Abstract
Utilization of the enterobactin siderophore by the respiratory pathogens Bordetella pertussis and Bordetella bronchiseptica is dependent on the BfeA outer membrane receptor. This study determined that production of BfeA was increased significantly in iron-starved bacteria upon supplementation of cultures with enterobactin. A 1.01-kb open reading frame, designated bfeR, encoding a predicted positive transcriptional regulator of the AraC family was identified upstream and divergently oriented from bfeA. In iron-depleted cultures containing enterobactin, a Bordetella bfeR mutant exhibited markedly decreased BfeA receptor production compared to that of the wild-type strain. Additionally, B. pertussis and B. bronchiseptica bfeR mutants exhibited impaired growth with ferric enterobactin as the sole source of iron, demonstrating that effective enterobactin utilization is bfeR dependent. Transcriptional analysis using bfeA-lacZ reporter fusions in wild-type strains demonstrated that bfeA transcription was stimulated in iron-depleted conditions in the presence of enterobactin, compared to modest expression levels in cultures lacking enterobactin. In contrast, bfeA transcription in B. pertussis and B. bronchiseptica bfeR mutants was completely unresponsive to the enterobactin inducer. bfeA transcriptional analyses of a bfeA mutant demonstrated that induction by enterobactin did not require BfeA receptor-mediated uptake of the siderophore. These studies establish that bfeR encodes an enterobactin-dependent positive regulator of bfeA transcription in these Bordetella species.
Bordetella pertussis and Bordetella bronchiseptica are obligate respiratory pathogens of mammals. B. pertussis is the etiologic agent of whooping cough in its natural human host (7), while the highly genetically related species B. bronchiseptica has a broader host range (27) and is classically associated with swine atrophic rhinitis (59) and canine tracheobronchitis (60). Iron is a limiting nutrient for bacteria in the host environment, since it is located primarily intracellularly (28) and any extracellular iron is sequestered by host glycoproteins such as transferrin (51) and lactoferrin (47). This extremely limited availability of free iron requires pathogenic bacteria to utilize specialized uptake mechanisms to successfully compete with the host and other microorganisms for iron.
Of the known iron sources for B. pertussis and B. bronchiseptica, the genetic systems for utilization of only three have been characterized. The products of the bhuRSTUV genes are required for utilization of heme iron, with BhuR serving as the outer membrane heme receptor (63). Located upstream of bhuR, hurI encodes an extracytoplasmic function σ factor which activates transcription of the bhu genes under iron starvation conditions only in the presence of heme (64). Fur represses bhuRSTUV transcription indirectly, by repressing hurI transcription when intracellular iron concentrations are high (63, 64). B. pertussis and B. bronchiseptica also produce the macrocyclic dihydroxamate siderophore alcaligin (11, 40). Transcription of the alcaligin biosynthetic genes and the fauA gene, encoding the outer membrane ferric alcaligin receptor, is Fur repressible (31, 32); however, transcription is activated under iron starvation conditions by the AraC-like positive regulator AlcR in the presence of the alcaligin inducer (6, 12). B. pertussis and B. bronchiseptica are also capable of utilizing siderophores produced by other bacterial species (xenosiderophores), including enterobactin (5) and its breakdown product 2,3-dihydroxybenzoylserine (DHBS), ferrichrome, and desferrioxamine B (4). B. bronchiseptica has additionally been reported to obtain iron from coprogen, schizokinen, ferricrocin, vicibactin, ferrichrysin, ferrirubin, aerobactin, protochelin, and several pyoverdins (46).
Enterobactin is a catecholate siderophore consisting of a cyclic trimer of DHBS that is produced by a number of bacterial species, including certain members of the Enterobacteriaceae such as Escherichia coli (42), and some Streptomyces isolates (22). The iron binding affinity of enterobactin is one of the strongest among siderophores, with a formation constant of 1049 (37). Several bacterial pathogens other than B. pertussis and B. bronchiseptica are known to utilize enterobactin as a xenosiderophore, suggesting its potential importance in in vivo iron acquisition. Neisseria gonorrhoeae and Neisseria meningitidis rely on the FetA outer membrane receptor to transport ferric enterobactin (15). Enterobactin-mediated growth stimulation of Haemophilus parainfluenzae and Haemophilus paraphrophilus has also been reported (66), and the opportunistic pathogen Pseudomonas aeruginosa utilizes ferric enterobactin in a process that is dependent on the PfeA outer membrane receptor (19). Transcription of pfeA is enterobactin inducible and involves the function of the PfeR and PfeS two-component regulators (18, 20).
Beall and Sanden identified the iron-regulated B. pertussis bfeA gene, encoding a predicted outer membrane siderophore receptor which exhibited significant similarity to the ferric enterobactin receptors FepA of E. coli and PfeA of P. aeruginosa (5). Mutational analyses of B. pertussis and B. bronchiseptica demonstrated that enterobactin (5) and DHBS (4) utilization was BfeA dependent. Expression of a bfeA-phoA translational fusion in Bordetella cells was induced by iron limitation, and supplementation of cultures with enterobactin had no additional effect on fusion gene expression (5). A subsequent study by Thulasiraman and coworkers suggested that the Bordetella enterobactin transport system may be inducible, since pretreatment of iron-starved B. bronchiseptica with enterobactin enhanced cell surface binding and uptake of the siderophore (61).
In the present study, Bordetella BfeA production was found to be significantly increased in iron-starved cells when enterobactin was supplied exogenously. Two open reading frames (ORFs) predicted to encode ferric enterobactin utilization functions were identified adjacent to bfeA in B. pertussis and B. bronchiseptica. The bfeB gene encodes a putative hydrolase that may be involved in release of iron from internalized ferric enterobactin. The bfeR gene is predicted to encode a member of the AraC family of transcriptional regulators, and in this study, bfeR was demonstrated to be required for enterobactin-responsive transcription of bfeA.
MATERIALS AND METHODS
Bacterial strains and plasmids.
B. pertussis and B. bronchiseptica strains and recombinant plasmids are described in Table 1. E. coli DH5α [F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 endA1 recA1 hsdR17(rK− mK+) deoR thi-1 supE44 λ− gyrA96 relA1] (Invitrogen, Carlsbad, Calif.) was used as the donor strain in conjugation and as a host for routine cloning purposes. E. coli H1717 (aroB fhuF::λplacMu) was used in the Fur repressor titration assay (FURTA) and has been described previously (58). E. coli AN102 [araC14 leuB6(Am) secA206 (Azir) fhuA23 lacY1 proC14 tsx-67 fep-104 glnV44(AS) λ− trpE38 rpsL109(Strr) xylA5 mtl-1 thi-1] (17) was employed in the production of enterobactin for purification. Plasmid pGEM3Z (Promega, Madison, Wis.) served as a cloning vector in E. coli, and broad-host-range vectors pRK415 (33), pBBR1MCS (35), and pBBR1MCS-5 (34) were used in the construction of recombinant plasmids for Bordetella host strains. Plasmids pSS1129 (57) and pEG7 (16) were used for allelic exchange in B. pertussis and B. bronchiseptica, respectively. For conjugations, DNA processing and mobilization functions were provided by E. coli DH5α carrying pRK2013 (24).
TABLE 1.
