Abstract
Although only a few blood cells are infected during measles, this infectious disease is followed by acute immunosuppression, associated with high infant mortality. Measles virus nucleoprotein has been suggested to contribute to virus-induced inhibition of the immune response. However, it has been difficult to understand how this cytosolic viral protein could leave an infected cell and then perturb the immune response. Here we demonstrate that intracellularly synthesized nucleoprotein enters the late endocytic compartment, where it recruits its cellular ligand, the Fcγ receptor. Nucleoprotein is then expressed at the surfaces of infected leukocytes associated with the Fcγ receptor and is secreted into the extracellular compartment, allowing its interaction with uninfected cells. Finally, cell-derived nucleoprotein inhibits the secretion of interleukin-12 and the generation of the inflammatory reaction, both shown to be impaired during measles. These results reveal nucleoprotein egress from infected cells as a novel strategy in measles-induced immunosuppression.
Measles virus (MV) is among the most contagious pathogens for humans, still infecting more than 40 million people and causing the death of around 1 million a year (33). MV is responsible for an acute childhood disease which is widespread in developing countries, with sporadic outbreaks in industrialized countries despite vaccination. MV is a morbillivirus with a single-stranded, negative-sense RNA genome, encoding six structural proteins. The lipid bilayer contains two envelope glycoproteins, the hemagglutinin (H) and the fusion protein (F). The matrix protein (M) supplies an interface between the envelope and the virion core, composed of viral RNA, the nucleocapsid protein (N), the phosphoprotein (P), and the large protein (L). The function of the N protein appears to be packaging and protection of the viral genomic nucleic acid and formation of a replication complex, along with the P and L proteins (20). N is the most abundant of the viral proteins, synthesized on free ribosomes and folded in the cytoplasm, where it binds viral RNA and forms intracellular inclusions (17). This 60-kDa protein has the capacity to self-assemble into nucleocapsids on cellular RNA as well, in the absence of any of the other MV gene products (43). MV infection is initiated by the attachment of the virus via MV H to one of its specific receptors, CD46 or CD150, followed by virus-cell fusion and release of the nucleocapsid into the cytoplasm (20, 46).
MV causes a profound suppression of the immune system that permits opportunistic infections, leading to high infant morbidity and mortality. The immune abnormalities that are most evident are in the cellular arm of the immune response and include disappearance of the delayed-type hypersensitivity responses (45, 49), impaired proliferation of peripheral blood lymphocytes (PBLs) (23), and allospecific cytotoxicity (15). Type 2 polarization of cytokine responses occurs during late stages of measles: the production of interleukin 4 (IL-4) increases and that of IL-2 and gamma interferon (IFN-γ) decreases (21). Production of the proinflammatory cytokine IL-12 is markedly suppressed in patients with measles, providing a unifying mechanism for many of the immunological abnormalities associated with measles (3). Finally, a number of immunological alterations observed during natural measles also occur after vaccination with attenuated MV (25).
In spite of the fact that less than 1% of peripheral blood cells are infected during the course of acute measles (10), the severe suppression of immune responses can last for weeks (6). This observation suggests that, as with human immunodeficiency virus (HIV) infection (42), MV-induced immunosuppression may be caused by an indirect immunopathogenic mechanism. Several recent studies are in agreement with this hypothesis, suggesting that MV proteins could inhibit the immune response in the absence of MV replication in vitro (26, 36, 41) as well as in vivo (31). It has been demonstrated that MV N binds via its C-terminal part to the receptor for the Fc portion of immunoglobulin G (IgG), Fc gamma receptor type II (FcγRII/CD32) (31). The binding of recombinant N to FcγR inhibits antibody (Ab) production by human B cells in vitro (36) and impairs dendritic cell function (31). Because N is synthesized in the cytoplasm of infected cells (17, 20), the availability of this protein to bind FcγRII in the extracellular environment and cause immunosuppression was uncertain, and thus the pathophysiological significance of this interaction has not been established.
