Abstract
Premise of the study:
Herbarium specimens may provide a record of past environmental conditions, including heavy metal pollution. To explore this potential, we compared concentrations of copper, lead, and zinc in historical and new collections from four sites in Rhode Island, USA.
Methods:
We compared historical specimens (1846 to 1916) to congener specimens collected in 2015 at three former industrial sites in Providence, Rhode Island, and one nonindustrial site on Block Island. Leaf material was prepared by UltraWAVE SRC Microwave Digestion, and heavy metal concentrations were measured by inductively coupled plasma–atomic emission spectroscopy.
Results:
Heavy metal concentrations in the historical and new specimens were measurable for all elements tested, and levels of copper and zinc were comparable in the historical and 2015 collections. By contrast, the concentration of lead declined at all sites over time. Significant variability in heavy metal concentration was observed between taxa, reflecting their varied potential for elemental accumulation.
Discussion:
It seems clear that herbarium specimens can be used to evaluate past levels of pollution and assess local environmental changes. With careful sampling effort, these specimens can be a valuable part of environmental science research. Broadening the possible applications for herbarium collections in this way increases their relevance in an era of reduced funding for collections-based research.
Keywords: heavy metals, herbarium specimens, Rhode Island
Humans have radically altered biogeochemical cycling of heavy metals in the environment (Nriagu and Pacyna, 1988), and the negative health implications of heavy metal pollution have made this the focus of a growing body of research (Ho and El-Khaiary, 2009). Urban industrial centers are sites of particular concern and interest, because they are often impacted by high levels of pollution in areas of concentrated population. The city of Providence, Rhode Island, USA, is one such example. It was historically a center of industrial manufacturing, particularly jewelry and textile production (RIDEM, 1998). The first textile mills in Rhode Island, powered by flowing water, were built along the Seekonk River in the late eighteenth and early nineteenth centuries. Over time, this river valley became a center of the Industrial Revolution in the northeastern United States. In addition, various manufacturing companies were operating in the city of Providence. One example is the Gorham Manufacturing Company, a leading producer of jewelry and silverware in the United States since the mid-eighteenth century (Marlow, 2016). Jewelry and textile industries, along with metal smelting and incineration of effluent and fuel, created a local source of both air and water pollution by copper (Cu), lead (Pb), and zinc (Zn), among other metals (Nixon, 1991). Evidence for these emissions and deposits has been documented in sediment cores taken from Narragansett Bay, south of Providence. The cores show Cu, Pb, and Zn have been present in elevated quantities since the mid to late 1800s (Goldberg et al., 1977). More recently, industrial activity in Rhode Island has declined, and the Clean Air and Clean Water acts have sought to mitigate the negative health and environmental impacts of this industrial past. However, Providence County alone contains eight Environmental Protection Agency–designated Superfund sites and 88 hazardous waste sites that represent the modern legacy of this industrial past (Rose, 2008). Documenting the history of heavy metal accumulation is a crucial element of understanding the current environmental status of the northeastern United States.
Plants have been used as indicators of heavy metal pollution for several decades (Smith, 1972). They may accumulate atmospheric deposits on their leaves or incorporate metals from soil and groundwater into their tissues (Yoon et al., 2006; McKone and Maddalena, 2007; Rascio and Navari-Izzo, 2011; Bhargava et al., 2012; Fantozzi et al., 2013). Accumulation depends on the species of plant, as well as plant physiology and surroundings (Rodríguez Martín et al., 2015). Based on their relative ability to exclude or take up heavy metals, plants can be categorized as excluders, accumulators, or hyperaccumulators (Bhargava et al., 2012). Hyperaccumulation is hypothesized to be an adaptation to defend against pathogens and herbivores (Fones et al., 2016).
Herbarium specimens provide data for a wide range of studies beyond their traditional utility for comparative taxonomy and systematics research. The New York Botanical Garden Herbarium (NY) has documented uses of their specimens including projects that tracked the spread of invasive species, monitored population trends of rare plants, chronologic sites for conservation, seed identification, ethnobotanical studies, and studies of nickel-, cobalt-, and selenium-accumulating plants in New Zealand (Holmgren, 1977). A more recent review of the use of herbarium specimens (Lavoie, 2013) documented their use in studies focused on biogeographical patterns, collection biases, plant diseases, and climate change–induced impacts on plant distribution. Herbarium specimens have also been part of studies investigating changes to atmospheric ozone concentrations (Ryan et al., 2009) and are critical components of studies examining changing patterns of chronological events (Primack et al., 2004; Calinger et al., 2013; Everill et al., 2014; Hart et al., 2014; Park and Schwartz, 2015; Davis et al., 2015). All these studies indicate a growing interest in using herbarium collections for environmental and ecological research.
Using herbarium specimens as indicators of historical heavy metal pollution is a potentially valuable modern use of these collections and a pressing area of environmental science research. Previous studies have compared heavy metal concentrations in historical and contemporary specimens of moss (Herpin et al., 1997; Weiss et al., 1999; Shotbolt et al., 2007; Saxena et al., 2008; Ochota and Stebel, 2013), lichen (Minganti et al., 2014), and woody plants (Petrova et al., 2014; Rodríguez Martín et al., 2015). To the best of our knowledge, only one other study (Peñuelas and Filella, 2002) has used herbaceous plants to detect pollution levels in historical specimens. The study found increasing levels of vanadium concentrations, a byproduct of fossil fuel combustion, in the specimens they tested (collected between 1920 and 1985). Concentrations of other elements (chromium, iron, lead, cadmium, titanium, barium, strontium, and aluminum) increased until the 1960s, and then gradually decreased as environmental regulations started to take effect (Peñuelas and Filella, 2002).
Using herbarium specimens in this way is challenging because even closely related species may have different potential for accumulation of heavy metals (Rodríguez Martín et al., 2015). This makes comparisons between distantly related clades subject to uncontrolled variation. However, if this is taken into account, it should be possible to investigate broad temporal and spatial trends by comparing historical and new herbarium specimens collected from the same locations and, by so doing, take advantage of the huge amount of data stored in the world’s botanical repositories. Here we seek to build upon an existing body of work, with greater emphasis on site-specific changes in pollution, and with a particular focus on herbaceous plants. To accomplish this, we measured the concentrations of Cu, Pb, and Zn in historical and modern plant specimens from three former industrial sites within the city of Providence, Rhode Island, and one site on Block Island, a nonindustrial island 21 km off the coast of Rhode Island. Specifically, we addressed two questions: (1) Has the concentration of Cu, Pb, and Zn changed over time when comparing herbarium specimens collected during the height of industrial activity in Providence and the modern, nonindustrial era? This question examines how changes in Rhode Island’s economic base and stricter environmental regulations have affected levels of pollution through time. (2) Do the concentrations of Cu, Pb, and Zn at each time period vary between the four sites? Here we are primarily interested in comparisons between the industrial sites in Providence and the nonindustrial Block Island site. Much of the industrial era pollution resulted directly from waste dumping that contaminated soils and waterways. Because there is little reason to believe this occurred on Block Island, we are interested in possible effects of past and present atmospheric pollution on this island environment.
We were particularly interested in demonstrating the value of herbarium specimens as indicators of past pollution. In an era of reduced funding for collections-based research, broadening the scope of herbarium research is important to ensure these collections remain a valuable tool for modern scientific research even as resources diminish for natural history collections. This is particularly important for smaller collections that are especially vulnerable to administrative cuts, but that often have a strong regional focus and contain locally collected specimens suitable for studies such as ours.
