Abstract
The duck hepatitis B virus (DHBV) polymerase (P) is translated by de novo initiation from a downstream open reading frame (ORF) that partially overlaps the core (C) ORF on the bicistronic pregenomic RNA (pgRNA). The DHBV P AUG is in a poor context for translational initiation and is preceded by 14 AUGs that could intercept scanning ribosomes, yet P translation is unanticipatedly rapid. Therefore, we assessed C and P translation in the context of the pgRNA. Mutating the upstream C ORF revealed that P translation was inversely related to C translation, primarily due to occlusion of P translation by ribosomes translating C. Translation of the pgRNA was found to be cap dependent, because inserting a stem-loop (BamHI-SL) that blocked >90% of scanning ribosomes at the 5′ end of the pgRNA greatly inhibited C and P synthesis. Neither mutating AUGs between the C and P start sites in contexts similar to that of the P AUG nor blocking ribosomal scanning by inserting the BamHI-SL between the C and P start codons greatly altered P translation, indicating that most ribosomes that translate P do not scan through these sequences. Finally, optimizing the P AUG context did not increase P translation. Therefore, the majority of the ribosomes that translate P are shunted from a donor region near the 5′ end of the pgRNA to an acceptor site at or near the P AUG, and the shunt acceptor sequences may augment initiation at the P AUG.
Hepadnaviruses are small, enveloped, hepatotropic DNA viruses that replicate by reverse transcription (reviewed in reference 11). Reverse transcription occurs through an RNA intermediate, the pregenomic RNA (pgRNA), that is also a bicistronic mRNA encoding the viral C and P proteins. C is the viral capsid protein, and P is an enzyme with DNA polymerase and RNaseH activities that synthesizes the virus genome within cytoplasmic core particles.
The organization of the duck hepatitis B virus (DHBV) pgRNA is shown in Fig. 1. It has an 118-nucleotide-long 5′ leader that contains a stable RNA stem-loop (ɛ, ΔG = −10.4 kcal/mol [1]) which is an essential signal for encapsidation and reverse transcription in DHBV (12, 14, 23, 29, 30). The bicistronic DHBV pgRNA contains the P open reading frame (ORF) located 544 nucleotides downstream of the start site for the overlapping out-of-frame C ORF. Thirteen start codons are located between the initiating AUGs for C and P (C1 and P1, respectively), and four of these AUGs are in translation initiation contexts [“Kozak sequences” (15, 16)] similar or identical to that of the P1 AUG (Table 1). The positions of these four AUGs on the pgRNA relative to the C1 and P1 sites are shown in Fig. 1. Despite being located in a very unfavorable position on the pgRNA, we found that the DHBV P is synthesized relatively rapidly and in large excess over the amount minimally required for encapsidation and reverse transcription (32).
FIG. 1.
DHBV pgRNA. Top, relative positions of the C and P ORFs (shaded boxes), ɛ, cap, and poly(A) tail are shown on the pgRNA. Bottom, the positions of the AUG codons and the insertion sites for the BamHI-SL used in this study are shown on an enlarged 5′ segment of the pgRNA.
TABLE 1.
Initiation context of C and P AUGs
| Codon | Nucleotide position | Initiation context |
|---|---|---|
| Consensus context | (A/G)CCAUGG | |
| C1 | 2647 | UAUAUGG |
| C7 | 2732 | GAGAUGC |
| C8 | 2741 | AAGAUGC |
| C9 | 2783 | AACAUGU |
| C10 | 2845 | GGUAUGC |
| P1 | 170 | GAGAUGC |
| P2 | 299 | UCUAUGG |
| P3 | 461 | CAAAUGA |
P is synthesized by de novo translation initiation at the P1 AUG of the P ORF (3, 26). The mechanism by which ribosomes find the P1 AUG on the bicistronic mRNA is not completely clear. Bicistronic messages are rare in eukaryotes because ribosomes usually locate the initiating AUG by binding to the 5′ cap of the mRNA and then scanning 5′ to 3′ to the first AUG in a good context (reviewed in references 17 and 18). The most important determinants of a favorable translation context are a G following the AUG codon and a purine (preferably A) preceding it by three nucleotides. Translation normally begins at the first AUG of an mRNA, and downstream ORFs are very poorly translated because the scanning ribosomes never reach them. However, there are at least three mechanisms for translation initiation at internal AUGs (reviewed in references 10, 19, 22, and 25). First, if the initial AUG codon is not in an acceptable initiation context, the ribosomes will bypass it and initiate translation at a downstream AUG in a favorable context. This mechanism is called leaky scanning. Variations of leaky scanning can be elaborate and may involve backwards scanning or translation of upstream ORFs followed by reinitiation of translation, but they all involve continuous passage of the ribosome along the message from the cap to the downstream AUG. A second mechanism, internal entry, involves cap-independent recognition of the mRNA by binding of ribosomes to an internal ribosome entry site (IRES), which may be followed by local scanning to identify an appropriate AUG. A third mechanism, ribosomal shunting, employs features of both scanning and internal entry. In shunting, ribosomes bind to the 5′ end of the message and then scan discontinuously, skipping sections of the message between the shunt donor and acceptor sites.
