Abstract
BACKGROUND
Adolescents and young adults with cancer have inferior survival outcomes compared with younger pediatric patients and older adult patients. Lack of insurance may partly explain this disparity. The objective of this study was to identify associations between insurance status and both advanced-stage cancer and cancer-specific mortality.
METHODS
Using the Surveillance, Epidemiology, and End Results (SEER) 18 registries, 57,981 patients ages 15 to 39 years were identified who were diagnosed between 2007 and 2010 and had complete insurance and staging information. Multinomial logistic regression models were used to identify associations between insurance type and disease stage, with the models adjusted for sex, age, and race. Cox proportional hazards models were used to estimate cancer-specific mortality.
RESULTS
Overall, 84% of patients were aged ≥25 years, 64% were women, and 79% were privately insured. Compared with patients who had private insurance, those who had nonprivate insurance tended to present with more advanced-stage disease and to die more quickly and more commonly from their cancer. Patients ages 25 to 39 years who had Medicaid coverage or no insurance had 3.2 times and 2.4 times higher odds of having stage IV disease, respectively, than privately insured patients (95% confidence interval [CI], 3.0–3.5 times higher odds and 2.1–2.6 times higher odds, respectively). Among those with stage I/II and III/IV cancers, the risk of death was 2.9 times greater (95% CI, 2.2–3.9 times greater) and 1.7 times greater (95% CI, 1.5–1.9 times greater), respectively, than the risk for privately insured patients. Patients who died from stage III/IV cancers survived at least 2 months longer if they had private insurance.
CONCLUSIONS
Among young adults, insurance status is independently associated with advanced-stage cancer and the risk of death from cancer, even for patients who have low-stage disease. Broader insurance coverage and access to health care may improve some of the disparate outcomes of adolescents and young adults with cancer.
Keywords: adolescents, young adults, pediatrics, AYA, cancer, health insurance, cancer disparities, Affordable Care Act, health care reform
INTRODUCTION
Adolescents and young adults (AYAs) (ages 15–39 years) with cancer have not experienced the same improvement in survival rates as younger pediatric patients and older adult patients,1,2 and cancer remains the leading cause of death from disease in this age group.3 Possible explanations for this disparity include limited access to medical care and health insurance, leading to delays in diagnosis and presentation with later stage (and less curable) disease.4 Indeed, AYAs represent the age group in the United States with the lowest percentage of health insurance coverage.5,6
The Patient Protection and Affordable Care Act (ACA) has the potential to improve insurance coverage for AYAs; young adults may remain on their parents’ insurance plan until age 26 years. Likewise, the program’s emphasis on coordinated models of care, including patient-centered medical homes and integrated behavioral health, may promote patient well being among AYAs, whose care is often fragmented both during and after cancer.7 During the first year of the ACA (2010–2011), the proportion of insured individuals ages 19 to 25 years increased by approximately 3%.8 However, the impact of the ACA among young adults in their later 20s and 30s has yet to be determined. During the same period, the percentage of uninsured adults ages 25 to 34 years has remained relatively unchanged.8,9 Furthermore, <33% of these young adults state that they are likely to enroll in available insurance plans, and 57% disapprove of the ACA, stating that insurance premiums remain cost-prohibitive.10
In 2007, the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute began collecting insurance information from the hospital records of all patients with incident cancer. Recent studies among AYAs with cancer have established a positive relation between lack of insurance and delayed diagnosis11 as well as metastatic disease.4,12 Furthermore, evidence suggests that insured young adults are more likely to receive definitive cancer treatment and are less likely to die from their disease.4 Our overall objectives were to validate these findings using the population-based SEER data available from the era before passage of the ACA. Specifically, we aimed to describe the associations between insurance status and incremental stage of disease at diagnosis and to determine whether insurance status was associated with a later risk of death from cancer among patients with lower stage (eg, more traditionally curable) disease. We hypothesized that nonprivate insurance (eg, Medicaid or lack of insurance) would be associated with higher stages at diagnosis and proportionately higher rates of death from cancer. These findings could underscore the implications of the ACA among young adults who no longer qualify for coverage under their parents’ plans.
MATERIALS AND METHODS
Patient Selection
We conducted a retrospective analysis of data from 18 population-based cancer registries that contribute to the SEER Program.13 Patients were included if they were diagnosed with invasive cancer from 2007 to 2010 and if they were ages 15 to 39 years at their diagnosis. Patients were grouped by common AYA malignancies: thyroid cancer, breast cancer, lymphoma (Hodgkin lymphoma and non-Hodgkin lymphoma), female genitourinary (GU) (including cervical) cancers, male GU (including testicular germ-cell) cancers, skin cancer, colon cancer, and bone/soft-tissue sarcoma (excluding Kaposi sarcoma).14 An additional category of “other” solid tumors captured the remaining patients for whom staging studies were applicable (eg, head and neck, upper gastrointestinal, lung, liver, kidney, and nonpelvic germ-cell tumors). Patients with leukemia and brain tumors were excluded from the analyses, because their diagnosis did not include American Joint Committee on Cancer (AJCC) staging assessments. We identified 70,044 potentially eligible records. Of these, 538 were excluded for in situ (stage 0) disease, 8110 were excluded because staging information was unavailable, and 3145 were excluded because insurance information was unavailable, leaving a total of 57,981 records for complete analyses.
