Abstract
Rhodospirillum rubrum possesses a putative intracellular poly(3-hydroxybutyrate) (PHB) depolymerase system consisting of a soluble PHB depolymerase, a heat-stable activator, and a 3-hydroxybutyrate dimer hydrolase (J. M. Merrick and M. Doudoroff, J. Bacteriol. 88:60-71, 1964). In this study we reinvestigated the soluble R. rubrum PHB depolymerase (PhaZ1). It turned out that PhaZ1 is a novel type of PHB depolymerase with unique properties. Purified PhaZ1 was specific for amorphous short-chain-length polyhydroxyalkanoates (PHA) such as native PHB, artificial PHB, and oligomer esters of (R)-3-hydroxybutyrate with 3 or more 3-hydroxybutyrate units. Atactic PHB, (S)-3-hydroxybutyrate oligomers, medium-chain-length PHA, and lipase substrates (triolein, tributyrin) were not hydrolyzed. The PHB depolymerase structural gene (phaZ1) was cloned. Its deduced amino acid sequence (37,704 Da) had no significant similarity to those of intracellular PHB depolymerases of Wautersia eutropha or of other PHB-accumulating bacteria. PhaZ1 was found to have strong amino acid homology with type-II catalytic domains of extracellular PHB depolymerases, and Ser42, Asp138, and His178 were identified as catalytic-triad amino acids, with Ser42 as the putative active site. Surprisingly, the first 23 amino acids of the PHB depolymerase previously assumed to be intracellular revealed features of classical signal peptides, and Edman sequencing of purified PhaZ1 confirmed the functionality of the predicted cleavage site. Extracellular PHB depolymerase activity was absent, and analysis of cell fractions unequivocally showed that PhaZ1 is a periplasm-located enzyme. The previously assumed intracellular activator/depolymerase system is unlikely to have a physiological function in PHB mobilization in vivo. A second gene, encoding the putative true intracellular PHB depolymerase (PhaZ2), was identified in the genome sequence of R. rubrum.
Polyhydroxyalkanoates (PHA) are bacterial storage compounds that can be accumulated to as much as 90% of cellular dry weight during unbalanced growth in the form of inclusion bodies (for recent reviews, see references 7 and 30). Because of their thermoplastic properties and biodegradability, PHA have attracted considerable academic and industrial interest during the last 2 decades. About 150 hydroxyalkanoic acids other than 3-hydroxybutyrate (3HB) have been identified as constituents of PHA (23, 45, 46). The most frequently occurring PHA in bacteria is poly(3-hydroxybutyrate) (PHB), which has been commercialized under the trade name BIOPOL. Recently, a novel class of biopolymers, with thioester linkages instead of oxoester bonds, has been found in Wautersia (Ralstonia) eutropha (50) and has been classified as polythioesters (27, 28).
Investigation of the biodegradation of PHA should distinguish between intracellular and extracellular degradation (for a recent review, see reference 20). Intracellular degradation is the active mobilization (hydrolysis) of the polymer by the accumulating bacterium itself. In the case of extracellular degradation, PHA is utilized by means of extracellular enzymes that are secreted by PHA-degrading microorganisms. The source of extracellular polymers is PHA released by accumulating bacteria after death. PHA in vivo and outside of the bacteria are present in two different conformations. In vivo, polymer molecules are in the amorphous “rubbery” state (highly mobile chains in a disordered conformation), and PHA granules are covered by a ≈4-nm-thick surface layer. The surface layer of isolated PHB granules consists of proteins and phospholipids (3, 26, 31, 44), which are damaged or lost upon extraction of the polymer from the cell (12, 13, 33), and the polyester chains tend to adopt an ordered helical conformation and to develop a crystalline phase. This polymer is referred to as denatured (crystalline) PHA (5, 6, 32). Extracellular PHB is a partially crystalline polymer with an amorphous fraction (glass transition temperature [Tg], ∼ 0°C) and a crystalline fraction that melts in the range of 170 to 180°C (41). In this communication, PHA in the partially crystalline form are called denatured PHA (dPHA), whereas the same polyesters in the native state (i.e., in the intracellular granules with an intact surface layer) are referred to as nPHA. The same notation is used to differentiate PHA depolymerases according to their ability to hydrolyze dPHA (dPHA depolymerases) or nPHA (nPHA depolymerases). Some dPHA depolymerases are able to hydrolyze nPHA also, but all currently known nPHA depolymerases are specific for the amorphous form of the polymer and do not hydrolyze dPHA.
Many extracellular dPHA depolymerases (EC 3.1.1.75 and EC 3.1.1.76) have been characterized at the molecular level during the past decade (for a recent summary, see reference 20), but relatively little is known about nPHA depolymerases (40). Recently, an unusual extracellular nPHB depolymerase (PhaZ7) of Paucimonas lemoignei (PhaZ7), which was specific for nPHB and was unable to hydrolyze dPHB, was described (15). For W. eutropha, the presence of several isoenzymes of intracellular nPHB depolymerases (PhaZ1 to PhaZ3) has been proposed (16, 24, 38-40, 53), and recently, a fourth (intracellular) PHB depolymerase gene was identified on the pHG1 megaplasmid of W. eutropha H16 (42). Intracellular nPHB depolymerases of W. eutropha are not related to extracellular dPHB depolymerases with respect to amino acid sequence but share significant amino acid similarities with each other and with other putative intracellular PHB depolymerases found in the databases (10, 38, 53). None of the currently known extracellular or intracellular PHB depolymerases requires any proteins as cofactors. However, Rhodospirillum rubrum appeared to be an exception. R. rubrum was the first bacterium in which degradation of nPHB granules had been intensively investigated (32): due to the high rate of in vitro self-hydrolysis of nPHB granules isolated from R. rubrum, the authors used nPHB granules isolated from Bacillus megaterium to investigate PHB hydrolysis by R. rubrum components. They found that hydrolysis of nPHB to 3HB required three components. The first component was a soluble (intracellular), heat-sensitive depolymerase that could be enriched from soluble cell extracts. However, efficient hydrolysis of nPHB granules in vitro by soluble PHB depolymerase required pretreatment of PHB granules with a heat-stable second component called the activator that was also present in soluble cell extracts. The third component was a dimer hydrolase responsible for hydrolysis of the primary degradation products of PHB (i.e., dimers and oligomers of 3HB) to 3HB. Interestingly, the action of the activator in the PHB depolymerase reaction could be replaced by mild trypsin treatment of nPHB. However, the activator was not a protease, and it activated PHB granules by a mechanism different from that of trypsin. Recently, the activator ApdA was purified (18) and its function was studied (17). It turned out that ApdA in R. rubrum in vivo is a PHB-bound molecule with all the features of a phasin (44). In this study we continued our investigation of the R. rubrum depolymerase system by analysis of the soluble PHB depolymerase.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. R. rubrum was grown photoheterotrophically in PYI medium as described recently (17, 18). Only small amounts of PHB were produced in this medium. For PHB production and isolation of PHB depolymerase, bacteria of a PYI culture were transferred (0.05 to 0.1 volumes) to mineral salts medium (MSM) supplemented with 0.16% (wt/vol) acetate (17) and incubated in the light at 29 to 30°C. Late-stationary-phase cells contained significant amounts of PHB depolymerase activity. For induction of alkaline phosphatase (AP), phosphate-buffered MSM was replaced with 100 mM Tris-HCl and yeast extract was omitted.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Source or referencea |
|---|---|---|
| Strains | ||
| B. megaterium | Source of PHB | DSMZ32 |
| B. cereus | Source of PHB | DSMZ31 |
| C. violaceum | Source of PHV, PHASCL | DSMZ30191 |
| E. coli JM109 | Cloning strain | 52 |
| E. coli DH5α | Cloning strain | 14 |
| E. coli S17-1 | Mobilizing strain | 43 |
| E. coli HMS174 | Host for phaP and/or phaCBA | 22 |
| P. oleovorans | Source of PHAMCL | ATCC 29347 |
| P. putida KT224 | Source of PHAMCL | |
| R. rubrum S1 | Wild type | DSMZ467 |
| R. rubrum SmRif | Streptomycin- and rifampin-resistant mutant of S1 | 17 |
| W. eutropha H16 | Source of PHB | DSMZ428 |
| Plasmids | ||
| pJoe890 | Stem-loop cloning vector | Gift from J. Altenbuchner, Stuttgart, Germany |
| pJoe4036 | Rhamnose-inducible vector for His-tagging | 47 |
| pECFP | ecfp | BD Clontech |
| pBBR1-MCS2 | Broad-host-range vector | 25 |
| pJM9238 | phaCBA | 22 |
| pBluescript KS(−) | Cloning vector | Stratagene |
| pBluescript KS::phaP | phaP | 22 |
| pSN2213 | pBluescript KS(−)::2.1-kbp SalI::phaZ1 | This study |
| pSN2384 | pBBR1-MCS2::phaZ1-ecfp | This study |
| pSN2388 | pBBR1-MCS2::Plac-phaZ1-ecfp | This study |
| pSN2552 | pBBR1-MCS2::phaZ1-3x-gly-ecfp | This study |
| pSN2564 | pBBR1-MCS2::Plac-phaZ1-3x-gly-ecfp | This study |
| pSN2558 | pBBR1-MCS2::phaZ1-6x-gly-ecfp | This study |
| pSN2565 | pBBR1-MCS2::Plac-phaZ1-6x-gly-ecfp | This study |
DSMZ, Deutsche Sammlung für Mikroorganismen und Zellkulturen GmbH.
