Abstract
Alginate, an exopolysaccharide produced by Pseudomonas aeruginosa, provides the bacterium with a selective advantage that makes it difficult to eradicate from the lungs of cystic fibrosis (CF) patients. Previous studies identified a gene, algX, within the alginate biosynthetic gene cluster on the P. aeruginosa chromosome. By probing cell fractions with anti-AlgX antibodies in a Western blot, AlgX was localized within the periplasm. Consistent with these results is the presence of a 26-amino-acid signal sequence. To examine the requirement for AlgX in alginate biosynthesis, part of algX in P. aeruginosa strain FRD1::pJLS3 was replaced with a nonpolar gentamicin resistance cassette. The resulting algXΔ::Gm mutant was verified by PCR and Western blot analysis and was phenotypically nonmucoid (non-alginate producing). The algXΔ::Gm mutant was restored to the mucoid phenotype with wild-type P. aeruginosa algX provided on a plasmid. The algXΔ::Gm mutant was found to secrete dialyzable oligouronic acids of various lengths. Mass spectroscopy and Dionex chromatography indicated that the dialyzable uronic acids are mainly mannuronic acid dimers resulting from alginate lyase (AlgL) degradation of polymannuronic acid. These studies suggest that AlgX is part of a protein scaffold that surrounds and protects newly formed polymers from AlgL degradation as they are transported within the periplasm for further modification and eventual transport out of the cell.
Cystic fibrosis (CF) represents the most common deadly inherited disease in Caucasians; it occurs in 1 in 2,500 to 1 in 3,000 births each year (9). The disease is caused by a defect in the gene encoding the CF transmembrane conductance regulator (CFTR) (32, 48, 49). CFTR normally functions as a cyclic AMP-regulated chloride channel of epithelial cells lining the airways, intestine, and exocrine glands (9, 29). The genetic defect in CFTR causes abnormal chloride and water transport in the epithelia, which ultimately results in intestinal obstruction, male infertility, pancreatic insufficiency, increased levels of electrolytes in sweat, liver problems, and, more importantly, inadequate mucociliary clearance in the lungs, which facilitates chronic respiratory infections caused by Pseudomonas aeruginosa (9). In fact, the leading cause of death in CF patients is pulmonary failure due to inflammation and persistent respiratory infections caused by alginate-producing strains of P. aeruginosa (9, 38, 45, 60).
Alginate, a copolymer of mannuronic and guluronic acids (12), is an exopolysaccharide that makes it very difficult to eradicate P. aeruginosa from the lungs of CF patients due to its ability to shield the bacteria from antibiotics (2, 24) and host defenses (2, 3, 34, 54). Alginate also plays a role in the formation of microcolonies in vitro (40) and can act as an adhesin (11), which facilitates bacterial colonization of the respiratory tract.
Four main regions of the P. aeruginosa chromosome contain genes involved in alginate biosynthesis and regulation. The alginate biosynthetic gene cluster (17, 47, 56), containing algD, alg8, alg44, algK, algE, algG, algX, algL, algI, algJ, algF, and algA, is located at 34 min on the bacterial chromosome and acts as an operon (8) controlled by the algD promoter. algC, located at 10 min on the bacterial chromosome and regulated by its own promoter (21, 65), is involved in alginate biosynthesis as well as lipopolysaccharide biosynthesis.
The alginate switch (algU, mucA, mucB, mucC, and mucD), located at 68 min, is responsible for the conversion of P. aeruginosa to the alginate-producing forms found predominantly in lungs of CF patients (10, 36, 37, 56). algU (also named algT) encodes a protein (AlgU or σ22) with significant similarity to the alternative RNA polymerase sigma factor σE from Escherichia coli and positively regulates the transcription of itself, algR, algB, algD, and algC (56, 63). AlgU's activity is inhibited by MucA and MucB, which act as anti-σ factors on AlgU, resulting in a nonmucoid phenotype. MucA and MucB negatively affect the stability of AlgU in the cell (37). Spontaneous mutations in mucA or mucB represent the major mechanism of conversion from the nonmucoid to the mucoid phenotype. Regulatory genes algR, algP, and algQ, at 9 min, and algB, found at 13 min, are important for transcriptional activation of the alginate biosynthetic genes (17, 56).
The initial steps of alginate biosynthesis are well understood. It occurs in the cytoplasm through a series of steps involving AlgA (a bifunctional enzyme known as a phosphomannose isomerase and GDP-mannose pyrophosphorylase), AlgC (phosphomannomutase), and AlgD (GDP-mannose dehydrogenase), which convert fructose-6-phosphate to GDP-mannuronic acid, the subunit making up alginate (56). However, many of the subsequent steps in alginate biosynthesis remain to be elucidated.
An unidentified transporter moves GDP-mannuronic acid or an alginate intermediate across the inner membrane into the periplasmic space. The location and protein(s) involved in polymerization are unknown, but Alg8 and Alg44, two hydrophobic proteins, are thought to be involved (35). A role for Alg8 in polymerization has been suggested due to its resemblance to β-glycosyltransferases (52). After polymerization, the polymer undergoes a series of modification steps. The mannuronic acid residues are partially epimerized into guluronic acid by AlgG (mannuronan C5 epimerase) in the periplasm (14), and some of the mannuronic acid residues are acetylated by the action of proteins AlgF, AlgI, and AlgJ (15, 16). The mature alginate polymer consists of random polymannuronic acid (poly[M]) and poly(MG) (G, guluronic acid) blocks with some of the mannuronic acid residues acetylated. This polymer is thought to be transported out of the cell with the aid of AlgE, an outer membrane protein that seems to act as an anion channel (46). AlgL, an alginate lyase, (53), has been shown to be necessary for alginate production, although its role in alginate biosynthesis is not known (39). Recent work indicates that AlgK and AlgG may be part of a periplasmic protein complex which protects the newly formed polymer from AlgL degradation in the periplasm (19, 27).