Bordetella strains and recombinant plasmids
| Strain or plasmid | Description | Reference |
|---|---|---|
| Strains | ||
| UT25 | B. pertussis; wild-type clinical isolate | 23 |
| UT25Sml | B. pertussis; spontaneous streptomycin resistant derivative of UT25 | 8 |
| PM9 | B. pertussis UT25Sml; ΔbfeR | This study |
| B013N | B. bronchiseptica; spontaneous nalidixic acid resistant derivative of swine isolate B013 | 2 |
| BRM24 | B. bronchiseptica B013N; ΔbfeR | This study |
| BRM25 | B. bronchiseptica B013N; bfeA::pSS18 | This study |
| Plasmids | ||
| pKP1 | pUC19 derivative containing the 4.1-kb bfeR bfeA genetic region from B. pertussis strain 82 | 5 |
| p3Z100 | pGEM3Z with 0.4-kb EcoRI insert fragment containing the bfeA-bfeR intergenic region | This study |
| p3Z105 | pGEM3Z with 0.4-kb EcoRI-BamHI insert fragment containing the bfeA-bfeR intergenic region | This study |
| p3ZFBS | pGEM3Z containing an E. coli consensus Fur-binding site | 63 |
| pSS11 | pSS1129 with 1.1-kb EcoRI-HindIII insert containing B. pertussis DNA region with the 594-bp PstI fragment of bfeR deleted | This study |
| pSS18 | pSS1129 with 1.2-kb HindIII-BamHI insert containing internal region of B. bronchiseptica B013N bfeA gene | This study |
| pBB31 | pBBR1MCS with 1.7-kb SalI-StuI insert containing B. pertussis strain 82 bfeR gene from pKP1 | This study |
| pBB36 | pBBR1MCS-5 with 1.7-kb SalI-StuI insert containing B. pertussis strain 82 bfeR gene from pKP1 | This study |
| pBB37 | pBBR1MCS with 2.9-kb PstI insert containing B. pertussis strain 82 bfeA gene from pKP1 | This study |
| pEG7.2 | pEG7 with 3.8-kb BamHI sacBR and 1.4-kb BamHI-HindIII ΔbfeR insert fragments | This study |
| pRK40 | pRK415 with 3.3-kb EcoRI-HindIII trp′-′lacZ insert fragment | 64 |
| pRK43 | pRK40 with 0.4-kb EcoRI bfeA′-bfeR′ intergenic region from p3Z100; bfeR-lacZ transcriptional fusion | This study |
| pRK44 | pRK40 with 0.4-kb EcoRI-HindIII bfeR′-bfeA′ intergenic region from p3Z105; bfeA-lacZ transcriptional fusion | This study |
| pMP1 | pMP220 with 0.4-kb EcoRI-BamHI bfeR′-bfeA′ intergenic region from p3Z105; bfeA-lacZ transcriptional fusion | This study |
Culture conditions.
B. bronchiseptica and E. coli strains were grown on Luria-Bertani (LB) agar or in LB broth (50), and Bordet-Gengou (BG) agar (7) was used for B. pertussis plate cultures. Stainer-Scholte (SS) medium (56), modified as described previously (52) and deferrated by treatment with Chelex 100 (Bio-Rad, Richmond, Calif.) (2), was used as a chemically defined medium for growth of B. pertussis and B. bronchiseptica strains. Iron-replete SS cultures contained 36 μM FeSO4, and iron-restricted SS medium lacked added iron. SS medium was supplemented with 0.5 mM MgCl2 and 0.2 mM CaCl2 following treatment with Chelex 100. Lactose MacConkey agar was used for growth of E. coli strains in the FURTA (58). For enterobactin production, E. coli AN102 was grown in T medium (54) supplemented with the following: leucine, 50 μg/ml; proline, 50 μg/ml; tryptophan, 50 μg/ml; thiamine · HCl, 5 μg/ml; and glucose, 0.2%. Bacterial growth was measured densitometrically with a spectrophotometer or a Klett-Summerson colorimeter fitted with a no. 54 filter (Klett Manufacturing Co., Long Island City, N.Y.). Antibiotics were added to media at the following concentrations: ampicillin, 100 μg/ml; chloramphenicol, 30 μg/ml; gentamicin, 10 μg/ml; kanamycin, 50 μg/ml; streptomycin, 100 μg/ml; and tetracycline, 15 μg/ml. Siderophores were added to SS medium at the following concentrations: enterobactin, 3.25 μM; alcaligin, 5 μM; ferrichrome, 10 μM; desferrioxamine B, 10 μM; and 2,3-dihydroxybenzoic acid (DHBA), 10 μM. Hemin chloride, ferrichrome, and DHBA were purchased from Sigma-Aldrich (St. Louis, Mo.). Desferrioxamine B (Desferal) was purchased from Ciba Agrochemicals AG (Basel, Switzerland).
Genetic methods.
Standard genetic methods were used as described previously (50). Conjugal transfer of plasmids to Bordetella recipient strains was achieved by triparental mating as described previously (8). Genomic DNA was purified with the genomic-tip system (Qiagen, Valencia, Calif.). DNA hybridization probes were labeled with [α32-P]dCTP (ICN Radiochemicals, Irvine, Calif.) by use of the random primers DNA labeling system (Invitrogen, Carlsbad, Calif.). Oligonucleotide primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, Iowa).
Nucleotide sequence data were from sequence determinations by this laboratory or from the genome sequences of B. pertussis Tohama I, B. bronchiseptica strain RB50, and Bordetella parapertussis strain 12822 produced by the Bordetella Sequencing Group at The Wellcome Trust Sanger Institute (http://www.sanger.ac.uk/Projects/B_pertussis). The nucleotide sequences determined in this laboratory were obtained by primer walking on both DNA strands. Nucleotide sequencing services were provided by the Advanced Genetic Analysis Center at the University of Minnesota.
Nucleotide sequence analyses were conducted by using the Lasergene sequence analysis software package (DNASTAR, Inc., Madison, Wis.). Database searches were accomplished by using the BLAST servers provided by the Sanger Centre and the National Center for Biotechnology Information at the National Library of Medicine. Deduced amino acid sequences were analyzed for the presence of conserved patterns at the ExPASy web site (http://us.expasy.org/tools/#pattern) with the InterPro Scan server or by the Conserved Domain Database and Search Service (reverse position-specific BLAST) algorithm at the National Center for Biotechnology Information. Amino acid sequence alignments were performed by the CLUSTAL or Jotun-Hein method with the MegAlign software module of the Lasergene program. The putative BfeB signal sequence cleavage site was predicted by using the SignalP server at the Center for Biological Sequence Analysis (http://www.cbs.dtu.dk/services/SignalP/index.html).
Enterobactin purification.
Enterobactin purification was based on the protocol of Neilands and Nakamura (41). E. coli AN102 was cultured in 1 l of T medium for 14 h. Bacteria were removed from the culture fluid by centrifugation, and the resulting supernatant was extracted three times with ethyl acetate. The organic-phase extracts were pooled and reduced in volume with a Rotavapor R-114 condensing apparatus (Büchi Labortechnik AG, Flawil, Switzerland). Extracts were washed with 0.1 M citrate buffer at pH 5.5 to remove charged species, followed by drying over anhydrous MgSO4. The volume of washed extract was reduced a second time, after which enterobactin was precipitated by the addition of hexanes. The yield of enterobactin was determined by measuring the optical density at 316 nm of a methanolic solution. Purified enterobactin was stored at 4°C as a methanolic solution.
Construction of Bordetella mutants.
The 1.7-kb SalI-BamHI DNA fragment containing the B. pertussis bfeR gene from pKP1 was subcloned to vector pGEM3Z (p3Z94); a 594-bp internal fragment of bfeR was removed from p3Z94 by partial PstI digestion and religation to engineer an in-frame deletion mutation, which was confirmed by nucleotide sequencing. The 1.1-kb EcoRI-HindIII fragment containing the ΔbfeR allele was subcloned to suicide vector pSS1129 (pSS11) and delivered to B. pertussis UT25Sm1 by conjugation. Following allelic exchange, mutant PM9 was isolated and confirmed to be ΔbfeR by Southern hybridization.
The B. bronchiseptica BRM24 bfeR mutant was constructed by allelic exchange using SacB counterselection based on the method of Akerley et al. (1). Briefly, primers 5′bfeRsac (5′-GGCCGGATCCGGTCGCCGTTCCAGCCATAG-3′) and 3′bfeRsac (5′-GGCCAAGCTTGCACGCGCTCAAGGAACTGG-3′) were used to PCR amplify the ΔbfeR allele from B. pertussis mutant PM9, incorporating BamHI and HindIII restriction endonuclease sites at the 5′ and 3′ ends of the product, respectively. The ΔbfeR allele, as well as the 3.8-kb BamHI fragment from pEG18.3 (1; P. Cotter, personal communication) containing sacBR and a kanamycin resistance gene, were ligated to BamHI-linearized pEG7, resulting in plasmid pEG7.2. Plasmid pEG7.2 was delivered to B. bronchiseptica B013N; kanamycin-resistant cointegrants were subsequently grown in LB broth without selection, followed by plating on LB agar containing 5% sucrose. Isolates that had undergone allelic exchange were sucrose resistant and were confirmed to carry the ΔbfeR allele by PCR analysis with primers 5′bfeRdetect (5′-GACCCAGACTCCTCCAC-3′) and bfedP (5′-ATGCGGCGCGAAAATCAG-3′).
