Abstract
Ongoing aerobic metabolism in nongrowing cells may generate oxidative stress. It is shown here that the levels of thiobarbituric acid-reactive substances (TBARSs), which measure fragmentation products of oxidized molecules, increased strongly at the onset of starvation for phosphate (Pi). This increase in TBARS levels required the activity of the histone-like nucleoid-structuring (H-NS) protein. TBARS levels weakly increased further in ΔahpCF mutants deficient in alkyl hydroperoxide reductase (AHP) activity during prolonged metabolism of glucose to acetate. Inactivation of pyruvate oxidase (PoxB) activity decreased the production of acetate by half and significantly increased the production of TBARS. Overall, these data suggest that during incubation under aerobic, Pi starvation conditions, metabolic flux is diverted from the pyruvate dehydrogenase (PDH) complex (NAD dependent) to PoxB (NAD independent). This shift may decrease the production of NADH and in turn the adventitious production of H2O2 by NADH dehydrogenase in the respiratory chain. The residual low levels of H2O2 produced during prolonged incubation can be scavenged efficiently by AHP. However, high levels of H2O2 may be reached transiently at the onset of stationary phase, primarily because H-NS may delay the metabolic shift from PDH to PoxB.
Escherichia coli possesses numerous mechanisms aimed at protecting and repairing cell constituents exposed to toxic agents. Defense mechanisms in growing cells are generally transient responses that allow rapid adaptation to stress. However, in nature, bacteria may alternate between growing and nongrowing states because of nutritional starvation. Survival of starved cells poses a specific problem because of the low capacity of stationary-phase cells to synthesize proteins. Starved cells can at least in part resolve this problem by accumulating RpoS (σs) at the entry into stationary phase. The induction of the numerous genes of the RpoS regulon helps to protect nongrowing cells against various stresses (14, 16).
Moreover, it has been suggested that metabolic fluxes may be redistributed in starved cells, which may decrease the endogenous production of reactive oxygen species (ROS). For instance, cells incubated under aerobic, glucose starvation conditions shift from aerobic to fermentative metabolism in an ArcA-dependent manner (e.g., pyruvate dehydrogenase synthesis is decreased, whereas pyruvate formate-lyase synthesis is increased) (24). Such a shift may decrease the production of NADH and the respiratory chain-mediated generation of ROS (24, 28). In the aerobic respiratory chain, NADH dehydrogenase II produces superoxide and hydrogen peroxide (H2O2) by autooxidation of its reduced flavin adenine dinucleotide cofactor (20). H2O2 is primarily detoxified by the alkyl hydroperoxide reductase (AHP) complex (AhpCF), which efficiently scavenges low levels of H2O2 but is inefficient against high levels of H2O2 (27).
In contrast to cells incubated under aerobic, glucose starvation conditions which shift from aerobic to anaerobic metabolism, cells incubated under aerobic, Pi starvation conditions may continue to metabolize glucose through aerobic metabolism, which may generate ROS (11). The cellular levels of ROS, which are not diluted by growth any more, may hence steadily increase in Pi-starved cells. This idea is supported by the finding that the colony-forming ability of ahp mutants decreases after 2 days of incubation under aerobic, Pi starvation conditions (22). However, these data might indicate that Pi-starved cells are primarily exposed to ROS at the exit of rather than during stationary phase (5). It has been shown that ahp mutants accumulate DNA damage and fragmentation products of oxidized molecules, measured as thiobarbituric acid-reactive substances (TBARSs), when they exit stationary phase and enter the lag phase in fresh Luria broth (LB) medium (12). Therefore, TBARS measurements were used in this study to determine whether oxidative stress occurs in cells when they are incubated under aerobic, Pi starvation conditions.
MATERIALS AND METHODS
Bacterial strains.
The Escherichia coli K-12 strains are described in Table 1. Mutations were introduced into strains by P1 transduction (21). For strains carrying the leuB and argE3(Oc) mutations, cultures were routinely checked for the absence of Leu+ contaminants and Arg+ suppressors, which can grow in old cultures by consuming nutrients excreted into the medium (11).
TABLE 1.