Conjugation.
R. rubrum SmRif and Escherichia coli S17-1 containing the plasmid of interest were grown in PYI and Luria-Bertani medium, respectively. A mixture of 500 μl of fresh PYI medium and 250 μl each of the donor and recipient cultures was spotted onto a PYI agar plate and incubated at 30°C overnight. The bacteria were resuspended in PYI medium and diluted with PYI medium (1:100 or 1:1,000), and 100-μl portions of each dilution were plated on selection agar (PYI agar supplemented with 600 μg of streptomycin/ml, 100 μg of rifampin/ml, and 20 μg of kanamycin/ml). Red colonies of R. rubrum transconjugants appeared after incubation under air in the dark at 30°C for 1 to 2 weeks. Selected colonies were isolated by repeated transfers on selective medium and finally grown in liquid culture on PYI medium or acetate MSM.
Isolation of nPHB and dPHB.
dPHB and poly(3-hydroxyvalerate) (dPHV) were isolated from accumulating cells of W. eutropha H16 and Chromobacterium violaceum by sodium hypochlorite digestion and subsequent solvent extraction of the dried polymer with acetone-diethyl ether (2:1) (15, 21). Poly(3-hydroxyoctanoate) (PHO) and poly(3-hydroxyoctanoate-co-3-hydroxydecanoate) [P(HO-co-HD)] were isolated from Pseudomonas oleovorans and Pseudomonas putida, respectively, by chloroform extraction and repeated methanol precipitation. nPHB granules with intact surface layers were prepared from crude extracts (French press) of W. eutropha by two subsequent 50 mM Tris-HCl (pH 8)-buffered glycerol density gradient centrifugations. nPHA granules of C. violaceum, B. megaterium, Bacillus cereus, and R. rubrum cells that had accumulated PHA, and of recombinant E. coli harboring the PHB biosynthetic genes (phaCAB) with or without phaP were isolated by the same procedure. Artificial PHA granules coated with sodium dodecyl sulfate (SDS), cetyltrimethylammonium bromide (CTAB), or sodium cholate were prepared from solutions of the respective polymer in chloroform by ultrasonication with 10-fold volumes of aqueous SDS, CTAB, or cholate solutions (19). The PHA content in lyophilized cells was determined by gas chromatography after conversion of PHA into the respective methyl esters by methanolysis and with benzoate methyl ester as an internal standard.
Assay of enzyme activities.
The activity of nPHB depolymerase was assayed photometrically at 650 nm (35). The assay mixture contained 100 mM Tris-HCl (pH 9.0), 1 mM MgCl2, and nPHB granules (500 μg/ml) that had been activated by trypsin (0.6 μg/ml; SERVA, Heidelberg, Germany) for 10 min at 40°C. One unit was defined as hydrolysis of 1 μg of PHB per min. Alternatively, PHB depolymerase activity was assayed by titration of the released acid as described recently (15). For assay of 3HB oligomer hydrolysis, 2 mg of the oligomer dissolved in 0.5 ml of dichloromethane was used to coat the bottom and ≈5 mm of the inside walls of the reaction vessel by evaporation, resulting in a thin film of the oligomer. A 4-ml volume of distilled water was added, and the pH was adjusted to pH 7.5 or 9.0 by using the titristat method at 40°C as described above. One unit of PHB depolymerase activity (titristat mode) is the release of 1 μmol of acid per min. Significant spontaneous hydrolysis occurred with oligomers, and this endogenouos hydrolysis rate had to be determined for each type of oligomer separately.
NAD+-dependent malate dehydrogenase (MDH) activity was determined in 100 mM potassium phosphate buffer containing 0.23 mM NADH and 0.5 mM oxaloacetate by monitoring the oxidation of NADH at 340 nm and 30°C. AP activity was assayed by monitoring the release of p-nitrophenol from p-nitrophenyl phosphate at 410 nm in 0.9 M Tris-HCl, pH 8. One unit of activity corresponds to the release of 1 nmol of p-nitrophenol per min.
Preparation of protoplasts of R. rubrum and cell fractionation.
R. rubrum cells grown photoheterotrophically on Tris-MSM without yeast extract were harvested by centrifugation and resuspended in ice-cold 100 mM Tris-HCl (pH 7.5) containing 20% (wt/vol) sucrose, 1 mM EDTA, and 1 mg of lysozyme/ml. About 90% of the cells formed protoplasts, visible microscopically by rounding of the cells within 1 h of incubation at room temperature (RT). Protoplasts were collected by centrifugation. Crude extracts were prepared by ultrasonic treatment of R. rubrum cells before and after lysozyme treatment.
Purification of nPHB depolymerase from R. rubrum.
One hundred eighty-four grams of MSM-acetate-grown R. rubrum cells was resuspended in 20 mM potassium phosphate buffer (pH 7.0) (KPP) and disrupted by a combined cell lysis protocol (45 min at 8°C in 20 mM KPP [pH 7.0]-5 mM EDTA-20% [wt/vol] sucrose-0.2 mg of lysozyme/ml; addition of NaCl up to 156 mM; freeze-thawing at −70 and +37°C after addition of 10 volumes of KPP and sonication at 4°C). Debris was removed by centrifugation (30,000 × g). The soluble supernatant was fractionated by NH4(SO4)2 precipitation (0 to 15% [wt/vol] saturation) followed by a second precipitation step (50% saturation). The pellet was resuspended in 20 mM KPP, dialyzed against KPP, and concentrated by ultrafiltration (YM10). The concentrate (155 ml) was loaded on a DEAE-Sephacel column (bed volume, 300 ml; 1 ml/min; eluent, 20 mM KPP). A final purification step was performed by successive runs on a Mono S HR 5/5 column (bed volume, 1 ml) with 25 mM 2-(N-morpholino)ethanesulfonic acid (pH 5.9) (MES)-1 mM CaCl2 as the equilibration buffer. After removal of contaminants by an initial linear gradient from 0 to 12 mM NaCl, PhaZ1 was eluted isocratically at 12 mM NaCl in equilibration buffer (0.4 ml/min).
MS.
Matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectrometry (MS) analysis was performed on a GSGfuture MS with time lag focusing and a 337-nm UV laser (GSG Mess- und Analysengeräte Vertriebsgesellschaft mbH). Desalted PhaZ1 samples (1 μl = 0.36 μg) or peptides generated by protease cleavage were embedded in 1 volume of a mixture of 3,5-dimethoxy-4-hydroxycinnamic acid and acetonitrile-0.1% (wt/vol) trifluoroacetic acid (TFA) (40:60, vol/vol) and dried at 21°C. The system was calibrated with carbonic anhydrase B (28,980 Da). Measurement was performed at 20 kV.
Glycosylation assay.
A digoxigenin (DIG) Glycan Detection kit immunoassay (Boehringer Mannheim) was used for qualitative detection of sugars in glycoconjugates. Assays were performed as described by the manufacturer except for the protein immobilization step on polyvinylidene difluoride membranes that was performed before oxidation of sugars. Creatinase was used as a negative control. PhaZ2 and PhaZ5 (both P. lemoignei) and transferrin served as positive controls.
