Abstract
Streptococcus suis serotype 2 (SS2) is a major porcine and human pathogen which causes arthritis, meningitis, and septicemia. Streptococcus suis nuclease A (SsnA) is a recently discovered deoxyribonuclease (DNase), which has been demonstrated to contribute to escape killing in neutrophil extracellular traps (NETs). To further determine the effects of ssnA on virulence, the ssnA deletion mutant (ΔssnA) and its complemented strain (C-ΔssnA) were constructed. The ability of ΔssnA mutant to interact with human laryngeal epithelial cell (Hep-2) was evaluated and it exhibited dramatically decreased ability to adhere to and invade Hep-2 cells. This mutation was found to exhibit significant attenuation of virulence when evaluated in CD1 mice, suggesting ssnA plays a critical role in the pathogenesis of SS2. Finally, we found that immunization with the ΔssnA mutant triggered both antibody responses and cell-mediated immunity, and conferred 80% protection against virulent SS2 challenge in mice. Taken together, our results suggest that ΔssnA represents an attractive candidate for designing an attenuated live vaccine against SS2.
Introduction
Streptococcus suis (S. suis) is a major swine pathogen responsible for a huge economic cost in the worldwide pig industry [1]. It is also a severe threat to human health by causing severe systemic infection [2–3]. Based on the capsular polysaccharide, 35 S. suis serotypes have been described. S. suis serotype 2 (SS2) is considered an emerging zoonotic agent, causing a variety of diseases which include arthritis, meningitis, septicemia, and even acute death in pigs and humans [4–5]. The first case of S. suis-caused human infection was diagnosed in Denmark in 1968 [6]. So far, SS2 has been reported in more than 20 countries and caused approximately 700 human infections [7–8]. In particular, two large outbreaks have taken place in China in 1998 and 2005 [9–10]. Despite various virulence factors of S. suis have been identified, limited knowledge on the pathogenesis of S. suis impedes the attempts to control this organism [11]. S. suis infection remains a serious infectious disease challenge to global public health and food safety. Therefore, there needs to carry out more researches to identify new virulence factors and understand S. suis infection mechanism.
It is known that many pathogenic bacteria produce secreted deoxyribonucleases (DNases), which are regarded as virulence factors [12–13]. More specifically, DNases have been shown to be involved in biofilm maturation and bacterial growth [14–15]. Recent studies implied that secreted DNases contribute to pathogenesis by enhancing evasion of the innate immune response [13, 16]. Streptococcus suis nuclease A (SsnA) is a recently discovered cell wall—anchored extracellular DNase, which present in most of the serotypes and clinical isolates analyzed. The study has confirmed that the ssnA gene is expressed during all the growth process of S. suis, and reacts with S. suis immune sera confirmed that SsnA is expressed in vivo [17]. SsnA could be a good vaccine formulation against S. suis, as it is well exposed and approachable to antibodies, being also very immunogenic [18–19]. Although the characterization of DNase activity of ssnA and its immunogenicity have been previously reported, the actual contribution of this DNase on S. suis virulence remains to be verified.
In this study, an attenuated SS2 strain was developed by deleting the ssnA gene of the GD01 strain. Our results showed that ssnA is a pathogenicity-associated determinant of S. suis involved in growth, adhesion and invasion to host cell. The ΔssnA mutant represents a promising vaccine candidate of SS2 with comparable safety in murine animal models.
Materials and Methods
Ethics statement
All animal procedures were approved by the Ethics Committee of Institute of Animal Health, Guangdong Academy of Agricultural Sciences according to Guangdong Province Laboratory Animal Management Regulations—2010. The license number was SYXK(Yue) 2011–0116. All efforts were made to minimize suffering. Humane endpoints used during the animal survival study were: rapid weight loss of >20% of body weight, poor physical appearance (reduced mobility, rough coat and depression), rapid breathing, swollen eyes, and joint tumefaction. Following infection, the healthy status of animals was evaluated every 8 h and there were not unexpected deaths. Animals that reached humane endpoints were euthanized through complete exsanguination via cardiac puncture under general anesthesia with inhaled 2% isoflurane.
Bacterial strains, culture conditions, and plasmids
The bacterial strains and plasmids used in this study are describes in Table 1. SS2 wild-type (WT) strain GD01 was isolated from the brain of a sick pig in Guangdong, China. S. suis was grown in tryptic soy broth (TSB) or plated on tryptic soy agar (TSA) plates (Difco, Detroit, MI, USA) plus 5% bovine sera at 37°C. E. coli strains were cultured in Luria-Bertani (LB) broth or on LB agar plates. Antibiotics were used as follow: 100 μg/mL of spectinomycin (Spc), 5 μg/mL of chloramphenicol (Cm), or 8 μg/mL of erythromycin (Erm).
Table 1. Bacterial strains and plasmids used in this study.
