Abstract
A variety of amino acid substitutions in the protease and Gag proteins have been reported to contribute to the development of human immunodeficiency virus type 1 (HIV-1) resistance to protease inhibitors. In the present study, full-length molecular infectious HIV-1 clones were generated by using HIV-1 variants isolated from heavily drug-experienced and therapy-failed AIDS patients. Of six full-length infectious clones generated, four were found to have unique insertions (TGNS, SQVN, AQQA, SRPE, APP, and/or PTAPPA) near the p17/p24 and p1/p6 Gag cleavage sites, in addition to the known resistance-related multiple amino acid substitutions within the protease. The addition of such Gag inserts mostly compromised the replication of wild-type HIV-1, whereas the primary multidrug-resistant HIV infectious clones containing inserts replicated significantly better than those modified to lack the inserts. Western blot analyses revealed that the processing of Gag proteins by wild-type protease was impaired by the presence of the inserts, whereas that by mutant protease was substantially improved. The present study represents the first report clearly demonstrating that the inserts seen in the proximity of the Gag cleavage sites in highly multi-PI resistant HIV-1 variants restore the otherwise compromised enzymatic activity of mutant protease, enabling the multi-PI-resistant HIV-1 variants to remain replication competent.
Currently available combination chemotherapy with reverse transcriptase inhibitors (RTIs) and protease inhibitors (PIs) for human immunodeficiency virus type 1 (HIV-1) infection and AIDS have been shown to suppress the replication of HIV-1 and extend the life expectancy of HIV-1-infected individuals (8, 41). In the course of treatment, however, drug-resistant HIV-1 variants often emerge, which has been a major factor contributing to treatment failure (8, 16, 26, 27).
HIV-1 also develops high levels of resistance against multiple antiviral drugs by accumulating a variety of amino acid substitutions near (and beyond) the active sites of target viral enzymes (5, 20, 35-37), whereas such multiple mutations can often compromise the enzymatic functions of the viral protease and reverse transcriptase (RT) (7, 10, 17, 24, 34, 39). In the case of HIV-1 resistance to an RTI, amino acid changes in the polymerase are virtually fully responsible for the viral acquisition of resistance to RTIs. Indeed, the introduction of such amino acid changes into the polymerase can generally convert a wild-type HIV-1 to a nucleoside reverse transcriptase inhibitor (NRTI)-resistant HIV-1 variant (21). However, in the case of HIV-1 resistance to PIs, the mere introduction of amino acid substitutions seen within the viral protease of PI-resistant variants to wild-type HIV-1 in many cases results in impaired replication competence of the virus (4, 7, 21, 33). Indeed, when HIV-1 develops resistance to PIs, the virus is known to add further amino acid substitutions often located outside the protease that do not confer resistance on HIV-1 per se but improve the otherwise compromised catalytic functions of protease (3, 18). For example, several amino acid substitutions have been seen in the cleavage sites of the Gag proteins in HIV-1 resistant to PIs (6, 9, 25, 43). These substitutions have been shown to compensate for the reduced catalytic activity of mutant proteases. Moreover, certain amino acid substitutions in noncleavage sites have been shown to contribute to the development of high levels of viral resistance to multiple PIs (15).
The addition of certain amino acids can also contribute to the development of viral resistance. Winters et al. identified a 6-bp insert between codons 69 and 70 of the RT gene in HIV-1 isolated from NRTI-treated patients and conducted elegant site-directed mutagenesis studies showing that the insert alone confers on HIV-1 reduced susceptibility to multiple NRTIs (40). Peters et al. have also recently identified the duplication of a proline-rich motif, Ala-Ala-Pro (APP), in the PTAP motif of the Gag protein in HIV-1 variants isolated from patients with AIDS receiving NRTIs, including didanosine (ddI), stavudine (d4T), zidovudine (AZT), and lamivudine (3TC), and have shown that this addition could improve assembly and packaging at membrane locations, resulting in increased infectivity and viral resistance to NRTIs (28).
In the present study, we identified unique insertions (TGNS, SQVN, AQQA, SRPE, APP, and/or PTAPPA) near the p17/p24 and p1/p6 Gag cleavage sites, in addition to the known resistance-related multiple amino acid substitutions within the protease in full-length molecular infectious multidrug-resistant HIV-1 (HIVMDR) clones generated from HIV-1 variants isolated from patients with AIDS who had received 7 to 11 anti-HIV-1 drugs over 24 to 81 months and had lost response to any existing antiviral drugs (except for tenofovir and enfuvirtide at the time). Virologic and biochemical studies demonstrated that whereas these inserts mostly compromise the enzymatic functions of the wild-type protease, they restore the Gag processing by the mutant protease and enable PI-resistant HIV variants to remain replication competent.
MATERIALS AND METHODS
Patients.
Patients with AIDS were enrolled into a randomized clinical study of amprenavir (APV) and abacavir (ABC) (11, 42). The clinical characteristics of the patients are presented in Table 1. Other medications, including antiretroviral therapies, were permitted with the exception of other PIs (indinavir [IDV], ritonavir [RTV], and saquinavir [SQV]), non-NRTIs (nevirapine [NPV] and delavirdine), and agents available on expanded access, which were prohibited for the first 12 weeks of therapy with APV. These patients were put on the study under an investigational new drug application by Critical Care Medicine Department, Clinical Center, National Institutes of Health. For the present study, 20 patients were randomly chosen from the enrollees who had failed the APV-plus-ABC therapy.
TABLE 1.
Patient profiles
| Patient | No. of CD4 cells/mm3 | No. of HIV RNA copies/ml | Duration (mo) of antiviral therapy | Anti-HIV-1 agents receiveda |
|---|---|---|---|---|
| B | 361 | 247,000 | 64 | AZT, ddI, ddC, d4T, 3TC, ABC, IDV, RTV, SQV, APV, DLV |
| C | 3 | 554,000 | 46 | AZT, ddI, ddC, d4T, 3TC, ABC, IDV, RTV, SQV, APV |
| G | 568 | 60,000 | 81 | AZT, ddI, ddC, d4T, 3TC, ABC, IDV, SQV, APV, DLV |
| EV | 185 | 68,000 | 24 | AZT, 3TC, ABC, RTV, SQV, APV, EFV |
| ES | 8 | 42,000 | 34 | AZT, ddI, ddC, d4T, 3TC, ABC, IDV, RTV, APV, EFV, HU |
| EY | 140 | 10,000 | 36 | AZT, ddI, d4T, 3TC, ABC, IDV, RTV, APV, EFV, HU |
ddC, zalcitabine; EFV, efavirenz; HU, hydroxyurea. Levels of HIV RNA in serum were determined by using a branched DNA assay.
Cells and viruses.
MT-4 and PM1 cells were grown in RPMI 1640-based culture medium supplemented with 10% fetal calf serum (HyClone, Logan, Utah), 50 U of penicillin/ml, and 50 μg of streptomycin/ml. Peripheral blood mononuclear cells (PBMC) obtained from healthy donors were stimulated by phytohemagglutinin (PHA) in RPMI 1640-based medium containing interleukin-2 (5 ng/ml; R&D Systems, Minneapolis, Minn.) for 2 days (PHA-PBMC) before HIV-1 exposure. Clinical HIV-1 strains were isolated as previously described (37) by culturing PBMC obtained from patients with AIDS. Thus obtained HIV-1 isolates were passaged once or twice in PHA-PBMC.
Generation of full-length molecular HIVMDR clones.
