Abstract
A unique opportunity for the study of the role of serial passage and cross-species transmission was offered by a series of experiments carried out at the Tulane National Primate Research Center in 1990. To develop an animal model for leprosy, three black mangabeys (BkMs) (Lophocebus aterrimus) were inoculated with lepromatous tissue that had been serially passaged in four sooty mangabeys (SMs) (Cercocebus atys). All three BkMs became infected with simian immunodeficiency virus from SMs (SIVsm) by day 30 postinoculation (p.i.) with lepromatous tissue. One (BkMG140) died 2 years p.i. from causes unrelated to SIV, one (BkMG139) survived for 10 years, whereas the third (BkMG138) was euthanized with AIDS after 5 years. Histopathology revealed a high number of giant cells in tissues from BkMG138, but no SIV-related lesions were found in the remaining two BkMs. Four-color immunofluorescence revealed high levels of SIVsm associated with both giant cells and T lymphocytes in BkMG138 and no detectable SIV in the remaining two. Serum viral load (VL) showed a significant increase (>1 log) during the late stage of the disease in BkMG138, as opposed to a continuous decline in VL in the remaining two BkMs. With the progression to AIDS, neopterin levels increased in BkMG138. This study took on new significance when phylogenetic analysis unexpectedly showed that all four serially inoculated SMs were infected with different SIVsm lineages prior to the beginning of the experiment. Furthermore, the strain infecting the BkMs originated from the last SM in the series. Therefore, the virus infecting BkMs has not been serially passaged. In conclusion, we present the first compelling evidence that direct cross-species transmission of SIV may induce AIDS in heterologous African nonhuman primate (NHP) species. The results showed that cross-species-transmitted SIVsm was well controlled in two of three BkMs for 2 and 10 years, respectively. Finally, this case of AIDS in an African monkey suggests that the dogma of SIV nonpathogenicity in African NHP hosts should be reconsidered.
Cross-species transmission of lentiviruses from nonhuman primates (NHPs) to humans is the root source of the AIDS pandemic which has become a major public health problem worldwide (http://www.unaids.org/). Major research efforts with the aim to control the disease have led to an understanding of human immunodeficiency virus (HIV) epidemiology and pathogenesis. It is presently acknowledged that cross-species transmissions to humans of simian immunodeficiency virus (SIV) from chimpanzees (SIVcpz) in central Africa and of SIV from sooty mangabeys (SIVsm) in West Africa generated HIV types 1 and 2 (HIV-1 and -2), respectively (13, 16, 26). Although progress made in the study of in vivo HIV pathogenesis has led to concepts allowing partial control of the infection, the mechanisms of HIV emergence in the human population and the phenomena associated with lentivirus adaptation to new hosts following cross-species transmission are still poorly understood.
This is not a theoretical debate, since studies in Africa have revealed the existence of a plethora of SIVs infecting more than 35 NHP hosts and whose prevalence is 50 to 75% in adult wild monkeys (2, 14, 37, 69). These viruses have a high propensity for cross-species transmission to other NHPs (9, 35, 51, 73, 75) or to humans (13, 26). However, humans in central and West Africa have been exposed to these viruses for centuries, but HIV emerged only in the second half of the 20th century (40). Therefore, assessment of the true risk of human exposure to SIV-infected NHPs for the emergence of new HIVs needs to be carefully evaluated. Such assessments are hampered by a lack of understanding of discrete mechanisms of viral adaptation to the new host after cross-species transmission.
When SIVs are transmitted to new NHP hosts, the outcome of the infection varies widely. In the wild, cross-species transmissions of SIVagm to the yellow baboon (Papio cynocephalus), chacma baboons (Papio ursinus), and patas monkeys (Erythrocebus patas) have been reported to be nonpathogenic for these heterologous species (9, 35, 75). However, in each case, the follow-up was too short for proper evaluation of these cross-species transmissions.
In other instances, cross-species transmission to other NHP species may result in increased pathogenicity and induction of immunosuppression. SIVsm has been reported to induce AIDS in rhesus macaques (42, 48, 51); together with SIVagm, SIVlhoest, SIVsun, and SIVagm, this virus also induces AIDS in pig-tailed macaques (32, 33, 48; V. M. Hirsch, Abstr. Keystone Symp. Twenty Years of HIV Research: from Discovery to Understanding, 2003). However, preliminary studies suggested that SIVmnd-2 from mandrills and SIVrcm from red-capped mangabeys were minimally pathogenic when transmitted to rhesus or cynomolgous macaques (28, 68, 71). Pig-tailed macaques have not yet been infected with either of these two viruses.
HIV-1 generated the unprecedented AIDS pandemic that is characterized by severe immunosuppression. HIV-2 also generates immunosuppressive disease in its new human host. In their natural hosts, both SIVcpz and SIVsm appear to be nonpathogenic in most instances (1, 11, 44, 61, 63, 67). Extensive studies of the emergence of HIV and its simian sources revealed, however, that different cross-species transmissions have had extremely unbalanced outcomes. At least three cross-species transmissions of SIVcpz from chimpanzees to humans occurred during the last century, resulting in the emergence of different HIV-1 groups, namely, M (major), O (outlier), and N (non-M, non-O). However, only group M caused a pandemic, whereas group O is responsible for a minority of cases in Cameroon (3) and group N was detected in only six individuals, also in Cameroon (62). The same is true for HIV-2: of seven known HIV-2 lineages, only A and B are epidemic (13, 18, 27). In contrast, groups C to G (13, 27, 79) are nonepidemic strains that are weakly pathogenic, replicate poorly in infected humans, and are found only within the range of sooty mangabeys (SMs) or in persons who emigrated from western Africa (14, 27).
In summary, the field of SIV adaptation to new hosts is poorly explored and understood. Moreover, not only are the mechanisms of SIV cross-species transmission unknown, but the findings are rife with contradictions.
The opportunity to study the role of serial passage and cross-species transmission was presented by a series of experiments carried out at the Tulane National Primate Research Center (TNPRC) in the 1980s with the aim to develop an animal model for leprosy (30, 78). In February 1990, a group of three black mangabeys (BkMs; Lophocebus aterrimus) received a Mycobacterium leprae suspension by both intradermal (i.d.) and intravenous (i.v.) routes. The M. leprae infection had been serially passaged in four SMs. The leprosy tissue homogenate from the last SM in the series was used to inoculate the three BkMs. Retrospective studies showed that although the BkMs did not develop clinical leprosy, they became infected with SIVsm. The initial importance of the study was dismissed because it was believed that a pathogenic variant of SIVsm had been selected by the four serial passages (J. Heeney, B. Gormus, P. Marx, G. Koopman, P. ten Haaft, and G. Baskin, 17th Annu. Symp. Nonhum. Primate Models AIDS, abstr. 109, 1999). However, as we demonstrate here, the SIVsm transmitted to the BkMs was not a serially passaged virus but came from the last SM in the series. Therefore, our study shows that SIVsm has intrinsic pathogenic potential in BkMs upon cross-species transmission.
We present here the natural history of SIVsm experimental infection of BkMs. We show that inadvertent cross-species transmission of SIVsm resulted in the development of immunosuppression and AIDS in one BkM and in the clearance of SIVsm infection in the remaining two. Virologic, immunologic, and histopathologic characteristics of SIVsm disease in BkMs showed AIDS. Our results show that SIV cross-species transmission into new African hosts may have different clinical outcomes among individuals, suggesting that the selection of a pathogenic SIV in a new species is an unpredictable event.
MATERIALS AND METHODS
Animals.
The three BkMs used in this study were imported from central Africa and housed at the TNPRC in accordance with the Guide for the Care and Use of Laboratory Animals (51a) and the Animal Welfare Act. The protocols and procedures were approved by the TNPRC Institutional Animal Care and Use Committee. The BkMs were adults, between 9 and 10 years old, when inoculated with lepromatous tissue. One BkM was male (BkMG139), while the remaining two were females (BkMG138 and BkMG140); each weighed 8 to 10 kg. All three BkMs were negative for SIV when included in the protocol, as shown by both Western blot (WB) and PCR analyses. All three were simian T-lymphotropic virus antibody negative prior to inoculation, as demonstrated by enzyme-linked immunosorbent assay. All three BkMs were clinically healthy at the time of inoculation. None of the three BkMs was assigned to any other project following the M. leprae study.
The source animal for the leprosy experiments carried out from 1980 to 1990 at the TNPRC (30) was SMA015, a mangabey diagnosed as naturally infected with M. leprae in 1979 that was SIVsm seronegative. Subsequently, inoculated SMs were used as a source of M. leprae for new SMs (SMA015→SMA022→SMD177→SMF102→SMG930) and rhesus macaques. SMA015 was brought to the TNPRC colony from the New Iberia Research Center (NIRC), New Iberia, La.
Inoculations.
Monkeys were inoculated with M. leprae by combined i.d. and i.v. routes. Inoculation was done in February 1990. The inoculum for the BkMs was a saline suspension of a leproma from SMG930. Nonulcerated dermal lepromatous nodules were aseptically collected into cold phosphate-buffered saline. Tissues were cut into small pieces and, after fat removal, homogenized in a Dounce homogenizer, as previously described (30). The homogenate was passed through sterile gauze and centrifuged at 500 × g for 5 min at 4°C. Acid-fast bacilli (AFB) in the supernatant were counted, and morphological indices were determined as described previously (30). The final AFB suspension contained 5.9 × 107 AFB/ml with a mean index of 10%. Each BkM was inoculated i.d. with 3.5 ml distributed over nine sites and with 6.5 ml i.v. via the saphenous vein.