| Strains/plasmid | Characteristics/Functions | Source/Reference |
|---|---|---|
| Bacterial strains | ||
| E. coli DH5a | Cloning vehicle: clone the upstream and downstream flanking regions of ssnA | Invitrogen |
| E. coli BL21(DE3) | For expressing the recombinant plasmids | Invitrogen |
| S. suis GD01 | Serotype 2, clinical isolated virulent strain | Laboratory collection |
| ΔssnA | The deletion mutant of ssnA with background of GD01 | This study |
| C-ΔssnA | Complemented strain of ssnA | This study |
| Plasmids | ||
| pMD19-T | Cloning vector; AmpR | TaKaRa |
| pSET4s | E. coli–S. suis shuttle vector thermosensitive suicide; SpcR | Takamatsu et al., 2001 |
| pSET4sΔssnA | A recombinant vector with the background of pSET4s, designed to knockout ssnA; SpcR | This study |
| pAT18 | A plasmid carrying an erythromycin resistance rRNA methylase (Erm) gene expression cassette | Trieu-Cuot et al., 1991 |
| pAT18-ssnA | A recombinant vector with the background of pAT18, designed to complementation ssnA; ErmR | This study |
Construction of the mutant and functional complementation strains
Primers used for amplification (Table 2) in this study were ordered from the Invitrogen (Shanghai, China). The thermosensitive plasmid pSET4s was used for the substitution of the ssnA with the Cm in S. suis strain. The upstream (865 bp) and downstream (624 bp) fragments flanking regions of ssnA were amplified from SS2 WT strain GD01 genomic DNA using PCR with two pairs of primers: P1-F/P1-R and P2-F/P2-R. The Cm was amplifed with specific primer Cm-F/Cm-R. The PCR products were digested using the corresponding restriction enzymes and inserted between the Sal I and Sca I site of pSET4s to generate the recombinant plasmid pSET4sΔssnA. This plasmid was confirmed by DNA sequencing and restriction analysis, and then was electrotransformed into GD01 strain. The temperature sensitive replication of this vector enabled allelic replacement of ssnA with Cm in the SS2 chromosome to generate the mutant strain ΔssnA [20]. Allelic exchange mutagenesis of ssnA was confirmed by PCR with four primer pairs (SS-16S-F/SS-16S-R, Cm-F/Cm-R, Spc-F/Spc-R, and P3-F/P3-R) and sequence analysis.
Table 2. Oligonucleotide primers used in this study.
| Primers | Primers sequence (5’-3’)* | Amplification for |
|---|---|---|
| P1-F | gagtcgactcaaactgtctgggaga | Upstream border of ssnA |
| P1-R | gcggatccataaaactcctttttc | |
| P2-F | gaggatcctcattttttgagcttg | Downstream border of ssnA |
| P2-R | gcgagctcgctccaggtaaaactt | |
| Cm-F | gcggatcctaattcgatgggttccgagg | CmR expression cassette |
| Cm-R | tctggatccgaaaacactagagcttgatg | |
| Spc-F | gtgttcgtgaatacatgttata | SpcR expression cassette |
| Spc-R | gttttctaaaatctgattacca | |
| P3-F | gttaaacaaaatgaatatggcacc | the part of upstream homologous arm region, the intermediate region, and the part of down homologous arm region of ssnA |
| P3-R | attgacagccaaaatctgattggc | |
| SS-16S-F | cagtatttaccgcatggtagatat | S.suis 16S rRNA |
| SS-16S-R | gtaagataccgtcaagtgagaa | |
| P4-F | gcggaattctgttaaacaaaatgaatat | ssnA coding sequence and its promoter |
| P4-R | gcggatccttaggattctttttg |
* Underlined nucleotides denote enzyme restriction sites.
To obtain a complementary strain, the fragment containing the ssnA gene and its upstream promoter was amplified with primer pair (P4-F and P4-R), and then was cloned into the shuttle vector pAT18 [21] to generate the plasmid pAT18-ssnA. This plasmid was introduced into ΔssnA to obtain the function complemented strain C-ΔssnA by Erm resistance screening in TSA plates.
Growth characteristic
The SS2 WT GD01, ΔssnA, and C-ΔssnA strains were separately inoculated in 5 mL TSB containing 5% bovine sera, and incubated at 37°C for 12 h. Then the cultures were diluted to the optical density at 600 nm (OD600) of 0.6 and inoculated into 100 mL TSB. OD600 values for three cultures were determined using spectrophotometer (Bio-Rad, Hercules, CA, USA) at 1 h intervals. Uninoculated media served as the blank control.
Cell adhesion and invasion assays
The human laryngeal epithelial cell (Hep-2) line was cultivated as previously described [22]. Briefly, cells were maintained as a monolayer in 24-well tissue culture plates. S. suis cultures (WT, ΔssnA, and C-ΔssnA) were pelleted and washed three times with PBS (pH 7.4). Cells were infected with 106 CFU/mL bacterial suspension at 37°C with 5% CO2 for 2 h. Then the plates were rinsed with PBS, and 100 μL PBS containing 0.25% trypsin/0.02% EDTA was added to lyse cells at 37°C for 10 min. The lysates were diluted 10-fold and placed onto TSA agar to calculate viable bacteria.
For the invasion assay, the minimal inhibitory concentration (MIC) values of three strains were determined as the lowest concentration of antimicrobial agent that prevented visible growth. Then 100 μg/mL gentamycin (MIC = 16 μg/mL) was added to the Hep-2 cells to kill non-invaded bacteria, prior to lysing the cells.
Effect of ssnA on virulence in CD1 mice
The virulence of the WT GD01, mutant strain ΔssnA, and C-ΔssnA were determined by 50% lethal dose (LD50) value. Ninety-six six-week-old female CD1 mice were randomly divided into 16 groups. The WT, ΔssnA, and C-ΔssnA strains were cultured at 37°C and then diluted to appropriate concentration. The mice were infected intraperitoneally (i.p.) with 500 μL of the bacterial suspension (Table 3). The mice injected only with PBS were served as negative controls. The mortality of mice was recorded for 7 days, and the LD50 values were calculated using the method of Karber [23].
Table 3. Determination of LD50 in CD1 mice infected with the WT GD01, ΔssnA, and C-ΔssnA strains.
| Strains | Challenge dose (CFU) | Number dead | Value of LD50 (CFU) | Fold attenuationc a |
|---|---|---|---|---|
| 1.3×107 | 0 | |||
| 2.0×107 | 1 | |||
| Wild-type (GD01) | 4.0×107 | 3 | 4.42×107 | 1 |
| 8.0×107 | 4 | |||
| 1.3×108 | 6 | |||
| 2.0×108 | 1 | |||
| 4.0×108 | 2 | |||
| Mutant (ΔssnA) | 8.0×108 | 4 | 6.17×108 | 14 |
| 1.6×109 | 4 | |||
| 3.2×109 | 6 | |||
| 4.0×107 | 1 | |||
| 8.0×107 | 2 | |||
| C-ΔssnA | 1.2×108 | 4 | 7.63×107 | 2 |
| 1.6×108 | 6 | |||
| 2.0×108 | 6 |
a Fold attenuation normalised to the wild-type strain.
