Abstract
The osa (oncogenic suppressive activity) gene of the IncW group plasmid pSa is sufficient to suppress tumorigenesis by Agrobacterium tumefaciens. osa confers oncogenic suppression by inhibiting VirE2 protein export. This result is similar, but not identical, to that of oncogenic suppression by the IncQ plasmid RSF1010. We conducted a series of experiments to compare oncogenic suppression by these two systems. Agrobacterium strains harboring plasmids containing osa are more able to effect oncogenic suppression than are similar strains containing various RSF1010 derivatives. When osa is present within a donor Agrobacterium strain that also carries a derivative of RSF1010, the transfer of RSF1010 derivatives to recipient bacteria and their establishment in plants are blocked. Oncogenic suppression is still effected when the osa gene is integrated into the Agrobacterium chromosome, suggesting that it is the osa gene product that is active in suppression and that suppression does not require a protein-nucleic acid intermediate like that described for IncQ plasmids. Extracellular complementation experiments with tobacco leaf disks indicated that Osa blocks stable transfer of RSF1010 to plant cells by inhibiting transfer of VirE2, which is essential for the transfer of RSF1010 into plant cells, and not by inhibiting the actual transfer of RSF1010 itself. Our results suggest that Osa and RSF1010 cause oncogenic suppression by using different mechanisms.
When coresident with the Ti (tumor-inducing) plasmid in Agrobacterium tumefaciens, the IncW plasmid pSa can inhibit the genetic transformation of plant cells (11, 23). The osa (oncogenic suppressive activity) gene of pSa is sufficient to inhibit plant transformation (7, 9). This phenomenon, called oncogenic suppression, resembles fertility inhibition of conjugative plasmid transfer by plasmids of different incompatibility groups (16, 24, 27). A possible explanation for oncogenic suppression is that osa inhibits the transfer of the Agrobacterium transfer DNA (T-DNA) to plant cells. However, it was demonstrated recently that osa does not affect the transfer of T-DNA; rather, osa inhibits the export of the single-stranded DNA binding protein VirE2 (19).
The IncQ plasmid RSF1010 can also cause oncogenic suppression (4, 34). Although RSF1010 is not self-transmissible, other plasmids, including the Ti plasmid, can mobilize it between Agrobacterium cells (3, 8). The Ti plasmid can also mobilize RSF1010 to plant cells; this transfer depends upon the type IV secretion system encoded by the virB/virD4 genes (6, 34). Thus, RSF1010 conjugative transfer between Agrobacterium cells resembles T-DNA transfer from Agrobacterium to plant cells. Stahl et al. (33) reported that an RSF1010 nucleic acid-protein conjugative intermediate is required for oncogenic suppression of Agrobacterium. They suggested that this intermediate may compete with the VirD2-T-strand complex and/or VirE2 for the VirB/D4 export apparatus. In accordance with this model, Ward et al. (34) showed that overexpression of virB9, virB10, and virB11 from an octopine-type Ti plasmid could reverse oncogenic suppression by RSF1010.
Although osa causes oncogenic suppression by blocking VirE2 but not T-DNA transfer to plant cells, RSF1010 apparently inhibits both VirE2 export and (to a lesser extent) T-DNA export (4). In addition, oncogenic suppression by osa cannot be reversed by overexpression of the virB9, virB10, and virB11 genes from the nopaline-type Ti plasmid pTiC58 (19). We therefore sought to determine whether there is a fundamental difference between the mechanisms of oncogenic suppression by RSF1010 and pSa or whether the differences previously noted were merely quantitative.
The lack of reversal of oncogenic suppression by osa when additional copies of virB9, virB10, and virB11 are expressed in Agrobacterium, along with our finding that a conjugative intermediate is not required for oncogenic suppression by osa, suggests that a distinctive mechanism of oncogenic suppression is conferred by Osa.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Table 1 describes the various A. tumefaciens strains and plasmids used in this study. A. tumefaciens was grown at 30°C in either YEP rich medium or AB-sucrose minimal medium (21). When appropriate, the following antibiotics were used: rifampin (10 μg/ml), carbenicillin (100 μg/ml in solid medium and 50 μg/ml in liquid medium); kanamycin (25 μg/ml), spectinomycin (100 μg/ml), erythromycin (150 μg/ml), and tetracycline (2 μg/ml in liquid medium and 10 μg/ml in solid medium).
TABLE 1.
