Abstract
We previously demonstrated that mink cells undergo apoptosis after MCF13 murine leukemia virus (MLV) infection. In this study, we observed that virus-infected mink epithelial cells had significantly larger amounts of steady-state levels of MCF13 MLV envelope precursor protein (gPr80env) than did Mus dunni fibroblasts, which are resistant to virus-induced cytopathicity. Infection of mink cells with the noncytopathic NZB-9 MLV did not result in the accumulation of gPr80env. MCF13 MLV infection of mink cells produced low cell surface expression of envelope glycoprotein and less efficient spread of infectious virus. Western blot analysis of mink epithelial cells infected with MCF13 MLV showed an increase in GRP78/BiP, which was not observed for either mink cells infected with NZB-9 MLV or M. dunni fibroblasts infected with MCF13 MLV. MCF13 MLV infection of mink cells also resulted in a significant upregulation of CHOP/GADD153. These results indicate that the accumulation of MCF13 MLV gPr80env triggers endoplasmic reticulum stress, which may mediate apoptosis in mink epithelial cells.
A high correlation between viral pathogenicity and the ability to produce cytopathic effects has been observed for retroviruses that generate different types of disease, including neoplasia, neurodegeneration, and immunodeficiency (1, 4, 16, 17, 22, 25, 29). We have observed a similar phenomenon for mink cell focus-forming murine leukemia virus (MCF13 MLV), which induces apoptosis in thymic lymphocytes of inoculated AKR mice during the preleukemic period (31). Upon testing of cultured cell lines, we observed that mink cells were similarly susceptible to MCF13 MLV-induced cell killing (18). It has been shown that cell killing by pathogenic retroviruses such as FeLV-FAIDS, ts-1 MoMLV, MuLV-NT40, and FrCasE MLV strongly correlates with the inefficient processing of the precursor Env glycoprotein, resulting in its intracellular accumulation in a cell type-specific manner (11, 15, 21, 25). A recent study of the neurovirulent FrCasE MLV further showed that the accumulation of gPr80env activated endoplasmic reticulum (ER) stress in virus-infected microglial cells (3). Because prolonged ER stress can result in apoptosis (24), we undertook this study to determine whether a similar mechanism is functioning in the induction of cell killing by MCF13 MLV. A more complete understanding of the role that apoptosis may play in tumorigenesis and the cellular responses to it should provide insights into the early stages of this disease process.
MCF13 MLV gPr80env accumulates in cells that undergo apoptosis.
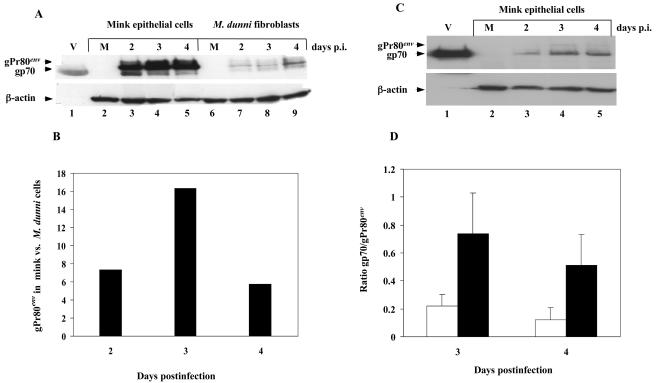
We previously showed that mink epithelial cells were susceptible to MCF13 MLV-induced cell killing, whereas other types of cells, such as Mus dunni fibroblasts, were resistant (18). To determine whether there is a correlation between Env precursor accumulation and virus-induced cell killing, we compared intracellular levels of gPr80env in mink epithelial cells and M. dunni fibroblasts after infection with MCF13 MLV at a multiplicity of infection (MOI) of 3. Infectious titers for viruses used in this study were determined for each cell line by an indirect immunofluorescence focus assay (26). Western blot analysis of cellular extracts with a monoclonal antibody (MAb) that detects the envelope protein of various MLVs (MAb 83A25) (8) showed significantly greater amounts of steady-state levels of gPr80env in MCF13 MLV-infected mink cells than in M. dunni fibroblasts (Fig. 1A). Quantification of gPr80env bands with a Kodak EDAS 120 scanner and software showed that mink cells had 5- to 17-fold greater amounts of this protein than did M. dunni fibroblasts at different times postinfection (p.i.) (Fig. 1B). At all times postinfection, the percentage of virus-infected mink cells was either comparable to or lower than that of M. dunni fibroblasts (Fig. 2B).
FIG. 1.