Determination of viable bacteria invasion in susceptible tissues
Thirty female CD1 mice were classified into three groups and injected i.p. with 500 μL of GD01, ΔssnA, or C-ΔssnA (5 × 106 CFU). Five mice inoculated with PBS were served as controls. One control and three infected mice were euthanased at 3, 5, and 7 day post-infection to assess the bacterial invasion of WT, ΔssnA, or C-ΔssnA strains in infected tissues. Blood samples (10 μL) collected from the tail vein and homogenized spleen, liver, and brain samples (0.05 g/ tissue) were plated on TSA plates.
Competitive-infection assay
A 1:1 mixture of GD01 and ΔssnA in 200 μL TSB was inoculated i.p. into six mice. After inoculation for 8 h, the blood samples from the infected mice were collected and diluted to plate onto TSA plates. The competitive index (CI) was calculated as follow: CI = [CFU of mutant strain in blood/CFU of WT strain in blood] / [CFU of mutant strain in original inoculum/CFU of WT strain in original inoculum].
Immunization and challenge
The protection assay was carried out in the CD1 mouse model. Forty-five six-week-old female CD1 mice were randomly assigned to 3 groups of 15 each. Group 1 were immunized with 108 CFU of ΔssnA in 200 μL PBS. Group 2 were immunized with the SS2 inactivated vaccine, and Group 3 were injected with 200 μL of PBS served as a negative control. All animals were vaccinated twice with 2-week interval.
At 28 days post-immunization, blood samples were obtained by tail vein bleeding, and then all animals were challenged i.p. with 6.0×109 CFU (5 × LD50) of GD01 in 500 μL of PBS. At 14 days post-challenge, the surviving mice were euthanized.
Determination of antibody levels
The antigen-specific serum IgG levels were detected using an indirect enzyme-linked immunosorbent assay (ELISA). Briefly, microtitre plates were coated with 5 μg/100 μL of the killed bacteria SS2 at 4°C overnight. Following washing and blocking, serial dilutions of sera were added and incubated for 2 h at 37°C. After washing five times, the plates were added with anti-mouse IgG (1: 5000) horseradish peroxidase (HRP)-conjugated antibodies (Sigma, USA) for 1 h at 37°C. Upon washing, 100 μL/well of TMB was added for color reaction. The reaction was stopped by adding 50 μL 2 M H2SO4 to each well. OD450 nm was measured using an ELISA plate reader (Bio-Rad, USA).
Lymphocyte proliferation
On 14 days post the second immunization, three mice from each group were sacrificed and spleens were isolated under sterile condition. Splenocyte pellets were collected by centrifuge and resuspended in complete DMEM medium (105 cells/mL) (Hyclone, Logan, UT, USA). Lymphocytes were cultured in 96 well culture plates and incubated with the heat-killed SS2 strain (5 × 108 CFU/mL) for 72 h at 37°C with 5% CO2. Cultures were made in medium along as a negative control, or containing 5 μg/well of concanavalin A (ConA) (Sigma, USA) as a positive control. Lymphoproliferation assays were carried out with MTT reagent using a lymphocyte proliferation kit (Promega, USA) following the manufacturer’s protocol. The absorbance at 490 nm was measured using an ELISA reader.
Cytokines detection assay
Quantitative analyses of interleukins (IL)-10, IL-4, IL-2, and interferon(IFN)-γ were performed with cytokine detection kits, following manufacturer’s instructions (R & D, Minneapolis, MN, USA).
Statistical analysis
The data were analyzed with Excel software and presented as the mean ± SD. One-way analysis of variance followed by Student’s t-test was used to analyze the difference between two groups. Differences among three groups were analyzed using ANOVA. Statistical significance was defined at p < 0.05, and highly statistical significance was defined at p < 0.01.
Results
Construction of ΔssnA mutant strain and complemented strain
In order to determine whether the function of the ssnA was crucial for virulence in S. suis, we deleted the ssnA gene of SS2 GD01 strain using a double-crossover homologous recombination approach (Fig 1A). The deletion of the ssnA was confirmed by DNA sequencing (data not shown) and PCR (Fig 1B), verifying that the ssnA mutant was successfully constructed. The S. suis 16S rRNA fragment (294 bp) was detectable in both of the WT and mutant strains. The primer P3-F/P3-R amplified a bigger band in the WT strain, because the sequence of ssnA is longer than that of the Cm resistance cassette. The primers Cm-F/Cm-R amplified a 1056 bp fragment in the ΔssnA strain, while did not obtain a band from WT strain. The Spc fragment was undetectable in both of strains (data not shown). The complemented strain construction method was transformation of GD01 with plasmid pAT18-ssnA, and C-ΔssnA was identified by Erm-resistance screening.
Fig 1. Construction of the ΔssnA mutant strain and complementation strain.