Generation of internal peptides of PhaZ1 by digestion with Lys-C and trypsin.
One hundred fifty micrograms of purified PhaZ1 was desalted on a YM10 membrane and concentrated by lyophilization. The protein was digested by endoproteinase Lys-C (sequencing grade; Sigma-Aldrich) for 12 h at 37°C. The assay mixture for reduction (12 h, 45°C) contained 25 μl of urea (8 M in 0.4 M NH4HCO3 purified by an Amberlite MB ion exchanger) and 5 μl of dithiothreitol (DTT; 45 mM). A 5-μl volume of 100 mM iodoacetamide was added for alkylation (15 min). After dilution with Tris-HCl (pH 8.5)-1 mM EDTA (to 115 μl), 16.5 μl of acetonitrile was added. Digestion was started by addition of 50 μl (2.5 μg) of Lys-C. PhaZ1 was digested completely within 12 h. For digestion with trypsin, 125 μg of protein was separated by SDS-14% (wt/vol) polyacrylamide gel electrophoresis (PAGE) and visualized by negative staining with zinc (9). The 35-kDa band of interest was cut out, homogenized, destained by repetitive washing in 25 mM Tris-100 mM glycine-100 mM DTT (pH 8.0), and incubated twice, for 15 min each time, in NH4HCO3 at RT, followed by 30 min of incubation at 60°C in 350 μl of urea (8 M in 0.4 M NH4HCO3) and 70 μl of DTT (45 mM). A 70-μl volume of 100 mM iodoacetamide was added at RT for 15 min. After 20 min in 50% (vol/vol) acetonitrile-100 mM NH4HCO3, the gel was shrunk in 150 μl of acetonitrile. The vacuum-dried matrix was swollen for 15 min in 50 μl of digestion mixture (25 mM NH4HCO3, 12.5 μg of trypsin), and 25 mM NH4HCO3 was added to 300 μl. PhaZ1 was digested for 12 h at 37°C, and the peptides generated were eluted by shrinking (addition of 0.1% [wt/vol] TFA-acetonitrile up to a final acetonitrile concentration of 60% and sonication). All fragments generated were separated by high-performance liquid chromatography (HPLC) using a Pharmacia LKB μ-Separation unit with an RP C18 Hypersil ODS column (150 by 2.1 mm; eluent A, 0.1% [wt/vol] TFA in water; eluent B, 0.085% [wt/vol] TFA in acetonitrile; two-step linear gradient from 7 to 50% [vol/vol] B within 105 min and 50 to 100% B within 20 min at 0.1 ml/min). Peaks containing internal fragments of protease recognized by MALDI-TOF analysis were discarded. Remaining peaks or peptide fractions were subjected directly to N-terminal amino acid sequencing.
HPLC analysis.
Normal-phase HPLC as described in reference 2 was used to examine the products of ester hydrolysis by nPHA depolymerase. In brief, the samples were titrated to pH 7.0, and after addition of 1 volume of ice-cold acetone, the mixture was frozen immediately at −70°C. Samples were lyophilized, resolved in 5 ml of CH2Cl2-0.1% (vol/vol) acetic acid, and concentrated 10-fold by evaporation. Methylation was performed by adding diethyl ether-diazomethane. After evaporation of the solvent at RT, methyl esters were diluted in 100 μl of dichloromethane and separated on a Lichrosorb Si60 column (250 by 4 mm; inner diameter, 7 μm; Chrompack/VARIAN, Darmstadt, Germany) by isocratic elution with n-hexane-2-propanol (96:4, vol/vol) at 3 ml/min. UV peaks were identified by comparison with reference esters kindly provided by P. Waser and B. Bachmann from D. Seebach's lab (ETH Zürich).
Cloning of the nPHB depolymerase structural gene phaZ1.
DNA was manipulated by standard procedures. Chromosomal DNA of R. rubrum (461 bp) was PCR amplified by using degenerate oligonucleotides (5′-CTNGCNATYGAYGCIGAYGAYGT and 5′-GTNAGRAANGCRTGNCCIGCYTT) derived from internal peptide fragments (LAIDADHV, AGHAFLT) of PhaZ1, and the PCR product was used as a probe for cloning a 2.1-kbp genomic SalI DNA fragment by colony hybridization of a genomic library of R. rubrum DNA in E. coli DH5α. The 2.1-kbp SalI fragment was subcloned into pBluescript KS(−), yielding pSN2113, and its DNA was sequenced.
Nucleotide sequence accession number.
The sequence of phaZ1 has been deposited in GenBank under accession no. AY061637.
RESULTS
Purification and biochemical characterization of the soluble PHB depolymerase from R. rubrum.
R. rubrum was grown photoheterotrophically at 30°C on PYI medium and on MSM supplemented with acetate (0.16%) or succinate (0.2%). Low doubling times (td, 4.2 h) and high cell densities (optical density at 600 nm, ≈10) were obtained on PYI medium, but PHB depolymerase activity on PYI medium was poor regardless of the time point of sampling. The use of MSM with acetate or succinate resulted in significantly slower growth of R. rubrum (td, 8.5 h [acetate] or 7.8 h [succinate]) and a lower cell yield (optical density, ≈1.5 to 1.9), but PHB depolymerase activity was high. A specific PHB depolymerase activity of 200 to 300 U/mg was obtained for soluble extracts of acetate-grown cells from early-stationary phase. Soluble PHB depolymerase activity was purified from 184 g of cells as described in Materials and Methods. About 1 mg of purified PHB depolymerase (PhaZ1) with a specific activity of 74,000 U/mg (13% yield, 430-fold purification) was obtained. SDS-PAGE revealed that isolated PhaZ1 was electrophoretically pure (apparent molecular mass, 35 ± 3 kDa) even after the gel was overloaded and the sensitive silver-staining method was used (data not shown). The protonated depolymerase was determined by MALDI-TOF MS to have a molecular mass of 35,247 ± 85 Da, close to the value determined by SDS-PAGE analysis.
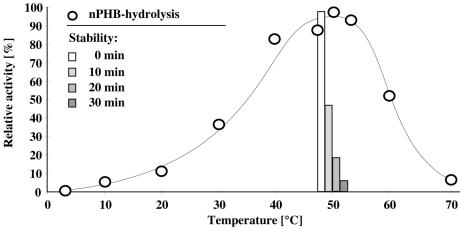
Purified PhaZ1 was stained for glycoproteins. As controls, the purified extracellular PHB depolymerases PhaZ2 and PhaZ5 (both positive) as well as PhaZ7 (negative) (all from P. lemoignei [4, 15]), transferrin (positive), and creatinase (negative) were used. The controls reacted as expected, but no evidence for the presence of carbohydrates was obtained for the R. rubrum PHB depolymerase (data not shown). An isoelectric point of 9.5 ± 0.5 was determined for PhaZ1 by isoelectric focusing. The temperature optimum of purified PhaZ1 was 50°C. At 50°C or above, PhaZ1 was very unstable and rapidly lost its activity (Fig. 1). PhaZ1 was partially stable at RT but also lost about 50% of its activity within 12 h of incubation in potassium phosphate buffer (pH 7). During storage on ice, the enzyme lost 25% activity within 24 h. Addition of ammonium sulfate, sucrose, or glycerol (5% each) partially stabilized the enzyme. For long-term storage, freezing at −20 or −70°C was necessary. The pH optimum of PhaZ1 was determined in succinate-NaOH (pH 3.5 to 5.0), potassium phosphate (pH 5.0 to 7.5), Tris-HCl (pH 7.5 to 10), and glycine-NaOH buffer (pH 10.0 to 12.0). The highest activity was obtained in Tris-HCl at pH 9.0 ± 0.5 (data not shown). PHB depolymerase activity was partially dependent on the presence of divalent cations (Table 2): CaCl2 or MgCl2 increased the activity to 130 or 140% at 1 mM, respectively, but decreased the activity at 5 mM (both to 75%) or at 10 mM (to 40 or 35%, respectively). EDTA partially inhibited PhaZ1, but a 5 mM concentration was necessary to obtain more than 50% inhibition. However, even 20 mM EDTA did not completely inhibit PHB depolymerase activity (80% inhibition). Monovalent cations such as Na+ or K+ (1 to 10 mM) slightly increased activity up to 140%. At higher concentrations (100 mM), an inhibitory effect (75 to 85% residual activity) was found for NaCl and KCl.