Specimen collection.
The end point of this experiment was the development of clinical leprosy; therefore, sampling was designed to achieve this aim. Serum samples were collected at day 30 postinoculation (p.i.), every 15 days during the first 120 days p.i., every 3 months during the first 2 years p.i., and then twice yearly to year 5 p.i. The last sample was obtained from BkMG139 10 years p.i. at necropsy. The animals were anesthetized with 10 mg of ketamine HCl/ml. Seven to ten milliliters of whole blood was collected from each monkey with no anticoagulant. Serum aliquots were stored at −70°C prior to their use for reverse transcription-PCR and viral load (VL) testing.
Anti-SIVsm antibody detection.
Antibody responses to SIVsm were monitored by an SIV WB assay (ZeptoMetrix Corporation, Buffalo, N.Y.), according to the manufacturer's instructions.
Dynamic evaluation of SIVsm VL.
Due to the nature of the available samples, VL was measured with serum that had been stored at −70°C and thawed only once before testing. Quantification was done by a branched-DNA (bDNA) assay (SIVmac RNA bDNA assay; Bayer Diagnostics, Berkeley, Calif.). This method uses overlapping probes covering the entire pol region of three consensus lineages of viruses from macaques (based on SIVmac239, SIVmac32H, SIVmac251, SIVmacRESIVMXX, SIVmac1A11AA, SIVmac142, SIVmne, and SIVstm clone 37.16) and SMs (based on SIVsmM7, SIVsmH4; SIVsmH9, SIVsmPBj14, clones 4.41 and 1.5; SIVsmPBj6, clone 6; SIVsmPGm, clone PGm5.3; SIVsmF236). There are 101 sites for these probes. For those sites having a significant amount of degeneracy in the consensus, two probes (one SIVmac and one SIVsm) are used for the same site. In total, there are 152 probes in this assay (Charlene Wong, personal communication). Parallel testing of serum and plasma from SHIV89.6P-infected rhesus macaques was done to compare the reliabilities of VL measurements of sera.
Neopterin quantification.
Neopterin is a soluble marker of immune activation that was reported to have predictive value for the progression to AIDS in HIV-infected patients (22). Serum neopterin levels were measured by a quantitative competitive enzyme-linked immunosorbent assay (ELItest; Brahms Diagnostica, Berlin, Germany, supplied by ALPCO Diagnostics, Windham, N.H.), according to the manufacturer's instructions. The minimum detection level was 2 nM.
Flow cytometry.
To quantify CD4+-, CD8+-, and CD20+-lymphocyte subsets, samples from BkMs were analyzed by flow cytometry using fresh samples collected at selected time points between days 0 and 850 p.i. A whole-blood staining procedure was used for flow cytometric analysis to quantify the CD4+ (OKT4; Ortho Diagnostics)-, CD8+ (Leu-2a; Becton Dickinson)-, CD2+ (T-11; Coulter)-, and CD20+ (B-1; Coulter)-lymphocyte subsets. Cells were stained with directly conjugated monoclonal antibodies for 30 min at room temperature. Erythrocytes were lysed and leukocytes were fixed by use of the Q-Prep or Multi Q-Prep instruments and reagents (Coulter). Data were acquired and analyzed with an EPICS 541 flow cytometer and software (Coulter).
Necropsy.
All BkMs either died or were euthanized, and a detailed AIDS necropsy was performed in each case, including the archiving of frozen tissues in optimum-cutting-temperature media, to evaluate the state of the disease. The tissues collected were also fixed in 10% buffered formalin for 48 h, embedded in paraffin, and stained by the conventional hematoxylin-eosin technique. Complete histopathology evaluations were performed with all BkMs. To identify collagen deposition in lymph nodes (LNs), a modified trichrome stain was used, as described previously (41).
Immunohistochemistry.
An immunohistochemical determination for the SIV antigen was performed with formalin-fixed, paraffin-embedded material from all three animals by use of an avidin-biotin complex enzyme technique and a p27 antibody (Mardex Diagnostics, Carlsbad, Calif.).
To determine the phenotype of the SIV-infected cells, double- and triple- immunofluorescence staining techniques were used. Briefly, a 1-h incubation either with macrophage (HAM56; DAKO Corporation, Carpinteria, Calif.) and anti-SIV (p27) or with T cells (CD3; DAKO Corporation), macrophage (HAM56), and anti-SIV (p27) primary antibody was performed, followed by incubation with secondary antibodies conjugated with Alexa fluorochromes 488, 568, and 633. The nuclei were stained with ToPro-3 for the double-staining technique and BoPro-1 for the triple-staining technique, and sections were mounted with antiquenching solution (Sigma, St. Louis, Mo.). Corresponding negative control sections were stained with irrelevant isotype antibodies. Also, LNs from a SIVsm-negative SM and a noninfected macaque were used as negative controls for the SIV staining (data not shown).
The relative proportion between CD4+ and CD8+ T cells was measured with a double-immunofluorescence technique that combined CD3 (Pan T cell; DAKO Corporation) and CD8 (clone 1A5; Novocastra, Newcastle-upon-Tyne, United Kingdom) antibodies.
Slides were examined with a Leica (Wetzlar, Germany) True confocal laser-scanning microscope equipped with three lasers that span from the visible to the far-red side of the spectrum. Differential interference contrast for the observation of nonstained specimens during fluorescent confocal image collection was used.
Virus characterization.
SIVsm strains infecting the three BkMs and the SMs involved in the serial passage of M. leprae were sequenced. Viral RNA extractions were done with 280 μl of serum kept at −70°C. Nested reverse transcription-PCRs were performed to amplify fragments from the gag (793 bp in the p24-encoding region), pol (602 bp in the integrase-encoding region), and env (438 bp in the gp36 gene) genes, as previously described (13, 17, 27).
PCR products were purified using a QIAquick gel extraction kit or a PCR purification kit (QIAGEN, Valencia, Calif.) and sequenced by direct sequencing and dye terminator methodologies (ABI PRISM Big Dye terminator cycle sequencing ready reaction kit with AmpliTaq FS DNA polymerase [Applied Biosystems, Foster City, Calif.]) on an automated sequencer (ABI 373, stretch model; Applied Biosystems).
Phylogenetic analysis.
The gag, pol, and env nucleotide sequence alignments for SIVsmmB670, SIVsmmF236, and SIVsmSL92b were obtained from the Los Alamos National Laboratory HIV Sequence Database (http://hiv-web.lanl.gov). Newly derived SIVsm sequences were aligned by use of the CLUSTALW (72) profile alignment option. The resulting alignments were adjusted manually where necessary. Regions of ambiguous alignment and all gap-containing sites were excluded. Pairwise matrixes based on resulting sequence alignements were generated with the DNADIST program of the PHYLIP package version 3.56 (23). Tree topology was inferred by neighbor joining (64) with the Kimura two-parameter distance matrix (PHYLIP) and a transition/transversion ratio of 2. Bootstrap analysis was performed with the SEQBOOT (1,000 resamplings), DNADIST, NEIGHBOR, and CONSENSE programs (PHYLIP package). Phylogenetic analysis was also performed by the maximum-likelihood method, using the DNAML program (23) (data not shown). The tree outlier was SIVsmSL92b (not shown in trees) (12, 14).
The ratio of synonymous to nonsynonymous substitutions (ds/dn) were calculated using SNAP (Synonymous/Non-synonymous Analysis Program) (25) from the Los Alamos HIV Database (http://hiv-web.lanl.gov). SNAP calculates synonymous and nonsynonymous substitution rates based on a set of codon-aligned nucleotide sequences, which is based on a method described by Nei and Gojobori (52) that incorporates a statistic developed by Ota and Nei (57).
Nucleotide sequence accession numbers.
The nucleotide sequences of the gag, pol, and env sequences from SIVsm infecting SMs and BkMs were deposited in GenBank under accession numbers AY674001 to AY674049.
RESULTS
Unintentional infection of BkMs with SIVsm by experimental exposure to M. leprae.
SIVsm infection of BkMs at the TNPRC resulted after experimental exposure to M. leprae nonulcerated lepromatous nodules by combined i.d. and i.v. routes.
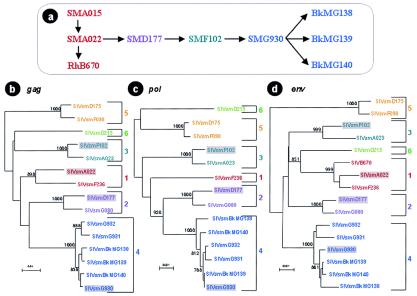
The M. leprae inoculum administered to BKMs had been serially passaged as follows: SMA015→SMA022→SMD177→SMF102→SMG930→BkMG138, BkMG139, and BkMG140 (Fig. 1a).
FIG. 1.
(a) Diagram of the serial passage of leprosy lesions between SMs and BkMs. (b to d) Phylogenetic relationships between SIVsm strains infecting the SMs involved in serial passage of M. leprae and of BkMs inadvertently infected with SIVsm during the leprosy experiments. The analysis of gag (640-bp) (b), pol (588-bp) (c), and env (396-bp) (d) gene fragments revealed that all these viruses cluster in different SIVsm lineages and that BkMs were infected with the virus strain naturally infecting SMG930, the final SM in the series. Trees were constructed using the neighbor-joining method with 1,000 replicates. The bootstrap values at the nodes represent the numbers of replicates. Only significant values (>750) are shown. Each lineage is shown in the same color: lineage 1, red; lineage 2, violet; lineage 3, green; lineage 4, blue.