Collected Variables
All variables were collected from available SEER data. Our 2 primary outcomes of interest were stage of disease at diagnosis and survival. The primary exposure of interest was insurance type, defined in SEER as the primary insurance carrier or method of payment at the time of initial diagnosis, which was coded as uninsured, any Medicaid (public insurance), insured (private insurance, including military coverage), insured (no specifics), or unknown. Because all patients were diagnosed before age 65 years, we assumed none were Medicare eligible. Additional collected variables were: age group (collected by SEER in 5-year intervals of ages 15–19 years, 20–24 years, 25–29 years, 30–34 years, and 35–39 years), sex, race (white, black, other), SEER registry site, and cause of death (cancer-related vs other). Treatment data, including radiation therapy and surgery at the primary cancer site, were collected and identified as highly inconsistent. In addition, SEER does not collect chemotherapy treatment data. For these reasons, treatment variables were not included in our analyses.
Statistical Analyses
Raw data were extracted from the SEER*Stat program and imported into the Stata 12 software system (Stata-Corp, College Station, Tex). Descriptive statistics were used for each variable. Multinomial logistic regression models with robust standard errors were used to detect associations between insurance status and incremental increases in disease stage at diagnosis. Cox proportional hazards models with robust standard errors were used to detect the hazard ratio of death from cancer based on insurance status; the proportional hazard assumption was tested on the basis of Schoenfeld residuals after fitting all Cox models. Kaplan-Meier survival methods were used to assess differences in cancer-specific mortality by insurance status. Events were defined as cancer-related deaths, and patients who did not experience a cancer-related death were censored at the time of their death from other causes or at their last known follow-up. Survival was defined as the time from initial diagnosis to the date of death or censoring, and the median survival was calculated for subsets of patients who died from cancer. We considered sex, race, and age as potential effect modifiers. On the basis of likelihood ratio testing, age <25 years modified the relation between insurance status and disease stage, and sex modified the relation between insurance status and the risk of death from cancer (Pinteraction < .05). Therefore, we stratified our analyses to describe patients who would benefit from an extension of parental insurance coverage under the ACA (ages 20–24 years) to those ages 25 to 39 years. With respect to confounding, all models were adjusted for age, sex, race, and insurance type, except as indicated (eg, analyses for patients with breast cancer were limited to women). In addition to analyzing data for all cancers combined, we conducted cancer-specific analyses for patients with breast cancer, cervical cancer, lymphoma, male GU cancers, and skin cancer, because these tumor types are 1) amenable to screening and/or early detection by routine medical care and 2) present at various stages of disease. (Thyroid cancers were not included in the cancer-specific analyses, because the vast majority of those patients present with stage I disease.) Finally, because our analyses suggested that patients who were “insured (private)” and “insured (no specifics)” were similar, we collapsed those 2 variables into a single category of “private insurance.” Also, because our analyses suggested that the hazard ratios were similar for Medicaid patients and uninsured patients, we further collapsed them into a single category of “nonprivate insurance,” and we used the collapsed variables for our survival analyses to compare the private insurance group with the nonprivate insurance group.
RESULTS
Of the 57,981 patients who were included in our analyses, slightly more than half (54%) had stage I disease; 20% had stage II disease, 14% had stage III disease, and 12% had stage IV disease (Table 1). Most patients (84%) were aged ≥25 years, 64% were women, and 80% were white. Seventynine percent of patients were privately insured at the time of diagnosis, 15% were covered by Medicaid, and only 7% were uninsured. The most common diagnoses were thyroid and breast cancers followed by lymphomas, female and male GU cancers, and skin cancers.
TABLE 1.