algX was previously cloned and sequenced (39) and found to be located in the alginate biosynthetic operon between algG and algL. By transposon mutagenesis, merodiploid analysis, and complementation studies, AlgX was determined to be necessary for alginate production, although its exact role was unclear (39). The present study seeks to gain more information about AlgX. First, its subcellular location was determined by cell fractionation studies, Western blot analysis, and N-terminal amino acid sequencing. Second, an algX nonpolar deletion mutant was created by allelic exchange, and the alginate produced by this mutant was characterized by membrane dialysis, biochemical analyses, mass spectroscopy (MS), and Dionex chromatography.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Bacterial strains and plasmids used in this study are described in Table 1. Strains were routinely cultured in LB broth or on LB agar plates (Difco Laboratories, Detroit, Mich.) at 37°C with antibiotics as needed. For some studies, special culture media and conditions were used, and these are described further below. Antibiotics were used at the following concentrations: carbenicillin, 100 μg/ml for E. coli and 300 μg/ml for P. aeruginosa; gentamicin, 15 μg/ml for E. coli and 250 μg/ml for P. aeruginosa; kanamycin, 30 μg/ml for E. coli; tetracycline, 25 μg/ml for E. coli and 100 μg/ml for P. aeruginosa.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Phenotype and/or genotypea | Reference or source |
|---|---|---|
| P. aeruginosa strains | ||
| FRD1 | Alg+ CF isolate; contains mutation in mucA | 42 |
| FRD1::pJLS3 | Prototrophic, Alg+ CF isolate; algD operon controlled by tac promoter; Cbr | D. Ohman |
| FRD2 | Spontaneous nonmucoid strain derived from FRD1 | 43 |
| 2-2 | algX deletion mutant derivative of FRD1::pJLS3; Gm cassette replaces part of algX; Cbr | This study |
| E. coli strains | ||
| DH10B | F−mcrAΔ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu)7697 galU galK rpsL(Strr) endA1 nupG fhuA::IS2 | Invitrogen |
| DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 51 |
| JM109 | recA1 supE44 endA1 hsdR17 gyrA96 relA1 thi Δ(lac-proAB) F′ [traD36 proAB+lacIqlacZΔM15] | 51 |
| HB101 | supE44 hsdS20(r−B m−B) recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 | 51 |
| HMS174(DE3)pLysS | F−recA hsdR (rk12− mk12+) Rifr (DE3) pLysS Cmr | Novagen |
| Plasmids | ||
| pALG2-3.3HindIII/XbaI | Apr; 3.3-kb HindIII-XbaI fragment cloned into pBluescript SK(−); contains part of algG, all of algX, and part of algL | This study |
| pBSSK(−)pALG2-2.9ΔX | Apr; 2.9-kb HindIII-XbaI fragment cloned into pBluescript SK(−); contains part of algG, algX with a 384-bp XcmI fragment deleted from its 3′ end, and part of algL | This study |
| pAR4 | Cbr Gmr; pBSSK(−)pALG2-2.9ΔX with 40-bp EcoRI fragment deleted within algX just upstream of area with 384-bp XcmI fragment replaced with 700-bp Gm nonpolar cassette | This study |
| pAR5 | Cbr Gmr; 3.6-kb HindIII/XbaI fragment from pAR4 blunted and ligated into pEX100T previously cut with SmaI | This study |
| pAR6 | Tcr; 1.6-kb HindIII/KpnI PCR fragment containing wild-type algX cloned into pRK415 | This study |
| pSJ12 | Apr Gmr; 700-bp SmaI fragment containing a nonpolar Gm cassette cloned into pBluescript II KS(−) | 28 |
| pEX100T | bla (Cbr Apr) sacB oriT | 55 |
| pRK415 | Broad-host-range expression vector; contains lac promoter; Tcr; mob+ | 30 |
| pRK2013 | Kmr ColE1-Tra(RK2)+ | 13 |
| pNLS20 | pRK415 vector carrying 2.5-kb fragment containing algX and oriented for its expression | N. Schiller |
| pSM7 | pET21a vector with algX for expression of AlgX-His; inserted via NdeI/XhoI sites | This study |
| pET-21a | Expression vector; Apr; T7lac promoter; product contains His tag at C-terminal end | Novagen |
| pBluescript SK(−) | Apr; ColE1 origin | Stratagene |
Abbreviations: Alg+, alginate producing; Cbr, carbenicillin resistance; Cmr, chloramphenicol resistance; Apr, ampicillin resistance; Tcr, tetracycline resistance; mob+, mobilizable plasmid; Kmr, kanamycin resistance.
DNA manipulations.
Restriction endonucleases were purchased from New England Biolabs (Beverly, Mass.), Gibco BRL (Gaithersburg, Md.), or Promega (Madison, Wis.). Genomic DNA was isolated with Promega's chromosomal DNA isolation kit. DNA fragments were isolated from agarose gels with either the QIAquick gel extraction kit or the MinElute gel extraction kit (QIAGEN, Valencia, Calif.). Plasmids were transferred into E. coli by chemical transformation (41) or electroporation (51) with minor modifications or with Electrocomp Gene Hogs E. coli (Invitrogen, Carlsbad, Calif.). Plasmid DNA was isolated from E. coli with the Qiaprep spin miniprep kit (QIAGEN). DNA primers were purchased from Sigma Genosys (The Woodlands, Tex.) or Integrated DNA Technologies, Inc. (Coralville, Iowa). PCR was performed with PLATINUM Pfx DNA polymerase (Invitrogen) and 10 mM deoxynucleoside triphosphate mixture (Gibco BRL).
Expression and purification of AlgX-His.
algX was cloned into pET-21a (Novagen, Madison, Wis.) in frame with and upstream of the vector His6 codons, creating pSM7, and transformed into HMS174(DE3)pLysS (kindly provided by Steve Monday). Cultures were grown in LB broth containing 50 μg of carbenicillin/ml and 34 μg of chloramphenicol/ml at 37°C until the optical density at 600 nm (OD600) reached 0.6; after induction with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 2 h, the bacteria were harvested by centrifugation and resuspended in binding buffer (5 mM imidazole, 0.5 M NaCl, 20 mM Tris-HCl [pH 7.9]). Bacterial cells were disrupted by sonication and then centrifuged at 20,000 × g for 15 min. The pellet was washed with binding buffer, recentrifuged, and resuspended in 10 ml of binding buffer containing 6 M urea to solubilize inclusion bodies and placed on ice for 1 h. Any remaining insoluble material was removed by centrifugation at 39,000 × g for 20 min. The supernatant was filtered with a 0.45-μm-pore-size filter and loaded onto a 2.5-ml nickel chelation resin column (Novagen). The column was washed with 25 ml of binding buffer containing 6 M urea, followed by a solution containing 11 ml of binding buffer with 6 M urea and 4.1 ml of wash buffer (60 mM imidazole, 0.5 M NaCl, 20 mM Tris-HCl [pH 7.9]) with 6 M urea. AlgX-His was eluted in 1-ml fractions with 15 ml of elution buffer (1 M imidazole, 0.5 M NaCl, 20 mM Tris-HCl [pH 7.9]) containing 6 M urea. Refolding of AlgX-His was accomplished by adding dithiothreitol (DTT) to 50 mM and dialyzing at 4°C against progressively lower concentrations of urea and DTT until the urea and DTT concentrations were 0.16 M and 1 mM, respectively. The protein was then stored at −20°C.
Polyclonal anti-AlgX-His antibody production.
Rabbits were immunized with 100 μg of AlgX-His in complete Freund's adjuvant (Sigma-Aldrich, St. Louis, Mo.), with booster injections containing 64 μg of AlgX-His in incomplete Freund's adjuvant (Sigma-Aldrich). The serum was monitored for anti-AlgX antibody titer by Western blot analysis. To remove any nonspecific antibodies, the immune serum was preabsorbed before use with a suspension of proteins from strain FRD2 prepared by acetone precipitation by a protocol described by Harlow and Lane (23).
Cell fractionation, assessment of cross-contamination, and Western blot analysis.
Cultures of strain FRD1 were grown at 37°C for 22 h in phosphate-deficient media (7) to induce production of alkaline phosphatase, the periplasmic marker (18). The cells were diluted 1:2 with 0.01 M Tris-HCl (pH 8.4) and centrifuged at 30,100 × g for 1 h. The cytoplasmic and periplasmic fractions were obtained by a modified version of Wood's procedure (6, 62). Cells were resuspended in a solution containing 0.5 mg of lysozyme/ml, 40 mM Tris-HCl (pH 8.0), 0.5 M sucrose, and 4 mM EDTA and incubated in a 30°C water bath, with gentle shaking, for 60 min to form spheroplasts. MgCl2 was added to 10 mM after the initial 2 min. The solution was centrifuged at 12,100 × g at 4°C for 15 min to pellet the spheroplasts, leaving the periplasmic proteins in the supernatant. The spheroplasts were washed in a solution containing 0.5 M sucrose and 40 mM Tris-HCl (pH 8.0) to remove any residual supernatant, recentrifuged, and resuspended in 3 ml of 10 mM Tris-HCl (pH 8.0)-10 mM MgCl2. After sonication to break up the spheroplasts, the suspension was centrifuged at 77,600 × g for 3 h at 4°C to separate the cytoplasmic proteins in the supernatant from the total membrane fraction in the pellet.