In this study we analyzed the intracellular mechanism allowing the egress of cytosolic N, which induces immunosuppression. We demonstrated the existence of the particular intracellular pathway allowing the delivery of MV N to the cell membrane and its secretion, leading to the inhibition of IL-12 production and inflammatory reactions. These results demonstrate the remarkable role of the cell membrane translocation of MV N in the induction of immunosuppression, providing new insight into the MV-induced inhibition of the immune response and a novel concept in the pathogenesis of measles.
MATERIALS AND METHODS
Cells, virus, and recombinant N.
PBLs and T lymphocytes were purified from blood of healthy donors as described previously (13). Tonsillar lymphocytes were isolated by density gradient centrifugation, using Ficoll/Hypaque, and B lymphocytes were purified by rosetting with sheep red blood cells. The IIA1.6 B-cell line transfected with human FcγRIIb1 was provided by J. Van De Winkel (48). P815-N and P815-M cells were obtained by transfection of P815 mastocytoma cells with MV N and MV M DNA, respectively, and the L-NP line was produced by transfecting murine L fibroblasts with MV N DNA (5). All cell cultures were performed in RPMI 1640 medium supplemented with 10% fetal calf serum, 10 mM HEPES, 2 mM glutamine, 5 × 10−5 M 2β-mercaptoethanol, and 50 μg of gentamicin/ml at 37°C under 5% CO2. For transwell experiments, 2 × 105 P815-M and P815-N cells separated by a 0.4-μm-pore-size membrane (Costar, Corning, N.Y.) were incubated for 48 h. Treatment with brefeldin A (BFA) and monensin (BD Bioscience, San Diego, Calif.) was performed according to the manufacturer's instructions.
Wild-type MV strains G954, G945, and G943 (28) were propagated on PBLs. A recombinant MV Edmonston strain in which the H and F glycoproteins were replaced by G protein of vesicular stomatitis virus (MGV) (44) and the molecularly cloned Edmonston strain (EDtag) were provided by M. Billeter (Zurich, Switzerland). MV Edmonston (VR-24) from the American Type Culture Collection and recombinant MV Edmonston strains were propagated on Vero fibroblasts. Viruses were inactivated by UV irradiation at 254 nm. A recombinant vaccinia virus expressing MV N was produced as described previously (50).
Human PBLs, previously activated with 2 μg of phytohemagglutinin (PHA) (Sigma, Saint Louis, Mo.)/ml and 5 U of IL-2 (Roche Diagnostics, Mannheim, Germany)/ml for 48 h, were incubated with the different viruses (0.01 to 1 PFU/cell) for 2 h, and then 106 cells/ml were cultivated in the presence of IL-2 (R&D Systems, Abingdon, United Kingdom) for different times. Tonsillar lymphocytes and B cells were infected with MV for 2 h and then activated by culturing 2 × 106 cells/ml with mitomycin C-inactivated, CD40-L-transfected fibroblasts (2 × 105 cells/ml) for 48 h.
Recombinant MV N (525 amino acids) was produced in a baculovirus system and purified on a cesium chloride (CsCl) gradient as described elsewhere (31, 36).
Flow cytometry.
For flow cytometry analyses, cells were obtained after Ficoll separation and labeled with the biotinylated anti-N antibody Cl.25 or Cl.120, recognizing the C- or N-terminal domain of N, respectively (7), and/or anti-H Cl.55, or anti-M monoclonal antibody (MAb) 8910 (Chemicon), or fluorescein isothiocyanate (FITC)-conjugated anti-CD32 (Pharmingen, San Diego, Calif.), followed by either streptavidin conjugated to phycoerythrin (Pharmingen) or goat anti-mouse IgG conjugated to FITC (Immunotech, Marseille, France). Irrelevant biotinylated mouse IgG2a or an FITC-conjugated goat anti-mouse antibody (Immunotech) was used as a negative control. All flow cytometry analyses were carried out on a FACScan (Becton Dickinson, Le Pont de Claix, France).