Patient Characteristics
| No. of Patients (%)a
|
|||||
|---|---|---|---|---|---|
| Characteristic | Stage I, N = 31,438 | Stage II, N = 11,479 | Stage III, N = 8270 | Stage IV, N = 6794 | Total, N = 57,981 |
| Age at diagnosis, y | |||||
| 15–19 | 1459 (5) | 767 (7) | 493 (6) | 495 (7) | 3216 (6) |
| 20–24 | 3277 (10) | 1111 (10) | 848 (10) | 713 (10) | 5949 (10) |
| 25–29 | 5898 (19) | 1682 (15) | 1274 (15) | 997 (15) | 9851 (17) |
| 30–34 | 8396 (27) | 2715 (24) | 2049 (25) | 1607 (24) | 14,767 (25) |
| 35–39 | 12,408 (39) | 5202 (45) | 3606 (44) | 2982 (44) | 24,198 (42) |
| Sex | |||||
| Female | 21,140 (67) | 7879 (69) | 4933 (60) | 3414 (50) | 37,366 (64) |
| Male | 10,298 (33) | 3600 (31) | 3337 (40) | 3380 (50) | 20,615 (36) |
| Race | |||||
| White | 25,969 (84) | 8823 (78) | 6325 (77) | 4883 (72) | 46,000 (80) |
| Black | 2130 (7) | 1483 (13) | 1104 (13) | 1156 (17) | 5873 (10) |
| Other | 2707 (9) | 1079 (9) | 794 (10) | 716 (11) | 5296 (9) |
| Missing | 632 | 94 | 47 | 39 | 812 |
| Insurance status | |||||
| Private | 26,411 (84) | 8939 (78) | 5796 (70) | 4403 (65) | 45,549 (79) |
| Medicaid | 3261 (10) | 1852 (16) | 1721 (21) | 1682 (25) | 8516 (15) |
| Uninsured | 1766 (6) | 688 (6) | 753 (9) | 709 (10) | 3916 (7) |
| Cancer-type | |||||
| Thyroid | 10.461 (33) | 117 (1) | 17 (<1) | 49 (1) | 10,644 (18) |
| Breastb | 2835 (9) | 4633 (40) | 2052 (25) | 679 (10) | 10,199 (18) |
| Lymphoma | 1985 (6) | 2930 (26) | 1328 (16) | 1996 (29) | 8239 (14) |
| Female genitourinary | 4115 (13) | 513 (4) | 1016 (12) | 435 (6) | 6079 (10) |
| Male genitourinary | 4079 (13) | 788 (7) | 937 (11) | 23 (<1) | 5827 (10) |
| Skin | 4419 (14) | 462 (4) | 547 (7) | 160 (2) | 5588 (10) |
| Colon | 532 (2) | 712 (6) | 1152 (14) | 876 (13) | 3272 (6) |
| Bone/soft-tissue sarcoma | 499 (2) | 452 (4) | 238 (3) | 515 (8) | 1704 (3) |
| Other | 2513 (8) | 872 (8) | 983 (12) | 2061 (30) | 6429 (11) |
Total percentages may not add up to 100% because of rounding errors.
These included 26 male patients with breast cancer.
In analyses of all cancer types stratified by age, both Medicaid coverage and a lack of insurance were associated independently with increasing disease stage at the time of diagnosis (Table 2). For example, among patients ages 25 to 39 years, those with Medicaid coverage had 3.2 times (95% confidence interval [CI], 3.0–3.5 times) higher odds and those with no insurance had 2.4 times (95% CI, 2.1–2.6 times) higher odds of presenting with stage IV disease compared with privately insured patients.
TABLE 2.
Odds Ratio of Higher Stage Cancer Compared With Stage I Given Nonprivate Insurance Coveragea
| Characteristic | Insurance Typeb
|
||||
|---|---|---|---|---|---|
| Private No. (%) | Medicaid
|
Uninsured
|
|||
| No. (%) | OR [95% CI] | No. (%) | OR [95% CI] | ||
| Ages 20–24 y | |||||
| Total no. Stage | 4419 | 953 | — | 577 | — |
| I | 2623 (59) | 381 (40) | 1.0 [—] | 273 (47) | 1.0 [—] |
| II | 815 (18) | 187 (20) | 1.5 [1.2–1.8] | 109 (19) | 1.3 [1.0–1.6] |
| III | 545 (12) | 195 (20) | 2.4 [1.9–2.9] | 108 (19) | 1.7 [1.3–2.2] |
| IV | 436 (10) | 190 (20) | 2.7 [2.2–3.3] | 87 (15) | 1.9 [1.3–2.3] |
| Ages 25–39 y | |||||
| Total no. Stage | 38,720 | 6871 | — | 3225 | — |
| I | 22,627 (58) | 2630 (38) | 1.0 [—] | 1445 (45) | 1.0 1.0 [—] |
| II | 7529 (19) | 1512 (22) | 1.7 [1.6–1.8] | 558 (17) | 1.2 [1.1–1.3] |
| III | 4919 (13) | 1388 (20) | 2.4 [2.3–2.6] | 622 (19) | 2.0 [1.8–2.2] |
| IV | 3645 (9) | 1341 (20) | 3.2 [3.0–3.5] | 600 (19) | 2.4 [2.1–2.6] |
Abbreviations: CI, confidence interval; OR, odds ratio.