The inner and outer membranes were isolated from cultures of FRD1 grown in phosphate-deficient media for 22 h by the protocols described by Hancock and Nikaido (22), resuspended in 1 ml of 10 mM Tris-HCl (pH 8.4), and stored at −20°C until use. Cross-contamination of the subcellular fractions was determined by assaying each fraction for the following markers: isocitrate dehydrogenase, a cytoplasmic marker (20), lactate dehydrogenase, an inner membrane marker (57), alkaline phosphatase, a periplasmic marker (18), and lipopolysaccharide (LPS), an outer membrane marker (25). As percentages of the total amount found in all fractions, 70, 79, and 48% of the isocitrate dehydrogenase, lactate dehydrogenase, and alkaline phosphatase activities, respectively, were found in the cytoplasm, inner membrane, and periplasm, respectively. LPS was detected mainly in the outer membrane fraction, with trace levels detected in the inner membrane and periplasmic fractions.
The four cell fractions (periplasmic, cytoplasmic, and outer and inner membrane) were resolved on a sodium dodecyl sulfate (SDS)-10% polyacrylamide gel and transferred to a nitrocellulose membrane. Membranes were blocked overnight in 0.01 M phosphate-buffered saline (PBS), pH 7.4, containing 0.1% Tween and 5% nonfat dried milk (T-PBS-5% milk) at 4°C, rinsed in the same solution, and then probed with an anti-AlgX polyclonal rabbit antibody (1:2,000 dilution in T-PBS-5% milk) for 2 h at room temperature. The membranes were washed in T-PBS-5% milk, incubated with a donkey anti-rabbit immunoglobulin G-horseradish peroxidase-linked antibody (1:2,000 dilution in T-PBS-5% milk) for 1 h at room temperature, and rinsed. The ECL Western blotting kit and ECL Hyperfilm (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.) were used to detect AlgX via luminescence.
Amino-terminal sequencing of AlgX.
Previous studies had demonstrated that AlgX overexpressed with JM109(pNLS20) was localized predominantly in the periplasm, similar to what was found for FRD1. Therefore, to produce enough AlgX for amino-terminal sequencing analysis, cultures of JM109(pNLS20) were grown in LB broth containing 25 μg of tetracycline/ml. At an OD600 of 0.5, the culture was induced with 1 mM IPTG for 3 h. The cells were collected by centrifugation, and the periplasmic fraction was obtained as described above. A 50 to 60% ammonium sulfate cut on the periplasmic fraction was resolved on an SDS-10% polyacrylamide gel electrophoresis gel. After electrophoresis, the proteins were transferred onto an Immobilon-P transfer membrane for 2.5 h at 65 mA. The membrane was washed in distilled water, air dried completely, and stained with 0.25% Coomassie R-250 in 50% methanol for 1 min. The AlgX protein band was located, and then the membrane was destained with 50% methanol-10% acetic acid and rinsed with 100 ml of 50% methanol. The AlgX protein band was cut and subjected to N-terminal sequencing with the Perkin-Elmer Applied Biosystems model 492 sequencer by the Department of Environmental Toxicology at the University of California, Riverside.
Construction and confirmation of algXΔ::Gm.
pBSSK(−)pALG2-2.9ΔX, containing 530 bp of algG, 1,422 bp of algX with a 384-bp XcmI area deleted from its 3′ end, and 1,315 bp of algL, was digested with EcoRI, removing a 40-bp piece just upstream of the XcmI-deleted area of algX. This was ligated to an ∼700-bp SmaI nonpolar gentamicin resistance (Gm) cassette obtained from pSJ12 (28) with the Gm promoter in the same orientation as algG, algX, and algL, creating pAR4. pAR4 was digested with HindIII and XbaI, releasing an ∼3.6-kb DNA fragment containing part of algG, algXΔ::Gm, and part of algL, which was ligated into pEX100T cut with SmaI, creating pAR5.
Triparental matings were used to mobilize pAR5 from E. coli into P. aeruginosa strain FRD1::pJLS3 with the aid of helper plasmid pRK2013 (13) as described previously (39). Merodiploids resulting from a single-crossover homologous recombination event were selected for on LA-PIA-Gm250 (a 1:1 mixture of L agar and Pseudomonas isolation agar [Difco Laboratories] containing 250 μg of gentamicin/ml). Single colonies were then grown in LB broth for 18 h and plated onto LA-PIA-Gm250 containing 7.5% sucrose to select for colonies that had undergone double crossovers, leading to gene replacement. Possible algXΔ::Gm mutant colonies were subcultured, and PCR was used to verify the replacement of chromosomal algX with algXΔ::Gm. Primer a (5′CTGTTCCGCACCACCTACGAC3′) is specific to the 5′ end of algX, which is common to both FRD1::pJLS3 and the algXΔ::Gm mutant; primer b (5′CAGGGAAAGGAACTGCTGGTC3′) is in the reverse orientation and is specific to a sequence within the XcmI-deleted region of algX; primer c (5′GATCGTCACCGTAATCTGCTTGC3′) is also in the reverse orientation and is specific to a sequence within the Gm cassette.
Western blot analysis was also used to further verify that gene replacement had occurred. Mutant algXΔ::Gm strains were streaked on LA-PIA-Gm250 with and without 1 mM IPTG, and harvested colonies were resuspended in 0.01 M PBS (pH 7.4) to an OD600 of 0.5. One milliliter of the cell suspension was pelleted by centrifugation, and the pellet was washed twice and resuspended in Laemmli buffer, followed by boiling for 5 min. The samples were resolved on a SDS-10% polyacrylamide gel, transferred to a nitrocellulose membrane, and subjected to Western blot analysis using anti-AlgX polyclonal antibodies as described above.
Complementation of the algXΔ::Gm mutant.
For complementation of the algXΔ::Gm mutant, P. aeruginosa algX was amplified by PCR with pALG2-3.3HindIII/XbaI as the template and a HindIII site (underlined) was introduced at the 5′ end with primer 1A (5′AAAAAAGCTTCAGGACAAGGCGGTGCTGATC 3′) and a KpnI site (underlined) was introduced at the 3′ end with primer 1B (5′AAAAGGTACCCTGGCTGACCTGGCTGGCG 3′). The PCR product was cloned into pRK415 previously cut with HindIII and KpnI such that algX was under control of the lac promoter, forming pAR6. The construct was then transferred from E. coli to P. aeruginosa algXΔ::Gm strain 2-2 by triparental mating as described earlier. Potential transconjugants were plated onto LA-PIA-Tc100 plates at 37°C until colonies appeared. The colonies were then scored for alginate production by streaking them on LA-PIA-Tc100-1 mM IPTG and looking for the characteristic mucoid phenoptype. PCR and Western blot analysis were used as previously described to verify that complementation had occurred.
Uronic acid assays.
Fifteen milliliters of modified alginate promoting (MAP) medium, an alginate-promoting medium (14) containing 1 mM IPTG and antibiotics (as needed), was inoculated with 1 ml of a test strain overnight culture, and the strain was grown at 37°C for 22 h. Ten milliliters of the culture was centrifuged at 10,000 × g for 1 h, and 7 ml of the supernatant was dialyzed against an equal volume of 10 mM Tris-HCl (pH 7.6) overnight for equilibrium dialysis with Spectra/Por 6 membrane tubing (Fisher Scientific, Pittsburgh, Pa.) with a molecular mass cutoff of either 10,000 or 1,000 Da. The uronic acid content of material from inside and outside of the bag was determined by the carbazole assay (33). For extensive dialysis, samples in the dialysis bags were then placed in 1 liter of 10 mM Tris-HCl (pH 7.6) for 3 h. The dialysis bags were transferred to fresh buffer overnight, and the uronic acid concentration remaining in the dialysis bag was determined. Uronic acid concentrations were determined from a standard curve using Macrocystis pyrifera alginate (Sigma-Aldrich).
Measuring alginate lyase activity.