Bacterial strains
| Strain | Genotype | Source or reference |
|---|---|---|
| ENZ1203 | F−thr-1(Am) leuB6 Δ(argF-lac)U169 glnV44 sulA211 hisG4(Oc) rpsL31 argE3(Oc) thi-1 rpoS+ | 22 |
| ENZ1257 | ENZ1203 sulA::lacZ | 22 |
| ENZ1399 | ENZ1257 ΔahpCF::kan | 22 |
| ENZ1643 | ENZ1257 poxB176::lacZ Cmr | P1(YYC912) × ENZ1257 |
| ENZ1645 | ENZ1203 poxB176::lacZ Cmr | P1(YYC912) × ENZ1203 |
| ENZ1646 | ENZ1203 ΔahpCF::kan | P1(ENZ1399) × ENZ1203 |
| ENZ1667 | ENZ1203 hns::neo | P1(MC4100hns) × ENZ1203 |
| MC4100hns | hns::neo | 30 |
| YYC912 | poxB176::lacZ Cmr | 7 |
Media and culture conditions.
The minimal medium used for liquid cultures was essentially the morpholinepropanesulfonic acid (MOPS) medium described by Neidhardt et al. (23), which notably contained 40 mM MOPS, 86 mM NaCl, 9.5 mM NH4Cl, 5 mM K2HPO4 and 20 mM glucose, supplemented with five vitamins (0.02 mM thiamine, 0.02 mM calcium pantothenate, 0.02 mM p-aminobenzoic acid, 0.02 mM p-hydroxybenzoic acid, and 0.02 mM 2,3-dihydroxybenzoic acid) and six amino acids (0.8 mM leucine, 0.4 mM threonine, 0.2 mM histidine, 0.4 mM arginine, 0.4 mM isoleucine, and 0.6 mM valine) (pH 7.2). The Pi- and glucose-limiting media contained 0.1 mM K2HPO4 plus 9.8 mM KCl and 3 mM glucose, respectively (11). Strains were grown in MOPS medium for 24 h, diluted 1:200 into MOPS medium at time zero, and incubated further with aeration in a water bath rotary shaker (Aquatron Infors HT, 150 rpm) at 37°C, unless otherwise indicated. Culture optical density at 600 nm (OD600) was measured with a Jasco V-530 spectrophotometer in cells of 1-cm path length. The pH of the media was determined at ≈25°C.
Levels of glucose and by-products.
The concentrations of glucose (glucose HK assay; Sigma), pyruvate (Roche), and glutamate, formate, acetate, and d-and l-lactate (Boehringer Mannheim/R-Biopharm) were assayed with enzymatic tests according to the instructions of the manufacturers.
Determination of TBARS concentration.
Cells were centrifuged at 12,000 × g for 15 min, suspended in phosphate buffer (pH 7.0) containing 0.1 mM butylated hydroxytoluene (Sigma) as an antioxidant, and disrupted by one passage through a French pressure cell at 108 Pa. The extract was clarified by centrifugation at 27,000 × g for 20 min, and the supernatant was centrifuged for 90 min at 230,000 × g (k = 96, rotor type 70.1Ti; Beckman) at 4°C. The pellet fraction, which contained cytoplasmic membrane vesicles, was suspended in phosphate buffer containing 0.1 mM butylated hydroxytoluene, precipitated with 10% (wt/vol) trichloroacetic acid, incubated with 4.6 mM thiobarbituric acid (Sigma) in 50% (vol/vol) glacial acetic acid plus 0.1 mM butylated hydroxytoluene for 1 h at 95°C, mixed with 1 ml of butanol, and centrifuged at 11,000 × g for 3 min. The absorbance of the butanol phase was determined at 532 nm. The concentration of TBARS (ɛ532 = 156 mM−1 cm−1) was expressed in nanomoles per milligram of protein (6, 13). Protein content was determined by the Bradford dye-binding assay (Bio-Rad) with bovine serum albumin as the standard.
RESULTS AND DISCUSSION
TBARS levels increase significantly in cells incubated under Pi starvation conditions.
Cells incubated for 4 days under glucose and Pi starvation conditions exhibited TBARS levels 2.5- and 8-fold higher than those of exponentially growing cells, respectively (Table 2, Fig. 1). These data suggest that ROS levels may eventually increase more strongly in Pi- than in glucose-starved cells.