B. bronchiseptica bfeA mutant BRM25 was constructed by insertion mutagenesis. Oligonucleotide primers 5′bfeAdsrpt (5′-GCGAAGCTTGCGAGGGCGTCATCAACCAG-3′) and 3′bfeAdsrpt (5′-GCGGGATCCGGCCGTCGCCGTTCAGTTCG-3′) were used to PCR amplify a 1.2-kb internal fragment of the B. bronchiseptica B013N bfeA gene, incorporating HindIII and BamHI termini. The PCR product was ligated to pSS1129, creating pSS18, which was delivered to the chromosome of B. bronchiseptica B013N by conjugal transfer. Mutant BRM25 was confirmed to be completely defective in enterobactin-mediated growth stimulation.
Construction of lacZ fusion plasmids.
A 401-bp DNA segment corresponding to the B. pertussis Tohama I nucleotide sequence positions 3076925 to 3077326 (annotated genome sequence, Sanger Centre) was amplified from a B. pertussis UT25Sm1 chromosomal DNA template by PCR with primers designed to introduce EcoRI restriction sites for cloning: bfe1 (5′-GGCCGAATTCTCTCCTCGGCGGTGATGAC-3′) and bfe2 (5′-GGCCGAATTCCGGTGCGCGTGGAGGAGT-3′). The PCR product, containing the bfeR-bfeA intergenic region, predicted promoter elements, and 5′ coding sequences of the genes, was cloned as an EcoRI fragment (p3Z100) and subsequently subcloned to pRK40, yielding the bfeR-lacZ transcriptional fusion plasmid pRK43. The bfeA-lacZ transcriptional fusion plasmid, pRK44, was similarly constructed by cloning the same 0.4-kb region (in reverse orientation) from plasmid p3Z105. The correct bfeR-lacZ and bfeA-lacZ fusion junctions of pRK43 and pRK44, respectively, were confirmed by nucleotide sequencing. For B. pertussis studies, the 0.4-kb bfeR-bfeA intergenic region from p3Z105 was subcloned as an EcoRI-XbaI DNA fragment to pMP220 (55), resulting in the bfeA-lacZ transcriptional fusion plasmid pMP1.
Fur titration assays.
The E. coli FURTA reporter strain H1717 was used to assess the in vivo Fur binding function of the cloned B. pertussis bfeR-bfeA intergenic region. H1717(p3Z100) was cultured on lactose MacConkey agar supplemented with 30 μM Fe(NH4)2(SO4)2. After incubation for ∼18 h, the LacZ+ phenotype of each strain was used to assess Fur repressor binding as described previously (58). Strains H1717(p3ZFBS) (63) and H1717(pGEM3Z) served as the positive and negative controls, respectively.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting.
B. bronchiseptica strains were harvested by centrifugation after 24 h of growth in SS medium under iron-replete or iron-depleted conditions in the presence or absence of exogenously added siderophores. For total protein analysis, bacterial suspensions in 50 mM HEPES (pH 7.4) were normalized based on optical density and incubated in solubilization buffer at 100°C for 6 min, followed by clarification of the sample by centrifugation. Bacterial cells were disrupted by using a French pressure cell and fractionated as described previously (9, 32). Sample volumes corresponding to approximately 0.1 optical density unit of bacterial culture were subjected to electrophoresis on 7.5 or 10% denaturing polyacrylamide gels containing 3% urea as described previously (52).
For immunoblotting, proteins resolved by electrophoresis were transferred to a nitrocellulose membrane and processed as described previously (62). Detection of BfeA was accomplished by using a mouse antiserum (1:500 dilution) raised to E. coli FepA (3), a horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G secondary antibody (1:2,000 dilution) (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.), and 4-chloro-1-naphthol as the chromogen.
Enterobactin bioassays.
B. bronchiseptica strains were grown for 24 h on LB agar, and bacteria were seeded into molten LB agar (50°C) containing 100 μg of ethylenediaminedi-(o-hydroxyphenyl)acetic acid (EDDA) per ml as described previously (11). After the agar solidified, 5-mm-diameter wells cut into the agar were filled with 50 μl of aqueous solutions of purified enterobactin or alcaligin. Alcaligin was purified as described previously (11). Following overnight incubation, the diameters of the growth zones were measured. The values reported are the means from triplicate bioassays and are representative of at least two experiments.
Enterobactin growth stimulation of B. pertussis was monitored in agar bioassays by using a modification of a method described previously (63). Pertussis LB bioassay agar was modified to contain 2 g of randomly methylated β-cyclodextrin (Cyclodextrin Technologies Development Inc., High Springs, Fla.) per liter. B. pertussis strains were grown on BG agar for 48 h, followed by culture for 24 h in SS medium containing 18 μM FeSO4. Bacteria were harvested by centrifugation and washed once with SS basal medium lacking iron. The strains were then seeded into Pertussis LB molten agar containing 50 μg of EDDA per ml, feeding solutions were added to wells cut into the agar, and the plates were incubated for 36 to 48 h. The growth zone values reported are the means from triplicate bioassays and are representative of at least two experiments.
β-Galactosidase assays.
B. bronchiseptica strains carrying pRK40 lacZ fusion derivatives were grown on LB agar for 24 h and used to inoculate iron-replete SS medium. After 24 h of growth, bacteria were washed once with SS basal medium and subcultured at a 1:50 dilution into iron-replete or iron-depleted SS medium containing siderophores as appropriate. Bacteria were assayed after 18 h for the production of β-galactosidase by the method of Miller (39) as modified by Brickman et al. (14). Enzymatic activity was calculated by using the formulation of Miller (39), with the results reported as the mean values from triplicate cultures ± one standard deviation. All results are representative of at least two experiments.
β-Galactosidase assays with B. pertussis strains were performed essentially as described for B. bronchiseptica, with the following exceptions. B. pertussis strains carrying pMP1 or the pMP220 fusion plasmid vector were grown on BG agar, followed by cultivation in iron-replete SS medium for 24 to 48 h. Washed bacteria were then subcultured to an optical density (600 nm) of ∼0.1 in iron-replete or iron-depleted SS medium. After 14 h of growth, enterobactin was added to one set of parallel iron-depleted cultures. Bacteria were analyzed for the production of β-galactosidase 8 h after the addition of enterobactin. The reported values are the means from triplicate assays and are representative of at least two experiments.
RESULTS
BfeA production is enterobactin inducible.
To investigate potential positive regulation of bfeA in Bordetella spp., production of BfeA was assessed by immunoblot analysis. Since BfeA exhibits 50% amino acid sequence identity to the E. coli FepA enterobactin receptor, a FepA-specific mouse antiserum was tested for reactivity to BfeA in B. pertussis and B. bronchiseptica cells. Total protein preparations from B. pertussis UT25 grown under iron-replete and iron-depleted conditions in SS medium exhibited low levels of reactivity with the FepA-specific antiserum. However, when the bacteria were iron starved in the presence of enterobactin, a strongly immunoreactive, high-molecular-mass protein (∼80 kDa) was produced (Fig. 1A). B. bronchiseptica B013N also produced an immunoreactive protein of similar size when grown under iron-depleted conditions with exogenously added enterobactin (Fig. 1B). Induced production of the 80-kDa protein appeared to be specifically responsive to enterobactin, since supplementation of cultures with the native siderophore alcaligin (Fig. 1B), the xenosiderophores ferrichrome and desferrioxamine B, or the enterobactin precursor DHBA (Fig. 1C) did not stimulate production of the protein. The enterobactin-inducible 80-kDa protein was localized to the outer membrane protein fraction of B. bronchiseptica cells (Fig. 2A).
FIG. 1.