Accumulation of MCF13 MLV gPr80env in mink epithelial cells. (A) Western blot analysis of cellular extracts at indicated times p.i. from mink epithelial cells (ATCC CCL64) (lanes 3-5) or M. dunni fibroblasts (ATCC CRL2017) (lanes 7-9) infected with MCF13 MLV at an MOI of 3. M refers to protein extract from mock-infected cells, which were treated only with Polybrene (lanes 2 and 6). Lane 1 (V) consists of an aliquot of purified virions of MCF13 MLV produced by M. dunni fibroblasts. gpPr80env and gp70 bands are indicated by arrowheads. β-Actin was detected as a loading control for cellular extracts. (B) Ratio of gPr80env present in mink cells relative to M. dunni fibroblasts was calculated from densitometry of protein bands in the Western blot in Fig. 1A. (C) Western blot analysis of cell extracts from mink epithelial cells infected with either NZB-9 MLV at an MOI of 3 (lanes 3-5) or mock infected (M, lane 2). (D) Mean ratios of gp70 relative to gPr80env for mink epithelial cells (open bars) and M. dunni fibroblasts (filled bars) are shown. Mean values and standard deviations were calculated from the results of two independent experiments. The differences between mink cells and M. dunni fibroblasts at days 3 and 4 were significant (P = 0.03 using Student's t test).
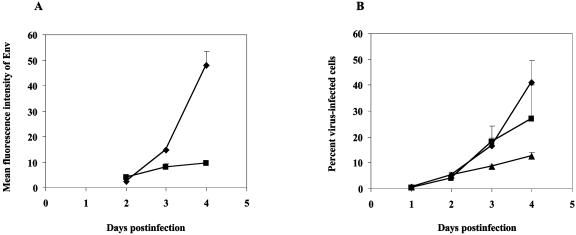
FIG. 2.
Mink epithelial cells have low cell surface expression of MCF13 MLV Env and inefficient virus spread. (A) Mean values and standard deviations of mean fluorescence intensity of Env expression as detected by flow cytometry analysis of mink epithelial cells (▪) and M. dunni fibroblasts (♦) after MCF13 MLV infection over time were calculated from two independent experiments. (B) Percent mink epithelial cells infected with either MCF13 (▴) or NZB-9 MLV (▪) and percent M. dunni fibroblasts infected with MCF13 MLV(♦). Values represent means and standard deviations calculated from the results of two independent experiments.
gPr80env is processed by cellular proteases to the surface glycoprotein gp70 and transmembrane subunit p15E, which are subsequently expressed at the cell surface (5). To assess the amount of gp70 compared to that of gPr80env in mink epithelial and M. dunni cells, we quantified the gp70 bands in Fig. 1A by densitometry. This analysis revealed that at days 3 and 4 p.i., gPr80env was in significantly greater excess over gp70 in mink cells than in M. dunni fibroblasts (Fig. 1D). These results suggest that there is less efficient processing of MCF13 MLV gPr80env in mink epithelial cells, which results in its accumulation. This idea is supported by an earlier study by Famulari and Jelalian (9), which demonstrated by pulse-chase analysis that gPr80env of a related MCF MLV is inefficiently processed in mink cells compared with that of either an ecotropic or xenotropic virus.
To test whether the inefficient processing of gPr80env in mink epithelial cells is virus specific, we performed a similar analysis with the noncytopathic NZB-9 MLV (Fig. 1C). Unlike our results with MCF13 MLV, gPr80env was barely detectable for mink cells infected with NZB-9 MLV, although gp70 was easily seen. These results indicate that the defective processing of MCF13 MLV gPr80env in mink epithelial cells is unique to this virus and not due to a cellular deficiency.
Mink cells have low cell surface expression of envelope glycoprotein and less efficient spread of infectious virus.
To verify that gPr80env is inefficiently processed in mink cells, we compared the expression of the MCF13 MLV envelope glycoprotein on the surface of mink cells and M. dunni fibroblasts. To assess the amount of Env expressed on the surface of these cells at different times after MCF13 MLV infection, we calculated the mean fluorescence intensity by flow cytometric analysis of secondary immunofluorescence binding to MAb 83A25 (Fig. 2A). Cell analysis was performed using a Becton Dickinson FACScan flow cytometer and CELLQuest software (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). From days 2 to 4 p.i., we detected an approximately 20-fold increase of Env expression on the surface of M. dunni fibroblasts. In contrast, for virus-infected mink cells the increase in Env expression over this time period was only threefold.