Chang and colleagues have reported that DHBV P translation initiation is independent of C translation (2). However, these experiments were limited in three important ways. First, a reporter construct in which the lacZ gene was fused to the P gene downstream of the C:P overlap was used. This construct was employed to permit detection of P translation products because sensitive P-specific antibodies were unavailable. Second, their construct produced an mRNA that lacked ɛ and most of the 5′ untranslated region (UTR) of the pgRNA. Finally, COS-7 cells (African green monkey kidney cells) were used rather than avian cells competent for virus production, such as the chicken hepatoma cell line LMH (4). Therefore, with the advantage of highly specific monoclonal antibodies against DHBV P (31), we decided to study P and C translation from the native pgRNA in LMH cells to mimic the natural conditions of DHBV C and P translation as closely as possible.
MATERIALS AND METHODS
Plasmids.
D1.5G is an overlength DHBV3 (27) expression construct containing a 5′ duplication of nucleotides 1658 to 3021 in pBluescript(−) (Stratagene). Transfection of D1.5G into LMH cells leads to production of infectious virions (4). Mutations in D1.5G are summarized in Table 2. Coding sequences for an RNA stem-loop (BamHI-SL) with a predicted free energy of −69.2 kcal/mol were generated by synthesizing five copies of a BamHI linker (CGCGGATCCGCG) flanked by appropriate restriction sites. The insertion sites of the stem-loop are listed in Table 2. As a control to verify the ability of BamHI-SL to intercept scanning ribosomes, it was inserted at the EcoRI site upstream of the green fluorescent protein (GFP) ORF in pCIHAC-GFP (Promega).
TABLE 2.
Plasmids employed
| Plasmid name | Descriptiona |
|---|---|
| D1.5G | Over-length expression vector for wild-type DHBV3 |
| D1.5G-C1AUG+ | Initiation context of C1 AUG optimized from UAUAUGG to ACCAUGG |
| D1.5G-C1AUG− | C1 AUG destroyed by mutating A2647T |
| D1.5G-dlC2 | C expression eliminated by a frameshift from deleting the 5′ copy of nt 2848-2851 |
| D1.5G-dlC2, C1AUG+ | D1.5G-dlC2 with the C1AUG+ mutation |
| D1.5G-C-C7,8AUGKO | C7 AUG destroyed by mutating A2732T and C8 AUG destroyed by mutating A2741T in the C1 AUG− background |
| D1.5G-C-C9AUGKO | C9 AUG eliminated by mutating A2783T in the C1 AUG− background |
| D1.5G-C-C10AUGKO | C10 AUG eliminated by mutating A2845T in the C1 AUG− background |
| pCIHAC-GFP | CMV-driven GFP reporter construct |
| pCIHAC-GFP-SLEcoRI | Insertion of BamHI-SL at the EcoRI site upstream of GFP in pCIHAC-GFP |
| D1.5G-C-SLNsiI | Insertion of BamHI-SL at the NsiI site of D1.5G-C1AUG− |
| D1.5G-C-SLEcoRI | Insertion of BamHI-SL at the EcoRI site of D1.5G-C1AUG− |
| D1.5G-SL5′ | Insertion of BamHI-SL at nt 2539 adjacent to the pgRNA start site |
| D1.5G-P1AUGKO | P1 AUG destroyed by mutating A170T |
| D1.5G-P1,2AUGKO | P1 and P2 destroyed by mutating A170T and A299T |
| D1.5G-P1AUG+ | Initiation context of P1 AUG optimized from GAGAUGC to GCCAUGG |
nt, nucleotide.
Cell culture and transfection.
LMH cells were used for all experiments. Cells were seeded on 60-mm dishes at a density of 1.2 × 106 cells per plate 24 h prior to transfection. LMH cells were cotransfected with test plasmids and pCMV-MP1-lacZ that carries the β-galactosidase gene for normalizing transfection efficiency. Transfections employed FuGENE (Roche) according to the manufacturer's instructions. Cells were harvested on day 1 posttransfection by lysis in 0.75× radioimmunoprecipitation assay buffer on ice for 10 min followed by clarification at 12,000 × g for 10 min at 4°C.