A. tumefaciens strains and plasmids used in this study
| Strain or plasmida | Relevant characteristics | Antibiotic resistanceb | Reference or source |
|---|---|---|---|
| Strains | |||
| A136 | Nononcogenic derivative of C58; lacks pTi | Rif | 35 |
| A208 | Oncogenic nopaline-type strain; contains pTiT37 | Rif | 31 |
| A281 | Oncogenic agropine-type strain; contains pTiBo542 | Rif | 31 |
| A348 | Oncogenic octopine-type strain; contains pTiA6 | Rif | 14 |
| At221 | Tn3-HoHoI insertion in virE2; A348mx358 | Car, Rif | 32 |
| At333 | UIA143 | Ery | 12 |
| At902 | LBA4404(pUCD3960) | Rif, Spe | 19 |
| At903 | LBA4404(pUCD5533) | Rif, Spe | 19 |
| At906 | At221(pUCD3960) | Car, Spe | 19 |
| At907 | At221(pUCD5533) | Car, Spe | 19 |
| At928 | A208(pBISN1) | Kan, Rif | 19 |
| At931 | A208(pBISN1, pUCD3960) | Car, Kan, Rif, Spe | 19 |
| At932 | A208(pBISN1, pUCD5533) | Car, Kan, Rif, Spe | 19 |
| At986 | A208(pBISN1, pJB31) | Kan, Spe | This study |
| At998 | A136(pJW323) | Kan, Rif | This study |
| At999 | A348(pJW323) | Kan, Rif | This study |
| At1000 | A208(pJW323) | Kan, Rif | This study |
| At1001 | A281(pJW323) | Kan, Rif | This study |
| At1002 | A136(pUCD5533) | Car, Rif, Spe | This study |
| At1003 | A348(pUCD5533) | Car, Rif, Spe | This study |
| At1004 | A208(pUCD5533) | Car, Rif, Spe | This study |
| At1005 | A281(pUCD5533) | Car, Rif, Spe | This study |
| At1006 | A348(pJW323, pUCD5533) | Car, Kan, Rif, Spe | This study |
| At1007 | A348(pJW323, pUCD3960) | Car, Kan, Rif, Spe | This study |
| At1008 | A136(pUCD3960) | Car, Rif, Spe | This study |
| At1009 | A348(pUCD3960) | Car, Rif, Spe | This study |
| At1011 | A281(pUCD3960) | Car, Rif, Spe | This study |
| At1016 | A136(pSa) | Chl, Kan, Rif, Spe, Suf | This study |
| At1017 | A348(pSa) | Chl, Kan, Rif, Spe, Suf | This study |
| At1018 | A208(pSa) | Chl, Kan, Rif, Spe, Suf | This study |
| At1019 | A281(pSa) | Chl, Kan, Rif, Spe, Suf | This study |
| At1020 | A136(pSa::neo) | Chl, Kan, Neo, Rif, Spe, Sul | This study |
| At1021 | A348(pSa::neo) | Chl, Kan, Neo, Rif, Spe, Sul | This study |
| At1022 | A208(pSa::neo) | Chl, Kan, Neo, Rif, Spe, Sul | This study |
| At1023 | A281(pSa::neo) | Chl, Kan, Neo, Rif, Spe, Sul | This study |
| At1055 | A348(pML122, pUCD3960) | Car, Gen, Kan, Rif, Spe | This study |
| At1056 | A348(pML122, pUCD5533) | Car, Gen, Kan, Rif, Spe | This study |
| At1057 | A136(pML122) | Gen, Kan, Rif | This study |
| At1058 | A348(pML122) | Gen, Kan, Rif | This study |
| At1059 | A208(pML122) | Gen, Kan, Rif | This study |
| At1060 | A281(pML122) | Gen, Kan, Rif | This study |
| At1076 | A136(pJB31) | Rif, Spe | This study |
| At1077 | A348(pJB31) | Rif, Spe | This study |
| At1078 | A208(pJB31) | Rif, Spe | This study |
| At1079 | A281(pJB31) | Rif, Spe | This study |
| At1080 | A281::osa | Rif, Spe | This study |
| At1081 | A348::vector | Rif, Spe | This study |
| At1084 | A348::osa | Rif, Spe | This study |
| At1096 | A348ΔvirB | Rif | 1 |
| At1111 | At1096::osa | Rif, Spe | This study |
| At1112 | At1096::vector | Rif, Spe | This study |
| At1113 | A208(pBISN1, pED9) | Kan, Rif, Tet | This study |
| At1114 | A208(pBISN1, pED9, pUCD3960) | Car, Kan, Rif, Spe, Tet | This study |
| At1115 | A208(pBISN1, pED9, pUCD5533) | Car, Kan, Rif, Spe, Tet | This study |
| At1116 | A208(pBISN1, pED9, pJB31) | Kan, Rif, Spe, Tet | This study |
| At1148 | At1081(pML122) | Gen, Kan, Rif, Spe | This study |
| At1149 | At1084(pML122) | Gen, Kan, Rif, Spe | This study |
| At1160 | UIA143(pTiA6) | Ery, Rif | 5 |
| At1181 | UIA143(pTiA6, pE1649) | Ery, Spe, Tet | This study |
| At1182 | UIA143(pTiA6, pE1650) | Ery, Spe, Tet | This study |
| At1312 | At902(pJW323) | Kan, Rif, Spe | This study |
| At1313 | At903(pJW323) | Kan, Rid, Spe | This study |
| At1314 | At906(pJW323) | Car, Kan, Spe | This study |
| At1315 | At907(pJW323) | Car, Kan, Spe | This study |
| At1368 | LBA1251(pTiA6, pML122, pUCD3960) | Car, Gen, Rif, Spe, Tet, | This study |
| At1369 | LBA1251(pTiA6, pML122, pUCD5533) | Car, Gen, Rif, Spe, Tet | This study |
| LBA1251 | C58 chromosome, lacks pTi and pAtC58 | Rif | Paul Hooykaas |
| LBA4404 | Disarmed octopine-type strain; lacks T-DNA | Rif | 28 |
| Plasmids | |||
| pAD1361 | Complete virB operon plus virGN54D | Car, Tet | 10 |
| pBISN1 | T-DNA binary vector; contains a nos-nptII and superpromoter-gusA intron gene | Kan | 26 |
| pJB31 | RSF1010 derivative | Spe | 2 |
| pJW323 | RSF1010 derivative, contains a nos-nptII plant-selectable marker | Kan | 34 |
| pML122 | RSF1010-derived broad-host-range vector | Gen, Tet | 18 |
| pE578 | IncP plasmid carrying the pgl/picA locus as an EcoRI fragment from the A. tumefaciens C58 chromosome | Tet | 30 |
| pE1649 | PstI fragment containing the osa and spectinomycin resistance genes cloned into pE578 | Spe, Tet | This study |
| pE1650 | PstI fragment containing a spectinomycin resistance gene cloned into pE578; vector control for osa construction | Spe, Tet | This study |
| pED9 | virB, virB10, and virB11 genes under the control of the pTiA6 virB promoter | Tet | 34 |
| pPH1JI | IncP plasmid; used for eviction of other IncP plasmids during marker exchange mutagenesis | Gen | 15 |
| pSa | Oncogenic suppressive plasmid | Chl, Kan, Spe, Sul | 23 |
| pSa::neo | Neomycin phosphotransferase gene disruption of the osa gene of pSa | Chl, Kan, Neo, Spe, Sul | 19 |
| pUCD3960 | osa gene under the control of an npt promoter in pUCD105 | Car, Spe | 19 |
| pUCD5533 | Asp718-blunted derivative of pUCD105 | Car, Spe | 19 |
Laboratory stock designations: A, E. W. Nester; At, S. B. Gelvin; UCD, C. I. Kado; AD, A. Das.