FIG. 1.
Influence of temperature on activity and stability of purified soluble PHB depolymerase. Purified PhaZ1 was assayed at different temperatures (circles). For determination of temperature stability, purified PhaZ1 (0.6 μg) was incubated in assay buffer at 50°C for the times indicated before residual activity was determined (columns).
TABLE 2.
Inhibition of PhaZ1-dependent nPHB hydrolysis by detergents, specific hydrolase inhibitors, and redox active agents, and influence of ions
| Agenta | Relative activityb (%) at the following concn: | |||
|---|---|---|---|---|
| Detergentsc | 0.0001% | 0.001% | 0.05% | 0.1% |
| Tween 20 | 72 | 6 | 10 | 3 |
| Tween 80 | 57 | 5 | 4 | 3 |
| Triton X-100 | 80 | 55 | 3 | 3 |
| SDS | 98 | 89 | 45 | 2 |
| Ions | 1 mM | 5 mM | 10 mM | 100 mM |
| MgCl2 | 140 | 75 | 40 | NP |
| CaCl2 | 130 | 75 | 35 | NP |
| NaCl | — | 140 | 130 | 87 |
| KCl | — | 140 | 130 | 75 |
| Chelator | 1 mM | 5 mM | 10 mM | 20 mM |
| EDTA | 79 | 48 | 24 | 21 |
| EDTA + CaCl2 (2.5 mM) | 88 | — | — | — |
| EDTA + MgCl2 (2.5 mM) | 86 | — | — | — |
| Serine hydrolase inhibitors | 0.01 mM | 0.1 mM | 1 mM | 10 mM |
| DFP | 49 | 10 | 9 | — |
| PMSF (corrected by effect of 2-propanol) | >99 | — | 94 | 89 |
| DDSC (corrected by effect of 2-propanol) | >99 | — | 98 | 98 |
| 2-propanol (vol/vol) | 91 | 86 | 62 | 46 |
| Redox active agents | 1 mM | 4 mM | ||
| 2-Mercaptoethanol | 14 | 11 | ||
| DTE | 16 | 6 | ||
| DTT | 20 | 0 | ||
| DTNB | 96 | 92 | ||
| DTT + DTNB (equimolar) | 37 | — | ||
DTE, 1,4-dithioerythritol; DTNB, dithionitrobenzoate.
—, the value has not been determined; NP, precipitation was observed, but determination of activity was not possible.
For Tween 20, Tween 80, and Triton X-100, concentrations are expressed in percentages (volume/volume); for SDS, concentrations are expressed as percentages (weight/volume).
Substrate specificity of PhaZ1.
Purified R. rubrum PHB depolymerase was specific for native granules of short-chain-length PHA (nPHASCL) such as nPHB or nPHV regardless of their bacterial origin (Table 3). Even nPHB granules isolated from recombinant E. coli harboring the PHB biosynthetic genes of W. eutropha with or without the phasin PhaP were a suitable substrate for PhaZ1. However, it was strictly necessary to activate the granules either with ApdA (17, 18) or with trypsin before the depolymerase reaction was started. Without this activation, only a reduced rate of hydrolysis, varying with different batches of nPHB granules from 5 to 20% of the rate obtained after activation, was obtained. Medium-chain-length PHA (PHAMCL) such as PHO were not hydrolyzed. Denatured (paracrystalline) PHA were not a substrate of the R. rubrum depolymerase regardless of whether PHASCL or PHAMCL were used and regardless of whether ApdA or trypsin had been added or not. Apparently, PhaZ1 is specific for amorphous PHASCL. When artificial (amorphous) PHA granules, in which the in vivo proteinaceous or membranous surface layer has been replaced by surfactants (SDS, cholate, and CTAB were tested), were used as a substrate, only those PHASCL that were coated with negatively charged surfactants (SDS or cholate) were hydrolyzed. CTAB-coated artificial PHB granules were not hydrolyzed. Interestingly, artificial granules did not require activation by ApdA or by trypsin, indicating that the proteins of the surface layer of nPHB are the target of the in vitro activation process (17, 18, 34). Other potential substrates such as poly(6-hydroxyhexanoate), lipase substrates (triolein, tributyrin, olive oil), casein, Cucurbita cutin, or p-nitrophenyl fatty acids with various chain lengths of the fatty acid moiety were not hydrolyzed significantly (Table 3). Atactic PHB, which is an amorphous random copolymer of (S)- and (R)-3HB, was not hydrolyzed even if the highly sensitive titristat method was used.
TABLE 3.
Substrate specificity of purified PHB depolymerase PhaZ1
| Substrate | Activitya
|
||
|---|---|---|---|
| Without pretreatment | With pretreatment
|
||
| ApdA | Trypsin/ovomucoid | ||
| nPHA granules isolated from: | |||
| R. eutropha H16 | 660b | 2,800b | 3,100b |
| E. coli (phaCAB + phaP) | 104b | 560b | 1,600b |
| E. coli (phaCAB) | 95b | 490b,d | 1,900b |
| B. megaterium | − | + | ++ |
| B. cereus | − | + | ++ |
| nPHV granules from C. violaceum | − | + | ++ |
| nPHO granules from P. oleovorans with or without 1 % (wt/vol) SDS | <0.01b | <0.01b | <0.01b |
| Atactic PHB [poly(R-, S-3-HB)] | <0.01b | <0.01b | <0.01b |
| dPHA (isotactic, paracrystalline): PHB, PHV, PHO, P(3HO-3HD) | <0.01b | <0.01b | <0.01b |
| Artificial (amorphous) PHA | |||
| PHB coated with SDS or sodium cholate | ++ (Cholate) | ++ (Cholate) | + (Cholate) |
| + (SDS) | + (SDS) | + (SDS) | |
| PHV coated with SDS or sodium cholate | + (Cholate) | + (Cholate) | + (Cholate) |
| + (SDS) | + (SDS) | + (SDS) | |
| PHO-latex; P(HO-co-HD)-latex | <0.01b | ||
| PHB, PHV coated with CTAB | <1b | <5b | <1b |
| Other hydrolase substrates: | |||
| Poly(6-hydroxyhexanoate) | <0.01b | <0.01b | |
| Triolein, tributyrin, olive oil | <0.01b | <0.01b | |
| Casein; N-α-benzoyl-l-arginine-4-nitranilide | − | − | |
| Cutin (cucumber)b (80-h incubation time) | <0.01b | <0.01b | <0.01b |
| p-Nitrophenyl estersc | <0.01c | <0.01c | |
Values are units per milligram. Unless otherwise noted, turbidimetric assays were performed; 1 U mg−1 = 1 μg of polymer min−1 mg−1 Symbols:-, <1 U/mg; +, >5 U/mg; ++, >100 U/mg.
Titration was performed; 1 U mg−1 = 1 μmol of acid min−1 mg−1.
Turbidimetric assays (with and without detergents) were performed; values are micromoles per minute per milligram. p-Nitrophenyl ester substrates are as follows: p- nitrophenyl acetate, p-nitrophenyl butyrate, p-nitrophenyl hexanoate (p-nitrophenyl caproate), p-nitrophenyl octanoate (p-nitrophenyl caprylate), p-nitrophenyl decanoate, p- nitrophenyl hexadecanoate (p-nitrophenyl palmitate).
A lag phase was observed (18).
In order to study the substrate specificity of PhaZ1 with respect to the spatial configuration of the ester linkages, oligomers with different compositions of (S)-3HB and (R)-3HB were used (Table 4). Oligomers and atactic PHB were gifts from D. Seebach's lab (Zürich, Switzerland) and M. Scandola's lab (Bologna, Italy), respectively. The reaction was assayed by the titristat method, and the end products were analyzed by HPLC. Oligomers that consisted only of (S)-3HB units or isotactic oligomers {[(R)-3HB-(S)-3HB]4} were not hydrolyzed at all. Oligomers of (R)-3HB with 4 to 8 3HB units were hydrolyzed at similar rates (1,400 to 2,000 U/mg) to a mixture of dimers and trimers. Monomers and tetramers were also identified as products, but only in trace amounts. The (R)-3HB trimer was hydrolyzed at a significantly lower rate than higher oligomers (340 U/mg) to an equimolar mixture of the dimer and 3HB. The (R)-3HB dimer was not a substrate of PhaZ1. 3HB octamers consisting of three (S)-3HB and 5 adjacent (R)-3HB units were hydrolyzed to a mixture of dimers, trimers, and tetramers, suggesting that only (R)-(R) linkages were hydrolyzed. When cyclic oligomers [(R)-3HB pentamers and hexamers] and end-protected linear oligomers were used, hydrolysis rates comparable to those of the unprotected linear counterparts were obtained (Table 4). We conclude that R. rubrum PHB depolymerase is highly specific for oligomers or polymers of 3HB in the (R) configuration and has significant endohydrolase activity.