Reaction mixtures contained 100 μl of the enzyme reaction buffer (30 mM Tris-HCl, 9 mM MgCl2 [pH 7.5]), 100 μl of MAP with or without Flavobacterium multivorum alginate lyase (Sigma-Aldrich), and 50 μl of the culture supernatant and were incubated at 37°C for 2 min. The thiobarbituric acid assay of Weissbach and Hurwitz (61) was used to quantify the unsaturated residues formed at the nonreducing ends of the lyase-cleaved polymers or in secreted uronic acids.
Production of P. aeruginosa alginate oligomers for MS and Dionex studies.
Cultures were grown in shake flasks in PIA medium containing (per liter) 20 g of bacteriological peptone, 1.4 g of MgCl2, 5 g of NaCl, 10 g of K2SO4, and 20 ml of 87% glycerol. Gentamicin (225 μg/ml) was added when cultivating strain 2-2. IPTG was added to a final concentration of 1 mM. The flasks were then incubated at 25°C for 48 h in an orbital shaker.
Preparation of dimer and trimer standards.
Unsaturated mannuronan dimer and trimer standards for Dionex and MS analyses were prepared by lyase degradation (mannuronic acid-specific lyase from abalone; Sigma-Aldrich) of poly(M) (19), followed by preparative gel filtration. An unsaturated dimer [(MG)n] containing both mannuronic acid (M) and guluronic acid (G) was prepared by lyase degradation (guluronic acid-specific lyase from a Klebsiella sp.) of polyalternating alginate. Guluronic acid-specific lyase from Klebsiella was purified as described earlier (1, 44). This alginate was prepared in vitro by incubating poly(M) with the recombinantly produced Azotobacter vinelandii mannuronan C5 epimerase AlgE4 (26). The conditions used for lyase degradation and preparative gel filtration were essentially as described earlier (4, 5).
Electrospray ionization MS and HPAEC-PAD (Dionex).
For MS analyses culture supernatants and standards were diluted in 5 mM ammonium acetate, pH 9, and analyzed with direct infusion (0.6 ml/h) into an Agilent MSDTrap SL mass spectrometer equipped with an electrospray ion source and operated in negative-ion mode. Drying gas flow was 5 liters/min, drying gas temperature was 325°C, and nebulizer pressure was 15 lb/in2. The capillary voltage was 3,500 V, with end plate offset of −500 V. For MS/MS studies the parent ions were isolated and fragmented with a fragmentation voltage of 1 V, with helium as the collision gas.
For high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD; Dionex) analyses the culture supernatants and standards were diluted in H2O. The analyses were performed by the same protocol and with the same equipment described earlier (5).
RESULTS
AlgX is located in the periplasm and contains a signal sequence.
A hydrophilicity plot of AlgX (not shown) indicated that it is predominantly hydrophilic, with no extensive hydrophobic domains. Its amino-terminal end displays the characteristics of a typical signal sequence: a short region consisting of polar basic amino acids, followed by a longer stretch of hydrophobic amino acids, followed by a short region with higher-polarity amino acids (59). Thus, AlgX was predicted to be localized in the periplasm along with some of the other alginate biosynthetic proteins. To test this hypothesis, FRD1 was grown and subcellular fractions were isolated as described in Materials and Methods.
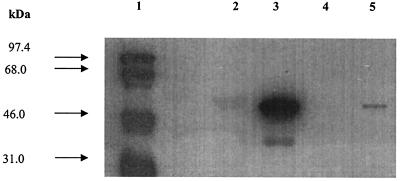
The subcellular fractions were resolved on a SDS-10% polyacrylamide gel; proteins were then transferred to a nitrocellulose membrane and probed with anti-AlgX polyclonal antibodies. AlgX was detected predominantly in the periplasm, and a small amount was also seen in the outer membrane fraction (Fig. 1). The localization of AlgX in the periplasm strongly suggested the presence of a signal peptide in the protein. To confirm this, AlgX was isolated from the periplasm of strain JM109/pNLS20 and subjected to amino-terminal sequencing as described in Materials and Methods. The first six amino acid residues of the mature, periplasmic AlgX were Ala-Asp-Pro-Gly-Ala-Ala, corresponding to amino acids 27 to 32 of the predicted amino acid sequence of AlgX (39). The first 26 amino acid residues represent the signal sequence for AlgX, and the cleavage site of the signal peptide followed the “(−3, −1) rule” of von Heijne (58). Therefore, the mature AlgX protein has a predicted molecular mass of 53 kDa and is localized predominantly in the periplasm.
FIG. 1.
Localization of P. aeruginosa AlgX by Western blot analysis. Subcellular fractions of strain FRD1 were resolved on an SDS-10% polyacrylamide gel; proteins were transferred to a nitrocellulose membrane, probed with anti-AlgX polyclonal antibodies, and visualized on ECL hyperfilm. Lane 1, molecular mass markers; lane 2, cytoplasmic membrane fraction; lane 3, periplasm sample; lane 4, inner membrane fraction; lane 5, outer membrane fraction. Note that the lane between lanes 1 and 2 contains a rainbow marker (Amersham Pharmacia Biotech, Inc.).
A nonpolar algX deletion mutation results in loss of alginate production.
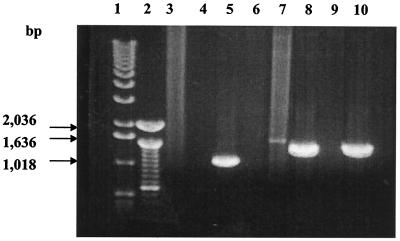
To determine the role that AlgX plays in alginate biosynthesis, an algX deletion mutation was created in FRD1::pJLS3 (kindly provided by Dennis Ohman). In this strain, the alginate biosynthetic operon is under tac promoter control and is “off” until induced by the addition of IPTG. A nonpolar gentamicin resistance cassette was used to replace part of algX. One representative candidate algX deletion mutant (identified as strain 2-2) was selected for further study. FRD1::pJLS3 and isolate 2-2 were streaked onto LA-PIA plates with appropriate antibiotics, and 1 mM IPTG was added to induce alginate synthesis. Whereas FRD1::pJLS3 produces alginate upon IPTG induction (as expected), isolate 2-2 was phenotypically nonmucoid, suggesting that a functional AlgX is required for alginate synthesis. Primers a and b and a and c (see Materials and Methods) were used to amplify PCR products with genomic DNA isolated from FRD1::pJLS3 and isolate 2-2 as templates. Primers a and b would be expected to produce a DNA fragment of 1,109 bp when algX is present, while primers a and c should produce a DNA fragment of 1,391 bp only when algXΔ::Gm is present. As shown in Fig. 2, PCR amplification of genomic DNA from strain 2-2 did not produce a wild-type algX PCR product of 1,109 bp when primers a and b were used (lane 9) but did produce an expected mutant product of 1,391 bp when primers a and c were used (lane 10), thus confirming the replacement of algX with algXΔ::Gm in strain 2-2.
FIG. 2.
algX chromosomal deletion mutant 2-2 was verified via PCR with primers a and b and a and c. Genomic DNA was isolated from FRD1::pJLS3 and strain 2-2, and the presence of algX or algXΔ::Gm was identified by PCR with primers a and b and a and c. Lane 1, 1-kb DNA ladder; lane 2, 100-bp DNA ladder; lanes 3 and 4, no-DNA control with a and b and a and c primers, respectively; lanes 5 and 6, wild-type FRD1::pJLS3 DNA with a and b and a and c primers, respectively; lanes 7 and 8, plasmid construct pAR5 with a and b and a and c primers, respectively; lanes 9 and 10, algX deletion mutant 2-2 DNA with a and b and a and c primers, respectively. DNA markers were purchased from Gibco BRL.