Car, carbenicillin; Chl, chloramphenicol; Ery, erythromycin; Gen, gentamicin; Kan, kanamycin; Neo, neomycin; Rif, rifampicin; Spe, spectinomycin; Sul, sulfonamide; Tet, tetracycline.
Tumorigenesis assays.
Kalanchöe daigremontiana plants were used for tumorigenesis assays on leaves. A. tumefaciens cells were grown to the stationary phase, washed, and resuspended in 0.9% NaCl to cell densities of 109, 1010, and 1011 cells/ml. Ten-microliter portions of each cell suspension were inoculated onto multiple wound sites on the leaves. Visible tumors developed approximately 2 weeks after inoculation, and well-developed tumors were photographed approximately 4 weeks after inoculation.
Tobacco leaf disk assays.
Disks from axenically grown Nicotiana tabacum cv. Wisconsin 38 were cut with a cork borer and inoculated with A. tumefaciens as previously described (19). Two days after inoculation, disks were moved to selective medium containing 100 μg of timentin per ml to kill Agrobacterium. To select for tumors, disks were incubated on MS medium (25) lacking phytohormones. To select for kanamycin-resistant calli, disks were incubated on MS medium containing phytohormones (callus-inducing medium) and 100 μg of kanamycin per ml. To assay for β-glucuronidase activity, disks were sampled, stained with 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (X-Gluc) overnight, and then destained in 70% ethanol (17). For extracellular complementation experiments, equal concentrations of bacteria (RSF1010 donor or VirE2 donor) were mixed and used to infect tobacco leaf disks for 2 days. Leaf disks were then transferred to callus-inducing medium containing timentin to kill Agrobacterium and kanamycin to select for kanamycin-resistant calli.
Bacterial conjugation.
Octopine-type Agrobacterium strain A348 (At6) containing a kanamycin-resistant derivative of RSF1010, pML122 (provided by Eugene Nester), with or without an osa gene construction was used as a donor. As mentioned below, strain A348 with or without the osa gene integrated into the chromosomal pgl/picA locus was also used as a donor. pTi-cured nopaline-type Agrobacterium strain UIA143 (At333, erythromycin resistant) with or without pTiA6 and with or without osa was used as a recipient. Both donor and recipient cells were grown at 30°C in YEP medium overnight. The next day, cells were transferred to glucose-containing induction medium (pH 5.6) with or without 100 μM acetosyringone to obtain a density of 2.5 × 108 cells/ml. Cells were grown at 22°C for 6 h. Donor and recipient cells were mixed at a ratio of 7:1, and 10 μl of the cell mixture was spotted onto AB glucose agar induction medium (pH 5.6) containing or lacking 100 μM acetosyringone. The conjugation plates were incubated at 20°C for 3 days. One milliliter of 0.9% NaCl was used to wash and resuspend the conjugation mixture from the plates. Tenfold serial dilutions were made in 0.9% NaCl, and 100-μl portions of appropriate dilutions were plated on AB-sucrose medium containing both erythromycin and kanamycin to select for transconjugants that harbored pML122. Donor and recipient cells were quantified by plating the bacteria on AB-sucrose medium containing rifampin and on AB-sucrose medium containing erythromycin, respectively.
Chromosomal integration of the osa gene.
A PstI fragment containing both osa and the spectinomycin resistance gene from pUCD3960 (7) or a control fragment lacking the osa gene from pUCD5533 (19) was cloned into the PstI site of plasmid pE578 between the pgl and picA genes of A. tumefaciens (20), generating pE1649 and pE1650, respectively. Plasmids pE1649 and pE1650 were electroporated into various Agrobacterium strains (A136, A348, A208, and A281). Spectinomycin- and tetracycline-resistant transformants were selected. Subsequently, plasmid pPH1JI (gentamicin resistant) was introduced by conjugation into each of these Agrobacterium strains, and transconjugants which were resistant to gentamicin and spectinomycin and sensitive to tetracycline were selected. Total DNA from the colonies was isolated and subjected to EcoRI digestion and gel electrophoresis and blotted onto nylon membranes. A 3.1-kbp EcoRI fragment containing the pgl/picA locus was used as a hybridization probe to confirm integration of the osa gene into the Agrobacterium chromosome.