To generate full-length molecular infectious HIVMDR clones from multidrug-resistant clinical HIV-1 isolates, the PCR-mediated recombination (PMR) method was used (12). First, we amplified an upstream proviral DNA fragment (5′ DNA fragment, 5,337 bp) and a downstream proviral DNA fragment (3′ DNA fragment, 5,042 bp), both of which shared an overlapping sequence (730 bp), by using the primer pair 5LTR (5′-GGG GAC AAG TTT GTA CAA AAA AGC AGG CT TGG AAG GGC TAA TTT GGT CCC AAA AAA GAC-3′) plus pol-2 (5′-GTC TAC TTG TGT GCT ATA TCT CTT TTT CCT CC-3′) and the primer pair pol-1 (5′-GCA TTC CCT ACA ATC CCC AAA G-3′) plus 3LTR (5′-GGG GAC CAC TTT GTA CAA GAA AGC TGG GTT GCT AGA GAT TTT CCA CAC TGA CTA AAA GG-3′), respectively. The DNA recombination sequence, attB, was tagged at the 5′ ends of 5LTR and 3LTR for subsequent cloning. Thus obtained 5′ and 3′ DNA fragments were joined by using PMR. The PMR reaction was performed using the standard condition for ExTaq polymerase (Takara, Kyoto, Japan) with 40 pmol of attB1 (5′-GGG GAC AAG TTT GTA CAA AAA AGC AGG CT-3′) and attB2 (5′-GGG GAC CAC TTT GTA CAA GAA AGC TGG GT-3′) adapter primers and the 5′ and 3′ DNA fragments (100 ng each) in a 50-μl reaction solution. Thermal cycling was carried out as follows: 95°C for 2 min, followed by 15 cycles of 95°C for 10 s, 55°C for 30 s, and 68°C for 8 min, and followed finally by 68°C for 10 min. Thus amplified attB-flanked full-length HIV-1 was cloned into pcDNA3.1 according to the manufacturer's instructions (Gateway Cloning System; Invitrogen, Carlsbad, Calif.).
Generation of molecular HIVNL4-3 clones containing a Gag insert.
To generate molecular infectious HIV-1 clones carrying the wild-type protease plus an insert, site-directed mutagenesis by using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.) was performed as described previously (15). In brief, each desired insert (9 to 18 nucleotides) was introduced into the BssHII-SmaI fragment of the wild-type HIVNL4-3 (nucleotides 712 to 2591), which encodes Gag and protease, by site-directed mutagenesis. The fragment was subsequently introduced into pHIVNLSma, which had been created to have a SmaI site by changing two nucleotides (2590 and 2593) of pHIVNL4-3. The generated molecular HIV-1 clones containing Ala-Gln-Gln-Ala (AQQA), Pro-Thr-Ala-Pro-Pro-Ala (PTAPPA), Thr-Gly-Asn-Ser (TGNS), Ala-Pro-Pro (APP), Ser-Arg-Pro-Glu (SRPE), and Ser-Gln-Val-Asn (SQVN) were designated HIVAQQA, HIVPTAPPA, HIVTGNS, HIVAPP, HIVSRPE, and HIVSQVN, respectively.
Generation of molecular HIVMDR clones lacking a Gag insert.
Primers that lack the insert sequence were designed to generate molecular HIVMDR clones lacking a Gag insert. Primers del17-1 (5′-GCA GCT GAC ACA GGA AAC AAC AGC CAG GTC AGC CAA AAT TAC-3′), del17-2 (5′-GTA ATT TTG GCT GAC CTG GCT GTT GTT TCC TGT GTC AGC TGC-3′), del17-3 (5′-GAG CAA AAC AAA AGT AAG AAA AAG GCA CAG CAA GCA GCA GCT GAC-3′), and del17-4 (5′-GTC AGC TGC TGC TTG CTG TGC CTT TTT CTT ACT TTT GTT TTG CTC-3′) were used to delete the inserts near the p17/p24 cleavage site, whereas del6-1 (5′-CTT CAG AGC AGA CCA GAG CCA ACA GCC CCA CCA GAA GAG AGC-3′), del6-2 (5′-GCT CTC TTC TGG TGG GGC TGT TGG CTC TGG TCT GCT CTG AAG-3′), del6-3 (5′-CAG AGC AGA CTA GAG CCA ACA GCC CCA CCA GCA GAG AGC TTC AGC-3′), and del6-4 (5′-CCT GAA GCT CTC TGC TGG TGG GGC TGT TGG CTC TAG TCT GCT CTG-3′) were used to delete the inserts near the p1/p6 cleavage site. Thus obtained 5′ and 3′ DNA fragments were joined by using PMR as described above.
Determination of the nucleotide sequences of the plasmids containing full-size molecular HIV-1 clones confirmed that each molecular clone generated had the desired mutations but no unintended mutations. Each recombinant plasmid was transfected into COS-7 cells with Lipofectamine 2000 reagent (Invitrogen), and the infectious virions thus obtained were harvested 48 h after transfection and stored at −80°C until use. In order to determine virus titers, PHA-PBMC (15,000 cells/well) in 96-well flat-bottom microtiter culture plates (Costar, Cambridge, Mass.) were exposed to each virus preparation that had been serially diluted. Culture supernatants were examined for the amounts of p24 Gag on day 7 of culture by using a commercially available radioimmunoassay kit (Dupont/NEN Research Products, Boston, Mass.). When the amounts of p24 Gag were <0.6 ng/ml, cultures were defined to be negative for the virus, and the 50% tissue culture infective dose (TCID50) was determined by the method of Reed and Muench (32). All titration assays were performed in six replicates.
Generation of HIVNL4-3 carrying mutated protease with or without Gag inserts.
To conduct experiments to examine the possible effects of the inserts identified in Gag on the proteolytic activity of mutated protease and the viral fitness, two infectious clones (HIVB and HIVES) that have two inserts near the p17/p24 and p1/p6 cleavage sites were chosen. First, an EagI site was introduced into pHIVNLSma by changing two nucleotides (2215 and 2216) as described above, generating pHIVNLEag/Sma. Using a pair of primers containing the EagI and SmaI sites, PCR products were generated with HIVB and HIVES as templates, followed by digestion by both EagI and SmaI, thus generating the EagI-SmaI fragments for both HIVB and HIVES. Each of the HIVB and HIVES EagI-SmaI fragments was introduced into pHIVNLEag/Sma, generating HIVNL/B-Pr and HIVNL/ES-Pr, respectively. Subsequently, each pair of insertions (TGNS plus APP or AQQA plus PTAPPA) was introduced into HIVNL/B-Pr and HIVNL/ES-Pr, thus generating HIVNL/B-Pr/TGNS-APP and HIVNL/ES-Pr/AQQA-PTAPPA.
Replication kinetic assay.
MT-4 cells (5 × 105) or PHA-PBMC (1.5 × 106) were exposed to each infectious virus preparation (30 TCID50 in 1 ml of culture medium) for 2 h, washed twice with phosphate-buffered saline (PBS), and cultured in 1.5 ml of complete medium. Culture supernatants were harvested every 3 days, and the amounts p24 Gag were determined
CHRA.
The competitive HIV replication assay (CHRA) was performed as previously reported (15, 22) with minor modifications. In brief, two titrated infectious clones to be examined in the assay were combined and added to freshly prepared MT-4 cells (3 × 105) or PM1 cells (3 × 105). To ensure that the two infectious clones to be compared were of an approximately equal infectivity, a fixed amount (30 TCID50) of one infectious clone was combined with three different amounts (15, 30, and 60 TCID50) of the other infectious clone. On day 1, one-third of infected cells were harvested and washed twice with PBS, and cellular DNA was purified. The purified DNA was subjected to nested PCR and sequencing as described below. The HIV-1 coculture that best approximated a 50:50 mixture on day 1 was further propagated; the remaining cultures were discarded. Every 4 to 10 days, the cell supernatant of the virus coculture (1 ml) was transferred to fresh uninfected cells (1.5 × 105 MT-4 cells or 1.5 × 105 PM1 cells in 1 ml), 8 ml of fresh culture medium was added on the following day, and a half of the medium was replenished with an equal volume of fresh culture medium every 3 to 4 days. The cells harvested at the conclusion of each passage were subjected to DNA extraction and then to direct DNA sequencing of the proviral DNA, and a viral population change was determined as previously reported (15, 22).