To verify that the mutation was nonpolar on downstream genes within the biosynthetic operon, attempts to restore alginate production by direct complementation with AlgX were conducted. For these experiments, algX from FRD1 was amplified by PCR and cloned into pRK415 and the resulting construct, pAR6, was introduced into strain 2-2 by triparental mating. Alginate production was successfully restored in isolate 2-2 after complementation with pAR6, indicating that downstream alginate biosynthetic genes were unaffected by the presence of algXΔ::Gm. Quantitative analyses of alginate production demonstrated that complementation of strain 2-2 with P. aeruginosa algX resulted in alginate production at a level of 67% of that for FRD1::pJLS3 (data not shown).
To further prove that algX expression was disrupted in mutant strain 2-2, the production of AlgX was examined by Western blot analysis. As expected, algX expression in FRD1::pJLS3 was detected only when this strain was induced with IPTG. In contrast, AlgX was not detected in strain 2-2 with or without IPTG, indicating that algX expression was disrupted in this construct. In complementation studies, AlgX was detected in isolate 2-2(pAR6) induced with IPTG; further, both the 1,109-bp algX PCR product obtained with primers a and b and the 1,391-bp algXΔ::Gm PCR product obtained with primers a and c were detected in strain 2-2(pAR6) (data not shown).
A nonpolar algX deletion mutant secretes uronic acid oligomers.
Recent studies have shown that the inactivation of algK (28) or algG (27) within the alginate biosynthetic operon results in phenotypically nonmucoid colonies and the release of dialyzable uronic acid oligomers from cells. To determine whether our nonmucoid algXΔ::Gm mutant behaved similarly, culture supernatants of the parental strain, FRD1::pJLS3, FRD2 (a nonmucoid derivative of FRD1), and isolate 2-2 were tested for uronic acid content and approximate size by using dialysis membranes with either 10- or 1-kDa molecular mass cutoffs (Table 2). IPTG-induced FRD1::pJLS3, which is phenotypically mucoid, produced nondialyzable uronic acid polymers upon equilibrium and exhaustive dialysis. FRD2, our negative control, did not produce any detectable uronic acid (data not shown). In contrast, IPTG-induced 2-2 was phenotypically nonmucoid but produced dialyzable uronic acid. With 10-kDa pore dialysis membranes, approximately equal amounts of uronic acid were detected inside and outside of the dialysis bag after equilibrium dialysis, whereas, after exhaustive dialysis, most of the uronic acid in the dialysis bag had been dialyzed away. With 1-kDa pore dialysis membranes, after equilibrium dialysis approximately 61% of the uronic acid produced by strain 2-2 remained in the bag while 39% was outside of the bag (Table 2). After extensive dialysis, 24% of the uronic acid was still in the dialysis bag while 76% had dialyzed away. These results suggest that, in the absence of AlgX, oligouronides are secreted and may have a mixture of sizes. To gain a better understanding of the nature of these oligouronides, further experiments were performed.
TABLE 2.
Amounts and relative sizes of uronic acid secreted by FRD1::pJLS3 and isolate 2-2c
| Strain | UA level (mg/ml) with:
|
|||
|---|---|---|---|---|
| 10-kDa pore membranes
|
1-kDa pore membranes
|
|||
| UAs in/out of dialysis baga | UAs after exhaustive dialysisb | UAs in/out of dialysis bag | UAs after exhaustive dialysis | |
| FRD1::pJLS3 | 0.21/0 | 0.26 | 0.20/0 | 0.26 |
| 2-2 | 0.17/0.17 | 0.01 | 0.20/0.13 | 0.08 |
The MAP culture supernatants of FRD1::pJLS3 (parental mucoid) and 2-2 (algXΔ::Gm) were collected via centrifugation, and 7 ml was dialyzed against an equal volume of 10 mM Tris-HCl (pH 7.6) overnight for equilibrium dialysis. Samples from inside and outside of the bag were taken and tested for uronic acid content by the carbazole assay.
After equilibrium dialysis, each bag was subjected to exhaustive dialysis as described in Materials and Methods. A sample of the inside of the dialysis bag was taken and tested for uronic acids by the carbazole assay.
Data are from one representative experiment repeated several times. UAs, uronic acids.
The uronic acid oligomers secreted by an algX mutant are products of alginate lyase degradation of an alginate polymer.
In a recent paper (27), the uronic acid oligomers secreted from algK and algG mutants were shown to be the result of degradation of the newly formed alginate polymer by alginate lyase (AlgL) within the periplasm. We tested whether the same was true for this algX mutant. The uronic acids found in the culture supernatants from the 2-2 isolate (algX mutant) were tested for the presence of unsaturated bonds at the nonreducing ends of an alginate polymer that could be the result of lyase activity. As a control, the secreted alginate polymer of IPTG-induced FRD1::pJLS3 was tested, and, as expected, only trace amounts of unsaturated bonds were present (Table 3). However, treatment of this alginate with lyase increased the relative amount of unsaturated bonds approximately threefold. As a second control, we examined the alginate produced by Macrocystis pyrifera. Untreated, it contained no unsaturated bonds; however, alginate lyase treatment increased the relative amount of unsaturated bonds to an OD value of 0.29. In contrast, the uronic acid oligomers produced by strain 2-2 had a relatively large amount of unsaturated bonds (OD548 = 0.53) without the addition of exogenous alginate lyase. This strongly suggests that the oligouronides secreted by our algXΔ::Gm mutant are the result of alginate polymerization and subsequent degradation by the lyase action of AlgL resident within the periplasm.
TABLE 3.
Detection of unsaturated ends resulting from alginate lyase activitya
| Alginate source | Genotype | AlgL added | Unsaturated ends (OD548) |
|---|---|---|---|
| FRD1::pJLS3 | algX+ | − | 0.03 |
| + | 0.10 | ||
| Macrocystis pyrifera | NA | − | 0 |
| + | 0.29 | ||
| 2-2 | Mutant algX | − | 0.53 |
MAP culture supernatants of FRD1::pJLS3 and 2-2 were used as the source of alginate. The alginate concentrations for each sample were determined by uronic acid assays. Macrocystis pyrifera alginate was obtained from Sigma. The alginate of each sample (approximately 17 μg) was tested for unsaturated uronic acid products as evidence of endogenous alginate lyase activity by the thiobarbituric acid assay. Some samples were treated with AlgL (alginate lyase from Flavobacterium multivorum). The values for unsaturated ends represent relative amounts of unsaturated ends. The experiment was repeated several times. Data shown are one depiction of representative results obtained. NA, not applicable.
MS and Dionex chromatography analyses of 2-2 culture supernatant show that these secreted oligomers are mainly mannuronic acid dimers.
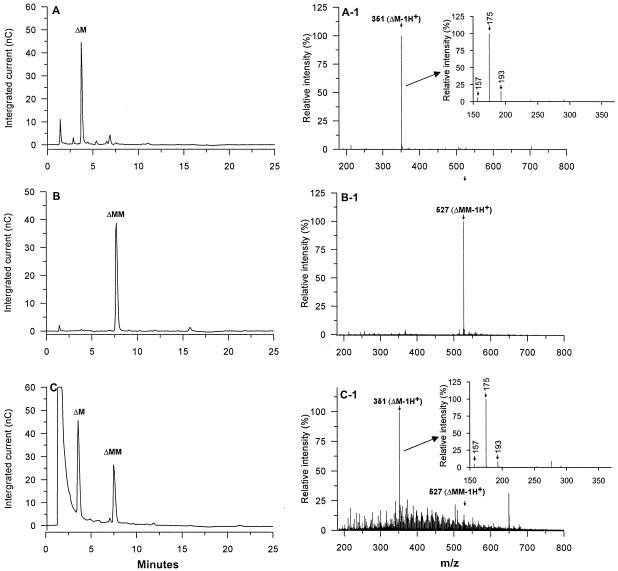
The oligouronides secreted by the 2-2 isolate were also examined by Dionex chromatography and MS. MS is not quantitative in terms of giving a correct representation of the relative amounts of various oligouronides produced; Dionex chromatography solves this problem and serves as an independent way of confirming the MS data. Standards for these experiments were first prepared by making dimers and trimers from mannuronan. This was done by degrading the polymer with a mannuronic acid-specific alginate lyase from abalone, followed by preparative gel filtration. Dionex chromatography of these fractions showed that both oligouronides became reasonably pure, as shown in Fig. 3A (dimer) and B (trimer), and their identities were further verified by MS (Fig.3A-1 and B-1, respectively). Note that the dimer fraction corresponds to ΔM-1H+ (mannuronic acid with one proton subtracted) with an m/z value of 351, while the trimer (ΔMM-1H+) has an m/z value of 527. These numbers fit if one assumes unsaturated nonreducing ends, which is a predicted outcome of the lyase reaction.