RESULTS
A. tumefaciens strains containing the IncW plasmid pSa show stronger oncogenic suppression than strains containing many derivatives of the IncQ plasmid RSF1010 show when they are inoculated onto Kalanchöe plants.
Both the osa gene and the IncQ plasmid RSF1010 confer oncogenic suppression upon Agrobacterium. We therefore tested several strains of Agrobacterium harboring various pSa or RSF1010 derivatives to determine the strength of oncogenic suppression conferred by IncW and IncQ plasmids. Agrobacterium strains A136 (lacking a Ti plasmid), A208 (containing the nopaline-type Ti plasmid pTiT37), A348 (containing the octopine-type Ti plasmid pTiA6), and A281 (containing the supervirulent agropine-type Ti plasmid pTiBo542) were used to test oncogenic suppression on leaves of Kalanchoë plants by the IncW plasmid pSa, a plasmid carrying the osa gene (pUCD3960), or derivatives of the IncQ plasmid RSF1010 (pML122, pJB31, and pJW323). Figure 1 shows the results of oncogenic suppression of A. tumefaciens A348 by pSa, osa, and pJW323. osa either on its parental plasmid pSa or on the pTAR-based plasmid pUCD3960 was more complete in oncogenic suppression than the RSF1010 derivatives pJB31 and pJW323 were. Similar results were obtained with A. tumefaciens A208 and A281 (data not shown). When the osa gene was disrupted by a neomycin cassette (pSa::neo), no oncogenic suppression was observed (data not shown). These results indicate that Agrobacterium strains harboring pSa or the osa gene are less virulent than Agrobacterium strains harboring various derivatives of RSF1010 are and that therefore osa may be a more potent inhibitor of virulence than RSF1010 is.
FIG. 1.
Oncogenic suppression of A. tumefaciens strains by pSa, osa, and RSF1010. (A) Template for inoculation of Kalanchoë leaf wound sites. The number of bacteria inoculated into each wound is indicated. (B) Inoculation with A. tumefaciens A348 containing plasmids. Leaf 1, At1003; leaf 2, At1017; leaf 3, At1009; leaf 4, At999. The leaves were photographed 1 month after inoculation.
osa inhibits the establishment of RSF1010 in plant cells by inhibiting the transfer of VirE2 protein.
A. tumefaciens can transfer the RSF1010 derivative pJW323, carrying a plant-active nptII gene, to plant cells. Although pJW323 partially inhibits tumorigenesis by A. tumefaciens, the infected plant cells may become kanamycin resistant as a result of stable expression of the nptII gene (34). We introduced pJW323 into A. tumefaciens strains containing either osa on plasmid pUCD3960 or the vector control plasmid pUCD5533 and infected tobacco leaf disks to determine whether osa inhibits the transfer of RSF1010 from A. tumefaciens to plant cells. The presence of osa, but not the presence of the vector control plasmid, in A. tumefaciens suppressed formation of both tumors and kanamycin-resistant calli (Fig. 2). When we infected tobacco leaf disks with an A. tumefaciens strain containing pJW323 plus the vector control plasmid, some small tumors still developed on disks incubated on plant medium lacking phytohormones (Fig. 2A). As a result of transfer of pJW323, some kanamycin-resistant calli developed on kanamycin-containing callus-inducing medium (Fig. 2B). These data are consistent with our findings that the presence of pJW323 in A. tumefaciens only partially inhibits tumorigenesis on Kalanchöe (Fig. 1) and further indicate that osa can inhibit the establishment of RSF1010 in plant cells.
FIG. 2.
osa inhibits transformation of tobacco leaf disks by RSF1010. Tobacco leaf disks were inoculated with various A. tumefaciens strains for 2 days and then were transferred to MS medium containing timentin and either lacking phytohormones (A) or containing phytohormones and kanamycin (B). The plates were photographed 1 month after infection. A136, no pTi; A348, A136 containing pTiA6; A348(pJW323, vector), At1006; A348(pJW323, osa), At1007.