β-Galactosidase assay.
The amount of protein employed for sodium dodecyl sulfate-polyacrylamide gel electrophoresis for Western analyses was normalized for transfection efficiency based on β-galactosidase levels. β-Galactosidase levels were measured by adding cell lysate to 0.1 M sodium phosphate buffer (pH 7.5) to make a total volume of 150 μl. An equal volume of substrate mixture containing 3 μl of 100× Mg2+ solution (0.1 M MgCl2, 4.5 M β-mercaptoethanol)-66 μl of 1× O-nitrophenyl-β-d-galactopyranoside (4 mg/ml in 0.1 M sodium phosphate buffer [pH 7.5]) was added to the diluted cell lysate, and the tubes were incubated at 30°C for 30 min. The reaction was stopped by adding 0.5 ml of 1 M sodium bicarbonate, and the optical density was read at 420 nm.
Western and Northern blotting.
Cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Immobilon-P (Millipore) membranes. P was detected with anti-DHBV P monoclonal antibody mAb11 (epitope between amino acids 46 to 77 [31]) except for Fig. 5A, where mAb6 and mAb22 were used simultaneously (epitopes between amino acids 182 and 202 and 138 and 202, respectively; data not shown). C was detected using a rabbit polyclonal antibody raised against the full C protein (gift of J. Summers). Monoclonal antibody JL-8 (BD Biosciences Clontech) was used for detection of GFP expression in LMH cells. Following incubation with the appropriate immunoglobulin G-alkaline phosphatase conjugate (Promega), proteins were visualized by incubation with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Promega) according to the manufacturer's instructions. Scanned Western blots were quantitated with the Imagequant software package (Molecular Dynamics).
FIG. 5.
Effects of altering AUG codons P1 to P3 on P translation. LMH cells were transfected with D1.5G derivatives containing mutations in the first three AUG codons of the P ORF, and P levels were measured by Western analysis of cell lysates on day 1 posttransfection. (A) Local scanning at the 5′ end of the P ORF. The P1 or P1 plus P2 AUGs were destroyed in D1.5G, and translation from the downstream AUGs was measured by Western blotting. The positions of P products initiated from the P1, P2, and P3 AUGs are indicated at left, and the positions of two cross-reacting cellular bands are indicated on the right. The P bands are less intense than in other figures because the monoclonal antibodies needed to detect products from P3 (mAb6 and mAb22) are not as sensitive as mAb11. pBS, empty vector control. (B) Optimizing the P AUG initiaion context does not alter P translation. The P AUG was optimized in D1.5G, and P translation was measured by Western blotting; pBS, empty vector control.
RNA was isolated from transfected cells by using Tri Reagent (Molecular Research Center). The pgRNA was detected by Northern blotting as described previously (28), using 32P-labeled monomeric DHBV DNA as a probe.
RESULTS
DHBV P translation is inversely related to C translation.
Translation from the P1 AUG is not dependent upon C translation when assayed using lacZ reporter constructs lacking ɛ (2). We tested this result in a physiological context by studying P expression from the natural pgRNA in LMH cells, using anti-P monoclonal antibodies. Two D1.5G derivatives were constructed with mutations that either optimized the C1 AUG context to maximize C expression or destroyed the C1 AUG (Table 2).
The C1 AUG mutant plasmids were transfected into LMH cells, cells were harvested on day 1 posttransfection, and C and P were detected by Western blotting. Eliminating C synthesis by destroying C1 resulted in about a twofold increase in translation of P compared to translation in cells transfected with wild-type plasmid (Fig. 2B, lane 3). Optimizing the initiation context of the C1 AUG increased C synthesis (Fig. 2A, lane 4) and also led to a ∼4-fold reduction in P translation compared to that for the wild type (Fig. 2B, lane 4). Therefore, P translation was inversely correlated with C translation and could be modulated ∼8-fold by manipulating the C1 AUG.
FIG. 2.
Altering the C1 AUG alters both C and P translation. LMH cells were transfected with D1.5G derivatives containing mutations in the C ORF, and C (panel A) and P (panel B) levels were measured by Western analysis of cell lysates on day 1 posttransfection. pBS, empty vector control.