We also examined the spread of infectious MCF13 MLV produced by mink cells in a time course study (Fig. 2B). The percentage of virus-infected mink cells was significantly lower than that of M. dunni fibroblasts at days 3 and 4 p.i., indicating that there is a lower efficiency of virus spread in mink cells. In contrast, we observed efficient spread of NZB-9 in mink cells. These results support the idea that the MCF13 MLV envelope glycoprotein is inefficiently processed in mink epithelial cells, which results in its concomitant low cell surface expression and, likewise, low production of infectious virus.
MCF13 MLV infection triggers ER stress in mink epithelial cells.
Disruption in protein processing produced, for example, by the inhibition of N-linked glycosylation or inadequate transport from ER to the Golgi results in the accumulation of misfolded or wild-type protein in the lumen of the ER, which triggers ER stress (10, 28). It has been shown that one of the effects of ER stress is the transcriptional upregulation of ER resident chaperone proteins, such as GRP78/BiP (13, 14). Under conditions of prolonged ER stress, cells can undergo apoptosis, which is partially dependent upon an increase in transcription factors, such as CHOP/GADD153 (C/EBP homologous protein/growth arrest and DNA damage) (19, 24, 28). Because of these observations we examined whether the large amount of MCF13 MLV gPr80env that accumulates in mink cells could trigger ER stress.
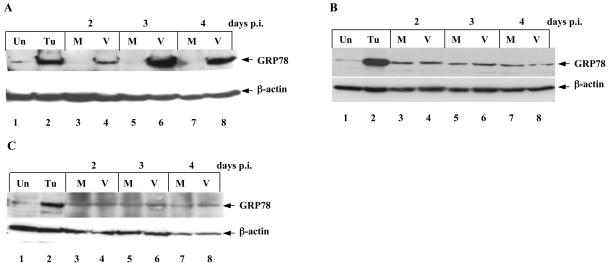
To analyze GRP78 in mink cells infected with MCF13 MLV, we performed a time course analysis by Western blotting (Fig. 3A, lanes 3 to 8). At all time points p.i., we detected significantly more GRP78 in virus-infected cells relative to mock-infected cells. The maximum difference was 125-fold at day 3 p.i. Positive controls for ER stress consisted of cell extracts from mink epithelial cells exposed to tunicamycin (Fig. 3 and 4, lanes 2). For comparison, we assessed GRP78 levels in mink epithelial cells infected with NZB-9 MLV, which did not produce high levels of unprocessed glycoprotein. For these cells, we did not detect a significant difference in GRP78 levels compared with mock-infected cells (Fig. 3B, lanes 3 to 8). In addition, we detected no difference in GRP78 levels in M. dunni fibroblasts after MCF13 MLV infection compared with mock-infected cells (Fig. 3C, lanes 3 to 8).
FIG. 3.
MCF13 MLV upregulates GRP78 in mink epithelial cells. Western blot analysis of cellular extracts from (A) mink cells infected with MCF13 MLV (V, lanes 4, 6, and 8) or mock infected (M, lanes 3, 5, and 7). (B) Mink cells infected with NZB-9 MLV (V, lanes 4, 6, and 8) or mock infected (M, lanes 3, 5, and 7). (C) M. dunni fibroblasts infected with MCF13 MLV (V, lanes 4, 6, and 8) or mock infected (M, lanes 3, 5, and 7). Protein extracts from cells exposed to 1 μg of tunicamycin per ml for 24 h (Tu, lanes 2) or untreated (Un, lanes 1) were used for control for the ER stress response. The Western blots are representative of results from at least two independent experiments.
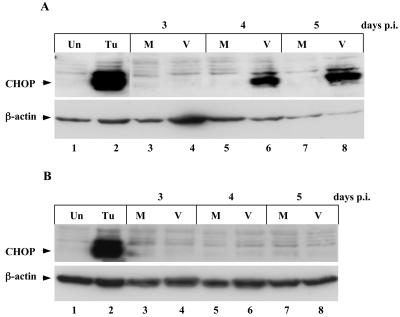
FIG. 4.
CHOP induction in mink epithelial cells by MCF13 MLV infection. (A) Western blot analysis of total cellular extracts from mink cells infected with MCF13 MLV (V, lanes 4, 6, and 8) or mock infected (M, lanes 3, 5, and 7). (B) Analysis of mink cells infected with NZB-9 MLV (V, lanes 4, 6, and 8) or mock infected (M, lanes 3, 5, and 7). Cell extracts from mink cells exposed to tunicamycin (Tu, lanes 2) or untreated (Un, lanes 1) were controls for ER stress.