MATERIALS AND METHODS
Sample collection
Samples were taken from historical specimens in the Brown University Herbarium (BRU) from four Rhode Island sites. New specimens, from the same four sites, were collected in the summer of 2015. The availability of specimens in the herbarium was a limiting factor in determining the study sites. The four sites we included in the analysis (Fig. 1)—Cove Lands, Mashapaug Pond, Seekonk River, and Block Island—were selected because of their land use history and because they are well represented in the BRU collection. The first three sites are located within the city of Providence in formerly industrial areas historically exposed to substantial levels of pollution. The Seekonk River site is located on the East Side of Providence, on the western bank of the Seekonk River. Cove Lands refers to the land surrounding what is currently the Providence train station, specifically an empty lot immediately between the northbound train tracks and the Moshassuck River. On the west side of Providence at Mashapaug Pond, the former site of the Gorham Silver Manufacturing Company, we collected at two small parks on the southwest side of the pond. Block Island, 21 km off the coast of Rhode Island, was historically a farming and fishing community that provided a nonindustrial site for our study. We collected on the southern portion of the island, in Rodman’s Hollow Nature Preserve, a glacial outwash basin containing a maritime shrub–land plant community.
Fig. 1.
(A) Location of Rhode Island in the northeastern United States and (B) locations of the three formerly industrial sites in Providence and one nonindustrial site on Block Island.
From these four sites, 50 historical specimens were selected from the herbarium, collected between 1846 and 1916. This time period corresponds with the main growth period of Providence’s industrial era. We attempted to account for the possible confounding effects of phylogeny on heavy metal accumulation potential by selecting a set of specimens that included some close relatives across all sites. In the end, this effort was not entirely successful, primarily because the element analysis required destructive sampling from a portion of each specimen. As a result, we were only able to include species well represented in our collection, and it was not always possible to locate the same species in the field in 2015. Given this constraint, our strongest phylogenetic control came from examining the genus Plantago L., from which we were able to sample specimens from both time periods from across all study sites. We were also able to sample across all sites in both time periods from within the family Asteraceae. We recognize that this is a large clade and is likely to include wide variation in metal accumulation potential, making this a rather weak phylogenetic control.
Using the list of available historical specimens as a guide, we visited each site to make new collections for comparisons of contemporary heavy metal concentrations. We attempted to match the historical and contemporary species, but when this was not possible we collected congeneric or confamilial specimens. Three to five individuals of each species were collected at each site. Voucher specimens for all new collections were deposited in BRU (Appendix 1). Approximately 0.5–1.0 g of leaf material was removed from each specimen. For both historical and recently collected specimens, we pooled leaf material from two to five specimens of the same genus, or the same species when possible, from each location to ensure adequate material for analysis without removing too much tissue from any individual specimen.
Sample preparation
Sample material was freeze-dried for 48 h and then ground with a mortar and pestle. The resulting fine powder was acid-digested in the Milestone UltraWAVE SRC Microwave Digestion System (Milestone Inc., Shelton, Connecticut, USA) to dissolve the organic material and make heavy metals available for measurement following the Environmental Protection Agency (EPA) Method 3051A (EPA, 2007a). This method was designed for digesting the organic components of soils and sediments, and we adapted it for use on plant material, using the UltraWAVE Microwave Digestion System instead of a standard microwave because it allowed us to use a smaller quantity of leaf material, reducing the amount of material removed from each specimen. We used 0.2 g of each sample, along with National Institute of Standards and Technology Standard Reference Material (NIST) 1575 (pine needles), 1515 (apple leaves), and reagent blanks. Samples were combined with 5 mL of 70% double-distilled nitric acid in a preweighed glass test tube with a Teflon cap. The samples were loaded into the UltraWAVE, pressurized to 40 bars, and heated to 175°C for 15 min. After cooling, approximately 2.5 mL of the digested samples was passed through a 0.45-µm Millipore Millex-HV syringe filter (EMD Millipore, Billerica, Massachusetts, USA) and diluted by a factor of 5 with 18.2 Mohm-cm deionized water. The specific dilution for each sample was determined gravimetrically.
Inductively coupled plasma–atomic emission spectroscopy
Inductively coupled plasma–atomic emission spectroscopy (ICP-AES) was used to measure levels of Cu, Pb, and Zn in the samples. Calibration curves were generated for the metals of interest for the expected ranges of 0–0.1 μg/g for Cu and Pb and 0–1.0 μg/g for Zn. Samples that had been acid-digested and diluted as previously described were measured on a Jobin Yvon JY2000 sequential optical emission spectrometer (Horiba Instruments, Irvine, California, USA), along with Inorganic Ventures EPA Quality Control Standard IV-28 (QC-28; Inorganic Ventures, Christiansburg, Virginia, USA) interspersed between every 10 samples to assess the precision and accuracy of the machine.
The measured values of the blanks, QC-28, and apple and pine standards are shown in Appendix 2. Based on analyses of these blanks, all of which contained <0.02 μg/g of the measured metals, the detection limit of Cu, Pb, and Zn was calculated to be 1 μg/kg. Similarly, the QC-28 measurements were consistently close to 0.085 μg/g, confirming the reliability of the UltraWAVE Digestion and ICP-AES.
In addition to measuring the concentrations of Cu, Pb, and Zn with the ICP-AES, we considered studying other metals such as mercury (Hg), cadmium (Cd), and chromium (Cr). The Milestone DMA-80 Mercury Analyzer was used to measure the Hg concentration of the dried leaf material following EPA Method 7473 (EPA, 2007b). A subset of the historical samples was run twice, and in each case we encountered difficulties. This was attributed to high levels of Hg in samples due to treatment of mercuric chloride insecticide that saturated the column and detector. Mercuric chloride was a common treatment in the past for herbarium collections to reduce insect damage to the specimens (Oyarzun et al., 2007; Purewal et al., 2008). Other metals, including Cd and Cr, were found in measurable concentrations following additional fivefold dilution using a Thermo Scientific XSERIES 2 inductively coupled plasma–mass spectrometer (ICP-MS) (Thermo Fisher Scientific, Waltham, Massachusetts, USA), but the high variability observed on our blanks caused us to discount these results.
Statistical analysis
To account for the nonnormality of our data, we used nonparametric Wilcoxon and Kruskal–Wallis rank sums tests to compare elemental concentrations for all taxa combined at each site between the historical and 2015 specimens to address question 1. To investigate changes in elemental concentration in Plantago, we considered each site to be an independent sample (n = 4 for each time period) and used Wilcoxon and Kruskal–Wallis rank sums tests to compare heavy metal concentrations between the historical Plantago specimens and 2015 collections. By focusing on a single genus, we gained some degree of phylogenetic control to account for likely variations in metal accumulation potential across taxa. To address question 2, we compared mean concentrations of elements of all taxa across all sites for each time period separately with analysis of variance (ANOVA). All analyses were performed in JMP version 11 (SAS Institute, Cary, North Carolina, USA).
RESULTS
Of the 50 historical herbarium specimens we analyzed from BRU, 48 recorded the year of collection, which ranged from 1846 to 1916 (Appendix S1 (201.3KB, pdf) ). Most were collected in the decade from 1889 to 1899, a period of high activity in BRU. The oldest specimens were collected along the Seekonk River. In addition to the historical specimens, we also analyzed 22 new collections from the same four study sites, collected in 2015.
Overall, we sampled from 10 plant families: Apiaceae, Asteraceae, Cyperaceae, Juncaceae, Plantaginaceae, Poaceae, Polygonaceae, Scrophulariaceae, Verbenaceae, and Violaceae (Table 1). Members of the Asteraceae were represented at all sites. Plantago was the only genus represented at each site in both the historical and 2015 collections and was our best opportunity to control for phylogenetic differences in the potential of different taxa to accumulate heavy metals.
Table 1.