Immunoblot analysis of an enterobactin-inducible protein in Bordetella species. Bacterial strains were grown in iron (Fe)-replete (+) or iron-depleted (−) SS medium. Total cellular proteins were analyzed by using a FepA-specific antiserum as described in Materials and Methods. (A) B. pertussis UT25; (B and C) B. bronchiseptica B013N. Siderophores (Sid): Ent, enterobactin; Alc, alcaligin; Fer, ferrichrome; Des, desferrioxamine B; DHBA, 2,3-dihydroxybenzoic acid. The arrowheads indicate the dominant FepA cross-reactive protein.
FIG. 2.
Immunoblot analysis of BfeA production in B. bronchiseptica. BfeA was detected by using the FepA-specific antiserum. (A) B. bronchiseptica B013N grown in iron (Fe)-replete (+) or iron-depleted (−) SS medium in the presence (+) or absence (−) of enterobactin (Ent). Bacterial lysates were processed to yield soluble (cytoplasmic and periplasmic proteins) (S), inner membrane (IM), and outer membrane (OM) protein fractions. (B) Lysates of B. bronchiseptica strains cultured in iron-replete or iron-depleted SS medium with or without enterobactin supplementation. Lanes 1, 2, 7, and 8, wild-type strain B013N; lanes 3, 4, 9, and 10, bfeA mutant BRM25(pBBR1MCS) (plasmid vector control); lanes 5, 6, 11, and 12, BRM25(pBB37) (bfeA+ plasmid).
A B. bronchiseptica bfeA mutant that cannot utilize ferric enterobactin (data not shown) failed to produce the protein (Fig. 2B). Complementation of the mutant with bfeA in trans restored production of the protein, demonstrating its identity as BfeA. The complemented mutant exhibited enhanced production of BfeA in iron-depleted cultures either lacking or containing enterobactin, compared with the wild-type strain; this increased BfeA production is most likely due to bfeA multicopy effects. Stimulation of BfeA production in both the wild-type and complemented bfeA mutant strains occurred only under iron-restricted growth conditions in the presence of enterobactin, since enterobactin-supplemented iron-replete cultures failed to produce detectable BfeA. Taken together, these results indicate that maximal production of the BfeA enterobactin receptor by B. pertussis and B. bronchiseptica requires that the bacteria sense iron starvation as well as the presence of the enterobactin siderophore.
Identification of additional predicted enterobactin utilization genes.
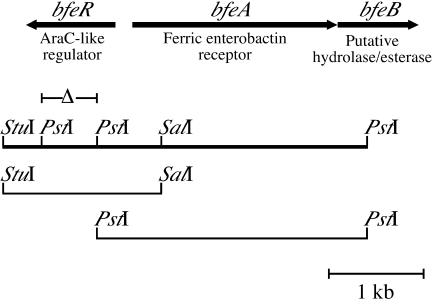
Enhanced production of BfeA in Bordetella cells in response to enterobactin suggested the involvement of a siderophore-inducible transcriptional regulatory mechanism. The DNA regions surrounding bfeA were analyzed for the presence of ORFs encoding potential regulatory functions. A 1.01-kb ORF, designated bfeR, was identified in both B. pertussis and B. bronchiseptica, divergently oriented from bfeA (Fig. 3). Examination of the nucleotide sequence of B. parapertussis strain 12822 (Sanger Centre) revealed that this Bordetella species also contained the bfeA and bfeR genes. A comparison of the bfeR ORFs from B. pertussis and B. bronchiseptica demonstrated that the genes are greater than 99% identical at the nucleotide level. Database searches identified numerous AraC family transcriptional regulators bearing significant similarity to the 336-amino-acid BfeR protein, with the greatest similarity residing in the putative C-terminal DNA binding domain that is conserved among AraC-like proteins (25). Comparison of the C-terminal 86-amino-acid sequence of BfeR with the C termini of other AraC-like proteins revealed striking similarity with members of the iron acquisition subfamily of AraC regulators (Fig. 4). BfeR has 43% identity to the Bordetella AlcR transcriptional activator of the alcaligin biosynthetic (12) and receptor (9) genes in B. pertussis and B. bronchiseptica. Furthermore, it is 31% identical to the P. aeruginosa PchR regulator of pyochelin system genes (30) and 30% identical to YbtA, a transcriptional regulator of the yersiniabactin biosynthesis and receptor genes in Yersinia pestis (21). AraC itself is 24% identical to BfeR at the C terminus. Because BfeR exhibited strong similarity to members of the iron subfamily of AraC-like proteins, it was hypothesized that BfeR may mediate enterobactin-responsive transcriptional activation of bfeA in a manner analogous to activation of fauA transcription in Bordetella cells by alcaligin and AlcR (9).
FIG. 3.
Genetic organization of the Bordetella enterobactin utilization gene cluster. The arrows depict the spatial limits and transcriptional orientations of the bfe genes. The known or proposed function of each gene product is designated. The positions of relevant restriction enzyme sites are indicated; bfeR mutants were constructed by deletion of the 594-bp PstI fragment (Δ). Cloned B. pertussis DNA regions: StuI-SalI, plasmids pBB31 and pBB36; PstI-PstI, plasmid pBB37.
FIG. 4.
BfeR sequence alignment with selected members of the AraC family of transcriptional regulators. Proteins exhibiting significant similarity to the deduced amino acid sequence of BfeR were identified as described in Materials and Methods. The 86-amino-acid C-terminal portion of BfeR was aligned with the analogous regions of B. pertussis AlcR (GenBank accession number AF018255), P. aeruginosa PchR (L11657), Y. pestis YbtA (U50452), and E. coli AraC (NP_414606). Identical amino acids are boxed, and the conserved helix-turn-helix motifs are bracketed.
Downstream of bfeA, in the same transcriptional orientation, is an ORF designated bfeB. Since only two nucleotides separate the deduced stop codon of bfeA from the start codon of bfeB, bfeB is predicted to be cotranscribed and translationally coupled with bfeA. BfeB shares 31% amino acid sequence identity with the predicted IroE hydrolase of Salmonella enterica. In S. enterica, iroE is located in the iroBCDEN gene cluster, which is involved in utilization of several catechol siderophores, including enterobactin (48).
Enterobactin-mediated growth stimulation requires bfeR.
To test whether bfeR encodes a positive regulator that controls expression of the bfeA ferric enterobactin receptor gene, B. bronchiseptica and B. pertussis mutants carrying 594-bp in-frame deletion mutations in bfeR were constructed and analyzed in growth stimulation bioassays with agar containing a nonutilizable iron chelator. Wild-type B. bronchiseptica exhibited concentration-dependent growth stimulation in the presence of enterobactin, showing well-defined growth zones even at the lowest concentration of enterobactin tested (Table 2). In contrast, the isogenic bfeR mutant BRM24, carrying the plasmid vector control pBBR1MCS, displayed markedly reduced enterobactin-dependent growth stimulation. There was a ∼34% reduction in growth stimulation observed at the highest concentration of enterobactin, while lower concentrations failed to stimulate any visible growth of BRM24. Genetic complementation of BRM24 by using the bfeR+ plasmid pBB31 (Fig. 2) restored enterobactin-mediated growth stimulation to wild-type levels. Similarly, B. pertussis bfeR mutant PM9 exhibited a defective enterobactin utilization phenotype which was complemented to wild-type levels by bfeR provided in trans (Table 3). The Bordetella bfeR mutants were fully capable of obtaining iron via other sources, such as hemin and alcaligin. These results demonstrate that bfeR is required for maximal enterobactin-dependent growth stimulation of B. bronchiseptica and B. pertussis.
TABLE 2.
Growth stimulation of B. bronchiseptica strains by enterobactin
| Strain | Genotype | Diam of growth zone (mm) surrounding wells containing the indicated siderophore concentration (μM)
|
|||
|---|---|---|---|---|---|
| Enterobactin
|
Alcaligin (310) | ||||
| 6.5 | 3.25 | 1.625 | |||
| B013N | bfeR+ | 20 | 18 | 16 | 15 |
| BRM24(pBBR1MCS) | ΔbfeR | 13 | —a | — | 15 |
| BRM24(pBB31) | ΔbfeR/bfeR+ | 22 | 18 | 15 | 14 |
—, no growth stimulation detected.
