Abstract
The tropism of human immunodeficiency virus type 1 for chemokine receptors plays an important role in the transmission of AIDS. Although CXCR4-tropic virus is more cytopathic for T cells, CCR5-tropic strains are transmitted more frequently in humans for reasons that are not understood. Phenotypically immature myeloid dendritic cells (mDCs) are preferentially infected by CCR5-tropic virus, in contrast to mature mDCs, which are not susceptible to infection but instead internalize virus into a protected intracellular compartment and enhance the infection of T cells. Here, we define a mechanism to explain preferential transmission of CCR5-tropic viruses based on their interaction with mDCs and sensitivity to neutralizing antibodies. Infected immature mDCs differentiated normally and were found to enhance CCR5-tropic but not CXCR4-tropic virus infection of T cells even in the continuous presence of neutralizing antibodies. Infectious synapses also formed normally in the presence of such antibodies. Infection of immature mDCs by CCR5-tropic virus can therefore establish a pool of infected cells that can efficiently transfer virus at the same time that they protect virus from antibody neutralization. This property of DCs may enhance infection, contribute to immune evasion, and could provide a selective advantage for CCR5-tropic virus transmission.
Dendritic cells (DCs) normally circulate throughout tissues and lymphoid organs, where they capture antigens and process them for presentation to the immune system (reviewed in reference 40). DCs also capture both CCR5- and CXCR4-tropic viruses efficiently and transmit them to T cells (19, 21, 26). The envelope (Env) glycoprotein of human immunodeficiency virus type 1 (HIV-1) (gp120) is highly glycosylated, and virus attachment to DCs is mediated largely through mannose-specific C-type lectin receptor DC-SIGN (12, 19, 27, 43, 44, 49). Virus bound to DC-SIGN is internalized into distinct cellular compartments and can remain infectious for several days. HIV-1 uses a specific cell contact, termed the infectious synapse (31), to facilitate delivery to CD4+ lymphocytes, resulting in enhanced viral replication in DC-CD4+ lymphocyte cocultures. Geijtenbeek et al. observed that HIV does not infect monocyte-derived DCs, but the virus is captured by DC-SIGN and transferred to CD4+ T cells by a mechanism called trans-infection. Other reports have suggested that immature DCs must be infected to transmit virus (5, 20, 45), although it has been difficult to demonstrate productive infection of DCs both in vitro (3, 6-8, 19, 21) and in vivo (38). Monoclonal antibodies (MAbs) can block both virus entry and transmission of HIV from monocyte-derived DCs to T cells during trans-infection, but it is unclear whether such MAbs can inhibit transfer after productive infection of immature DCs or by mature DCs.
The aim of the present study was to characterize the transmission of CXCR4- and CCR5-tropic viruses to immature and mature myeloid DCs (mDCs) and to define the sensitivity of DC-mediated virus infection to neutralizing antibodies. Our findings confirm and extend previous observations: immature mDCs are shown to be preferentially infected by CCR5-tropic virus (5, 20, 35, 45), whereas mature mDCs are not susceptible to infection by either type of virus. Infected immature mDCs differentiated normally and enhanced CCR5-tropic but not CXCR4-tropic virus infection of T cells even in the continuous presence of neutralizing antibodies, indicating that mDCs confer resistance to HIV-1 inactivation through this previously unrecognized mechanism of antibody resistance.
MATERIALS AND METHODS
Cell preparation and analysis.
Human T-cell leukemia cell lines A3R5 (a subline of CEM expressing both CCR5 and CXCR4) and MT-2 expressing CXCR4 were kindly provided by John Mascola. mDCs and autologous CD4+ T cells were purified from elutriated monocytes and lymphocytes prepared from healthy adult donors by a two-step procedure consisting of automated leukapheresis and counterflow centrifugal elutriation at the Transfusion Medicine Department of Warren Grant Magnuson Clinical Center, National Institutes of Health, Bethesda, Md. (1). Blood mDCs were isolated from the elutriated monocyte fraction by deletion of cells expressing BDCA-4 and CD9 by using microbeads (Miltenyi Biotech, Auburn, Calif.) and positive selection by using antibodies to CD1c (Miltenyi Biotech). CD4+ T cells were isolated from the lymphocyte fraction by negative selection with a CD4+ T-cell isolation kit (Miltenyi Biotech). mDCs CD4+ T lymphocytes, and the T-cell lines were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin. Primary CD4+ T cells were stimulated with phytohemagglutinin A (PHA; 10 μg/ml; Calbiochem)-interleukin-2 (IL-2; 20 U/ml; PeproTech) for 24 h and maintained in medium supplemented with IL-2 (20 U/ml). mDCs were cultured in medium containing granulocyte-macrophage colony-stimulating factor (10 ng/ml; PeproTech). To initiate differentiation of DCs, cells were treated with poly(I-C) (50 ng/ml; Sigma) for 48 h (9). Antibodies to CD11c and CD14 (BD Pharmingen) were used to assess the purity of DCs and antibodies to CD40, CD80, CD86, and HLA-DR (BD Pharmingen) were used to characterize the differentiation of DCs by flow cytometry.
