Abstract
BRLF1 (R) is one of two Epstein-Barr virus (EBV) immediate-early proteins that mediate the switch from the latent to the lytic form of viral replication. In this report, we show that R induces expression of the cellular C-mer gene in a variety of cell lines. C-mer expression was detected in lymphoblastoid cells immortalized with wild-type EBV but not in lymphoblastoid cells immortalized with an EBV that had BRLF1 deleted. Oral hairy leukoplakia tongue tissue, which contains the lytic form of EBV replication, also has enhanced C-mer expression. C-mer is a receptor tyrosine kinase activated by the ligand Gas6. C-mer is required for phagocytosis of apoptotic debris by monocytes/macrophages and retinal pigment epithelial cells and is capable of producing an antiapoptotic signal. Modulation of the C-mer signal transduction cascade by a variety of different approaches did not alter the ability of R to induce lytic EBV gene transcription. Therefore, C-mer activation may be important for some other aspect of lytic EBV infection.
The Epstein-Barr virus (EBV) is a human gammaherpesvirus that causes infectious mononucleosis and is associated with a variety of different B-cell and epithelial cell tumors. EBV can infect cells in either a latent or a lytic form (28, 39). While EBV infection in B cells is usually latent, viral infection of normal epithelial cells in vivo results in lytic infection (28, 39) and asymptomatic shedding of infectious virus in the saliva of immunocompetent hosts. In some immunosuppressed patients, lytic infection results in an epithelial lesion on the lateral border of the tongue, oral hairy leukoplakia (OHL), which contains lytically replicating EBV (20, 28, 39).
The two EBV immediate-early proteins, BRLF1 (R) and BZLF1 (Z), are transcription factors that mediate the switch from latent to lytic infection (8, 13, 24, 25, 27, 30, 47). Expression of either Z or R is sufficient to initiate EBV lytic viral gene expression in latently infected cells (5, 7, 14, 38, 40, 46, 51, 54). R activates certain early viral promoters (such as the SM and BHRF1 promoters) through a direct DNA binding mechanism (21, 37) but stimulates the immediate-early Z promoter by an indirect mechanism involving phosphatidylinositol 3-kinase and stress mitogen-activated protein kinase pathway activation (1, 9). The effect of R on cellular gene expression has not been as well studied. However, we recently found that R activates expression of the cellular fatty acid synthase (FAS) gene and that FAS activity is important for R-mediated induction of lytic viral gene expression (29).
C-mer is a transmembrane protein belonging to the Mer/Axl/Tyro3 receptor tyrosine kinase family. Signal transduction by all members is initialized by a ligand, Gas6 (4, 18, 19, 22, 31, 36). C-mer is primarily expressed in monocytes/macrophages and certain epithelial cell types, with the highest C-mer mRNA levels detected in kidney, testis, ovary, prostate, lung, and peripheral blood monocytes (18, 19). Although C-mer is not expressed in normal B cells and T cells, C-mer expression is also detected in certain neoplastic T-cell and B-cell lines (18) and is ectopically expressed in the majority of childhood acute lymphocytic leukemias (D. K. Graham et al., unpublished data).
C-mer mutations are found in three kindreds with familial retinitis pigmentosa, a disease leading to blindness in which specialized phagocytic retinal pigment epithelial cells are unable to ingest the shed apoptotic tips of photoreceptor cells (10, 16, 48). The C-mer-knockout mouse also develops a lupus-like autoimmune disorder due to the inability of monocytes to clear apoptotic cellular debris, resulting in the development of autoantibodies (6). The C-mer ligand, Gas6, the gene for which was originally identified as a cellular gene induced by growth arrest, is expressed constitutively by a variety of cell types (4, 12, 32, 36). Activation of C-mer results in increased phosphatidylinositol-3 kinase, extracellular signal-related kinase, and p38 kinase activity, and in some conditions it enhances NF-κB activity (17, 22). C-mer may also coordinate cytoskeletal changes through its effects on Vav1, Rac1, Cd42, and RhoA (31). Furthermore, activation of the C-mer pathway is sufficient to rescue certain interleukin-3 (IL-3)-dependent cell lines from the apoptosis normally induced by IL-3 withdrawal (17, 22).
Here we report that R activates expression of the cellular C-mer gene in a variety of cell lines in vitro. Furthermore, whereas C-mer expression was detected in lymphoblastoid cells immortalized with wild-type EBV, no C-mer expression was detected in lymphoblastoid cells immortalized with a virus having BRLF1 deleted. C-mer was also overexpressed in OHL lesions. Thus, R activates C-mer expression in host cells during the lytic form of EBV infection. However, modulation of the C-mer signal transduction cascade by a variety of different approaches did not alter the ability of R to induce lytic EBV gene transcription. Therefore, the functional significance of C-mer activation during the lytic form of EBV infection remains unknown, but it could possibly enhance the ability of infected cells to digest apoptotic debris or resist apoptosis.
MATERIALS AND METHODS
Cell lines and viruses.