To determine if C translation inhibits P translation by intercepting scanning ribosomes and/or by occluding initiation at the P AUG by ribosomes translating the overlapping C ORF, we introduced a frameshift mutation in the C ORF that truncates C translation upstream of the P1 AUG in wild-type D1.5G (D1.5G-dlC2) and in D1.5G-C1AUG+ (D1.5G-dlC2,C1AUG+). Blocking C translation upstream of the P AUG increased P translation as efficiently as did eliminating the C1 AUG (Fig. 2B, compare lanes 3 and 6), and truncating C translation completely relieved suppression of P translation from optimizing the C1 AUG context (Fig. 2B, lanes 4 and 5). Furthermore, introducing the C1AUG+ mutation to intercept more scanning ribosomes before they reached P1 decreased P translation only slightly when the C ORF was truncated (dlC2,C1AUG+) (Fig. 2B, compare lane 6 to lane 5), compared to its large detrimental effect on P translation when C translation could proceed through the P1 AUG (C1AUG+; Fig. 2B, compare lanes 2 and 4). Therefore, the inverse relationship between C and P synthesis was primarily due to occlusion of P translation by ribosomes translating the overlapping C ORF.
Translation of C and P is cap dependent.
To test whether translation of C and P is cap dependent, we inserted a very stable stem-loop (BamHI-SL; ΔG = −69.2 kcal/mol) constructed by linking five copies of a 12-nucleotide BamHI polylinker near the 5′ end of the pgRNA. The BamHI-SL is considerably more stable than is required to block scanning ribosomes (ΔG = −40 kcal/mol) (21), and hence, if it inhibited translation when inserted at the extreme 5′ end of a mRNA, it would indicate that the ribosomes bound to the 5′ end of the RNA and were blocked from proceeding along the message and initiating translation at downstream AUGs.
As a control to verify that the BamHI-SL could impede scanning ribosomes, we inserted it upstream of the GFP ORF in the plasmid pCIHAC-GFP. LMH cells were transfected with wild-type and BamHI-SL-containing pCIHAC-GFP plasmids, and cell lysates were observed for GFP expression 48 h posttransfection by Western blot analysis using anti-GFP antibody. Figure 3A shows that insertion of BamHI-SL upstream of the GFP ORF reduced GFP expression by more than 10-fold. Thus, the stem-loop blocked more than 90% of the scanning ribosomes from translating GFP.
FIG. 3.
Effects of blocking scanning ribosomes near the 5′ end of the pgRNA. The BamHI-SL was inserted upstream of the GFP ORF as a control or near the 5′ end of the pgRNA, plasmids were transfected into LMH cells, and protein expression from the downstream ORFs was measured by Western analysis of cell lysates. (A) GFP translation from pCIHAC-GFP vectors. (B) C translation from D1.5G and D1.5G-SL5′ in which the BamHI-SL was inserted ∼10 nucleotides downstream of the predominant pgRNA start site; pBS, empty vector control. (C) P translation from D1.5G and D1.5G-SL5′. Samples are the same as in panel B. (D) pgRNA levels in cells transfected with D1.5G and D1.5G-SL5′.
The BamHI-SL was inserted into D1.5G after DHBV nucleotide 2539 (D1.5G-SL5′), approximately 10 nucleotides downstream of the major pgRNA start site. The SL5′ mutation was expected to greatly decrease C and P synthesis based on the ability of the BamHI-SL to reduce GFP synthesis, assuming that the ribosomes that translate C and P scan from the cap to the C1 and P1 AUGs. LMH cells were transfected with D1.5G and D1.5G-SL5′, and cell lysates harvested on day 1 posttransfection were analyzed for C and P expression by Western blotting. The SL5′ mutation reduced both C and P translation roughly 5- to 10-fold (Fig. 3B and C, lanes 2 and 3), although Northern blots show that the mutation had no effect on pgRNA levels (Fig. 3D). This indicates that blocking ribosomal scanning within the first few nucleotides of the pgRNA blocked C and P translation and hence that translation of the large majority of C and P from the pgRNA is cap dependent.
P translation occurs by discontinuous scanning.
The relatively high translation rate of P from its bicistronic mRNA (32) and the presence of 13 AUGs between C1 and P1 argue against leaky scanning as the mechanism of P translation. Furthermore, the C7, C8, C9, and C10 AUGs have initiation contexts similar or identical to that of the P1 AUG (Table 1). These AUGs would be anticipated to intercept scanning ribosomes on the pgRNA as well as the P1 AUG, which would proportionately decrease the number of ribosomes reaching P1. If this were true, we would detect increased P expression from C7 to C10 AUG knockout mutants. Therefore, point mutations were introduced to change the C7, C8, C9, and C10 AUGs to UUG in D1.5G-C1AUG−. C translation is eliminated in this plasmid by mutating the C1 AUG to prevent possible complications from synthesizing mutant C proteins. LMH cells were transfected with D1.5G, D1.5G-C-C7/C8AUGKO, D1.5G-C-C9AUGKO, or D1.5G-C-C10AUGKO. Western blots of cell lysates harvested on day 1 posttransfection showed that the amount of P made from the C7 plus C8 or the C10 AUG knockout mutants was the same as the amount of P translated by the parental genome and that the amount made from the C9 knockout was slightly reduced (Fig. 4A). None of the AUG knockout mutants increased P synthesis as would be expected if they intercepted scanning ribosomes. These results suggest that the ribosomes are probably not scanning past the C7, C8, C9, or C10 AUGs, and this raises the possibility of a ribosomal shunt from the neighborhood of C1 to P1.