List of the species from historical and modern collections sampled from each site.
| Family/Species | Lifespana | BI | CL | MP | SR |
| Apiaceae | |||||
| Cicuta bulbifera | P | H | |||
| Cicuta maculata | P/B | H | |||
| Daucus carota | B | C | C | ||
| Sium suave | P | H | |||
| Asteraceae | |||||
| Artemisia biennis | B/A | H | |||
| Artemisia vulgaris | P | C | C | ||
| Erigeron annuus | A | C | |||
| Eurybia divaricata | P | C | C | ||
| Lactuca scariola | B/A | H | |||
| Lactuca sp. | — | C | |||
| Solidago rugosa | P | H/C | |||
| Symphyotrichum ericoides | P | H/C | |||
| Symphyotrichum laeve | P | H | |||
| Symphyotrichum lateriflorum | P | H | |||
| Symphyotrichum novae-angliae | P | H | |||
| Symphyotrichum tenuifolium | P | H | |||
| Cyperaceae | |||||
| Carex blanda | P | C | |||
| Carex muhlenbergii | P | H | |||
| Juncaceae | |||||
| Juncus acuminatus | P | H | |||
| Juncus articulatus | P | ||||
| Juncus tenuis | P | C | |||
| Plantaginaceae | |||||
| Plantago lanceolata | P/B/A | C | H | ||
| Plantago major | P | C | H/C | C | |
| Plantago media | P | H | |||
| Plantago patagonica | A | H | |||
| Plantago rugelii | P | H/C | H/C | ||
| Plantago sp. | — | H | |||
| Poaceae | |||||
| Spartina alterniflora | P | H/C | |||
| Polygonaceae | |||||
| Persicaria lapathifolia | A | H | H | ||
| Persicaria sp. | — | C | |||
| Polygonum aviculare | P/A | H | H | ||
| Polygonum hydropiperoides | P | H | |||
| Scrophulariaceae | |||||
| Veronica arvensis | A | H | |||
| Veronica peregrina | A | H | |||
| Verbenaceae | |||||
| Verbena bracteata | P/B/A | C | |||
| Violaceae | |||||
| Viola blanda | P | H | |||
| Viola cucullata | P | H | |||
| Viola sagittata | P | H | |||
| Viola sp. | — | C |
Note: BI = Block Island; C = contemporary 2015 collections; CL = Cove Lands; H = historical herbarium specimens; MP = Mashapaug Pond; SR = Seekonk River.
The lifespan denotes whether the species is perennial (P), biennial (B), or annual (A).
Question 1: Has the concentration of Cu, Pb, and Zn changed over time?
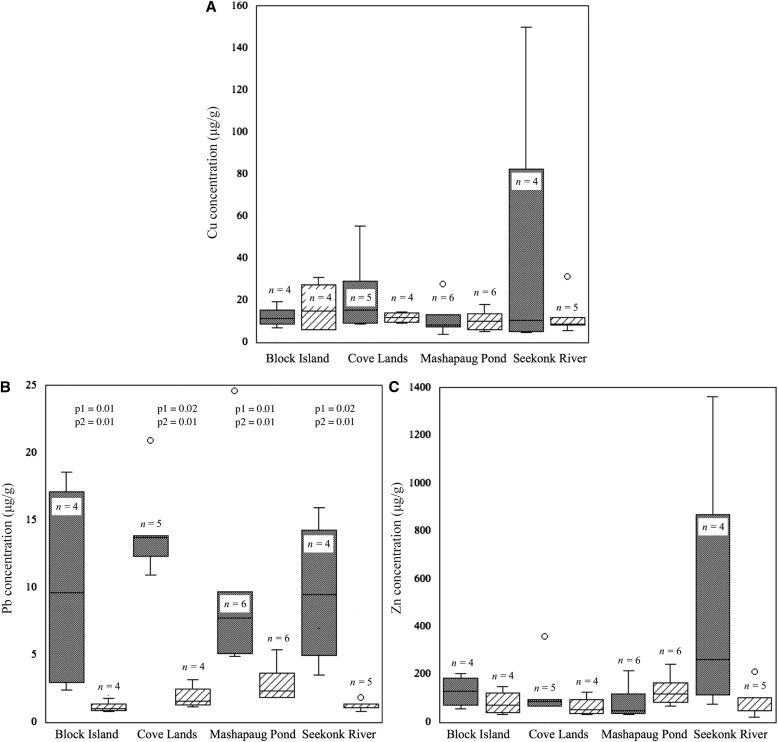
Based on Wilcoxon and Kruskal–Wallis tests, when considering all taxa pooled at each site, mean levels of Cu and Zn do not appear to have changed at any of the four study sites between the historical and 2015 collections (Fig. 2, Table 2). By contrast, we detected a significant decrease in Pb concentration at each site (Fig. 2, Table 2) based on Wilcoxon and Kruskal–Wallis rank sums tests. Raw data for these figures are given in Appendix 3.
Fig. 2.
Heavy metal concentrations (μg/g) of all species combined at each site. Historical specimens are shown with dotted fill; 2015 specimens are shown with diagonal lines. Box plots indicate first and third quartile and median values. Whiskers show range, except for outliers, which are shown as circles. Significant differences are indicated by P values based on Wilcoxon (p1) and Kruskal–Wallis rank sums tests (p2). Raw data can be found in Appendix 3. Duplicates were included as a single value (the average of the two measurements), and historical specimens treated with mercuric chloride insecticide were excluded.
Table 2.
Results of Wilcoxon and Kruskal–Wallis rank sums tests comparing historical (1846–1916) and 2015 specimens at the four study sites. The number of specimens for each element at each site for each time period is indicated on Fig. 2.
| Wilcoxon 2-sample rank sums test | Kruskal–Wallis 1-way rank sums test | ||||||
| Study site | Element | S | Z | P | χ2 | df | P |
| Block Island | Cu | 20 | −0.32 | 0.74 | 0.18 | 1 | 0.67 |
| Pb | 10 | −2.45 | 0.01* | 6.55 | 1 | 0.01* | |
| Zn | 17 | −0.96 | 0.34 | 1.14 | 1 | 0.29 | |
| Cove Lands | Cu | 17 | −0.61 | 0.54 | 0.54 | 1 | 0.46 |
| Pb | 10 | −2.33 | 0.02* | 6.00 | 1 | 0.01* | |
| Zn | 14 | −1.35 | 0.18 | 2.16 | 1 | 0.14 | |
| Mashapaug Pond | Cu | 42 | 0.40 | 0.69 | 0.23 | 1 | 0.63 |
| Pb | 23 | −2.48 | 0.01* | 6.56 | 1 | 0.01* | |
| Zn | 49 | 1.52 | 0.13 | 2.56 | 1 | 0.11 | |
| Seekonk River | Cu | 19 | −0.12 | 0.90 | 0.06 | 1 | 0.81 |
| Pb | 30 | 2.33 | 0.02* | 6.00 | 1 | 0.01* | |
| Zn | 26 | 1.35 | 0.18 | 2.16 | 1 | 0.14 | |
Note: df = degrees of freedom; S = score sum; Z = test statistic.
Significant differences (P < 0.05).
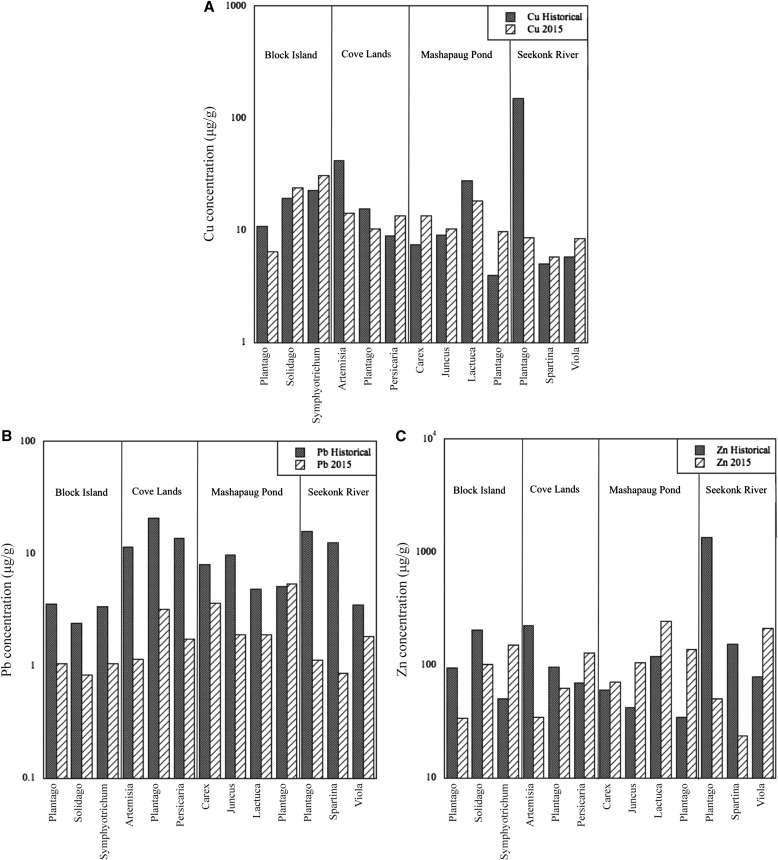
To provide some degree of phylogenetic control on the potential for heavy metal accumulation across taxa, we compared congeneric samples prepared from historical and 2015 collections (Fig. 3). These unreplicated comparisons cannot be tested statistically, but they do illustrate some broad trends that we also observed in the pooled comparisons. Concentrations of Cu and Zn did not show a strong pattern, increasing in some taxa and decreasing in others. By contrast, the concentrations of Pb decreased between the historical time period and 2015 in all taxa across all sites, except for Plantago at Mashapaug Pond.