HeLa is a cervical carcinoma cell line. Telomerase-immortalized human keratinocytes (TIK) were a gift from A. J. Klingelhutz at the University of Iowa and originally derived from human neonatal foreskin as previously described (35). TIK were maintained in keratinocyte-SFM medium (Gibco-BRL) with epidermal growth factor (EGF) and bovine pituitary extract added. RPE is a retinal pigment epithelial cell line. D98/HE-R-1 is an epithelial cell line formed by fusion of a HeLa cell subclone (D98) with the EBV-positive Burkitt lymphoma cell line P3HR/1. Akata (EBV positive or EBV negative) is a Burkitt lymphoma cell line (a gift from K. Takada). 293 is a human embryonic epithelial kidney cell line. EBV-positive versions of 293 cells, containing either wild-type virus or virus with BRLF1 deleted (R-KO) virus, were made as previously described (11, 14) and were a gift from H. J. Delecluse. Lymphoblastoid cell lines (LCLs) were derived from the same donor by using wild-type EBV (LCL-WT) or EBV having BRLF1 deleted (LCL-R-KO). For the production of stocks of virus with BRLF1 deleted, 5 × 106 293 R-KO cells were plated in 10 ml of RPMI 1640-10% fetal bovine serum-penicillin-streptomycin in a 100-mm-diameter dish on the day prior to transfection. The cells were transfected with expression vectors for the lytic EBV gene products BRLF1, BZLF1, BRRF1, and BALF4 with Lipofectamine 2000 according to the manufacturer's instructions. At 72 h posttransfection, supernatants were filtered through 0.45-μm-pore-size filters and viral stocks were stored at 4°C. For the generation of LCL R-KO cells, 3 × 106 peripheral blood mononuclear cells (provided by the laboratory of Susan Fiscus, University of North Carolina at Chapel Hill) were resuspended in 3 ml of R-KO virus and incubated overnight at 37°C. Seven milliliters of RPMI 1640-20% fetal bovine serum-penicillin-streptomycin was then added, and cyclosporine (Sigma) was added to achieve a final concentration of 500 ng/ml. Fifty percent of the medium was changed every 7 days until transformation into LCLs was apparent (at ∼3 to 4 weeks postinfection). All lymphoid cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum. Epithelial cell lines were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. All cell lines were cultured at 37°C in a 5% CO2 incubator.
Adenovirus vector construction and infection.
The EBV immediate-early gene BRLF1 or the control lacZ gene was inserted via cre-loxP-mediated recombination into an adenovirus type 5 derivative (under control of the immediate-early cytomegalovirus promoter) lacking the E1 and E3 genes to create adenovirus-BRLF1 (AdR) and adenovirus-LacZ (AdLacZ), as previously described (51). Virus stocks were grown in 293 cells and purified by double cesium chloride gradient followed by dialysis. TIK or HeLa, RPE, or 293 cells were plated in 150-mm-diameter plates 24 h prior to infection. Cells were infected with no adenovirus (mock infection), AdLacZ, or AdR at a multiplicity of infection (MOI) of 20. Cells were harvested 24 h postinfection for RNA preparation or whole-cell protein preparation. Some cells were treated with human recombinant Gas6 (150 nM; a gift from Brian Varnum, Amgen Corp.) for 10 min before harvest.
Plasmids.
The BRLF1 expression vector (SG5-R) contains the BRLF1 genomic sequence inserted in the pSG5 expression vector (Stratagene) under the control of the simian virus 40 promoter (a gift from Diane Hayward) (41). The EGFR/Mer chimera (C-mer) was constructed by combining the extracellular and transmembrane regions of the EGF receptor (EGFR) with the intracellular domain of C-mer inserted into the pLXSN vector (34) as previously described (22). Kinase-inactive C-mer (Mer-KD) was made by site-directed mutation of lysine 619 of the C-mer kinase domain to methionine (22).
Transfection.
HeLa and 293 cells were transfected with 10 μg of SG5-R or control SG5 plasmid DNA with the use of Lipofectamine 2000 (Invitrogen) according to the manufacturer's specifications. RNA was prepared 24 h posttransfection. Some 293 cells were treated with Gas6 24 h posttransfection, and whole-cell extracts were prepared 48 h posttransfection. EBV-positive D98/HE-R-1 cells were transfected with SG5 or SG5-R, in combination with C-mer or Mer-KD plasmid, by electroporation with 1,500 V from a Zapper electroporation unit (Medical Electronics Shop, University of Wisconsin). EGF (100 ng/ml) was added 24 h posttransfection, and whole-cell extracts were prepared 48 h posttransfection.
Northern blot analysis.
Ten micrograms of total RNA, purified using the Trizol reagent (Invitrogen) as specified by the manufacturer, was separated on a 1% agarose-formaldehyde gel and transferred to nylon membranes (Schleicher and Schuell). Membranes were prehybridized for 25 min at 68°C in QuikHyb hybridization buffer (Stratagene) and then hybridized with 32P-randomly radiolabeled C-mer probes for 1 h at 68°C. After hybridization, membranes were washed twice in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate at room temperature, and a final wash was carried out in 0.2× SSC-0.1% sodium dodecyl sulfate for 5 to 10 min at 60°C. The C-mer probes were generated by reverse transcriptase-PCR (RT-PCR) of 1 μg of total cellular RNA with the primers C-mer sense (5′-TCCGTAACAGCACTGCACAC-3′) and C-mer antisense (5′-GTGACGGCTGCAATCCTCAC-3′) followed by random labeling of the PCR product. A randomly labeled (Oligolabeling kit; Amersham) mouse glyceraldehyde phosphate dehydrogenase (GAPDH) probe (Ambion) was used as a control.
RT-PCR.
Total RNA was isolated from LCL (WT or R-KO) or Akata cells (EBV negative or EBV positive) treated with or without anti-human immunoglobulin G (IgG; Sigma; 0.1 mg/ml) for 24 h. RT-PCR was performed using a kit according to the instructions of the manufacturer (Promega). The same C-mer primers used to generate probes for Northern analysis above were used to synthesize the PCR products. PCR was run for 35 to 40 cycles at 95°C for 30 s, 58°C for 30 s, and 72°C for 30 s. Primers for β2-microglobin were 5′ TTCTGGCCTGGAGGGCATCC (forward) and 5′ ATCTTCAAACCTCCATGATG (reverse).
Immunoblot analysis.