Confocal and time-lapse microscopy.
Cells were fixed in 3.7% formaldehyde and, where indicated, permeabilized with 0.2% Triton X-100. Coverslips were mounted with Fluorsave reagent (Calbiochem). Cells were observed under an LSM 510 laser scanning confocal microscope using a 63× (NA 1.4) Zeiss Plan Neo Fluor objective. Available illumination sources were the 488-, 543-, and 633-nm lasers. The multitrack recording module was used for sequential acquisition of each channel before merging. Images were processed with Adobe Photoshop software (version 6.0; Southern Biotechnologies). The following antibodies were used: biotinylated anti-N Cl.25 and Cl.120, followed by rhodamine-conjugated streptavidin (Pharmingen) or cyanin 5-streptavidin (Caltag); the anti-M MAb 8910 (Chemicon) or anti-major histocompatibility complex (MHC) class I (34.1.2s), followed by cyanin 5-conjugated donkey anti-mouse IgG (Jackson); the anti-mouse FcγR Ab 2.4G2 (Pharmingen), followed by FITC-conjugated donkey anti-rat IgG (Jackson); as anti-human FcγRII, FITC-conjugated anti-CD32 (Pharmingen) or MAb KB61 (from K. Pulford Oxford, United Kingdom); as anti-Lamp-1, an anti-CD107a MAb conjugated to Cy-chrome (BD Biosciences) or a rabbit anti-Lamp1 serum (from S. Meresse, Marseille, France); as rabbit anti-cathepsin B, Ab-3 (Oncogene); as anti-p23, a rabbit anti-p23 serum (39) (from M. Rojo), followed by Alexa Fluor 488-conjugated donkey anti-rabbit IgG (Molecular Probes). Rafts were detected by using the FITC-conjugated B subunit of cholera toxin (Sigma).
For time-lapse microscopy, P815-N cells were labeled with Cl.120 and transferred in polylysine-coated glass-bottom dishes in α-MEM with 10% fetal calf serum and 20 mM HEPES, without bicarbonate (Life Technologies). Dishes were placed on a 37°C heated stage, and cells were imaged with a Zeiss 510 laser scanning microscope (Axiovert 100 M) and a Zeiss Plan-Apochromat 40 × (NA 1.0) objective. Meta Imaging Series 4.5 (Universal Imaging Corporation) was used to mount AVI movies from image stacks. Images extracted from stacks were processed with Adobe Premiere 5.1 software.
Electron microscopy.
Cells were fixed with periodate-paraformaldehyde-0.2% glutaraldehyde, washed with 0.4 M saccharose-0.1 M lysine-0.05 M Sorensen phosphate buffer (pH 7.4), partially dehydrated in graded ethanol, and embedded in LR White. Sections (thickness, 80 to 90 nm) were collected on 200-mesh nickel grids, incubated with MAb Cl.120 and an anti-mouse IgG antibody conjugated with either10- or 20-nm gold particles, contrasted with aqueous uranyl acetate and lead citrate, and observed with a JEOL (Tokyo, Japan) 1200EX electron microscope.
Isolation of microvesicles and Western blotting.