FIG. 4.
Mutating the pgRNA between C1 and P1 has minimal effect on P translation. LMH cells were transfected with D1.5G-C- derivatives, and P levels were measured by Western analysis of lysates on day 1 posttransfection; pBS, empty vector control. (A) Destroying AUG codons between C1 and P1 has no effect on P translation. (B) Blocking ribosomal scanning by inserting the BamHI-SL between C1 and P1 has little effect on P translation.
To test whether ribosomes scan along the pgRNA between C1 and P1, we inserted the BamHI-SL at the NsiI and EcoRI sites between the C1 and P1 AUGs in D1.5G-C1AUG− (C synthesis stopped by the C1 AUG knockout mutation). The insertion sites between C1 and P1 were chosen to be within the region implied by the AUG mutations to be skipped by the ribosomes. LMH cells were transfected with D1.5G plasmids containing BamHI-SL insertions at the NsiI and EcoRI sites, and lysates from cells harvested on day 1 posttransfection were analyzed for P expression by Western blotting. Inserting the stem-loop at the NsiI site decreased P translation by about twofold (Fig. 4B, lane 2), and inserting the stem-loop at the EcoRI site had almost no effect on P translation (Fig. 4B, lane 3). Therefore, inserting the BamHI linker stem-loop at either the NsiI site or the EcoRI site between C1 and P1 had little to no effect on P translation compared to the large effect it had on GFP expression in pCIHAC-GFP (Fig. 3A).
These data indicate that the ribosomes that initiate translation from the P1 AUG do not scan the entire distance between the cap and P1, and because P translation is cap dependent (Fig. 3C), the ribosomes must be shunted from a region upstream of the NsiI site to somewhere near P1.
Local ribosomal scanning around the P1 AUG.
Our results indicate that ribosomes that translate P travel to the P1 AUG discontinuously, and thus, we asked how the ribosomes moved in the vicinity of the P1 AUG. We introduced point mutations (AUG to UUG) at the P1 and P2 AUGs in D1.5G. LMH cells were transfected with wild-type and mutant plasmids, and P was detected by Western blotting of cell lysates harvested on day 1 posttransfection (Fig. 5A). When the P1 AUG was eliminated, P translation initiated from the P2 and P3 AUGs (Fig. 5A, lane 2). When the P1 and P2 AUGs were eliminated, P translation initiated from the P3 AUG (Fig. 5A, lane 3). Therefore, once ribosomes reach the vicinity of the P1 AUG, they appear to scan normally.
Effect of the P1 initiation context.
The P1 AUG is not in an optimal initiation context, but it can still efficiently intercept ribosomes that could initiate at the P2 or P3 AUG (Fig. 5A). This implies that the large majority of ribosomes reaching P1 initiate there. To test this hypothesis, we optimized the P1 initiation context in D1.5G to determine if this would increase translation of P. LMH cells were transfected with D1.5G-P1AUG+ and D1.5G, and P was detected by Western blotting of cell lysates harvested on day 1 posttransfection (Fig. 5B). Optimizing the P1 context had no effect on P translation compared to P translated from the wild-type plasmid. Hence, it appears that nearly all ribosomes reaching P1 AUG initiate there. This implies that the initiation context surrounding the P1 AUG may not be the only factor determining translation efficiency at P1.
DISCUSSION
Ribosomal shunting is a discontinuous mode of scanning that allows ribosomes to bypass RNA regions that have AUG codons and secondary structures inhibiting linear ribosomal migration. Translation of the human adenovirus late mRNAs (33), Sendai virus Y protein mRNA (5), Cauliflower mosaic virus (CaMV) 35S mRNA (8), papillomavirus E1 mRNA (24), and ORF1 of the rice tungro bacilliform virus (9) all occurs by ribosomal shunting.