In 1986 it was observed that such serial leprosy passage experiments efficiently transmitted SIVsm from naturally infected SMs to rhesus macaques, in which serial passages succeeded in adaptation of a pathogenic virus (B670) (6, 7, 51). We have retrospectively shown that this pathogenic SIVsmB670 strain belonged to the same lineage as other SIVsm strains known to be pathogenic in rhesus macaques (SIVsmF236, SIVsmPBj, and SIVsmPGM) or SMs (SIVsmE041) (19, 31, 45, 54). This lineage is presently called lineage 1, according to Ling et al. (44).
In order to investigate whether the development of AIDS in BkMG138 was due to enhancement of virulence from serial passage, SIVsm strains from these four SMs were characterized. Retrospective serological analysis showed that all but one of the SMs (SMA015) in this serial passage were SIVsm infected prior to leprosy or SIVsm exposure. To establish phylogenetic relationships between SIVsm strains infecting these four animals, gag, pol, and env gene fragments were sequenced and characterized. These analyses were done to evaluate the role of SIVsm serial passage in SIVsm pathogenesis in BkMs. Also, different gene fragments were analyzed to investigate the occurrence of recombinant viruses as a result of experimental SIVsm exposure. Five lineages (lineages 1 to 5) of SIVsm have been previously shown to cocirculate in the TNPRC colony, and their degree of diversity is markedly similar to that reported for the different HIV-1 subtypes (44). This high diversity offers a unique opportunity to trace divergent viruses in the colony and the correlates of SIVsm transmission and serial passage.
The analysis of gag (Fig. 1b), pol (Fig. 1c), and env (Fig. 1d) gene fragments of the SIVsm strains infecting SMA022, SMD177, SMF102, and SMG930 revealed that SIVsm from each one clustered in a different lineage: thus, SIVsmA022 clustered with lineage 1 strains, SIVsmD177 clustered with lineage 2 strains, SIVsmF102 clustered with lineage 3 strains, and SIVsmG930 clustered with lineage 4 strains (Fig. 1b to d). Analysis of the phylogenetic clustering across the genome failed to reveal any recombination between the naturally infecting strains and viruses involved in the serial passage.
The epidemiology of SIVsm in this group of SMs also supports the phylogenetic data. SMG930 was transferred to the TNPRC from the NIRC at the same time as SMG932 and SMG931. Our retrospective analysis showed that all three animals were seropositive upon arrival at the TNPRC. Sequence analyses showed that these three SMs from the NIRC and the three BkMs had SIVsm strains belonging to the same lineage 4 (Fig. 1b to d). Our sequence characterization of SIVsm diversity in the TNPRC, as well as in the Yerkes National Primate Research Center, failed to reveal any other lineage 4 viruses. The most probable origin of lineage 4 viruses was one of the infected SMs in the NIRC colony. All three SMs brought to the TNPRC from the NIRC (SMG930, SMG931, and SMG932) had served as controls in a kuru experiment (J. Hardcastle, personal communication), which may explain the close relationship between these three SIVsm strains.
In conclusion, virus characterization showed that all the monkeys involved in the serial passage of M. leprae used to infect the BkMs were already SIVsm infected prior to M. leprae inoculation and that the virus inoculum used to infect BkMs was not adapted through serial passages in SMs during leprosy experiments. Thus, the development of AIDS in this heterologous species reflects an intrinsic pathogenic potential of lineage 4 of SIVsm.
Clinical and serological data.
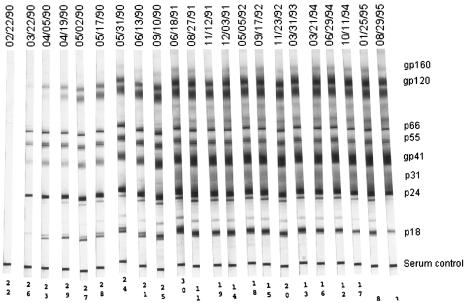
None of the BkMs showed clinical signs of leprosy. Instead, all three became SIVsm infected and seroconverted by day 30 postinfection. Figure 2 shows the evolution of WB profiles of SIVsm infection in BkMG138. The seroconversion pattern of early samples is suggested by the low intensity of pol bands with intense p24 and gp160 reactivities. Anti-gp105 antibodies were not detected in the sample collected at day 30 p.i. The full serological profile was observed from day 90 p.i. on. During the evolution, there was no loss of WB antibodies for BkMG138. Similar WB profiles were observed for the remaining two BkMs (data not shown).
FIG. 2.
SIVsm-specific antibody in BkMG138 after inoculation of lepromatous tissues.
BkMG140 died with nephritis by day 835 p.i. A detailed AIDS necropsy was performed and failed to show signs of AIDS or leprosy. BkMG139 was euthanized in December 2000, at the age of 20 years; this animal had no signs of immunodeficiency or leprosy. BkMG138 was euthanized after 5 years 6 months (by day 2000 p.i.) with chronic intermittent diarrhea of approximately 2 years' duration, which responded only transiently to treatment. Forty-eight hours prior to death, the animal presented with hypoproteinemia, dehydration, and anorexia. The animal had significant weight loss (>10%) by the time of death.
Histological examination.
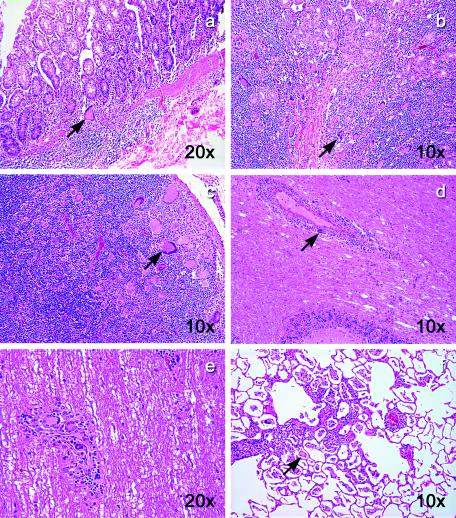
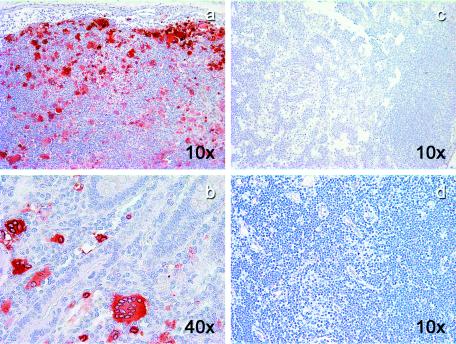
Microscopically, a large number of multinucleated giant cells were observed in multiple tissues from BkMG138. Numerous syncytia were present in the intestine (Fig. 3a and b), LNs (Fig. 3c), brain (Fig. 3d), spinal cord (Fig. 3e), and lung (Fig. 3f), suggesting a diagnosis of SIV-related giant cell disease, which is frequently seen in SIVmac-infected rhesus macaques with terminal AIDS (6, 7) but is a rare finding in natural African NHP hosts of SIV (49).
FIG. 3.
Giant cell disease in BkMG138. Hematoxylin-eosin stain revealed a large number of giant cells in the colon (a), duodenum (b), LNs (c), brain (d), spinal cord (e), and lung (f). The magnifications are shown on each panel.
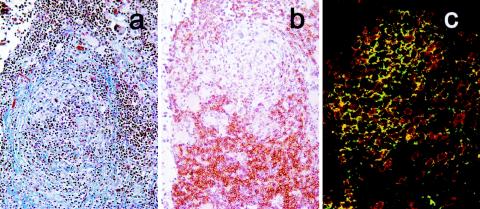
LN architecture was severely disrupted in BkMG138. Atrophic lymphoid follicles (with decreased numbers of follicular center cells and a poorly defined mantle zone) and fibrosis of the LN were obvious (Fig. 4a). Collagen deposition in LN is frequent in AIDS patients and was reported to be correlated with CD4+-T-cell depletion (65; D. Douek, Abstr. 21st Annu. Symp. Nonhum. Primate Models AIDS, abstr. 4, 2003). The majority of the lymphoid follicles were absent, being replaced by large areas of SIVsm-infected cells (Fig. 5a). The majority of the parafollicular lymphoid population was represented by CD8+ T cells, a feature unique to the LN pathology in AIDS patients (60) (Fig. 4b). The same high CD8+-T-cell proportion was observed in the remaining regions of the LNs. The double immunofluorescence for CD3 and CD8 showed that the majority of the lymphocytes in the LNs (Fig. 4c) and intestine (data not shown) were CD8+ T cells and thus confirmed the CD4+-T cell-depletion in the two sites.
FIG. 4.
Pathology of LNs in BkMG138, which developed AIDS. (a) Atrophic lymphoid follicles and fibrosis (modified trichrome stain); collagen is stained in blue. (b) Immunohistochemistry for CD8+ T cells showing that they predominate in parafollicular areas. (c) Confocal microscopy showing indirect proof of paucity of CD4+ T cells. The CD4+-T-cell depletion is indirectly shown by the high proportion of CD3 (red) and CD8 (green) double-positive cells.