All cancer types were included; estimates were adjusted for insurance type, race, patient age, and sex.
Total percentages may not add up to 100% because of rounding errors.
The trends were similar in analyses stratified by cancer type; insurance was independently associated with stage at diagnosis for patients with breast cancer, cervical cancer, lymphoma, male GU cancers, skin cancer, colon cancer, and other cancers (Table 3). Among women who were ages 25 to 39 years at the time of their diagnosis, those with Medicaid coverage had had 4.2 times (95% CI, 3.4–5.1 times) higher odds and those with no insurance had 3.1 times (95% CI, 2.1–4.7 times) higher odds of presenting with stage IV disease than those with private insurance. Men with GU cancers had 4.1 times (95% CI, 1.4–12.7 times) higher odds of presenting with stage IV disease if they had Medicaid coverage compared with private insurance.
TABLE 3.
Odds Ratio of Higher Stage Cancer Compared With Stage I, Given Nonprivate Insurance Coverage, by Cancer-Typea
| Cancer Type | Insurance Type
|
||||
|---|---|---|---|---|---|
| Private No. (%) | Medicaid
|
Uninsured
|
|||
| No. (%) | OR [95% CI] | No. (%) | OR [95% CI] | ||
| Breast cancerb | |||||
| Total No. Stage | 8086 | 1595 | — | 339 | — |
| I | 2460 (30) | 259 (16) | 1.0 [—] | 65 (19) | 1.0 [—] |
| II | 3682 (46) | 718 (45) | 1.8 [1.6–2.1] | 153 (45) | 1.6 [1.2–2.1] |
| III | 1513 (19) | 422 (26) | 2.6 [2.2–3.1] | 83 (25) | 2.1 [1.5–2.9] |
| IV | 431 (5) | 196 (13) | 4.2 [3.4–5.1] | 38 (11) | 3.1 [2.1–4.7] |
| Cervical cancerb | |||||
| Total no. Stage | 2957 | 1042 | — | 366 | — |
| I | 2293 (78) | 603 (58) | 1.0 [—] | 232 (63) | 1.0 [—] |
| II | 199 (7) | 138 (13) | 2.7 [2.1–3.4] | 34 (9) | 1.8 [1.3–2.4] |
| III | 346 (12) | 202 (19) | 2.2 [1.8–2.7] | 65 (18) | 1.8 [1.3–2.4] |
| IV | 119 (4) | 99 (10) | 3.2 [2.4–4.3] | 35 (10) | 3.0 [2.0–4.4] |
| Lymphoma | |||||
| Total no. Stage | 4197 | 832 | — | 524 | — |
| I | 1,168 (28) | 184 (22) | 1.0 [—] | 113 (22) | 1.0 [—] |
| II | 1,449 (36) | 240 (29) | 1.1 [0.9–1.3] | 131 (25) | 1.0 [0.7–1.2] |
| III | 625 (15) | 133 (16) | 1.3 [1.0–1.7] | 111 (21) | 1.8 [1.4–2.3] |
| IV | 955 (23) | 275 (33) | 1.8 [1.5–2.2] | 169 (33) | 1.8 [1.4–2.3] |
| Male GUc | |||||
| Total no. Stage | 3302 | 416 | — | 484 | — |
| I | 2465 (75) | 212 (51) | 1.0 [—] | 329 (68) | 1.0 [—] |
| II | 457 (14) | 70 (17) | 1.9 [1.4–2.5] | 60 (12) | 1.1 [0.8–1.4] |
| III | 365 (11) | 130 (31) | 4.1 [3.2–5.2] | 91 (18) | 1.8 [1.4–2.4] |
| IV | 15 (<1) | 4 (1) | 4.1 [1.4–12.7] | 4 (1) | 2.8 [0.9–8.7] |
| Skin cancer | |||||
| Total no. Stage | 4297 | 213 | — | 220 | — |
| I | 3521 (82) | 102 (48) | 1.0 [—] | 136 (62) | 1.0 [—] |
| II | 335 (8) | 37 (17) | 3.8 [2.6–5.7] | 24 (11) | 1.9 [1.2–3.0] |
| III | 351 (8) | 44 (21) | 4.5 [3.1–6.5] | 41 (19) | 2.9 [2.0–4.3] |
| IV | 90 (2) | 30 (14) | 12.2 [7.6–19.7] | 128 (3) | 5.3 [3.1–9.0] |
| Colon cancer | |||||
| Total no. Stage | 2264 | 514 | — | 278 | — |
| I | 413 (18) | 51 (10) | 1.0 [—] | 35 (13) | 1.0 [—] |
| II | 519 (23) | 96 (19) | 1.5 [1.0–2.2] | 67 (24) | 1.4 [1.9–2.2] |
| III | 766 (34) | 203 (39) | 2.1 (1.5–2.2] | 98 (35) | 1.4 [0.9–2.2] |
| IV | 566 (25) | 164 (32) | 2.3 [1.6–3.2] | 78 (28) | 1.6 [1.0–2.4] |
| Other cancer | |||||
| Total no. Stage | 4149 | 1088 | — | 518 | — |
| I | 1797 (43) | 328 (30) | 1.0 [—] | 147 (28) | 1.0 [—] |
| II | 575 (14) | 138 (13) | 1.3 [1.1–1.6] | 62 (12) | 1.4 [1.0–1.8] |
| III | 614 (15) | 154 (14) | 1.4 [1.1–1.7] | 89 (17) | 1.8 [1.4–2.4] |
| IV | 1163 (28) | 468 (43) | 2.2 [1.9–2.6)] | 220 (43) | 2.3 [1.8–2.9] |
Abbreviations: CI, confidence interval; GU, genitourinary; OR, odds ratio.