Shunting of the ribosomes that translate DHBV P was demonstrated by blocking ribosomal scanning at various positions on the pgRNA with the BamHI-SL and measuring the effect on P translation. We found that P translation is cap dependent, because P synthesis was strongly inhibited by inserting the BamHI-SL ∼10 nucleotides downstream from the dominant pgRNA start site (Fig. 3C). This observation precludes IRES-mediated translation for the majority of P translation because IRES translation is cap independent. We also found that blocking ribosome scanning between C1 and P1 by inserting the BamHI-SL at the NsiI and EcoRI sites had little to no effect on P translation (Fig. 4B). Further evidence against continuous scanning came from the observation that although the C7, C8, C9, and C10 AUGs are predicted to intercept scanning ribosomes, eliminating them had had no effect on P translation (Fig. 4A). Thus, ribosomes bind to the mRNA cap and are transferred discontinuously to the vicinity of the P1 AUG, bypassing at least some of the 13 start codons between C1 and P1 AUGs; this mechanism is diagramed in Fig. 6. However, because inserting the BamHI-SL near the 5′ end of the pgRNA did not totally abolish P synthesis, it remains possible that a small fraction of P may be translated by other mechanisms.
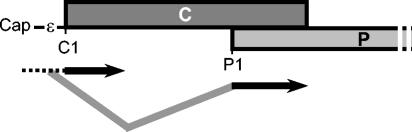
FIG. 6.
Shunting in DHBV P translation. Ribosomes bind to the 5′ end of the pgRNA and scan toward C1. The majority of the ribosomes that translate P shunt to the vicinity of P1, where they initiate P translation. The donor and acceptor sites are unknown. Dashed line, scanning ribosomes; gray lines, shunting ribosomes; black arrows, ribosomes translating C or P.
The molecular mechanism of ribosomal shunting is poorly understood. Ribosomal shunting is believed to be promoted by cis-acting elements within a structured region of the 5′ UTR (the donor site) that direct the scanning complex to the acceptor site, where scanning resumes. However, no defined sequence or structure has been universally associated with shunting. In CaMV, ribosomal shunting is facilitated by two cis-acting elements, a short ORF followed by an RNA element called stem section 1, that are present in the 600-nucleotide-long 5′ UTR (8). In the human adenovirus late mRNAs, the tripartite leader contains a group of three elements of split complementarity to both RNA strands of the stem in the 3′ hairpin of the 18S rRNA (34). The 18S sequence complementarity augments the activity of a basal shunting element present in the tripartite leader that by itself promotes ∼5% shunting activity. Translation of the human adenovirus late mRNA by ribosomal shunting is about ∼10-fold higher than translation by shunting observed in CaMV, Sendai virus, and papillomavirus mRNAs, all of which lack the sequence complementarity to the 18S rRNA (34). The ability of such complementarity to facilitate ribosomal shunting suggests that shunting may involve either specific RNA structural features or a prokaryotic-like interaction between mRNA and rRNA. The 5′ UTR of the DHBV pgRNA showed no obvious sequence complementarity to the human or chicken 18S rRNA, and the only confirmed secondary structure in the DHBV pgRNA 5′ UTR is ɛ, but disrupting folding of ɛ did not alter translation of either of the C and P ORFs (data not shown). Therefore, we cannot speculate on the specific shunt mechanism used by DHBV.
The inverse correlation of DHBV C and P translation observed in Fig. 2B was primarily due to inhibition of P translation by ribosomes translating the overlapping C ORF. This is because truncating translation of the C ORF upstream of P1 relieved the suppression of P translation by C and reduced the ability of alteration of the C1 AUG to affect P translation. The occlusion could result either from the ribosomes translating C sterically interfering with initiation at P1 or from the ribosomes disrupting RNA secondary structures or protein-RNA complexes needed for initiation at P1. The existence of ribosomal occlusion on the pgRNA indicates that P and C are largely translated from the same pool of pgRNA molecules, because if P and C were translated from distinct pools of molecules, there would be no ribosomes translating the upstream C ORF to interfere with P translation. Therefore, this observation argues against the possibility that the pgRNA may adopt alternate conformations that exclusively support either P or C translation.
Chang and colleagues (2) previously analyzed DHBV P translation by using a reporter construct in which the lacZ gene was fused to P downstream of the C:P overlap and which lacked most of the DHBV 5′UTR. Expression of this construct was analyzed in COS-7 cells rather than in LMH cells. They found that translation from the P1 AUG in this construct was minimally influenced by alterations in C translation. The differences in P translation from the natural pgRNA and from the P/lacZ-reporter construct may be due to (i) differences in cell types used in the two experiments or (ii) differences in the structure of the mRNA from which P was translated. We suspect these differing results are primarily due to deleting the shunt-donor sequences in the reporter construct. If so, the relatively efficient expression of the P/lacZ fusion protein from this plasmid would imply that the shut acceptor sequences may possess an IRES-like activity when the shunt donor sequences are disabled.