To determine whether CHOP levels are upregulated in mink cells infected with MCF13 MLVs, we performed Western blot analysis of cellular extracts (Fig. 4). Beginning at day 4 p.i., we detected a significant increase in CHOP levels compared with mock-infected cells (Fig. 4A, lanes 3 to 8). This increase in CHOP protein coincides with the appearance of apoptosis in mink cells after virus infection (30). A similar increase in CHOP levels was not detectable in mink epithelial cells after NZB-9 MLV infection (Fig. 4B, lanes 3 to 8). These results strongly support the idea that the induction of apoptosis by MCF13 MLV occurs through a mechanism that involves ER stress.
We do not yet know what property of the MCF13 MLV recombinant Env precursor is responsible for its accumulation. It is intriguing that a major determinant of the pathogenicity of MCF MLVs is the env gene (12, 23), which results from recombination of ecotropic and polytropic endogenous MLV sequences (2, 6, 7). For other retroviruses, it has been shown that Env accumulation can occur as a result of a single amino acid change in surface glycoprotein or altered protein glycosylation (20, 27). We have also observed that MCF13 MLV is able to superinfect mink cells, which may be a contributing factor to the high levels of Env precursor protein present in these cells.
Acknowledgments
The authors would like to thank Xixia Luo and Xiaoqing Zhao for their assistance in many of the experiments. We also acknowledge T. R. Reddy and T. Holland for their helpful comments on the manuscript. We are grateful to Eric Van Buren for his help at the Wayne State University and Karmanos Cancer Institute Flow Cytometry Core Facility.
This work was supported by Public Health Service grant CA44166 to F.K.Y. from the National Institutes of Health.
REFERENCES
- 1.Bonzon, C., and H. Fan. 1999. Moloney murine leukemia virus-induced preleukemic thymic atrophy and enhanced thymocyte apoptosis correlate with disease pathogenicity. J. Virol. 73:2434-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chattopadhyay, S. K., M. W. Cloyd, D. L. Linemeyer, M. R. Lander, E. Rands, and D. R. Lowy. 1982. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature 295:25-31. [DOI] [PubMed] [Google Scholar]
- 3.Dimcheff, D. E., S. Askovic, A. H. Baker, C. Johnson-Fowler, and J. L. Portis. 2003. Endoplasmic reticulum stress is a determinant of retrovirus-induced spongiform neurodegeneration. J. Virol. 77:12617-12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donahue, P. R., S. L. Quackenbush, M. V. Gallo, C. M. deNoronha, J. Overbaugh, E. A. Hoover, and J. I. Mullins. 1991. Viral genetic determinants of T-cell killing and immunodeficiency disease induction by the feline leukemia virus FeLV-FAIDS. J. Virol. 65:4461-4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Einfeld, D. 1996. Maturation and assembly of retroviral glycoproteins. Curr. Top. Microbiol. Immunol. 214:133-176. [DOI] [PubMed] [Google Scholar]
- 6.Elder, J. H., J. W. Gautsch, F. C. Jensen, R. A. Lerner, J. W. Hartley, and W. P. Rowe. 1977. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc. Natl. Acad. Sci. USA 74:4676-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans, L. H., and F. G. Malik. 1987. Class II polytropic murine leukemia viruses (MuLVs) of AKR/J mice: possible role in the generation of class I oncogenic polytropic MuLVs. J. Virol. 61:1882-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans, L. H., R. P. Morrison, F. G. Malik, J. Portis, and W. J. Britt. 1990. A neutralizable epitope common to the envelope glycoproteins of ecotropic, polytropic, xenotropic, and amphotropic murine leukemia viruses. J. Virol. 64:6176-6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Famulari, N. G., and K. Jelalian. 1979. Cell surface expression of the env gene polyprotein of dual-tropic mink cell focus-forming murine leukemia virus. J. Virol. 30:720-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hampton, R. Y. 2000. ER stress response: getting the UPR hand on misfolded proteins. Curr. Biol. 10:R518-R521. [DOI] [PubMed] [Google Scholar]
- 11.Hansen, R., S. Czub, E. Werder, J. Herold, G. Gosztonyi, H. Gelderblom, S. Schimmer, S. Mazgareanu, V. ter Meulen, and M. Czub. 2000. Abundant defective viral particles budding from microglia in the course of retroviral spongiform encephalopathy. J. Virol. 74:1775-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holland, C. A., J. W. Hartley, W. P. Rowe, and N. Hopkins. 1985. At least four viral genes contribute to the leukemogenicity of murine retrovirus MCF 247 in AKR mice. J. Virol. 53:158-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorgensen, M. M., P. Bross, and N. Gregersen. 2003. Protein quality control in the endoplasmic reticulum. APMIS Suppl. 2003:86-91. [PubMed] [Google Scholar]