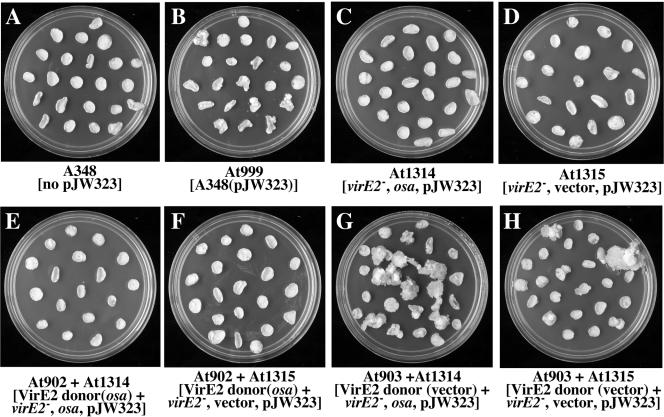
We next introduced pJW323 into virE2 mutant and VirE2 donor Agrobacterium strains harboring either osa or a vector control plasmid and infected tobacco leaf disks with various combinations of Agrobacterium strains in extracellular complementation experiments. Two days after cocultivation with various bacterial combinations, leaf disks were transferred to either plant basal medium lacking phytohormones to detect tumor formation (data not shown) or callus-inducing medium containing kanamycin to detect the formation of kanamycin-resistant calli as a result of RSF1010 transfer. The results of experiments to investigate kanamycin-resistant callus formation are shown in Fig. 3. A. tumefaciens A348 containing pJW323 (At999) alone was able to transfer RSF1010 into plant cells to form kanamycin-resistant calli on 59% of the leaf disks (Fig. 3A and B). The VirE2 donor strain LBA4404 (At1313), but not virE2 mutant strain At1314 or At1315 containing RSF1010, also incited kanamycin-resistant calli (Fig. 3C and D) (38% of the leaf disks with At1313 compared with no leaf disks with At1314 or At1315). These results indicate that VirE2 is required for establishment of RSF1010 in plant cells, as previously described (4). The presence of osa in strain At1312 completely blocked the formation of kanamycin-resistant calli. Kanamycin-resistant callus formation could be restored by coinfecting leaf disks with a mixture of a VirE2 donor (At903) and either At1314 (64% of the disks) or At1315 (29% of the disks) (the At903 strain provided VirE2 and the At1314 and At1315 strains provided RSF1010) (Fig. 3G and H). On the other hand, when VirE2 export was blocked by osa in A. tumefaciens At902, neither At1313 nor At1314 induced formation of any kanamycin-resistant calli (Fig. 3E and F). Taken together, these results indicate that osa does not block RSF1010 transfer per se to plant cells but rather inhibits VirE2 transfer that is required for formation of kanamycin-resistant calli after RSF1010 transfer.
FIG. 3.
osa blocks RSF1010 transfer from A. tumefaciens to plants by inhibiting the transfer of VirE2. Tobacco leaf disks were inoculated with various A. tumefaciens strains for 2 days and then were transferred to MS medium containing timentin, phytohormones, and kanamycin. The plates were photographed 1 month after infection.
osa inhibits virB-mediated conjugative transfer of RSF1010 between Agrobacterium cells.
A. tumefaciens can transfer RSF1010 derivatives between bacterial cells in a virB-dependent manner (3). To determine whether osa inhibits conjugative transfer of RSF1010 between Agrobacterium cells, we introduced pML122 into the rifampin-resistant strain A. tumefaciens A348. pML122 is a derivative of RSF1010 that contains a kanamycin resistance marker (18). We also introduced into this strain either a plasmid that contains osa or a control plasmid and mated the resulting strains (At1055 and At1056, respectively) with the erythromycin-resistant strain UIA143, selecting for kanamycin- and erythromycin-resistant bacteria. Table 2 shows that the conjugative transfer of pML122 depended upon the vir gene inducing the compound acetosyringone, as previously reported (3, 13). Conjugative transfer of pML122 was markedly decreased (by 4 orders of magnitude) when osa was present in the Agrobacterium donor strain.
TABLE 2.
osa inhibits the vir gene-dependent conjugative transfer of RSF1010a
| Strain | Acetosyringone induction | Transfer frequency (transconjugant/donor) |
|---|---|---|
| A348(pML122, vector) (At1056) | − | <1.9 × 10−12 |
| + | 1.3 × 10−7 | |
| A348(pML122, osa) (At1055) | − | <2.2 × 10−12 |
| + | <4.1 × 10−11 |
A. tumefaciens A348 was used as the donor, and A. tumefaciens UIA143 was used as the recipient. The results are representative of five independent experiments.
To determine whether osa can function in the recipient bacterial cell to inhibit the conjugative transfer of RSF1010, we introduced into A. tumefaciens UIA143(pTiA6) a plasmid carrying osa or an empty vector control plasmid and used these strains as recipients in mating experiments. Our results indicate that although osa could inhibit the acetosyrongone-dependent transfer of RSF1010 when it was present in donor cells, it did not inhibit conjugative transfer when it was present in the recipient cells (data not shown).
A conjugative intermediate is not required for oncogenic suppression and inhibition of RSF1010 transfer between bacteria by osa.
To date, all evidence for the oncogenic suppressive effect of osa and the inhibition of RSF1010 conjugative transfer by osa has involved A. tumefaciens strains harboring osa on a plasmid. Stahl et al. (33) reported that a nucleic acid-protein conjugative intermediate of RSF1010 is required for the oncogenic suppressive effect of RSF1010. In order to determine whether oncogenic suppression by osa requires a plasmid DNA-Osa protein complex as a conjugative intermediate, we constructed A. tumefaciens strains containing the osa gene inserted into the pgl/picA locus (31) of the A. tumefaciens C58 chromosome. Rong et al. (30) previously demonstrated that disruption of this chromosomal locus does not affect tumorigenesis. We used strains containing the osa gene either on a plasmid or integrated into the chromosome and vector control strains to inoculate wound sites on Kalanchöe leaves. Figure 4 shows that osa suppressed the oncogenicity of A. tumefaciens strains A348 and A281 regardless of whether the osa gene was located on a plasmid [A348(osa) and A281(osa)] or integrated into the chromosome [A348::osa (At1084) and A281::osa (At1080)]. The presence in these strains of an empty plasmid vector either as a separate replicon or integrated into the chromosome did not result in oncogenic suppression. These results suggest that expression of the osa gene from a chromosomal locus is sufficient to cause oncogenic suppression even of the highly oncogenic strain A. tumefaciens A281. The A. tumefaciens strains containing osa integrated into the chromosome do not contain a plasmid that is transfer competent under the conditions used. Because no transfer-competent relaxation complex can be formed in these strains, our results suggest that the Osa protein itself directly effects oncogenic suppression. It is highly unlikely that oncogenic suppression is mediated by osa mRNA because disruption of the Osa open reading frame results in loss of oncogenic suppression (data not shown).