TABLE 2.
TBARS levels increase in cells incubated for 4 days under aerobic, Pi starvation conditionsa
| Medium | Growth phase | OD600 | Mean TBARS concn, nmol/mg, ± SD (fold increase) |
|---|---|---|---|
| MOPS | Exponential | 0.7 | 0.06 ± 0.01 |
| Glucose limiting | Stationary | 0.7 | 0.15 ± 0.02 (2.5) |
| Pi limiting | Stationary | 0.8 | 0.48 ± 0.04 (8) |
Strain ENZ1257 was inoculated into 100 ml of MOPS (5 mM Pi plus 20 mM glucose), glucose-limiting (5 mM Pi plus 3 mM glucose), or Pi-limiting (0.1 mM Pi plus 20 mM glucose) medium at time zero and incubated further with aeration in 500-ml Erlenmeyer flasks. When cells were either in exponential growth phase or in stationary phase, i.e., on day 4 of incubation under glucose or Pi starvation conditions (starved cells entered stationary phase after ≈10 h of incubation), the OD600 of the cultures and TBARS concentrations were determined. TBARS values are the means of two experiments. The values relative to those in exponentially growing cells are indicated in parentheses.
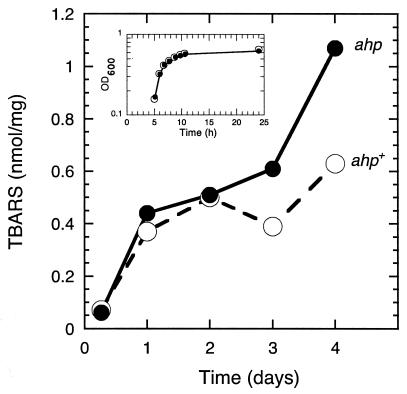
FIG. 1.
TBARS levels as a function of time of incubation under aerobic, Pi starvation conditions in ahp+ and ahp mutant cells. Strains ENZ1257 (ahp+) (○) and ENZ1399 (ENZ1257 ΔahpCF) (•) were inoculated into 100 ml of Pi-limiting medium (0.1 mM Pi plus 20 mM glucose) (time zero) and incubated under aerobic conditions in 500-ml Erlenmeyer flasks. TBARS concentrations were determined between days 1 and 4 of incubation (OD600 ≈ 0.7). The values for exponentially growing cells were obtained from cells grown for 6.5 h (OD600 ≈ 0.7) in MOPS medium (5 mM Pi plus 20 mM glucose). The data are from a representative experiment from three separate trials. Variations between experiments were ≤20%. The inset shows the growth curves of ahp+ and ahp mutant cells in Pi-limiting medium.
TBARS levels depend on the amount of glucose metabolized in ahp cells.
Inactivation of the ahpCF genes increased by ≈1.9-fold the levels of TBARS in cells incubated for 4 days in Pi-limiting medium initially containing 20 mM glucose (from 0.48 nmol/mg in ahp+ cells to 0.90 nmol/mg in ahp mutants; Tables 2 and 3). These data suggest that one role of AHP is to protect cell constituents against ROS produced during Pi starvation.
TABLE 3.
TBARS levels increase as a function of the amount of glucose consumed in ahp mutants starved of Pi for 4 daysa
| Initial glucose concn (mM) | No. of expt | OD600 | Glucose (mM) | Mean TBARS concn (nmol/mg) ± SD |
|---|---|---|---|---|
| 5 | 2 | 0.6 | ≤0.4 | 0.52 ± 0.04 |
| 10 | 2 | 0.7 | ≤0.4 | 0.66 ± 0.03 |
| 20 | 4 | 0.7 | ≤0.4 | 0.90 ± 0.07 |
| 30 | 4 | 0.7 | 4.2 ± 0.1 | 1.09 ± 0.10 |
| 40 | 2 | 0.7 | 14.3 ± 0.1 | 0.93 ± 0.10 |
Strain ENZ1399 (ENZ1257 ΔahpCF) was inoculated into 100 ml of Pi-limiting medium (0.1 mM Pi) containing increasing concentrations of glucose, and incubated further under aerobic conditions in 500-ml Erlenmeyer flasks. On day 4 of incubation, the OD600 of the cultures, glucose concentrations in the spent medium, and TBARS levels were determined.