TABLE 4.
Hydrolysis of 3HB oligomers by PhaZ1
| Oligomersa | Ab | Bc | Product spectra (HPLC)d |
|---|---|---|---|
| 3HB oligomers | |||
| R2 | 0 | <0.01 | No products of hydrolysis detectable |
| R3 | 0.7 | 340 | Monomer, dimer |
| R4 | 1.1 | 1,430 | Dimer (traces: monomer and trimer) |
| R5 | 1.3 | 1,970 | Dimer, trimer (traces: monomer) |
| R6 | 1.8 | 1,220 | Dimer, trimer, (traces: tetramer) |
| R8 | 3.1 | 930 | Dimer, trimer (traces: monomer) |
| R4S4 | + | Monomer, dimer, trimer, pentamer | |
| S4R4 | ++ | Monomer, dimer, pentamer | |
| R5S3 | ++ | Dimer, trimer, tetramer (traces: monomer) | |
| S3R5 | ++ | Dimer, trimer, tetramer (traces: monomer, pentamer) | |
| R6S2 | ++ | Dimer, trimer (traces: monomer, tetramer) | |
| S2R6 | ++ | Dimer, trimer, tetramer (traces: monomer,) | |
| R7S1 | ++ | Dimer, trimer (traces: monomer, tetramer) | |
| S1R7 | ++ | Dimer, trimer, tetramer (traces: monomer) | |
| [RS]4 | <0.01 | No products of hydrolysis detectable | |
| S4 | <0.01 | No products of hydrolysis detectable | |
| Bn-R4-t-butyl (protected) | 1,390 | ND | |
| Bn-S4-t-butyl (protected) | <0.01 | ND | |
| Cyclic oligomers (oligolides) | |||
| oR1S3 | <0.01 | No products of hydrolysis detectable | |
| oR5 | 2,300 | Dimer, trimer | |
| oR6 | 1,840 | Dimer, trimer (traces: monomer, tetramer) | |
| nPHB (trypsin activated) | 3,100 | Monomer, dimer, trimer |
R8, (R)-3HB octamer; R4S4, 3HB octamer consisting of [(R)-3HB]4 at the hydroxy terminus and [(S)-3HB]4 at the carboxy terminus; [RS]4, isotactic 3HB octamer consisting of four (R)-3HB-(S)-3HB building blocks; oR6, cyclic (R)-3HB hexamer; Bn, benzoyl.
Equivalents of ester bonds cleaved within 24 h. After 2 h of continuous titration in a titristat, acid released was neutralized at 5-h intervals, accompanied by addition of fresh PhaZ1 in H2O.
Specific activity corrected by spontaneous hydrolysis of the substrate. Values are in units per milligram; 1 U mg−1 = 1 μmol of acid min−1 mg of protein−1. Symbols represent qualitative results derived from end point titration: ++, >1,000 U/mg; +, >100 U/mg.
ND, not determined.
Inhibitors.
Phenylmethylsulfonyl fluoride (PMSF) and dodecanesulfonyl chloride (DDSC) had hardly any inhibitory effect, even at 10 mM (Table 2). However, diisopropylfluorophosphate (DFP) almost completely inhibited PHB depolymerase activity at 1 mM, suggesting that PhaZ1 is a serine hydrolase. Variation of the inhibitor concentration showed a Ki of 0.1 mM for DFP (data not shown). Reducing agents such as DTT or 2-mercaptoethanol (4 mM) strongly inhibited the depolymerase reaction. Reoxidation by addition of dithionitrobenzoate in at least equimolar concentration to the inhibitor partially restored the activity and indicated the presence of essential disulfide bonds in PhaZ1. Detergents such as Tween, Triton, or SDS almost completely inhibited the reaction at 0.1%. Other potential inhibitors such as KCN (10 mM), NaN3 (10 mM), or p-mercuribenzoate (4 mM) did not significantly influence the depolymerase reaction (degree of inhibition, <10%) (data not shown).
Partial amino acid sequencing of purified PHB depolymerase and cloning of the PHB depolymerase structural gene.
Purified PHB depolymerase was digested with trypsin or Lys-C, and the peptide fragments generated were separated by HPLC analysis. Six peptide sequences and the N-terminal amino acid sequence of the untreated PHB depolymerase were determined by Edman sequencing. In total, 95 amino acids could be identified (Fig. 2). Two peptide sequences were used for generation of specific oligonucleotides. A DNA fragment of about 460 bp could be amplified by using the oligonucleotides as primers and chromosomal DNA as a template. This DNA fragment was used as a homologous DNA probe to clone a 2,112-kbp SalI chromosomal DNA fragment harboring the complete PHB depolymerase structural gene phaZ1. Expression of phaZ1 in E. coli led to significant growth inhibition and increased polysaccharide production by the cells. The DNA sequence of the 2,112-bp SalI fragment was determined for both DNA strands.
FIG. 2.
Amino acid sequence of soluble PHB depolymerase of R. rubrum PhaZ1 and alignment with extracellular PHB depolymerases harboring catalytic type II domains. PhaZAspTP4, Acidovorax sp. (accession no. AB015309.1); PhaZDac, Delftia acidovorans (accession no. AB003186); PhaZCsp, Comamonas sp. (accession no. AAA87070); PhaZCte, Comamonas testosteroni (accession no. AB000508); PhaZLspHS, Lepthotrix sp. strain HS (Caldimonas manganoxidans) (accession no. AB038647); PhaZSex, Streptomyces exfoliatus (accession no. U58990); PhaZRr, Rhodospirillum rubrum (accession no. AY061637). Amino acids that have been determined by Edman degradation of purified PhaZ1 or isolated peptides of PhaZ1 of R. rubrum are underlined. Alignment was performed with Clustalw 1.7 (gonnet series [49]). Increasing darkness of shading indicates increasing degrees of identity among PHB depolymerases at the respective position. Boldfaced amino acids indicate the putative catalytic triad and the oxyanion pocket amino acids. Asterisks, hydrophobic residues. Values in the consensus line represent phylogenetic tree scores for conserved residues; a lower value is better (i.e., there is less evolutionary cost).
Characterization of the PHB depolymerase gene (phaZ1) and of its deduced gene product.
The 2,112-bp DNA fragment (G+C content, 61.7%) contained one large open reading frame of 1,089 bp including a potential ribosome binding site (GGAGGA) 8 bases ahead of the ATG start codon. The deduced amino acid sequence of the open reading frame contained all amino acid sequences of PhaZ1 that had been determined by Edman degradation of isolated peptide fragments, confirming that the PHB depolymerase gene (phaZ1) of R. rubrum had been cloned (Fig. 2). Surprisingly, the Edman degradation-determined sequence of the N terminus of PhaZ1 was identical to Asp24 to Val53 of the gene-deduced amino acid sequence. Indeed, the first 23 amino acids of the DNA-deduced sequence revealed features of classical signal peptides, and a signal peptidase cleavage site was predicted between Ala23 and Asp24 by use of the SignalP algorithm (36). BLAST analysis revealed no significant amino acid homology of PhaZ1 to intracellular PHB depolymerases of W. eutropha or to other (putative) intracellular PHB depolymerases. However, significant homologies (30 to 35% identity) to extracellular PHB depolymerases of different bacteria were found (Fig. 2 and 3). The homology was restricted to the type-II catalytic domain of classical PHB depolymerases (20). No evidence for the presence of a linker domain or a substrate-binding domain, which are located C-terminal of the catalytic domain of classical PHB depolymerases, was obtained for PhaZ1. These results suggest that the cloned gene did not encode an intracellular but, more likely, a new type of extracellular PHB depolymerase. Comparison of the amino acid sequence of PhaZ1 with those of other extracellular PHB depolymerases showed that regions around amino acids that had been identified as catalytic active amino acids of extracellular PHA depolymerases were highly conserved in extracellular PHA depolymerases and also appeared in R. rubrum PhaZ1 (Fig. 2) (20). These regions comprise the PHB depolymerase (lipase) box [G(I/L)S19/42(S/A)G] (subscript numbers give numbering for the mature protein and the preprotein, respectively), the aspartate box (GxxD115/138xxV) (where x is an unknown amino acid), the histidine (H155/178), and the oxyanion pocket (H250/273GC).