Fig. 3.
Heavy metal concentration (μg/g) in historical and 2015 specimens, grouped by site, comparing congeneric specimens between the historical time period and 2015 at each site. Samples were pooled for each taxon at each site and time period so each bar represents a single value, not a mean value.
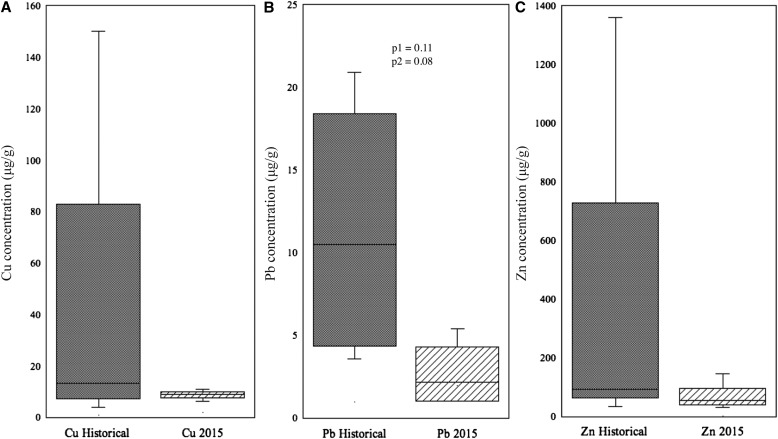
Plantago was the only genus collected at all sites in both time periods, making it a useful phylogenetically controlled comparison of heavy metal concentrations at our study sites between the historical and 2015 time periods. We pooled leaf tissue of Plantago at each site and considered each pool to be an independent sample (n = 4 for each time period). Treating the four sites from each time period as replicate measurements, we observed a marginally significant decrease in Pb between the two time periods and an overall reduction in the range of measurements of Cu, Pb, and Zn from the historical time period to 2015 (Fig. 4, Table 3).
Fig. 4.
Concentration (μg/g) of Cu (A), Pb, (B), and Zn (C) of Plantago specimens from the historical and 2015 time periods. Samples from all sites were pooled for each time period (n = 4 for each site at each time period). Marginally significant differences in Pb concentrations are indicated by P values based on Wilcoxon (p1) and Kruskal–Wallis rank sums tests (p2).
Table 3.
Results of Wilcoxon and Kruskal–Wallis rank sums tests comparing historical (1846–1916) and 2015 Plantago specimens. Each study site includes pooled leaf tissue from several specimens and represents one replicate (n = 4) for each time period.
| Wilcoxon 2-sample rank sums test | Kruskal–Wallis 1-way rank sums test | |||||
| Element | S | Z | P | χ2 | df | P |
| Cu | 15 | −0.72 | 0.47 | 0.75 | 1 | 0.39 |
| Pb | 12 | −0.59 | 0.11 | 3.00 | 1 | 0.08 |
| Zn | 16 | −0.43 | 0.67 | 0.33 | 1 | 0.56 |
Note: df = degrees of freedom; S = score sum; Z = test statistic.
Question 2: Do the concentrations of Cu, Pb, and Zn at each time period vary between the four sites?
We compared heavy metal concentrations of all taxa combined at each study site for each time period separately. For the historical time period included in our study, ANOVA indicated no significant differences in elemental concentrations measured between the four study sites (Table 4). Likewise, in 2015, concentrations of Cu and Zn did not vary between sites. By contrast, there was a significant difference in Pb levels between sites in 2015 (Fig. 2). Post hoc comparisons using the Tukey HSD test indicated that Pb concentrations were significantly lower at the Block Island and Seekonk River sites when compared to Mashapaug Pond (P = 0.050 and 0.047, respectively). The Cove Lands site Pb concentrations were intermediate between the Mashapaug Pond and Seekonk River sites.
Table 4.
Analysis of variance comparing heavy metal composition between sites at each time period. Analysis combines all sampled taxa at each site.
| Historical time period | 2015 | |||||||
| Element | df | Sum of squares | F ratio | P value | df | Sum of squares | F ratio | P value |
| Cu | 3 | 3.31e+9 | 0.99 | 0.42 | 3 | 9.94e+7 | 0.47 | 0.71 |
| Pb | 3 | 8.03e+7 | 0.62 | 0.61 | 3 | 1.05e+7 | 3.92 | 0.03* |
| Zn | 3 | 4.74e+10 | 2.08 | 0.15 | 3 | 1.30e+10 | 1.16 | 0.36 |
Note: df = degrees of freedom.
Significant difference between sites at one time period.
DISCUSSION
With careful sampling effort, herbarium specimens may be a valuable resource for studying historical levels of environmental heavy metal pollution. Tree specimens have been studied in this way (Rodríguez Martín et al., 2015), as well as lichens and mosses (Herpin et al., 1997; Weiss et al., 1999; Shotbolt et al., 2007; Saxena et al., 2008; Ochota and Stebel, 2013; Minganti et al., 2014). Our study demonstrates that herbaceous vascular plants may also be used for this purpose. However, the varied potential of different taxa to accumulate metals (Filipović-Trajković et al., 2012; Rodríguez Martín et al., 2015) and the different lifespans of perennials vs. annual herbaceous species result in a wide disparity of potential for heavy metal accumulation across taxa. These taxa-based differences are evident in the samples we analyzed; therefore, we juxtaposed close relatives to make spatio-temporal comparisons (Fig. 3). Indeed, many common weedy herbaceous species such as Plantago are documented metal accumulators (Dimitrova and Yurukova, 2005; Filipović-Trajković et al., 2012; Nadgórska-Socha et al., 2013), making them particularly good focal taxa for this type of study.
The goal of our study was to investigate levels of heavy metal concentration in old and new herbarium specimens as a proxy for changing pollution levels in the environment. We strived to create parallels between historical and modern collections at each site as well as phylogenetic overlap between sites. However, because BRU is a relatively small collection (Whitfeld et al., 2014), we ended up with less overlap than originally intended. As a result, the variation in metal accumulation potential of the species we sampled limits our ability to draw strong conclusions about changes over time in pollution at our study sites. More detailed analysis could be achieved by filling in gaps with historical specimens from other collections and increasing the number of samples. Unfortunately, this was beyond the scope of the current study but provides opportunities for future research and collaboration.
The use of older herbarium collections presents other challenges. Here we highlight two difficulties we encountered that limited some aspects of our study: (1) locality data on older herbarium specimens is often vague or absent, making many specimens unsuitable for studies that aim for direct comparisons of specific sites. (2) The site itself may have changed, through ecological succession or land use change, so that species documented in the past may no longer be present. Both of these problems limit the choice of specimens that can be sampled when the primary goal is assessing change in heavy metal pollution through time. For example, in BRU there are approximately 12,000 specimens collected in Rhode Island during the time period corresponding to Providence’s industrial era. Of these specimens, 654 were collected from the sites we hoped to sample (291 from Block Island, 194 from Cove Lands, 77 from Mashapaug Pond, and 92 from Seekonk River). The element analysis required approximately 5 cm2 of plant material; only a subset of the available specimens had sufficient tissue, which further limited the number of specimens available for the study. As a result, our original goal of sampling closely related species across all sites was difficult to realize based on specimens from BRU alone. Specimens from other herbaria could be used to fill the gaps. When available, online databases that combine herbarium records from many regional herbaria provide valuable sources of information for finding additional specimens for analysis. Examples include the North American Network of Small Herbaria (http://nansh.org/portal/), the Consortium of Northeastern Herbaria (www.neherbaria.org), the Consortium of Midwest Herbaria (http://midwestherbaria.org/portal/index.php), and the Southwestern Biodiversity Network (http://swbiodiversity.org/seinet/index.php), to name just a few. These online databases provide an open access gateway into the wealth of accumulated data in the world’s herbarium collections.