TABLE 3.
Growth stimulation of B. pertussis strains by enterobactin
| Strain | Genotype | Diam of growth zone (mm) surrounding wells containing the indicated iron source concentration (μM)
|
|||
|---|---|---|---|---|---|
| Enterobactin
|
Hemin (100) | ||||
| 10 | 5 | 2.5 | |||
| UT25Sml | bfeR+ | 23 | 20 | 16 | 19 |
| PM9(pBBR1MCS-5) | ΔbfeR | 17 | —a | — | 19 |
| PM9(pBB36) | ΔbfeR/bfeR+ | 23 | 19 | 16 | 19 |
—, no growth stimulation detected.
Transcription of bfeA is enterobactin inducible.
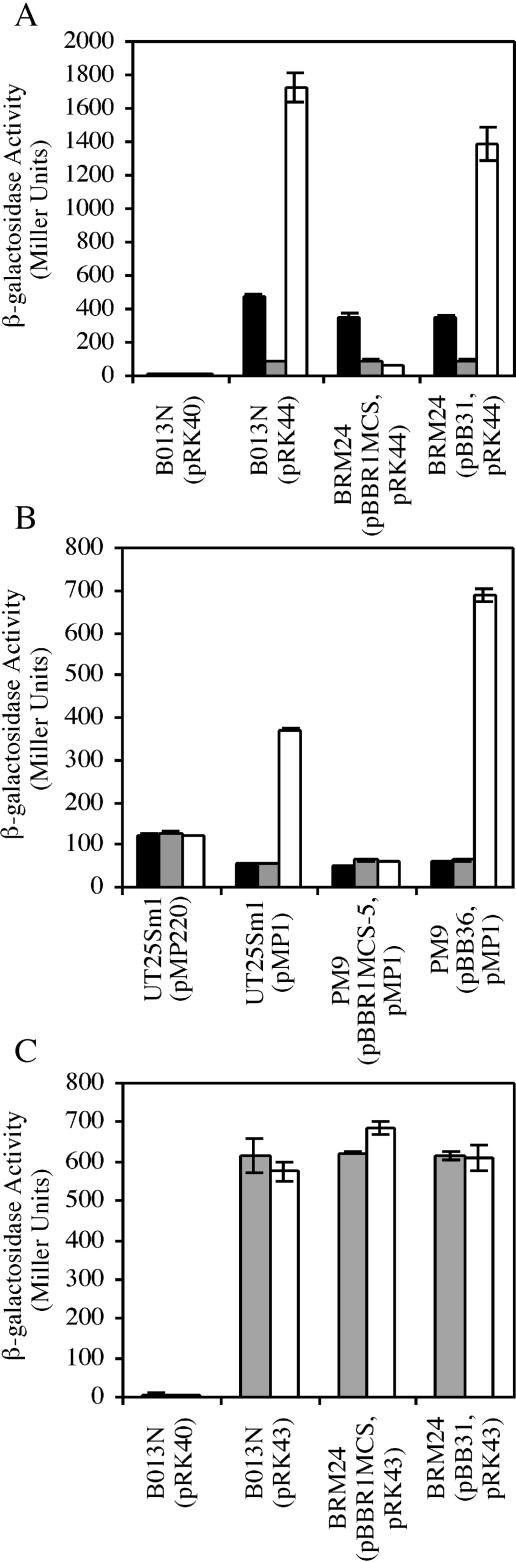
To assess the effect of enterobactin and iron on expression of Bordetella enterobactin utilization genes, bfeA-lacZ and bfeR-lacZ promoter fusions carried on low-copy-number plasmids were tested in wild-type B. bronchiseptica cells. Transcription of the bfeR-lacZ fusion (pRK43) was generally unaffected by the iron status of the cultures (Fig. 5A), and addition of enterobactin to parallel iron-replete or iron-depleted cultures did not result in any further increase in β-galactosidase activity. These results indicate that bfeR transcription is neither iron repressible nor enterobactin inducible under these conditions. However, the expression profile of B. bronchiseptica carrying the bfeA-lacZ fusion (pRK44) was markedly different, as indicated by the nearly 15-fold increase in transcription of bfeA when iron-depleted cultures were supplied with enterobactin. Enterobactin supplementation of iron-replete cultures did not result in increased bfeA transcription, in concordance with the immunoblot analysis (Fig. 2B). Similar to the results for B. bronchiseptica, bfeA-lacZ expression in wild-type B. pertussis was increased approximately sevenfold upon exposure of iron-depleted cultures to enterobactin (Fig. 6B). bfeA transcription appeared to be distinctly responsive to enterobactin, since ferrichrome, desferrioxamine B, and DHBA failed to induce transcription in B. bronchiseptica (Fig. 5B). Together these results demonstrate that maximal expression of bfeA requires both iron starvation and the presence of the enterobactin inducer. A decrease in bfeA-lacZ transcription was consistently observed when B. bronchiseptica(pRK44) was starved for iron, compared to transcription levels in bacteria grown under iron-replete conditions (see e.g., Fig. 5A). This is an unexpected result, since bacterial iron uptake genes are generally derepressed upon iron starvation. However, this pattern of expression has also been observed in the positively regulated Bordetella heme (64) and alcaligin (10, 12) systems. Although this result may suggest that BfeR represses bfeA transcription in the absence of the enterobactin inducer, results from bfeA expression experiments using bfeR mutants (Fig. 6A) do not support this notion.
FIG. 5.
Iron- and enterobactin-mediated regulation of bfeR-lacZ and bfeA-lacZ gene fusions in wild-type B. bronchiseptica strain B013N. Bars indicate β-galactosidase activities of cultures grown under the following conditions: black bars, iron replete; hatched black bars, iron replete and supplemented with enterobactin; gray bars, iron depleted; hatched open bars, iron depleted and supplemented with ferrichrome; horizontally striped bars, iron depleted and supplemented with desferrioxamine B; vertically striped bars, iron depleted and supplemented with DHBA; open bars, iron depleted and supplemented with enterobactin. (A) B013N(pRK40), plasmid vector control; B013N(pRK43), bfeR-lacZ; B013N(pRK44), bfeA-lacZ. (B) B013N(pRK40), plasmid vector control; B013N(pRK44), bfeA-lacZ. Error bars represent ±1 standard deviation from the means of independent triplicate determinations.
FIG. 6.
Analysis of bfeA and bfeR transcription in Bordetella ΔbfeR mutants. Strains were grown in SS medium and assayed for β-galactosidase activity. (A) Wild-type B. bronchiseptica strain B013N and strain BRM24 (ΔbfeR) carrying lacZ fusion plasmid pRK40 (plasmid vector control) or pRK44 (bfeA-lacZ). BRM24 was genetically complemented by using pBB31 (bfeR+); pBBR1MCS is the plasmid vector control. (B) Wild-type B. pertussis strain UT25Sm1 and ΔbfeR mutant strain PM9 carrying pMP220 (plasmid vector control) or pMP1 (bfeA-lacZ). PM9 was genetically complemented by using pBB36 (bfeR+); pBBR1MCS-5 is the plasmid vector control. (C) Wild-type B. bronchi-septica strain B013N and strain BRM24 (ΔbfeR) carrying lacZ fusion plasmid pRK40 (plasmid vector control) or pRK43 (bfeR-lacZ). BRM24 was genetically complemented by using pBB31 (bfeR+); pBBR1MCS is the plasmid vector control. Culture conditions: black bars, iron replete; gray bars, iron depleted; open bars, iron depleted and supplemented with enterobactin. Error bars represent ±1 standard deviation from the means of triplicate determinations.
Expression of bfeA is bfeR dependent.