FIG. 3.
Domain structure of PHASCL depolymerases. A schematic model of PHASCL depolymerases with different combinations of the catalytic domain, linker domain, and substrate-binding domain is shown. Abbreviations: Thr, threonine-rich region; Fn3, fibronectin type 3-like domain; Cad, cadherin-like domain; SBD, substrate-binding domain.
Localization of PHB depolymerase in the periplasm.
The unexpected finding of a functional signal peptide in R. rubrum PHB depolymerase prompted us to reinvestigate the subcellular localization of the enzyme. The R. rubrum PHB depolymerase had been described as an intracellular protein in the publication by Merrick and Doudoroff in 1964 (32). An assay of cell-free culture fluid from R. rubrum cells (grown photoheterotrophically on acetate-MSM or PYI medium and sampled at various phases of growth) for PHB depolymerase activity did not result in detection of any extracellular depolymerase activity, even if the culture fluid was concentrated 10-fold by ultrafiltration. In order to test for the presence of cell wall-associated activity, the assay was repeated with noncentrifuged R. rubrum cultures of different cell densities. No PHB depolymerase activity was detected. Controls in which purified PhaZ1 had been added to a culture of R. rubrum showed that PHB depolymerase activity can be detected principally in the presence of the cells. We concluded that PhaZ1 is not an extracellular enzyme in R. rubrum.
Attempts to obtain periplasmic extracts of R. rubrum by osmotic shock procedures without significant cell lysis were not successful (data not shown). Treatment of the cells with chloroform, a method that has been described as an easy and quick way to obtain periplasmic proteins (1), also did not work well with R. rubrum (data not shown). However, when washed R. rubrum cells were incubated at RT with 1 mg of lysozyme/ml in the presence of 1 mM EDTA and 5 to 20% sucrose, rapid rounding of the cells was observed by light microscopy within 60 min. We concluded that lysozyme-EDTA treatment in the presence of sucrose is a suitable method for solubilizing the cell wall and releasing the proteins of the periplasm. In order to identify the cell fraction in which PHB depolymerase was present, we analyzed cell-free culture fluid, the supernatant obtained after lysozyme-EDTA treatment (periplasmic fraction), and an ultrasonic extract of the remaining protoplasts for PHB depolymerase activity. MDH and AP were assayed as marker enzymes for the intracellular and periplasmic cell fractions, respectively. As shown in Table 5, about 90% of PHB depolymerase activity was found in the periplasmic fraction. The correctness of cell fractionation was confirmed by the presence of high levels of AP only in the supernatants of lysozyme-EDTA-treated cells and high levels of MDH activity only in the cell extracts of the remaining protoplasts. We concluded that PHB depolymerase is localized in the periplasm in vivo.
TABLE 5.
Distribution of PHB depolymerase (PhaZ1), MDH, and AP activities in cell fractions of R. rubrum
| Fraction | Activity (U [%]) of:
|
||
|---|---|---|---|
| PhaZ1 | MDH | AP | |
| Cell-free culture supernatant | <50 (<3) | 5.5 (5) | <35 (<3) |
| Supernatant of lysozyme-EDTA treatment | 1,600 (88) | 24 (20) | 1,100 (93) |
| Ultrasonic extract of pellet after lysozyme-EDTA treatment | 210 (12) | 88 (75) | 78 (7) |
DISCUSSION
Intracellular PHB depolymerases are necessary for mobilizing PHB, which has been accumulated during times of carbon excess, by converting the insoluble polymer into water-soluble products (3HB or 3HB oligomers) during times of starvation. Most PHB-accumulating bacteria, e.g., Paracoccus denitrificans (10) or W. eutropha (16, 24, 38, 53), have PHB depolymerases that apparently are bound to or associated with nPHB granules in vivo, and no activity, or only poor activity, can be detected in soluble cell extracts of W. eutropha in vitro (39). Isolated nPHB granules of W. eutropha, for example, show a slow but significant rate of self-hydrolysis and release 3HB if incubated in buffer at 30°C (16). This endogenous hydrolysis rate cannot be enhanced significantly if soluble cell extracts of W. eutropha are added. However, the same isolated PHB granules can be hydrolyzed easily if soluble cell extracts of R. rubrum are added (18, 32, 34). The rate of hydrolysis by R. rubrum extracts in vitro is about 2 orders of magnitude higher than the self-hydrolysis rate of isolated PHB granules with nothing added. Therefore, soluble PHB depolymerase of R. rubrum appeared to be a suitable model with which to investigate intracellular PHB degradation. Efficient in vitro hydrolysis of isolated W. eutropha nPHB granules by R. rubrum depolymerase requires pretreatment of the granules with an R. rubrum activator (ApdA) or with trypsin (32, 34). In a recent study, we found that in vivo, ApdA is a PHB-bound molecule that has all the features of a phasin and is similar to PhaP of W. eutropha (17, 18). The ability of ApdA to bind to PhaP-containing W. eutropha nPHB granules in vitro presumably disturbs the membranous or proteinaceous surface layer of nPHB in such a way that PHB molecules become partially exposed and PHB depolymerase can bind to the polymer surface. Treatment of nPHB granules with small amounts of trypsin partially removes phasins such as PhaP, thus leading to an effect similar to that of ApdA (i.e., rapid hydrolysis of nPHB by PHB depolymerase), although the mechanisms by which trypsin and ApdA activate PHB granules are clearly different from each other (17, 18).
In this study we purified and characterized the soluble nPHB depolymerase and its structural gene, phaZ1, from R. rubrum. The enzyme had properties similar to those of PhaZ7, the extracellular nPHB depolymerase of P. lemoignei (15), except that PhaZ7 does not require activation of nPHB in vitro. R. rubrum PHB depolymerase is related to extracellular dPHB depolymerases with respect to amino acid sequence and putative catalytic-triad amino acids (Ser19/42, Asp115/138, His155/178, His250/273 [oxyanion] [subscript numbers give numbering for the mature protein and the preprotein, respectively]) (Fig. 2). However, PhaZ1 differed from dPHB depolymerases by its inability to hydrolyze dPHA, by the absence of a linking domain and a substrate-binding-domain, and by its low stability. (Fig. 1 and 3) (20). On the other hand, R. rubrum PHB depolymerase is not related to intracellular PHB depolymerases of W. eutropha and P. denitrificans in terms of amino acid homology (10, 16, 24, 38, 40, 53). Therefore, R. rubrum PHB depolymerase represents a novel type of PHB depolymerase.
The most surprising and unexpected result was the identification of a functional signal peptide in the putative intracellular PHB depolymerase of R. rubrum. However, all attempts to detect any extracellular PHB depolymerase activity failed. In order to obtain direct evidence for the periplasmic localization of PhaZ1, we developed a procedure for preparation of protoplasts of R. rubrum; detection of PHB depolymerase activity and the distribution of MDH (an intracellular marker) and AP (a marker for periplasm) activities clearly showed that R. rubrum PhaZ1 is localized in the periplasm. Additional experiments, in which we constructed and expressed three individual gene fusions of phaZ1 with enhanced cyan fluorescent protein (phaZ1-ecfp)—-each fusion differing from the others only in the number of glycine-encoding spacers (none, three, or six) between phaZ1 and ecfp—showed no fluorescence either in the cytoplasm or at the surfaces of PHB granules (unpublished data). Since the same constructs were expressed in recombinant E. coli and showed PHB depolymerase activity, the nonfluorescent phenotype of R. rubrum transconjugants harboring the phaZ1-ecfp fusions is unlikely to be caused by spatial hindrance of the two domains. The results show that PhaZ1 apparently is not localized in the cytoplasm or at the surfaces of PHB granules. Since it is known that green fluorescent protein needs a reduced environment and cannot fold correctly in the periplasm (8, 48), the absence of fluorescence in R. rubrum harboring the phaZ1-ecfp fusions is consistent with a localization of PhaZ1 outside of the cytoplasmic membrane.