FIG. 5.
SIV immunohistochemistry in SIVsm-infected BkMs. Giant cells that appeared in BkMG138 were strongly positive for SIV in both LNs (a) and intestine (b). No evidence of SIV infection could be detected in LNs from BkMG139 (c) and BkMG140 (d).
Immunohistochemistry.
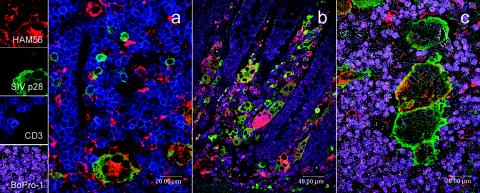
The anti-SIV stain revealed a large number of infected cells in LNs and intestine of BkMG138 (Fig. 4a and b) and none in BkMG139 (Fig. 4c) or BkMG140 (Fig. 4d). All giant cells were positive for SIVsm (Fig. 4a and b). The immunophenotyping done by double- and triple-immunofluorescence stainings determined that the majority of infected cells were macrophages (Fig. 6a to c), but in some areas a large number of infected T cells were also present (Fig. 6a). The in situ hybridization for SIV confirmed the presence of large amounts of SIV RNA in the giant cells (data not shown).
FIG. 6.
Confocal microscopy for triple- and quadruple-fluorescence immunohistochemistry: green (SIV), red (macrophage), blue (T lymphocytes), and magenta (nuclei) stains are evident in BkMG138 diagnosed with giant cell disease. (a) Triple staining of a LN. Large numbers of macrophages and T lymphocytes are infected. (b) Quadruple staining of the intestine from BkMG138. Large numbers of infected cells, mainly macrophages, are visible in the lamina propria. (c) Detail of a quadruple staining of the LN of BkMG138. A large giant cell heavily infected with SIVsm and harboring the macrophage marker HAM56 is visible.
Virological investigations.
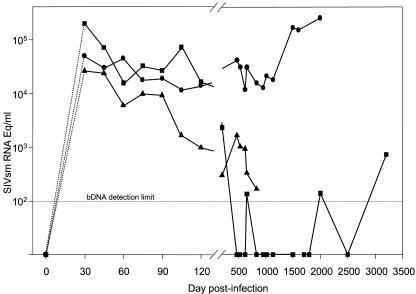
The dynamics of VL was investigated with serum samples. At least 10 specimens sampled at different time points after infection were tested for each BkM. These samples spanned the postacute and chronic phases of infection. The VL dynamics for the three BkMs is presented in Fig. 7. Set point VL values for BkMG138 ranged from 11,759 to 41,848 copies/ml. With the progression to AIDS, a significant increase in VL (>1 log) was observed at late time points. At necropsy, the VL was 253,500 copies/ml. A different picture was observed for the two other BkMs. BkMG139 controlled the VL, which decreased to an undetectable level by day 480 p.i. (Fig. 7). The VL of BkMG139 remained undetectable, with the exception of two insignificant levels of 130 copies/ml. These values are likely to be due to a higher sample background, thus being false positive by bDNA assay. For a sample obtained in November 1998, more than 8 years p.i., the VL was detectable, but at a very low level (700 copies/ml). A similar tendency for the clearance of viral replication was observed for BkMG140, whose VLs decreased below 1,000 copies/ml (Fig. 7). This animal died early p.i. from causes unrelated to AIDS. In order to evaluate the relationship between VL measured with plasma versus values obtained by quantification with sera, we tested plasma in parallel with sera from rhesus macaques experimentally infected with SHIV89.6P. Our results showed lower values of VL when sera were tested. These values were 40 to 80% lower than those obtained when plasma was tested. However, these differences were not statistically significant (data not shown).
FIG. 7.
Dynamics of serum VL in SIVsm-infected BkMs. Due to the sampling schedule, the peak VL could not be measured, and dashed lines covering the interval of days 0 to 30 p.i. are used to reflect this uncertainty. During the follow-up, the VL in BkMG138 (•) showed a significant increase (>1 log) with the progression to AIDS. In contrast, in BkMG139 (▪) and BkMG140 (▴), a tendency to clear viral infection was observed. VL was measured by a bDNA assay (detection limit, 125 eq/ml).
Neopterin testing.
Neopterin is a product of cytokine activity that was reported to have prognostic value for the progression to AIDS in HIV-infected patients. We have tested the dynamics of neopterin levels in the three BkMs in order to study the prognostic value of this marker in African NHPs and to develop a prognostic marker other than the VL. Neopterin levels were remarkably similar between the three BkMs during the initial stages of SIVsm infection, with values of 5.18 ± 1.03 nmol/liter in BkMG138 and 3.51 ± 0.354 and 3.68 ± 0.949 nmol/liter in BkMG139 and BkMG140, respectively. Then, correlated with disease progression, a large increase in neopterin was observed for BkMG138, with a 25-fold increase over the normal levels (103.65 nmol/liter). This increase in neopterin levels was delayed compared to the increase in VL levels (data not shown).
ds/dn ratio.
Evaluation of the ds/dn ratio gives an indication of the type of selection pressure that contributes to the evolution of viral sequences. A majority of synonymous mutations (ds/dn of >1) indicates the predominance of a purifying type of selection, which is associated with a preferential elimination of viruses with variant amino acids. Conversely, a majority of nonsynonymous mutations (ds/dn of <1) reflects the predominance of a diversifying type of selection, such as the pressure from the immune system which selects for viral escape variants.
The ds/dn ratio between sequences obtained from the BkMs at different time points was computed based on the method of Nei and Gojobori (52), incorporating a statistic developed by Ota and Nei (57). Time points selected for these analyses included the first available sample from each BkM (day 30 p.i.) and the last available sample (collected in 1995 for BkMG138, 1998 for BkMG139, and 1992 for BkMG140). We have also calculated the ds/dn ratios for the source SM (SMG930) on samples collected in 1980 and 1996. Analysis of BkM pairs of sequences revealed high ds/dn ratios in gag (18.66, 6.02, and 11.50 for BkMG138, BkMG139, and BkMG140, respectively) and the pol integrase region (16.12, 17.81, and 31.46 for BkMG138, BkMG139, and BkMG140, respectively) and lower ratios in the 436-bp fragment of the gp41 env (3.64, 5.29, and 8.80 for BkMG138, BkMG139, and BkMG140, respectively). No significant differences were observed between BkMG138, whose disease progressed to AIDS, and the remaining two BkMs. Such ds/dn ratios are indicative of a predominant purifying selection for viral fitness, which is usually the case in these regions of the lentivirus genome (4, 38). It was interesting that over a significantly longer period of time (16 years), the ds/dn ratios in the naturally SIVsm-infected SMG930 were significantly lower than those observed in BkMs (4.63 and 4.25 in gag and env, respectively, whereas this calculation was not possible for the pol sequences, since no nonsynonymous substitution was observed in this gene fragment), indicating a strong conservation of amino acid sequences for this virus. Altogether, these calculations showed a significant selective pressure in BkM following infection with SIVsmG930, irrespective of the status of their disease progression. The high ds/dn ratios observed in all three animals were probably due to the intervention of immune effectors in the postacute phase of SIVsmG930 infection.
Dynamics of CD4+ and CD8+ cells in SIVsm-infected BkMs.
Immunophenotyping was available only for selected samples spanning days 0 to 850 p.i. No cells from BkMs were stored, so a retrospective investigation could not be carried out. Thus, the lack of late samples hampered any definitive conclusion regarding the correlations between the onset of AIDS and CD4+ cell counts in BkMG138.
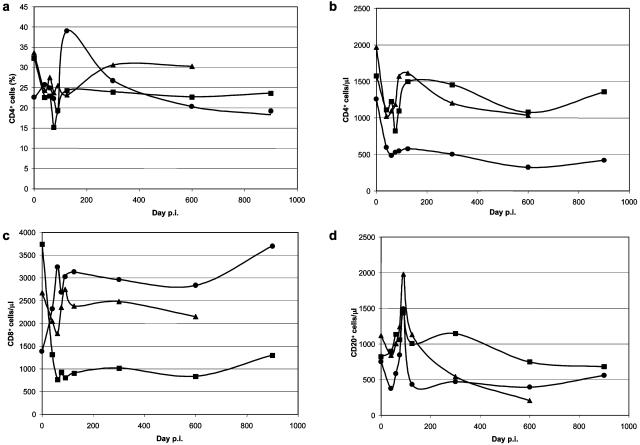
However, the short-term analysis of the immunophenotypic markers showed a significant depletion of CD4+ cells in BkMG138 during the primary infection with SIVsm. This CD4 depletion during the acute phase of infection was observed in all three BkMs, but the evolution of this depletion in BkMG138 was different from that in the remaining two. Thus, CD4+-T-cell depletion did not recover during the follow-up in BkMG138 (Fig. 8a and b). Also, CD8+-T-cell counts were higher in BkMG138 than in BkMG139 and BkMG140 (Fig. 8c). No significant effect of SIVsm infection on the dynamics of CD20 cells was observed (Fig. 8d).
FIG. 8.
Dynamics of peripheral immunophenotypic markers during SIVsm infection in BkMs. (a) Percentages of CD4+ cells; (b) numbers of CD4+ cells per microliter; (c) numbers of CD8+ cells per microliter; (d) numbers of CD20+ cells per microliter.Symbols: •, BkMG138; ▪, BkMG139; ▴, BkMG140.