Viruses.
Pseudotyped HIV IIIB and ADA lentivirus expressing luciferase were prepared by transient cotransfection of 293T cells with calcium phosphate (Invitrogen). Briefly, the packaging vector pMD8.2, pHR-Luciferase, and the envelope expressing vector pSVIII-ADA (10) or pRSV-IIIB were transiently transfected into 293T cells (33). Supernatants were harvested 48 and 72 h after transfection, filtered, and stored at −80°C. The virus concentration was determination by an enzyme-linked immunosorbent assay for the p24 antigen (Coulter). Green fluorescent protein (GFP)-Vpr-labeled HIV-1 was produced by transfection of HEK 293 T cells with pLAI provirus and the plasmid pEGFP-C3 (Clontech, Palo Alto, Calif.) containing the entire Vpr coding region fused to the carboxy terminus of eGFP (GFP-Vpr), which was a generous gift from Thomas Hope (30). Cells were washed at 16 to 20 h posttransfection and replenished with fresh medium. After 48 h, the supernatants were harvested, filtered through a 0.45-μm syringe filter, and concentrated. Briefly, 32 ml of supernatant was layered on 5 ml of Optiprep (Iodoxinal) medium (Invitrogen, Carlsbad, Calif.) and centrifuged at 50,000 × g for 1.5 h with a Surespin 630 rotor (Sorvall, Newtown, Conn.). The last 3 ml of supernatant remaining above the OptiPrep interface was collected and frozen at −80°C in 500-μl aliquots. Concentrated wild-type HIV stocks were prepared in peripheral blood mononuclear cells and were kindly provided by John Mascola and Mark Louder.
Flow cytometry.
For antibody neutralization assays, primary T cells were collected 48 h after incubation with infected DCs. These cells were stained with CD3 phycoerythrin (PE), CD11c APC, and ethidium bromide monoazide (EMA) for 10 min. EMA was cross-linked onto the cells by exposing them to a bright light source for 15 min. Cells were washed once, fixed and permeabilized with Cytoperm/Cytofix (BD Pharmingen) for 20 min. Cells were then stained for p24 Gag (KC-57 FITC; Coulter) for 20 min and washed once in 1× Perm/Wash buffer (BD Pharmingen). The percentage of T cells that received virus from DC without antibody ranged from 0.5 to 6% of T cells infected with HIV-1, as measured by p24 in the single-round replication assay, and varied according to the donor. The percent neutralization was defined as a reduction in the number of p24-Ag-positive cells compared to the number in control wells with no antibody (29).
To assess HIV infection and DC maturation, cells were stained with CD80 PE, CD11c APC, or EMA as described above. Fixed and permeabilized cells were then stained for p24 Gag. All flow cytometry was done by using a four-color FACSCalibur (BD Biosciences), and data analysis was done by using FloJo (Tree Star, Inc).
Lentivirus infections and luciferase assays.
mDCs were plated in 96-well White View plates (Packard) after isolation and either maintained in culture or differentiated and infected with lentiviruses as described above. For direct infections of DC, cells were incubated for another 48 h. To measure transfer of IIIB and ADA lentivirus, DC cells were mixed with T cells (MT2 or A3R5) for 48 h (26). Antibodies were added at various time points as described in the figure legends. After 48 h, the cells were lysed in the plates with 20 μl of cell lysis buffer (Promega) for 5 to 10 min. Then, 100 μl of luciferin substrate (Promega) was added to the wells with a multichannel pipette, followed by immediate assay for luminescence by using a 96-well plate luminometer (Packard).
Confocal microscopy.
mDCs (105) isolated from human elutriated monocytes were plated onto 12-well plates. After 24 to 48 h, they were infected with HIV-1 labeled with Vpr-GFP for 30 min. Cells were washed, detached with trypsin-EDTA, washed again, and added to T cells (3 × 104 cells/well) plated onto eight-well coverslip slides (Nalge Nunc). Uptake, polarization, and transfer were assessed in the presence of b12 or a control antibody starting immediately after the addition of infected mDCs to T cells by confocal microscopy. Images of live cells were obtained every 2 min for 1 h. Multiple fields were sampled, and representative images were recorded. The DC-T-cell synapses in the presence of control or b12 antibody were quantified as follows: the number of DCs labeled with the HIV-1 Vpr-GFP virus and the numbers of DC-T cell synapses in each of the 10 fields were counted and are represented as a ratio of DC-T cell synapses versus total labeled DCs. There were 168 labeled cells in the control antibody, and 12 of them formed synapses with the T cells (7.1%). In the b12 group, there were 172 labeled cells, 14 of which formed synapses with T cells (8.1%) (P = 0.5 [Student t test]).