Western blot analysis.
To analyze whether HIV-1 polyproteins in molecular HIV-1 clones were cleaved by the viral protease, Western blot analysis with the lysates of HIV-1-producing cells and cell-free virions was conducted. Briefly, at 48 or 72 h after transfection with plasmid preparations, COS-7 cells were washed with PBS and lysed in M-per solution (Pierce, Rockford, Ill.), and the cell lysates were subjected to Western blotting. Culture supernatants containing virions were harvested 48 h after transfection, filtered through 0.22-μm-pore-size Millex-GV membranes (Millipore, Bedford, Mass.), and centrifuged at 20,000 × g for 4 h to pellet virions, which were then lysed in M-per solution.
In the assay, samples were normalized based on the amounts of p24 Gag and subjected to electrophoresis on sodium dodecyl sulfate-15% polyacrylamide gel (Bio-Rad, Hercules, Calif.), followed by electroblotting onto nitrocellulose membranes. The HIV-1 Gag proteins were visualized with SuperSignal WestPico (Pierce) by using anti-p24 Gag antiserum or anti-p6 monoclonal antibody (Advanced Biotechnologies, Inc., Columbia, Md.). The anti-p6 monoclonal antibody in general does not recognize the p6 protein of HIVNL4-3 (9). Thus molecular HIV-1 clones with or without each insert containing the BssHII-SmaI fragment that encodes the entire gag-pol region of HIVHXB, the p6 protein of which can be recognized by the antibody, were generated for Western blot analysis of the wild-type HIVNL4-3-based clones.
The percent signal density of Gag products was analyzed on a Macintosh computer by using the NIH Image Program (developed at the U.S. National Institutes of Health <http://rsb.info.nih.gov/nih-image/>) and the percent density of p24 Gag (% densityp24) was determined by use of the following formula: % densityp24 = 100 × (the density of the p24 Gag signal)/(the cumulated density of all Gag signals).
Determination of nucleotide sequences.
Determination of nucleotide sequences of HIV-1 was performed as described previously (15). In brief, high-molecular-weight DNA was extracted from HIV-1-infected cells by using a QIAamp DNA minikit (Qiagen, Valencia, Calif.), and the Gag and protease-encoding regions of HIV-1 were amplified by using nested-PCR with AmpliTaq DNA polymerase (Applied Biosystems, Foster City, Calif.). The primers used were Seq1 (5′-GTA TGG GCA AGC AGG GAG CTA GAA CGA TTC-3′) and Seq2 (5′-GGG TAT TAC TTC TGG GCT GAA AGC CTT CTC) for the first PCR of the p17/p24 Gag cleavage site, Seq3 (5′-TGT AAA ACG GCC AGT TGT AGA CAA ATA CTG GGA CAG CTA CAA CCA-3′) and Seq4 (5′-CAG GAA ACA GCT ATG ACC CTT TTA CCC ATG CAT TTA AAG TTC TAG GTG-3′) for the second PCR of the p17/p24 Gag cleavage site, Seq5 (5′-AGG GCT GTT GGA AAT GTG GAA AGG AAG G-3′) and Seq6 (5′-TCT TCT GTC AAT GGC CAT TGT TTA AC) for the first PCR of the p1/p6 Gag cleavage site and protease-encoding region, and Seq7 (5′-TGT AAA ACG ACG GCC AGT TAG GGA AGA TCT GGC CTT CC-3′) and Seq8 (5′-CAG GAA ACA GCT ATG ACC TAC TGG TAC AGT CTC AAT AGG-3′) for the second PCR of the p1/p6 Gag cleavage site and PR encoding region. The products of the second PCR were directly sequenced by using M13 forward and reverse dye-labeled primers with an Applied Biosystems model 3100 automated DNA sequencer.
RESULTS
Examination of clinical profiles of patients with AIDS.
We first determined the sequence of the protease-encoding region of 20 primary HIV-1 strains isolated from patients with AIDS who were enrolled in salvage therapy clinical trials in the Clinical Center of the National Cancer Institute (11, 42). We chose six primary strains that harbored the highest numbers of drug resistance-associated amino acid substitutions in the RT and protease and generated full-length molecular infectious clones. The clinical profiles of the six patients are given in Table 1. These individuals had received 7 to 11 anti-HIV-1 drugs over 24 to 81 months and no longer responded to any existing antiviral drugs (except for tenofovir and enfuvirtide at the time), showing low CD4+ counts ranging from 3 to 568 cells/mm3 and moderately to high levels of viremia (1 × 104 to 5.54 × 105 RNA copies/ml). As shown in Table 2, all of the HIV-1 strains isolated from these patients were found to be highly resistant to all anti-HIV-1 drugs used (four NRTIs and five PIs) in the present study.
TABLE 2.
Sensitivity of HIV-1 isolated from heavily drug-experienced individuals against NRTIs and PIs
| Virus | IC50a (μM) (fold change)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| AZT | ddI | 3TC | ABC | IDV | RTV | SQV | NFV | APV | |
| HIV-1wtb | 0.0060 (1) | 0.20 (1) | 0.22 (1) | 0.13 (1) | 0.017 (1) | 0.055 (1) | 0.0090 (1) | 0.030 (1) | 0.015 (1) |
| HIV-1B | 0.42 (70) | 2.8 (14) | >100 (>455) | 5.0 (22) | >1 (>77) | >1 (18) | 0.25 (28) | >1 (>33) | 0.25 (17) |
| HIV-1C | 0.42 (70) | 1.6 (8) | >100 (>455) | 4.4 (34) | 0.39 (23) | >1 (18) | 0.039 (4) | 0.40 (13) | 0.31 (21) |
| HIV-1G | 0.54 (90) | 1.2 (6) | >100 (>455) | 2.2 (17) | 0.17 (10) | >1 (18) | 0.033 (4) | 0.095 (3) | 0.22 (15) |
| HIV-1ES | 0.75 (125) | 4.3 (>22) | 0.46 (2) | 4.3 (33) | >1 (>77) | >1 (18) | 0.040 (4) | 0.24 (8) | 0.73 (49) |
| HIV-1EV | 0.53 (88) | 4.9 (>25) | >100 (>455) | 5.0 (22) | 0.86 (51) | >1 (18) | 0.50 (56) | 0.60 (20) | 0.55 (37) |
| HIV-1EY | 0.72 (120) | 1.5 (>8) | 0.97 (4) | 1.6 (12) | 0.33 (19) | 0.62 (11) | 0.18 (20) | 0.18 (6) | 0.61 (40) |
The IC50 values were determined by using PHA-PBMC exposed to HIV-1s (50 TCID50 dose/105 PBMC) and the inhibition of p24 Gag protein production as an endpoint. All drug sensitivities were determined in triplicate. The values shown are representative of two or three separate experiments. The numbers in parentheses represent the fold changes of the IC50 values against each isolate compared to the IC50 values against HIV-1wt. See Table 1, footnote a, and Materials and Methods for other abbreviations. NFV, nelfinavir.
HIV-1wt was a pretherapy clinical HIV-1 strain, HIV-1ERS104pre (36).
Inserts identified in the Gag of HIVMDR were duplicates.
