Abstract
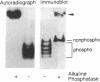
The insulin-like growth factors (IGF-I and IGF-II) are present in extracellular fluids bound to specific IGF-binding proteins (IGFBPs). We and others have reported varying biologic activity of different preparations of IGFBP-1 that appeared to have identical amino acid sequences and molecular sizes. This observation prompted us to determine whether IGFBP-1 undergoes posttranslational modifications. Immunoprecipitation was used to show that Chinese hamster ovary cells (transfected with a human IGFBP-1 cDNA construct) and human hepatoma (HepG2) cells secrete 32P-labeled IGFBP-1 following incubation with [32P]orthophosphate. Phospho amino acid analysis of 32P-labeled IGFBP-1 revealed only phosphoserine residues. A method was developed that could separate nonphosphorylated IGFBP-1 from four or five phosphorylated isoforms. Using this technique we demonstrated that human amniotic fluid and human fetal serum contain a large proportion of nonphosphorylated IGFBP-1, as well as phosphorylated forms. In contrast, HepG2 cells and human decidual cells secrete predominantly the phosphorylated isoforms. These observations suggest that IGFBP-1 is secreted as a phosphoprotein and is subsequently dephosphorylated in vivo. Binding studies showed that the phosphorylated IGFBP-1 secreted by HepG2 cells has a 6-fold higher affinity for IGF-I than it does after dephosphorylation. We conclude that IGFBP-1 is phosphorylated and that this phosphorylation is a physiologically important posttranslational modification.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard J., Baxter R., Binoux M., Clemmons D., Drop S., Hall K., Hintz R., Rechler M., Rutanen E., Schwander J. On the nomenclature of the IGF binding proteins. Acta Endocrinol (Copenh) 1989 Nov;121(5):751–752. doi: 10.1530/acta.0.1210751. [DOI] [PubMed] [Google Scholar]
- Baxter R. C., Martin J. L., Wood M. H. Two immunoreactive binding proteins for insulin-like growth factors in human amniotic fluid: relationship to fetal maturity. J Clin Endocrinol Metab. 1987 Sep;65(3):423–431. doi: 10.1210/jcem-65-3-423. [DOI] [PubMed] [Google Scholar]
- Brewer M. T., Stetler G. L., Squires C. H., Thompson R. C., Busby W. H., Clemmons D. R. Cloning, characterization, and expression of a human insulin-like growth factor binding protein. Biochem Biophys Res Commun. 1988 May 16;152(3):1289–1297. doi: 10.1016/s0006-291x(88)80425-x. [DOI] [PubMed] [Google Scholar]
- Busby W. H., Hossenlopp P., Binoux M., Clemmons D. R. Purified preparations of the amniotic fluid-derived insulin-like growth factor-binding protein contain multimeric forms that are biologically active. Endocrinology. 1989 Aug;125(2):773–777. doi: 10.1210/endo-125-2-773. [DOI] [PubMed] [Google Scholar]
- Busby W. H., Jr, Klapper D. G., Clemmons D. R. Purification of a 31,000-dalton insulin-like growth factor binding protein from human amniotic fluid. Isolation of two forms with different biologic actions. J Biol Chem. 1988 Oct 5;263(28):14203–14210. [PubMed] [Google Scholar]
- Busby W. H., Snyder D. K., Clemmons D. R. Radioimmunoassay of a 26,000-dalton plasma insulin-like growth factor-binding protein: control by nutritional variables. J Clin Endocrinol Metab. 1988 Dec;67(6):1225–1230. doi: 10.1210/jcem-67-6-1225. [DOI] [PubMed] [Google Scholar]
- Butler W. T. The nature and significance of osteopontin. Connect Tissue Res. 1989;23(2-3):123–136. doi: 10.3109/03008208909002412. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- D'Ercole A. J., Drop S. L., Kortleve D. J. Somatomedin-C/insulin-like growth factor I-binding proteins in human amniotic fluid and in fetal and postnatal blood: evidence of immunological homology. J Clin Endocrinol Metab. 1985 Oct;61(4):612–617. doi: 10.1210/jcem-61-4-612. [DOI] [PubMed] [Google Scholar]
- Feige J. J., Bradley J. D., Fryburg K., Farris J., Cousens L. C., Barr P. J., Baird A. Differential effects of heparin, fibronectin, and laminin on the phosphorylation of basic fibroblast growth factor by protein kinase C and the catalytic subunit of protein kinase A. J Cell Biol. 1989 Dec;109(6 Pt 1):3105–3114. doi: 10.1083/jcb.109.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg P. O., Martin S. C. Plasmin digestion of human fibrinogen previously phosphorylated by protein kinase C or dephosphorylated by alkaline phosphatase in vitro. Thromb Res. 1990 Apr 15;58(2):119–127. doi: 10.1016/0049-3848(90)90169-d. [DOI] [PubMed] [Google Scholar]