Immunoblot analysis of total cell extracts was performed as previously described (29) with the following antibodies: anti-EBV BRLF1 (Argene; 1:100) and anti-EBV-BMRF1 (Capricorn Products, Inc.; 1:100). For C-mer detection, whole-cell extracts were precipitated either with 1 μg of anti-C-mer polyclonal antibodies (31) or with wheat germ agglutinin-Sepharose (WGA-Sepharose; Sigma) beads overnight. The proteins were dissolved from beads, and an immunoblot analysis was performed to detect total C-mer (anti-C-mer antibody; 1:1,000) or phosphorylated C-mer (antiphosphotyrosine antibody; 1:400; BD Transduction Laboratories) expression.
Patients and tissues.
Biopsy specimens of OHL were obtained from human immunodeficiency virus-seropositive patients identified at the University of North Carolina Hospitals. Biopsy specimens of tissue from the lateral tongue border of human immunodeficiency virus-negative healthy subjects without leukoplakia were used as controls. Specimens were immediately frozen and stored at −80°C. A portion of each biopsy specimen was fixed in formalin and sent to UNC Hospitals Department of Pathology for histopathologic diagnosis. The presence of EBV within the OHL biopsy specimens was confirmed by detection of the terminal restriction enzyme fragments on Southern blots (49).
Immunofluorescence.
Frozen tissue sections were cut to 5-μm thickness and placed on poly-l-lysine-coated slides. Tissue sections and cell lines were fixed in a chilled 1:1 mixture of methanol and acetone; blocked with 20% normal goat serum; stained with a primary antibody for FAS (BD Biosciences; 1:20), Z (Argene; 1:40), or R (Argene; 1:20) or with an isotype control antibody; and then stained with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (50). Slides were mounted with coverslips by using Vectashield (Vector Laboratories) and then subjected to confocal microscopy.
Affymetrix GeneChip analysis.
Telomerase-immortalized human keratinocytes were plated at a cell density of 2 × 107 cells per 150-mm-diameter dish and then either mock infected or infected with adenovirus expressing LacZ or R at an MOI of 17. The cells were harvested 48 h later, and total RNA was obtained using the RNeasy kit (Qiagen). Microarray analysis was performed with Affymetrix GeneChip as previously described (29).
RESULTS
R activates C-mer expression.
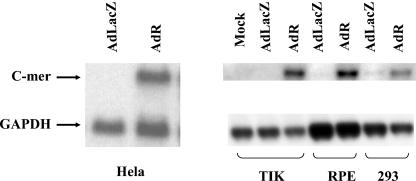
In preliminary experiments, human TIK were mock infected or infected with adenovirus vectors expressing R (AdR) or LacZ (AdLacZ) constructed as previously described (51). RNA was harvested 24 h after infection and analyzed by microarray analysis as previously described (29). The results indicated that cells infected with the AdR vector had sevenfold-higher expression of C-mer than did cells which were mock infected or infected with the AdLacZ vector (data not shown). To confirm that R induces C-mer expression, Northern blot analysis was performed to quantitate the level of C-mer message in a series of cell lines (HeLa cells, TIK, RPE cells, and 293 cells) that were infected with the control AdLacZ vector or the AdR vector (MOI of 20). RNA was isolated 24 h postinfection and analyzed by Northern blotting with probes specific for c-Mer or the housekeeping gene GAPDH. Compared to AdLacZ-infected cells, cells infected with AdR had significantly more C-mer mRNA in all cell lines tested (Fig. 1). These results indicate that infection of cells with the AdR vector results in enhanced C-mer mRNA expression.
FIG. 1.
An R adenovirus vector increases C-mer mRNA expression. HeLa, RPE, and 293 cells and TIK were infected with AdR or AdLacZ (MOI of 20); RNA was isolated 24 h postinfection, and Northern blot analysis was performed to detect C-mer or GAPDH mRNA expression.
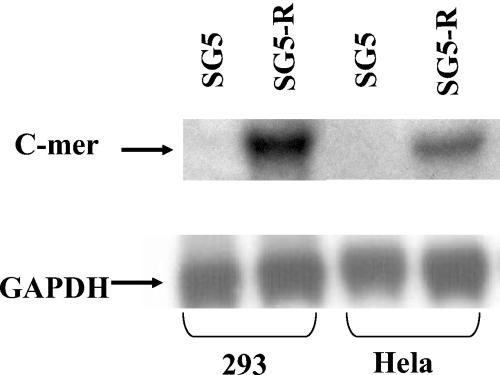
To determine if R activates C-mer expression in the absence of any adenovirus gene products, HeLa and 293 cells were transfected with an R expression vector (SG5-R) or the SG5 control vector (Stratagene). RNA was harvested 24 h after transfection, and Northern blot assays were performed. In both HeLa and 293 cells, the transfected R vector increased the level of C-mer RNA compared to the control vector (Fig. 2). Thus, R activates C-mer expression in the absence of adenovirus help.
FIG. 2.
Transfected R increases C-mer mRNA expression. HeLa and 293 cells were transfected with a vector expressing R (SG5-R) or a control vector (SG5); RNA was isolated 24 h posttransfection, and Northern blot analysis was performed to detect C-mer and GAPDH expression.
C-mer mRNA is activated by lytic EBV infection in Burkitt lymphoma cells and LCLs.
