Abstract
Latent Epstein-Barr virus (EBV) is reactivated by 12-O-tetradecanoylphorbol-13-acetate (TPA) in EBV-infected cells. In this study, we found that TPA up-regulated phosphorylation of p38, a mitogen-activated protein kinase, and activated c-myc mRNA in EBV-positive epithelial GT38 cells. The EBV immediate-early gene BZLF1 mRNA and its product ZEBRA protein were induced following TPA treatment. Protein kinase C inhibitors, 1-(5-isoquinolinesulphonyl)-2, 5-dimethylpiperazine (H7) and staurosporine, inhibited the induction of p38 phosphorylation and the activation of c-Myc by TPA. The p38 inhibitor SB203580 blocked both p38 phosphorylation and ZEBRA expression by TPA. Pretreatment of GT38 cells with the nitric oxide (NO) donor S-nitroso-N-acetylpenicillamine inhibited p38 phosphorylation and c-Myc activation by TPA, suggesting that NO may inhibit EBV reactivation via both p38 and c-Myc. By using short interfering RNA (siRNA) targeting either p38 or c-myc, we found that p38 or c-myc siRNA specifically inhibited expression of the respective gene and also suppressed the induction of ZEBRA and EBV early antigen. The interferon (IFN)-responsive gene expression tests ruled out the possibility that the antiviral effect of siRNA is dependent on IFN. Our present study demonstrates for the first time that either p38 or c-myc siRNA can efficiently inhibit TPA-induced EBV reactivation in GT38 cells, indicating that p38- and/or c-myc-associated signaling pathways may play critical roles in the disruption of EBV latency by TPA.
Epstein-Barr virus (EBV) is a human herpesvirus that infects B cells and epithelial cells to establish latent infection. EBV is associated with a variety of cancers, including Burkitt's lymphoma (BL) (41), nasopharyngeal carcinoma (57), and gastric carcinoma (44). Upon primary infection, EBV infects epithelial cells, where it undergoes lytic replication, and B cells, where it usually remains latent (32, 41). Expression of the EBV immediate-early proteins BZLF1 (ZEBRA and Zta) or BRLF1 (Rta) is sufficient to convert EBV infection from its latent to lytic form (7, 50). Latency can be disrupted by certain compounds, such as 12-O-tetradecanoylphorbol-13-acetate (TPA) (12, 13, 14, 56), which induces EBV reactivation via NF-κB and AP-1 as regulated by protein kinase C (PKC) and mitogen-activated protein kinase (MAPK) (13).
MAPKs, which consist of extracellular signal-regulated kinases 1 and 2, p38, and stress-activated protein kinase 1/c-Jun NH2-terminal kinase, are central components of signal transduction pathways in the regulation of cell proliferation and differentiation, cytokine production, and apoptosis (15). Several species of viruses can induce the activation of MAPK in infected cells, such as human immunodeficiency virus type 1 (27, 30), herpes simplex virus type 1 (54), and encephalomyocarditis virus (18). In addition, the Ras/MEK/MAPK pathway contributes to EBV reactivation (43) and replication of herpes simplex virus type 2 (46). p38 kinase activation is also necessary for efficient disruption of EBV latency from the endogenous viral genome in B cells by surface immunoglobulin cross-linking (1). However, the precise role of p38 in EBV reactivation has not been elucidated.
The c-myc proto-oncogene encodes a nuclear transcriptional factor that contains a basic helix-loop-helix leucine zipper domain and binds to the cis element CACGTG when dimerized with another nuclear factor, Max. Through regulating expression of various target genes, c-myc is actively involved in the control of cellular proliferation, differentiation, and apoptosis (39). c-myc is a key downstream target of the EBV latency-associated gene EBNA2 in infected B lymphocytes (3), and induction of c-myc expression by latency-associated genes likely plays a crucial role in promoting cell cycle progression (21, 40). Although latency-associated EBV genes can induce c-myc expression, these genes are not expressed in EBV-associated tumor in immunocompetent individuals (21). Instead, c-myc is translocated in BL (36) or overexpressed through other mechanisms in nasopharyngeal carcinoma (33). It is well known that TPA reactivates latent EBV in infected cells (13, 56) and activates c-myc in resting lymphocytes (23), T cells (35), chronic lymphocytic leukemia cells (28), and BL cells (8). However, the relationship between c-myc activation and EBV reactivation is not well understood. Therefore, study of the involvement of c-myc in EBV reactivation would be valuable to a further exploration of the mechanisms underlying EBV latency.
RNA interference (RNAi) is a highly conserved mechanism found in almost all eukaryotes and is believed to serve as an antiviral defense mechanism. The molecular details are becoming clear due to combined genetic and biochemical approaches (51, 48). On entry into the cells, the double-stranded RNA (dsRNA) is cleaved by an RNase III-like enzyme, Dicer, into short interfering RNAs (siRNAs) (4, 17, 25, 26, 55). The siRNAs are incorporated into a multisubunit protein complex, the RNA-induced silencing complex, which directs the siRNA to the appropriate mRNA. This complex, when activated, can specifically silence or downregulate gene expression. RNAi has been used to study gene function in multiple model organisms, including plants (52), flies (24), Caenorhabditis elegans (11), and mice (53). However, in most mammalian cells, dsRNAs longer than 30 nucleotides activate an interferon (IFN) response, leading to nonspecific degradation of RNA transcripts and a general shutdown of host cell protein translation (47). This nonspecific effect can be circumvented by the use of synthetic siRNA that are 21 nucleotides long with short 3′ overhangs (9). The synthetic siRNA has been shown to induce homology-dependent degradation of cognate mRNA and has been used to knock down expression of endogenous and heterologous genes in mammalian cell lines (5, 16, 19, 29, 38). RNAi interferes with the replication of a number of animal viruses including human immunodeficiency virus (19, 31, 38), hepatitis C virus (22), and gammaherpesviruses (20). However, the effect of RNAi on EBV has not been reported.
The aim of this study was to determine whether p38 and c-myc play a role in TPA-induced EBV reactivation. Here, we demonstrated that TPA-induced EBV reactivation in GT38 cells is dependent on PKC-mediated phosphorylation of p38 and c-myc activation and that the suppression of p38 phosphorylation by the specific inhibitor inhibited ZEBRA induction. Furthermore, we found that the RNAi efficiently inhibited TPA-induced ZEBRA expression and EBV early antigen (EA) through interference with either p38 or c-myc expression, suggesting that p38 and c-myc play key roles in the reactivation of EBV. siRNA targeting to either p38 or c-myc is sufficient to efficiently interfere with EBV reactivation by TPA in EBV-infected GT38 cells.