RESULTS
HIV Infection in mDCs.
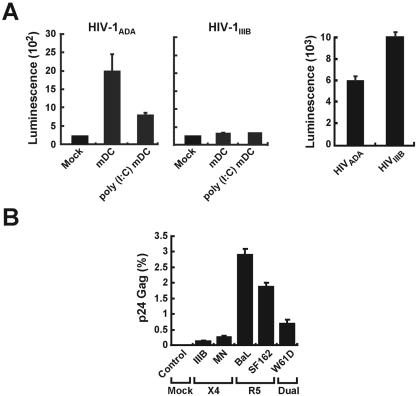
To characterize mDC infection, immature mDCs were isolated and infected with virus. Cells with an immature phenotype, i.e., ≥90% CD3−, CD14−, CD15−, CD19−, CD56−, and CD11c+ HLA-DR+, were isolated and characterized. Treatment of these cells with poly(I-C) resulted in ≥95% mDCs that upregulated CD40, CD80, CD86, and HLA-DR glycoprotein expression consistent with a mature phenotype, as previously described (4, 22, 39). Immature or poly(I-C)-treated mDCs were transduced with luciferase-expressing HIV vectors (33) that use CCR5- (HIV-1ADA) or CXCR4-tropic (HIV-1IIIB) envelopes (Env). Immature mDCs were more readily tranduced by CCR5-tropic virus than were poly(I-C)-treated mDCs (Fig. 1A, left panel), but neither cell type could be transduced with CXCR4-tropic HIV-1IIIB virus (Fig. 1A, middle panel), though T cells were readily infected by both viruses (Fig. 1A, right panel). To confirm this finding in additional strains and with replication-competent virus, wild-type HIV CCR5-tropic or CXCR4-tropic virus strains were prepared and used to infect immature mDCs. Virus replication was assessed by using a single-step growth assay for intracellular Gag protein described previously (29). In contrast to CXCR4 and a dualtropic virus, CCR5-tropic HIV-1 infected immature mDCs (Fig. 1B).
FIG. 1.
HIV infection of mDCs. (A) Direct viral infection by replication-defective HIV reporter viruses of mDCs (left and middle) and T cells (A3R5; right). Untreated mDCs and poly(I-C)-treated mDCs (3 × 104 cells) were infected with either HIV-1ADA (left) or HIV-1IIIB (middle) (25 ng of p24) for 2 h. A3R5 T cells (3 × 104 cells) were also infected with HIV-1ADA or HIV-1IIIB (25 ng of p24) for 2 h (right). Cells washed three times and maintained in cell culture were collected 2 days later for luciferase assay. (B) Direct viral infection of mDCs by CXCR4-tropic (X4), CCR5-tropic (R5), and dualtropic (Dual), HIV-1 isolates. mDCs (3 × 105/well) isolated from a single donor elutriated monocytes (see Materials and Methods) were mock infected or infected with the indicated HIV-1 strains for 12 h, washed, incubated for 48 h, and analyzed by fluorescence-activated cell sorting for intracellular p24 after gating to exclude EMA and to select for CD11c cells.
Maturation of mDCs after HIV infection.
The ability of infected immature mDCs to differentiate was assessed with wild-type CCR5-tropic HIV-1BaL. Cells infected with HIV-1BaL but treated with zidovudine (AZT) served as a negative control, and an aliquot of each was treated with poly(I-C) for 48 h after HIV-1 infection. Cells in each group were analyzed by flow cytometry for intracellular HIV-1 p24, CD11c, and either CD80 or CD40 DC maturation markers after dead cells were excluded by their affinity for EMA. Immature DCs infected with HIV were able to mature and display the characteristic dendritic cell maturation markers CD40 and CD80 (Fig. 2) comparably to HIV-1-infected AZT-treated mDCs in both poly(I-C)-treated (right lower panel) and untreated groups (right upper panel). Notably, immature DCs infected with HIV that were subsequently matured with poly(I-C) were able to transfer the virus to T cells more efficiently than immature infected DCs (Fig. 3A).
FIG. 2.
Infection with HIV-1BaL does not prevent maturation of mDCs by poly(I-C). mDCs (3 × 105/well) isolated from a single donor elutriated monocytes (see Materials and Methods) were infected with HIV-1BaL (2 μg of p24/ml). The control groups were treated with AZT (10 μM; left top and bottom panels) to prevent HIV replication. One set of control and HIV-infected cells (left lower panel, second lower panel) were matured 48 h after infection with poly(I-C) (50 μg/ml). To determine the relationship between infection and maturation, CD80 and CD40 expression (in separate experiments) were assessed in the Gag+ population compared to the Gag− population in untreated (right upper) and poly(I-C)-treated (right lower) groups of DCs.