Microvesicles released in the supernatants of P815-N and P815-M cells in culture were isolated by differential centrifugation as described elsewhere (9). Briefly, cells were washed by centrifugation and recultured in fresh medium for 24 h. Cell cultures were centrifuged for 10 min at 200 × g (pellet P1). The supernatant was removed and centrifuged for 10 min at 500 × g (pellet P2). This was repeated once (the pooled pellets are referred to as P2). Supernatants were sequentially centrifuged at 2,000 × g twice for 15 min (the pooled pellets are referred to as P3), once at 10,000 × g for 30 min (pellet P4), and once at 70,000 × g for 60 min (pellet P5), by using an SW28 rotor (Beckman Instruments, Inc., Fullerton, Calif.). P1 contained the cells, whereas P5 was enriched in microvesicles. P5 was then washed with phosphate-buffered saline (PBS) once at 70,000 × g for 60 min. For further purification of microvesicles, P5 was layered on top of a 0 to 20% linear CsCl-3 mM imidazole (pH 7.4) gradient in an SW40 tube (Beckman Instruments). Gradients were centrifuged at 35,000 × g for 20 h. Fractions (1 ml) were collected from the top of the tube. To collect membranes from these fractions, they were diluted with 12 ml of 3 mM imidazole (pH 7.4) and centrifuged for 60 min at 35,000 × g by using an SW40 rotor (Beckman Instruments). The pellets were solubilized in nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer for FcγR, CD81, and N detection or submitted to an enzyme-linked immunosorbent assay (ELISA) for lysobisphosphatidic acid (LBPA) detection.
For Western blotting, proteins were separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and transferred to an Immobilon-P membrane (Millipore). The following reagents were used: anti-N Cl.25 or Cl.120 followed by a polyclonal anti-IgG antibody and labeled with 0.1 μg of 125I-labeled protein G (Zymed Laboratorie)/ml; anti-CD32, anti-CD81 (both from Pharmingen), and rabbit-polyclonal anti-exosomes (from S. Mecheri, Paris, France), followed, respectively, by a polyclonal anti-rat, anti-hamster, or anti-rabbit antibody coupled with horseradish peroxidase (Pharmingen). Labeling was visualized and/or quantified by using a phosphorimager (Bio-Rad) and/or enhanced chemiluminescence system.
ELISA.
THP-1 or RAW 264.7 cells (106/ml) were activated with 200 U of human or mouse IFN-γ (Roche)/ml, respectively, for 16 h and then stimulated with lipopolysaccharide (LPS) (1 μg/ml) (Difco Laboraotries) for 36 h. IL-12 (p40) concentrations in the culture supernatants of THP-1 cells were determined with a Quantikine kit (R&D Systems, Minneapolis, Minn.), and that for RAW 264.7 supernatants was determined by an ELISA performed with reagents supplied by M. Gately, as described elsewhere (16).
LBPA quantification was performed by ELISA. After centrifugation on a CsCl gradient, fractions were collected and resuspended in 20 mM HEPES (pH 7.4)-150 mM NaCl (HEPES buffer) and incubated overnight on a 96-well plate. After addition of 3% bovine serum albumin (BSA) in HEPES buffer for 2 h and washes with Tris buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl), fractions were incubated with the anti-LBPA Ab 6C4 (27) for 2 h in HEPES buffer supplemented with 1% BSA. After extensive washes with Tris buffer, a biotin-conjugated anti-IgG Ab was added for 1.5 h, followed by streptavidin-conjugated alkaline phosphatase in HEPES buffer supplemented with 1% BSA for 1 h. After washes with Tris buffer, 0.25 mM UMP1 (Sigma-Aldrich) in 1 M diethanolamine-1 mM MgCl2 was added as a substrate for 10 to 20 min. Fluorescence was measured (excitation wavelength, 365 nm; emission wavelength, 450 nm) with a plate reader (Spectra Max Gemini XS; Bucher).
Mice and assay for contact hypersensitivity.
Eight-week-old DBA-2 mice were purchased from Charles River (L'Arbresle, France). Contact hypersensitivity to dinitrofluorobenzene (DNFB) was determined as described elsewhere (31), and the protocol was approved by the Institutional Animal Care Committee (ENS-Lyon, Lyon, France). Briefly, DNFB was applied to shaved ventral skin, and 5 days later, mice received 10 μl of a nonirritant concentration of DNFB applied to the left ear and the solvent alone on the right ear. Ear thickness was monitored before challenge and 24 h after challenge.
RESULTS
MV N is expressed on the surfaces of infected PBLs.