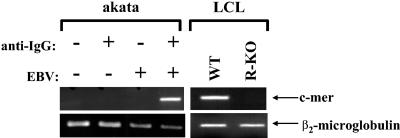
To determine if lytic EBV infection results in increased C-mer expression in B cells, EBV-positive Burkitt lymphoma cells (Akata) were switched from the latent to lytic form of infection by cross-linking the B-cell surface receptor with anti-IgG (0.1 mg/ml; Sigma) as previously described (45). Total RNA was isolated 24 h postinduction, and RT-PCR was performed to detect C-mer or β2-microglobin gene expression. As shown in Fig. 3, in the absence of B-cell receptor activation, no C-mer expression was detected in Akata cells. Following anti-IgG treatment, C-mer RNA expression was dramatically increased, while that of a housekeeping gene, β2-microglobin, was not affected. Anti-IgG treatment did not induce C-mer expression in EBV-negative Akata cells. The level of C-mer expression was also compared in LCLs (from the same donor) derived with wild-type virus and virus having BRLF1 deleted (R-KO; a gift from H.-J. Delecluse) (14). C-mer expression was detected in LCLs derived with wild-type EBV but not in the LCLs derived from the virus having BRLF1 deleted (Fig. 3). These results suggest that lytic EBV infection enhances C-mer expression in at least some EBV-infected B-cell lines.
FIG. 3.
C-mer is activated by lytic EBV induction in B cells. EBV-positive or EBV-negative Akata Burkitt lymphoma cells were treated with or without anti-IgG (0.1 mg/ml) for 24 h to induce the lytic form of EBV infection (left panel). Total RNA was harvested, and RT-PCR analysis was performed to detect C-mer or β2-microglobin RNA expression. C-mer expression was also examined in LCLs derived from the same donor with wild-type virus (WT) or virus having BRLF1 deleted (R-KO) (right panel). Samples without RT did not produce detectable C-mer expression (data not shown).
R activates C-mer protein expression.
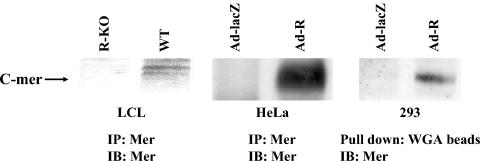
To determine if R also activates C-mer protein expression, HeLa cells were infected with the AdLacZ or AdR vectors (MOI of 20), and whole-cell extracts were immunoprecipitated 24 h postinfection with a C-mer polyclonal antibody (31), followed by immunoblot analysis with the same antibody. C-mer protein expression was detected in HeLa cells infected with the AdR vector but not the AdLacZ vector (Fig. 4, middle panel). A similar experiment was performed in 293 cells. In this experiment, extracts were incubated overnight in WGA-Sepharose (Sigma) beads to enrich for the heavily glycosylated C-mer protein as previously described (15), glycoproteins were then dissociated from the beads, and an immunoblot analysis was performed to detect C-mer. C-mer protein expression was enhanced in 293 cells infected with the AdR vector over that in cells infected with the control vector (Fig. 4, right panel). Most importantly, C-mer protein expression was observed in LCLs obtained with the wild-type virus but not in LCLs obtained with the virus having BRLF1 deleted (Fig. 4, left panel).
FIG. 4.
R increases C-mer protein expression. HeLa cells and 293 cells were infected with AdR or AdLacZ (MOI of 20), and protein extracts were harvested 24 h postinfection. Protein extracts were also obtained from LCLs derived from the same donor with wild-type virus (WT) or virus having BRLF1 deleted (R-KO). HeLa and LCL extracts were immunoprecipitated (IP) and immunoblotted (IB) with a C-mer polyclonal antibody. 293 cell extracts were first pulled down by using WGA-Sepharose beads and then immunoblotted with a C-mer antibody.
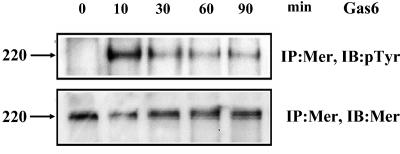
Gas6 phosphorylates the C-mer induced by R in HeLa cells.
To determine if the C-mer protein induced by R expression in HeLa cells can be efficiently phosphorylated in the presence of Gas6, HeLa cells were infected with the AdLacZ or AdR vector (MOI of 20). Twenty-four hours postinfection, cells were treated with 150 nM human recombinant Gas6 for various lengths of time (0, 10, 30, 60, and 90 min) at 37°C. Cell extracts were then immunoprecipitated with the C-mer polyclonal antibody, followed by immunoblotting with antiphosphotyrosine antibody to detect phosphorylated C-mer or with the C-mer antibody to detect total C-mer. HeLa cell extracts infected with the AdLacZ vector had no detectable C-mer protein and no detectable C-mer phosphorylation in the presence of the Gas6 ligand (data not shown). In contrast, Gas6 treatment of HeLa cells infected with the AdR vector resulted in tyrosine phosphorylation of C-mer, which peaked at 10 min and then decreased gradually over time (Fig. 5). These results indicate that the C-mer protein induced by R is capable of responding to the Gas6 ligand.
FIG. 5.
Gas6 phosphorylates C-mer induced by R in HeLa cells. HeLa cells infected with AdLacZ or AdR (MOI of 20) for 24 h were treated with recombinant Gas6 for 0, 10, 30, 60, and 90 min. Cell extracts were immunoprecipitated (IP) with a C-mer antibody and then immunoblotted (IB) with the same C-mer antibody to measure total C-mer protein or immunoblotted with a p-Tyr antibody to measure phosphorylated C-mer. The AdLacZ-infected cells had no detectable C-mer protein (data not shown).