FIG. 3.
Effect of antibody exposure on HIV-1 trans-infection. (A) HIV-1BaL-infected immature DCs transfer the virus more effectively to T cells upon maturation. mDCs (3 × 105/well) isolated from a single donor (elutriated monocytes) were mock infected or infected with HIV-1BaL (2 μg of p24/ml). One set of mock-infected and HIV-infected mDCs was matured 48 h after infection with poly(I-C) (50 μg/ml). After 48 h, primary PHA-IL-2-stimulated autologous CD4+ T cells were added to both mock-infected and HIV-1-infected immature and mature mDCs, followed by incubation in the presence of indinavir (1 μM) for another 48 h. Cells were then assayed for intracellular Gag by flow cytometry. (B) Poly(I-C)-treated mDCs were infected with HIV-1BaL for 2 h, washed five times, and incubated with the indicated concentrations of 2F5 (left) or b12 (right) and primary PHA-IL-2-stimulated autologous CD4+ T cells for 30 min or 48 h. In the control group, the virus was preincubated with antibody for 30 min prior to infection of CD4+ T cells for 2 h. Cells were maintained in indinavir (1 μM), and p24 Gag in CD3+ CD11c− cells was assayed by fluorescence-activated cell sorting 48 h later. Percent neutralization was defined as the reduction in the number of p24-Ag-positive cells compared to the number in control wells with no antibody (29).
Effect of antibody exposure on trans-infection.
To determine whether virus sequestration by mature mDCs could affect the sensitivity of HIV to neutralization by antibodies, virus transfer studies were next performed in mature mDCs with two well-characterized potent broadly neutralizing antibodies, 2F5 and b12, that inactivate both primary and laboratory-adapted HIV-1 isolates (11, 15, 34, 42). b12 binds to gp120 and blocks the virus from attaching to CD4 or chemokine receptors (37, 50, 51). 2F5 recognizes an epitope on the transmembrane subunit gp41 (32, 36) and does not prevent HIV-1 cell attachment but inhibits HIV entry, presumably by interfering with fusion process (34). Poly(I-C)-treated mDCs were exposed to HIV-1BaL for 2 h, washed, and incubated with 2F5 or b12 for 30 min or 48 h with autologous PHA-IL-2-stimulated T cells. Compared to a control group incubated with 2F5 antibody prior to infection, virus neutralization was almost completely ineffective at the time of transfer by mDCs, and b12 efficacy was also substantially reduced (Fig. 3B). In this assay, a fraction of the virus is transferred to the T cells by formation of the infectious synapse at any 30-min interval, and therefore the virus became more sensitive to neutralization over time. However, a percentage of this CCR5-tropic virus remained resistant, 19% with 2F5 and 18% with b12, even after 48 h of antibody exposure (Fig. 3B). This finding was observed in independent experiments on DCs and T cells from different donors, suggesting that CCR5-tropic virus can escape neutralization in DCs.
Effect of antibody exposure on cell-mediated transfer from HIV-1-infected mDCs.
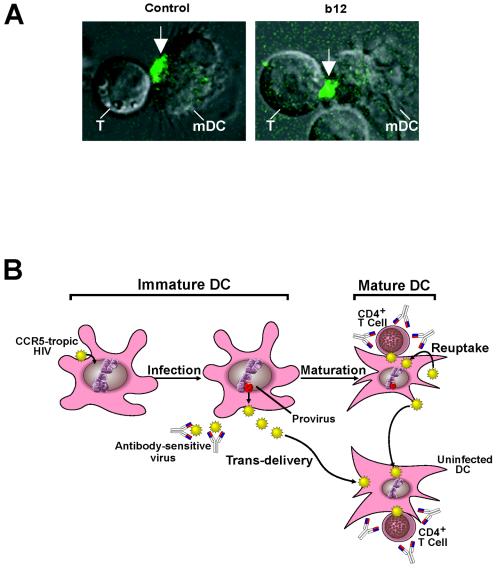
During trans-infection, a significant fraction of virus was not inactivated even after 48 h of antibody exposure in a single-round replication assay. The effect of broadly neutralizing antibodies on viral replication in primary human DCs and T cells over extended times was therefore assessed. In one set of experiments, CXCR4-tropic virus HIV-1IIIB, CCR5-tropic virus HIV-1BaL, or dualtropic virus HIV-189.6 were neutralized with b12 for an hour before they infected T cells (Fig. 4A) or mature mDCs (Fig. 4B). In another set of experiments, these viruses were used to infect mature mDCs washed and incubated with T cells in the presence of b12 continuously (Fig. 4C). In contrast to virus incubated with antibody before infection in the absence of mDC, CCR5-tropic virus, which appeared to be completely neutralized 2 days after infection, as previously described (18), unexpectedly grew exponentially after an initial lag phase (Fig. 4A versus 4C, middle panel). In contrast, CXCR4-tropic virus was completely inactivated by the b12 antibody (Fig. 4A versus 4C, left panel). A dualtropic virus, HIV-189.6, behaved similarly to the CXCR4-tropic virus but was incompletely inactivated by b12 in the presence of infected mDCs (Fig. 4A versus 4C, right panel). When the virus is neutralized by b12 antibody, it is unable to infect T cells, but the same virus, when passaged through DCs, was able to establish a productive infection upon transfer to T cells after an initial lag phase (Fig. 4A versus 4B). When the ability of b12 antibody to reduce “infectious synapse” formation was analyzed with a GFP-labeled HIV (31), no inhibition was seen (Fig. 5A, 7.1% [control] versus 8.1% [b12]; P = 0.5). Taken together, these data suggest that infection of mDCs by CCR5-tropic virus, in combination with uptake by these cells, provides a mechanism for evading the antibody response and enhance infection of T cells.