To determine whether enterobactin-inducible bfeA transcription was dependent on bfeR, β-galactosidase assays were performed on Bordetella strains carrying low-copy-number bfeA-lacZ fusion plasmids. Transcription of bfeA-lacZ (pRK44) in B. bronchiseptica bfeR mutant BRM24 carrying the plasmid vector control pBBR1MCS grown in the presence of enterobactin was 11-fold less than that in wild-type B013N grown under the same conditions (Fig. 6A). Thus, the bfeR mutation resulted in decreased bfeA transcription to levels comparable to those achieved in the absence of the enterobactin inducer. Iron-starved BRM24(pRK44) carrying the complementing bfeR+ plasmid pBB31 demonstrated β-galactosidase activity that was restored to near-wild-type levels in the presence of enterobactin. Similarly, bfeA-lacZ expression in B. pertussis bfeR mutant PM9 was also unresponsive to enterobactin; complementation of PM9 with bfeR in trans not only restored enterobactin-inducible bfeA transcriptional activation but resulted in enhanced expression (Fig. 6B). Genetic complementation of Bordetella bfeR mutants not only confirms that bfeA transcription is bfeR dependent but also indicates that there is a stringent requirement for the enterobactin inducer, even when bfeR is present in multicopy.
The inability of bfeR mutants to activate bfeA transcription in response to enterobactin was predicted to result in a corresponding decrease in ferric enterobactin receptor abundance. Whereas abundant amounts of BfeA were detected in samples from iron-starved wild-type B. bronchiseptica cells exposed to enterobactin (Fig. 7, lane 3), the bfeR mutant strain produced negligible levels (Fig. 7, lane 6). The bfeR mutant was complemented by pBB31 (bfeR+), resulting in near-wild-type levels of BfeA production (Fig. 7, lane 9). The reduced production of BfeA correlates with the observed defects in enterobactin growth stimulation and bfeA transcription in Bordetella bfeR mutants, confirming the role of BfeR as a positive regulator of enterobactin-inducible bfeA expression.
FIG. 7.
Immunoblot analysis of BfeA production in B. bronchiseptica ΔbfeR mutant BRM24. Bacterial strains were grown in iron (Fe)-replete (+) or iron-depleted (−) medium in the presence (+) or absence (−) of enterobactin (Ent). Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and BfeA was identified by using the FepA-specific antiserum. Lanes 1 to 3, wild-type strain B013N; lanes 4 to 6, BRM24(pBBR1MCS) (plasmid vector control); lanes 7 to 9, BRM24(pBB31) (bfeR+ plasmid). The arrowhead indicates the position of the BfeA immunoreactive protein.
bfeR transcription is not autoregulated.
Analysis of bfeR-lacZ expression in wild-type B. bronchiseptica established that transcription of bfeR was not enterobactin inducible. To determine whether bfeR transcription is autoregulated, transcriptional activity of the pRK43 bfeR-lacZ fusion plasmid was assayed in bfeR mutant BRM24. The β-galactosidase activities in both wild-type B013N and BRM24 were similar under iron-depleted conditions, regardless of the presence of enterobactin (Fig. 6C). Providing bfeR in multicopy (pBB31) had no additional effect on β-galactosidase activity levels. These results demonstrate that bfeR transcription is neither enterobactin responsive nor bfeR dependent.
Transcription of bfeA is BfeA independent.
BfeR is a member of the AraC family of transcriptional regulators, which characteristically require activation by their cognate inducer molecules. Since both bfeR and enterobactin are required for transcriptional activation of bfeA and the BfeA receptor is required for ferric enterobactin uptake, the involvement of BfeA in the induction of bfeA transcription was evaluated (Fig. 8). Expression levels of the bfeA-lacZ fusion (pRK44) in both wild-type B. bronchiseptica and the isogenic bfeA mutant strain BRM25 were essentially the same, indicating that high-affinity transport of enterobactin by the BfeA receptor is not required for enterobactin-mediated induction of bfeA transcription.
FIG. 8.
Analysis of bfeA-lacZ transcription in B. bronchiseptica bfeA mutant BRM25. Wild-type strain B013N and bfeA mutant strain BRM25 were grown in SS medium and assayed for β-galactosidase activity. Strains harbored plasmid pRK40 (plasmid vector control) or pRK44 (bfeA-lacZ). Error bars represent ±1 standard deviation from the means of independent triplicate determinations. Culture conditions: black bars, iron replete; gray bars, iron depleted; open bars, iron depleted and supplemented with enterobactin.
DISCUSSION
Beall and Sanden first determined that B. pertussis and B. bronchiseptica could utilize enterobactin and identified the iron-repressible bfeA gene encoding the receptor for ferric enterobactin (5) and DHBS (4). These investigators assessed enterobactin-inducible expression of a bfeA-phoA translational fusion in Bordetella cells and found no evidence of positive regulation (5). The reason for the apparent discordant results between that study and the present work remains unknown. However, the discrepancy could be due to differences in expression of the translational bfeA-phoA fusion versus the lacZ transcriptional fusions used in our studies. The results of our studies are consistent with another report that suggested enterobactin-inducible production of the B. bronchiseptica enterobactin receptor (61).
BfeR is a member of the iron subfamily of AraC transcriptional regulators, which are encoded by Fur-repressible genes and whose functions require activation by the cognate iron source. Potential Fur repressor DNA-binding sites had previously been predicted upstream of bfeA in B. pertussis (5), and we have demonstrated functional Fur binding to the bfeA-bfeR intergenic region in E. coli by using the Fur titration assay (58) (data not shown). Our studies demonstrate that expression of bfeA in Bordetella cells is stimulated in the presence of enterobactin and that this induction requires both iron starvation and bfeR. Downstream of bfeA is the bfeB ORF, encoding a deduced secreted product significantly similar to the S. enterica IroE protein. BfeB and IroE are predicted to be members of the α/β superfamily of hydrolases (COG2819) and have the conserved GXSXGG esterase protein family signature (InterPro Accession IPR000801, pfam00756) which is also present in the Fes protein. In E. coli, the Fes esterase is required for the release of iron from enterobactin following transport to the cytoplasm (13, 36); therefore, BfeB may provide a similar function to Bordetella cells.
Many bacteria possess transport systems for siderophores synthesized by other species, and microbial flora found on host surfaces may provide xenosiderophores to pathogens. Of the known siderophores, enterobactin has one of the highest ferric stability constants (37), and in a given environment it may be capable of removing the iron bound to other microbial siderophores, thereby limiting their iron-chelating utility to the producing microbes. Other bacterial species that utilize enterobactin as a xenosiderophore include Yersinia enterocolitica (53), P. aeruginosa (45), H. parainfluenzae and H. paraphrophilus (66), Vibrio cholerae (38, 49, 67), and N. meningitidis and N. gonorrhoeae (15). The ability of obligate respiratory pathogens such as B. pertussis and N. meningitidis to utilize enterobactin is intriguing, given that most known enterobactin-producing organisms reside in the intestinal tract. Bacterial receptors required for enterobactin uptake may also be used to transport other catechol siderophores. For example, in S. enterica, both FepA and IroN serve as receptors for enterobactin and DHBS; however, FepA is also required for myxochelin C transport, and IroN is required for uptake of corynebactin (48) and salmochelin (29). Thus, the enterobactin receptors of some pathogens may also be used for the uptake of other catechol siderophores that may be present in the host.
P. aeruginosa activates transcription of the pfeA enterobactin receptor gene by a two-component regulatory system involving the PfeS histidine kinase and PfeR response regulator proteins, which respond to the presence of enterobactin (18, 20). Expression of one of the enterobactin receptor genes of V. cholerae, irgA (38), is positively regulated by the LysR family regulator IrgB (26). Adjacent to the V. cholerae vctA enterobactin receptor gene is an ORF, VCA0231, predicted to encode an AraC-like regulator that may be involved in transcriptional control of vctA (38). Together with our observations, these reports indicate that mechanistically different regulators may be involved in transcriptional control of enterobactin receptor genes. To date, only the systems of P. aeruginosa and Bordetella spp. have been demonstrated to be inducible by enterobactin.