DISCUSSION
AIDS in an African NHP host following SIV cross-species transmission.
Our study provides the first compelling evidence that direct cross-species transmission of a naturally occurring SIV to another African NHP species has the ability to induce AIDS in the recipient monkey species. The experiments described here resulted from the inadvertent transmission of SIVsm to three BkMs. One of three BkMs developed AIDS after 5 years. The disease was characterized by weight loss, chronic diarrhea, and giant cell disease. Although BkMG138 did not present the classical clinical picture of immunosuppression in macaques characterized by opportunistic infections and lymphomas (6, 47), the immunodeficiency was strongly supported by the clinical presentation. The earliest definition of a clinical case of AIDS in humans (“the Bangui definition”) (http://www.who.int/hiv/strategic/en/bangui1985report.pdf) defined the clinical case of AIDS based on major and minor criteria. The diagnostic of AIDS is established in the presence of one minor and two major signs. According to this definition, BkMG138 presented with AIDS based on two major criteria (weight loss of >10% and chronic diarrhea for >1 month) and one minor criterion (generalized lymphadenopathy). Clinical presentation was corroborated by laboratory investigations showing giant cell disease, a pathological condition which is present only in immunosuppressed macaques (6, 7). Laboratory data, such as the dynamics of VL, LN pathology, and neopterin levels, also supported the diagnosis of AIDS.
Interestingly, in the remaining two animals, no clinical or biological signs of immunosuppression were observed, and moreover, the analysis of the dynamics of VL revealed a tendency for clearing SIV infection, as reported for SIV transmission in other heterologous hosts (15, 28, 36, 68). These VL results were confirmed by immunohistochemistry and in situ hybridization showing high concentrations of SIVsm in the LNs and intestine of BkMG138 and no evidence of SIVsm infection in the same tissues from BkMG139 and BkMG140.
Cases of immunosuppression after experimental transmission of SIVs, HIV-1, and HIV-2 into African NHPs have already been described. Thus, HIV-2 has been reported to induce AIDS in baboons following direct transmission (5) and to display increased pathogenicity following serial passages (46). Also, a subset of chimpanzees infected with HIV-1 was reported to develop AIDS after a mean incubation period of 13 years (56). In both cases, NHPs received human viruses. HIVs are pathogenic viruses of simian origin, which were already adapted into a new host following cross-species transmission. The same is true for SIVsmPBj, which, after adaptation in pig-tailed macaques, was shown to induce disease in its natural SM host (24).
SIVs are pathogenic in natural African NHP hosts of SIVs.
This case of AIDS adds further support to the hypothesis that SIVs have pathogenic potential in African NHPs. Current views are that African NHPs are adapted to SIV and do not develop AIDS. Such reports have been published regarding natural infection of African green monkeys with SIVagm (8, 10, 29, 53), of SMs with SIVsm (11, 24, 39, 61, 67), or of chimpanzees with SIVcpz (63). However, all of these viruses retained their pathogenic potential, as shown by the outcome of the disease following experimental or accidental cross-species transmission (26, 33, 48, 51). Moreover, recent reports have shown that cases of AIDS may occur in African NHPs (45, 49, 58, 74). Since these cases are rare and were documented after a long period of persistent infection, it was proposed that the incubation period of SIV infection generally exceeds the normal life span of monkeys in the wild (58). To explain the lack of pathogenicity of SIVs in African NHP hosts, several studies showing a lack of activation or of bystander pathology associated with SIV infections were carried out (11, 59, 67, 76). Recently, it was also determined that African NHP hosts display significantly lower levels of CD4+ CCR5+ CD45RAneg cells (I. Pandrea et al., submitted for publication), which were shown to be the major target cells of lentiviruses in humans and macaques (77).
The occurrence of AIDS in BkMs should redirect our current interpretation concerning the outcome of SIV infection in African NHP hosts. BkMs were recently shown to carry a specific lentivirus which is highly divergent from SIVsm (S. Saragosti, personal communication), so the mechanisms of adaptation to lentiviral infection should have evolved in this species like they did in other African monkeys. However, after SIVsm cross-species transmission, one of the infected BkMs developed AIDS after an incubation period of 5 years. Also, our study showed that the transmission of SIVsm to a heterologous host species varies widely in pathogenic outcome, since the two other BkMs effectively controlled the infection. The different outcomes of SIVsm infection in the three BkMs may be a result of the viral inoculum being heterogenous, and thus each monkey had received different quasispecies of lineage 4 virus. However, VL testing in the postacute phase of SIVsm infection showed that the viruses replicated to high levels in all three at the initial stages of infection, in the absence of immune memory effectors. Therefore, the ability of the two BkMs that did not develop AIDS to control cross-species-transmitted SIV may be related to restrictions of cross-species transmission or to effective immune control. Sequencing of virus fragments did not reveal significant differences in virus genes in the postacute phase of infection (data not shown).
Serial passage was previously thought to be the major factor behind increased pathogenicity of SIVs following cross-species transmission to a new host. However, this study proved that the virus strain associated with AIDS in BkMG138 had not been serially passaged prior to its administration to the BkMs. It is also noteworthy that this study provides the first evidence that a SIVsm belonging to a lineage other than lineage 1 can be pathogenic in a heterologous species. All the infections of rhesus macaques with viruses naturally infecting SMs involved viruses from lineage 1 (24, 51, 54). Pathogenic infections following experimental transmission to macaques were also observed following infection with SIVmac viruses. However, the source SM virus for this SIVmac lineage is not yet known, and also one should note that viruses in the SIVmac group had been heavily passaged in macaques before the selection of the pathogenic strains SIVmac251 and SIVmac239 (47). The SIVsm strains belonging to lineage 1 seem to display a very high pathogenic potential in heterologous hosts. Thus, SIVsmmPBj and SIVsmmB670 have been shown to be pathogenic in macaques following direct transfer from the naturally infected SMs (24, 51). Even for these pathogenic viruses, serial passage was shown to contribute to an increased pathogenicity (31, 34).
All three SMs brought to the TNPRC from the NIRC (SMG930, SMG931, and SMG932) had served as controls in a kuru experiment (J. Hardcastle, personal communication), which may explain the close relationship between these three SIVsm strains. One may thus question whether it is possible that the SIVsm was serially passaged in these monkeys during kuru experiments. In order to rule out this possibility, we have tested samples from the SMs in the NIRC collected over a 10-year period. In this study, we were able to show SIVsm transmission within the NIRC colony. However, SMG930 was SIVsm infected by the time of the first sample collection (data not shown).
Technical limitations.
As mentioned before, this is a retrospective study examining animals in an experiment originally designed to induce leprosy in BkMs. Therefore, the schedule and the type of sampling were not the most suitable for characterizing the natural history of SIVsm infection in BkMs. However, a major strength of this study is that samples were stored under ideal conditions over years, which permitted robust results. For the evaluation of the comparative dynamics of VL in BkMs, we used sera sampled at the set point of VL (day 30 p.i.) and during the chronic phase of the infection. The sampling schedule did not cover the peak of VL during the primary infection. This would have provided interesting information concerning early SIVsm replication in BkMs. However, the dynamic evaluation of the VL during the postacute and chronic phases of SIVsm infection of BkMs was done for a large number of samples. Also, although plasma VL is the best predictor of disease progression and of the in vivo dynamics (43, 50), it is still a surrogate marker of viral replication, since part of the viral RNA quantified by these methods may represent fragments of virus or nonreplicative forms. Moreover, in human clinical practice, it is estimated that testing VLs based on sera will generate levels of VL representing 30 to 80% of the plasma VL levels, but these differences are not statistically significant (66). A comparison between SHIV89.6P VL levels in serum and plasma showed that serum VLs are 40 to 80% lower than plasma VLs (data not shown). These differences are not statistically significant. Therefore, in order to correctly evaluate the dynamics of viral replication, sera may be used if the following two conditions are fulfilled: (i) the same type of sample is used to test the dynamics of VL, and (ii) only good-quality samples are chosen for testing (stored at −70°C and not thawed prior to testing). The serum samples originating from the BkMs included in this study fulfilled both these criteria.
Quantification was done by bDNA assay. Although this assay was not designed based on sequences of the divergent lineage 4 viruses, we assumed that the quantification by bDNA assay resulted in a correct estimation of VLs in BkMs. The bDNA assay is designed to map the entire polymerase region by the use of a cocktail of overlapping probes. In HIV-1 monitoring, viral diversity may significantly influence the performance of different commercial VL quantification kits. The bDNA assay has a better sensitivity than the gag-based Roche Monitor assay for the quantification of divergent HIV-1 group M viruses, which may differ in up to 20% of the pol sequences (F. Damond, C. Apetrei, D. Descamps, F. Brun-Vezinet, and F. Simon, Letter, AIDS 13:286-288, 1999). This range of genetic distances is similar to those observed between different SIVsm lineages cocirculating in primate centers in the United States (44). Therefore, it is unlikely that the bDNA assay underestimated the VLs in BkMs infected with lineage 4 viruses. As shown above, this assay was designed to quantify SIVmac, SIVsmB670, and SIVstm, three divergent lineages of SIVsm. We have tested the dynamics of VLs in more than 20 SMs infected with SIVsm strains belonging to different lineages and observed no significant difference between lineage 4 viruses and the remaining SIVsm lineages circulating in the TNPRC SM colony (C. Apetrei, unpublished data). When the SIVsmBkMG138 serum VL set point values were compared, they were slightly higher than those observed in chronically infected SMs (data not shown) but lower than those reported in an SIVsm-infected SM whose disease had progressed to AIDS (45).