- 14.Lee, A. S. 2001. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem. Sci. 26:504-510. [DOI] [PubMed] [Google Scholar]
- 15.Lynch, W. P., W. J. Brown, G. J. Spangrude, and J. L. Portis. 1994. Microglial infection by a neurovirulent murine retrovirus results in defective processing of envelope protein and intracellular budding of virus particles. J. Virol. 68:3401-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch, W. P., S. Czub, F. J. McAtee, S. F. Hayes, and J. L. Portis. 1991. Murine retrovirus-induced spongiform encephalopathy: productive infection of microglia and cerebellar neurons in accelerated CNS disease. Neuron 7:365-379. [DOI] [PubMed] [Google Scholar]
- 17.Meyaard, L., S. A. Otto, R. R. Jonker, M. J. Mijnster, R. P. Keet, and F. Miedema. 1992. Programmed death of T cells in HIV-1 infection. Science 257:217-219. [DOI] [PubMed] [Google Scholar]
- 18.Nanua, S., and F. K. Yoshimura. 2004. Differential cell killing by lymphomagenic murine leukemia viruses occurs independently of p53 activation and mitochondrial damage. J. Virol. 78:5088-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pahl, H. L. 1999. Signal transduction from the endoplasmic reticulum to the cell nucleus. Physiol. Rev. 79:683-701. [DOI] [PubMed] [Google Scholar]
- 20.Poss, M. L., S. W. Dow, and E. A. Hoover. 1992. Cell-specific envelope glycosylation distinguishes FIV glycoproteins produced in cytopathically and noncytopathically infected cells. Virology 188:25-32. [DOI] [PubMed] [Google Scholar]
- 21.Poss, M. L., S. L. Quackenbush, J. I. Mullins, and E. A. Hoover. 1990. Characterization and significance of delayed processing of the feline leukemia virus FeLV-FAIDS envelope glycoprotein. J. Virol. 64:4338-4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rojko, J. L., J. R. Hartke, C. M. Cheney, A. J. Phipps, and J. C. Neil. 1996. Cytopathic feline leukemia viruses cause apoptosis in hemolymphatic cells. Prog. Mol. Subcell. Biol. 16:13-43. [DOI] [PubMed] [Google Scholar]
- 23.Rowe, W. P., and J. W. Hartley. 1983. Genes affecting mink cell focus-inducing (MCF) murine leukemia virus infection and spontaneous lymphoma in AKR F1 hybrids. J. Exp. Med. 158:353-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutkowski, D. T., and R. J. Kaufman. 2004. A trip to the ER: coping with stress. Trends Cell Biol. 14:20-28. [DOI] [PubMed] [Google Scholar]
- 25.Shikova, E., Y. C. Lin, K. Saha, B. R. Brooks, and P. K. Wong. 1993. Correlation of specific virus-astrocyte interactions and cytopathic effects induced by ts1, a neurovirulent mutant of Moloney murine leukemia virus. J. Virol. 67:1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sitbon, M., J. Nichio, H. Wehrly, D. Lodmell, and B. Chesebro. 1985. Use of a focal immunofluorescence assay on live cells for quantitation of retroviruses: distinction of host range classes in virus mixture and virological cloning of dual-tropic murine leukemia viruses. Virology 141:110-118. [DOI] [PubMed] [Google Scholar]
- 27.Szurek, P. F., P. H. Yuen, J. K. Ball, and P. K. Wong. 1990. A Val-25-to-Ile substitution in the envelope precursor polyprotein, gPr80env, is responsible for the temperature sensitivity, inefficient processing of gPr80env, and neurovirulence of ts1, a mutant of Moloney murine leukemia virus TB. J. Virol. 64:467-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welihinda, A. A., W. Tirasophon, and R. J. Kaufman. 1999. The cellular response to protein misfolding in the endoplasmic reticulum. Gene Expr. 7:293-300. [PMC free article] [PubMed] [Google Scholar]
- 29.Weller, S. K., and H. M. Temin. 1981. Cell killing by avian leukosis viruses. J. Virol. 39:713-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshimura, F. K., T. Wang, and S. Nanua. 2001. Mink cell focus-forming murine leukemia virus killing of mink cells involves apoptosis and superinfection. J. Virol. 75:6007-6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshimura, F. K., T. Wang, F. Yu, H. R. Kim, and J. R. Turner. 2000. Mink cell focus-forming murine leukemia virus infection induces apoptosis of thymic lymphocytes. J. Virol. 74:8119-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]