MATERIALS AND METHODS
Cell line and reagents.
Cells of the GT38 cell line are EBV-positive epithelial cells derived from human gastric tissue (49). The cells were cultured in RPMI 1640 medium, supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). Cells were incubated in an atmosphere of 95% air and 5% CO2. For EBV reactivation, cells were treated with 20 ng of TPA (Sigma Chemical Co., St. Louis, Mo.) per ml. 1-(5-Isoquinolinesulphonyl)-2,5-dimethylpiperazine (H7) and staurosporine were purchased from Sigma. SB203580 and S-nitroso-N-acetylpenicillamine (SNAP) were obtained from Calbiochem (Bioscience, Inc., La Jolla, Calif.) and dissolved in dimethyl sulfoxide just before being added to the culture medium.
Preparation of RNA and Northern blot analysis.
Total RNA was prepared from cells by using an ISOGEN kit (Nippongene Inc., Tokyo, Japan) according to the manufacturer's protocol as described previously (13, 14). The RNA was dissolved in diethyl pyrocarbonate-treated water and stored at −20°C. For all RNA samples, the ratio of the optical density at 260 nm to the optical density at 280 nm was >1.50.
Northern blot analysis was carried out as described previously (13, 14). Total RNA samples were denatured in 50% formamide and 2.2 M formaldehyde at 57°C for 15 min. Aliquots containing 20 μg of total RNA were loaded into 1% agarose gel containing 2.2 M formaldehyde and were electrophoresed with running buffer containing 50 mM MOPS (morpholinepropanesulfonic acid) acetate (pH 7.0), 10 mM sodium acetate, and 1 mM EDTA. The RNA was then transferred onto Hybond N+ membranes (Amersham, Buckinghamshire, United Kingdom) by capillary transfer and UV autocross-linked. Membranes were treated with prehybridization buffer for 6 to 24 h at 42°C. The solution was then replaced with fresh hybridized solution containing the [α-32P]dCTP-labeled probe of c-myc (6) at a final concentration of 106 cpm/ml, and hybridization continued for 24 h at 42°C. The blots were washed three times at 65°C in 1× SSPE (0.18 M NaCl, 10mM NaH2PO4, and 1mM EDTA [pH 7.7])-0.1% sodium dodecyl sulfate (SDS) for 10 min and then exposed to Kodak X-AR film (Eastman Kodak Co., Rochester, N.Y.) at −80°C for 1 to 3 days. Northern blot membranes were stripped by boiling in 5 mM EDTA-0.1% SDS. They were then rehybridized with a specific probe for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (13) to control for variations in the amount of RNA per lane. Each Northern blot was representative of at least three separate experiments performed at different times, unless otherwise specified.
RT-PCR analysis.
Reverse transcription (RT)-PCR was performed essentially as previously described (13). The sequences of sense and antisense primers used for amplification of specific human 2′,5′-oligoadenylate synthetase 1 (2,5 OAS1; 40 kDa) cDNA were 5′-TGGCTGAATTACCCATGCTT-3′ (sense) and 5′-TGGACAAGGGATGTGAAAAT-3′ (antisense) to amplify a 265-bp product. The PCR primers for 2,5 OAS2 (71 kDa) were 5′-TTAAATGATAATCCCAGCCC-3′ (sense) and 5′-AAGATTACTGGCCTCGCTGA-3′ (antisense) to amplify a 424-bp product; β-tubulin primers were 5′-TGGATCTAGAACCTGGGACCAT-3′ (sense) and 5′-ACCATGTTGACTGCCAACTTGC-3′ (antisense). The amplified PCR products were analyzed after electrophoresis in a 1.2% agarose gel to compare relative induction levels of 2,5 OAS.
RNA interference assays.
The 19-nucleotide p38 SMARTpool siRNA was purchased from Dharmacon Research, Inc. (Lafayette, Colo.). With SMARTpool, four individual siRNAs targeting the same p38 gene were combined into one pool by using a proprietary pooling algorithm of five parameters. The individual p38 siRNA sequences were as follows: 5′-GCAAGAAACUAUAUUCAGU-3′, 5′-GAACUGCGGUUACUUAAAC-3′, 5′-GAACUUGCGAAUGUAUUU-3′, and 5′-CAAGGUCUCUGGAGGAAUU-3′. The scrambled p38 siRNA containing nonspecifically pooled duplex was obtained from Dharmacon Research, Inc. Silencer c-myc siRNA kits were obtained from Ambion Inc. (Austin, Tex.). The c-myc siRNA sequence is derived from the 3′ untranslated region of the human c-myc mRNA sequence. The scrambled c-myc negative control siRNAs, which have no significant homology to any known gene sequences from mouse, rat, or human, were obtained from Ambion Inc. According to the protocol of the manufacturer, the siRNA duplexes were resuspended with siRNA universal dilution buffer at a concentration of 20 μM. Cells were seeded in 12-well plates at a density of 5 × 104 to 7 ×104 cells per well, incubated for 24 h, and then transfected with 100 nM p38 or c-myc siRNAs by using 2 μl of Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, Calif.) according to the manufacturer's optimized protocol; the transfected cells were then incubated for 48 to 72 h at 37°C.
Western blot analysis.
Western blot analysis was performed on cell lysates as described previously (13). Protein samples (20-μg) were separated in SDS-10% polyacrylamide gel and electrophoretically transferred onto polyvinylidene difluoride membranes (Millipore Co., Bedford, Mass.), by using the semidry transfer blot system. The membrane was blocked with 5% skim milk in Tris-buffered saline containing 0.1% Tween 20 and subsequently incubated with primary antibody. Immunoblottings were performed by using the following antibodies: a 1:100 dilution of anti-c-Myc and a 1:200 dilution of anti-β-actin (Santa Cruz Biotechnology, Inc., Calif.); a 1:1,000 dilution of anti-phospho-c-Myc, anti-phospho-p38, and anti-p38 (New England, Biolabs Inc., Beverly, Mass.); and a 1:100 dilution of anti-ZEBRA monoclonal antibodies (developed in our laboratory). After washing, the membrane was incubated with peroxidase-conjugated goat anti-rabbit or anti-mouse immunoglobulin G antibody (Amersham Biosciences Co.), and the specific bands were detected by the Amersham ECL enhanced chemiluminescence technique system.
Immunofluorescence analysis.