Despite some limitations, we were able to highlight patterns and broad trends in heavy metal accumulation between the two time periods under investigation and between our study sites in Rhode Island.
Question 1: Has the concentration of Cu, Pb, and Zn changed over time?
When we combined specimens from across the plant phylogeny, including all major angiosperm lineages (monocots, early diverging eudicots, asterids, rosids), Cu and Zn accumulation appeared to be steady or showed no clear trend between historical and 2015 specimens (Fig. 2). Previous studies (Tomašević et al., 2004) documented increasing levels of Cu and Zn on leaves in city parks in Belgrade, due to increasing urbanization. That we observed no clear trend in concentrations of these elements suggests that despite the general decrease in industrial activity in Providence, concomitant increases in automobile traffic and other sources of pollution have continued emissions of these metals.
We did observe a significant decrease in Pb accumulation between historical and 2015 collections both when all taxa were combined at each time period (Fig. 2, Table 2) and when we compared specimens within a single genus (Fig. 3). This suggests a widespread decline in Pb uptake at multiple sites around Rhode Island. Previous studies have observed a similar decline in Pb concentrations in other locations and attributed this to the introduction of unleaded gasoline (Peñuelas and Filella, 2002; Minganti et al., 2014). However, all of the historical specimens we used were collected before 1925, the year that lead was added to gasoline, so emissions from burning gasoline cannot explain the reduction in Pb that we observed (Needleman, 2004). Instead, the decrease in Pb concentration in our 2015 specimens is more likely due to a general decline in industrial manufacturing in Providence and more recent mitigation efforts.
Furthermore, we were able to use specimens of Plantago that were collected at the four sites during both time periods to control phylogenetic variation in bioaccumulation. This provided a more rigorous temporal comparison of the region and indicated a marginally significant decrease in Pb concentrations over time (Table 3). The range of Cu, Pb, and Zn concentration was also noticeably lower in the 2015 samples (Fig. 4). This may be because the historical specimens of Plantago were collected over several years (1890–1892) when there may have been variation in pollution levels. The larger range in concentration in the historical specimens may also reflect different levels of point-source pollution, e.g., dumping of industrial waste into waterways, compared to the more dispersed effects of atmospheric pollution.
Question 2: Do the concentrations of Cu, Pb, and Zn at each time period vary between the four sites?
We did not observe spatial differences in heavy metal concentrations in the historical specimens, even when comparing industrial sites in the city of Providence to the nonindustrial Block Island site. Historically, levels of contamination from both point and atmospheric sources were high throughout the region (Nixon, 1991; Rose, 2008). Widespread pollution is evidenced by elevated levels of heavy metals measured in sediment cores from Narragansett Bay, between Providence and Block Island (Goldberg et al., 1977). Block Island, located 21 km off the southern coast of Rhode Island, does not have a history of industrial activity. Agricultural and other human activities on the island may have left a legacy of pollution, but the elevated heavy metal concentrations we observed at this site during the historical time period were most likely from atmospheric sources generated on the mainland. By 2015, a general decline in mainland heavy metal emissions appears to have reduced Pb levels on Block Island. By contrast, the Seekonk River was formerly the site of several manufacturing plants, and from 1871 to 1920, unprocessed effluent was released from the Providence sewer system into the river (Nixon, 1991). Our collection site has been a public park since 1866 (Cady, 1957), and recent mitigation efforts replaced much of the contaminated soil from the area. These management efforts and a general decline in industrial activity along the river have significantly reduced heavy metal contamination in the area. High Pb measurements from specimens collected at Mashapaug Pond likely reflect activities at the Gorham Manufacturing Company silver factory, located on the northeast side of the pond between 1897 and 1985 (Providence Redevelopment Authority, 2012; Marlow, 2016). Remediation efforts began on the contaminated soil in 2000 in the area immediately surrounding the former factory site, which now is the site of Alvarez High School. However, lingering pollutants, including heavy metals, have been found in the sediments and water of Mashapaug Pond (Providence Redevelopment Authority, 2012), and there have been no remediation efforts on the southwest side of the pond, where we collected, therefore Pb levels remain high.
In addition to documenting changes in heavy metal concentrations in Rhode Island, the goal of this project was to showcase the potential of historical herbarium specimens in providing retrospective data for studies of environmental pollution. Despite the challenges of sampling from a small collection, we were still able to demonstrate significant decreases in Pb levels in the old and new plant specimens we analyzed across all four of our study sites. This shows that herbarium specimens have the potential to be an effective tool for comparing past and present pollution levels, to build a more complete picture of changes in environmental health. With careful selection of specimens that include detailed locality information, there is great potential for herbarium specimens to be an important component of environmental science research. Taking advantage of online herbarium portals to locate additional specimens would greatly facilitate collaborative efforts, increase replication, and improve statistical power. Overall, this approach expands the scope of herbarium collections beyond their traditional uses in taxonomy and systematics, making them relevant to a broader range of scientific studies. Perhaps such an expansion will allow herbarium collections to fulfill a wider potential to botany, ecology, and environmental science (Lavoie, 2013). We advocate strongly for broadening the relevance of herbarium collections in an era when general funding for organismal biology and, in particular, for museum collections, is declining.
Supplementary Material
Appendix 1.
List of herbarium specimens sampled. All specimens are located in the Brown University Herbarium (BRU), Providence, Rhode Island, USA.