TBARS levels measured on day 4 of incubation in ahp mutants increased from ≈0.5 to ≈1.0 nmol/mg as a function of the concentration of glucose added in the range from 5 to 30 mM (Table 3). TBARS levels remained steady at ≈0.5 nmol/mg in ahp+ cells incubated under the same conditions (data not shown). Therefore, AHP may help scavenge ROS produced during prolonged incubation in Pi-limiting medium in the presence of excess glucose (≈3 mM glucose was consumed at the entry into stationary phase by ≈10 h of incubation; data not shown).
However, when ahp mutants were incubated for 4 days in Pi-limiting medium initially containing 30 or 40 mM glucose, similar levels of TBARS were produced and similar amounts of glucose were consumed (≈26 mM) (Table 3). Therefore, the ROS generated during incubation in Pi-limiting medium may primarily be produced endogenously through glucose metabolism rather than by autooxidation of glucose present in the medium (27).
Overall, these data suggest that metabolism of glucose in Pi-starved cells may generate ROS. However, consumption of glucose had little effect on biomass formation (OD600) (Table 3), which suggests that metabolism may primarily be achieved by overflow metabolism, i.e., by excretion of by-products.
AHP prevents a late increase in TBARS levels.
TBARS levels were measured in ahp+ and ahp mutant strains as a function of the time of incubation in Pi-limiting medium containing 20 mM glucose (Fig. 1). TBARS levels increased in roughly two steps. First, a strong increase (≈8-fold) occurred during the transition from exponential growth to stationary phase (day 1 of incubation) in both ahp+ and ahp mutant cells. Second, a weaker increase (≈2-fold) preferentially occurred between day 1 and day 4 of incubation in ahp mutants.
The differential roles of AHP suggest that the levels of H2O2 reached at the onset of Pi starvation may be too high to be scavenged efficiently by AHP (27). However, between days 1 and 4 of incubation, when the rate of production of H2O2 may be reduced, AHP can scavenge the low levels of H2O2 accumulated in Pi-starved cells as a result of glucose metabolism.
TBARS levels increase strongly in poxB mutants.
In Pi-starved cells, glucose was primarily metabolized to acetate, which was excreted into the incubation medium. On day 5 of incubation in Pi-limiting medium initially containing 40 mM glucose, acetate levels reached 29.4 mM and the pH of the medium decreased from ≈7.2 to ≈4.8 (Table 4); the concentrations of pyruvate, lactate, aspartate, glutamate and formate in the incubation medium were lower than 0.4 mM (Table 4 and data not shown).
TABLE 4.
Metabolic pattern in cells starved of Pi for 5 daysa
| Strain | OD600 | Final pH | Mean concn (mM) ± SD
|
||
|---|---|---|---|---|---|
| Glc | Ace | Pyr | |||
| ENZ1257 | 0.7 | 4.8 | 11.6 ± 0.2 | 29.4 ± 1.8 | 0.31 ± 0.01 |
| ahp | 0.7 | 5.1 | 13.2 ± 0.6 | 24.0 ± 0.1 | 0.08 ± 0.01 |
| poxB | 0.8 | 4.7 | 14.3 ± 0.6 | 13.0 ± 0.1 | 3.9 ± 0.1 |
Strains ENZ1257, ENZ1399 (ENZ1257 ΔahpCF), and ENZ1643 (ENZ1257 poxB) were inoculated into 50 ml of Pi-limiting medium (0.1 mM Pi plus 40 mM glucose) and incubated further under aerobic conditions in 500-ml Erlenmeyer flasks. On day 5 of incubation, the OD600 and pH of the cultures and the concentrations in the culture supernatants (adjusted to pH 7) of glucose (Glc), acetate (Ace), and pyruvate (Pyr) were determined. Data are the means of two experiments.
The low concentration of formate in the spent medium indicates that pyruvate formate-lyase played no significant role in the metabolism of pyruvate in Pi-starved cells. Therefore, the metabolism of pyruvate should occur through aerobic enzymes: the pyruvate dehydrogenase (PDH) complex (AceEF Lpd) or pyruvate oxidase (PoxB) or both (10).