Approximately 2 × 104 cells were seeded in Lab-Tek eight-well chamber slides (Nalge Nunc International, Naperville, Ill.). After a 24-h incubation, the cells were transfected with 100 nM concentrations of the siRNAs according to the procedure described above. At 2 days posttransfection, the cells were treated with or without TPA (20 ng/ml) for 72 h, washed twice with phosphate-buffered saline (PBS), and then fixed with acetone for 10 min at room temperature. Thereafter, cells were incubated with anti-EA antibodies, which were derived from a patient's serum, at a final dilution of 1:40 for 45 min at 37°C. After washing with PBS, fluorescein isothiocyanate-conjugated anti-human antibodies (Organon Teknica Co., Durham, N.C.) were added and incubated for another 45 min at 37°C. The slides were washed and mounted with 1:1 glycerol-PBS and finally were examined under a fluorescence microscope. For each determination, at least 1,000 cells were counted.
Colorimetric (MTT) assay for cell proliferation.
Cell growth was assayed by incorporation of MTT [3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyl tetrazolium bromide] dye (Chemicon International, Inc., Temecula, Calif.). Cells were seeded on 96-well plates (Corning Inc., Corning, N.Y.) at a density of 5 ×103 cells/100 μl per well. At the indicated times, 10 μl of MTT solution (5 mg/ml) was added to each well, and the plates were incubated for 4 h at 37°C. One hundred microliters of isopropanol with 0.04 N HCl was added to each well and mixed by repeated pipetting. Absorbance was measured at a test wavelength of 570 nm and a reference wavelength of 655 nm with a microplate reader (model 550; Bio-Rad, Richmond, Calif.).
Statistical analysis.
The results for Northern and Western blotting analyses were quantified according to a method described previously (13). Data are expressed as means ± standard deviations. The data shown were mean values of at least three different experiments. A Student's t test was used to compare the results. A P value of less than 0.01 is considered statistically significant.
RESULTS
Effects of TPA on the expression and/or activation of BZLF1 or BRLF1, c-myc, and p38 in GT38 cells.
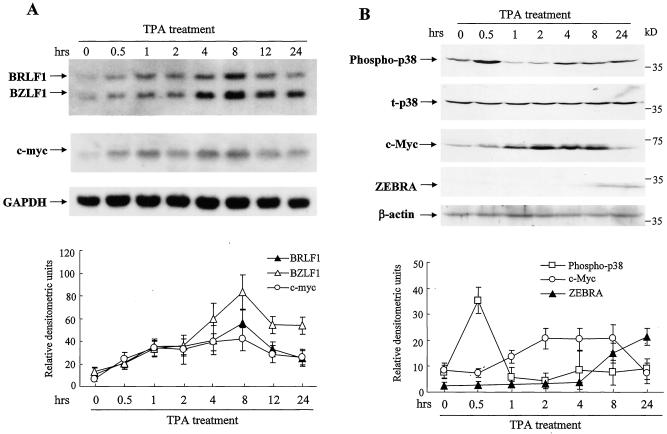
We previously reported that the expression of EBV immediate-early genes BZLF1 and BRLF1 in GT38 cells was induced by TPA (13). To further investigate cellular genes that are associated with EBV reactivation by TPA, we focused on p38 and c-myc expression. GT38 cells were incubated with 20 ng of TPA per ml from 0 to 24 h. BZLF1 and BRLF1 mRNAs were induced at 0.5 h and reached a maximum level at 8 h. The c-myc mRNA level began to increase at 0.5 h, reached maximum at 8 h, and then declined at 12 h after TPA stimulation (Fig. 1A). To determine whether activation of p38 or/and c-myc is associated with EBV reactivation, we used Western blot analysis to examine p38 phosphorylation and c-myc activation in GT38 cells treated with TPA (Fig. 1B). Phosphorylation of p38 reached a maximum at 0.5 h, decreased to a lower level at 1 h, and returned to the basal level at 4 to 24 h. TPA treatment did not alter the expression levels of total p38. The expression of c-Myc was increased at 1 h, reached a plateau at 2 to 8 h, and then decreased at 24 h after TPA treatment. ZEBRA was detected at 8 and 24 h. In the parallel samples without TPA treatment, the phosphorylation of p38, the activation of c-myc, and ZEBRA expression remained essentially unchanged at different time points (data not shown). These results indicated that TPA is able to induce the concomitant activation of p38 and c-myc during EBV reactivation in GT38 cells.
FIG. 1.
TPA induces c-myc and p38 gene expression and EBV reactivation. GT38 cells were incubated with TPA (20 ng/ml). (A) Total RNAs were isolated from the cells at indicated times, and the expression levels of BZLF1, BRLF1, and c-myc genes were analyzed by Northern blotting. GAPDH was analyzed as an internal control standard. (B) Cell lysates were isolated at indicated times, and the phosphorylation of p38, total p38 (t-p38), c-Myc, and ZEBRA were analyzed by Western blotting with anti-phospho-p38, anti-p38, anti-c-Myc, and anti-ZEBRA antibodies. Total p38 and β-actin were analyzed as internal control standards. The relative densitometric units of BRLF1, BZLF1, and c-myc to GAPDH (panel A) and of phospho-p38 to total p38 and c-Myc and ZEBRA to β-actin (panel B) were quantified by a phosphorimage system. The data represent three independent experiments, and the standard deviations are shown.
Effects of p38 and PKC inhibitors on p38 phosphorylation and expression of c-myc and ZEBRA.