| Species | Collection year | Location | Geographic coordinates | Accession no. |
| Artemisia biennis Willd. | 1889, 1892, 1900 | Cove Lands, Providence, RI, USA | 41°49′47.4″N, 71°24′41.9″W | PBRU00021762, PBRU00021761, PBRU00021760 |
| Artemisia vulgaris L. | 2015 | Cove Lands, Providence, RI, USA | 41°49′47.4″N, 71°24′41.9″W | PBRU00030884 |
| Artemisia vulgaris | 2015 | Seekonk River, Providence, RI, USA | 41°49′52.9″N, 71°22′45.0″W | PBRU00030757 |
| Carex blanda Dewey | 2015 | Mashapaug Pond, Providence, RI, USA | 41°47′22.7″N, 71°26′06.2″W | PBRU00030890 |
| Carex muhlenbergii Kunth ex Boott | 1890, 1892 | Mashapaug Pond, Providence, RI, USA | 41°47′22.7″N, 71°26′06.2″W | PBRU00002532, PBRU00002539 |
| Cicuta bulbifera L. | 1895, 1895, 1895 | Mashapaug Pond, Providence, RI, USA | 41°47′22.7″N, 71°26′06.2″W | PBRU00024137, PBRU00024139, PBRU00024140 |
| Cicuta maculata Lam. | 1892 | Block Island, New Shoreham, RI, USA | 41°09′30.9″N, 71°35′28.7″W | PBRU00024154 |
| Daucus carota L. | 2015 | Block Island, New Shoreham, RI, USA | 41°09′30.9″N, 71°35′28.7″W | PBRU00030753 |
| Daucus carota | 2015 | Mashapaug Pond, Providence, RI, USA | 41°47′22.7″N, 71°26′06.2″W | PBRU00030886 |
| Erigeron annuus (L.) Pers. | 2015 | Cove Lands, Providence, RI, USA | 41°49′47.4″N, 71°24′41.9″W | PBRU00030893 |
| Eurybia divaricata (L.) G. L. Nesom | 2015 | Mashapaug Pond, Providence, RI, USA | 41°47′22.7″N, 71°26′06.2″W | PBRU00030887 |
| Eurybia divaricata | 2015 | Seekonk River, Providence, RI, USA | 41°49′52.9″N, 71°22′45.0″W | PBRU00030750 |
| Juncus acuminatus Michx. | 1893, 1893 | Mashapaug Pond, Providence, RI, USA | 41°47′22.7″N, 71°26′06.2″W | PBRU00011674, PBRU00011704 |
| Juncus articulatus L. | 1892 | Mashapaug Pond, Providence, RI, USA | 41°47′22.7″N, 71°26′06.2″W | PBRU00011715 |
| Juncus tenuis Willd. | 2015 | Mashapaug Pond, Providence, RI, USA | 41°47′22.7″N, 71°26′06.2″W | PBRU00030755 |
| Lactuca scariola L. | 1894, 1894, 1895 | Mashapaug Pond, Providence, RI, USA | 41°47′22.7″N, 71°26′06.2″W | PBRU00023243, PBRU00023246, PBRU00023250 |
| Lactuca L. sp. | 2015 | Mashapaug Pond, Providence, RI, USA | 41°47′22.7″N, 71°26′06.2″W | PBRU00030888 |
| Persicaria Mill. sp. | 2015 | Cove Lands, Providence, RI, USA | 41°49′47.4″N, 71°24′41.9″W | PBRU00030892 |
| Plantago lanceolata L. | Seekonk River, Providence, RI, USA | 41°49′52.9″N, 71°22′45.0″W | PBRU00021069 | |
| Plantago major L. | 1892, 2015 | Cove Lands, Providence, RI, USA | 41°49′47.4″N, 71°24′41.9″W | PBRU00020878, PBRU00030889 |
| Plantago major | 2015 | Block Island, New Shoreham, RI, USA | 41°09′30.9″N, 71°35′28.7″W | PBRU00030754 |
| Plantago media L. | 1890, 1892 | Cove Lands, Providence, RI, USA | 41°49′47.4″N, 71°24′41.9″W | PBRU00020889, PBRU00020890 |
| Plantago patagonica Jacq. | 1892 | Block Island, New Shoreham, RI, USA | 41°09′30.9″N, 71°35′28.7″W | PBRU00021073 |
| Plantago rugelii Decne. | 1892, 2015 | Block Island, New Shoreham, RI, USA | 41°09′30.9″N, 71°35′28.7″W | PBRU00021094, PBRU00030747 |
| Plantago rugelii | 1892, 2015 | Mashapaug Pond, Providence, RI, USA | 41°47′22.7″N, 71°26′06.2″W | PBRU00021096, PBRU00030756 |
| Plantago L. sp. | 1890 | Block Island, New Shoreham, RI, USA | 41°09′30.9″N, 71°35′28.7″W | PBRU00021100 |
| Plantago sp. | 2015 | Seekonk River, Providence, RI, USA | 41°49′52.9″N, 71°22′45.0″W | PBRU00030891 |
| Polygonum aviculare L. | 1893 | Mashapaug Pond, Providence, RI, USA | 41°47′22.7″N, 71°26′06.2″W | PBRU00018989 |
| Polygonum aviculare | 1899 | Cove Lands, Providence, RI, USA | 41°49′47.4″N, 71°24′41.9″W | PBRU00018988 |
| Polygonum hydropiperoides Michx. | 1892, 1892 | Block Island, New Shoreham, RI, USA | 41°09′30.9″N, 71°35′28.7″W | PBRU00019119, PBRU00019122 |
| Polygonum lapathifolium L. | 1892, 1900 | Cove Lands, Providence, RI, USA | 41°49′47.4″N, 71°24′41.9″W | PBRU00019145, PBRU00019142 |
| Polygonum lapathifolium | 1893 | Mashapaug Pond, Providence, RI, USA | 41°47′22.7″N, 71°26′06.2″W | PBRU00019151 |
| Sium cicutifolium Schrank | 1892 | Block Island, New Shoreham, RI, USA | 41°09′30.9″N, 71°35′28.7″W | PBRU00024281 |
| Solidago rugosa Mill. | 1892, 1892, 1916, 2015 | Block Island, New Shoreham, RI, USA | 41°09′30.9″N, 71°35′28.7″W | PBRU00023701, PBRU00023711, PBRU00023699, PBRU00030752 |
| Spartina alterniflora Loisel. | 1873, 1889, 2015 | Seekonk River, Providence, RI, USA | 41°49′52.9″N, 71°22′45.0″W | PBRU00014271, PBRU00014264, PBRU00030751 |
| Symphyotrichum ericoides (L.) G. L. Nesom | 1916, 1916, 2015 | Block Island, New Shoreham, RI, USA | 41°09′30.9″N, 71°35′28.7″W | PBRU00022377, PBRU00022378, PBRU00030748 |
| Symphyotrichum laeve (L.) Á. Löve & D. Löve | 1871 | Seekonk River, Providence, RI, USA | 41°49′52.9″N, 71°22′45.0″W | PBRU00022405 |
| Symphyotrichum lateriflorum (L.) Á. Löve & D. Löve | 1890 | Seekonk River, Providence, RI, USA | 41°49′52.9″N, 71°22′45.0″W | PBRU00022081 |
| Symphyotrichum novae-angliae (L.) G. L. Nesom | 1916 | Block Island, New Shoreham, RI, USA | 41°09′30.9″N, 71°35′28.7″W | PBRU00022193 |
| Symphyotrichum tenuifolium (L.) G. L. Nesom | 1889 | Seekonk River, Providence, RI, USA | 41°49′52.9″N, 71°22′45.0″W | PBRU00021988 |
| Verbena bracteata Lag. & Rodr. | 2015 | Cove Lands, Providence, RI, USA | 41°49′47.4″N, 71°24′41.9″W | PBRU00030885 |
| Veronica arvensis L. | 1890, 1891, 1892 | Cove Lands, Providence, RI, USA | 41°49′47.4″N, 71°24′41.9″W | PBRU00021128, PBRU00021133, PBRU00021124 |
| Veronica peregrina L. | 1892 | Cove Lands, Providence, RI, USA | 41°49′47.4″N, 71°24′41.9″W | PBRU00021163 |
| Viola blanda Willd. | Seekonk River, Providence, RI, USA | 41°49′52.9″N, 71°22′45.0″W | PBRU00015711 | |
| Viola cucullata Aiton | 1846 | Seekonk River, Providence, RI, USA | 41°49′52.9″N, 71°22′45.0″W | PBRU00015784 |
| Viola sagittata Aiton | 1891 | Seekonk River, Providence, RI, USA | 41°49′52.9″N, 71°22′45.0″W | PBRU00015922 |
| Viola L. sp. | 2015 | Seekonk River, Providence, RI, USA | 41°49′52.9″N, 71°22′45.0″W | PBRU00030749 |
Appendix 2.
Concentrations (μg/g) of copper, lead, and zinc measured by ICP-AES in the blanks and standards included with analysis of historical and new herbarium specimens. All measurements were above the detectable limit of 1 μg/kg.
| Sample IDa | Cu | Pb | Zn |
| Blanks | |||
| Mean | 0.00 | 0.00 | 0.01 |
| SD | 0.00 | 0.00 | 0.01 |
| EPA Quality Control Standard 28 | |||
| Reference | 0.10 | 0.10 | 0.10 |
| Mean | 0.08 | 0.08 | 0.09 |
| SD | 0.00 | 0.00 | 0.00 |
| Apple Standard | |||
| Reference | 5.64 ± 0.24 | 0.47 ± 0.2 | 12.50 ± 0.30 |
| Mean | 4.82 | 0.73 | 10.76 |
| SD | 0.20 | 0.13 | 0.16 |
| Pine Standard | |||
| Reference | 3.00 ± 0.30 | 10.80 ± 0.50 | n/a |
| Mean | 2.67 | 9.32 | 56.20 |
| SD | 0.23 | 0.37 | 3.48 |
“Reference” refers to mean and expected variation for the apple and pine standards.