For the AraC-like proteins that regulate iron acquisition genes, the mechanism of activation by their cognate inducers is unknown. In Y. pestis, YbtA-mediated transcriptional activation of yersiniabactin system genes is independent of the Psn yersiniabactin receptor (21), TonB, and the yersiniabactin permease (44), indicating that active transport of the yersiniabactin inducer is not required. Similarly, our results indicate that in Bordetella cells, BfeA-mediated uptake of the enterobactin inducer is not required for transcriptional activation of bfeA by BfeR. In contrast, the pyochelin receptor FptA was reported to be required for transcriptional activation of fptA by PchR in P. aeruginosa (30). This would suggest that the pyochelin inducer requires receptor-mediated transport into the cell or that binding of the siderophore to the receptor stimulates transcriptional activation. Receptor-dependent transcriptional activation also characterizes the E. coli ferric citrate transport system, which is controlled by a surface signaling mechanism through the action of the FecI extracytoplasmic function σ factor (43, 65). In the absence of the pyochelin and yersiniabactin inducers, the regulators PchR of P. aeruginosa (30) and YbtA of Y. pestis (21), respectively, repress transcription of pchR and ybtA. In contrast to those systems, but similar to the Bordetella alcR regulator gene (12), bfeR does not appear to be autoregulated.
The genetic systems of the three known Bordetella iron acquisition mechanisms (alcaligin, heme, and now enterobactin) are repressed by Fur under iron-replete growth conditions, and each is transcriptionally activated under iron starvation conditions in the presence of the cognate iron compound (9, 12, 64). The BfeR protein identified in this study functions as an enterobactin-dependent AraC-type activator of bfeA transcription and is the first example of a regulator involved in expression of Bordetella xenosiderophore transport genes. Positive transcriptional regulation is an economically favorable mechanism to prioritize gene expression of these Bordetella iron uptake systems by activating transcription only when the relevant iron source is present in the environment.
Acknowledgments
We thank Timothy Brickman for discussions and critical reading of the manuscript and Hari Krishnan for providing plasmid pMP220. We are grateful to Bernard Beall for sharing B. bronchiseptica strains and plasmid pKP1. We acknowledge Anna Strain and Yuqing Chen for construction of pKP1 subclones and Anita Garrett for technical support. We thank our colleagues at the Sanger Centre for providing access to Bordetella nucleotide sequences prior to annotation.
Support for this study was provided by University of Minnesota grant-in-aid 19473 and Public Health Service grant AI-31088 from the National Institute of Allergy and Infectious Diseases; M.T.A. was supported by Public Health Service grant T32 AI-07421 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Akerley, B. J., P. A. Cotter, and J. F. Miller. 1995. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell 80:611-620. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, S. K., and M. O. Clements. 1993. Isolation and characterization of Bordetella bronchiseptica mutants deficient in siderophore activity. J. Bacteriol. 175:1144-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong, S. K., C. L. Francis, and M. A. McIntosh. 1990. Molecular analysis of the Escherichia coli ferric enterobactin receptor FepA. J. Biol. Chem. 265:14536-14543. [PubMed] [Google Scholar]
- 4.Beall, B., and T. Hoenes. 1997. An iron-regulated outer-membrane protein specific to Bordetella bronchiseptica and homologous to ferric siderophore receptors. Microbiology 143:135-145. [DOI] [PubMed] [Google Scholar]
- 5.Beall, B., and G. N. Sanden. 1995. A Bordetella pertussis fepA homologue required for utilization of exogenous ferric enterobactin. Microbiology 141:3193-3205. [DOI] [PubMed] [Google Scholar]
- 6.Beaumont, F. C., H. Y. Kang, T. J. Brickman, and S. K. Armstrong. 1998. Identification and characterization of alcR, a gene encoding an AraC-like regulator of alcaligin siderophore biosynthesis and transport in Bordetella pertussis and Bordetella bronchiseptica. J. Bacteriol. 180:862-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bordet, J., and O. Gengou. 1906. Le microbe de la coqueluche. Ann. Inst. Pasteur (Paris) 20:731-741. [Google Scholar]
- 8.Brickman, T. J., and S. K. Armstrong. 1996. The ornithine decarboxylase gene odc is required for alcaligin siderophore biosynthesis in Bordetella spp.: putrescine is a precursor of alcaligin. J. Bacteriol. 178:54-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brickman, T. J., and S. K. Armstrong. 1999. Essential role of the iron-regulated outer membrane receptor FauA in alcaligin siderophore-mediated iron uptake in Bordetella species. J. Bacteriol. 181:5958-5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brickman, T. J., and S. K. Armstrong. 2002. Bordetella interspecies allelic variation in AlcR inducer requirements: identification of a critical determinant of AlcR inducer responsiveness and construction of an alcR(Con) mutant allele. J. Bacteriol. 184:1530-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brickman, T. J., J. G. Hansel, M. J. Miller, and S. K. Armstrong. 1996. Purification, spectroscopic analysis and biological activity of the macrocyclic dihydroxamate siderophore alcaligin produced by Bordetella pertussis and Bordetella bronchiseptica. Biometals 9:191-203. [DOI] [PubMed] [Google Scholar]
- 12.Brickman, T. J., H. Y. Kang, and S. K. Armstrong. 2001. Transcriptional activation of Bordetella alcaligin siderophore genes requires the AlcR regulator with alcaligin as inducer. J. Bacteriol. 183:483-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brickman, T. J., and M. A. McIntosh. 1992. Overexpression and purification of ferric enterobactin esterase from Escherichia coli. Demonstration of enzymatic hydrolysis of enterobactin and its iron complex. J. Biol. Chem. 267:12350-12355. [PubMed] [Google Scholar]
- 14.Brickman, T. J., B. A. Ozenberger, and M. A. McIntosh. 1990. Regulation of divergent transcription from the iron-responsive fepB-entC promoter-operator regions in Escherichia coli. J. Mol. Biol. 212:669-682. [DOI] [PubMed] [Google Scholar]
- 15.Carson, S. D., P. E. Klebba, S. M. Newton, and P. F. Sparling. 1999. Ferric enterobactin binding and utilization by Neisseria gonorrhoeae. J. Bacteriol. 181:2895-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotter, P. A., and J. F. Miller. 1997. A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Mol. Microbiol. 24:671-685. [DOI] [PubMed] [Google Scholar]
- 17.Cox, G. B., F. Gibson, R. K. Luke, N. A. Newton, I. G. O'Brien, and H. Rosenberg. 1970. Mutations affecting iron transport in Escherichia coli. J. Bacteriol. 104:219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dean, C. R., S. Neshat, and K. Poole. 1996. PfeR, an enterobactin-responsive activator of ferric enterobactin receptor gene expression in Pseudomonas aeruginosa. J. Bacteriol. 178:5361-5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dean, C. R., and K. Poole. 1993. Cloning and characterization of the ferric enterobactin receptor gene (pfeA) of Pseudomonas aeruginosa. J. Bacteriol. 175:317-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dean, C. R., and K. Poole. 1993. Expression of the ferric enterobactin receptor (PfeA) of Pseudomonas aeruginosa: involvement of a two-component regulatory system. Mol. Microbiol. 8:1095-1103. [DOI] [PubMed] [Google Scholar]
- 21.Fetherston, J. D., S. W. Bearden, and R. D. Perry. 1996. YbtA, an AraC-type regulator of the Yersinia pestis pesticin/yersiniabactin receptor. Mol. Microbiol. 22:315-325. [DOI] [PubMed] [Google Scholar]
- 22.Fiedler, H. P., P. Krastel, J. Muller, K. Gebhardt, and A. Zeeck. 2001. Enterobactin: the characteristic catecholate siderophore of Enterobacteriaceae is produced by Streptomyces species. FEMS Microbiol. Lett. 196:147-151. [DOI] [PubMed] [Google Scholar]
- 23.Field, L. H., and C. D. Parker. 1979. Differences observed between fresh isolates of Bordetella pertussis and their laboratory-passaged derivatives, p. 124-132. In C. R. Manclark and J. C. Hill (ed.), International Symposium on Pertussis. U.S. Department of Health, Education, and Welfare, Washington, D.C.