FIG. 4.
osa can confer oncogenic suppression when it is integrated into the A. tumefaciens chromosome. Wound sites on a Kalachöe leaf were inoculated with 109 cells of various A. tumefaciens strains, and the leaf was photographed 1 month later. A348, A136 containing pTiA6; A281, A136 containing pTiBo542; A348(osa), At1009; A281(osa), At1011; A348::osa, At1084; A281::osa, At1080; A348(vector), At1081; A136, no pTi.
We further tested whether the presence of osa within a chromosomal locus inhibits the conjugative transfer of RSF1010 between Agrobacterium cells. In these experiments, we used as a recipient an A. tumefaciens strain containing a Ti plasmid [UIA143(pTiA6) (= At1160)] because Bohne et al. (5) and Liu and Binns (22) showed that the use of such a strain increases the conjugation frequency of RSF1010 derivatives. Table 3 shows that when we used an Agrobacterium strain (At1149) carrying the osa gene integrated into the donor bacterial chromosome, conjugative transfer of RSF1010 (pML122) was inhibited to an extent similar to the extent observed when osa was present on a plasmid in the donor bacterium (Table 2).
TABLE 3.
Inhibition of RSF1010 transfer by chromosome-localized osa genesa
| Donor strain | Acetosyringone induction of donor | Conjugation frequency (per donor) | Conjugation frequency (per recipient)b |
|---|---|---|---|
| At1148 (A348::vector) | − | <4.72 × 10−10 | <3.05 × 10−10 |
| At1148 (A348::vector) | + | 1.50 × 10−5 | 9.95 × 10−6 |
| At1149 (A348::osa) | + | <1.01 × 10−9 | <3.83 × 10−10 |
The results are representative of three independent experiments:
The recipient strain was A. tumefaciens UIA143(pTiA6) (At1160).
Conjugative transfer of RSF1010 between Agrobacterium cells is inhibited by osa in the absence of plasmid pAtC58.
Many A. tumefaciens strains, including the strains used in the experiments described above, contain plasmid pAtC58. Chen et al. (8) showed that pAtC58 encodes a type IV secretion system, AvhB, that can promote the conjugative transfer of RSF1010 between Agrobacterium cells. We therefore sought to determine whether osa inhibited the conjugative transfer of RSF1010 in the absence of the AvhB transfer system.
A. tumefaciens LBA1251 lacks both a Ti plasmid and pAtC58. We introduced into this strain pTiA6, the RSF1010 derivative pML122, and either pUCD3960 (containing osa) or pUCD5533 (empty vector control for the presence of osa), generating A. tumefaciens At1368 and At1369, respectively. Our results indicated that in the absence of the AvhB type IV secretion system encoded by pAtC58, osa inhibited conjugative transfer of RSF1010 by more than 4 orders of magnitude (2.8 × 10−5 ± 0.6 × 10−5/recipient for At1369; <1 × 10−9/recipient for At1368). Thus, osa inhibits RSF1010 transfer directed by the Ti plasmid.
DISCUSSION
Plasmids of the IncW group (such as pSa) and the IncQ group (such as RSF1010) can suppress tumorigenesis by A. tumefaciens on many plant species. The RSF1010 derivatives pJB31 and pJW323 were shown previously to suppress tumorigenesis by the octopine-type strain A348 on N. tabacum (tobacco) and K. daigremontiana (4, 33), whereas pSa inhibited transformation of numerous plant species by both strain C58 (7, 9, 11, 23) and strain A208 (19), which contain a nopaline-type Ti plasmid, and by strains A348 (19) and 1D1 (11), which contain an octopine-type Ti plasmid. Oncogenic suppression by pSa was more effective on A. tumefaciens C58 than suppression of strain 1D1 was (11).
In this study we investigated the relative strengths of derivatives of pSa and RSF1010 in conferring oncogenic suppression upon various A. tumefaciens strains. By using different numbers of Agrobacterium cells as inocula on K. daigremontiana leaves, we observed different extents of suppression of these oncogenic Agrobacterium strains. We concluded that the presence of pSa, or just the osa gene of pSa, elicits greater oncogenic suppression than the presence of several derivatives of RSF1010, including pJB31 and pJW323, elicits. Plasmid pSa is present at a level of 2 or 3 copies per bacterial cell (Lee and Gelvin, unpublished data), whereas the derivatives of RSF1010 are present at levels of ∼20 copies per cell (4). Therefore, it is unlikely that the greater extent of oncogenic suppression effected by the osa gene than by the RSF1010 derivatives results from a higher copy number of the oncogenic suppressive entity.
The surprising finding of this study is that although oncogenic suppression of plant transformation by Osa involves inhibition of VirE2 but not T-DNA transfer, the effect of Osa on inhibition of RSF1010 conjugation may not involve VirE2. Previous studies indicated that mutation of virE2 does not affect conjugative transfer of RSF1010 between Agrobacterium cells (13). However, these studies were performed with Agrobacterium strains that contained pAtC58. It is possible that pAtC58 can express some function equivalent to that of VirE2. If so, this function per se cannot be required for RSF1010 conjugative transfer, because elimination of pAtC58 from the bacterium still permitted conjugative transfer of RSF1010.
