMed]
- 11. Chen L,, Huq S,, Gardner H,, de Fougerolles AR,, Barabino S,, Dana MR. Very late antigen 1 blockade markedly promotes survival of corneal allografts. Arch Ophthalmol. 2007; 125: 783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vogel DY,, Heijnen PD,, Breur M,, et al. Macrophages migrate in an activation-dependent manner to chemokines involved in neuroinflammation. J Neuroinflammation. 2014; 11: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dietrich T,, Bock F,, Yuen D,, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010; 184: 535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamagami S,, Dana MR. The critical role of lymph nodes in corneal alloimmunization and graft rejection. Invest Ophthalmol Vis Sci. 2001; 42: 1293–1298. [PubMed] [Google Scholar]
- 15. Gale NW,, Thurston G,, Hackett SF,, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002; 3: 411–423. [DOI] [PubMed] [Google Scholar]
- 16. Kerjaschki D,, Huttary N,, Raab I,, et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006; 12: 230–234. [DOI] [PubMed] [Google Scholar]
- 17. Geissler HJ,, Dashkevich A,, Fischer UM,, et al. First year changes of myocardial lymphatic endothelial markers in heart transplant recipients. Euro J Cardiothorac Surg. 2006; 29: 767–771. [DOI] [PubMed] [Google Scholar]
- 18. Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011; 17: 1371–1380. [DOI] [PubMed] [Google Scholar]
- 19. Liu YC,, Lwin NC,, Chan NS,, Mehta JS. Use of anterior segment optical coherence tomography to predict corneal graft rejection in small animal models. Invest Ophthalmol Vis Sci. 2014; 55: 6736–6741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.