If PhaZ1 is a periplasm-located enzyme and if PHB is an intracellularly accumulated storage polymer, what could be the function of a periplasm-located depolymerase? PhaZ1 has a very high specific activity with nPHB and oligomers of 3HB, and efforts to find other substrates failed (Table 3). Therefore, PHB and/or 3HB oligomers presumably are the physiological substrates of the enzyme. Since PhaZ1 cannot hydrolyze dPHB and since PhaZ1 is not secreted into the environment under any of the culture conditions tested, an extracellular function of PhaZ1 is unlikely. This conclusion is supported by the low stability of the enzyme. Storage at RT or on ice for 12 h resulted in 50 or 25% loss of activity, respectively. True extracellular PHB depolymerases are stable under such conditions (20). Even PhaZ7, the extracellular nPHB depolymerase of P. lemoignei, which is related to PhaZ1 of R. rubrum by the same specificity for amorphous PHB and also lacks linker and substrate binding domains, is very stable to physical and chemical stresses (15).
R. rubrum possesses stacks of invaginated intracellular cytoplasmic membranes during phototrophic growth (11, 29, 51). Therefore, the membrane surface and the volume of the periplasmic space are highly enlarged in these bacteria. One can speculate that such stacks of membranes might rearrange or fuse (e.g., in stationary phase) so that a portion of the periplasmic space and its proteins becomes intracellular. However, there is no experimental evidence for this assumption, and it is difficult to consider such a rearrangement physiologically useful. If PhaZ1 is localized in the periplasm and if the physiological function of PhaZ1 is that of a PHB depolymerase, can we be sure that PHB really is an intracellularly located polymer? To our knowledge, electron microscopic studies have never shown evidence for a localization of PHB outside the cytoplasm. However, the location of initiation of PHB granule formation is not known, and initiation of PHB biosynthesis might occur at or in the cytoplasmic membrane due to the hydrophobic environment that is necessary for full PHB synthase activity. Another explanation for the unexpected localization of PHB depolymerase could be that low-molecular-weight PHB, traces of which have been found in all living organisms (37), is the physiological substrate for PhaZ1. In that case, one would expect that similar depolymerase genes are present in the genomes of genome-sequenced organisms. However, we did not find any genes related to R. rubrum PhaZ1 in the database except for extracellular dPHB depolymerases. Taking the results of this and previous studies (17, 18) into consideration, the physiological relevance of an assumed activation process of nPHB granules (32) appears unlikely, and the remarkable effect of ApdA on PHB granules could be an in vitro artifact. The physiological function of PhaZ1 in vivo remains to be elucidated.
If PhaZ1 is not an intracellularly located protein, which enzyme is responsible for the mobilization of PHB during starvation? Microscopic analysis of R. rubrum cells showed that PHB can be degraded in stationary phase (data not shown). Inspection of the R. rubrum genome revealed the presence of an open reading frame whose product showed significant homologies to intracellular PHB depolymerases of W. eutropha and other PHB-accumulating bacteria (accession no. AY217774). No signal peptide was found in the deduced amino acid sequence. We assume that this gene (phaZ2) is the true intracellular PHB depolymerase of R. rubrum.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft.
We thank D. Seebach for providing 3HB oligomers and L. Focarete, M. Scandola, and M. Kowalczuk for providing atactic PHB. Cutin was a gift from D. Deising and is gratefully acknowledged. We thank D. Dennis for providing E. coli strains harboring PHB biosynthetic genes.
Footnotes
Dedicated to J. M. Merrick, who inspired us to investigate PHB metabolism in R. rubrum.
REFERENCES
- 1.Ames, G.F.-L., C. Prody, and S. Kustu. 1984. Simple, rapid, and quantitative release of periplasmic proteins by chloroform. J. Bacteriol. 160:1181-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann, B. M., and D. Seebach. 1999. Investigation of the cleavage of diastereomeric oligo(3-hydroxybutanoates) containing two to eight HB units. A model for stereoselectivity of PHB depolymerase from Alcaligenes faecalis T1. Macromolecules 32:1777-1784. [Google Scholar]
- 3.Boatman, E. S. 1964. Observations on the fine structure of spheroplasts of Rhodospirillum rubrum. J. Cell Biol. 20:297-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briese, B. H., B. Schmidt, and D. Jendrossek. 1994. Pseudomonas lemoignei has five depolymerase genes. A comparative study of bacterial and eucaryotic PHA depolymerases. J. Environ. Polym. Degrad. 2:75-87. [Google Scholar]
- 5.Cornibert, J., and R. H. Marchessault. 1975. Conformational isomorphism. A general 2(1) helical conformation for poly(β-alkanoates). Macromolecules 8:296-305. [Google Scholar]
- 6.De Koning, G. J. M., and P. J. Lemstra. 1992. The amorphous state of bacterial poly[(R)-3-hydroxyalkanoate] in vivo. Polymer 33:3304-3306. [Google Scholar]
- 7.Doi, Y., and A. Steinbüchel (ed.). 2001. Biopolymers, vol. 3a. Polyesters I: biological systems and biotechnological production. Wiley-VCH, Weinheim, Germany.
- 8.Feilmeier, B. J., G. Iseminger, D. Schroeder, H. Webber, and G. J. Phillips. 2000. Green fluorescent protein functions as a reporter for protein localization in Escherichia coli. J. Bacteriol. 182:4068-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Patron, C., M. Calero, P. Rodriguez-Collazo, J. R. Gracia, J. Madrazo, A. Musacchino, F. Soriano, R. Estrada, R. Frank, L. Castellanos-Serra, and E. Mendez. 1995. Protein reverse staining: high-efficiency microanalysis of unmodified proteins detected on electrophoresis gels. Anal. Biochem. 224:203-211. [DOI] [PubMed] [Google Scholar]
- 10.Gao, D., A. Maehara, T. Yamane, and S. Ueda. 2001. Identification of the intracellular polyhydroxyalkanoate depolymerase gene of Paracoccus denitrificans and some properties of the gene product. FEMS Microbiol. Lett. 196:159-164. [DOI] [PubMed] [Google Scholar]
- 11.Golecki, J. R., and J. Oelze. 1980. Differences in the architecture of cytoplasmic and intracytoplasmic membranes of three chemotrophically and phototrophically grown species of the Rhodospirillaceae. J. Bacteriol. 144:781-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griebel, R., Z. Smith, and J. M. Merrick. 1968. Metabolism of poly-β-hydroxybutyrate. I. Purification, composition, and properties of native poly-β-hydroxybutyrate granules from Bacillus megaterium. Biochemistry 7:3676-3681. [DOI] [PubMed] [Google Scholar]
- 13.Griebel, R. J., and J. M. Merrick. 1971. Metabolism of poly-β-hydroxybutyrate: effect of mild alkaline extraction on native poly-β-hydroxybutyrate granules. J. Bacteriol. 108:782-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 15.Handrick, R., S. Reinhardt, M. L. Focarete, M. Scandola, G. Adamus, M. Kowalczuk, and D. Jendrossek. 2001. A new type of thermoalkalophilic hydrolase of Paucimonas lemoignei with high specificity for amorphous polyesters of short chain-length hydroxyalkanoic acids. J. Biol. Chem. 276:36215-36224. [DOI] [PubMed] [Google Scholar]