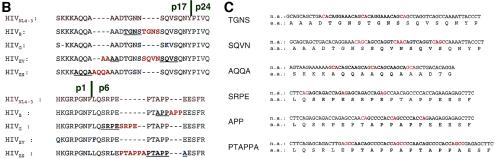
Determination of nucleotide sequences of these six molecular infectious HIVMDR clones revealed that they contained 12 to 23 amino acid changes in the RT compared to the consensus nucleotide sequence (Table 3). Most of these amino acid substitutions were reportedly associated with resistance to NRTIs and/or non-NRTIs (38). These HIVMDR clones also contained as many as 10 to 20 amino acid substitutions spread throughout the protease's amino acid sequence (Table 3). The nucleotide sequence determination of the entire gag region also revealed that each clone had a variety of amino acid substitutions spread throughout the Gag amino acid sequence, as shown in Fig. 1A. In addition to these amino acid substitutions, four of the six molecular clones were found to have amino acid insertions that were apparently clustered near the p17/p24 and p1/p6 cleavage sites (Fig. 1A). Interestingly, five of the six inserts had amino acid and nucleic acid sequences identical to those of the juxtapositioned stretches (Fig. 1B and C), strongly suggesting that the inserts represented duplicates, although the insert SQVNAAC differed from the adjacent stretch SQVSAGC. For example, TGNS in HIVB is a duplicate of the 5′-TGNS stretch, SRPE in HIVG is a duplicate of the 5′-SRPE stretch, and PTAPPA in HIVES is a duplicate of the 3′-PTAPPA stretch (Fig. 1B and C). It is noteworthy that for five of six duplicates, three sets of two to four bases (shown in red in Fig. 1C) were recognized in the 5′ end, center, and 3′ end of each of the duplicate-associated stretches (shown in boldface in Fig. 1C), although for the SQVN insert the four bases seen in the center differed from the other two pairs of four bases.
TABLE 3.
Amino acid substitutions in protease and RT
| Patient | Amino acid substitutionsa
|
|
|---|---|---|
| PR | RT | |
| B | L10I, K14R, L33I, M36I, M46I, I54V, K55R, I62V, L63P, A71V, G73S, V82A, L90M, I93L | V35I, M41L, K43E, S48T, D67DELETED, T69G, K70R, L74I, A98G, L100S, Q102K, K103N, M135I, V179I, M184V, G196E, T215F, K219Q, L227H |
| G | L10I, V11I, T12E, I15V, L19I, R40K, M46L, L63P, A71T, V82A, L90M | D67G, S68G, T69D, K70R, V118I, E122K, I135T, M184V, Q197K, T215F, D218E, K219Q |
| EV | L10V, T12E, G16A, L19I, K20R, L33F, E35D, M36I, M46I, I49V, F53L, I54V, K55R, G57K, D60E, I62V, L63P, A71V, V82A, L90M | M41L, K43E, E44A, T58A, D67E, S68G, L74V, V75M, L100I, Q102N, K104T, V118I, I135T, I142V, T165I, Q174N, Y181I, M184V, L210W, R211K, T215Y, H221Y, V245E |
| ES | L10I, M46L, K55R, I62V, L63P, I72L, G73C, V77I, I85V, L90M | M41L, K43E, E44D, D67N, T69D, L74V, K101E, V118I, E122K, D123E, Y181C, G190S, G196E, E203V, Q207E, L210W, R211K, L214F, T215Y, V245K |
Amino acids different from those of the consensus HIVNL4-3.
FIG. 1.
Amino acid and nucleic acid sequences of molecularly cloned HIVMDR. (A) Amino acid (AA) substitutions in the Gag protein and amino acid insertions clustered near the p17/p24 and p1/p6 cleavage sites in molecular infectious HIVMDR clones. (B) Deduced amino acids sequences near the Gag p17/p24 and p1/p6 cleavage sites. The consensus sequence of HIVNL4-3 is shown in brown. Amino acid insertions are shown in red, whereas amino acids different from those of the consensus HIVNL4-3 are shown in blue. The amino acid stretches corresponding to the inserts are shown aligned and underlined. (C) Nucleic acid sequences for the insertions. The stretches associated with the duplication are shown in boldface. Note the presence of three sets of two to four nucleotides located in the 5′ end, center, and 3′ end of the associated stretches (shown in red).
Gag inserts compromise or do not affect the replication of HIVNL4-3.
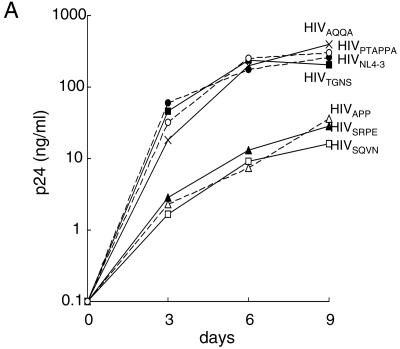
In order to examine whether these unique inserts affected the replication capability of HIV-1, we introduced each insert to a wild-type HIV-1 strain, HIVNL4-3, and determined the replication profile of resulting infectious clones. As shown in Fig. 2A, all six newly generated molecular clones with inserts were clearly capable of replicating in culture. Three molecular clones—HIVAQQA, HIVPTAPPA, and HIVTGNS—appeared to replicate comparably to the wild-type HIVNL4-3. Three other molecular clones—HIVAPP, HIVSRPE, and HIVSQVN—appeared to be less replication competent than HIVNL4-3. To confirm and corroborate these observations, we performed a CHRA in which two different clones were propagated in culture, and the percent population of each clone was determined, as previously reported (15, 22). Three molecular clones (HIVAPP, HIVSRPE, and HIVSQVN), which appeared to be less replication competent compared to HIVNL4-3, were readily outgrown by HIVNL4-3 in CHRA. HIVPTAPPA grew comparably to HIVNL4-3 by 4 weeks but then was outgrown by HIVNL4-3. Two other molecular clones, HIVTGNS and HIVAQQA replicated comparably to HIVNL4-3.
FIG. 2.
Replication profiles of the wild-type HIV-1 with or without an insert. (A) Replication kinetic assay with MT-4 cells was conducted with HIVNL4-3 and HIVNL4-3 clones with various inserts. The production of p24 Gag protein by MT-4 cells into the culture supernatants was monitored over 9 days. (B) Competitive HIV replication assay. Six pairs of HIVNL4-3 and HIVNL4-3 clones with an insert were propagated in MT-4 cells, and the percent proportion of each virus was determined over seven passages. When the cells harvested at the conclusion of the last passage were subjected to DNA extraction and direct DNA sequencing, no deletions or additional amino acid changes in the p17/p24 and p1/p6 Gag cleavage sites were identified.
Gag processing of HIVNL4-3 carrying an insert.
It was possible that the inserts introduced into the wild-type HIVNL4-3 impaired the activity of the wild-type protease of HIVNL4-3 to process the polyproteins, thereby largely compromising the replication of the molecular HIVNL4-3 clones containing an insert. Therefore, we performed a Western blot with anti-p24 antiserum and an anti-p6 monoclonal antibody by using the lysates of COS-7 cells producing insert-containing molecular HIV-1 clones and cell-free virions harvested on day 2 after transfection of COS-7 cells (Fig. 3). A dense p24 signal was detected in both the cell lysates and virion lysates of the wild-type HIVNL4-3 as examined with anti-p24 antiserum; however, all molecular clones carrying an insert produced substantially decreased amounts of p24 (Fig. 3) except for HIVTGNS and HIVAQQA. It is noteworthy that the two clones, HIVTGNS and HIVAQQA, which did not show a detectable difference in replication competence compared to HIVNL4-3 (Fig. 2A and B), had comparable amounts of p24 when both cell lysates and virions were examined (Fig. 3 and Table 4). HIVSQVN, HIVSRPE, and HIVAPP, which showed substantially decreased replication competence compared to HIVNL4-3 (Fig. 2A and B), had decreased p24 amounts compared to the wild type in the blotting of cell lysates. When analyzed by using densitometry, the decrease in the amounts of p24 in the case of APP added was by 45.1% (43.5 to 23.9% densityp24 [Table 4]). In the assay with HIVSQVN, HIVSRPE, and HIVAPP virions, significant amounts of noncleaved immature Gag protein precursors were also detected. HIVPTAPPA, which comparably replicated initially but was ultimately overgrown by HIVNL4-3 (Fig. 2B), appeared to contain a slightly lower amount of p24 in the blotting of cell lysates, and more substantial amounts of immature Gag proteins were seen in virions.