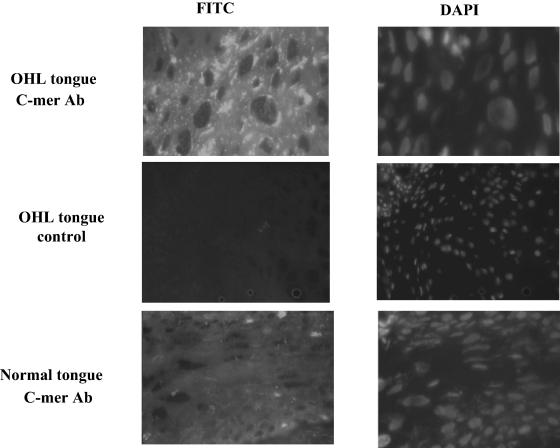
C-mer is overexpressed in epithelial cells of OHL lesions.
To determine the effect of lytic EBV infection in humans, C-mer expression was examined in tongue tissue derived from patients with OHL versus lateral tongue tissue derived from healthy individuals. OHL is a lesion along the lateral aspect of the tongue that contains lytic EBV infection (20, 53). BZLF1 expression was observed by immunohistochemistry in the condensed nuclei of koilocyte cells in the middle to upper strata in biopsy specimens of OHL lesions (data not shown), confirming that these cells contained the lytic form of EBV infection. Punctate cytoplasmic staining for C-mer was observed by immunofluorescence in the OHL tissue in the middle to upper strata within the stratum spinosum and stratum granulosum layer but was present at a much lower level in the control tongue tissue (Fig. 6). No C-mer expression was detected in OHL or healthy tongue stained with the secondary antibody only. These results indicate that C-mer expression is induced during lytic EBV infection in tongue epithelial cells.
FIG. 6.
C-mer expression is detected in OHL tongue tissue. Tongue biopsy specimens from healthy individuals (bottom panel) or OHL patients (top and middle panels) were incubated with either a C-mer monoclonal antibody (MAB891; R & D Systems; top and lower panels) or phosphate-buffered saline (middle panel), followed by incubation with an anti-mouse antibody conjugated with fluorescein isothiocyanate. C-mer expression was examined using fluorescence microscopy (left panels). 4′,6′-Diamidino-2-phenylindole (DAPI) staining was also performed on all specimens (right panels).
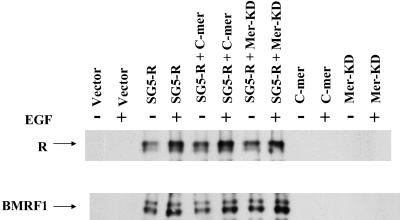
Modulation of C-mer activity does not affect R-mediated lytic EBV induction.
To determine whether C-mer activation plays a role in R-mediated lytic EBV gene expression, latently infected, EBV-positive D98/HE-R-1 cells were transfected with the control vector (SG5) or the R-expression vector (SG5-R), in the presence or absence of vectors encoding chimeric C-mer receptors, with either wild-type (C-mer) or kinase-dead (Mer-KD) C-mer (22). Both vectors contain the extracellular and transmembrane domains of the EGFR linked to the C-mer cytoplasmic domain: while one vector has an active C-mer tyrosine kinase, in the other vector lysine 619 of the kinase domain is mutated to methionine. The latter does not send a signal in the presence of the EGF ligand (22, 31). Transfected cells were treated with or without EGF (100 ng/ml) 24 h posttransfection. Cells were harvested 48 h posttransfection, and immunoblot analysis was performed to detect expression of the R protein, as well as the early lytic EBV protein BMRF1. As shown in Fig. 7, cells transfected with the R expression vector alone expressed the early protein BMRF1, as expected. Neither the wild-type nor the kinase-dead C-mer-EGFR chimera altered BMRF1 expression in the presence or absence of the EGF ligand (Fig. 7).
FIG. 7.
Modulation of C-mer activity does not affect R-mediated lytic gene expression. EBV-positive D98/HE-R-1 cells were transfected with the SG5 or SG5-R vectors, in combination with either a control vector, a vector that inhibits C-mer signaling in the presence of EGF (Mer-KD), or a vector that activates a C-mer-like pathway in the presence of EGF (C-mer). EGF was added 24 h after transfection to some conditions, and cells were harvested 48 h posttransfection for immunoblot analysis to detect lytic EBV gene expression.
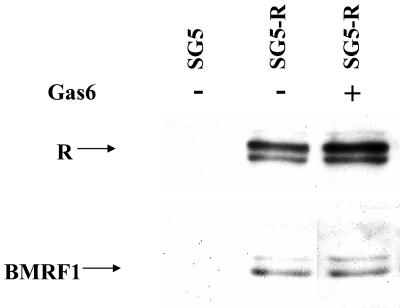
Recombinant Gas6 does not enhance R-mediated viral gene expression.
To further confirm that C-mer activation is not directly involved in the transcriptional effects of R, EBV-positive 293 cells were transfected with the control vector (SG5) or the R expression vector (SG5-R) in the presence or absence of recombinant Gas6 ligand (150 nM, added 24 h posttransfection). Cells were harvested 48 h posttransfection, and an immunoblot analysis was performed to detect R and BMRF1 expression (Fig. 8). The presence of the C-mer ligand, Gas6, did not significantly affect the ability of transfected R to induce early lytic EBV gene expression.
FIG. 8.
Gas6 does not affect R-mediated EBV lytic gene expression. EBV-positive 293 cells were transfected with the control vector, SG5, or SG5-R. Human recombinant Gas6 (150 nM) was added 24 h posttransfection, and cells were harvested 48 h posttransfection for immunoblot analysis to detect EBV lytic gene expression.
DISCUSSION
In this report, we demonstrate that the EBV immediate-early protein BRLF1 activates C-mer expression in a variety of cell lines in vitro. In addition, we show that C-mer expression is increased by induction of lytic EBV infection in the Burkitt lymphoma line Akata and that C-mer is expressed in lymphoblastoid cells obtained with wild-type EBV but not in lymphoblastoid cells obtained with the virus in which BRLF1 was deleted. C-mer is also expressed at a high level in OHL lesions. Thus, C-mer activation may be a common phenomenon during the lytic form of EBV replication. At this point, however, whether this C-mer activation plays an important role in EBV pathogenesis remains unknown.