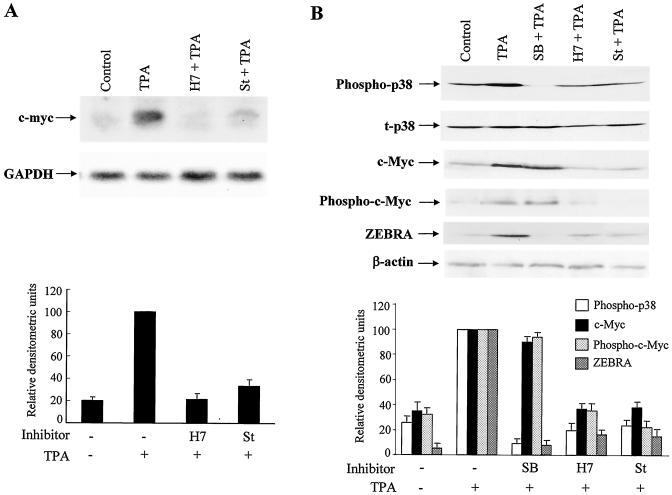
TPA is a potent activator of PKC (37). It was previously reported that TPA induced EBV reactivation via the PKC pathway in GT38 cells (13). To examine whether the activation of the c-myc gene is associated with TPA-induced PKC activation, GT38 cells were pretreated with potent PKC inhibitors, H7 and staurosporine, and the p38 inhibitor SB203580 for 1 h and then treated with TPA for 4 h (Fig. 2A). Consequently, the induction of c-myc mRNA by TPA was greatly inhibited by H7 pretreatment. Likewise, staurosporine also inhibited c-myc mRNA expression, although the inhibitory effect was weaker than that of H7. In contrast, the p38-specific inhibitor SB203580 did not affect the induction of c-myc mRNA by TPA (data not shown). To examine whether TPA induces EBV reactivation via the p38 and/or c-myc pathways as regulated by PKC, we performed immunoblotting analyses (Fig. 2B). H7 and staurosporine greatly inhibited the phosphorylation of p38 and activation of c-Myc and ZEBRA by TPA. SB203580 blocked the phosphorylation of p38 and ZEBRA expression by TPA but did not affect the expression of c-Myc. The protein levels of total p38 were not affected by SB203580, H7, or staurosporine. To examine whether p38 regulates the phosphorylation of c-Myc, we performed Western blot analyses by using anti-phospho-c-Myc antibodies. The phosphorylation pattern of c-Myc was similar to that of c-Myc activation. Both H7 and staurosporine inhibited the phosphorylation of c-Myc by TPA, but SB203580 had no effect on the phosphorylation of c-Myc. Taken together, these results indicated that PKC is an upstream effector of p38 and c-myc activation, which are essential for ZEBRA expression by TPA, and that the phosphorylation of p38 and c-myc may be in independent pathways regarding their roles in TPA-induced EBV reactivation.
FIG. 2.
Inhibitors of PKC and p38 specifically suppress the TPA-mediated expression levels of c-myc, p38, and ZEBRA. (A) GT38 cells were pretreated with a PKC inhibitor H7 (100 μM) or staurosporine (St; 0.1 μM) for 1 h, and then exposed to TPA (20 ng/ml) for 4 h. Total RNAs were extracted and analyzed for c-myc mRNA expression by Northern blotting. After stripping, the membrane was reprobed with GAPDH. (B) GT38 cells were pretreated with H7 or staurosporine (St) or SB203580 (SB; 10 μM) for 1 h and then treated with TPA. Cell lysates were extracted for p38, c-Myc, and ZEBRA at 0.5, 4, and 48 h, respectively, after TPA treatment. The phosphorylation of p38, total p38 (t-p38), c-Myc, phospho-c-Myc, ZEBRA, and β-actin were analyzed by Western blotting with anti-phospho-p38, anti-p38, anti-c-Myc, anti-phospho-c-Myc, anti-ZEBRA, or anti-β-actin antibodies. The relative densitometric units of c-myc to GAPDH (panel A) and of phospho-p38 to total p38, as well as c-Myc, phospho-c-Myc, and ZEBRA to β-actin (panel B) were quantified with a phosphorimage system. The data represent three independent experiments and the standard deviations are shown.
Effects of SNAP, an NO donor, on p38 phosphorylation and c-Myc activation by TPA in GT38 cells.
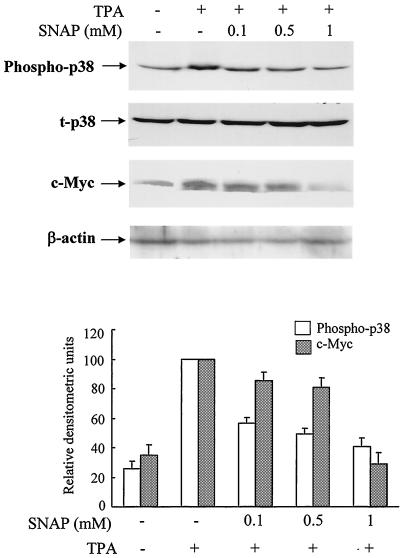
It was previously reported that NO downregulates the induction of EBV reactivation by TPA (13). To determine the effects of NO on p38 and/or c-myc activation, GT38 cells were treated with TPA in the absence or presence of the NO donor SNAP, which generates NO in an aqueous solution, resulting in the formation of peroxynitrite. The phosphorylation of p38 and expression of c-Myc were examined by immunoblotting analysis (Fig. 3). TPA-mediated phosphorylation of p38 and c-Myc activation were inhibited by SNAP in a dose-dependent manner; SNAP (0.1 mM) only has a slight inhibition effect, and the maximum inhibition was observed at a concentration of 1 mM SNAP. TPA-mediated induction of BZLF1 mRNA and ZEBRA protein were also inhibited by SNAP (reference 13 and data not shown). These results suggested that NO may inhibit TPA-induced EBV reactivation via the inhibition of the p38 and c-myc pathways.
FIG. 3.
TPA-induced phosphorylation of p38 and activation of c-Myc were inhibited by the NO donor SNAP. GT38 cells were pretreated with SNAP at indicated concentrations for 1 h prior to TPA (20 ng/ml) treatment. Cell lysates were prepared for phosphorylation of p38 and total p38 (t-p38) and of c-Myc at 0.5 and 4 h, respectively, after TPA treatment. Cell lysates were separated by SDS-PAGE and blotted onto membranes. Phosphorylated p38, p38, and c-Myc were detected by Western blotting with anti-phospho-p38, anti-p38, anti-c-Myc or anti-β-actin antibodies. The p38 and β-actin were used as internal standards. The relative densitometric units of phosphorylation of p38 and activation of c-Myc were determined to p38 and β-actin signals, respectively. The data represent three independent experiments and the standard deviations are shown.
siRNA targeting to either p38 or c-myc inhibits EBV reactivation in GT38 cells.