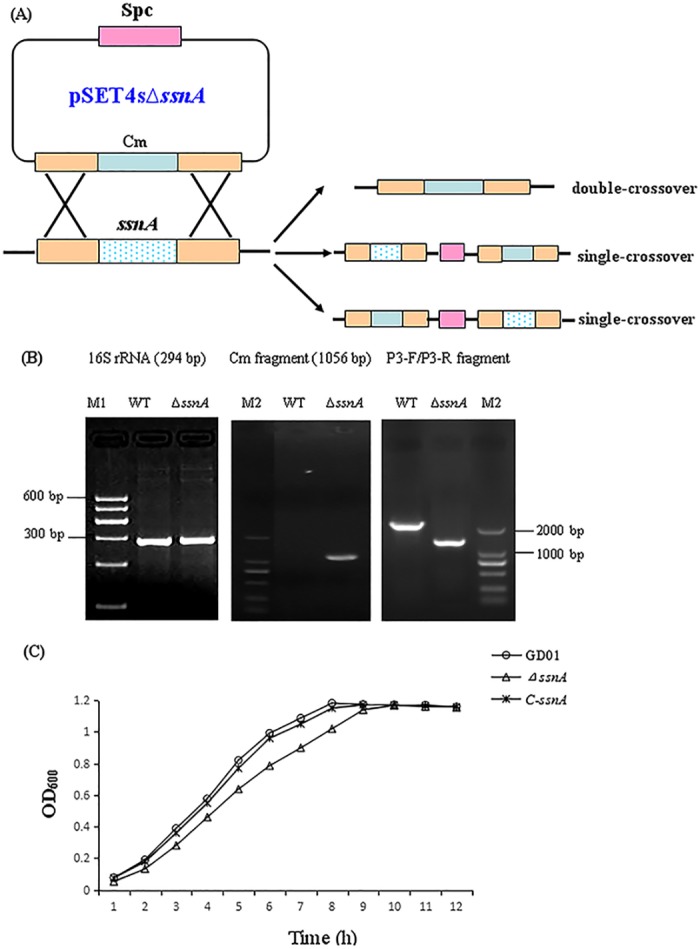
(A) Schematic of the strategy for the ssnA gene deletion in the SS2 GD01 strain via double-crossover recombination. The plasmid pSET4sΔssnA is used for the ssnA gene knockout. (B) PCR confirmation of the mutant strain. (C) Growth of the WT GD01, ΔssnA mutant, and its complementation.
SsnA is required for growth of SS2
Growth curves of the WT, ΔssnA mutant, and C-ΔssnA strains were determined at OD600, which showed that ΔssnA exhibited slightly lower growth than the WT and C-ΔssnA in the early-exponential phase and mid-exponential phase (Fig 1C). This result indicated that ssnA plays a role in the growth of SS2.
The ssnA of SS2 contributes to the adherence to and invasion of Hep-2 cells
To check whether the ssnA interacted with host cells, we used Hep-2 cells to compare the adherence and invasion abilities in the WT GD01, ΔssnA mutant, and complementation strains (Fig 2). In the adhesion assay, the numbers of adhesion bacterial in the extracellular and invasion to the intracellular were detected (Fig 2A). After 2 h of infection, the ΔssnA mutant exhibited the lowest level of adherence. Compared to the ΔssnA strain, the WT strain showed significantly increased adherence capability in Hep-2 cells (p < 0.01), whereas the level of adhesion was fully recovered in the complemented strain. The similar results were obtained in the invasion assay (Fig 2B).
Fig 2.
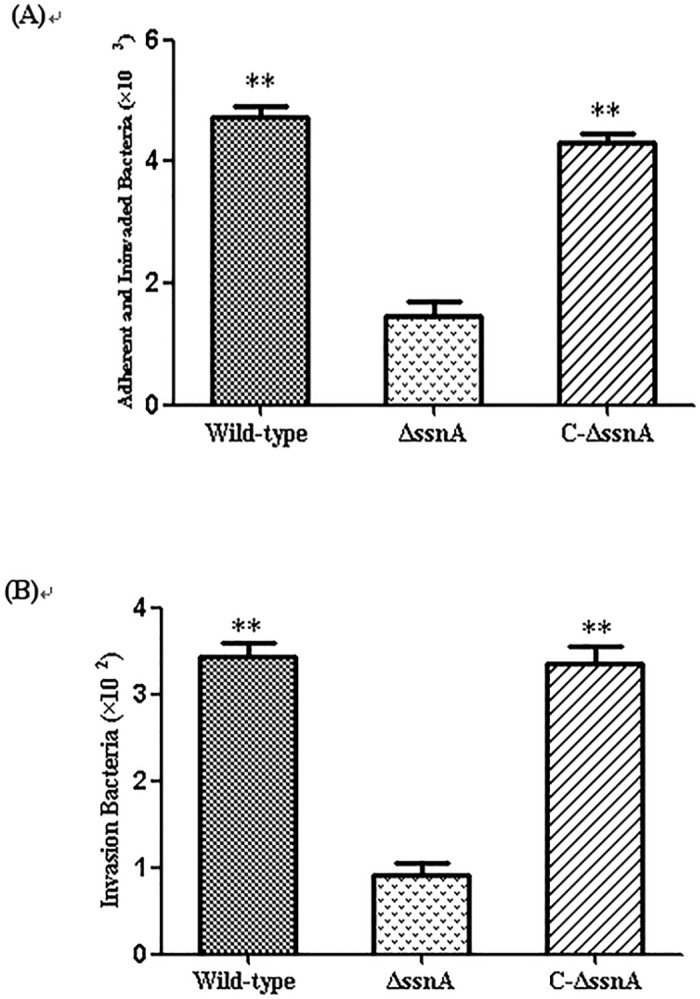
The WT, ΔssnA, and C-ΔssnA strains adhere to and invade (A), and invasion of (B) Hep-2 cells after 2 h of infection. The data are means and SD of three independent experiments, **P < 0.01.
The absence of ssnA affects SS2 virulence in mice
To evaluate the effect of ssnA on virulence, the CD1 mice were injected with various doses of GD01, ΔssnA, or C-ΔssnA. In the higher inoculum dose groups, infected mice showed obvious clinical signs of SS2 infection, including depression, irregular coat, swollen eyes, and rapid breathing. As shown in Table 3, the LD50 was 4.42 × 107, 6.17 × 108, and 7.63× 107 CFU for the WT, ΔssnA, and C-ΔssnA strains, respectively. Mutant ΔssnA was attenuated by 14-fold compared with the WT, indicating that ssnA deletion significantly reduced the virulence of SS2.