FIG. 3.
MS and Dionex analyses of standards and algX deletion mutant 2-2 culture supernatant. (A to C) Dionex analyses. (A-1 to C-1) MS analyses. Inserts A-1 and C-1 are MS/MS analyses of the m/z 351 ion. (A and B) Fractions from gel filtration (after lyase degradation of poly[M]). (C) Diluted broth from the culture supernatant of strain 2-2. m/z values represent the molecular weights divided by the numbers of charges after ionization in the mass spectrometer. Δ, unsaturated mannuronic acid; M, mannuronic acid; −1H+, one proton subtracted. Note that peak intensities in the Dionex analysis are proportional to the amounts of dimer and trimer (C), while this is not the case in the MS analysis (C-1).
The sample from the 2-2 isolate was then first analyzed by Dionex chromatography (Fig. 3C). There are two main peaks that correspond very well to the dimer (retention time, 4 min) and trimer (retention time, 8 min) standards, respectively. Furthermore, MS analyses of the culture supernatant from strain 2-2 also identified a major peak corresponding to the dimer and a less intense peak corresponding to the trimer (Fig. 3C-1). These analyses therefore confirmed that strain 2-2 produces oligomers similar to those recently reported for algG deletion mutants (19, 27). The MS experiments were also extended by carrying out MS/MS analyses (fragmentation) of the m/z 351 ion in the 2-2 supernatant (Fig. 3C-1) and in the dimer standard (Fig. 3A-1). The results showed that the same dominating fragment ions (m/z 157, 175, and 193) were derived from the parent ions in both cases. The ions of m/z 193 and 175 can be explained as representing products from cleavage of the glycosidic bond between the two monomers in the unsaturated dimer, but the m/z 175 ion can also be explained as the result of neutral loss of water from the m/z 193 ion. Additionally, a neutral loss of water from the m/z 175 ion will yield a fragment ion of m/z 157.
Another interesting question related to these experiments is whether epimerization takes place prior to degradation in strain 2-2. To analyze this, we prepared another standard by in vitro lyase degradation (guluronic acid-specific lyase from Klebsiella) of polyalternating alginate (MG)n, which resulted in generation of mainly unsaturated dimers containing mannuronic acid (M) and guluronic acid (G). Dionex analysis of this fraction showed that it did not overlap with any of the peaks in the 2-2 sample (not shown), indicating that epimerization does not take place in strain 2-2.
DISCUSSION
In previous studies, P. aeruginosa algX had been cloned, sequenced, and localized within the alginate biosynthetic operon (39). Although transposon mutagenesis and complementation studies strongly suggested that AlgX was necessary for alginate production, its exact function and cellular location were unknown. The objectives of this study were to determine AlgX's subcellular location and create and characterize an algX deletion mutant. Cell fractionation studies and Western blot analysis indicate that AlgX is found predominantly within the periplasm, with a small amount detected in the outer membrane fraction. Since 17% of the periplasmic marker (alkaline phosphatase) was also detected in the outer membrane fraction, this small amount of AlgX associated with the outer membrane could be due to minor cross-contamination of the outer membrane fraction with periplasmic proteins. The predominant localization of AlgX in the periplasm was consistent with a hydrophilicity plot created for AlgX (data not shown), which indicated that AlgX is predominantly a hydrophilic protein with no extensive hydrophobic domains. Amino-terminal sequencing of AlgX confirmed that the mature periplasmic form of AlgX has a 26-amino-acid signal sequence removed, resulting in a protein of 53 kDa.
We next created and characterized an algX nonpolar deletion mutant using FRD1::pJLS3. We deleted the last one-third of algX and replaced it with a nonpolar gentamicin resistance cassette. The resulting algXΔ::Gm mutants formed phenotypically nonmucoid colonies, confirming previous studies (39), which suggested that AlgX was necessary for alginate production.
To extend this information, we initially characterized an algXΔ::Gm mutant, which we called 2-2, by analyzing the cell culture supernatants for uronic acid using dialysis and the carbazole assay. A nondialyzable form of uronic acid suggests the presence of polymerized alginate, while a dialyzable form of uronic acid suggests the presence of alginate precursors or alginate lyase degradation products of alginate, as recently reported for nonpolar algG and algK deletion mutants (27). Our results indicate that the algX deletion mutant secretes short oligouronides of various sizes. In addition, thiobarbituric acid assays of culture supernatants from strain 2-2 demonstrated that these oligomers were unsaturated at the nonreducing end, indicating that they were the result of alginate lyase degradation; this is very similar to the results obtained for the algG and algK deletion mutants from P. aeruginosa (27) and an algG deletion mutant from Pseudomonas fluorescens (19). To obtain more-definitive information regarding the identity of the short oligomers secreted by 2-2, MS and Dionex analysis were performed; these studies further clarified the identity of these secreted oligomers as mainly dimers, with some trimers of mannuronic acid resulting from alginate lyase degradation. The results from the MS and Dionex analysis are consistent with the dialysis and carbazole assay studies and the thiobarbituric acid assays.
The presence of small oligomers (dimers with some trimers of mannuronic acid resulting from alginate lyase degradation) indicated that polymerization was still occurring and that the polymer was accessible to alginate lyase in this AlgX mutant and was thus degraded. Thus, AlgX appears to have a function similar to those of AlgG and AlgK in protecting the mannuronic acid polymer from alginate lyase degradation as the polymer is being shuttled within the periplasm for further modification and transported to the outer membrane for export. A model was recently proposed for alginate biosynthesis in P. fluorescens (19) in which it is hypothesized that some of the periplasmic proteins involved in alginate biosynthesis (AlgG and AlgK) form an alginate biosynthetic protein complex and protect the polymer from AlgL degradation. We speculate that AlgX may participate in a similar protein complex in P. aeruginosa, forming part of the protein scaffold that surrounds the polymer and protects it from periplasmic alginate lyase degradation. When one or more of these proteins is missing, part of the polymer becomes exposed and susceptible to lyase degradation. It is interesting that the oligouronides secreted by the algX deletion mutant did not contain any guluronic acid residues. It is uncertain if any of the dimers or trimers of mannuronic acid secreted by the algX deletion mutant were acetylated or not; there are no peaks in the MS data that suggest that the dimer or trimer was acetylated. However, we cannot exclude the possibility that the alginate oligomers were acetylated but that the bond is so weak that the acetyl groups were removed prior to MS. Perhaps, within this protein complex containing AlgG, AlgK, and AlgX, AlgX functions after polymerization but before epimerization and acetylation. AlgX may bind the mannuronic acid polymer and align it properly so that AlgG can then epimerize some of the mannuronic acid residues, followed by acetylation.