During Agrobacterium-mediated transformation of plants, VirE2 functions in the plant cell but probably not in the bacterium. A virE2 mutant Agrobacterium strain can still deliver T-strands to a plant cell (36) and effect transformation if it is coinoculated with a VirE2-producing Agrobacterium strain (29) or if it is inoculated onto a transgenic plant that produces VirE2 (19). In the plant cell, VirE2 likely protects the T-strand from nucleolytic degradation (36) and may be involved in targeting the T-complex to the plant nucleus (37). These functions would not be required for RSF1010 conjugative transfer between bacterial cells but would be required for RSF1010-mediated transformation of plants. Thus, inhibition of VirE2 export to plant cells could explain why Osa blocks plant transformation. The lack of importance of VirE2 in RSF1010 conjugative transfer would explain why Osa likely blocks conjugation by some process not involving VirE2.
Oncogenic suppression by Osa does not require the formation of a conjugative intermediate in the Agrobacterium cell. When we placed osa onto the Agrobacterium chromosome and eliminated any plasmid that could be transferred to the plant cell, oncogenic suppressive activity remained undiminished. This differs from the situation with RSF1010, where formation of a conjugative intermediate is required to effect oncogenic suppression (33). Therefore, although the consequences of the presence of the suppressive plasmids in the donor bacterial cells are the same, the suppressive mechanisms of the two systems likely differ. The Osa protein of the IncW plasmid pSa is sufficient to block the VirB/VirD4-mediated transfer of IncQ plasmid RSF1010 during bacterial conjugation. However, Osa does not block RSF1010 transfer from bacteria into plant cells. Rather, Osa blocks VirE2 export and subsequently the formation of kanamycin-resistant calli. Unlike RSF1010-mediated inhibition of T-DNA transfer, the inhibition of plant transformation by osa is a function of the Osa protein per se and is not associated with formation of a nucleic acid-protein complex. Thus, the oncogenic suppression by osa differs mechanistically from the suppression effected by RSF1010.
Acknowledgments
We thank Clarence I. Kado for useful discussions concerning our experiments and for reviewing a previous version of the manuscript. We also thank Eugene W. Nester, Stephen K. Farrand, Anath Das, Lois Banta, Paul J. J. Hooykaas, and Andrew N. Binns for providing various plasmids and Agrobacterium strains.
This work was supported in part by USDA grant 98-35304-6675 to S.B.G.
REFERENCES
- 1.Anderson, L. B., A. V. Hertzl, and A. Das. 1996. Agrobacterium tumefaciens VirB7 and VirB9 form a disulfide-linked protein complex. Proc. Natl. Acad. Sci. USA 93:8889-8894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaupre, C. E., J. Bohne, E. M. Dale, and A. N. Binns. 1997. Interactions between VirB9 and VirB10 membrane proteins involved in movement of DNA from Agrobacterium tumefaciens into plant cells. J. Bacteriol. 179:78-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beijersbergen, A., A. Den Dulk-Ras, R. A. Schilperoort, and P. J. J. Hooykass. 1992. Conjugative transfer by the virulence system of Agrobacterium tumefaciens. Science 256:1324-1327. [DOI] [PubMed] [Google Scholar]
- 4.Binns, A. N., C. E. Beaupre, and E. M. Dale. 1995. Inhibition of VirB-mediated transfer of diverse substrates from Agrobacterium tumefaciens by the IncQ plasmid RSF1010. J. Bacteriol. 177:4890-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohne, J., A. Yim, and A. N. Binns. 1998. The Ti plasmid increases the efficiency of Agrobacterium tumefaciens as a recipient in virB-mediated conjugal transfer of an IncQ plasmid. Proc. Natl. Acad. Sci. USA 95:7057-7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchanan-Wollaston, V., J. E. Passiatore, and F. Cannon. 1987. The mob and oriT mobilization functions of a bacterial plasmid promote its transfer to plants. Nature 328:172-175. [Google Scholar]
- 7.Chen, C.-Y., and C. I. Kado. 1994. Inhibition of Agrobacterium tumefaciens oncogenicity by the osa gene of pSa. J. Bacteriol. 176:5697-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, L., Y. Chen, D. W. Wood, and E. W. Nester. 2002. A new type IV secretion system promotes conjugal transfer in Agrobacterium tumefaciens. J. Bacteriol 184:4838-4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Close, T. J., and C. I. Kado. 1991. The osa gene of pSa encodes a 21.1-kilodalton protein that suppresses Agrobacterium tumefaciens oncogenicity. J. Bacteriol. 173:5449-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das, A., and Y.-H. Xie. 1998. Construction of transposon Tn3phoA: its application in defining the membrane topology of the Agrobacterium tumefaciens DNA transfer proteins. Mol. Microbiol. 27:405-414. [DOI] [PubMed] [Google Scholar]
- 11.Farrand, S., C. I. Kado, and C. R. Ireland. 1981. Suppression of tumorigenicity by the IncW R plasmid pSa in Agrobacterium tumefaciens. Mol. Gen. Genet. 181:44-51. [Google Scholar]