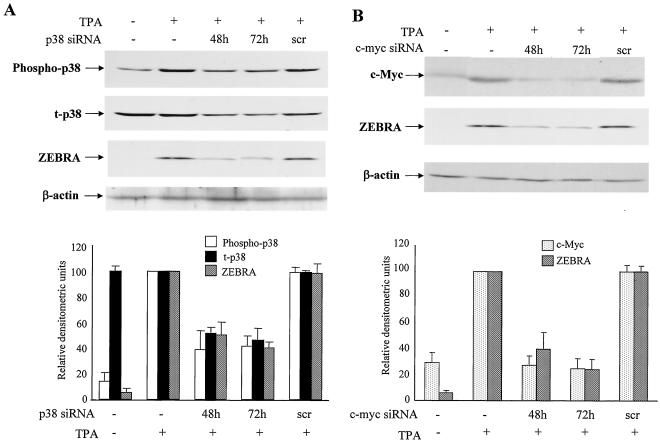
To further study the critical roles of p38 and c-Myc in EBV reactivation, we analyzed the loss of function of p38 and c-myc on EBV reactivation with RNAi methods. We transfected GT38 cells with p38 siRNAs by using Lipofectamine 2000 and then treated the cells with TPA (Fig. 4A). The phosphorylation of p38 was markedly downregulated by p38 siRNAs at 48 and 72 h after transfection. Expression of total p38 was also downregulated by the siRNA. TPA-mediated induction of ZEBRA expression was also inhibited by p38 siRNAs. Similarly, GT38 cells were transfected with c-myc siRNA, and thereafter the expression of c-Myc was examined by Western blot analysis (Fig. 4B). As a result, activation of c-Myc by TPA was inhibited by c-myc siRNA at 48 and 72 h posttransfection. ZEBRA induction by TPA was also inhibited by c-myc siRNA. To investigate the effect of siRNA targeted to p38 on c-myc expression and siRNA targeted to c-myc on p38 phosphorylation, GT38 cells were transfected with p38 or c-myc siRNA for 48 or 72 h and then treated with TPA. The phosphorylation of p38 was not affected by c-myc siRNA transfection, and p38 siRNA also had no effect on c-Myc activation (data not shown). These results demonstrated that siRNAs targeted to p38 and c-myc can efficiently inhibit TPA-induced ZEBRA expression in GT38 cells and that both the p38- and c-Myc-associated pathways are crucial for TPA-mediated EBV reactivation.
FIG. 4.
siRNAs targeting p38 and c-myc specifically inhibit the expression of respective genes and ZEBRA expression. (A) GT38 cells were transfected with p38 siRNA (0.1 μM) or scrambled siRNA (scr) and incubated for 48 and 72 h or for 72 h and then treated with TPA for 30 min for phosphorylated p38 and total p38 (t-p38) and 48 h for ZEBRA. The cell extracts were analyzed for phosphorylation of p38, total p38, ZEBRA, and β-actin by Western blotting. (B) GT38 cells were transfected with siRNA (0.1 μM) targeted to c-myc for 48 or 72 h or with scrambled (scr) siRNA for 72 h and then treated with TPA (20 ng/ml) for 4 or 48 h for the expression analysis of c-Myc and ZEBRA, respectively. Cell lysates were harvested from siRNA-transfected or untransfected GT38 cells at indicated times posttransfection. C-Myc and ZEBRA expression were analyzed by Western blotting by using anti-c-Myc, anti-ZEBRA, and anti-β-actin antibodies. The signals of phosphor-p38, t-p38, and ZEBRA (panel A) and of c-Myc and ZEBRA (panel B) were quantified by a phosphorimage system. The relative protein level was measured as ratio of β-actin protein. The data represent three independent experiments, and the standard deviations are shown.
p38 and c-myc siRNAs inhibit EA induction in GT38 cells.
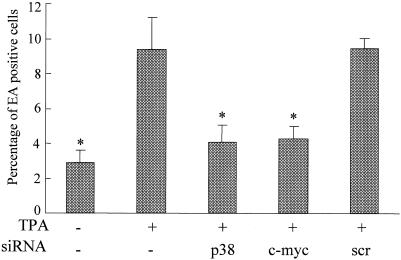
To further examine whether p38 or c-myc siRNA could inhibit EA expression in GT38 cells, the cells were transfected with p38 or c-myc siRNA and then treated with TPA at 48 h posttransfection. The expression of EA was analyzed by the immunofluorescence method (Fig. 5). EA was detected in 2.9% of untreated GT38 cells. After TPA treatment for 72 h, the percentage of EA-positive cells was increased to 9.4% (threefold increase; P = 0.001). However, the percentage of EA-positive cells was decreased to 4.1 and 4.3% (2.3-fold [P = 0.003] and 2.2-fold [P = 0.002], respectively) in cells transfected with p38 and c-myc siRNAs, respectively, whereas no significant reduction of EA-positive cells was observed in the scrambled siRNA-transfected cells (P = 0.468). These results indicated that both p38 and c-myc siRNAs can block TPA-mediated induction of EA in GT38 cells and strongly suggested that siRNAs targeted to p38 and c-myc are able to inhibit EBV reactivation.
FIG. 5.
p38 or c-myc siRNA blocks EA induction by TPA. GT38 cells were transfected with siRNA (0.1 μM) targeting p38 and c-myc or with scrambled (scr) siRNA. At 48 h posttransfection, cells were treated with TPA (20 ng/ml) for 72 h. EA-positive cells were determined by indirect immunofluorescence. The data shown represent three independent experiments. Bars represent standard deviations. (*, P < 0.01 versus the TPA-positive and siRNA-negative cells).
siRNA transfection neither activates IFN-induced 2,5 OAS induction nor induces cell-growth inhibition.
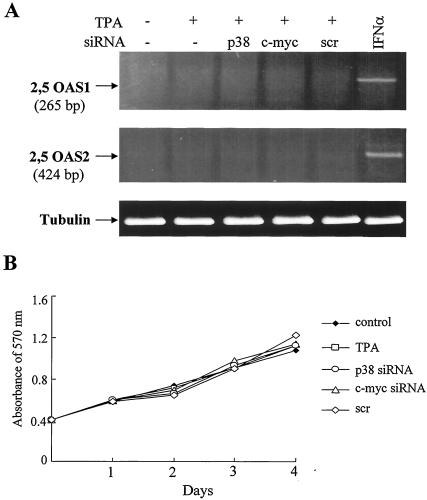
To rule out the possibility that the siRNA-mediated inhibition of EBV reactivation in GT38 cells could be mediated by dsRNA-induced activation of the IFN pathway, we analyzed the expression levels of 2,5 OAS1 and 2,5 OAS2 mRNA, known as the IFN-induced gene (22). The relative amounts of 2,5 OAS1 and 2,5 OAS2 transcripts were determined by RT-PCR. IFN-α induced the expression of 2,5 OAS1 and 2,5 OAS2 mRNAs in GT38 cells (Fig. 6A). However, 2,5 OAS1 mRNA was not detected in any group of cells that were untransfected or transfected with siRNA targeted to p38 or c-myc or with an irrelevant scrambled siRNA. Although low-level expression of 2,5 OAS2 mRNA was detected in untransfected GT38 cells and in GT38 cells transfected with p38, c-myc, or scrambled siRNAs, no difference was observed between untransfected cells and cells transfected with p38, c-myc, or scrambled siRNAs. We concluded that IFN induction is not involved in the decrease in the phosphorylation of p38 or the expression of c-myc and ZEBRA by the siRNAs.
FIG. 6.