Invasion ability of ΔssnA in susceptible tissues
To analyze the virulence attenuation conferred by ΔssnA, the invasion experiments in vivo were performed. The mice were infected with 5 × 106 CFU of the WT, ΔssnA, or complementation strain according to the LD50 assessment results. At different time-points, the viable bacteria were recovered from liver, spleen, brain, and blood. Compared with the WT-infected mice, bacterial counts in blood and organs of the ΔssnA-infected mice were significantly decreased (Fig 3). Furthermore, the abilities of the WT and ΔssnA strains to colonize the blood were evaluated by competitive-infection assay. Six mice were infected with the WT and ΔssnA in a 1:1 ratio. The CI value showed that the CFU of the mutant strain in vivo were significantly lower than that of the WT strain (Fig 4), indicating the ΔssnA had a decreased ability of invasion in blood. Taken together, above results suggested that ssnA is important for the pathogenesis of SS2.
Fig 3. Detection of the WT, ΔssnA, and C-ΔssnA strains invasion in blood and tissues.
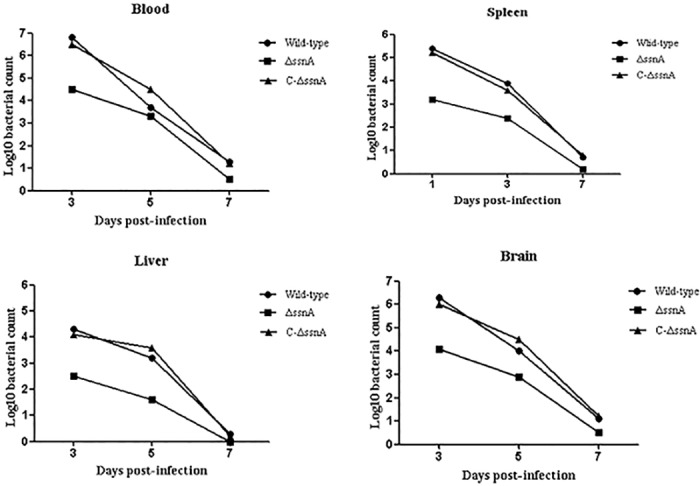
Blood (A), spleens (B), livers (C), and brains (D) were collected from infected CD1 mice and homogenized at 3, 5, and 7 days after infection.
Fig 4. The CI of ΔssnA against the WT strain in vivo.
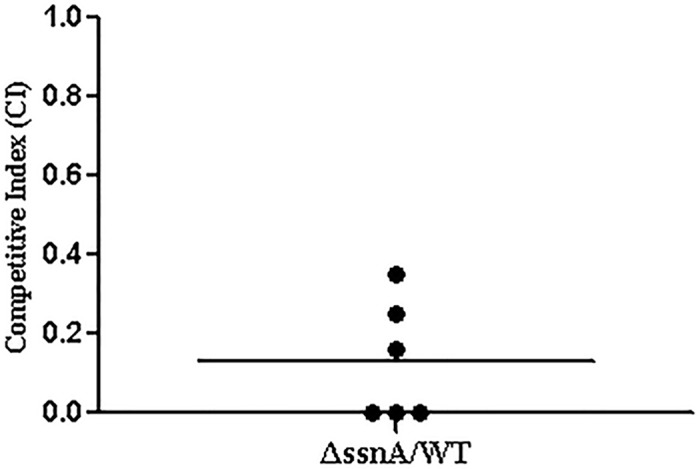
Six CD1 mice were infected i.p. with ΔssnA: WT at 1:1 ratio. At 8 h post inoculation, the value of CI in blood samples was calculated.
Evaluation of immune responses conferred by the ΔssnA mutant
Serum samples were collected from each group at 14 and 28 day post-immunization and the humoral responses induced by ΔssnA were detected by indirect ELISA. As shown in Fig 5, the mice vaccinated with the mutant and SS2 inactivated vaccine exhibited significant IgG antibody response (p < 0.01), whereas no specific serum IgG antibody against bacteria antigen were detected in the PBS group.
Fig 5. Production of SS2-specific IgG antibodies in immunized mice.
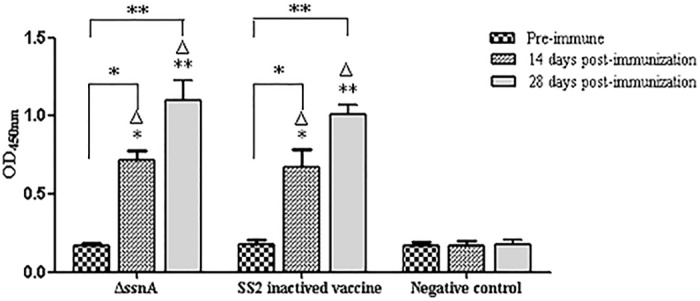
The CD1 mice were immunized with SS2 inactive vaccine, ΔssnA, or PBS, and antibody levels were detected at 14, and 28 days post-inoculation by indirect ELISA. The data are expressed as the mean ± SD, *P < 0.05 and **P < 0.01. Δ, versus the negative control.
At 28 day post-inoculation, splenocytes supernatants collected from immunized mice were assessed for lymphocyte proliferation to characterize the cellular immune response (Fig 6A). The splenocytes from both immunized groups stimulated with ConA and heat-killed S. suis strain produced significant proliferative T-cell immune response (p < 0.05), whereas no proliferative response in the negative control group.
Fig 6. The cellular immune responses induced in immunized mice.