FIG. 3.
Gag processing in HIVNL4-3 with or without an insert. The lysates of COS-7 cells producing HIVNL4-3 or HIV clones containing an insert and the virion lysates were examined for the Gag processing. The samples were prepared 48 h after transfection and subjected to Western blotting with anti-p24 antiserum and anti-p6 monoclonal antibody. The positions corresponding to the sizes of the fully cleaved mature proteins (p24 and p6) and the immature proteins (p55, p41, p37, and p15) are indicated by arrows.
TABLE 4.
p24 signal density of each virus in a Western blot
| Strain | Protease | Gag | Gag insertion at site:
|
Day posttransfection | % Densityp24a | Corresponding figure | |
|---|---|---|---|---|---|---|---|
| p17/p24 | p1/p6 | ||||||
| NL4-3 | WTb | WT | 2 | 43.5 | Fig. 3 | ||
| WT | WT | TGNS | 2 | 46.3 | Fig. 3 | ||
| WT | WT | SQVS | 2 | 31.7 | Fig. 3 | ||
| WT | WT | SRPE | 2 | 29.6 | Fig. 3 | ||
| WT | WT | APP | 2 | 23.9 | Fig. 3 | ||
| WT | WT | AQQA | 2 | 49.9 | Fig. 3 | ||
| WT | WT | PTAPPA | 2 | 37.1 | Fig. 3 | ||
| WT | WT | 3 | 73.5 | Fig. 5A | |||
| B | B | B | TGNS | APP | 3 | 10.8 | Fig. 5A |
| B | B | APP | 3 | 2.4 | Fig. 5A | ||
| B | B | TGNS | 3 | 4.7 | Fig. 5A | ||
| G | G | G | SRPE | 3 | 45.4 | Fig. 5A | |
| G | G | 3 | 29.1 | Fig. 5A | |||
| EV | EV | EV | SQVN | 3 | 14.3 | Fig. 5A | |
| EV | EV | 3 | 7.3 | Fig. 5A | |||
| ES | ES | ES | AQQA | PTAPPA | 3 | 53.7 | Fig. 5A |
| ES | ES | PTAPPA | 3 | 26.5 | Fig. 5A | ||
| ES | ES | AQQA | 3 | 14.6 | Fig. 5A | ||
| NL4-3 | B | WT | 2 | 10.5 | Fig. 6 | ||
| B | WT | TGNS | APP | 2 | 24.2 | Fig. 6 | |
| ES | WT | 2 | 19.9 | Fig. 6 | |||
| ES | WT | AQQA | PTAPPA | 2 | 33.2 | Fig. 6 | |
Calculated as 100 × (p24 signal density/total Gag product signal densities) in a Western blot of cell lysates with anti-p24 antisera. The values for % density are to be compared within the same figure.
WT, wild type.
When the cell lysates were examined with an anti-p6 monoclonal antibody, a significant amount of p6 was detected in HIVPTAPPA and a small amount of p6 seen in HIVAPP. In contrast, when virions were examined, substantial amounts of the p6 proteins were seen in all clones, although small amounts of immature proteins were seen in HIVSRPE, HIVAPP, and HIVPTAPPA. It is of note that although the PTAP motif within the p6 Gag has recently been shown to recruit the human protein Tsg101 to facilitate HIV-1 budding (14, 30) significant amounts of the mature p6 protein were seen in all of the virions, suggesting that none of the SRPE, APP, or PTAPPA inserts located near the PTAP motif blocked viral budding (Fig. 3).
We noted that the p15 (cell lysates) and p6 (virions) species in three clones—HIVSRPE, HIVAPP, and HIVPTAPPA—appeared to be of a slightly greater size(s) than those of other clones, presumably reflecting that these three clones contained the inserts within the p6 protein, thus being of a slightly larger size, although it is also possible that the p6 species in the three clones may represent undigested p1+p6 proteins.
Replication of HIVMDR with or without Gag inserts.
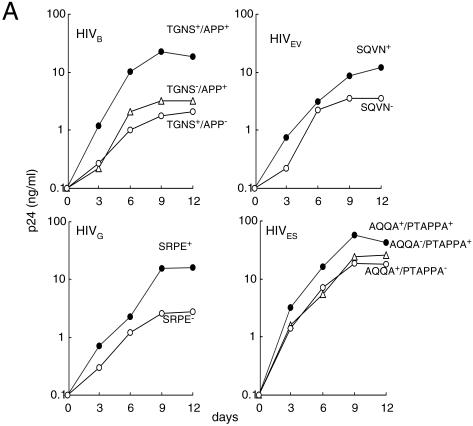
Next, we examined whether the Gag inserts affected the replication competence of the full-length primary HIVMDR clones. We deleted each insert from molecularly cloned clinical isolates by using the PMR method and propagated each of them in MT-4 cells or PM1 cells. HIVB, HIVG, and HIVES replicated well and caused significant cytopathic effects in MT-4 cells (data not shown). In contrast, HIVEV more efficiently propagated in PM1 cells than in MT-4 cells and did not induce cytopathic effects in MT-4 cells (data not shown). These data suggest that HIVB, HIVG, and HIVES are of the X4 lineage, whereas HIVEV is of the R5 lineage. As shown in Fig. 4A, all four primary HIVMDR clones appeared to propagate more efficiently than any Gag insert-deletant clones (i.e., clones with the Gag insert deleted) in culture over 12 days. To corroborate and extend this observation, we conducted the CHRA assay and compared the fitness of these molecular clones. The TGNS-deletant (TGNS−/APP+) and APP-deletant (TGNS+/APP−) clones were readily overgrown by the primary HIVB clone (TGNS+/APP+) (Fig. 4B). The SQVN-deletant (SQVN−) and SRPE-deletant (SRPE−) clones were also readily overgrown by the primary HIVEV (SQVN+) and HIVG (SRPE+) clones, respectively. Moreover, the AQQA-deletant and PTAPPA-deletant clones were overgrown by the primary HIVES (AQQA+/PTAPPA+). These data strongly suggest that each insert conferred replication advantage on primary HIVMDR clones.
FIG. 4.
Replication profiles of primary HIVMDR and insert-lacking infectious clones. (A) Replication profiles of primary molecular HIVMDR clones and insert-lacking infectious clones were determined in PHA-PBMC over 12 days. (B) CHRA. Six pairs of a primary HIVMDR clone plus an insert-lacking HIVMDR were examined (HIVB, HIVG, and HIVES in MT-4 cells and HIVEV in PM1 cells), and the percent proportion of each virus was determined.
Gag processing of HIVMDR with or without Gag inserts.
We sought to determine whether the inserts altered the Gag processing within the cells producing each HIVMDR by using the Western blotting technique with anti-p24 antiserum and anti-p6 monoclonal antibody. As shown in Fig. 5A, a dense p24 signal and lower amounts of immature polyproteins were detected in the wild-type HIVNL4-3. In contrast, all four HIVMDR variants had substantially lower % densityp24 values than HIVNL4-3 (Table 4). We found that, in all HIVMDR clones that were modified to lack an insert, even lower % densityp24 values than those of the original clones were identified (Fig. 5A and Table 4).
FIG. 5.
Gag processing in molecular HIVMDR clones and insert-lacking HIVMDR clones. COS-7 cells producing primary molecular HIVMDR clones and the corresponding insert-lacking HIVMDR clones were harvested at 72 h after transfection, and their cell lysates were subjected to Western blot analysis with anti-p24 antiserum (A) and an anti-p6 monoclonal antibody (B). The positions corresponding to the sizes of the fully cleaved mature proteins (p24 and p6) and the immature proteins (p55, p41, p37, and p15) are indicated by arrows.