Effects of siRNAs targeted to p38 and c-myc on the 2,5 OAS induction and cell growth. (A) GT38 cells were transfected with p38, c-myc, or scrambled (scr) siRNA. At 48 h posttransfection, cells were treated with TPA (20 ng/ml) for 48 h. As the positive control of 2,5 OAS, GT38 cells were treated with IFN (300 U/ml) for 6 h. The expression levels of 2,5 OAS1 and 2,5 OAS2 were determined by RT-PCR. β-tubulin mRNA was amplified from the same cDNA as an internal standard. (B) GT38 cells were plated in 96-well plates and incubated for 24 h and then transfected with siRNA duplexes targeted to c-myc or p38 or scrambled (scr) siRNA. Cell growth was analyzed by using MTT assays.
To confirm whether the RNAi-mediated inhibition of EBV reactivation was not due to siRNA transfection-induced cytotoxicity, cell growth was monitored by an MTT assay (Fig. 6B). TPA treatment did not affect cell growth, and the growth pattern of siRNA-transfected cells was quite similar to that of cells not treated with TPA or treated only with TPA. These results indicated that the suppression of EBV reactivation in the siRNA-transfected cells was not due to the cytotoxicity of p38 and c-myc siRNA in the cells.
DISCUSSION
It has been reported that EBV latency can be disrupted by TPA in the EBV-positive epithelial cell lines GT38 and GT39 and that the effect of TPA was mediated by the PKC- and MAPK-associated pathways (13). In this study, we focused on the roles of p38 MAPK and c-Myc that were suggested to be the downstream signaling molecules of PKC in the TPA-induced EBV reactivation in the GT38 cell line and found that TPA induced phosphorylation of p38 and c-myc activation.
We analyzed whether PKC activates p38 and c-myc during EBV reactivation. To address this question, we treated GT38 cells with the PKC inhibitors H7 and staurosporine. The phosphorylation of p38 and c-myc activation by TPA were abolished by the inhibitors. Furthermore, GT38 cells were treated with p38 inhibitor SB203580; consequently, both phosphorylation of p38 and expression of ZEBRA were simultaneously inhibited. These findings indicate that p38 and c-myc are downstream effectors of PKC on EBV reactivation and that those pathways may play important roles in regulating the stringency of viral latency.
Previous reports have shown that TPA induces BZLF1 expression by inhibiting inducible nitric oxide synthase expression in GT38 cells (13, 14). TPA-mediated induction of BZLF1 expression was abolished by PKC inhibitors, and TPA downregulated inducible nitric oxide synthase gene expression was reversed by PKC inhibitors (13). In order to extend the observation, in the present study we further examined the effects of the NO donor SNAP in the TPA-mediated EBV reactivation in GT38 cells and found that the phosphorylation of p38 and c-myc activation by TPA were inhibited by SNAP in a dose-dependent manner. This finding suggested that NO first downregulates the phosphorylation of p38 and c-Myc and then leads to the inhibition of BZLF1 expression.
c-myc can be activated by TPA in many kinds of cells including BL cells (8, 23, 28, 35). The normal c-myc allele in BL cells is activated by TPA. Based on these observations, we studied the effects of TPA on c-Myc activation and EBV reactivation and found that c-Myc was activated by TPA in GT38 cells and that c-myc activation is closely associated with BZLF1 and ZEBRA expression. These results suggested that activation of c-Myc may be important for efficient lytic EBV infection. The results that PKC inhibitors inhibited the activation of c-Myc suggested that PKC, as an upstream kinase of EBV reactivation, may directly and/or indirectly activate c-Myc and finally lead to EBV reactivation. SB203580 did not affect the activation and phosphorylation of c-Myc. This indicates that the phosphorylation of p38 and c-myc activation are in independent pathways. To our knowledge, this is the first report that provides direct evidence linking c-Myc activation to EBV reactivation.
Previous reports demonstrated that Zta downregulated c-Myc protein expression (42) and that the c-myc transfectants expressed low levels of BZLF1 protein and of its mRNA (10). These observations seem to contradict our present findings. However, in the report by Fais et al. (10) it was also shown that TPA could reinduce the expression of EBV lytic antigens. It indicated that c-myc transfectants did not completely lose the capacity to initiate the lytic cycle. Although the molecular mechanisms underlying this are not yet clearly understood, these observations reiterated the correlations existing between c-myc expression and regulation of the EBV lytic cycle.
We have demonstrated that siRNAs targeted to p38 and c-myc blocked TPA-mediated EBV reactivation in transfected GT38 cells. The p38 and c-myc siRNAs specifically inhibited expression of their respective proteins, whereas the scrambled siRNA, which has no significant homology with the targeting gene sequences, affected neither p38 nor c-Myc protein levels. ZEBRA and EA inductions were significantly inhibited by p38 and c-myc siRNAs but not by an irrelevant scrambled siRNA. siRNA designed against p38 had no effect on c-Myc expression; siRNA targeted against c-myc also did not affect the phosphorylation of p38 (data not shown). These results further confirmed that the TPA-induced phosphorylation of p38 and activation of c-myc belong to independent transduction pathways.
The percentages of EA-positive cells were also significantly reduced by p38 or c-myc siRNAs. To rule out the possibility that the inhibition may be due to the cytotoxicity of siRNA transfection, we showed by using an MTT assay that both p38 and c-myc siRNAs inhibited EA induction in a cytotoxicity-independent manner. This finding indicated that the inhibiting effects of siRNAs are not the result of cytotoxicity.
Recently, it has been reported that transfection of siRNAs probably cause IFN-mediated activation of the Jak-STAT pathway, and this effect is mediated by the dsRNA-dependent protein kinase PKR and 2,5 OAS, which are activated by siRNAs and are required for upregulation of IFN-β in response to siRNA (45). To further clarify this question, transcripts of the IFN-inducible 2,5 OAS gene were examined by RT-PCR in siRNA-transfected GT38 cells. Consequently, no induction of 2,5 OAS gene was found in cells transfected with p38 or c-myc siRNAs. These results ruled out the possibility of an IFN response in the downregulating effects of p38, c-myc, and ZEBRA by siRNA transfection. Thus, our results showed that RNAi could be used to study the ability of host genes to regulate EBV reactivation in this system. Recently, many viruses have been shown to be susceptible to inhibition by RNAi, suggesting that RNAi may play an adaptive antiviral role in controlling these viral infections (2, 19, 34, 38). In addition, some viruses have evolved mechanisms to evade RNAi. For example, flock house virus induces RNAi in Drosophila cells, yet the flock house virus B2 protein suppresses this antiviral effect (31), reflecting an attempt to escape from this endogenous antiviral host response.