We also conducted a BLAST search on AlgX to gain further insight into the role it might play in alginate biosynthesis. The search indicated that it has 49% identity with AlgX and 29% identity with AlgV (a homolog of P. aeruginosa AlgJ) from A. vinelandii, 52% identity with Pseudomonas syringae AlgX, and 31% identity with P. aeruginosa AlgJ. A. vinelandii is a soil bacterium that produces alginate as an exopolysaccharide in vegetatively growing cells, and alginate might be involved in cyst formation (50). P. syringae, a plant pathogen, produces alginate, which permits the bacterium to avoid host plant cell recognition, protects it against desiccation, and is involved in its epiphytic fitness and virulence (31, 64). Although AlgX from A. vinelandii has not been characterized, we have cloned and sequenced algX from P. syringae and have shown that AlgX proteins from these two bacteria are interchangeable (A. Robles-Price and N. L. Schiller, unpublished results). It is unknown whether AlgX has any additional roles in alginate biosynthesis comparable to those of AlgG, which epimerizes the mannuronic acid residues to guluronic acid. AlgX's high sequence similarity with AlgJ may represent a shared domain involved in binding similar substrates. Future investigations will include experiments to determine if AlgX is in fact part of a protein complex with AlgG and AlgK within the periplasm and to identify the alginate polymer precursor that interacts with AlgX.
REFERENCES
- 1.Aasen, I. M., K. Folkvord, and D. W. Levine. 1992. Development of a process for large scale chromatographic purification of an alginate lyase from Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 37:55-60. [Google Scholar]
- 2.Bayer, A. S., D. P. Speert, S. Park, J. Tu, M. Witt, C. C. Nast, and D. C. Norman. 1991. Functional role of mucoid exopolysaccharide (alginate) in antibiotic-induced and polymorphonuclear leukocyte-mediated killing of Pseudomonas aeruginosa. Infect. Immun. 59:302-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabral, D. A., B. A. Loh, and D. P. Speert. 1987. Mucoid Pseudomonas aeruginosa resists nonopsonic phagocytosis by human neutrophils and macrophages. Pediatr. Res. 22:429-431. [DOI] [PubMed] [Google Scholar]
- 4.Campa, C., S. Holtan, N. Nilsen, T. M. Bjerkan, B. T. Stokke, and G. Skjåk-Bræk. 2004. Biochemical analysis of the processive mechanism for epimerisation of alginate by mannuronan C-5-epimerase AlgE4. Biochem. J. 381:155-164. [DOI] [PMC free article] [PubMed]
- 5.Campa, C., A. Oust, G. Skjåk-Bræk, B. S. Paulsen, S. Paoletti, B. E. Christensen, and S. and Ballance. 2004. Determination of average degree of polymerization and distribution of oligosaccharides in a partially acid-hydrolysed homopolysaccharide: A comparison of four experimental methods applied to mannuronan. J. Chromatogr. 1026:271-281. [DOI] [PubMed] [Google Scholar]
- 6.Cha, J., and D. A. Cooksey. 1991. Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc. Natl. Acad. Sci. USA 88:8915-8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, K. J., J. M. Ingram, and J. W. Costerton. 1970. Release of alkaline phosphatase from cells of Pseudomonas aeruginosa by manipulation of cation concentration and of pH. J. Bacteriol. 104:748-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chitnis, C. E., and D. E. Ohman. 1993. Genetic analysis of the alginate biosynthetic gene cluster of Pseudomonas aeruginosa shows evidence for an operonic structure. Mol. Microbiol. 8:583-590. [DOI] [PubMed] [Google Scholar]
- 9.Davis, P. B., M. Drumm, and M. W. Konstan. 1996. Cystic fibrosis. Am. J. Respir. Crit. Care Med. 154:1229-1256. [DOI] [PubMed] [Google Scholar]
- 10.Deretic, V., M. J. Schurr, J. C. Boucher, and D. W. Martin. 1994. Conversion of Pseudomonas aeruginosa to mucoidy in cystic fibrosis: environmental stress and regulation of bacterial virulence by alternative sigma factors. J. Bacteriol. 176:2773-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doig, P., N. R. Smith, T. Todd, and R. T. Irvin. 1987. Characterization of the binding of Pseudomonas aeruginosa alginate to human epithelial cells. Infect. Immun. 55:864-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans, L. R., and A. Linker. 1973. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J. Bacteriol. 116:915-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figurski, D., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin, M. J., C. E. Chitnis, P. Gacesa, A. Sonesson, D. C. White, and D. E. Ohman. 1994. Pseudomonas aeruginosa AlgG is a polymer level alginate C5-mannuronan epimerase. J. Bacteriol. 176:1821-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franklin, M. J., and D. E. Ohman. 1993. Identification of algF in the alginate biosynthetic gene cluster of Pseudomonas aeruginosa which is required for alginate acetylation. J. Bacteriol. 175:5057-5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franklin, M. J., and D. E. Ohman. 1996. Identification of algI and algJ in the Pseudomonas aeruginosa alginate biosynthetic gene cluster which are required for alginate O acetylation. J. Bacteriol. 178:2186-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gacesa, P. 1998. Bacterial alginate biosynthesis—recent progress and future prospects. Microbiology 144:1133-1143. [DOI] [PubMed] [Google Scholar]
- 18.Garen, A., and C. Levinthal. 1960. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli: purification and characterization of alkaline phosphatase. Biochim. Biophys. Acta 38:470-483. [DOI] [PubMed] [Google Scholar]
- 19.Gimmestad, M., H. Sletta, H. Ertesvåg, K. Bakkevig, S. Jain, S. Suh, G. Skjåk-Bræk, T. E. Ellingsen, D. E. Ohman, and S. Valla. 2003. The Pseudomonas fluorescens AlgG protein, but not its mannuronan C-5-epimerase activity, is needed for alginate polymer formation. J. Bacteriol. 185:3515-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg, D. M., and G. Ellis. 1983. Isocitrate dehydrogenase, p. 183-190. In H. U. Bergmeyer (ed.), Methods of enzymatic analysis, vol. 3. Verlag Chemie, Deerfield Beach, Fla. [Google Scholar]
- 21.Goldberg, J. B., K. Hatano, and G. B. Pier. 1993. Synthesis of lipopolysaccharide O side chains by Pseudomonas aeruginosa PAO1 requires the enzyme phosphomannomutase. J. Bacteriol. 175:1605-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hancock, R. E. W., and H. Nikaido. 1978. Outer membranes of gram-negative bacteria: isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J. Bacteriol. 136:381-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 24.Hatch, R. A., and N. L. Schiller. 1998. Alginate lyase promotes diffusion of aminoglycosides through the extracellular polysaccharide of mucoid Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 42:974-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Høidal, H. K., H. Ertesvåg, G. Skjåk-Bræk, B. T. Stokke, and S. Valla. 1999. The recombinant Azotobacter vinelandii mannuronan C5-epimerase AlgE4 epimerizes alginate by a non-random attack mechanism. J. Biol. Chem. 274:12316-12322. [DOI] [PubMed] [Google Scholar]
- 27.Jain, S., M. J. Franklin, H. Ertesvåg, S. Valla, and D. E. Ohman. 2003. The dual roles of AlgG in C-5-epimerization and secretion of alginate polymers in Pseudomonas aeruginosa. Mol. Microbiol. 47:1123-1133. [DOI] [PubMed] [Google Scholar]
- 28.Jain, S., and D. E. Ohman. 1998. Deletion of algK in mucoid Pseudomonas aeruginosa blocks alginate polymer formation and results in uronic acid secretion. J. Bacteriol. 180:634-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jilling, T., and K. L. Kirk. 1997. The biogenesis, traffic, and function of the cystic fibrosis transmembrane conductance regulator. Int. Rev. Cytol. 272:193-241. [DOI] [PubMed] [Google Scholar]
- 30.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 31.Keith, L. M. W., and C. L. Bender. 1999. AlgT (s22) controls alginate production and tolerance to environmental stress in Pseudomonas syringae. J. Bacteriol. 181:7167-7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerem, B., J. M. Rommens, J. A. Buchanan, D. Markiewicz, T. K. Cox, A. Chakravarti, M. Buchwald, and L. C. Tsui. 1989. Identification of the cystic fibrosis gene: genetic analysis. Science 245:1073-1080. [DOI] [PubMed] [Google Scholar]
- 33.Knutson, C. A., and A. Jeanes. 1968. A new modification of the carbazole analysis: application to heteropolysaccharides. Anal. Biochem. 24:470-481. [DOI] [PubMed] [Google Scholar]
- 34.Learn, D. B., E. P. Brestel, and S. Seetharama. 1987. Hypochlorite scavenging by Pseudomonas aeruginosa alginate. Infect. Immun. 55:1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maharaj, R., T. B. May, S. Wang, and A. M. Chakrabarty. 1993. Sequence of the alg8 and alg44 genes involved in the synthesis of alginate by Pseudomonas aeruginosa. Gene 136:267-269. [DOI] [PubMed] [Google Scholar]
- 36.Martin, D. W., M. J. Schurr, M. H. Mudd, J. R. W. Govan, B. W. Holloway, and V. Deretic. 1993. Mechanism of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 90:8377-8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathee, K., C. J. McPherson, and D. E. Ohman. 1997. Posttranslational control of the algT (algU)-encoded s22 for expression of the alginate regulon in Pseudomonas aeruginosa and localization of its antagonist proteins MucA and MucB (AlgN). J. Bacteriol. 179:3711-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCubbin, M., and R. B. Fick, Jr. 1993. Pathogenesis of Pseudomonas lung disease in cystic fibrosis, p. 189-211. In R. B. Fick, Jr. (ed.), Pseudomonas aeruginosa the opportunist: pathogenesis and disease. CRC Press, Boca Raton, Fla.