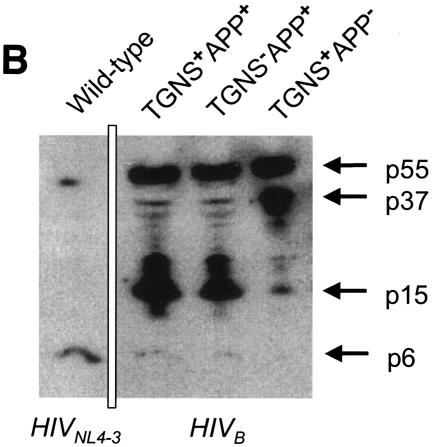
The anti-p6 monoclonal antibody did not recognize the p6 protein of HIVG, HIVEV, or HIVES; therefore, the profile of polyprotein processing was determined only for HIVB. As shown in Fig. 5B, HIV contained a smaller amount of the p6 protein and larger amounts of precursor proteins than did HIVNL4-3. It was found that when the TGNS or APP insert was deleted from HIVB, even lower amounts of p6 protein and similar or much greater amounts of precursor proteins were detected compared to HIVB.
Gag processing in HIVNL4-3 carrying a mutated protease with or without inserts.
Finally, in order to examine more directly the possible effects of the inserts identified in Gag on the proteolytic activity of mutated proteases and the viral fitness, we generated four additional infectious clones, HIVNL/B-Pr, HIVNL/B-Pr/TGNS-APP, HIVNL/ES-Pr, and HIVNL/ES-Pr/AQQA-PTAPPA. When the lysates of COS-7 cells producing each of these clones were tested for the Gag processing profiles by using a Western blot, a relatively smaller amount of p24 Gag (% densityp24 = 10.5%) was detected in the lysates of COS-7 cells producing HIVNL/B-Pr; however, the introduction of TGNS plus APP increased the p24 Gag amount (% densityp24 = 24.2%) (Fig. 6). The same was true for HIVNL/ES-Pr (% densityp24 = 19.9%) and HIVNL/ES-Pr/AQQA-PTAPPA (% densityp24 = 33.2%).
FIG. 6.
Gag processing in HIVNL4-3 carrying a mutated protease with or without inserts. COS-7 cells producing four molecular clones (left pair, HIVNL/B-Pr and HIVNL/B-Pr/TGNS-APP; right pair, HIVNL/ES-Pr and HIVNL/ES-Pr/AQQA-PTAPPA) were harvested at 48 h after transfection, and their cell lysates were subjected to Western blot analysis with anti-p24 antiserum.
These data, taken together, strongly suggest that the inserts had rendered the polyproteins more excisable by the mutated and functionally compromised proteases and improved the otherwise reduced replicative competence of the HIVMDR variants.
DISCUSSION
The prevalence of HIV-1 variants that contain the Gag inserts identified in the present study cannot be estimated from currently available data sets. In the present study, we examined HIV-1 variants isolated from 20 patients randomly chosen from those who had received a number of RTIs and PIs over long periods of time and were enrolled in the salvage therapy clinical trials (11, 42). Of these 20 HIV-1 isolates, 6 that harbored the greatest numbers of drug resistance-associated mutations were chosen, and a full-length molecular infectious clone was generated from each of the 6 isolates. Thus, it should not be surprising that Gag inserts were identified at such a high rate (four of the six clones) in the present study. It is noteworthy that the presence of Gag inserts is apparently not associated with drug resistance profiles of the clones (Table 2) or the numbers or positions of amino acid substitutions in the protease or RT (Table 3), although the number of the HIVMDR clones examined in the present study is rather small and an analysis of greater numbers of HIVMDR clones is warranted.
Although various inserts have been reported to occur around the p6 Gag's PTAP motif (13, 28), their exact amino acid sequences have not been well documented, and their frequency and role have been controversial. One study has shown that these insertions occurred with equal frequency in HIV-1 isolates from both drug-naive and drug-experienced patients (13), whereas other studies have shown that they are seen more frequently in HIV-1 from drug-experienced individuals (28). In the Los Alamos database (23), no insertions identical to those in the present study have been reported, although a number of other insertions have been seen. Of 114 wild-type HIV-1 isolates in the database, insertions of more than three amino acids occurred in four regions as follows: 5 (4.4%) near the p17/p24 cleavage site, 6 (5.3%) near the p2/p7 cleavage site, 6 (5.3%) near the p1/p6 cleavage site, and 15 (13.2%) within p6. Considering that the incidence of insertions identified in the present study is very high (four of the six clones as described above), the present insertions are likely to be related to the long-term exposure of HIV-1 to antiviral drugs including multiple PIs. Indeed, a recently reported longitudinal study by Ibe et al. (19) demonstrated that certain insertions such as APP were identified in wild-type viruses at low percentages; however, with antiviral therapy started, more virions were found to harbor such insertions, suggesting that these insertions represent “polymorphisms” that are associated with drug resistance.
Pettit et al. have shown that the proteolytic processing of the Gag precursor by the viral protease occurs in a sequential manner and that the rates of cleavage at the five major Gag cleavage sites, including the p17/p24 and p1/p6 sites, differ by as much as 400-fold when full-length Gag protein is digested with wild-type HIV-1 protease in vitro (29). Although it is not clear whether the polyprotein is cleaved at similar rates by mutated proteases, it appears that the sequence of processing or the catalytic rates are not associated with the acquisition of the inserts seen in the present study.
The mechanism of the HIV-1 acquisition of the inserts observed in the present study is not known from the data presented. An extensive body of literature has demonstrated that HIV-1 RT is substantially error prone (1, 2, 31); however, mutations at Gag cleavage sites are rather limited, since not much flexibility is allowed near the scissile bond and the cleavage site must remain generally hydrophobic for the cleavage by the protease. One can presume that with highly mutated and enzymatically malfunctioning protease developed, the acquisition of inserts rather than developing cleavage site mutations should have been an efficient strategy for the virus to improve the otherwise deteriorated viral fitness by increasing the accessibility of the mutated protease to the cleavage sites and/or enhancing the cleavage sensitivity of the polyprotein to the mutated enzyme. It is also of note that the nucleic acid sequences of certain inserts has reportedly substantial variability in spite of the observation that the resulting amino acid sequence is relatively restricted (40). However, five of the six inserts studied here had a nucleic acid sequence identical to that of the juxtapositioned stretch, a finding that also corroborates that the inserts likely occurred through duplications. In addition, since three sets of two to four bases (Fig. 1C) were recognized in the 5′ end, center, and 3′ end of each of the duplicate-associated stretches, it is likely that a slippage or dislocation of the primer with respect to the template during DNA synthesis by RT occurred, although the possibility of involvement of polymerase errors, recombination, hypermutation, and instability cannot be fully ruled out.
It is noteworthy that the 449-Leu→Phe mutation at the p1/p6 site first reported by Doyon et al. (9) and Zhang et al. (43) is seen relatively often in HIV-1 variants resistant to PIs, suggesting that the mutated and enzymatically malfunctioning proteases excise the polyprotein at the p1/p6 site least efficiently, thus resulting in the elimination of HIV-1 with a “wild-type p1/p6 site” and the propagation of HIV-1 that acquired the 449-Leu→Phe mutation. It is plausible that with the inserts near the p1/p6 cleavage site obtained, the mutated proteases of HIVB, HIVG, and HIVES could excise the polyprotein efficiently. In this respect, it is possible that HIVEV acquired the 449-Leu→Phe mutation to recover the catalytic activity and thus required no inserts close to the p1/p6 cleavage site. Similarly, the mutated proteases perhaps became less competent in excising the polyproteins at the p17/p24 cleavage site, and thus HIV-1 variants that acquired the insert(s) close to the site were presumably selected in the presence of the selection pressure imposed by PIs. It is also noteworthy that the introduction of the inserts to wild-type HIV-1 decreased the processing of polyproteins and viral fitness. We suggest that the presence of the inserts alters the conformation of the cleavage sites and limits the access of the wild-type protease to the cleavage sites and/or reduces the cleavage sensitivity of the polyprotein to the wild-type protease. This would explain why no insertions identical to those in the present study have been identified in wild-type HIV-1 isolates as described above.