In conclusion, our study demonstrates that p38 and c-myc play critical roles in TPA-induced EBV reactivation in EBV-positive epithelial GT38 cells and that RNAi provides an antiviral response in EBV-infected cells. Using the efficient RNAi method, we can identify essential genes involved in EBV reactivation and suppress EBV reactivation and replication in infected cells. RNAi may potentially represent a powerful therapy for EBV-associated disorders such as BL, nasopharyngeal carcinoma, gastric carcinoma, etc.
Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research and by Special Coordination Funds for Promoting Science and Technology (Scientific Research, grant C 13670298; Exploratory Research, grant 12877050; and Carcinogenesis, grant C 13214066) in Priority Areas both from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and from the Second-Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health and Welfare of Japan.
We thank Erik Flemington and Gary Chui for critical reading of the manuscript.
REFERENCES
- 1.Adamson, A. L., D. Darr, E. Holley-Guthrie, R. A. Johnson, A. Mauser, J. Swenson, and S. Kenney. 2000. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J. Virol. 74:1224-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adelman, Z. N., I. Sanchez-Vargas, E. A. Travanty, J. O. Carlson, B. J. Beaty, C. D. Blair, and K. E. Olson. 2002. RNA silencing of dengue virus type 2 replication in transformed C6/36 mosquito cells transcribing an inverted-repeat RNA derived from the virus genome. J. Virol. 76:12925-12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfieri, C., M. Birkenbach, and E. Kieff. 1991. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology 181:595-608. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein, E., A. A. Caudy, S. M. Hammond, and G. J. Hannon. 2001. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409:363-366. [DOI] [PubMed] [Google Scholar]
- 5.Caplen, N. J., S. Parrish, F. Imani, A. Fire, and R. A. Morgan. 2001. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc. Natl. Acad. Sci. USA 98:9742-9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaouchi, N., L. Arvanitakis, M. T. Auffredou, D. A. Blanchard, A. Vazquez, and S. Sharma. 1995. Characterization of transforming growth factor-beta 1 induced apoptosis in normal human B cells and lymphoma B cell lines. Oncogene 11:1615-1622. [PubMed] [Google Scholar]
- 7.Countryman, J., and G. Miller. 1985. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc. Natl. Acad. Sci. USA 82:4085-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eick, D., and G. W. Bornkamm. 1989. Expression of normal and translocated c-myc alleles in Burkitt's lymphoma cells: evidence for different regulation. EMBO J. 8:1965-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 10.Fais, F., G. Cutrona, M. Ulivi, S. Roncella, M. C. Gagliardi, P. Cornaglia-Ferraris, M. Rowe, V. Barnaba, and M. Ferrarini. 1996. Lymphoblastoid cells transfected with c-myc: downregulation of EBV-lytic antigens and impaired response of autologous CD4+ T cells in vitro. Int. J. Cancer. 68:810-816. [DOI] [PubMed] [Google Scholar]
- 11.Fire, A., S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver, and C. C. Mello. 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806-811. [DOI] [PubMed] [Google Scholar]
- 12.Flemington, E., and S. H. Speck. 1990. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J. Virol. 64:1227-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao, X., K. Ikuta, M. Tajima, and T. Sairenji. 2001. 12-O-tetradecanoylphorbol-13-acetate induces Epstein-Barr virus reactivation via NF-κB and AP-1 as regulated by protein kinase C and mitogen-activated protein kinase. Virology 286:91-99. [DOI] [PubMed] [Google Scholar]
- 14.Gao, X., M. Tajima, and T. Sairenji. 1999. Nitric oxide down-regulates Epstein-Barr virus reactivation in epithelial cell lines. Virology 258:375-381. [DOI] [PubMed] [Google Scholar]
- 15.Garrington, T. P., and G. L. Johnson. 1999. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 11:211-218. [DOI] [PubMed] [Google Scholar]
- 16.Garrus, J. E., U. K. von Schwedler, O. W. Pornillos, S. G. Morham, K. H. Zavitz, H. E. Wang, D. A. Wettstein, K. M. Stray, M. Cote, R. L. Rich, D. G. Myszka, and W. I. Sundquist. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55-65. [DOI] [PubMed] [Google Scholar]
- 17.Hannon, G. J. 2002. RNA interference. Nature 418:244-251. [DOI] [PubMed] [Google Scholar]
- 18.Hirasawa, K., A. Kim, H. S. Han, J. Han, H. S. Jun, and J. W. Yoon. 2003. Effect of p38 mitogen-activated protein kinase on the replication of encephalomyocarditis virus. J. Virol. 77:5649-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacque, J. M., K. Triques, and M. Stevenson. 2002. Modulation of HIV-1 replication by RNA interference. Nature 418:435-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia, Q., and R. Sun. 2003. Inhibition of gammaherpesvirus replication by RNA interference. J. Virol. 77:3301-3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaiser, C., G. Laux, D. Eick, N. Jochner, G. W. Bornkamm, and B. Kempkes. 1999. The proto-oncogene c-myc is a direct target gene of Epstein-Barr virus nuclear antigen 2. J. Virol. 73:4481-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapadia, S. B., A. Brideau-Andersen, and F. V. Chisari. 2003. Interference of hepatitis C virus RNA replication by short interfering RNAs. Proc. Natl. Acad. Sci. USA 100:2014-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly, K., B. H. Cochran, C. D. Stiles, and P. Leder. 1983. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell 35:603-610. [DOI] [PubMed] [Google Scholar]
- 24.Kennerdell, J. R., and R. W. Carthew. 1998. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell 95:1017-1026. [DOI] [PubMed] [Google Scholar]
- 25.Ketting, R. F., S. E. Fischer, E. Bernstein, T. Sijen, G. J. Hannon, and R. H. Plasterk. 2001. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 15:2654-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight, S. W., and B. L. Bass. 2001. A role for the RNase III enzyme DCR-1 in RNA interference and germ line development in Caenorhabditis elegans. Science 293:2269-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar, A., S. K. Manna, S. Dhawan, and B. B. Aggarwal. 1998. HIV-Tat protein activates c-Jun N-terminal kinase and activator protein-1. J. Immunol. 161:776-781. [PubMed] [Google Scholar]