- 24.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallegos, M. T., R. Schleif, A. Bairoch, K. Hofmann, and J. L. Ramos. 1997. AraC/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 61:393-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberg, M. B., S. A. Boyko, and S. B. Calderwood. 1991. Positive transcriptional regulation of an iron-regulated virulence gene in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 88:1125-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodnow, R. A. 1980. Biology of Bordetella bronchiseptica. Microbiol. Rev. 44:722-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gubler, C. J. 1956. Absorption and metabolism of iron. Science 123:87-90. [DOI] [PubMed] [Google Scholar]
- 29.Hantke, K., G. Nicholson, W. Rabsch, G. Winkelmann. 2003. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc. Natl. Acad. Sci. USA 100:3677-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinrichs, D. E., and K. Poole. 1996. PchR, a regulator of ferripyochelin receptor gene (fptA) expression in Pseudomonas aeruginosa, functions both as an activator and as a repressor. J. Bacteriol. 178:2586-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang, H. Y., and S. K. Armstrong. 1998. Transcriptional analysis of the Bordetella alcaligin siderophore biosynthesis operon. J. Bacteriol. 180:855-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang, H. Y., T. J. Brickman, F. C. Beaumont, and S. K. Armstrong. 1996. Identification and characterization of iron-regulated Bordetella pertussis alcaligin siderophore biosynthesis genes. J. Bacteriol. 178:4877-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 34.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 35.Kovach, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop II, and K. M. Peterson. 1994. pBBR1MCS: a broad-host-range cloning vector. BioTechniques 16:800-802. [PubMed] [Google Scholar]
- 36.Langman, L., I. G. Young, G. E. Frost, H. Rosenberg, and F. Gibson. 1972. Enterochelin system of iron transport in Escherichia coli: mutations affecting ferric-enterochelin esterase. J. Bacteriol. 112:1142-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loomis, L. D., and K. N. Raymond. 1991. Solution equilibria of enterobactin and metal-enterobactin complexes. Inorg. Chem. 30:906-911. [Google Scholar]
- 38.Mey, A. R., E. E. Wyckoff, A. G. Oglesby, E. Rab, R. K. Taylor, and S. M. Payne. 2002. Identification of the Vibrio cholerae enterobactin receptors VctA and IrgA: IrgA is not required for virulence. Infect. Immun. 70:3419-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 40.Moore, C. H., L. A. Foster, D. G. Gerbig, Jr., D. W. Dyer, and B. W. Gibson. 1995. Identification of alcaligin as the siderophore produced by Bordetella pertussis and B. bronchiseptica. J. Bacteriol. 177:1116-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neilands, J. B., and K. Nakamura. 1991. Detection, determination, isolation, characterization and regulation of microbial iron chelates, p. 1-14. In G. Winkelmann (ed.), Handbook of microbial iron chelates. CRC Press, Inc., Boca Raton, Fla.
- 42.O'Brien, I. G., and F. Gibson. 1970. The structure of enterochelin and related 2,3-dihydroxy-N-benzoylserine conjugates from Escherichia coli. Biochim. Biophys. Acta 215:393-402. [DOI] [PubMed] [Google Scholar]
- 43.Ochs, M., S. Veitinger, I. Kim, D. Welz, A. Angerer, and V. Braun. 1995. Regulation of citrate-dependent iron transport of Escherichia coli: fecR is required for transcription activation by FecI. Mol. Microbiol. 15:119-132. [DOI] [PubMed] [Google Scholar]
- 44.Perry, R. D., J. Abney, I. Mier, Jr., Y. Lee, S. W. Bearden, and J. D. Fetherston. 2003. Regulation of the Yersinia pestis Yfe and Ybt iron transport systems, p. 275-283. In M. Skurnik, J. A. Bengoechea, and K. Granfors (ed.), The genus Yersinia: entering the functional genomic era. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 45.Poole, K., L. Young, and S. Neshat. 1990. Enterobactin-mediated iron transport in Pseudomonas aeruginosa. J. Bacteriol. 172:6991-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pradel, E., and C. Locht. 2001. Expression of the putative siderophore receptor gene bfrZ is controlled by the extracytoplasmic-function sigma factor BupI in Bordetella bronchiseptica. J. Bacteriol. 183:2910-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Querinjean, P., P. L. Masson, and J. F. Heremans. 1971. Molecular weight, single-chain structure and amino acid composition of human lactoferrin. Eur. J. Biochem. 20:420-425. [DOI] [PubMed] [Google Scholar]
- 48.Rabsch, W., W. Voigt, R. Reissbrodt, R. M. Tsolis, and A. J. Baumler. 1999. Salmonella typhimurium IroN and FepA proteins mediate uptake of enterobactin but differ in their specificity for other siderophores. J. Bacteriol. 181:3610-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rutz, J. M., T. Abdullah, S. P. Singh, V. I. Kalve, and P. E. Klebba. 1991. Evolution of the ferric enterobactin receptor in gram-negative bacteria. J. Bacteriol. 173:5964-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 51.Schade, A. L., and L. Caroline. 1946. An iron-binding component of human blood plasma. Science 104:340-341. [DOI] [PubMed] [Google Scholar]
- 52.Schneider, D. R., and C. D. Parker. 1982. Effect of pyridines on phenotypic properties of Bordetella pertussis. Infect. Immun. 38:548-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schubert, S., D. Fischer, and J. Heesemann. 1999. Ferric enterochelin transport in Yersinia enterocolitica: molecular and evolutionary aspects. J. Bacteriol. 181:6387-6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simon, E. H., and I. Tessman. 1963. Thymidine-requiring mutants of phage T4. Proc. Natl. Acad. Sci. USA 50:526-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spaink, H. P., R. J. H. Okker, C. A. Wijffelman, E. Pees, and B. J. J. Lugtenberg. 1987. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol. Biol. 9:27-39. [DOI] [PubMed] [Google Scholar]
- 56.Stainer, D. W., and M. J. Scholte. 1970. A simple chemically defined medium for the production of phase I Bordetella pertussis. J. Gen. Microbiol. 63:211-220. [DOI] [PubMed] [Google Scholar]
- 57.Stibitz, S. 1994. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol. 235:458-465. [DOI] [PubMed] [Google Scholar]
- 58.Stojiljkovic, I., A. J. Baumler, and K. Hantke. 1994. Fur regulon in gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a Fur titration assay. J. Mol. Biol. 236:531-545. [DOI] [PubMed] [Google Scholar]
- 59.Switzer, W. P. 1956. Studies on infectious atrophic rhinitis. V. Concept that several agents may cause turbinate atrophy. Am. J. Vet. Res. 17:478-484. [PubMed] [Google Scholar]
- 60.Thompson, H., I. A. McCandlish, and N. G. Wright. 1976. Experimental respiratory disease in dogs due to Bordetella bronchiseptica. Res. Vet. Sci. 20:16-23. [PubMed] [Google Scholar]
- 61.Thulasiraman, P., S. M. Newton, J. Xu, K. N. Raymond, C. Mai, A. Hall, M. A. Montague, and P. E. Klebba. 1998. Selectivity of ferric enterobactin binding and cooperativity of transport in gram-negative bacteria. J. Bacteriol. 180:6689-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vanderpool, C. K., and S. K. Armstrong. 2001. The Bordetella bhu locus is required for heme iron utilization. J. Bacteriol. 183:4278-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vanderpool, C. K., and S. K. Armstrong. 2003. Heme-responsive transcriptional activation of Bordetella bhu genes. J. Bacteriol. 185:909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Hove, B., H. Staudenmaier, and V. Braun. 1990. Novel two-component transmembrane transcription control: regulation of iron dicitrate transport in Escherichia coli K-12. J. Bacteriol. 172:6749-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams, P., D. J. Morton, K. J. Towner, P. Stevenson, and E. Griffiths. 1990. Utilization of enterobactin and other exogenous iron sources by Haemophilus influenzae, H. parainfluenzae, and H. paraphrophilus. J. Gen. Microbiol. 136:2343-2350. [DOI] [PubMed] [Google Scholar]
- 67.Wyckoff, E. E., A. M. Valle, S. L. Smith, and S. M. Payne. 1999. A multifunctional ATP-binding cassette transporter system from Vibrio cholerae transports vibriobactin and enterobactin. J. Bacteriol. 181:7588-7596. [DOI] [PMC free article] [PubMed] [Google Scholar]