All models were limited to patients ages 25 to 39 years and were adjusted for insurance type, race, patient age, and sex. Total percentages may not add up to 100% because of rounding errors.
Only female patients were included.
Only male patients were included.
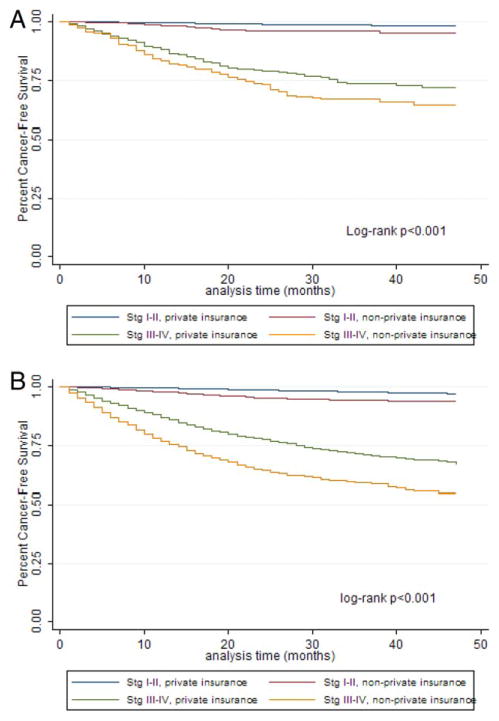
Insurance also was associated with relapse-free survival (Fig. 1). Patients who died from their cancers tended to have a shorter median survival if they had nonprivate insurance compared with patients who had private insurance (Table 4). For example, among women ages 25 to 39 with stage I/II and III/IV disease who died, the median survival was 18 months and 12 months, respectively, for those with private insurance compared with 14 months and 9 months, respectively, for those with nonprivate insurance. For men of the same age with stage I/II and III/IV disease, the median survival was 14 months and 9 months, respectively, among privately insured patients compared with 9 months and 7 months, respectively, among nonprivately insured patients. These trends were present in several subgroups.
Figure 1.
Kaplan-Meier curves illustrate survival according to insurance type for (A) patients ages 20 to 24 years and (B) patients ages 25 to 39 years. Stg indicates stage.
TABLE 4.
Median Survival Among Patients Who Died From Cancer and Hazard Ratio of Death From Cancer, Given Nonprivate Insurance or Nonwhite Racea
| Patient Group | Stage | Median Survival (95% CI), mob
|
HR of Death From Cancer With Nonprivate Insurance vs Private Insurance (95% CI)b | |
|---|---|---|---|---|
| Private Insurance | Nonprivate Insurance | |||
| Women and men ages 20–24 y, all cancers | I–II | 23 (20–25) | 18 (15–22) | 2.6 (1.7–3.9)c |
| III–IV | 18 (17–21) | 12 (10–15) | 1.2 (1.0–1.4)c | |
| Women ages 25–39 y, all cancers | I–II | 18 (17–22) | 14 (11–15) | 2.9 (2.3–3.5) |
| III–IV | 12 (11–13) | 9 (9–10) | 1.7 (1.5–1.9) | |
| Men ages 25–39 y, all cancers | I–II | 14 (11–16) | 9 (6–12) | 2.9 (2.2–3.9) |
| III–IV | 9 (9–10) | 7 (6–8) | 1.7 (1.5–1.8) | |
| Women ages 25–39 y, breast cancer | I–II | 23 (20–25) | 18 (15–22) | 1.9 (1.3–2.9) |
| III–IV | 18 (17–21) | 12 (10–15) | 2.0 (1.6–2.6) | |
| Women ages 25–39 y, cervical cancer | I–II | 17 (15–22) | 15 (12–17) | 2.7 (1.8–4.1) |
| III–IV | 13 (9–14) | 10 (9–12) | 1.2 (1.0–1.6) | |
| Women and men ages 25–39 y, lymphoma | I–II | 11 (9–15) | 7 (5–9) | 3.4 (2.3–4.9)c |
| III–IV | 8 (7–9) | 6 (5–7) | 2.6 (2.1–3.4)c | |
| Men ages 25–39 y, GU cancers | I–II | 13 (3–23) | 15 (2–23) | 3.8 (1.7–8.8) |
| III–IV | 10 (7–13) | 10 (5–15) | 1.1 (0.7–1.9) | |
| Women and men ages 25–39 y, skin cancer | I–II | 31 (16–36) | 20 (16–35) | 2.2 (0.7–7.4)c |
| III–IV | 11 (8–13) | 6 (5–11) | 2.1 (1.4–3.2)c | |
| Women and men ages 25–39 y, colon cancer | I–II | 17 (9–25) | 15 (8–22) | 2.7 (1.4–5.5)c |
| III–IV | 14 (13–15) | 11 (9–13) | 1.7 (1.4–2.0)c | |
| Women and men ages 25–39 y, other cancers | I–II | 13 (10–16) | 8 (6–12) | 1.9 (1.3–2.7)c,d |
| III–IV | 8 (7–8) | 6 (5–6) | 1.4 (1.3–1.6)c,d | |
Abbreviations: CI, confidence interval; GU, genitourinary; HR, hazard ratio.