- 28.Larsson, L. G., H. E. Gray, T. Totterman, U. Pettersson, and K. Nilsson. 1987. Drastically increased expression of MYC and FOS protooncogenes during in vitro differentiation of chronic lymphocytic leukemia cells. Proc. Natl. Acad. Sci. USA 84:223-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, N. S., T. Dohjima, G. Bauer, H. Li, M. J. Li, A. Ehsani, P. Salvaterra, and J. Rossi. 2002. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat. Biotechnol. 20:500-505. [DOI] [PubMed] [Google Scholar]
- 30.Li, C. J., Y. Ueda, B. Shi, L. Borodyansky, L. Huang, Y. Z. Li, and A. B. Pardee. 1997. Tat protein induces self-perpetuating permissivity for productive HIV-1 infection. Proc. Natl. Acad. Sci. USA 94:8116-8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, H., W. X. Li, and S. W. Ding. 2002. Induction and suppression of RNA silencing by an animal virus. Science 296:1319-1321. [DOI] [PubMed] [Google Scholar]
- 32.Li, Q. X., L. S. Young, G. Niedobitek, C. W. Dawson, M. Birkenbach, F. Wang, and A. B. Rickinson. 1992. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature 356:347-350. [DOI] [PubMed] [Google Scholar]
- 33.Lin, C. T., W. Y. Chan, W. Chen, H. M. Huang, H. C. Wu, M. M. Hsu, S. M. Chuang, and C. C. Wang. 1993. Characterization of seven newly established nasopharyngeal carcinoma cell lines. Lab. Investig. 68:716-727. [PubMed] [Google Scholar]
- 34.Lindenbach, B. D., and C. M. Rice. 2002. RNAi targeted an animal virus: news from the front. Mol. Cell 9:925-927. [DOI] [PubMed] [Google Scholar]
- 35.Lindsten, T., C. H. June, and C. B. Thompson. 1988. Multiple mechanisms regulate c-myc gene expression during normal T cell activation. EMBO J. 7:2787-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nesbit, C. E., J. M. Tersak, and E. V. Prochownik. 1999. MYC oncogenes and human neoplastic disease. Oncogene 18:3004-3016. [DOI] [PubMed] [Google Scholar]
- 37.Nishizuka, Y. 1984. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 308:693-698. [DOI] [PubMed] [Google Scholar]
- 38.Novina, C. D., M. F. Murray, D. M. Dykxhoorn, P. J. Beresford, J. Riess, S. K. Lee, R. G. Collman, J. Lieberman, P. Shankar, and P. A. Sharp. 2002. siRNA-directed inhibition of HIV-1 infection. Nat. Med. 8:681-686. [DOI] [PubMed] [Google Scholar]
- 39.Pelengaris, S., and M. Khan. 2003. The many faces of c-MYC. Arch. Biochem. Biophys. 416:129-136. [DOI] [PubMed] [Google Scholar]
- 40.Polack, A., K. Hortnagel, A. Pajic, B. Christoph, B. Baier, M. Falk, J. Mautner, C. Geltinger, G. W. Bornkamm, and B. Kempkes. 1996. c-myc activation renders proliferation of Epstein-Barr virus (EBV)-transformed cells independent of EBV nuclear antigen 2 and latent membrane protein 1. Proc. Natl. Acad. Sci. USA 93:10411-10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 42.Rodriguez, A., E. J. Jung, Q. Yin, C. Cayrol, and E. K. Flemington. 2001. Role of c-myc regulation in Zta-mediated induction of the cyclin-dependent kinase inhibitors p21 and p27 and cell growth arrest. Virology 284:159-169. [DOI] [PubMed] [Google Scholar]
- 43.Satoh, T., Y. Hoshikawa, Y. Satoh, T. Kurata, and T. Sairenji. 1998. The interaction of mitogen-activated protein kinases to Epstein-Barr virus activation in Akata cells. Virus Genes 18:57-64. [DOI] [PubMed] [Google Scholar]
- 44.Shibata, D., and L. M. Weiss. 1992. Epstein-Barr virus-associated gastric adenocarcinoma. Am. J. Pathol. 140:769-774. [PMC free article] [PubMed] [Google Scholar]
- 45.Sledz, C. A., M. Holko, M. J. de Veer, R. H. Silverman, and B. R. Williams. 2003. Activation of the interferon system by short-interfering RNAs. Nat. Cell Biol. 5:834-839. [DOI] [PubMed] [Google Scholar]
- 46.Smith, C. C., J. Nelson, L. Aurelian, M. Gober, and B. B. Goswami. 2000. Ras-GAP binding and phosphorylation by herpes simplex virus type 2 RR1 PK (ICP10) and activation of the Ras/MEK/MAPK mitogenic pathway are required for timely onset of virus growth. J. Virol. 74:10417-10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 48.Tabara, H., E. Yigit, H. Siomi, and C. C. Mello. 2002. The dsRNA binding protein RDE-4 interacts with RDE-1, DCR-1, and a DExH-box helicase to direct RNAi in C. elegans. Cell 109:861-871. [DOI] [PubMed] [Google Scholar]
- 49.Tajima, M., M. Komuro, and K. Okinaga. 1998. Establishment of Epstein-Barr virus-positive human gastric epithelial cell lines. Jpn. J. Cancer Res. 89:262-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takada, K., N. Shimizu, S. Sakuma, and Y. Ono. 1986. trans Activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J. Virol. 57:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tijsteran, M., R. F. Ketting, and R. H. Plasterk. 2002. The genetics of RNA silencing. Annu. Rev. Genet. 36:489-519. [DOI] [PubMed] [Google Scholar]
- 52.Vaucheret, H., C. Beclin, T. Elmayan, F. Feuerbach, C. Godon, J. B. Morel, P. Mourrain, J. C. Palauqui, and S. Vernhettes. 1998. Transgene-induced gene silencing in plants. Plant J. 16:651-659. [DOI] [PubMed] [Google Scholar]
- 53.Wianny, F., and M. Zernicka-Goetz. 2000. Specific interference with gene function by double-stranded RNA in early mouse development. Nat. Cell Biol. 2:70-75. [DOI] [PubMed] [Google Scholar]
- 54.Zachos, G., B. Clements, and J. Conner. 1999. Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J. Biol. Chem. 274:5097-5103. [DOI] [PubMed] [Google Scholar]
- 55.Zamore, P. D., T. Tuschl, P. A. Sharp, and D. P. Bartel. 2000. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 101:25-33. [DOI] [PubMed] [Google Scholar]
- 56.zur Hausen, H., F. J. O'Neill, U. K. Freese, and E. Hecker. 1978. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature 272:373-375. [DOI] [PubMed] [Google Scholar]
- 57.zur Hausen, H., H. Schulte-Holthausen, G. Klein, W. Henle, G. Henle, P. Clifford, and L. Santesson. 1970. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature 228:1056-1058. [DOI] [PubMed] [Google Scholar]