Analysis of the dynamics of VLs clearly showed differences in natural history of SIVsm-infected BkMs. A significant increase in VL was observed in late specimens in BkMG138, which was in marked contrast to the two other BkMs in which a lack of viral replication was observed by serial testing of VL. Immunohistochemistry confirmed the reliability of VL results, with no evidence of virus in the LNs of BkMG139 and BkMG140 and a high concentration of virus in BkMG138 (Fig. 5).
The analysis of early points revealed that SIVsm infection induced a CD4+ depletion which was not recovered during the chronic stages of the infection. Primary SIV infection in African NHP hosts was reported to induce transient CD4+ depletion during the acute phase, which is rapidly recovered and controlled in African green monkeys and mandrills (20, 55). This finding contrasts with the outcome of lentiviral infection in humans and macaques, in which the CD4+ depletion observed during the acute phase of the infection does not recover to normal levels (77). Interestingly, it was recently reported that in SIVsm-infected SMs, CD4+ levels never recover after the depletion observed during the acute infection (D. Zhou, A. Muthukumar, J. Milush, M. Paiardini, A. Barry, H. McClure, S. Staprans, M. Feinberg, G. Silvestri, and D. Sodora, 21st Annu. Symp. Nonhum. Primate Models AIDS, abstr. 22, 2003). Whether this is a characteristic of SIVsm infection or an aspect that is host dependent remains to be established.
Since our study did not provide direct evidence of CD4 depletion in BkMs with progression to AIDS, we investigated neopterin as a surrogate marker of activation. This marker is a product of cytokine activity and represents the biologic consequence of increased cytokine production and cellular responses (22). Neopterin levels do not correlate closely with CD4 changes, and it was considered that the two parameters represent essential but differing elements of disease pathology (21). One of the main reasons for testing the serum neopterin levels in the three BkMs was that, as previously reported, plasma VLs in African NHP hosts of SIVs do not appear to have a prognostic value. However, in HIV-infected patients, VL and neopterin levels were reported to have similar prognostic values (70). We have demonstrated very large increases in serum neopterin levels with the progression to AIDS in BkMG138. Nevertheless, the increase in neopterin levels occurred after the increase in VL (data not shown). However, our results provided a prognostic marker for the evaluation of disease progression that is useful in nonpathogenic models. Another marker of activation, β2-microglobulin, has been previously shown to increase with the progression to AIDS in a subset of chimpanzees (56).
In conclusion, this study documents the first case of AIDS in an African NHP host of SIV after cross-species transmission. Moreover, the data showed that the outcome of the cross-species-transmitted SIV infection could vary widely. This may eventually explain why only a few cross-species transmission events from NHP to humans were successful, in spite of the commonplace exposure of humans in central Africa to a plethora of SIVs (2). Also, this study provides new evidence of AIDS occurring in an African NHP, which suggest that SIVs are pathogenic in African monkeys. Finally, we developed a strategy for the systematic investigation of SIV infection in archival cases by the use of contemporary tools and alternative samples.
Acknowledgments
This work was supported by funds from grants RO1 AI-19301, RO1 AI-44596, and P51 RR000164 from the National Institutes of Health.
We thank Megan Mefford, Tessa Williams, Meredith Hunter, and Nora Dillon for technical assistance; Calvin Lanclos for flow cytometry analyses; and Johnny Hardcastle for helpful discussion. We also thank the veterinary and animal care staff of the TNPRC for their service and expertise.
REFERENCES
- 1.Ansari, A. A., N. Onlamoon, P. Bostik, A. E. Mayne, L. Gargano, and K. Pattanapanyasat. 2003. Lessons learnt from studies of the immune characterization of naturally SIV infected sooty mangabeys. Front. Biosci. 8:1030-1050. [DOI] [PubMed] [Google Scholar]
- 2.Apetrei, C., D. L. Robertson, and P. A. Marx. 2004. The history of SIVs and AIDS: epidemiology, phylogeny and biology of isolates from naturally infected non-human primates (NHP) in Africa. Front. Biosci. 9:225-254. [DOI] [PubMed] [Google Scholar]
- 3.Ayouba, A., P. Mauclere, P. M. Martin, P. Cunin, J. Mfoupouendoun, B. Njinku, S. Souquieres, and F. Simon. 2001. HIV-1 group O infection in Cameroon, 1986 to 1998. Emerg. Infect. Dis. 7:466-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balfe, P., P. Simmonds, C. A. Ludlam, J. O. Bishop, and A. J. Brown. 1990. Concurrent evolution of human immunodeficiency virus type 1 in patients infected from the same source: rate of sequence change and low frequency of inactivating mutations. J. Virol. 64:6221-6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnet, W. S., M. K. Krishna, B. G. Herndier, and J. A. Levy. 1994. An AIDS-like condition induced in baboons by HIV-2. Science 266:642-646. [DOI] [PubMed] [Google Scholar]
- 6.Baskin, G. B., L. N. Martin, S. R. Rangan, B. J. Gormus, M. Murphey-Corb, R. H. Wolf, and K. F. Soike. 1986. Transmissible lymphoma and simian acquired immunodeficiency syndrome in rhesus monkeys. J. Natl. Cancer Inst. 77:127-139. [PubMed] [Google Scholar]
- 7.Baskin, G. B., M. Murphey-Corb, E. A. Watson, and L. N. Martin. 1988. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV)/Delta. Vet. Pathol. 25:456-467. [DOI] [PubMed] [Google Scholar]
- 8.Beer, B., J. Denner, C. R. Brown, S. Norley, J. zur Megede, C. Coulibaly, R. Plesker, S. Holzammer, M. Baier, V. M. Hirsh, and R. Kurth. 1998. Simian immunodeficiency virus of African green monkeys is apathogenic in the newborn natural host. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 18:210-220. [DOI] [PubMed] [Google Scholar]
- 9.Bibollet-Ruche, F., A. Galat-Luong, G. Cuny, P. Sarni-Manchado, G. Galat, J. P. Durand, X. Pourrut, and F. Veas. 1996. Simian immunodeficiency virus infection in a patas monkey (Erythrocebus patas): evidence for cross-species transmission from African green monkeys (Cercopithecus aethiops sabaeus) in the wild. J. Gen. Virol. 77:773-781. [DOI] [PubMed] [Google Scholar]
- 10.Broussard, S. R., S. I. Staprans, R. White, E. M. Whitehead, M. B. Feinberg, and J. S. Allan. 2001. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J. Virol. 75:2262-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakrabarti, L. A., S. R. Lewin, L. Zhang, A. Gettie, A. Luckay, L. N. Martin, E. Skulsky, D. D. Ho, C. Cheng-Mayer, and P. Marx. 2000. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J. Virol. 74:1209-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakrabarti, L. A., A. Luckay, and P. A. Marx. 2001. A divergent simian immunodeficiency virus from sooty mangabey with an atypical Tat-TAR structure. AIDS Res. Hum. Retrovir. 17:1155-1165. [DOI] [PubMed] [Google Scholar]
- 13.Chen, Z., A. Luckay, D. L. Sodora, P. Telfer, P. Reed, A. Gettie, J. M. Kanu, R. F. Shadek, J. A. Yee, D. D. Ho, L. Zhang, and P. A. Marx. 1997. Human immunodeficiency virus type 2 (HIV-2) seroprevalence and characterization of a distinct HIV-2 genetic subtype from the natural range of simian immunodeficiency virus-infected sooty mangabeys. J. Virol. 71:3953-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, Z., P. Telfier, A. Gettie, P. Reed, L. Zhang, D. D. Ho, and P. A. Marx. 1996. Genetic characterization of new west African simian immunodeficiency virus SIVsm: geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J. Virol. 71:3617-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, Z., P. Telfer, P. Reed, L. Zhang, A. Gettie, D. D. Ho, and P. A. Marx. 1995. Isolation and characterization of the first simian immunodeficiency virus from a feral sooty mangabey (Cercocebus atys) in West Africa. J. Med. Primatol. 24:108-115. [DOI] [PubMed] [Google Scholar]
- 16.Corbet, S., M. C. Muller-Trutwin, P. Versmisse, S. Delarue, A. Ayouba, J. Lewis, S. Brunak, P. Martin, F. Brun-Vézinet, F. Simon, F. Barré-Sinoussi, and P. Mauclere. 2000. env sequences of simian immunodeficiency viruses from chimpanzees in Cameroon are strongly related to those of human immunodeficiency virus group N from the same geographic area. J. Virol. 74:529-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courgnaud, V., X. Pourrut, F. Bibollet-Ruche, E. Mpoudi-Ngole, A. Bourgeois, E. Delaporte, and M. Peeters. 2001. Characterization of a novel simian immunodeficiency virus from guereza colobus monkeys (Colobus guereza) in Cameroon: a new lineage in the nonhuman primate lentivirus family. J. Virol. 75:857-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damond, F., C. Apetrei, D. L. Robertson, S. Souquiere, A. Lepretre, S. Matheron, J. C. Plantier, F. Brun-Vezinet, and F. Simon. 2001. Variability of human immunodeficiency virus type 2 (HIV-2) infection in patients living in France. Virology 280:19-30. [DOI] [PubMed] [Google Scholar]
- 19.Dewhurst, S., J. E. Embretson, D. C. Anderson, J. I. Mullins, and P. N. Fultz. 1990. Sequence analysis and acute pathogenicity of molecularly cloned SIVsmm-PBj14. Nature 345:636-640. [DOI] [PubMed] [Google Scholar]
- 20.Diop, O. M., A. Gueye, M. Dias-Tavares, C. Kornfeld, A. Faye, P. Ave, M. Huerre, S. Corbet, F. Barre-Sinoussi, and M. C. Muller-Trutwin. 2000. High levels of viral replication during primary simian immunodeficiency virus SIVagm infection are rapidly and strong controlled in African green monkeys. J. Virol. 74:7538-7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahey, J. L., J. M. G. Taylor, R. Detels, B. Hofmann, R. Melmed, P. Nishanian, and J. V. Giorgi. 1990. The prognosis value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N. Engl. J. Med. 322:166-172. [DOI] [PubMed] [Google Scholar]
- 22.Fahey, J. L., J. M. G. Taylor, B. Manna, P. Nishanian, N. Aziz, J. V. Giorgi, and R. Detels. 1998. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. AIDS 12:1581-1590. [DOI] [PubMed] [Google Scholar]
- 23.Felsenstein, J. 1993. PHYLIP (phylogeny interference package), version 3.5c ed. Departments of Genetics, University of Washington, Seattle.