All models were stratified by disease stage and were adjusted for age, insurance type, and race.
Values in boldface indicate a statistically significant difference.
This model also was adjusted for sex.
This model included upper gastrointestinal, liver, kidney, lung, and other noncentral nervous system solid tumors.
The hazard ratio of death from cancer was consistently elevated for those who had nonprivate insurance compared with those who had private insurance (Table 4), particularly for some patients who had stage I or II disease. Among women ages 25 to 39 years with cervical cancer, for example, the hazard ratio for death from cancer was 2.7 (95% CI, 1.8–4.1) for those with stage I/II disease and 1.2 (95% CI, 1.0–1.6) for those with stage III/IV disease.
DISCUSSION
By using the population-based SEER 18 registry, we observed that public insurance or no insurance was independently associated with progressively higher odds of advanced-stage disease for nearly all cancer types. Furthermore, nonprivate insurance was associated with a higher risk of death from cancer, and the risk was highest among patients who had lower stage (and presumably more curable) disease.
Passage of the ACA in 2010 has brought increasing attention to the role of insurance in health outcomes among AYAs with cancer.4,6,11,12,15–19 Much of the current literature has focused on the expansion of parental coverage to offspring aged 26 years and speculation that this change (with its corresponding improved access to health care) will improve lag times in diagnosis and, in turn, will improve outcomes. Indeed, in a large sample of AYAs, Robbins and colleagues observed a definitive association between lack of insurance and distant-stage disease, particularly in cancers that were amenable to early detection with routine health care.12 Similarly, Aizer and colleagues demonstrated that uninsured young adults more commonly presented with metastatic disease, less commonly received definitive cancer treatment, and more commonly died from all causes.4 Investigators in the AYA Health Outcomes and Patient Experience (HOPE) study observed that lack of insurance was associated with inferior quality of life among newly diagnosed patients,19 and that patients ages 25 to 39 years were more likely to experience a period without insurance during the immediate survivorship period compared with those ages 15 to 19 years.17,18
Our data add evidence that insurance status is associated with cancer-specific mortality, even in patients with nonmetastatic disease. This finding underscores the ongoing medical needs during and after the cancer experience; individuals without insurance may lack appropriate treatment and/or surveillance. In addition, younger age among adults diagnosed with cancer is associated with greater financial distress and inability to afford cancer care, which translates into forgone medical care.20 Patients with lower disease stages may be disproportionately affected, perhaps because of perceptions that their risks of complications or recurrence are lower. Likewise, ongoing contact with health care providers may encourage better adherence to treatment and follow-up guidelines, factors that have been associated previously with poor outcomes among AYAs.21
There are several notable limitations to our current analyses. First, compared with the nonpopulation-based study by Robbins et al,12 we had considerably fewer patients, which may have underpowered our stratified analyses. Second, although the SEER Program provides an excellent resource for population-level data regarding incident cancers in the United States, the variables are crude. We were unable to assess the roles of finer sociodemographic characteristics (eg, income, education, access to medical care), and we opted not to include treatment variables in our analyses, because these often were inconsistent and/or incomplete. This lack of treatment data diminishes the strength of our findings, because we could not determine the degree to which the disparities in survival were caused by differences in treatment (which also may be related to patients’ insurance coverage). Third, our cross-sectional analysis could not detect causality; thus, we cannot conclude that lack of insurance necessarily leads to later diagnosis. Fourth, because we limited our analyses to those cancers for which AJCC stage defines risk, we did not include leukemia or brain tumors, both of which are highly prevalent in AYA populations. How insurance status relates to the presentation of these cancer types is unclear.