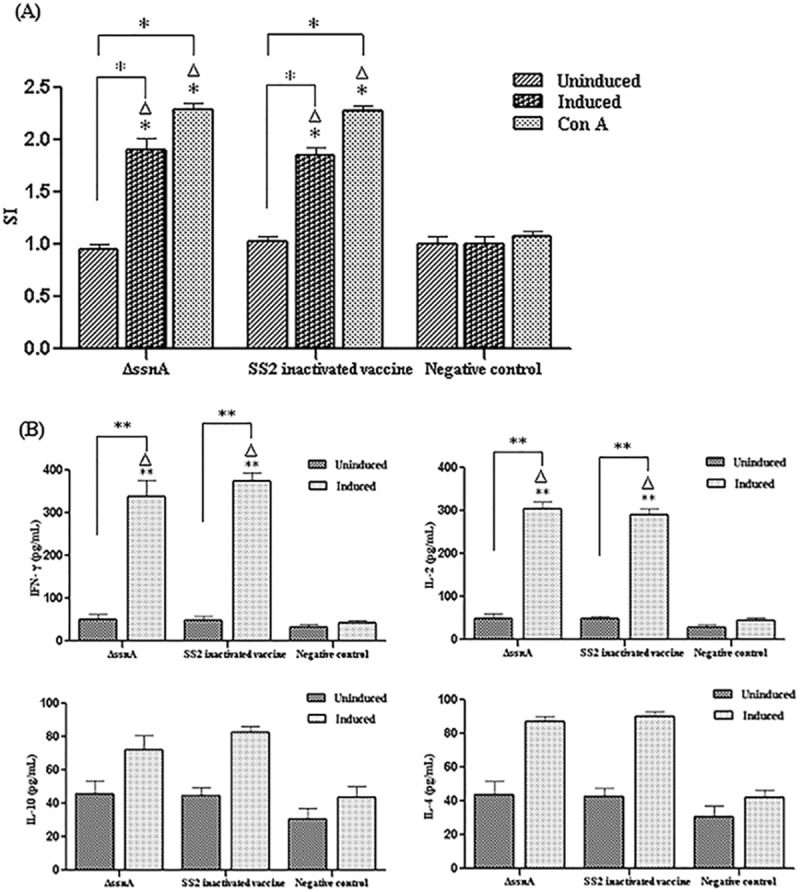
(A) Splenocytes from immunized mice were collected at 28 day post-first immunization and lymphocyte proliferation assay was evaluated using a cell proliferation kit with MTT reagent. (B) Production of cytokines in stimulated splenocytes was detected by ELISA. *P < 0.05. Δ, versus the negative control.
The levels of cytokines of splenocytes stimulated with either S. suis strain or medium alone are presented in Fig 6B. The Th1-type cytokine IFN- γ and IL-2 and the Th2-type cytokine IL-4 and IL-10 were assessed. Compared with the control group, the levels of IFN- γ and IL-2 were significantly high in the vaccine immunized groups (p < 0.01), which significantly greater than the IL-4 and IL-10 levels.
All above results suggested that the ssnA mutant induced both antibody and cell-mediated immunity with a dominant Th1 immune response.
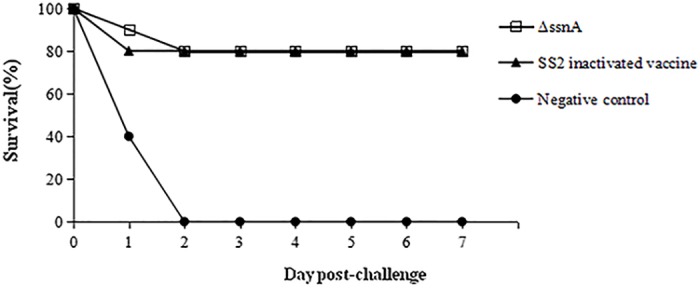
Protective efficacy against SS2 challenge in mice
To scrutinize the ability of the ΔssnA to protect against virulent SS2, the challenge study was performed and clinical symptoms of S. suis infection and mortality were observed. Compared with the negative group, the ΔssnA-vaccinated mice were significantly protected against SS2 challenge (Fig 7). All mice from control group showed obvious clinical signs, while in the vaccinated groups there were only a few mice showed temporal and slight depression. The ΔssnA mutant induced 80% protection against challenge with a dosage of 5-fold of the LD50 of WT SS2 in mice.
Fig 7. Survival curves of mice challenged with a high-virulent SS2 strain.
Discussion
DNases are common among prokaryotes. Intracellular bacterial DNases participate in replication, recombination or DNA repair to maintain chromosome and plasmid integrity. In contrast, extracellular DNases have been implicated as virulence factors in several species [24–26]. Nuclease activity in S. suis was first reported in 2004 [17]. The ssnA gene encoded a cell-associated DNase has been identified in SS2. Previous study has indicated that the amino-acid sequence of SsnA is high homology to the SpnA protein of Streptococcus pyogenes, which is a novel virulence factor of group A streptococcal [13]. Recent research has showed that SsnA is the first specific factor of neutrophil extracellular trap (NET) evasion [27], but the functional roles of this gene on virulence and immunogenicity were unknown. In present study, the ssnA mutant GD01ΔssnA and its complementation strain were constructed to investigate the effects of ssnA gene-deletion in S. suis and evaluate the potential of ΔssnA for developing attenuated vaccine. Based on the result of growth curves of the WT GD01, ΔssnA mutant, and C-ssnA strains, we found that the deletion of the ssnA has an effect on the growth of SS2 strain. Furthermore, we analyzed the role of ssnA in the process of SS2 infection in vitro and vivo.
Bacterial adherence to host cells is an important step toward cellular invasion, which conduce to break through the cell barrier, persistence, and penetration into deep tissues of the host [28]. The adhesion effect of S. suis on host epithelial cell is concerned with its invasion in the respiratory and organs of the infected host. Systemic infection of S. suis requires the circulating bacteria to effectively invade into different tissues and cells. In this study, we have demonstrated that ΔssnA has a markedly impaired interaction with Hep-2 in vitro. These findings provide evidence that ssnA may be associated with pathogenicity of S. suis.
To clarify the effect of ssnA on virulence, the infection experiment in vivo with the mice were performed. We chose the CD1 mouse as animal model for S. suis infections because it is has been confirmed effective and excellent [29]. In this study, comparing with the WT-infected group, the LD50 of ΔssnA mutant was significantly increased. Our results indicated that the ssnA gene play an important role on S. suis virulence. Bacteria invasion experiments in vivo were carried out to investigate the virulence attenuation of ΔssnA. A distinct difference of bacterial loads were detected from blood and the tissues of ΔssnA-infected mice compared to those of the mice infected with the WT strain. Interestingly, the clearance rate between the WT and mutant groups were no significant difference. Considering above results, the difference between WT and ΔssnA may come from the different invasiveness from abdominal cavity to blood. These indicated that ΔssnA is required during invasion in vivo, but not for bacterial survival in vivo. The role of ssnA on virulence were further investigated by infecting mice with the WT and mutant strain simultaneously to determine the CI, which is the ratio of bacteria CFU recovered from an individual strain in blood and determines the relative fitness of each strain to survive [30].