- Frauman A. G., Tsuzaki S., Moses A. C. The binding characteristics and biological effects in FRTL5 cells of placental protein-12, an insulin-like growth factor-binding protein purified from human amniotic fluid. Endocrinology. 1989 May;124(5):2289–2296. doi: 10.1210/endo-124-5-2289. [DOI] [PubMed] [Google Scholar]
- Heldin P., Hessel B., Humble E., Blombäck B., Engström L. Effect of phosphorylation in vitro of human fibrinogen with protein kinase C on thrombin-induced gelation. Thromb Res. 1987 Jul 1;47(1):93–99. doi: 10.1016/0049-3848(87)90244-1. [DOI] [PubMed] [Google Scholar]
- Hossenlopp P., Seurin D., Segovia-Quinson B., Hardouin S., Binoux M. Analysis of serum insulin-like growth factor binding proteins using western blotting: use of the method for titration of the binding proteins and competitive binding studies. Anal Biochem. 1986 Apr;154(1):138–143. doi: 10.1016/0003-2697(86)90507-5. [DOI] [PubMed] [Google Scholar]
- Kudryk B., Okada M., Redman C. M., Blombäck B. Biosynthesis of dog fibrinogen. Characterization of nascent fibrinogen in the rough endoplasmic reticulum. Eur J Biochem. 1982 Jul;125(3):673–682. doi: 10.1111/j.1432-1033.1982.tb06735.x. [DOI] [PubMed] [Google Scholar]
- Kübler D., Pyerin W., Bill O., Hotz A., Sonka J., Kinzel V. Evidence for ecto-protein kinase activity that phosphorylates Kemptide in a cyclic AMP-dependent mode. J Biol Chem. 1989 Aug 25;264(24):14549–14555. [PubMed] [Google Scholar]
- Kübler D., Pyerin W., Burow E., Kinzel V. Substrate-effected release of surface-located protein kinase from intact cells. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4021–4025. doi: 10.1073/pnas.80.13.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker R. H., Clemmons D. R. Insulin-like growth factor binding protein secretion by muscle cells: effect of cellular differentiation and proliferation. J Cell Physiol. 1988 Dec;137(3):505–512. doi: 10.1002/jcp.1041370316. [DOI] [PubMed] [Google Scholar]
- McGuire E. A., Peacock M. E., Inhorn R. C., Siegel N. R., Tollefsen D. M. Phosphorylation of vitronectin by a protein kinase in human plasma. Identification of a unique phosphorylation site in the heparin-binding domain. J Biol Chem. 1988 Feb 5;263(4):1942–1945. [PubMed] [Google Scholar]
- Nemir M., DeVouge M. W., Mukherjee B. B. Normal rat kidney cells secrete both phosphorylated and nonphosphorylated forms of osteopontin showing different physiological properties. J Biol Chem. 1989 Oct 25;264(30):18202–18208. [PubMed] [Google Scholar]
- Pyerin W., Burow E., Michaely K., Kübler D., Kinzel V. Catalytic and molecular properties of highly purified phosvitin/casein kinase type II from human epithelial cells in culture (HeLa) and relation to ecto protein kinase. Biol Chem Hoppe Seyler. 1987 Mar;368(3):215–227. doi: 10.1515/bchm3.1987.368.1.215. [DOI] [PubMed] [Google Scholar]
- Ritvos O., Ranta T., Jalkanen J., Suikkari A. M., Voutilainen R., Bohn H., Rutanen E. M. Insulin-like growth factor (IGF) binding protein from human decidua inhibits the binding and biological action of IGF-I in cultured choriocarcinoma cells. Endocrinology. 1988 May;122(5):2150–2157. doi: 10.1210/endo-122-5-2150. [DOI] [PubMed] [Google Scholar]
- Seydewitz H. H., Matthias F. R., Schöndorf T. H., Witt I. Increase in the degree of phosphorylation of circulating fibrinogen under thrombolytic therapy with urokinase. Thromb Res. 1987 May 1;46(3):437–445. doi: 10.1016/0049-3848(87)90131-9. [DOI] [PubMed] [Google Scholar]
- Seydewitz H. H., Witt I. Increased phosphorylation of human fibrinopeptide A under acute phase conditions. Thromb Res. 1985 Oct 1;40(1):29–39. doi: 10.1016/0049-3848(85)90347-0. [DOI] [PubMed] [Google Scholar]
- Seydewitz H. H., Witt I. The fraction of high molecular weight (HMW) fibrinogen and phosphorylated fibrinopeptide A in fetal fibrinogen. Thromb Res. 1989 Sep 15;55(6):785–790. doi: 10.1016/0049-3848(89)90309-5. [DOI] [PubMed] [Google Scholar]
- Singh K., DeVouge M. W., Mukherjee B. B. Physiological properties and differential glycosylation of phosphorylated and nonphosphorylated forms of osteopontin secreted by normal rat kidney cells. J Biol Chem. 1990 Oct 25;265(30):18696–18701. [PubMed] [Google Scholar]
- Thraikill K. M., Clemmons D. R., Busby W. H., Jr, Handwerger S. Differential regulation of insulin-like growth factor binding protein secretion from human decidual cells by IGF-I, insulin, and relaxin. J Clin Invest. 1990 Sep;86(3):878–883. doi: 10.1172/JCI114788. [DOI] [PMC free article] [PubMed] [Google Scholar]