Appendix 3.
ICP-AES measurements of copper, lead, and zinc (μg/g) in historical and contemporary herbarium specimens adjusted for dilution. Congeneric specimens from the same site were pooled for analysis.a,b
| Site | Historical samples | Cu | Pb | Zn | 2015 Samples | Cu | Pb | Zn | |
| Block Island | Cicuta maculata and Sium suave | 11.99 | 18.63 | 57.44 | Daucus carota | 6.03 | 1.78 | 49.72 | |
| Symphyotrichum ericoides* and S. novae-angliae | 22.92 | 3.42 | 50.59 | Symphyotrichum ericoides | 31.34 | 1.05 | 150.53 | ||
| Plantago patagonica, P. rugelii, and Plantago sp. | 10.95 | 3.62 | 94.32 | Plantago major, P. patagonica, and P. rugelii | 6.82 | 1.04 | 35.10 | ||
| Plantago major, P. patagonica, and P. rugeliib | 6.29 | 1.06 | 33.11 | ||||||
| Polygonum hydropiperoides | 6.95 | 15.68 | 167.83 | ||||||
| Solidago rugosa | 18.94 | 2.49 | 213.34 | Solidago rugosa | 24.02 | 0.83 | 101.44 | ||
| Solidago rugosab | 19.93 | 2.31 | 199.10 | ||||||
| Solidago rugosa* | 54.12 | 8.61 | 120.30 | ||||||
| Mean | 13.75 | 8.54 | 146.41 | Mean | 14.90 | 1.15 | 73.98 | ||
| SD | 15.74 | 6.77 | 65.93 | SD | 11.96 | 0.36 | 50.95 | ||
| Cove Lands | Artemisia biennis | 29.38 | 12.39 | 362.14 | Artemisia vulgaris | 14.48 | 1.15 | 34.76 | |
| Artemisia vulgaris | 55.53 | 10.95 | 90.50 | ||||||
| Plantago major and P. media | 15.79 | 20.95 | 97.26 | Plantago lanceolata and P. major | 11.13 | 3.23 | 66.59 | ||
| Plantago lanceolata and P. majorb | 9.62 | 3.17 | 59.63 | ||||||
| Persicaria lapathifolia and Polygonum aviculare | 8.67 | 13.97 | 71.36 | Persicaria sp. | 13.66 | 1.75 | 128.43 | ||
| Persicaria lapathifolia and Polygonum aviculareb | 9.29 | 13.76 | 69.14 | ||||||
| Veronica arvensis and V. peregrina | 9.41 | 15.31 | 96.55 | ||||||
| Verbena bracteata | 9.29 | 1.43 | 44.00 | ||||||
| Mean | 21.34 | 14.56 | 131.16 | Mean | 11.64 | 2.15 | 66.68 | ||
| SD | 18.51 | 3.47 | 113.82 | SD | 2.35 | 0.99 | 36.73 | ||
| Mashapaug Pond | Cicuta bulbifera | 13.24 | 24.67 | 40.69 | Daucus carota | 6.07 | 2.82 | 84.46 | |
| Carex muhlenbergii | 7.57 | 8.13 | 61.07 | Carex blanda | 13.64 | 3.66 | 71.06 | ||
| Juncus acuminatus and J. articulatus | 9.17 | 9.76 | 42.45 | Juncus tenuis | 10.38 | 1.91 | 104.95 | ||
| Lactuca scariola | 14.31 | 4.87 | 122.91 | Lactuca sp. | 18.37 | 1.93 | 246.72 | ||
| Lactuca scariolab | 41.40 | 4.93 | 118.27 | ||||||
| Plantago rugelii | 3.99 | 5.14 | 34.70 | Plantago rugelii | 10.54 | 5.40 | 146.36 | ||
| Plantago rugeliib | 9.10 | 5.41 | 129.73 | ||||||
| Eurybia divaricata | 5.57 | 1.88 | 166.54 | ||||||
| Persicaria lapathifolia | 7.84 | 7.50 | 219.00 | ||||||
| Mean | 13.93 | 9.28 | 91.30 | Mean | 10.53 | 3.29 | 135.69 | ||
| SD | 12.61 | 7.04 | 67.21 | SD | 4.43 | 1.58 | 59.41 | ||
| Seekonk River | Symphyotrichum laeve, S. racemosum, and S. tenuifolium | 15.60 | 6.47 | 378.71 | Artemisia vulgaris | 31.65 | 1.39 | 105.02 | |
| Plantago lanceolata | 150.18 | 15.96 | 1363.26 | Plantago major | 8.58 | 1.08 | 48.96 | ||
| Plantago majorb | 8.92 | 1.18 | 51.50 | ||||||
| Spartina alterniflora | 5.25 | 14.22 | 184.10 | Spartina alterniflora | 5.89 | 0.87 | 23.79 | ||
| Spartina alterniflorab | 4.83 | 10.99 | 123.03 | ||||||
| Viola blanda, V. cucullata, and V. sagittata | 5.86 | 3.53 | 79.78 | Viola sp. | 8.55 | 1.86 | 213.43 | ||
| Eurybia divaricata | 12.16 | 1.12 | 105.29 | ||||||
| Mean | 36.35 | 10.24 | 425.78 | Mean | 12.63 | 1.25 | 91.33 | ||
| SD | 63.79 | 5.21 | 536.39 | SD | 9.53 | 0.34 | 68.17 |
Indicates herbarium sheet from which the sample was taken was labeled as “poisoned” with mercuric chloride insecticide. These samples were not included in statistical analyses.
Comparisons between time periods (shown in boldface) were included in Fig. 3.
Duplicate measurements (used as quality control checks) taken from the same sample.
LITERATURE CITED
- Bhargava A., Carmona F. F., Bhargava M., Srivastava S. 2012. Approaches for enhanced phytoextraction of heavy metals. Journal of Environmental Management 105: 103–120. [DOI] [PubMed] [Google Scholar]
- Cady J. H. 1957. The civic and architectural development of Providence, 1636–1950. Akerman Standard Press, Providence, Rhode Island, USA. [Google Scholar]
- Calinger K. M., Queenborough S., Curtis P. S. 2013. Herbarium specimens reveal the footprint of climate change on flowering trends across north-central North America. Ecology Letters 16: 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. C., Willis C. G., Connolly B., Kelly C., Ellison A. M. 2015. Herbarium records are reliable sources of phenological change driven by climate and provide novel insights into species’ phenological cueing mechanisms. American Journal of Botany 102: 1599–1609. [DOI] [PubMed] [Google Scholar]
- Dimitrova I., Yurukova L. 2005. Bioindication of anthropogenic pollution with Plantago lanceolata (Plantaginaceae): Metal accumulation, morphological and stomatal leaf characteristics. Phytologia Balcanica 11: 89–96. [Google Scholar]
- Environmental Protection Agency. 2007a. Method 3051A: Microwave assisted acid digestion of sediments, sludges, soils, and oils. Website https://www.epa.gov/hw-sw846/sw-846-test-method-3051a-microwave-assisted-acid-digestion-sediments-sludges-soils-and-oils [accessed 2 December 2016].
- Environmental Protection Agency. 2007b. Method 7473: Mercury in solids and solutions by thermal decomposition, amalgamation, and atomic absorption spectrophotometry. Website https://www.epa.gov/hw-sw846/sw-846-test-method-7473-mercury-solids-and-solutions-thermal-decomposition-amalgamation-and [accessed 2 December 2016].