FIG. 4.
mDCs infected with HIV-1BaL confer resistance to antibody neutralization. Wild-type HIV-1IIIB, HIV-1BaL, or HIV-189.6 (12 ng of p24) was exposed to anti-gp120 antibody, b12 (50 μg/ml), for 60 min before infection of T cells (A) or mDCs (B) (4 × 104 cells each) for 2 h. (C) Alternatively, these viruses were used to infect mDCs (4 × 104 cells each) for 2 h. Cells were washed five times to remove virus and either incubated with T cells alone (105 cells) (B) or treated with b12 (50 μg/ml) and T cells (105 cells) (C). b12 was added every 60 h and left in the DC-T-cell mixture for the duration of the experiments (C). At the appropriate time, cell supernatants were collected, and p24 ELISA was performed as instructed by the manufacturer (Coulter). The data are representative of duplicate experiments.
FIG. 5.
Confocal imaging of the infectious synapse and implications of mDC transmission of CCR5-tropic HIV to T cells. (A) Neutralizing b12 antibody does not inhibit formation of infectious synapses. Virus infection of mDCs was performed with a Vpr-GFP-labeled virus vector described previously (31) and assessed by confocal microscopy (see Materials and Methods; control versus b12, P = 0.5) with representative cells. The arrow indicates a concentrated region of labeled virus. (B) Model of HIV infection of immature mDCs, internalization, and transfer by mature mDCs that confers resistance to antibody neutralization.
DISCUSSION
Inactivation of HIV-1 by neutralizing antibodies at the time of initial exposure would provide a potent mechanism to inhibit HIV infection in vivo and would be a desirable feature of an immune response elicited by a highly effective AIDS vaccine, but HIV has evolved a number of mechanisms to evade broadly neutralizing antibodies. For example, HIV Env can evade this response through carbohydrate and variable loop masking, conformational changes that protect highly conserved, receptor binding structures, and its high degree of genetic variability (47). Here we report that immunoglobulin G neutralizing antibodies can block CCR5-tropic HIV-1 entry into myeloid DCs, but once the virus is internalized through DC-SIGN by the antigen-presenting cell, it provides a previously unrecognized mechanism of immune evasion to neutralizing antibodies that may also be integral to the strategy of HIV spread and persistence. The enhancement of infection and the protection from neutralizing antibodies provided by the DCs help the virus to efficiently infect host T cells (Fig. 4A versus C).
Recently, McDonald et al. have described the formation of an infectious synapse, which provides both a structure and a mechanism to explain the enhancement of T-cell infection by DCs (31). The present study is consistent with this model and further suggests that such a synapse is poorly accessible to neutralizing antibodies (Fig. 5A). In the seven viruses tested (two CXCR4-tropic, three CCR5-tropic, and two dualtropic), it was found that once CCR5-dependent viruses establish DC infection, even the most potent broadly neutralizing antibodies are limited in their ability to prevent the T-cell spread of infection (Fig. 4C). This phenomenon is likely reflective of primary HIV-1 strains that are also generally resistant to antibody neutralization (34). These findings suggest that in infected individuals, mDCs may serve as a reservoir and immune therapies for HIV will need to prevent or reduce the infection of these cells to be highly effective.