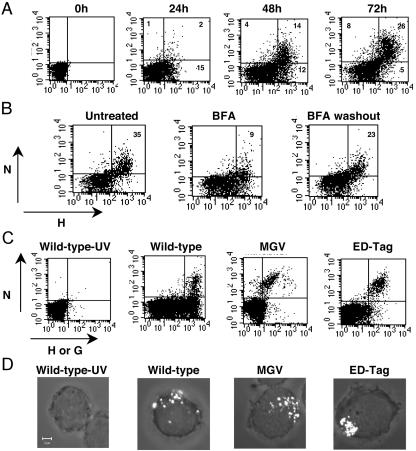
Human PBLs were infected with MV, and the expression of MV proteins N and H was monitored for 3 days (Fig. 1A). Surprisingly, viable infected cells were found to express at their surfaces not only the viral glycoprotein H but also N, known as a cytosolic viral protein (20). This was not the case with the other intracellular protein, M, which has never been detected on the surfaces of MV-infected cells (data not shown). Although N is synthesized before H and is synthesized in larger amounts during the MV replication cycle (20), H arrived on the cell surface before N. H was expressed on the membranes of 17% of lymphocytes at 24 h after infection. N was detected on the cell membrane 48 h after infection, thus suggesting different kinetics of plasma membrane translocation and indicating that N and H may use different pathways to reach the cell surface. When infected PBLs were incubated with BFA, which is capable of blocking intracellular membrane trafficking, the percentage of N-positive cells was dramatically decreased, returning to the initial level 2 h after the removal of BFA (Fig. 1B). Similar results were obtained with monensin, another intracellular transport inhibitor (data not shown), showing that the capacity of N to reach the cell membrane is associated with intracellular transport.
FIG. 1.
MV N is expressed at the cell membranes of infected PBLs. (A) Human PBLs were infected with MV strain Edmonston (MOI, 0.5), and the cell surface expression of N and H was analyzed by immunocytometry at different times after infection. The percentage of positive cells is given in the corner of each quadrant. (B) PBLs were infected with MV Edmonston; 48 h later, they were incubated with BFA (1 μg/ml) for 4 h; afterwards they were either stained or washed thoroughly, incubated for an additional 2 h at 37°C, and stained. (C) PBLs were infected with wild-type MV strain G954 (MOI, 0.1), previously inactivated or not by UV irradiation, or with the H- and F-deficient recombinant MV strain (MGV) or its molecularly cloned control (EDtag) (MOI, 0.5). N and H expression was analyzed 48 h postinfection. An isotype control for anti-N staining was used in all experiments; less than 0.5% of cells were positive with this control. (D) Immunofluorescence staining of N on nonpermeabilized human PBLs infected with wild-type MV, merged with transmitted light image.
MV replication was necessary for the surface expression of N, as evidenced by the fact that PBLs incubated with UV-inactivated MV expressed neither N nor H at the cell membrane (Fig. 1C). PBLs were then infected with one of several different wild-type MV strains—G954 (Fig. 1C), G945, or G943 (data not shown)-or with the recombinant virus MGV or the corresponding molecularly cloned control EDtag. In all cases, N was observed to be expressed on the surfaces of infected PBLs. Analysis of cells by immunofluorescent microscopy revealed that N was clustered in focused regions of the plasma membrane (Fig. 1D). Identical results were obtained with two different anti-N MAbs recognizing either the C-terminal or the N-terminal part of N (data not shown), indicating that epitopes on both domains are accessible at the cell membrane. Taken together, these observations demonstrate that N is transported from the cytoplasm to the cell membrane independently of MV glycoproteins and the type of MV strain used for infection.
N expression at the plasma membrane requires FcγRII.