- Everill P. H., Primack R. B., Ellwood E. R., Malaas E. K. 2014. Determining past leaf-out times of New England’s deciduous forests from herbarium specimens. American Journal of Botany 101: 1293–1300. [DOI] [PubMed] [Google Scholar]
- Fantozzi F., Monaci F., Blanusa T., Bargagli R. 2013. Holm Oak (Quercus ilex L.) canopy as interceptor of airborne trace elements and their accumulation in the litter and topsoil. Environmental Pollution 183: 89–95. [DOI] [PubMed] [Google Scholar]
- Filipović-Trajković R., Ilić Z. S., Šunić L., Andjelković S. 2012. The potential of different plant species for heavy metals accumulation and distribution. Journal of Food Agriculture and Environment 10: 959–964. [Google Scholar]
- Fones H. N., McCurrach H., Mithani A., Smith J. A. C., Preston G. M. 2016. Local adaptation is associated with zinc tolerance in Pseudomonas endophytes of the metal-hyperaccumulator plant Noccaea caerulescens. Proceedings of the Royal Society of London. Series B, Biological Sciences 283: 20160648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg E. D., Gamble E., Griffen J. J., Koide M. 1977. Pollution history of Narragansett Bay as recorded in its sediments. Estuarine and Coastal Marine Science 5: 549–561. [Google Scholar]
- Hart R., Salick J., Ranjitkar S., Xu J. 2014. Herbarium specimens show contrasting phenological responses to Himalayan climate. Proceedings of the National Academy of Sciences, USA 111: 10615–10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpin U., Markert B., Weckert V., Berlekamp J., Friese K., Siewers U., Lieth H. 1997. Retrospective analysis of heavy metal concentrations at selected locations in the Federal Republic of Germany using moss material from a herbarium. Science of the Total Environment 205: 1–12. [Google Scholar]
- Ho Y. S., El-Khaiary M. I. 2009. Metal research trends in the environmental field. In L. K. Wang, J. P. Chen, Y. T. Hung, and N. K. Shammas [eds.], Heavy metals in the environment, 1–12. CRC Press, Boca Raton, Florida, USA. [Google Scholar]
- Holmgren P. K. 1977. Uses of the New York Botanical Garden’s systematics collections for solution of problems of human health, food resources, environmental quality, and location of natural resources. Bulletin of the American Association of Botanical Gardens 11: 2–13. [Google Scholar]
- Lavoie C. 2013. Biological collections in an ever changing world: Herbaria as tools for biogeographical and environmental studies. Perspectives in Plant Ecology, Evolution and Systematics 15: 68–76. [Google Scholar]
- Marlow T. W. 2016. Socioenvironmental succession: The case of Rhode Island. Master’s thesis, Brown University, Providence, Rhode Island, USA. [Google Scholar]
- McKone T., Maddalena R. L. 2007. Plant uptake of organic pollutants from soil: A critical review of bioconcentration estimates based on models and experiments. Environmental Toxicology and Chemistry 26: 2494–2504. [DOI] [PubMed] [Google Scholar]
- Minganti V., Drava G., De Pellegrini R., Modenesi P., Malaspina P., Giordani P. 2014. Temporal trends (1981–2007) of trace and rare earth elements in the lichen Cetraria islandica (L.) Ach. from Italian herbaria. Chemosphere 99: 180–185. [DOI] [PubMed] [Google Scholar]
- Nadgórska-Socha A., Ptasiński B., Kita A. 2013. Heavy metal bioaccumulation and antioxidative responses in Cardaminopsis arenosa and Plantago lanceolata leaves from metalliferous and non-metalliferous sites: A field study. Ecotoxicology (London, England) 22: 1422–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman H. 2004. Lead poisoning. Annual Review of Medicine 55: 209–222. [DOI] [PubMed] [Google Scholar]
- Nixon S. 1991. A history of metal inputs to Narragansett Bay. Narragansett Bay Project, University of Rhode Island, Kingston, Rhode Island, USA. [Google Scholar]
- Nriagu J. O., Pacyna J. M. 1988. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 333: 134–139. [DOI] [PubMed] [Google Scholar]
- Ochota P., Stebel A. 2013. Preliminary studies on possibility of using the herbarium specimens of mosses in the assessment of heavy metal pollution in Katowice (Silesian Upland, Poland). Časopis Slezskeho Zemskeho Muzea Opava (A) 62: 59–64. [Google Scholar]
- Oyarzun R., Higueras P., Esbrí J. M., Pizarro J. 2007. Mercury in air and plant specimens in herbaria: A pilot study at the MAF Herbarium in Madrid (Spain). Science of the Total Environment 387: 346–352. [DOI] [PubMed] [Google Scholar]
- Park I. W., Schwartz M. D. 2015. Long-term herbarium records reveal temperature-dependent changes in flowering phenology in the southeastern USA. International Journal of Biometeorology 59: 347–355. [DOI] [PubMed] [Google Scholar]
- Peñuelas J., Filella I. 2002. Metal pollution in Spanish terrestrial ecosystems during the twentieth century. Chemosphere 46: 501–505. [DOI] [PubMed] [Google Scholar]
- Petrova S., Yurukova L., Velcheva I. 2014. Possibilities of using deciduous tree species in trace element biomonitoring in an urban area (Plovdiv, Bulgaria). Atmospheric Pollution Research 5: 196–202. [Google Scholar]
- Primack D., Imbres C., Primack R. B., Miller-Rushing A. J., Del Tredici P. 2004. Herbarium specimens demonstrate earlier flowering times in response to warming in Boston. American Journal of Botany 91: 1260–1264. [DOI] [PubMed] [Google Scholar]
- Providence Redevelopment Authority. 2012. Letter to community members about ongoing cleanup at Jorge Alvarez High School site. 2. Providence Redevelopment Authority, Providence, Rhode Island, USA. [Google Scholar]
- Purewal V., Colston B., Röhrs S. 2008. Developing a simple screening method for the identification of historic biocide residues on herbarium material in museum collections. X-Ray Spectrometry 37: 137–141. [Google Scholar]
- Rascio N., Navari-Izzo F. 2011. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Science 180: 169–181. [DOI] [PubMed] [Google Scholar]
- Rhode Island Department of Environmental Management. 1998. Narragansett Bay water quality: Status and trends. Rhode Island Department of Environmental Management, Providence, Rhode Island, USA. [Google Scholar]
- Rodríguez Martín J. A., De Arana C., Ramos-Miras J. J., Gil C., Boluda R. 2015. Impact of 70 years urban growth associated with heavy metal pollution. Environmental Pollution 196: 156–163. [DOI] [PubMed] [Google Scholar]
- Rose A. 2008. Toxics in Rhode Island: A town by town profile. Toxics Action Center, Providence, Rhode Island, USA. Website http://www.toxicsaction.org/sites/default/files/tac/information/TAC-toxics-in-rhode-island.pdf [accessed 2 December 2016]. [Google Scholar]
- Ryan K. G., Burne A., Seppelt R. D. 2009. Historical ozone concentrations and flavonoid levels in herbarium specimens of the Antarctic moss Bryum argenteum. Global Change Biology 15: 1694–1702. [Google Scholar]
- Saxena D., Srivastava K., Singh S. 2008. Retrospective metal data of the last 100 years deduced by moss, Barbula sp. from Mussoorie city, Garhwal Hills, India. Current Science 94: 901–904. [Google Scholar]
- Shotbolt L., Büker P., Ashmore M. R. 2007. Reconstructing temporal trends in heavy metal deposition: Assessing the value of herbarium moss samples. Environmental Pollution 147: 120–130. [DOI] [PubMed] [Google Scholar]
- Smith W. H. 1972. Lead and mercury burden of urban woody plants. Science 176: 1237–1238. [DOI] [PubMed] [Google Scholar]
- Tomašević M., Rajšić S., Đorđević D., Tasić M., Krstić J., Novaković V. 2004. Heavy metals accumulation in tree leaves from urban areas. Environmental Chemistry Letters 2: 151–154. [Google Scholar]
- Weiss D., Shotyk W., Kramers J. D., Gloor M. 1999. Sphagnum mosses as archives of recent and past atmospheric lead deposition in Switzerland. Atmospheric Environment 33: 3751–3763. [Google Scholar]
- Whitfeld T. J. S., McCauley K. M., Edwards E. J. 2014. Calling attention to the new Brown University Herbarium. Rhodora 116: 232–233. [Google Scholar]
- Yoon J., Cao X., Zhou Q., Ma L. Q. 2006. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Science of the Total Environment 368: 456–464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.