C-mer is a member of the Mer/Axl/Tyro3 receptor tyrosine kinase family. C-mer is normally expressed in monocytes and certain types of epithelial cells (18) and plays a role in phagocytosis of apoptotic debris (23, 43). Loss of C-mer function in humans results in a form of progressive blindness, retinitis pigmentosa, due to the failure of retinal pigment epithelial cells to ingest the shed apoptotic outer segments of photoreceptor cells, and in the rat and mouse, absence of C-mer leads to retinal degeneration (10, 16, 48). In addition, C-mer functions include the regulation of cytoskeletal elements and the sending of an antiapoptotic signal not accompanied by cellular proliferation (6, 43).
Our results suggest that lytic EBV infection increases the level of C-mer in host cells that do not otherwise express significant amounts of C-mer. While we did not detect significant C-mer expression in uninfected lateral tongue epithelial cells, C-mer was highly expressed in OHL lesions that are lytically infected with EBV. An LCL immortalized with wild-type EBV had C-mer expression, whereas an LCL derived with the virus in which BRLF1 was deleted (from the same host) did not express detectable C-mer. In addition, whereas latently infected EBV-positive Akata Burkitt lymphoma cells did not express detectable C-mer, when these cells were switched to the lytic form of EBV infection, C-mer expression was detected. Furthermore, R expression in HeLa cells (which do not express C-mer in the absence of R) resulted in C-mer tyrosine phosphorylation in the presence of the C-mer ligand Gas6.
Together, these results indicate that host cells containing the lytic form of EBV infection may have a greatly enhanced response to the Gas6 ligand. The Gas6 ligand, although originally described as a growth arrest gene, is expressed constitutively by a number of cell types (4, 12, 32, 36). However, exactly how this induction of the C-mer pathway during lytic EBV infection contributes to viral pathogenesis remains unknown. We explored the hypothesis that the C-mer signal transduction pathway contributes to R transcriptional effects but were unable to show by a variety of different approaches that this pathway alters R-mediated lytic EBV gene transcription.
Another potential role of C-mer activation during lytic EBV infection would be inhibition of cellular apoptosis. Activation of the C-mer pathway has been shown to inhibit cellular apoptosis mediated by withdrawal of IL-3 (17, 22), and thus C-mer activation could potentially inhibit apoptosis in response to viral infection, as well. Many viruses encode proteins that inhibit cellular apoptosis, thereby allowing the virus time to replicate its genome prior to the death of the host cell. EBV encodes an early gene product, BHRF1, which is a homologue of Bcl-2 and inhibits apoptosis (2, 26). The combination of C-mer activation and BHRF1 expression may be more efficient than either effect alone in protecting the virus from apoptosis during lytic EBV replication.
Finally, it is also possible that C-mer activation by R allows the virally infected cell to ingest apoptotic debris. Ingestion of apoptotic cellular debris might enhance the availability of limiting substrates required for efficient lytic viral replication. Whether C-mer activation could enhance the phagocytosis capacity of the primary target cells for EBV, B cells, and epithelial cells is not clear. However, there is increasing evidence that EBV also lytically infects monocytes and macrophages (42), in which C-mer activation could potentially dysregulate phagocytosis and possibly contribute to the EBV-associated hemophagocytic clinical syndrome (44).
In addition to the other phenotypic effects, C-mer-knockout mice produce much more tumor necrosis factor alpha (TNF-α) in response to lipopolysaccharide challenge than do wild-type mice (3), suggesting that C-mer has a role in down-regulating production of TNF-α. As TNF-α is a potent antiviral cytokine, as well as an activator of the host immune response (33, 52), inhibition of TNF-α production during lytic EBV infection would be expected to benefit the virus. The other EBV immediate-early gene protein, BZLF1, was recently shown by our laboratory to down-regulate expression of the major tumor necrosis factor receptor in host cells (35). Activation of C-mer by R, in combination with the Z effect, could allow EBV to evade TNF-α during lytic reactivation or primary infection.
Acknowledgments
This work was supported by grants 2-R01-CA58853 and P01-CA19014 from the National Institutes of Health.
We thank S. Diane Hayward for plasmids, Brian Varnum for Gas6, H.-J. Delecluse for the EBV with BRLF1 deleted, and the UNC Gene Therapy Core for preparing the adenovirus vectors.