Several groups have shown that DC-mediated trans-infection can be inhibited by both neutralizing antibodies and fusion inhibitors with different R5 isolates (18, 25). The findings here are consistent with previous work; however, there has been no analysis of the effect of DCs on neutralizing antibodies in long-term DC-T-cell cocultures. The present study shows that even in the continuous presence of neutralizing antibodies, mDCs confer resistance to HIV-1 inactivation by known, broadly neutralizing antibodies. This finding contrasts with the effect of peptide-based fusion inhibitors, which inhibit viral replication during trans-infection (25), suggesting that these lower-molecular-weight, more-diffusible antagonists are able to gain access to virus in DCs. The present study also points to at least two additional mechanisms by which mDCs may enhance HIV infection and transmission. The ability of CCR5-tropic viruses to infect immature DCs, although not highly productive (Fig. 1B), allows the development of a reservoir of infected mDCs that infect T cells efficiently upon maturation (Fig. 1B, 3A, and 4B). Although previous studies have addressed the infectivity of monocyte-derived DCs by HIV (13, 14, 16-18, 20, 21, 23, 46), it has not been evident why CCR5-tropic virus remains more highly transmissible than the CXCR4-tropic virus. One explanation is suggested by preferential infection of immature mDCs by CCR5-tropic virus, which may serve as a cellular “Trojan horse” that initiates a persistent infection (Fig. 5B). This property, as well as the enhanced infectivity, suggests that DCs may play a major role in HIV pathogenesis and transmission. These findings also suggest that a preventive vaccine should be able to elicit robust antibodies to inactivate the virus before the antigen-presenting cell can internalize it. Finally, it has been recognized that DC bind to other viruses, such as Ebola virus (2, 28), dengue virus (41), cytomegalovirus (24), and severe acute respiratory syndrome virus (48), through DC-SIGN or related receptors; this mechanism of viral uptake and protection from antibodies may be relevant to other infectious diseases.
Acknowledgments
We thank Tina Suhana and Ati Tislerics for help with manuscript preparation, John Mascola and Mark Louder for advice and assistance with neutralization assays, Karen Stroud for figure preparation, and members of the Nabel lab for helpful discussions.
REFERENCES
- 1.Abrahamsen, T. G., C. S. Carter, E. J. Read, M. Rubin, H. G. Goetzman, E. F. Lizzio, Y. L. Lee, P. A. Pizzo, and T. Hoffman. 1991. Stimulatory effect of counterflow centrifugal elutriation in large-scale separation of peripheral blood monocytes can be reversed by storing the cells at 37°C. J. Clin. Apheresis 6:48-53. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez, C. P., F. Lasala, J. Carrillo, A. L. Corbi, A. L. Corbi, and R. Delgado. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76:6841-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayehunie, S., E. A. Garcia-Zepeda, J. A. Hoxie, R. Horuk, T. S. Kupper, A. D. Luster, and R. M. Ruprecht. 1997. Human immunodeficiency virus-1 entry into purified blood dendritic cells through CC and CXC chemokine coreceptors. Blood 90:1379-1386. [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 5.Blauvelt, A., H. Asada, M. W. Saville, V. Klaus-Kovtun, D. J. Altman, R. Yarchoan, and S. I. Katz. 1997. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J. Clin. Investig. 100:2043-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron, P., M. Pope, A. Granelli-Piperno, and R. M. Steinman. 1996. Dendritic cells and the replication of HIV-1. J. Leukoc. Biol. 59:158-171. [DOI] [PubMed] [Google Scholar]
- 7.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383-387. [DOI] [PubMed] [Google Scholar]
- 8.Canque, B., Y. Bakri, S. Camus, M. Yagello, A. Benjouad, and J. C. Gluckman. 1999. The susceptibility to X4 and R5 human immunodeficiency virus-1 strains of dendritic cells derived in vitro from CD34+ hematopoietic progenitor cells is primarily determined by their maturation stage. Blood 93:3866-3875. [PubMed] [Google Scholar]
- 9.Cella, M., M. Salio, Y. Sakakibara, H. Langen, I. Julkunen, and A. Lanzavecchia. 1999. Maturation, activation and protection of dendritic cells induced by double-stranded RNA. J. Exp. Med. 189:821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choe, H., M. Farzan, Y. Sun, N. Sullivan, B. Rollins, P. D. Ponath, L. Wu, C. R. Mackay, G. LaRosa, W. Newman, N. Gerard, C. Gerard, and J. Sodroski. 1996. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85:1135-1148. [DOI] [PubMed] [Google Scholar]