Taken together, the results presented here establish that amino acid insertions in the proximity of Gag cleavage sites improve the otherwise compromised replication of HIV-1 variants that are highly resistant to multiple PIs. Further characterization of the factors related to the emergence of these insertions and biochemical studies of insertion-containing Gag polyproteins may open a new avenue to the intervention of HIV-1 highly resistant to multiple PIs.
Acknowledgments
We thank Shigeyoshi Harada, Zhi-Ming Zhen, and Robert Yarchoan for helpful discussions and/or critical reading of the manuscript.
S.T. was a recipient of the support from the Japanese Foundation for AIDS Prevention. This study was supported in part by a grant from the Research for the Future Program (JSPS-RFTF 97L00705) of the Japan Society for the Promotion of Science; a Grant-in-Aid for Scientific Research (Priority Areas) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Monbu-Kagakusho); and the Grant for Promotion of AIDS Research from the Ministry of Health, Welfare, and Labor of Japan (Kosei-Rohdosho).
REFERENCES
- 1.Abbotts, J., K. Bebenek, T. A. Kunkel, and S. H. Wilson. 1993. Mechanism of HIV-1 reverse transcriptase. Termination of processive synthesis on a natural DNA template is influenced by the sequence of the template-primer stem. J. Biol. Chem. 268:10312-10323. [PubMed] [Google Scholar]
- 2.Bebenek, K., J. D. Roberts, and T. A. Kunkel. 1992. The effects of dNTP pool imbalances on frameshift fidelity during DNA replication. J. Biol. Chem. 267:3589-3596. [PubMed] [Google Scholar]
- 3.Borman, A. M., S. Paulous, and F. Clavel. 1996. Resistance of human immunodeficiency virus type 1 to protease inhibitors: selection of resistance mutations in the presence and absence of the drug. J. Gen. Virol. 77:419-426. [DOI] [PubMed] [Google Scholar]
- 4.Brenner, B. G., J. P. Routy, M. Petrella, D. Moisi, M. Oliveira, M. Detorio, B. Spira, V. Essabag, B. Conway, R. Lalonde, R. P. Sekaly, and M. A. Wainberg. 2002. Persistence and fitness of multidrug-resistant human immunodeficiency virus type 1 acquired in primary infection. J. Virol. 76:1753-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condra, J. H., W. A. Schleif, O. M. Blahy, L. J. Gabryelski, D. J. Graham, J. C. Quintero, A. Rhodes, H. L. Robbins, E. Roth, M. Shivaprakash, D. Titus, T. Yang, H. Tepplert, K. E. Squires, P. J. Deutsch, and E. A. Emini. 1995. In vivo emergence of HIV-1 variants resistant to multiple protease inhibitors. Nature 374:569-571. [DOI] [PubMed] [Google Scholar]
- 6.Cote, H. C., Z. L. Brumme, and P. R. Harrigan. 2001. Human immunodeficiency virus type 1 protease cleavage site mutations associated with protease inhibitor cross-resistance selected by indinavir, ritonavir, and/or saquinavir. J. Virol. 75:589-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croteau, G., L. Doyon, D. Thibeault, G. McKercher, L. Pilote, and D. Lamarre. 1997. Impaired fitness of human immunodeficiency virus type 1 variants with high-level resistance to protease inhibitors. J. Virol. 71:1089-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Department of Health and Human Services Panel on Clinical Practices for Treatment of HIV Infection. 2003. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [Online.] http://aidsinfo.nih.gov/guidelines/adult/archive/AA_071403.html. [DOI] [PubMed]
- 9.Doyon, L., G. Croteau, D. Thibeault, F. Poulin, L. Pilote, and D. Lamarre. 1996. Second locus involved in human immunodeficiency virus type 1 resistance to protease inhibitors. J. Virol. 70:3763-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ermolieff, J., X. Lin, and J. Tang. 1997. Kinetic properties of saquinavir resistant mutants of human immunodeficiency virus type 1 protease and their implications in drug resistance in vivo. Biochemistry 36:12364-12370. [DOI] [PubMed] [Google Scholar]
- 11.Falloon, J., S. Piscitelli, S. Vogel, B. Sadler, H. Mitsuya, M. F. Kavlick, K. Yoshimura, M. Rogers, S. LaFon, D. J. Manion, H. C. Lane, and H. Masur. 2000. Combination therapy with amprenavir, abacavir, and efavirenz in human immunodeficiency virus (HIV)-infected patients failing a protease-inhibitor regimen: pharmacokinetic drug interactions and antiviral activity. Clin. Infect. Dis. 30:313-318. [DOI] [PubMed] [Google Scholar]
- 12.Fang, G., B. Weiser, A. Visosky, T. Moran, and H. Burger. 1999. PCR mediated recombination: a general method applied to construct chimeric infectious molecular clones of plasma-derived HIV-1 RNA. Nat. Med. 5:239-242. [DOI] [PubMed] [Google Scholar]
- 13.Gallego, O., C. de Mendoza, A. Corral, and V. Soriano. 2003. Changes in the human immunodeficiency virus p7-p1-p6 gag gene in drug-naive and pretreated patients. J. Clin. Microbiol. 41:1245-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrus, J. E., U. K. von Schwedler, O. W. Pornillos, S. G. Morham, K. H. Zavitz, H. E. Wang, D. A. Wettstein, K. M. Stray, M. Cote, R. L. Rich, D. G. Myszka, and W. I. Sundquist. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55-65. [DOI] [PubMed] [Google Scholar]
- 15.Gatanaga, H., Y. Suzuki, H. Tsang, K. Yoshimura, M. F. Kavlick, K. Nagashima, R. J. Gorelick, S. Mardy, C. Tang, M. F. Summers, and H. Mitsuya. 2002. Amino acid substitutions in Gag protein at non-cleavage sites are indispensable for the development of a high multitude of HIV-1 resistance against protease inhibitors. J. Biol. Chem. 277:5952-5961. [DOI] [PubMed] [Google Scholar]
- 16.Grabar, S., C. Pradier, E. Le Corfec, R. Lancar, C. Allavena, M. Bentata, P. Berlureau, C. Dupont, P. Fabbro-Peray, I. Poizot-Martin, and D. Costagliola. 2000. Factors associated with clinical and virological failure in patients receiving a triple therapy including a protease inhibitor. AIDS 14:141-149. [DOI] [PubMed] [Google Scholar]
- 17.Gulnik, S. V., L. I. Suvorov, B. Liu, B. Yu, B. Anderson, H. Mitsuya, and J. W. Erickson. 1995. Kinetic characterization and cross-resistance patterns of HIV-1 protease mutants selected under drug pressure. Biochemistry 34:9282-9287. [DOI] [PubMed] [Google Scholar]
- 18.Ho, D. D., T. Toyoshima, H. Mo, D. J. Kempf, D. Norbeck, C. M. Chen, N. E. Wideburg, S. K. Burt, J. W. Erickson, and M. K. Singh. 1994. Characterization of human immunodeficiency virus type 1 variants with increased resistance to a C2-symmetric protease inhibitor. J. Virol. 68:2016-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibe, S., N. Shibata, M. Utsumi, and T. Kaneda. 2003. Selection of human immunodeficiency virus type 1 variants with an insertion mutation in the p6gag and p6pol genes under highly active antiretroviral therapy. Microbiol. Immunol. 47:71-79. [DOI] [PubMed] [Google Scholar]
- 20.Iversen, A. K., R. W. Shafer, K. Wehrly, M. A. Winters, J. I. Mullins, B. Chesebro, and T. C. Merigan. 1996. Multidrug-resistant human immunodeficiency virus type 1 strains resulting from combination antiretroviral therapy. J. Virol. 70:1086-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kavlick, M. F., and H. Mitsuya. 2001. The emergence of drug-resistant HIV-1 variants and impact on antiretroviral therapy of HIV-1 infection. p. 279-312. In E. De Clarcq (ed.), Art of antiretroviral therapy. American Society for Microbiology, Washington, D.C.