The PDH complex is primarily used during aerobic growth to catalyze the oxidative decarboxylation of pyruvate to acetyl coenzyme A (acetyl-CoA) with the concomitant reduction of NAD. Since the rate of synthesis of the AceF subunit of the PDH complex decreases by approximately twofold at the onset of Pi starvation (29), a decrease in PDH activity might account at least in part for the apparent decrease in the production of H2O2 during prolonged incubation under Pi starvation conditions.
PoxB can catalyze the oxidative decarboxylation of pyruvate directly to acetate with the concomitant reduction of flavin adenine dinucleotide and ubiquinone, bypassing NADH oxidation by the respiratory chain (10). It has been suggested that PoxB, which is strictly regulated by RpoS, could play a beneficial role at very low growth rates and at the entry into stationary phase under microaerobic conditions, when neither PDH nor pyruvate formate-lyase can function efficiently (1, 7).
Inactivation of poxB caused significant changes in the metabolic pattern of cells incubated for 5 days in Pi-limiting medium initially containing 40 mM glucose (Table 4). Whereas the total amount of glucose consumed by poxB mutants (25.7 mM) was only slightly lower than that consumed by poxB+ cells (28.4 mM), the amount of acetate excreted into the culture medium by poxB mutants (13.0 mM) was 2.2-fold lower than that excreted by poxB+ cells (29.4 mM) (the concentrations of acetate remained steady between days 4 and 6 in poxB mutants, whereas they continued to increase for 6 days up to ≈33 mM in poxB+ cells; data not shown). In contrast, the amount of pyruvate excreted by poxB mutants was 12.5-fold higher than that excreted by poxB+ cells (Table 4).
These data suggest that (i) at least half of the pyruvate that is metabolized to acetate in Pi-starved cells is metabolized through PoxB rather than through the PDH complex-phosphotransacetylase-acetate kinase pathway and (ii) part of the pyruvate that is not metabolized in poxB mutants is excreted directly into the medium.
Inactivation of poxB caused a dramatic increase in TBARS levels during prolonged incubation in Pi-limiting medium initially containing 30 mM glucose (Table 5). Whereas poxB+ and poxB mutant cells exhibited similar levels of TBARS during the exponential growth phase (0.06 nmol/mg) and on day 1 of incubation (≤0.45 nmol/mg), TBARS levels increased sharply to 1.79 nmol/mg on day 4 of incubation in poxB mutants, while TBARS levels barely increased in poxB+ cells (0.46 nmol/mg) (Table 5). Under the same conditions (on day 4 of incubation in Pi-limiting medium containing 30 mM glucose), TBARS levels increased to 1.09 nmol/mg in ahp mutants (Table 3).
TABLE 5.
TBARS levels in poxB mutantsa
| Strain | Mean TBARS concn (nmol/mg) ± SD
|
||
|---|---|---|---|
| Exponential | Day 1 | Day 4 | |
| ENZ1203 | 0.06 ± 0.01 | 0.41 ± 0.01 | 0.46 ± 0.01 |
| poxB | 0.06 ± 0.01 | 0.45 ± 0.03 | 1.79 ± 0.13 |
Strains ENZ1203 and ENZ1645 (ENZ1203 poxB) were inoculated into 100 ml of MOPS containing 30 mM glucose and 5 mM Pi (exponential) or 0.1 mM Pi (day 1 and day 4). Data are the means of two experiments.
Overall, these data suggest that in poxB mutants, although part of the pyruvate that is not metabolized through PoxB is excreted, part may be metabolized through the PDH complex in addition to the amount normally metabolized in poxB+ cells, which may increase the production of NADH and eventually the production of H2O2 by the respiratory chain. Therefore, by diverting part of the metabolic flux from the PDH complex, PoxB may protect Pi-starved cells against oxidative stress.
H-NS modulates the production of TBARS.