Finally, the insurance variable within SEER is relatively new (data collection began in 2007); therefore, it is subject to misclassification. For example, we observed that 93% of AYAs were insured, either publicly or privately. This percentage is higher than that reported in other population-based data, which suggested that 72% to 78% of AYAs were insured9 and may reflect retroactive enrollment in Medicaid at the time of cancer diagnosis.22,23 Retroactive Medicaid enrollment may be more frequent among patients with more serious (or advanced-stage) cancers, thereby creating implicit bias and falsely elevating the associations described in this analysis. Indeed, we observed higher odds of stage IV disease among Medicaid patients than among uninsured patients in our sample. Rather than conclude that private insurance is more “protective” than Medicaid, it may be more appropriate to assume that any lack of insurance at the time of diagnosis is associated with increased risk and that patients who are coded as “Medicaid-insured” were truly uninsured before their diagnoses. For these reasons, we and others12 have pooled Medicaid and uninsured patients together for statistical analyses. In contrast, Aizer and colleagues compared privately insured and Medicaid-insured patients with all uninsured patients and reported results similar to ours. The correct classification of insurance data in SEER remains unclear; future, prospective cohort studies may better elucidate specific risks with insurance type.
Our findings validate reports that insurance status among AYAs is a strong independent risk factor both for advanced disease at the time of their cancer diagnosis4,11,12,24 and for cancer mortality.4 These findings are particularly true for cancers that are amenable to early detection with screening and/or physical examinations (ie, cervical cancer, lymphoma, breast cancer, and skin cancer). Unfortunately, adolescence and young adulthood represent the ages at which individuals are least likely to have a “usual place to go” for routine medical care.25 The finding that patients in our sample with lower stage disease had proportionately higher risk of cancer mortality underscores the importance of routine medical care. Lower stage disease is often highly curable, and patients with lower stage disease could avoid cancer-related deaths.
This finding has implications for the broader application of the ACA in the United States. Although we have made strides to improve coverage for individuals up to age 26 years by extending access to parental plans, individuals in their late 20s and 30s remain the least likely to be insured and often have the highest premiums.16 Therefore, outreach programs must endeavor to enroll older young adults. Health care providers, including physicians, nurses, social workers, and patient navigators, need to be trained in the enrollment and benefits of the ACA, as well as individual state health care exchanges where they exist, to successfully advise AYAs with cancer. Because the ACA prohibits the exclusion of pre-existing conditions, AYA survivors may also enroll independently, thereby promoting long-term surveillance and treatment of late effects.
Health insurance status in young adults may explain some of their disparate outcomes. Limited or no insurance is strongly associated with advanced-stage disease and death. Public health initiatives that endeavor to broaden and improve insurance coverage for young adults have the potential to minimize disease burden and optimize the societal contribution of AYAs in the United States.
Acknowledgments
FUNDING SUPPORT
No specific funding was disclosed.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Bleyer A. The adolescent and young adult gap in cancer care and outcome. Curr Probl Pediatr Adolesc Health Care. 2005;35:182–217. doi: 10.1016/j.cppeds.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Bleyer A, O’Leary M, Barr R, Ries LAG, editors. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975–2000. Bethesda, MD: National Cancer Institute; 2006. NIH Pub. No. 06–5767. [Google Scholar]
- 3.Heron M. Deaths: leading causes for 2010. Natl Vital Stat Rep. 2013;62:1–96. [PubMed] [Google Scholar]
- 4.Aizer AA, Falit B, Mendu ML, et al. Cancer-specific outcomes among young adults without health insurance. J Clin Oncol. 2014;32:2025–2030. doi: 10.1200/JCO.2013.54.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeNavas-Walt C, Proctor BD, Smith JC. Income, Poverty, and Health Insurance Coverage in the United States: 2011. Washington, DC: US Census Bureau; 2012. [Google Scholar]
- 6.Bleyer WA. Potential favorable impact of the Affordable Care Act of 2010 on cancer in young adults in the United States. Cancer J. 2010;16:563–571. doi: 10.1097/PPO.0b013e3181ff6509. [DOI] [PubMed] [Google Scholar]