- 24.Fultz, P. N., H. M. McClure, D. C. Anderson, and W. M. Switzer. 1989. Identification and biological characterization of an acutely lethal variant of simian immunodeficiency virus from sooty mangabeys (SIV/SMM). AIDS Res. Hum. Retrovir. 5:397-409. [DOI] [PubMed] [Google Scholar]
- 25.Ganeshan, S., R. E. Dickover, B. T. M. Korber, Y. J. Bryson, and S. M. Wolinsky. 1997. Human immunodeficiency virus type 1 genetic evolution in children with different rates of development of disease. J. Virol. 71:663-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao, F., E. Bailes, D. L. Robertson, Y. Chen, C. M. Rodenburg, S. F. Michael, L. B. Cummins, L. O. Arthur, M. Peeters, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1999. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397:436-441. [DOI] [PubMed] [Google Scholar]
- 27.Gao, F., L. Yue, D. L. Robertson, S. C. Hill, H. Hui, R. J. Biggar, A. E. Neequaye, T. M. Whelan, D. D. Ho, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1994. Genetic diversity of human immunodeficiency virus type 2: evidence for distinct sequence subtypes with differences in virus biology. J. Virol. 68:7433-7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Georges-Courbot, M. C., C. Y. Lu, M. Makuwa, P. Telfer, R. Onanga, G. Dubreuil, Z. Chen, S. M. Smith, A. Georges, F. Gao, B. H. Hahn, and P. A. Marx. 1998. Natural infection of a household pet red-capped mangabey (Cercocebus torquatus torquatus) with a new simian immunodeficiency virus. J. Virol. 72:600-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein, S., I. Ourmanov, C. R. Brown, B. E. Beer, W. R. Elkins, R. Plishka, A. Buckler-White, and V. M. Hirsch. 2000. Wide range of viral load in healthy African green monkeys naturally infected with simian immunodeficiency virus. J. Virol. 74:11744-11753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gormus, B. J., K. Xu, G. B. Baskin, L. N. Martin, R. P. Bohm, J. L. Blanchard, P. A. Mack, M. S. Ratterree, M. H. McClure, W. M. Meyers, and G. P. Walsh. 1995. Experimental leprosy in monkeys. I. Sooty mangabey monkeys: transmission, susceptibility, clinical and pathological findings. Lepr. Rev. 66:96-104. [DOI] [PubMed] [Google Scholar]
- 31.Hirsch, V., D. Adger-Johnson, B. Campbell, S. Goldstein, C. Brown, W. R. Elkins, and D. C. Montefiori. 1997. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543-3. J. Virol. 71:1608-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirsch, V. M., B. J. Campbell, E. Bailes, R. Goeken, C. Brown, W. R. Elkins, M. Axthelm, M. Murphey-Corb, and P. M. Sharp. 1999. Characterization of a novel simian immunodeficiency virus (SIV) from L'Hoest monkeys (Cercopithecus l'hoesti): implications for the origin of SIVmnd and other primate lentiviruses. J. Virol. 73:1036-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirsch, V. M., G. Dapolito, P. R. Johnson, W. R. Elkins, W. T. London, R. J. Montali, S. Goldstein, and C. Brown. 1995. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J. Virol. 69:955-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holterman, L., R. Dubbes, J. Mullins, G. Learn, H. Niphuis, W. Koornstra, G. Koopman, E. M. Kuhn, A. Wade-Evans, B. Rosenwirth, J. Haaijman, and J. Heeney. 2001. Characteristics of a pathogenic molecular clone of an end-stage serum-derived variant of simian immunodeficiency virus (SIVF359). J. Virol. 75:9328-9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin, M. J., J. Rogers, J. E. Phillips-Conroy, J. S. Allan, R. C. Desrosiers, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1994. Infection of a yellow baboon with simian immunodeficiency virus from African green monkeys: evidence for cross-species transmission in the wild. J. Virol. 68:8454-8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson, P. R., S. Goldstein, W. T. London, A. Fomsgaard, and V. M. Hirsch. 1990. Molecular clones of SIVsm and SIVagm: experimental infection of macaques and African green monkeys. J. Med. Primatol. 19:279-286. [PubMed] [Google Scholar]
- 37.Jolly, C., J. E. Phillips-Conroy, T. R. Turner, S. Broussard, and J. S. Allan. 1996. SIVagm incidence over two decades in a natural population of Ethiopian grivet monkeys (Cercopithecus aethiops aethiops). J. Med. Primatol. 25:78-83. [DOI] [PubMed] [Google Scholar]
- 38.Kasper, P., P. Simmonds, K. E. Schneweis, R. Kaiser, B. Matz, J. Oldenburg, H. H. Brackmann, and E. C. Holmes. 1995. The genetic diversification of the HIV type 1 gag p17 gene in patients infected from a common source. AIDS Res. Hum. Retrovir. 11:1197-1201. [DOI] [PubMed] [Google Scholar]
- 39.Kaur, A., R. M. Grant, R. E. Means, H. McClure, M. Feinberg, and R. P. Johnson. 1998. Diverse host responses and outcomes following simian immunodeficiency virus SIVmac239 infection in sooty mangabeys and rhesus macaques. J. Virol. 72:9597-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korber, B., M. Muldoon, J. Theiler, F. Gao, R. Gupta, A. Lapedes, B. H. Hahn, S. Wolinsky, and T. Bhattacharya. 2000. Timing the ancestor of the HIV-1 pandemic strains. Science 288:1789-1796. [DOI] [PubMed] [Google Scholar]
- 41.Lamps, L. W., M. P. Bronner, C. L. Vnencak-Jones, K. T. Tham, H. R. Mertz, and M. A. Scott. 1998. Optimal screening and diagnosis of microsporidia in tissue stains. A comparison of polarization, special strains, and molecular techniques. Am. J. Clin. Pathol. 109:404-410. [DOI] [PubMed] [Google Scholar]
- 42.Letvin, N. L., M. D. Daniel, P. K. Sehgal, R. C. Desrosiers, R. D. Hunt, L. M. Waldron, J. J. MacKey, D. K. Schmidt, L. V. Chalifoux, and N. W. King. 1985. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science 230:71-73. [DOI] [PubMed] [Google Scholar]
- 43.Lifson, J. D., M. A. Nowak, S. Goldstein, J. L. Rossio, A. Kinter, G. Vasquez, T. A. Wiltrout, C. Brown, D. Schneider, L. Wahl, A. L. Lloyd, J. Williams, W. R. Elkins, A. S. Fauci, and V. M. Hirsch. 1997. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J. Virol. 71:9508-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ling, B., M. L. Santiago, S. Meleth, B. Gormus, H. M. McClure, C. Apetrei, B. H. Hahn, and P. A. Marx. 2003. Noninvasive detection of new simian immunodeficiency virus lineages in captive sooty mangabeys: ability to amplify virion RNA from fecal samples correlates with viral load in plasma. J. Virol. 77:2214-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ling, B., C. Apetrei, I. Pandrea, R. S. Veazey, A. A. Lackner, B. Gormus, and P. A. Marx. 2004. Classic AIDS in a sooty mangabey after an 18-year natural infection. J. Virol. 78:8902-8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Locher, C. P., S. A. Witt, B. G. Herndier, N. W. Abbey, K. Tenner-Racz, P. Racz, N. B. Kiviat, K. K. Murthy, K. Brasky, M. Leland, and J. A. Levy. 2003. Increased virus replication and virulence after serial passage of human immunodeficiency virus type 2 in baboons. J. Virol. 77:77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mansfield, K. G., N. W. Lerche, M. B. Gardner, and A. A. Lackner. 1995. Origins of simian immunodeficiency virus infection in macaques at the New England Regional Primate Research Center. J. Med. Primatol. 24:116-122. [DOI] [PubMed] [Google Scholar]
- 48.McClure, H. M., D. C. Anderson, P. N. Fultz, A. A. Ansari, E. Lockwood, and A. Brodie. 1989. Spectrum of disease in macaque monkeys chronically infected with SIV/SMM. Vet. Immunol. Immunopathol. 21:13-24. [DOI] [PubMed] [Google Scholar]