- 39.Monday, S. R., and N. L. Schiller. 1996. Alginate synthesis in Pseudomonas aeruginosa: the role of AlgL (alginate lyase) and AlgX. J. Bacteriol. 178:625-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nivens, D. E., D. E. Ohman, J. Williams, and M. J. Franklin. 2001. Role of alginate and its O acetylation in formation of Pseudomonas aeruginosa microcolonies and biofilms. J. Bacteriol. 183:1047-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohman, D. E. 1988. Experiments in gene manipulation. Prentice Hall, Inc., Englewood Cliffs, N.J.
- 42.Ohman, D. E., and A. Chakrabarty. 1981. Genetic mapping of chromosomal determinants for the production of the exopolysaccharide alginate in a Pseudomonas aeruginosa cystic fibrosis isolate. Infect. Immun. 33:142-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohman, D. E., J. B. Goldberg, J. A. L. Flynn, and S. K. Powell. 1985. Genetics of exopolysaccharide production by mucoid Pseudomonas aeruginosa. Antibiot. Chemother. 36:13-22. [DOI] [PubMed] [Google Scholar]
- 44.Ostgaard, K., S. H. Knutsen, N. Dyrset, and I. M. Aasen. 1993. Production and characterization of guluronate lyase from Klebsiella pneumoniae for applications in seaweed biotechnology. Enzyme Microb. Technol. 15:756-763. [DOI] [PubMed] [Google Scholar]
- 45.Pier, G. B. 1985. Pulmonary disease associated with Pseudomonas aeruginosa in cystic fibrosis: current status of the host-bacterium interaction. J. Infect. Dis. 151:575-580. [DOI] [PubMed] [Google Scholar]
- 46.Rehm, B. H. A., G. Boheim, J. Tommassen, and U. K. Winkler. 1994. Overexpression of algE in Escherichia coli: subcellular localization, purification, and ion channel properties. J. Bacteriol. 176:5639-5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rehm, B. H. A., and S. Valla. 1997. Bacterial alginates: biosynthesis and applications. Appl. Microbiol. Biotechnol. 48:281-288. [DOI] [PubMed] [Google Scholar]
- 48.Riordan, J. R., J. M. Rommens, B. Kerem, N. Alon, R. Rozmahel, Z. Grzelczak, J. Zielenski, S. Lok, N. Plavsic, J. L. Chou, M. L. Drumm, M. C. Iannuzzi, F. S. Collins, and L. C. Tsui. 1989. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066-1073. [DOI] [PubMed] [Google Scholar]
- 49.Rommens, J. M., M. C. Iannuzzi, B. Kerem, M. L. Drumm, G. Melmer, M. Dean, R. Rozmahel, J. L. Cole, D. Kennedy, N. Hidaka, M. Zsiga, M. Buchwald, J. R. Riordan, L. C. Tsui, and F. S. Collins. 1989. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science 245:1059-1065. [DOI] [PubMed] [Google Scholar]
- 50.Sadoff, H. L. 1975. Encystment and germination in Azotobacter vinelandii. Bacteriol. Rev. 39:516-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 52.Saxena, I. M., R. M. Brown, M. Fevre, R. A. Geremia, and B. Henrissat. 1995. Multidomain architecture of β-glycosyltransferases: implications for mechanism of action. J. Bacteriol. 177:1419-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schiller, N. L., S. R. Monday, C. M. Boyd, N. T. Keen, and D. E. Ohman. 1993. Characterization of the Pseudomonas aeruginosa alginate lyase gene (algL): cloning, sequencing, and expression in Escherichia coli. J. Bacteriol. 175:4780-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwarzmann, S., and J. R. Boring. 1971. Antiphagocytic effect of slime from a mucoid strain of Pseudomonas aeruginosa. Infect. Immun. 3:762-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schweizer, H. P., and T. T. Hoang. 1995. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 158:15-22. [DOI] [PubMed] [Google Scholar]
- 56.Shankar, S., R. W. Ye, D. Schlictman, and A. M. Chakrabarty. 1995. Exopolysaccharide alginate synthesis in Pseudomonas aeruginosa: enzymology and regulation of gene expression. Adv. Enzymol. Relat. Areas Mol. Biol. 70:221-255. [DOI] [PubMed] [Google Scholar]
- 57.Vassault, A. 1983. Lactate dehydrogenase, p. 118-126. In H. U. Bergmeyer (ed.), Methods of enzymatic analysis, vol. 3. Verlag Chemie, Deerfield Beach, Fla. [Google Scholar]
- 58.von Heijne, G. 1984. How signal sequences maintain cleavage specificity. J. Mol. Biol. 173:243-251. [DOI] [PubMed] [Google Scholar]
- 59.von Heijne, G. 1985. Signal sequences: the limits of variation. J. Mol. Biol. 184:99-105. [DOI] [PubMed] [Google Scholar]
- 60.Warner, J. O. 1992. Immunology of cystic fibrosis. Br. Med. Bull. 48:893-911. [DOI] [PubMed] [Google Scholar]
- 61.Weissbach, A., and J. Hurwitz. 1959. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli. J. Biol. Chem. 234:705-709. [PubMed] [Google Scholar]
- 62.Wood, P. M. 1978. Periplasmic location of the terminal reductase in nitrite respiration. FEBS Lett. 92:214-218. [DOI] [PubMed] [Google Scholar]
- 63.Wozniak, D. J., and D. E. Ohman. 1994. Transcriptional analysis of the Pseudomonas aeruginosa genes algR, algB, and algD reveals a hierarchy of alginate gene expression which is modulated by algT. J. Bacteriol. 176:6007-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu, J., A. Peñaloza-Vázquez, A. M. Chakrabarty, and C. L. Bender. 1999. Involvement of the exopolysaccaharide alginate in the virulence and epiphytic fitness of Pseudomonas syringae pv. syringae. Mol. Microbiol. 33:712-720. [DOI] [PubMed] [Google Scholar]
- 65.Zielinski, N. A., A. M. Chakrabarty, and A. Berry. 1991. Characterization and regulation of the Pseudomonas aeruginosa algC gene encoding phosphomannomutase. J. Biol. Chem. 266:9754-9763. [PubMed] [Google Scholar]