Compared with vaccines based on an inactivated bacteria or recombinant protein, attenuated live vaccines are advantageous because they are able to induce good immunity and confer long-term protection. Therefore, we evaluated the attenuated live vaccine potential of the S. suis ΔssnA mutant. The mice vaccinated with the ΔssnA strain could elicit a high IgG level. In general, vaccine antigen induced antibody level is usually in accordance with its protection rate. In the later challenge study, the mice from two vaccines immunized groups showed higher protection rates than that of the control group. Our antibody level detective results were coordinated with the challenge experiments results, indicating the ΔssnA provided good protective efficacy.
Cell-mediated immunity to vaccination is essential to control infection in the host. In our study, significant lymphoproliferative responses of the immunized mice were detected by MTT assay. Th1 cells are in charge of regulating cellular immune and involved in host defense by producing TNF-α, IL-2, and IFN-γ, while Th2 cells are responsible for coordinating humoral immunity, are involved in host defense by secreting IL-4, IL-5, IL-10, and IL-13 [31]. Our results of the cytokines secretion showed the dominance of Th1 immune response induced by the ΔssnA. Recent study has also demonstrated that S. suis double mutant vaccine will evoke strong Th1 responses [32].
To successfully colonize the host cell and stimulate immune responses is a necessary condition for good attenuated live vaccine candidate [33]. Our researches indicated that the ΔssnA mutant maybe an appropriate attenuated vaccine candidate for S. suis on account of its ability to adhere to and invade epithelial cells, decreased virulence, and the ability to induce protective immune response upon infection.
In summary, our results suggest that the ΔssnA mutant is capable of inducing significant humoral and cell-mediated immune, and confers effective protection in CD1 mouse model against SS2 infection. Therefore, ΔssnA could potentially be used for an attenuated live vaccine development and further evaluation will be performed in pig.
Acknowledgments
We are grateful to professor Daisuke Takamatsu (National Institute of Animal Health, Japan) for supplying plasmid pSET4s and Shu-zhang Feng (The Academy of Military Medical Sciences of China) for supplying plasmid pAT18.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by Guangzhou Science and technology planning project of China 1563000807 to ML; The Presidential Foundation of Guangdong Academy of Agricultural Science of China 201437 to ML. The funders played a role in study design, data collection and analysis, and preparation of the manuscript.
References
- 1.Staats JJ, Feder I, Okwumabua O, Chengappa MM. Streptococcus suis: past and present. Vet Res Commun. 1997; 21: 381–407. [DOI] [PubMed] [Google Scholar]
- 2.Wertheim HF, Nghia HD, Taylor W, Schultsz C. Streptococcus suis: an emerging human pathogen. Clin Infect Dis. 2009; 48: 617–625. 10.1086/596763 [DOI] [PubMed] [Google Scholar]
- 3.Gottschalk M, Xu J, Calzas C, Segura M. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiol. 2010; 5: 371–391. 10.2217/fmb.10.2 [DOI] [PubMed] [Google Scholar]
- 4.Higgins R, Gottschalk M. Distribution of Streptococcus suis capsular types in 1999. Can Vet J. 2000; 41: 414 [PMC free article] [PubMed] [Google Scholar]
- 5.Su Y, Yao W, Perez-Gutierrez ON, Smidt H, Zhu WY. Changes in abundance of Lacto-bacillus spp. and Streptococcus suis in the stomach, jejunum and ileum of piglets after weaning. FEMS Microbiol Ecol. 2008; 66: 546–555. 10.1111/j.1574-6941.2008.00529.x [DOI] [PubMed] [Google Scholar]
- 6.Perch B, Kristjansen P, Skadhauge K. Group R streptococci pathogenic for man. Two cases of meningitis and one fatal case of sepsis. Acta Pathol Microbiol Scand. 1968; 74:69–76. [PubMed] [Google Scholar]
- 7.Mai NT, Hoa NT, Nga TV, Linh le D, Chau TT, Sinh DX, et al. Streptococcus suis meningitis in adults in Vietnam. Clin Infect Dis. 2008; 46: 659–667. 10.1086/527385 [DOI] [PubMed] [Google Scholar]
- 8.Zheng B, Zhang Q, Gao J, Han H, Li M, Zhang J, et al. Insight into interaction of metal ions with TroA form Streptococcus suis. PLoS ONE. 2011; 6: e19510 10.1371/journal.pone.0019510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang J, Wang C, Feng Y, Yang W, Song H, Chen Z, et al. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLOS Med. 2006; 3: e151 10.1371/journal.pmed.0030151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lun ZR, Wang QP, Chen XG, Li AX, Zhu XQ. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect Dis. 2007; 7: 201–209. 10.1016/S1473-3099(07)70001-4 [DOI] [PubMed] [Google Scholar]
- 11.Fittipaldi N, Segura M, Grenier D, Gottschalk M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol. 2012; 7: 259–279. 10.2217/fmb.11.149 [DOI] [PubMed] [Google Scholar]
- 12.Bendjennat M, Blanchard A, Loutfi M, Montagnier, Bahraoui E. Role of Mycoplasma penetrans endonuclease P40 as a potential pathogenic determinant. Infect Immun. 1999; 67:4456–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang A, Khemlani A, Kang H, Proft T. Functional analysis of Streptococcus pyogenes nuclease A (SpnA), a novel group A streptococcal virulencefactor. Mol Microbiol. 2011; 79: 1629–1642. 10.1111/j.1365-2958.2011.07550.x [DOI] [PubMed] [Google Scholar]