- 49.McClure, H. M., D. C. Anderson, T. P. Gordon, A. A. Ansari, P. N. Fultz, S. A. Klumpp, P. Emau, and M. Isahakia. 1992. Natural simian immunodeficiency virus infection in nonhuman primates. Top. Primatol. 3:425-438. [Google Scholar]
- 50.Mellors, J. W., C. R. Rinaldo, P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167-1170. [DOI] [PubMed] [Google Scholar]
- 51.Murphey-Corb, M., L. N. Martin, S. R. Rangan, G. B. Baskin, B. J. Gormus, R. H. Wolf, W. A. Andes, M. West, and R. C. Montelaro. 1986. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature 321:435-437. [DOI] [PubMed] [Google Scholar]
- 51a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 52.Nei, M., and T. Gojobori. 1986. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 3:418-426. [DOI] [PubMed] [Google Scholar]
- 53.Norley, S. G., G. Kraus, J. Ennen, J. Bonilla, H. Konig, and R. Kurth. 1990. Immunological studies of the basis for the apathogenicity of simian immunodeficiency virus from African green monkeys. Proc. Natl. Acad. Sci. USA 87:9067-9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Novembre, F. J., J. De Rosayro, S. P. O'Neil, D. C. Anderson, S. A. Klumpp, and H. M. McClure. 1998. Isolation and characterization of a neuropathogenic simian immunodeficiency virus derived from a sooty mangabey. J. Virol. 72:8841-8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Onanga, R., C. Kornfeld, I. Pandrea, J. Estaquier, S. Souquiere, P. Rouquet, V. Poaty-Mavoungou, O. Bourry, S. M'Boup, F. Barre-Sinoussi, F. Simon, C. Apetrei, P. Roques, and M. C. Muller-Trutwin. 2002. High viral replication contrasts with moderate changes in CD4+ and CD8+ cell numbers during the early phase of SIVmnd-1 experimental infection in Mandrillus sphinx. J. Virol. 76:10256-10263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Neil, S. P., F. J. Novembre, A. B. Hill, C. Suwyn, C. E. Hart, T. Evans-Strickfaden, D. C. Anderson, J. deRosayro, J. G. Herndon, M. Saucier, H. M. McClure. 2000. Progressive infection in a subset of HIV-1-positive chimpanzees. J. Infect. Dis. 182:1051-1062. [DOI] [PubMed] [Google Scholar]
- 57.Ota, T., and M. Nei. 1994. Variance and covariance of the numbers of synonymous and nonsynonymous substitutions per site. Mol. Biol. Evol. 11:613-619. [DOI] [PubMed] [Google Scholar]
- 58.Pandrea, I., R. Onanga, P. Rouquet, O. Bourry, P. Ngari, E. J. Wickings, P. Roques, and C. Apetrei. 2001. Chronic SIV infection ultimately causes immunodeficiency in African non-human primates. AIDS 14:2461-2462. [DOI] [PubMed] [Google Scholar]
- 59.Pandrea, I., R. Onanga, C. Kornfeld, P. Rouquet, O. Bourry, S. Clifford, K. Abernethy, L. T. W. White, P. T. Telfer, P. Ngari, M. C. Müller-Trutwin, P. Roques, P. A. Marx, F. Simon, and C. Apetrei. 2003. High levels of SIVmnd-1 replication in chronically infected mandrills. Virology 317:119-127. [DOI] [PubMed] [Google Scholar]
- 60.Raphael, M., P. Pouletty, M. Cavaille-Coll, W. Rozenbaum, A. Homond, L. Nonnenmacher, A. Delcourt, J. C. Gluckman, and P. Debre. 1985. Lymphadenopathy in patients at risk for acquired immunodeficiency syndrome. Histopathology and histochemistry. Arch. Pathol. Lab. Med. 109:128-132. [PubMed] [Google Scholar]
- 61.Rey-Cuille, M. A., J. L. Berthier, M. C. Bomsel-Demontoy, Y. Chaduc, L. Montagner, A. G. Hovanessian, and L. A. Chakrabarti. 1998. Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J. Virol. 72:3872-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roques, P., D. L. Robertson, S. Souquiere, C. Apetrei, E. Nerrienet, F. Barre-Sinoussi, M. C. Muller-Trutwin, and F. Simon. 2004. Phylogenetic characteristics of three new HIV-1 N strains and the implications for the origin of group N. AIDS 18:1371-1381. [DOI] [PubMed] [Google Scholar]
- 63.Rutjens, E., S. Balla-Jhagjhoorsingh, E. Verschoor, W. Bogers, G. Koopman, and J. Heeney. 2003. Lentivirus infections and mechanisms of disease resistance in chimpanzees. Front. Biosci. 8:d1134-d1145. [DOI] [PubMed] [Google Scholar]
- 64.Saitou, N., and M. Nei. 1985. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 65.Schacker, T. W., P. L. Nguyen, G. J. Beilman, S. Wolinsky, M. Larson, C. Reilly, and A. T. Haase. 2002. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J. Clin. Investig. 110:1133-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schupbach, J. 2003. Human immunodeficiency virus, p. 1253-1280. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. American Society for Microbiology, Washington, D.C.
- 67.Silvestri, G., D. L. Sodora, R. A. Koup, M. Paiardini, S. P. O'Neil, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18:441-452. [DOI] [PubMed] [Google Scholar]
- 68.Smith, S. M., M. Makuwa, F. Lee, A. Gettie, C. Russo, and P. A. Marx. 1998. SIVrcm infection of macaques. J. Med. Primatol. 27:94-98. [DOI] [PubMed] [Google Scholar]
- 69.Souquiere, S., F. Bibollet-Ruche, D. L. Robertson, M. Makuwa, C. Apetrei, R. Onanga, C. Kornfeld, J. C. Plantier, F. Gao, K. Abernethy, L. J. White, W. Karesh, P. Telfer, E. J. Wickings, P. Mauclere, P. A. Marx, F. Barre-Sinoussi, B. H. Hahn, M. C. Muller-Trutwin, and F. Simon. 2001. Wild Mandrillus sphinx are carriers of two types of lentivirus. J. Virol. 75:7086-7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spijkerman, I. J., M. Prins, J. Goudsmit, P. J. Veugelers, R. A. Coutinho, F. Miedema, and F. de Wolf. 1997. Early and late HIV-1 RNA level and its association with other markers and disease progression in long-term AIDS-free homosexual men. AIDS 11:1383-1388. [DOI] [PubMed] [Google Scholar]
- 71.Takehisa, J., Y. Harada, N. Ndembi, I. Mboudjeka, Y. Taniguchi, C. Ngansop, S. Kuate, L. Zekeng, K. Ibuki, T. Shimada, B. Bikandou, Y. Yamaguchi-Kabata, T. Miura, M. Ikeda, H. Ichimura, L. Kaptue, and M. Hayami. 2001. Natural infection of wild-born mandrills (Mandrillus sphinx) with two different types of simian immunodeficiency virus. AIDS Res. Hum. Retrovir 17:1143-1154. [DOI] [PubMed] [Google Scholar]
- 72.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tomonaga, K., J. Katahira, M. Fukasawa, M. A. Hassan, M. Kawamura, H. Akari, T. Miura, T. Goto, M. Nakai, M. Suleman, M. Isahakia, and M. Hayami. 1993. Isolation and characterization of simian immunodeficiency virus from African white-crowned mangabey monkeys (Cercocebus torquatus lunulatus). Arch. Virol. 129:77-92. [DOI] [PubMed] [Google Scholar]
- 74.Traina-Dorge, V., J. Blanchard, L. Martin, and M. Murphey-Corb. 1992. Immmunodeficiency and lymphoproliferative disease in an African green monkey dually infected with SIV and STLV-1. AIDS Res. Hum. Retrovir. 8:97-100. [DOI] [PubMed] [Google Scholar]
- 75.Van Rensburg, E. J., S. Engelbrecht, J. Mwenda, J. D. Laten, B. A. Robson, T. Stander, and G. K. Chege. 1998. Simian immunodeficiency viruses (SIVs) from eastern and southern Africa: detection of a SIVagm variant from a chacma baboon. J. Gen. Virol. 79:1809-1814. [DOI] [PubMed] [Google Scholar]
- 76.Veazey, R., B. Ling, I. Pandrea, H. McClure, A. Lackner, and P. Marx. 2003. Decreased CCR5 expression on CD4+ T cells of SIV-infected sooty mangabeys. AIDS Res. Hum. Retroviruses 19:227-233. [DOI] [PubMed] [Google Scholar]
- 77.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 78.Wolf, R. H., B. J. Gormus, L. N. Martin, G. B. Baskin, G. P. Walsh, W. M. Walsh, and C. H. Binford. 1985. Experimental leprosy in three species of monkeys. Science 227:529-531. [DOI] [PubMed] [Google Scholar]
- 79.Yamaguchi, J., S. G. Devare, C. A. Brennan. 2000. Identification of a new HIV-2 subtype based on phylogenetic analysis of full-length genomic sequence. AIDS Res. Hum. Retroviruses 16:925-930. [DOI] [PubMed] [Google Scholar]