- 7.Andrews CM, Darnell JS, McBride TD, Gehlert S. Social work and implementation of the Affordable Care Act. Health Soc Work. 2013;38:67–71. doi: 10.1093/hsw/hlt002. [DOI] [PubMed] [Google Scholar]
- 8.Mendes E. Uninsured Rate for US Young Adults Still Down From Past. Washington, DC: Gallop Inc; Jul, 2013. [Accessed March 9, 2014]. Available at: http://www.gallup.com/poll/163604/uninsured-rate-young-adults-down-past.aspx. [Google Scholar]
- 9.Cohen RA, Martinez ME. Health Insurance Coverage: Early Release of Estimates from the National Health Interview Survey, January–March 2011. Hyattsville, MD: CDC/National Center for Health Statistics; Sep, 2011. [Accessed March 9, 2014]. Available at: www.cdc.gov/nchs/data/nhis/earlyrelease/insur201206.pdf. [Google Scholar]
- 10.Columbia Broadcasting Company (CBS) Poll: 41 Percent of Uninsured Young Adults Uncertain About Enrolling in Obamacare. [Accessed 03/09/2014];Cost of Health Plans Weighing Heavily on Decisions of Young Adults. 2013 Dec; Available at: http://newyork.cbslocal.com/2013/12/09/poll-41-percent-of-uninsured-young-adults-uncertain-about-enrolling-in-obamacare/
- 11.Martin S, Ulrich C, Munsell M, Taylor S, Lange G, Bleyer A. Delays in cancer diagnosis in underinsured young adults and older adolescents. Oncologist. 2007;12:816–824. doi: 10.1634/theoncologist.12-7-816. [DOI] [PubMed] [Google Scholar]
- 12.Robbins AS, Lerro CC, Barr RD. Insurance status and distant-stage disease at diagnosis among adolescent and young adult patients with cancer aged 15 to 39 years: National Cancer Data Base, 2004 through 2010. Cancer. 2014;120:1212–1219. doi: 10.1002/cncr.28568. [DOI] [PubMed] [Google Scholar]
- 13.National Cancer Institute. [Accessed February 28, 2014];Surveillance, Epidemiology and End Results (SEER) Program. Available at. www.seer.cancer.gov.
- 14.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, LIVESTRONG Young Adult Alliance. [Accessed October 3, 2012];Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults With Cancer: Report for the Adolescent and Young Adult Oncology Progress Review Group. Available at: http://planning.cancer.gov/library/AYAO_PRG_Report_2006_FINAL.
- 15.Bleyer A, Ulrich C, Martin S. Young adults, cancer, health insurance, socioeconomic status, and the Patient Protection and Affordable Care Act. Cancer. 2012;118:6018–6021. doi: 10.1002/cncr.27685. [DOI] [PubMed] [Google Scholar]
- 16.Wilensky GR. Policy decisions have consequences: sometimes unintended ones [comment] J Clin Oncol. 2014;32:1994–1995. doi: 10.1200/JCO.2014.55.6712. [DOI] [PubMed] [Google Scholar]
- 17.Keegan TH, Tao L, DeRouen MC, et al. Medical care in adolescents and young adult cancer survivors: what are the biggest access-related barriers? J Cancer Surviv. 2014;8:282–292. doi: 10.1007/s11764-013-0332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsons HM, Schmidt S, Harlan LC, et al. Young and uninsured: insurance patterns of recently diagnosed adolescent and young adult cancer survivors in the AYA HOPE study. Cancer. 2014;120:2352–2360. doi: 10.1002/cncr.28685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith AW, Bellizzi KM, Keegan TH, et al. Health-related quality of life of adolescent and young adult patients with cancer in the United States: the Adolescent and Young Adult Health Outcomes and Patient Experience study. J Clin Oncol. 2013;31:2136–2145. doi: 10.1200/JCO.2012.47.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119:3710–3717. doi: 10.1002/cncr.28262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg AR, Macpherson CF, Kroon L, Johnson R. Rethinking adherence: a proposal for a new approach to risk assessment. J Adolesc Young Adult Oncol. 2013;2:83–86. doi: 10.1089/jayao.2012.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradley CJ, Gardiner J, Given CW, Roberts C. Cancer, Medicaid enrollment, and survival disparities. Cancer. 2005;103:1712–1718. doi: 10.1002/cncr.20954. [DOI] [PubMed] [Google Scholar]
- 23.Stuber J, Bradley E. Barriers to Medicaid enrollment: who is at risk? Am J Public Health. 2005;95:292–298. doi: 10.2105/AJPH.2002.006254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith EC, Ziogas A, Anton-Culver H. Association between insurance and socioeconomic status and risk of advanced stage Hodgkin lymphoma in adolescents and young adults. Cancer. 2012;118:6179–6187. doi: 10.1002/cncr.27684. [DOI] [PubMed] [Google Scholar]
- 25.National Center for Health Statistics, Centers for Disease Control and Prevention. Early Release of Selected Estimates Based on Data From the 2012 National Health Interview Survey. Hyattsville, MD: CDC/National Center for Health Statistics; Jun, 2013. [Accessed March 7, 2014]. Available at: http://www.cdc.gov/nchs/nhis/released201306.htm. [Google Scholar]