REFERENCES
- 1.Adamson, A. L., D. Darr, E. Holley-Guthrie, R. A. Johnson, A. Mauser, J. Swenson, and S. Kenney. 2000. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J. Virol. 74:1224-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellows, D. S., B. N. Chau, P. Lee, Y. Lazebnik, W. H. Burns, and J. M. Hardwick. 2000. Antiapoptotic herpesvirus Bcl-2 homologs escape caspase-mediated conversion to proapoptotic proteins. J. Virol. 74:5024-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camenisch, T. D., B. H. Koller, H. S. Earp, and G. K. Matsushima. 1999. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J. Immunol. 162:3498-3503. [PubMed] [Google Scholar]
- 4.Chen, J., K. Carey, and P. J. Godowski. 1997. Identification of Gas6 as a ligand for Mer, a neural cell adhesion molecule related receptor tyrosine kinase implicated in cellular transformation. Oncogene. 14:2033-2039. [DOI] [PubMed] [Google Scholar]
- 5.Chevallier-Greco, A., E. Manet, P. Chavrier, C. Mosnier, J. Daillie, and A. Sergeant. 1986. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 5:3243-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, P. L., R. Caricchio, V. Abraham, T. D. Camenisch, J. C. Jennette, R. A. Roubey, H. S. Earp, G. Matsushima, and E. A. Reap. 2002. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J. Exp. Med. 196:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Countryman, J., and G. Miller. 1985. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc. Natl. Acad. Sci. USA 82:4085-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox, M. A., J. Leahy, and J. M. Hardwick. 1990. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J. Virol. 64:313-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darr, C. D., A. Mauser, and S. Kenney. 2001. Epstein-Barr virus immediate-early protein BRLF1 induces the lytic form of viral replication through a mechanism involving phosphatidylinositol-3 kinase activation. J. Virol. 75:6135-6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Cruz, P. M., D. Yasumura, J. Weir, M. T. Matthes, H. Abderrahim, M. M. LaVail, and D. Vollrath. 2000. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 9:645-651. [DOI] [PubMed] [Google Scholar]
- 11.Delecluse, H. J., T. Hilsendegen, D. Pich, R. Zeidler, and W. Hammerschmidt. 1998. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc. Natl. Acad. Sci. USA 95:8245-8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dirks, W., D. Rome, F. Ringel, K. Jager, R. A. MacLeod, and H. G. Drexler. 1999. Expression of the growth arrest-specific gene 6 (GAS6) in leukemia and lymphoma cell lines. Leuk. Res. 23:643-651. [DOI] [PubMed] [Google Scholar]
- 13.Farrell, P. J., D. T. Rowe, C. M. Rooney, and T. Kouzarides. 1989. Epstein-Barr virus BZLF1 trans-activator specifically binds to a consensus AP-1 site and is related to c-fos. EMBO J. 8:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feederle, R., M. Kost, M. Baumann, A. Janz, E. Drouet, W. Hammerschmidt, and H. J. Delecluse. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 19:3080-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng, W., D. Yasumura, M. T. Matthes, M. M. LaVail, and D. Vollrath. 2002. Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J. Biol. Chem. 277:17016-17022. [DOI] [PubMed] [Google Scholar]
- 16.Gal, A., Y. Li, D. A. Thompson, J. Weir, U. Orth, S. G. Jacobson, E. Apfelstedt-Sylla, and D. Vollrath. 2000. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat. Genet. 26:270-271. [DOI] [PubMed] [Google Scholar]
- 17.Georgescu, M. M., K. H. Kirsch, T. Shishido, C. Zong, and H. Hanafusa. 1999. Biological effects of c-Mer receptor tyrosine kinase in hematopoietic cells depend on the Grb2 binding site in the receptor and activation of NF-κB. Mol. Cell. Biol. 19:1171-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham, D. K., G. W. Bowman, T. L. Dawson, W. L. Stanford, H. S. Earp, and H. R. Snodgrass. 1994. Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell Growth Differ. 5:647-657. [PubMed] [Google Scholar]
- 19.Graham, D. K., T. L. Dawson, D. L. Mullaney, H. R. Snodgrass, and H. S. Earp. 1995. Cloning and developmental expression analysis of the murine c-mer tyrosine kinase. Oncogene 10:2349-2359. [PubMed] [Google Scholar]
- 20.Greenspan, J. S., D. Greenspan, E. T. Lennette, D. I. Abrams, M. A. Conant, V. Petersen, and U. K. Freese. 1985. Replication of Epstein-Barr virus within the epithelial cells of oral “hairy” leukoplakia, an AIDS-associated lesion. N. Engl. J. Med. 313:1564-1571. [DOI] [PubMed] [Google Scholar]
- 21.Gruffat, H., E. Manet, A. Rigolet, and A. Sergeant. 1990. The enhancer factor R of Epstein-Barr virus (EBV) is a sequence-specific DNA binding protein. Nucleic Acids Res. 18:6835-6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guttridge, K. L., J. C. Luft, T. L. Dawson, E. Kozlowska, N. P. Mahajan, B. Varnum, and H. S. Earp. 2002. Mer receptor tyrosine kinase signaling: prevention of apoptosis and alteration of cytoskeletal architecture without stimulation or proliferation. J. Biol. Chem. 277:24057-24066. [DOI] [PubMed] [Google Scholar]
- 23.Hall, M. O., A. L. Prieto, M. S. Obin, T. A. Abrams, B. L. Burgess, M. J. Heeb, and B. J. Agnew. 2001. Outer segment phagocytosis by cultured retinal pigment epithelial cells requires Gas6. Exp. Eye Res. 73:509-520. [DOI] [PubMed] [Google Scholar]