- 11.Conley, A. J., J. A. Kessler, L. J. Boots, J. S. Tung, B. A. Arnold, P. M. Keller, A. R. Shaw, and E. A. Emini. 1994. Neutralization of divergent human immunodeficiency virus type 1 variants and primary isolates by IAM-41-2F5, an anti-gp41 human monoclonal antibody. Proc. Natl. Acad. Sci. USA 91:3348-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis, B. M., S. Scharnowske, and A. J. Watson. 1992. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 89:8356-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donaghy, H., B. Gazzard, F. Gotch, and S. Patterson. 2003. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood 101:4505-4511. [DOI] [PubMed] [Google Scholar]
- 14.Donaghy, H., A. Pozniak, B. Gazzard, N. Qazi, J. Gilmour, F. Gotch, and S. Patterson. 2001. Loss of blood CD11c+ myeloid and CD11c− plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood 98:2574-2576. [DOI] [PubMed] [Google Scholar]
- 15.D'Souza, M. P., D. Livnat, J. A. Bradac, S. H. Bridges, et al. 1997. Evaluation of monoclonal antibodies to human immunodeficiency virus type 1 primary isolates by neutralization assays: performance criteria for selecting candidate antibodies for clinical trials. J. Infect. Dis. 175:1056-1062. [DOI] [PubMed] [Google Scholar]
- 16.Engering, A., S. J. van Vliet, T. B. Geijtenbeek, and Y. van Kooyk. 2002. Subset of DC-SIGN+ dendritic cells in human blood transmits HIV-1 to T lymphocytes. Blood 100:1780-1786. [DOI] [PubMed] [Google Scholar]
- 17.Folcik, R. M., J. D. Merrill, Y. Li, C. J. Guo, S. D. Douglas, S. E. Starr, and W. Z. Ho. 2001. HIV-1 infection of placental cord blood monocyte-derived dendritic cells. J. Hematother. Stem Cell Res. 10:609-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frankel, S. S., R. M. Steinman, N. L. Michael, S. R. Kim, N. Bhardwaj, M. Pope, M. K. Louder, P. K. Ehrenberg, P. W. Parren, D. R. Burton, H. Katinger, T. C. VanCott, M. L. Robb, D. L. Birx, and J. R. Mascola. 1998. Neutralizing monoclonal antibodies block human immunodeficiency virus type 1 infection of dendritic cells and transmission to T cells. J. Virol. 72:9788-9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 20.Granelli-Piperno, A., E. Delgado, V. Finkel, W. Paxton, and R. M. Steinman. 1998. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72:2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Granelli-Piperno, A., V. Finkel, E. Delgado, and R. M. Steinman. 1999. Virus replication begins in dendritic cells during the transmission of HIV-1 from mature dendritic cells to T cells. Curr. Biol. 9:21-29. [DOI] [PubMed] [Google Scholar]
- 22.Granucci, F., I. Zanoni, S. Feau, and P. Ricciardi-Castagnoli. 2003. Dendritic cell regulation of immune responses: a new role for interleukin 2 at the intersection of innate and adaptive immunity. EMBO J. 22:2546-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gummuluru, S., V. N. KewalRamani, and M. Emerman. 2002. Dendritic cell-mediated viral transfer to T cells is required for human immunodeficiency virus type 1 persistence in the face of rapid cell turnover. J. Virol. 76:10692-10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halary, F., A. Amara, H. Lortat-Jacob, M. Messerle, T. Delaunay, C. Houles, F. Fieschi, F. Arenzana-Seisdedos, J. F. Moreau, and J. Dechanet-Merville. 2002. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity 17:653-664. [DOI] [PubMed] [Google Scholar]
- 25.Ketas, T. J., I. Frank, P. J. Klasse, B. M. Sullivan, J. P. Gardner, C. Spenlehauer, M. Nesin, W. C. Olson, J. P. Moore, and M. Pope. 2003. Human immunodeficiency virus type 1 attachment, coreceptor, and fusion inhibitors are active against both direct and trans infection of primary cells. J. Virol. 77:2762-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T-cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 27.Leonard, C. K., M. W. Spellman, L. Riddle, R. J. Harris, J. N. Thomas, and T. J. Gregory. 1990. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J. Biol. Chem. 265:10373-10382. [PubMed] [Google Scholar]
- 28.Lin, G., G. Simmons, S. Pohlmann, F. Baribaud, H. Ni, G. J. Leslie, B. S. Haggarty, P. Bates, D. Weissman, J. A. Hoxie, and R. W. Doms. 2003. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J. Virol. 77:1337-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mascola, J. R., M. K. Louder, C. Winter, R. Prabhakara, S. C. DeRosa, D. C. Douek, B. J. Hill, D. Gabuzda, and M. Roederer. 2002. Human immunodeficiency virus type 1 neutralization measured by flow cytometric quantitation of single-round infection of primary human T cells. J. Virol. 76:4810-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald, D., M. A. Vodicka, G. Lucero, T. M. Svitkina, G. G. Borisy, M. Emerman, and T. J. Hope. 2002. Visualization of the intracellular behavior of HIV in living cells. J. Cell Biol. 159:441-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295-1297. [DOI] [PubMed] [Google Scholar]