During the exponential growth phase, synthesis of the PDH complex is decreased, whereas synthesis of PoxB is increased in hns mutants compared to the hns+ parents (2, 15, 19). Therefore, a role in growing cells of the histone-like nucleoid-structuring protein H-NS may be to increase PDH activity (which gives rise to NADH and acetyl-CoA) and to decrease PoxB activity (which gives rise directly to acetate and eventually to acetyl-CoA through acetate kinase and acetyl-CoA synthetase, both activities requiring ATP) (1, 18). This specific role of H-NS, which may result primarily from its capacity to decrease RpoS levels in exponential phase (4), may help growing cells to sustain an efficient energetic metabolism and a rapid growth rate. In fact, the generation time of hns mutants was 3.5-fold higher than that of hns+ cells in MOPS medium (4) (Table 6). However, it was rather surprising that TBARS levels were threefold higher in hns mutants than in hns+ cells during the exponential growth phase (Table 6). This result may be explained at least in part by the recent finding that overexpression of poxB in PDH− mutants, which mimic the behavior of hns mutants, increases by ≈2-fold the specific rate of consumption of oxygen during growth (1). This is probably required to compensate for the higher consumption of ATP needed to metabolize further PoxB-derived acetate to acetyl-CoA (1). Higher activity of the tricarboxylic acid cycle enzymes and of the aerobic respiratory chain (8, 10) may eventually increase the production of ROS during growth in hns mutants.
TABLE 6.
H-NS modulates the production of TBARSa
| Strain | Pi (mM) | Growth phase (h of growth) | Glucose consumed (mM) | Mean TBARS concn, nmol/mg, ± SD (fold increase) |
|---|---|---|---|---|
| ENZ1203 | 5 | Exponential (7) | 3.3 | 0.05 ± 0.01 |
| ENZ1203 | 0.1 | Stationary (24) | 8.1 | 0.41 ± 0.02 (8.2) |
| hns | 5 | Exponential (22) | 2.1 | 0.15 ± 0.02 (3) |
| hns | 0.1 | Stationary (47) | 9.8 | 0.20 ± 0.03 (4) |
Strains ENZ1203 and ENZ1667 (ENZ1203 hns::neo) were inoculated (125 μl from overnight cultures in LB medium) into 100 ml of MOPS (5 mM Pi plus 20 mM glucose) or Pi-limiting medium (0.1 mM Pi plus 20 mM glucose) at time zero. Exponentially growing cells in MOPS medium were taken at an OD600 of 0.7 (hns+; time = 7 h; generation time ≈ 52 min) or at an OD600 of 0.4 (hns mutants; time = 22 h; generation time ≈ 3 h). TBARS values are the means of two experiments. The values relative to those in hns+ cells in the exponential growth phase are indicated in parentheses.
Whereas H-NS activity decreased TBARS levels during growth, H-NS activity increased TBARS levels at the onset of Pi starvation. TBARS levels increased strongly at the entry into stationary phase in hns+ cells (Table 6), while they barely increased in hns mutants (Table 6), although similar amounts of glucose were eventually consumed by both strains. These data may be explained simply by the hypothesis that at the entry into stationary phase, pyruvate metabolism occurs through the PDH complex in hns+ cells, whereas part of the metabolism may have already occurred through PoxB in hns mutants.
Overall, these data suggest that at the onset of Pi starvation, H-NS delays the shift from PDH to PoxB, which prolongs the production of NADH by the PDH complex and the production of ROS by NADH dehydrogenase II. The ≈4-fold increase in the rate of synthesis of H-NS at the onset of Pi starvation (29) may thus help to increase oxidative stress in Pi-starved cells.
Conclusion.
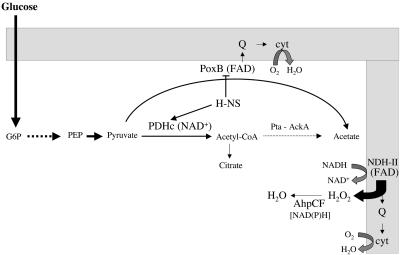
The data presented here provide the outlines to an understanding of how oxidative stress occurs in Pi-starved cells during the metabolism of glucose to acetate (Fig. 2). At the onset of stationary phase, H-NS may help to sustain a high metabolic flux through the PDH complex, which generates NADH and eventually ROS as a result of the activity of NADH dehydrogenase II in the respiratory chain. However, the rate of production of NADH may decrease rapidly, primarily because of diversion of the metabolic flux from PDH to PoxB. The residual low levels of H2O2 generated during prolonged metabolism of glucose may be efficiently scavenged by AHP.
FIG. 2.