The P1 AUG initiation context is weak (Table 1), yet optimizing the P1 context did not increase P translation (Fig. 5B), implying that the initiation context is not the only factor determining translation initiation at this site. It is possible that the P1 AUG is part of the shunt acceptor and that this may augment initiation from this codon. A similar but more extreme observation was made in the translation of the Sendai virus Y proteins, where mutating the initiating AUG to ACG did not alter translation of Y proteins (20). Another example of modulation of initiation codon usage by non-Kozak context flanking sequences is translation of ORF1 in rice tungro bacilliform virus, which occurs from an ATT initiation codon located 600 nucleotides from the cap and downstream of a number of short ORFs. It is possible that this non-AUG start codon is part of the actual shunt acceptor site, since it was barely recognized as a start site by scanning complexes (9). Our result with D1.5G-P1AUG+ (Fig. 5B) is consistent with the hypothesis that shunt acceptor sites may help ribosomes select appropriate start codons in addition to simply directing them to the appropriate region of an mRNA.
Here we show that the majority of DHBV P translation occurs by ribosomal shunting, and therefore, that the DHBV P translation mechanism is different from that of hepatitis B virus (HBV) P. For HBV, P translation occurs by more than one mechanism. The majority of HBV P (74%) is translated by reinitiation at the P1 AUG after translation termination of a minicistron that is translated from the second AUG between C and P ORFs (6, 7, 13). The remaining P translation is initiated via at least two mechanisms: (i) leaky scanning through the four AUGs between C and P ORFs and (ii) backward scanning to the P AUG after translation termination of the C ORF. The presence of 13 potential start codons between the C and P initiating AUGs in the DHBV P mRNA leader sequences is likely to be tolerated in DHBV due to the different mechanisms of translation initiation. It is intriguing that the two related viral species, HBV and DHBV, have evolved distinct mechanisms of translating the functionally homologous P gene.
Acknowledgments
We thank Maureen Donlin and Robert Schneider for helpful discussions and Jesse Summers for anticore antibodies.
This work was supported by grant number AI38447 from the NIH.
REFERENCES
- 1.Beck, J., H. Bartos, and M. Nassal. 1997. Experimental confirmation of a hepatitis B (HBV) epsilon-like bulge and loop structure in avian HBV RNA encapsidation signals. Virology 227:500-504. [DOI] [PubMed] [Google Scholar]
- 2.Chang, L. J., D. Ganem, and H. E. Varmus. 1990. Mechanism of translation of the hepadnaviral polymerase (P) gene. Proc. Natl. Acad. Sci. USA 87:5158-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, L. J., P. Pryciak, D. Ganem, and H. E. Varmus. 1989. Biosynthesis of the reverse transcriptase of hepatitis B viruses involves de novo translational initiation not ribosomal frameshifting. Nature (London) 337:364-368. [DOI] [PubMed] [Google Scholar]
- 4.Condreay, L. D., C. E. Aldrich, L. Coates, W. S. Mason, and T. T. Wu. 1990. Efficient duck hepatitis B virus production by an avian liver tumor cell line. J. Virol. 64:3249-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curran, J., and D. Kolakofsky. 1988. Scanning independent ribosomal initiation of the Sendai virus X protein. EMBO J. 7:2869-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fouillot, N., and J. M. Rossignol. 1996. Translational stop codons in the precore sequence of hepatitis B virus pre-C RNA allow translation reinitiation at downstream AUGs. J. Gen. Virol. 77:1123-1127. [DOI] [PubMed] [Google Scholar]
- 7.Fouillot, N., S. Tlouzeau, J. M. Rossignol, and O. Jean-Jean. 1993. Translation of the hepatitis B virus P gene by ribosomal scanning as an alternative to internal initiation. J. Virol. 67:4886-4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Futterer, J., Z. Kiss-Laszlo, and T. Hohn. 1993. Nonlinear ribosome migration on cauliflower mosaic virus 35S RNA. Cell 73:789-802. [DOI] [PubMed] [Google Scholar]