- 22.Kosalaraksa, P., M. F. Kavlick, V. Maroun, R. Le, and H. Mitsuya. 1999. Comparative fitness of multi-dideoxynucleoside-resistant human immunodeficiency virus type 1 (HIV-1) in an in vitro competitive HIV-1 replication assay. J. Virol. 73:5356-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuiken, C., B. Foley, B. P. M. Hahn, P. Marx, F. McCutchan, J. Mellors, J. Mullins, S. Wolinsky, and B. Kober. 2000. HIV Sequence compendium. Los Alamos National Laboratory, Los Alamos, N. Mex.
- 24.Mahalingam, B., J. M. Louis, C. C. Reed, J. M. Adomat, J. Krouse, Y. F. Wang, R. W. Harrison, and I. T. Weber. 1999. Structural and kinetic analysis of drug-resistant mutants of HIV-1 protease. Eur. J. Biochem. 263:238-245. [DOI] [PubMed] [Google Scholar]
- 25.Mammano, F., C. Petit, and F. Clavel. 1998. Resistance-associated loss of viral fitness in human immunodeficiency virus type 1: phenotypic analysis of protease and gag coevolution in protease inhibitor-treated patients. J. Virol. 72:7632-7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitsuya, H., and J. W. Erickson. 1999. Discovery and development of antiretroviral therapeutics for HIV infection, p. 751-780. In T. C. Merigan, J. G. Bartlet, and D. Bolognes (ed.), Textbook of AIDS medicine. The Williams & Wilkins Co., Baltimore, Md.
- 27.Paredes, R., A. Mocroft, O. Kirk, A. Lazzarin, S. E. Barton, J. van Lunzen, T. L. Katzenstein, F. Antunes, J. D. Lundgren, and B. Clotet. 2000. Predictors of virological success and ensuing failure in HIV-positive patients starting highly active antiretroviral therapy in Europe: results from the EuroSIDA study. Arch. Intern. Med. 160:1123-1132. [DOI] [PubMed] [Google Scholar]
- 28.Peters, S., M. Munoz, S. Yerly, V. Sanchez-Merino, C. Lopez Galindez, L. Perrin, B. Larder, D. Cmarko, S. Fakan, P. Meylan, and A. Telenti. 2001. Resistance to nucleoside analog reverse transcriptase inhibitors mediated by human immunodeficiency virus type 1 p6 protein. J. Virol. 75:9644-9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettit, S. C., M. D. Moody, R. S. Wehbie, A. H. Kaplan, P. V. Nantermet, C. A. Klein, and R. Swanstrom. 1994. The p2 domain of human immunodeficiency virus type 1 Gag regulates sequential proteolytic processing and is required to produce fully infectious virions. J. Virol. 68:8017-8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pornillos, O., S. L. Alam, D. R. Davis, and W. I. Sundquist. 2002. Structure of the Tsg101 UEV domain in complex with the PTAP motif of the HIV-1 p6 protein. Nat. Struct. Biol. 9:812-817. [DOI] [PubMed] [Google Scholar]
- 31.Preston, B. D., B. J. Poiesz, and L. A. Loeb. 1988. Fidelity of HIV-1 reverse transcriptase. Science 242:1168-1171. [DOI] [PubMed] [Google Scholar]
- 32.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 33.Rose, R. E., Y. F. Gong, J. A. Greytok, C. M. Bechtold, B. J. Terry, B. S. Robinson, M. Alam, R. J. Colonno, and P. F. Lin. 1996. Human immunodeficiency virus type 1 viral background plays a major role in development of resistance to protease inhibitors. Proc. Natl. Acad. Sci. USA 93:1648-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schock, H. B., V. M. Garsky, and L. C. Kuo. 1996. Mutational anatomy of an HIV-1 protease variant conferring cross-resistance to protease inhibitors in clinical trials: compensatory modulations of binding and activity. J. Biol. Chem. 271:31957-31963. [DOI] [PubMed] [Google Scholar]
- 35.Shafer, R. W., M. J. Kozal, M. A. Winters, A. K. Iversen, D. A. Katzenstein, M. V. Ragni, W. A. Meyer, III, P. Gupta, S. Rasheed, R. Coombs, and T. C. Merigan. 1994. Combination therapy with zidovudine and didanosine selects for drug-resistant human immunodeficiency virus type 1 strains with unique patterns of pol gene mutations. J. Infect. Dis. 169:722-729. [DOI] [PubMed] [Google Scholar]
- 36.Shirasaka, T., M. F. Kavlick, T. Ueno, W. Y. Gao, E. Kojima, M. L. Alcaide, S. Chokekijchai, B. M. Roy, E. Arnold, R. Yarchoan, and H. Mitsuya. 1995. Emergence of human immunodeficiency virus type 1 variants with resistance to multiple dideoxynucleosides in patients receiving therapy with dideoxynucleosides. Proc. Natl. Acad. Sci. USA 92:2398-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shirasaka, T., R. Yarchoan, M. C. O'Brien, R. N. Husson, B. D. Anderson, E. Kojima, T. Shimada, S. Broder, and H. Mitsuya. 1993. Changes in drug sensitivity of human immunodeficiency virus type 1 during therapy with azidothymidine, dideoxycytidine, and dideoxyinosine: an in vitro comparative study. Proc. Natl. Acad. Sci. USA 90:562-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanford University Medical Center. Stanford HIV drug resistance database. [Online.] http://hivdb.stanford.edu. Stanford University Medical Center, Stanford, Calif.
- 39.Ueno, T., T. Shirasaka, and H. Mitsuya. 1995. Enzymatic characterization of human immunodeficiency virus type 1 reverse transcriptase resistant to multiple 2′,3′-dideoxynucleoside 5′-triphosphates. J. Biol. Chem. 270:23605-23611. [DOI] [PubMed] [Google Scholar]
- 40.Winters, M. A., K. L. Coolley, Y. A. Girard, D. J. Levee, H. Hamdan, R. W. Shafer, D. A. Katzenstein, and T. C. Merigan. 1998. A 6-basepair insert in the reverse transcriptase gene of human immunodeficiency virus type 1 confers resistance to multiple nucleoside inhibitors. J. Clin. Investig. 102:1769-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeni, P. G., S. M. Hammer, C. C. Carpenter, D. A. Cooper, M. A. Fischl, J. M. Gatell, B. G. Gazzard, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, M. Schechter, R. T. Schooley, M. A. Thompson, S. Vella, and P. A. Volberding. 2002. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society-USA Panel. JAMA 288:222-235. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimura, K., R. Kato, K. Yusa, M. F. Kavlick, V. Maroun, A. Nguyen, T. Mimoto, T. Ueno, M. Shintani, J. Falloon, H. Masur, H. Hayashi, J. Erickson, and H. Mitsuya. 1999. JE-2147: a dipeptide protease inhibitor (PI) that potently inhibits multi-PI-resistant HIV-1. Proc. Natl. Acad. Sci. USA 96:8675-8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, Y. M., H. Imamichi, T. Imamichi, H. C. Lane, J. Falloon, M. B. Vasudevachari, and N. P. Salzman. 1997. Drug resistance during indinavir therapy is caused by mutations in the protease gene and in its Gag substrate cleavage sites. J. Virol. 71:6662-6670. [DOI] [PMC free article] [PubMed] [Google Scholar]