Diagram showing the proposed pathways for the metabolism of pyruvate in Pi-starved cells. Abbreviations: Q, ubiquinone; G6P, glucose 6-phosphate; cyt, cytochrome quinol oxidase; FAD, flavin adenine dinucleotide; PEP, phosphoenolpyruvate; PDHc, PDH complex; Pta, phosphotransacetylase; AckA, acetate kinase.
There is evidence that the PoxB shunt-AHP antioxidant activity integrated system may be specific to Pi-starved cells. First, even though glucose-starved cells accumulate RpoS and induce the expression of poxB (although at levels approximately twice lower than in Pi-starved cells; unpublished data), metabolism of glucose could occur primarily through an aerobic phosphoenolpyruvate-glyoxylate cycle (9), which may generate ROS (Table 2) (22), and eventually fermentative pathways, which may prevent the generation of ROS (17, 24). However, the increase in oxidative protein damage in glucose-starved cells results primarily from the synthesis of aberrant proteins rather than from an increase in ROS levels (3). Second, nitrogen-starved cells accumulate low levels of RpoS and poorly induce poxB (unpublished data). However, nitrogen-starved cells could prevent the generation of ROS by diverting glucose metabolism towards products such as glycogen (26). Finally, sulfur-starved cells may be exposed to oxidative stress as a result of the reduction of Fe3+ to Fe2+ by cystine, which increases the production of hydroxyl radicals through the Fenton reaction (25). Therefore, it appears that each type of metabolic perturbation may generate oxidative stress through different mechanisms, and cells may respond to these perturbations by specific strategies which are just beginning to be revealed.
Acknowledgments
This research was supported by the Fondation pour la Recherche Médicale.
I acknowledge Véronique Grand, Irina Rakotobe, Valentine Trotter, Lilia Todorov, and Sandrine Rivier for help with some of the experiments. I thank John Cronan and Takeshi Mizuno for donating strains.
Footnotes
This paper is dedicated to the memory of Benjamin Moreau (1977-2000).
REFERENCES
- 1.Abdel-Hamid, A. M., M. M. Attwood, and J. R. Guest. 2001. Pyruvate oxidase contributes to the aerobic growth efficiency of Escherichia coli. Microbiology 147:1483-1498. [DOI] [PubMed] [Google Scholar]
- 2.Atlung, T., and H. Ingmer. 1997. H-NS: a modulator of environmentally regulated gene expression. Mol. Microbiol. 24:7-17. [DOI] [PubMed] [Google Scholar]
- 3.Ballesteros, M., Å. Fredriksson, J. Henriksson, and T. Nyström. 2001. Bacterial senescence: protein oxidation in non-proliferating cells is dictated by the accuracy of the ribosomes. EMBO J. 20:5280-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barth, M., C. Marschall, A. Muffler, D. Fischer, and R. Hengge-Aronis. 1995. Role for the histone-like protein H-NS in growth phase-dependent and osmotic regulation of σs and many σs-dependent genes in Escherichia coli. J. Bacteriol. 177:3455-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogosian, G., and E. V. Bourneuf. 2001. A matter of bacterial life and death. EMBO Rep. 2:770-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, R. K., and F. J. Kelly. 1996. Peroxides and other products, p. 110-131. In N. A. Punchard and F. J. Kelly (ed.), Free radicals: a practical approach. IRL Press, New York, N.Y.
- 7.Chang, Y.-Y., A.-Y. Wang, and J. E. Cronan, Jr. 1994. Expression of Escherichia coli pyruvate oxidase (PoxB) depends on the sigma factor encoded by the rpoS (katF) gene. Mol. Microbiol. 11:1019-1028. [DOI] [PubMed] [Google Scholar]
- 8.Cronan, J. E., Jr., and D. LaPorte. 1996. Tricarboxylic acid cycle and glyoxylate bypass, p. 206-216. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, ASM Press, Washington, D.C.