- 24.Hardwick, J. M., P. M. Lieberman, and S. D. Hayward. 1988. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J. Virol. 62:2274-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardwick, J. M., L. Tse, N. Applegren, J. Nicholas, and M. A. Veliuona. 1992. The Epstein-Barr virus R transactivator (Rta) contains a complex, potent activation domain with properties different from those of VP16. J. Virol. 66:5500-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson, S., D. Huen, M. Rowe, C. Dawson, G. Johnson, and A. Rickinson. 1993. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc. Natl. Acad. Sci. USA 90:8479-8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenney, S., E. Holley-Guthrie, E. C. Mar, and M. Smith. 1989. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J. Virol. 63:3878-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2573. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 29.Li, Y., J. Webster-Cyriaque, C. C. Tomlinson, M. Yohe, and S. Kenney. 2004. Fatty acid synthase expression is induced by the Epstein-Barr virus immediate-early protein, BRLF1, and is required for lytic viral gene expression. J. Virol. 78:4197-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lieberman, P. M., J. M. Hardwick, and S. D. Hayward. 1989. Responsiveness of the Epstein-Barr virus NotI repeat promoter to the Z transactivator is mediated in a cell-type-specific manner by two independent signal regions. J. Virol. 63:3040-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahajan, N. P., and H. S. Earp. 2003. An SH2 domain-dependent, phosphotyrosine-independent interaction between Vav1 and the Mer receptor tyrosine kinase: a mechanism for localizing guanine nucleotide-exchange factor action. J. Biol. Chem. 278:42596-42603. [DOI] [PubMed] [Google Scholar]
- 32.Manfioletti, G., C. Brancolini, G. Avanzi, and C. Schneider. 1993. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol. Cell. Biol. 13:4976-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mestan, J., W. Digel, S. Mittnacht, H. Hillen, D. Blohm, A. Moller, H. Jacobsen, and H. Kirchner. 1986. Antiviral effects of recombinant tumor necrosis factor in vitro. Nature 323:816-819. [DOI] [PubMed] [Google Scholar]
- 34.Miller, A. D., and G. J. Rosman. 1989. Improved retroviral vectors for gene transfer and expression. BioTechniques 7:980-982. [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison, T. E., A. Mauser, A. Klingelhutz, and S. C. Kenney. 2004. Epstein-Barr virus immediate-early protein BZLF1 inhibits tumor necrosis factor alpha-induced signaling and apoptosis by downregulating tumor necrosis factor receptor 1. J. Virol. 78:544-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagata, K., K. Ohashi, T. Nakano, H. Arita, C. Zong, H. Hanafusa, and K. Mizuno. 1996. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J. Biol. Chem. 271:30022-30027. [DOI] [PubMed] [Google Scholar]
- 37.Quinlivan, E. B., E. Holley-Guthrie, M. Norris, D. Gutsch, S. L. Bachenheimer, and S. C. Kenney. 1993. Direct BRLF1 binding is required for cooperative BZLF1/BRLF1 activation of the Epstein-Barr virus early promoter, BMRF1. Nucleic Acids Res. 21:1999-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ragoczy, T., L. Heston, and G. Miller. 1998. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J. Virol. 72:7978-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 40.Rooney, C., N. Taylor, J. Countryman, H. Jenson, J. Kolman, and G. Miller. 1988. Genome rearrangements activate the Epstein-Barr virus gene whose product disrupts latency. Proc. Natl. Acad. Sci. USA 85:9801-9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarisky, R. T., Z. Gao, P. M. Lieberman, E. D. Fixman, G. S. Hayward, and S. D. Hayward. 1996. A replication function associated with the activation domain of the Epstein-Barr virus Zta transactivator. J. Virol. 70:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savard, M., C. Belanger, M. Tardif, P. Gourde, L. Flamand, and J. Gosselin. 2000. Infection of primary human monocytes by Epstein-Barr virus. J. Virol. 74:2612-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott, R. S., E. J. McMahon, S. M. Pop, E. A. Reap, R. Caricchio, P. L. Cohen, H. S. Earp, and G. K. Matsushima. 2001. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411:207-211. [DOI] [PubMed] [Google Scholar]
- 44.Su, I. J., C. H. Wang, A. L. Cheng, and R. L. Chen. 1995. Hemophagocytic syndrome in Epstein-Barr virus-associated T-lymphoproliferative disorders: disease spectrum, pathogenesis, and management. Leuk. Lymphoma 19:401-406. [DOI] [PubMed] [Google Scholar]
- 45.Takada, K. 1984. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt lymphoma lines. Int. J. Cancer 33:27-32. [DOI] [PubMed] [Google Scholar]
- 46.Takada, K., N. Shimizu, S. Sakuma, and Y. Ono. 1986. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J. Virol. 57:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urier, G., M. Buisson, P. Chambard, and A. Sergeant. 1989. The Epstein-Barr virus early protein EB1 activates transcription from different responsive elements including AP-1 binding sites. EMBO J. 8:1447-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vollrath, D., W. Feng, J. L. Duncan, D. Yasumura, P. M. D'Cruz, A. Chappelow, M. T. Matthes, M. A. Kay, and M. M. LaVail. 2001. Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc. Natl. Acad. Sci. USA 98:12584-12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Webster-Cyriaque, J., and N. Raab-Traub. 1998. Transcription of Epstein-Barr virus latent cycle genes in oral hairy leukoplakia. Virology 248:53-65. [DOI] [PubMed] [Google Scholar]
- 50.Webster-Cyriaque, J., J. Middeldorp, and N. Raab-Traub. 2000. Hairy leukoplakia: an unusual combination of transforming and permissive Epstein-Barr virus infections. J. Virol. 74:7610-7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westphal, E. M., A. Mauser, J. Swenson, M. G. Davis, C. L. Talarico, and S. C. Kenney. 1999. Induction of lytic Epstein-Barr virus (EBV) infection in EBV-associated malignancies using adenovirus vectors in vitro and in vivo. Cancer Res. 59:1485-1491. [PubMed] [Google Scholar]
- 52.Wong, G. H. W., and D. V. Goeddel. 1986. Tumor necrosis factors alpha and beta inhibit virus replication and synergize with interferons. Nature 323:819-822. [DOI] [PubMed] [Google Scholar]
- 53.Young, L. S., R. Lau, M. Rowe, G. Niedobitek, G. Packham, F. Shanahan, D. T. Rowe, D. Greenspan, J. S. Greenspan, and A. B. Rickinson. 1991. Differentiation-associated expression of the Epstein-Barr virus BZLF1 transactivator protein in oral hairy leukoplakia. J. Virol. 65:2868-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zalani, S., E. Holley-Guthrie, and S. Kenney. 1996. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc. Natl. Acad. Sci. USA 93:9194-9199. [DOI] [PMC free article] [PubMed] [Google Scholar]