- 12.Farrand, S. K., S. P. O'Morchoe, and J. McCutchan. 1989. Construction of an Agrobacterium tumefaciens C58 recA mutant. J. Bacteriol. 171:5314-5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fullner, K. J., and E. W. Nester. 1996. Temperature affects the T-DNA transfer machinery of Agrobacterium tumefaciens. J. Bacteriol. 178:1498-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garfinkel, D. J., R. B. Simpson, L. W. Ream, F. F. White, M. P. Gordon, and E. W. Nester. 1981. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell 27:143-153. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch, P. R., and J. E. Beringer. 1984. A physical map of pPH1JI and pJB4JI. Plasmid 12:139-141. [DOI] [PubMed] [Google Scholar]
- 16.Jacob, A. E., J. A. Shapiro, L. Yamamoto, D. L. Smith, S. N. Cohen, and D. E. Berg. 1977. Plasmids studied in Escherichia coli and other enteric bacteria, p. 607-638. In A. I. Bukhari, J. A. Shapiro, and S. L. Adhya (ed.), DNA insertion elements, plasmids and episomes. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 17.Jefferson, R. A. 1987. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol. Biol. Rep. 5:387-405. [Google Scholar]
- 18.Labes, M., A. Puhler, and R. Simon. 1990. A new family of RSF1010-derived expression and lac-fusion broad-host-range vectors for gram-negative bacteria. Gene 89:37-46. [DOI] [PubMed] [Google Scholar]
- 19.Lee, L.-Y., S. B. Gelvin, and C. I. Kado. 1999. pSa causes oncogenic suppression of Agrobacterium by inhibiting VirE2 protein export. J. Bacteriol. 181:186-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, L.-Y., J. M. Humara, and S. B. Gelvin. 2001. Novel constructions to enable the integration of genes into the Agrobacterium tumefaciens C58 chromosome. Mol. Plant-Microbe Interact. 14:577-579. [DOI] [PubMed] [Google Scholar]
- 21.Lichtenstein, C., and J. Draper. 1986. Genetic engineering of plants, p. 67-119. In D. M. Glover (ed.), DNA cloning: a practical approach, vol. 2. IRL Press, Washington, D.C. [Google Scholar]
- 22.Liu, Z., and A. N. Binns. 2003. Functional subsets of the VirB type IV transport complex proteins involved in the capacity of Agrobacterium tumefaciens to serve as a recipient in virB-mediated conjugal transfer of plasmid RSF1010. J. Bacteriol. 185:3259-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loper, J. E., and C. I. Kado. 1979. Host range conferred by the virulence-specifying plasmid of Agrobacterium tumefaciens. J. Bacteriol. 139:591-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meynell, E., G. G. Meynell, and N. Datta. 1968. Phylogenetic relationship of drug resistance factors and other transmissible bacterial plasmids. Bacteriol. Rev. 32:55-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murashige, T., and F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473-497. [Google Scholar]
- 26.Narasimhulu, S. B., X.-B. Deng, R. Sarria, and S. B. Gelvin. 1996. Early transcription of Agrobacterium T-DNA genes in tobacco and maize. Plant Cell 8:873-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsen, R. H., and P. L. Shipley. 1975. RP1 properties and fertility inhibition among P, N, W, and X incompatibility group plasmids. J. Bacteriol. 123:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ooms, G., P. J. J. Hooykaas, R. J. M. Van Veen, P. Van Beelan, T. J. G. Regensburg-Tuink, and R. A. Schilperoort. 1982. Octopine Ti-plasmid deletion mutants of Agrobacterium tumefaciens with emphasis on the right side of the T-region. Plasmid 7:15-29. [DOI] [PubMed] [Google Scholar]
- 29.Otten, L., H. DeGreve, J. Leemans, R. Hain, P. Hooykaas, and J. Schell. 1984. Restoration of virulence of vir region mutants of Agrobacterium tumefaciens strain B6S3 by coinfection with normal and mutant Agrobacterium strains. Mol. Gen. Genet. 195:159-163. [Google Scholar]
- 30.Rong, L., S. J. Karcher, K. O'Neal, M. C. Hawes, C. D. Yerkes, R. K. Jayaswal, C. A. Hallberg, and S. B. Gelvin. 1990. picA, a novel plant-inducible locus on the Agrobacterium tumefaciens chromosome. J. Bacteriol. 172:5828-5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sciaky, D. A., A. L. Montoya, and M.-D. Chilton. 1978. Fingerprints of Agrobacterium Ti plasmids. Plasmid 1:238-253. [DOI] [PubMed] [Google Scholar]
- 32.Stachel, S. E., and E. W. Nester. 1986. The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens. EMBO J. 5:1445-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stahl, L. E., A. Jacobs, and A. N. Binns. 1998. The conjugal intermediate of plasmid RSF1010 inhibits Agrobacterium tumefaciens virulence and VirB-dependent export of VirE2. J. Bacteriol. 180:3933-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward, J. E., E. M. Dale, and A. N. Binns. 1991. Activity of the Agrobacterium T-DNA transfer machinery is affected by virB gene products. Proc. Natl. Acad. Sci. USA 88:9350-9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson, B., T. C. Currier, M. P. Gordon, M.-D. Chilton, and E. W. Nester. 1975. Plasmid required for virulence of Agrobacterium tumefaciens. J. Bacteriol. 123:255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yusibov, V. M., T. R. Steck, V. Gupta, and S. B. Gelvin. 1994. Association of single-stranded transferred DNA from Agrobacterium tumefaciens with tobacco cells. Proc. Natl. Acad. Sci. USA 91:2994-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziemienowicz, A., T. Merkle, F. Schoumacher, B. Hohn, and L. Rossi. 2001. Import of Agrobacterium T-DNA into plant nuclei: two distinct functions of VirD2 and VirE2 proteins. Plant Cell 13:369-383. [DOI] [PMC free article] [PubMed] [Google Scholar]