- 16.Handrick, R., S. Reinhardt, and D. Jendrossek. 2000. Mobilization of poly(3-hydroxybutyrate) in Ralstonia eutropha. J. Bacteriol. 182:5916-5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handrick, R., S. Reinhardt, D. Schultheiss, T. Reichart, D. Schüler, V. Jendrossek, and D. Jendrossek. 2004. Unraveling the function of the Rhodospirillum rubrum activator of polyhydroxybutyrate (PHB) degradation: the activator is a PHB-granule-bound protein (phasin). J. Bacteriol. 186:2466-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Handrick, R., U. Technow, T. Reichart, S. Reinhardt, T. Sander, and D. Jendrossek. 2004. The activator of the Rhodospirillum rubrum PHB depolymerase is a polypeptide that is extremely resistant to high temperature (121 degrees C) and other physical or chemical stresses. FEMS Microbiol. Lett. 230:265-274. [DOI] [PubMed] [Google Scholar]
- 19.Horowitz, D. M., and J. K. M. Sanders. 1994. Amorphous, biomimetic granules of polyhydroxybutyrate: preparation, characterization, and biological implications. J. Am. Chem. Soc. 116:2695-2702. [Google Scholar]
- 20.Jendrossek, D., and R. Handrick. 2002. Microbial degradation of polyhydroxyalkanoates. Annu. Rev. Microbiol. 56:403-432. [DOI] [PubMed] [Google Scholar]
- 21.Jendrossek, D., B. Müller, and H. G. Schlegel. 1993. Cloning and characterization of the poly(hydroxyalkanoic acid)-depolymerase gene locus, phaZ1, of Pseudomonas lemoignei and its gene product. Eur. J. Biochem. 218:701-710. [DOI] [PubMed] [Google Scholar]
- 22.Kidwell, J., H. E. Valentin, and D. Dennis. 1995. Regulated expression of the Alcaligenes eutrophus pha biosynthesis genes in Escherichia coli. Appl. Environ. Microbiol. 61:1391-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, Y. B., and R. W. Lenz. 2001. Polyesters from microorganisms. Adv. Biochem. Eng. Biotechnol. 71:51-79. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi, T., M. Shiraki, T. Abe, A. Sugiyama, and T. Saito. 2003. Purification and properties of an intracellular 3-hydroxybutyrate-oligomer hydrolase (PhaZ2) in Ralstonia eutropha H16 and its identification as a novel intracellular poly(3-hydroxybutyrate) depolymerase. J. Bacteriol. 185:3485-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 26.Lundgren, D. G., R. Alper, C. Schnaitman, and R. H. Marchessault. 1965. Characterization of poly-β-hydroxybutyrate extracted from different bacteria. J. Bacteriol. 89:245-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lütke-Eversloh, T., K. Bergander, H. Luftmann, and A. Steinbüchel. 2001. Biosynthesis of poly(3-hydroxybutyrate-co-3-mercaptobutyrate) as a sulfur analogue to poly(3-hydroxybutyrate) (PHB). Biomacromolecules 2:1061-1065. [DOI] [PubMed] [Google Scholar]
- 28.Lütke-Eversloh, T., K. Bergander, H. Luftmann, and A. Steinbüchel. 2001. Identification of a new class of biopolymer: bacterial synthesis of a sulfur-containing polymer with thioester linkages. Microbiology 147:11-19. [DOI] [PubMed] [Google Scholar]
- 29.Madigan, M., J. C. Cox, and H. Gest. 1982. Photopigments in Rhodopseudomonas capsulata cells grown anaerobically in darkness. J. Bacteriol. 150:1422-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madison, L. L., and G. W. Huisman. 1999. Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol. Mol. Biol. Rev. 63:21-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer, F., M. H. Madkour, U. Pieper-Fürst, R. Wieczorek, M. Liebergesell, and A. Steinbüchel. 1996. Electron microscopic observations on the macromolecular organization of the boundary layer of bacterial PHA inclusion bodies. J. Gen. Appl. Microbiol. 42:445-455. [Google Scholar]
- 32.Merrick, J. M., and M. Doudoroff. 1964. Depolymerization of poly-β-hydroxybutyrate by intracellular enzyme system. J. Bacteriol. 88:60-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merrick, J. M., D. G. Lundgren, and R. M. Pfister. 1965. Morphological changes in poly-β-hydroxybutyrate granules associated with decreased susceptibility to enzymatic hydrolysis. J. Bacteriol. 89:234-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merrick, J. M., R. Steger, and D. Dombroski. 1999. Hydrolysis of native poly(hydroxybutyrate) granules (PHB), crystalline PHB, and artificial amorphous PHB granules by intracellular and extracellular depolymerases. Int. J. Biol. Macromol. 25:129-134. [DOI] [PubMed] [Google Scholar]
- 35.Müller, B., and D. Jendrossek. 1993. Purification and properties of poly(3-hydroxyvaleric acid) depolymerase from Pseudomonas lemoignei. Appl. Microbiol. Biotechnol. 38:487-492. [Google Scholar]
- 36.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. A neural network method for identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Int. J. Neural Syst. 8:581-599. [DOI] [PubMed] [Google Scholar]
- 37.Reusch, R. N. 2000. Transmembrane ion transport by polyphosphate/poly-(R)-3-hydroxybutyrate complexes. Biochemistry (Moscow) 65:280-295. [PubMed] [Google Scholar]
- 38.Saegusa, H., M. Shiraki, C. Kanai, and T. Saito. 2001. Cloning of an intracellular poly[d(−)-3-hydroxybutyrate] depolymerase gene from Ralstonia eutropha H16 and characterization of the gene product. J. Bacteriol. 183:94-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saito, T., K. Takizawa, and H. Saegusa. 1995. Intracellular poly(3-hydroxybutyrate) depolymerase in Alcaligenes eutrophus. Can. J. Microbiol. 41(Suppl. 1):187-191. [Google Scholar]
- 40.Saito, T., and T. Kobayashi. 2001. Intracellular degradation of PHAs, p. 24-40. In A. Steinbüchel and Y. Doi (ed.), Biopolymers, vol. 3b. Wiley-VCH, Weinheim, Germany. [Google Scholar]
- 41.Scandola, M., M. Pizzoli, G. Ceccorulli, A. Cesaro, S. Paoletti, and L. Navarini. 1988. Viscoelastic and thermal properties of bacterial poly(d-(−)-β-hydroxybutyrate). Int. J. Biol. Macromol. 10:373-377. [Google Scholar]
- 42.Schwartz, E., A. Henne, R. Cramm, T. Eitinger, B. Friedrich, and G. Gottschalk. 2003. Complete nucleotide sequence of pHG1: a Ralstonia eutropha H16 megaplasmid encoding key enzymes of H2-based lithoautotrophy and anaerobiosis. J. Mol. Biol. 332:369-383. [DOI] [PubMed] [Google Scholar]
- 43.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 44.Steinbüchel, A., K. Aerts, W. Babel, C. Follner, M. Liebergesell, M. H. Madkour, F. Mayer, U. Pieper-Furst, A. Pries, H. E. Valentin, et al. 1995. Considerations on the structure and biochemistry of bacterial polyhydroxyalkanoic acid inclusions. Can. J. Microbiol. 41(Suppl. 1):94-105. [DOI] [PubMed] [Google Scholar]
- 45.Steinbüchel, A., and S. Hein. 2001. Biochemical and molecular basis of microbial synthesis of polyhydroxyalkanoates in microorganisms. Adv. Biochem. Eng. Biotechnol. 71:81-123. [DOI] [PubMed] [Google Scholar]
- 46.Steinbüchel, A., and H. E. Valentin. 1995. Diversity of microbial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 128:219-228. [Google Scholar]
- 47.Stumpp, T., B. Wilms, and J. Altenbuchner. 2000. Ein neues l-Rhamnose induzierbares Expressionssystem für Escherichia coli. Biospektrum 6:33-36. [Google Scholar]
- 48.Thomas, J. D., R. A. Daniel, J. Errington, and C. Robinson. 2001. Export of active green fluorescent protein to the periplasm by the twin-arginine translocase (Tat) pathway in Escherichia coli. Mol. Microbiol. 39:47-53. [DOI] [PubMed] [Google Scholar]
- 49.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaneechoutte, M., P. Kampfer, P., T. De Baere, E. Falsen, and G. Verschraegen. 2004. Wautersia gen. nov., a novel genus accommodating the phylogenetic lineage including Ralstonia eutropha and related species, and proposal of Ralstonia [Pseudomonas] syzygii (Roberts et al. 1990) comb. nov. Int. J. Syst. Evol. Microbiol. 54:317-327. [DOI] [PubMed] [Google Scholar]
- 51.Varga, A. R., and L. A. Staehelin. 1983. Spatial differentiation in photosynthetic and nonphotosynthetic membranes of Rhodopseudomonas palustris. J. Bacteriol. 154:1414-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 53.York, G. M., J. Lupberger, J. Tian, A. G. Lawrence, J. Stubbe, and A. J. Sinskey. 2003. Ralstonia eutropha H16 encodes two and possibly three intracellular poly[d-(−)-3-hydroxybutyrate] depolymerase genes. J. Bacteriol. 185:3788-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]