- 14.Mulcahy H, Charron-Mazenod L, Lewenza S. Pseudomonas aeruginosa produces an extracellular deoxyribonuclease that is required for utilization of DNA as a nutrient source. Environ Microbiol. 2010;12: 1621–1629. 10.1111/j.1462-2920.2010.02208.x [DOI] [PubMed] [Google Scholar]
- 15.Mann EE, Rice KC, Boles BR, Endres JL, Ranjit D, Chandramohan L, et al. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS ONE. 2009; 4:e5822 10.1371/journal.pone.0005822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berends ETM, Horswill AR, Haste NM, Monestier M, Nizet V, von Köckritz-Blickwede M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun. 2010; 2:576–586. 10.1159/000319909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontaine MC, Perez-Casal J, Willson PJ. Investigation of a novel DNase of Streptococcus suis serotype 2. Infect Immun. 2004; 72:774–781. 10.1128/IAI.72.2.774-781.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vela AI, Goyache J, Tarradas C, Luque I, Mateos A, Moreno MA, et al. Analysis of genetic diversity of Streptococcus suis clinical isolates from pigs in Spain by pulsed-field gel electrophoresis. J Clin Microbiol. 2003; 41: 2498–2502. 10.1128/JCM.41.6.2498-2502.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gómez-Gascón L, Cardoso-Toset F, Amarilla PS, Tarradas C, Carrasco L, Olaya-Abril A, et al. A new recombinant SsnA protein combined with aluminum hydroxide protects mouse against Streptococcus suis. Vaccine. 2014; 32: 6992–6999. 10.1016/j.vaccine.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 20.Takamatsu D, Osaki M, Sekizaki T. Thermosensitive suicide vectors for genereplacement in Streptococcus suis. Plasmid. 2001; 46: 140–148. 10.1006/plas.2001.1532 [DOI] [PubMed] [Google Scholar]
- 21.Trieu-Cuot P, Carlier C, Poyart-Salmeron C, Courvalin P. Shuttle vectors containing a multiple cloning site and a lacZα gene for conjugal transfer of DNA from Escherichia coli to gram-positive bacteria. Gene. 1991; 102: 99–104. [DOI] [PubMed] [Google Scholar]
- 22.Feng Y, Cao M, Shi J, Zhang H, Hu D, Zhu J, et al. Attenuation of Streptococcus suis virulence by the alteration of bacterial surface architecture. Sci Rep. 2012;2:710 10.1038/srep00710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Zhou R, Li T, Kang M, Wan Y, Xu Z, et al. Enhanced biofilm formation and reduced virulence of Actinobacillus pleuropneumoniae luxS mutant. MicrobPathog. 2008; 45:192–200. [DOI] [PubMed] [Google Scholar]
- 24.Aziz RK, Ismail SA, Park HW, Kotb M. Post-proteomic identification of a novel phage-encoded streptodornase, Sda1, in invasive M1T1 Streptococcus pyogenes. Mol Microbiol. 2004; 54: 184–197. 10.1111/j.1365-2958.2004.04255.x [DOI] [PubMed] [Google Scholar]
- 25.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006; 16:401–407. 10.1016/j.cub.2006.01.056 [DOI] [PubMed] [Google Scholar]
- 26.Okumura K, Kawsar HI, Shimizu T, Ohta T, Hayashi H, Shimizu T. Identification and characterization of a cell-wall anchored DNase gene in Clostridium perfringens. FEMS Microbiol Lett. 2005; 242: 281–285. 10.1016/j.femsle.2004.11.019 [DOI] [PubMed] [Google Scholar]
- 27.de Buhr N, Neumann A, Jerjomiceva N, von Köckritz-Blickwede M, Baums CG. Streptococcus suis DNase SsnA contributes to degradation of neutrophil extracellular traps (NETs) and evasion of NET-mediated antimicrobial activity. Microbiology. 2014; 160:385–395. 10.1099/mic.0.072199-0 [DOI] [PubMed] [Google Scholar]
- 28.Zhang B, Xu C, Zhang L, Zhou S, Feng S, He Y. Enhanced adherence to and invasion of PUVEC and PK-15 cells due to the overexpression of RfaD, ThyA and Mip in the DompP2 mutant of Haemophilus parasuis SC096 strain. Vet Microbiol. 2013; 162:713–723. 10.1016/j.vetmic.2012.09.021 [DOI] [PubMed] [Google Scholar]
- 29.Dominguez-Punaro MC, Segura M, Plante MM, Lacouture S, Rivest S, Gottschalk M. Streptococcus suis serotype 2, an important swine and human pathogen, induces strong systemic and cerebral inflammatory responses in a mouse model of infection. J Immunol. 2007; 179: 1842–1854. [DOI] [PubMed] [Google Scholar]
- 30.Hemsley C, Joyce E, Hava DL, Kawale A, Camilli A. MgrA, an orthologue of Mga, Acts as a transcriptional repressor of the genes within the rlrA pathogenicity islet in Streptococcus pneumoniae. J Bacteriol. 2003; 185:6640–6647. 10.1128/JB.185.22.6640-6647.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stabel JR, Barnhill A, Bannantine JP, Chang YF, Osman MA. Evaluation of protection in a mouse model after vaccination with Mycobacterium avium subsp. paratuberculois protein cocktails.Vaccine. 2012; 31:127–134. 10.1016/j.vaccine.2012.10.090 [DOI] [PubMed] [Google Scholar]
- 32.Hu J, You W, Wang B, Hu X, Tan C, Liu J, et al. Construction, characterization and evaluation of the protective efficacy of the Streptococcus suis double mutant strain ΔSsPep/ΔSsPspC as a live vaccine candidate in mice. Microbiol Res. 2015; 170:87–94. 10.1016/j.micres.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 33.Curtiss R 3rd. Bacterial infectious disease control by vaccine development. J Clin Invest. 2002; 110:1061–1066. 10.1172/JCI16941 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.