- 9.Fischer, E., and U. Sauer. 2003. A novel metabolic cycle catalyzes glucose oxidation and anaplerosis in hungry Escherichia coli. J. Biol. Chem. 278:46446-46451. [DOI] [PubMed] [Google Scholar]
- 10.Gennis, R. B., and V. Stewart. 1996. Respiration, p. 217-261. In F. C. Neidhardt, R. Curtiss, III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 11.Gérard, F., A.-M. Dri, and P. L. Moreau. 1999. Role of Escherichia coli RpoS, LexA and H-NS global regulators in metabolism and survival under aerobic, phosphate starvation conditions. Microbiology 145:1547-1562. [DOI] [PubMed] [Google Scholar]
- 12.González-Flecha, B., and B. Demple. 1997. Homeostatic regulation of intracellular hydrogen peroxide concentration in aerobically growing Escherichia coli. J. Bacteriol. 179:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffiths, H. R., and J. Lunec. 1996. Investigating the effects of oxygen free radicals on carbohydrates in biological systems, p. 185-199. In N. A. Punchard, and F. J. Kelly (ed.), Free radicals: a practical approach. IRL Press, New York, N.Y.
- 14.Hengge-Aronis, R. 1996. Regulation of gene expression during entry into stationary phase, p. 1497-1512. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular Biology. ASM Press, Washington, D.C.
- 15.Hengge-Aronis, R. 1999. Interplay of global regulators and cell physiology in the general stress response of Escherichia coli. Curr. Opin. Microbiol. 2:148-152. [DOI] [PubMed] [Google Scholar]
- 16.Huisman, G. W., D. A. Siegele, M. M. Zambrano, and R. Kolter. 1996. Morphological and physiological changes during stationary phase, p. 1672-1682. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 17.Koo B.-M., M.-J. Yoon, C.-R. Lee, T.-W. Nam, Y.-J. Choe, H. Jaffe, A. Peterkofsky, and Y.-J. Seok. 2004. A novel fermentation/respiration switch protein regulated by enzyme IIAGlc in Escherichia coli. J. Biol. Chem. 279:31613-31621. [DOI] [PubMed] [Google Scholar]
- 18.Kumari, S., E. J. Simel, and A. J. Wolfe. 2000. σ70 is the principal sigma factor responsible for transcription of acs, which encoded acetyl coenzyme A synthetase in Escherichia coli. J. Bacteriol. 182:551-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurent-Winter, C., S. Ngo, A. Danchin, and P. Bertin. 1997. Role of Escherichia coli histone-like nucleoid-structuring protein in bacterial metabolism and stress response. Identification of targets by two-dimensional electrophoresis. Eur. J. Biochem. 244:767-773. [DOI] [PubMed] [Google Scholar]
- 20.Messner, K. R., and J. A. Imlay. 1999. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J. Biol. Chem. 274:10119-10128. [DOI] [PubMed] [Google Scholar]
- 21.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Moreau, P. L., F. Gérard, N. W. Lutz, and P. Cozzone. 2001. Non-growing Escherichia coli cells starved for glucose or phosphate use different mechanisms to survive oxidative stress. Mol. Microbiol. 39:1048-1060. [DOI] [PubMed] [Google Scholar]
- 23.Neidhardt, F. C., P. L. Bloch, and D. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nyström, T., C. Larsson, and L. Gustafsson. 1996. Bacterial defense against aging: role of the Escherichia coli ArcA regulator in gene expression, readjusted energy flux and survival during stasis. EMBO J. 15:3219-3228. [PMC free article] [PubMed] [Google Scholar]
- 25.Park, S., and J. A. Imlay. 2003. High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J. Bacteriol. 185:1942-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preiss, J. 1996. Regulation of glycogen synthesis, p. 1015-1024. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 27.Seaver, L. C., and J. A. Imlay. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 183:7173-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Storz, G., and J. A. Imlay. 1999. Oxidative stress. Curr. Opin. Microbiol. 2:188-194. [DOI] [PubMed] [Google Scholar]
- 29.VanBogelen, R. A., E. Olson, B. L. Wanner, and F. C. Neidhardt. 1996. Global analysis of proteins synthesized during phosphorus restriction in Escherichia coli. J. Bacteriol. 178:4344-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamada, H., T. Yoshida, K. Tanaka, C. Sasakawa, and T. Mizuno. 1991. Molecular analysis of the Escherichia coli hns gene encoding a DNA-binding protein, which preferentially recognizes curved DNA sequences. Mol. Gen. Genet. 230:332-336. [DOI] [PubMed] [Google Scholar]