- 32.Muster, T., F. Steindl, M. Purtscher, A. Trkola, A. Klima, G. Himmler, F. Ruker, and H. Katinger. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263-267. [DOI] [PubMed] [Google Scholar]
- 34.Parren, P. W., M. Wang, A. Trkola, J. M. Binley, M. Purtscher, H. Katinger, J. P. Moore, and D. R. Burton. 1998. Antibody neutralization-resistant primary isolates of human immunodeficiency virus type 1. J. Virol. 72:10270-10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pope, M., S. Gezelter, N. Gallo, L. Hoffman, and R. M. Steinman. 1995. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J. Exp. Med. 182:2045-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purtscher, M., A. Trkola, G. Gruber, A. Buchacher, R. Predl, F. Steindl, C. Tauer, R. Berger, N. Barrett, A. Jungbauer, and. 1994. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 10:1651-1658. [DOI] [PubMed] [Google Scholar]
- 37.Roben, P., J. P. Moore, M. Thali, J. Sodroski, C. F. Barbas III, and D. R. Burton. 1994. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 68:4821-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stahl-Hennig, C., R. M. Steinman, K. Tenner-Racz, M. Pope, N. Stolte, K. Matz-Rensing, G. Grobschupff, B. Raschdorff, G. Hunsmann, and P. Racz. 1999. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science 285:1261-1265. [DOI] [PubMed] [Google Scholar]
- 39.Steinman, R. M., and K. Inaba. 1999. Myeloid dendritic cells. J. Leukoc. Biol. 66:205-208. [DOI] [PubMed] [Google Scholar]
- 40.Steinman, R. M., and M. C. Nussenzweig. 2002. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T-cell tolerance. Proc. Natl. Acad. Sci. USA 99:351-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tassaneetrithep, B., T. H. Burgess, A. Granelli-Piperno, C. Trumpfheller, J. Finke, W. Sun, M. A. Eller, K. Pattanapanyasat, S. Sarasombath, D. L. Birx, R. M. Steinman, S. Schlesinger, and M. A. Marovich. 2003. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 197:823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trkola, A., A. B. Pomales, H. Yuan, B. Korber, P. J. Maddon, G. P. Allaway, H. Katinger, C. F. Barbas III, D. R. Burton, and D. D. Ho, and. 1995. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J. Virol. 69:6609-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turville, S. G., J. Arthos, K. M. Donald, G. Lynch, H. Naif, G. Clark, D. Hart, and A. L. Cunningham. 2001. HIV gp120 receptors on human dendritic cells. Blood 98:2482-2488. [DOI] [PubMed] [Google Scholar]
- 44.Turville, S. G., P. U. Cameron, A. Handley, G. Lin, S. Pohlmann, R. W. Doms, and A. L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975-983. [DOI] [PubMed] [Google Scholar]
- 45.Weissman, D., Y. Li, J. Ananworanich, L. J. Zhou, J. Adelsberger, T. F. Tedder, M. Baseler, and A. S. Fauci. 1995. Three populations of cells with dendritic morphology exist in peripheral blood, only one of which is infectable with human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 92:826-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu, L., T. D. Martin, R. Vazeux, D. Unutmaz, and V. N. KewalRamani. 2002. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J. Virol. 76:5905-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]
- 48.Yang, Z.-Y., Y. Huang, L. Ganesh, K. Leung, W.-P. Kong, O. Schwartz, K. Subbarao, and G. J. Nabel. 2004. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the Spike glycoprotein and ehanced by dendritic cell transfer through DC-SIGN. J. Virol. 78:5642-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu, X., C. Borchers, R. J. Bienstock, and K. B. Tomer. 2000. Mass spectrometric characterization of the glycosylation pattern of HIV-gp120 expressed in CHO cells. Biochemistry 39:11194-11204. [DOI] [PubMed] [Google Scholar]
- 50.Zwick, M. B., R. Kelleher, R. Jensen, A. F. Labrijn, M. Wang, G. V. Quinnan, Jr., P. W. Parren, and D. R. Burton. 2003. A novel human antibody against human immunodeficiency virus type 1 gp120 is V1, V2, and V3 loop dependent and helps delimit the epitope of the broadly neutralizing antibody immunoglobulin G1 b12. J. Virol. 77:6965-6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zwick, M. B., A. F. Labrijn, M. Wang, C. Spenlehauer, E. O. Saphire, J. M. Binley, J. P. Moore, G. Stiegler, H. Katinger, D. R. Burton, and P. W. Parren. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892-10905. [DOI] [PMC free article] [PubMed] [Google Scholar]