- 9.Futterer, J., I. Potrykus, Y. Bao, L. Li, T. M. Burns, R. Hull, and T. Hohn. 1996. Position-dependent ATT initiation during plant pararetrovirus rice tungro bacilliform virus translation. J. Virol. 70:2999-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gale, J., S. L. Tan, and M. G. Katze. 2000. Translational control of viral gene expression in eukaryotes. Microbiol. Mol. Biol. Rev. 64:239-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganem, D., and R. J. Schneider. 2001. Hepadnaviridae: the viruses and their replication, p. 2923-2969. In D. M. Knipe (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 12.Hirsch, R. C., D. D. Loeb, J. R. Pollack, and D. Ganem. 1991. cis-acting sequences required for encapsidation of duck hepatitis B virus pregenomic RNA. J. Virol. 65:3309-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang, W. L., and T. S. Su. 1998. Translational regulation of hepatitis B virus polymerase gene by termination-reinitiation of an upstream minicistron in a length-dependent manner. J. Gen. Virol. 79:2181-2189. [DOI] [PubMed] [Google Scholar]
- 14.Junker-Niepmann, M., R. Bartenschlager, and H. Schaller. 1990. A short cis-acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO J. 9:3389-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozak, M. 1986. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell 44:283-292. [DOI] [PubMed] [Google Scholar]
- 16.Kozak, M. 1987. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 15:8125-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozak, M. 1991. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J. Biol. Chem. 266:19867-19870. [PubMed] [Google Scholar]
- 18.Kozak, M. 1999. Initiation of translation in prokaryotes and eukaryotes. Gene 234:187-208. [DOI] [PubMed] [Google Scholar]
- 19.Kozak, M. 2002. Pushing the limits of the scanning mechanism for initiation of translation. Gene 299:1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latorre, P., D. Kolakofsky, and J. Curran. 1998. Sendai virus Y proteins are initiated by a ribosomal shunt. Mol. Cell. Biol. 18:5021-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelletier, J., and N. Sonenberg. 1985. Insertion mutagenesis to increase secondary structure within the 5′ noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell 40:515-526. [DOI] [PubMed] [Google Scholar]
- 22.Pestova, T. V., V. G. Kolupaeva, I. B. Lomakin, E. V. Pilipenko, I. N. Shatsky, V. I. Agol, and C. U. T. Hellen. 2001. Molecular mechanisms of translation initiation in eukaryotes. Proc. Natl. Acad. Sci. USA 98:7029-7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollack, J. R., and D. Ganem. 1993. An RNA stem-loop structure directs hepatitis B virus genomic RNA encapsidation. J. Virol. 67:3254-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remm, M., A. Remm, and M. Ustav. 1999. Human papillomavirus type 18 E1 protein is translated from polycistronic mRNA by a discontinuous scanning mechanism. J. Virol. 73:3062-3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryabova, L. A., M. M. Pooggin, and T. Hohn. 2002. Viral strategies of translation initiation: ribosomal shunt and reinitiation. Prog. Nucleic Acid Res. Mol. Biol. 72:1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlicht, H. J., G. Radziwill, and H. Schaller. 1989. Synthesis and encapsidation of duck hepatitis B virus reverse transcriptase do not require formation of core-polymerase fusion proteins. Cell 56:85-92. [DOI] [PubMed] [Google Scholar]
- 27.Sprengel, R., C. Kuhn, H. Will, and H. Schaller. 1985. Comparative sequence analysis of duck and human hepatitis B virus genomes. J. Med. Virol. 15:323-333. [DOI] [PubMed] [Google Scholar]
- 28.Tavis, J. E., and D. Ganem. 1996. Evidence for the activation of the hepatitis B virus polymerase by binding of its RNA template. J. Virol. 70:5741-5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tavis, J. E., S. Perri, and D. Ganem. 1994. Hepadnavirus reverse transcription initiates within the stem-loop of the RNA packaging signal and employs a novel strand transfer. J. Virol. 68:3536-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang, G. H., and C. Seeger. 1993. Novel mechanism for reverse transcription in hepatitis B viruses. J. Virol. 67:6507-6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao, E., Y. Gong, N. Chen, and J. E. Tavis. 2000. The majority of duck hepatitis B virus reverse transcriptase in cells is nonencapsidated and is bound to a cytoplasmic structure. J. Virol. 74:8648-8657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao, E., and J. E. Tavis. 2003. Kinetics of synthesis and turnover of the duck hepatitis B virus reverse transcriptase. J. Biol. Chem. 278:1201-1205. [DOI] [PubMed] [Google Scholar]
- 33.Yueh, A., and R. J. Schneider. 1996. Selective translation initiation by ribosome jumping in adenovirus-infected and heat-shocked cells. Genes Dev. 10:1557-1567. [DOI] [PubMed] [Google Scholar]
- 34.Yueh, A., and R. J. Schneider. 2000. Translation by ribosome shunting on adenovirus and hsp70 mRNAs facilitated by complementarity to 18S rRNA. Genes Dev. 14:414-421. [PMC free article] [PubMed] [Google Scholar]