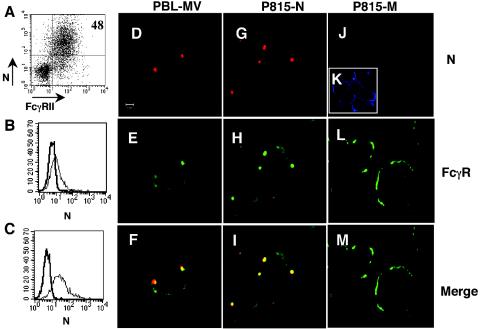
Cytofluorometric studies showed that not all MV-infected PBLs can express N at the membrane (Fig. 1), and this proportion was related to the percentage of cells expressing the N receptor, FcγRII. Analysis of infected human tonsillar lymphocytes revealed high N expression at the surfaces of those cells expressing FcγRII (Fig. 2A). This prompted us to analyze the requirement of FcγRII expression for export of N to the membrane. The murine B-cell line IIA1.6 and its FcγRIIb transfectant were infected with a recombinant vaccinia virus expressing MV N, and cells were analyzed for expression of N on their surfaces (Fig. 2B-C). Expression of N on the cell membrane was detected only for FcγRIIb-IIA1.6 cells. In accordance with these results, expression of N was not observed on the surfaces of cells lacking FcγR (N-transfected murine L fibroblasts, MV-infected Vero cells, and human T lymphocytes) but was readily detected on the FcγR-expressing human B-cell lines Raji and Daudi and the human monocyte line THP-1 when they were infected with MV (data not shown).
FIG. 2.
Delivery of MV N to the cell surface requires FcγR expression. (A) Tonsillar lymphocytes were activated by interaction with CD40-L and infected with MV Edmonston (MOI, 0.5), and cell membrane expression of N and FcγR was analyzed 48 h postinfection. (B) IIA1.6 cells or (C) IIA1.6 cells transfected with human FcγRIIb1 were infected with a recombinant vaccinia virus expressing MV N protein (MOI, 0.01) for 24 h and analyzed by immunocytometry for expression of N on cell membranes. (D to F) Nonpermeabilized human PBLs, (G to I) P815-N cells, or (J to M) P815-M cells were analyzed by confocal microscopy for cell surface expression of N (red) and FcγR (green). (K) M protein expression (blue) is detected after cell permeabilization. (F and I) Yellow color in the merged panels shows the colocalization of N and FcγR.
To confirm the association of these two proteins, MV-infected PBLs and cells of the FcγR-positive mastocytoma line P815 that had been transfected with either the MV n or the MV m gene were analyzed by confocal microscopy. When cells were labeled for both N and FcγR, cell surface N colocalized with FcγR both in infected PBLs (Fig. 2F) and in P815-N cells (Fig. 2I), producing the characteristic clusters in focused regions of the plasma membrane. After single labeling for FcγR, a similar type of FcγR staining in clusters was observed, in contrast to uniform staining in the absence of N (as in Fig. 2L), confirming the aggregation of N and FcγR at the cell surface and excluding the potential effect of the anti-N MAb (data not shown). When cells were labeled for the expression of other cell surface structures, such as sphingolipid-rich raft microdomains, no colocalization with N was observed, suggesting that formation of cell membrane N-FcγR aggregates does not occur in the raft microdomains (data not shown). Finally, the same pattern of uniform cell surface expression of MHC class I molecules was observed on both P815-N and P815-M cells, suggesting that formation of N-FcγR clusters on the cell membrane does not perturb the expression of the other surface proteins (data not shown). Altogether, these results point to the critical role of FcγR for the cell surface expression of N.
N enters the late endocytic compartment and binds FcγR.
We next analyzed the intracellular distribution of N by electron microscopy and immunogold labeling. The presence of N was detected in the cytoplasm and nuclei of P815-N cells, as described previously for different infected cell types (20), but also in the plasma membrane and in some intracellular vesicles (Fig. 3A and B), indicating translocation of N from the cytosol. Immunogold labeling for N, performed as a negative control on P815-M cells, showed a very low level of background staining (data not shown), confirming specific N expression in P815-N cells. However, N was not found in the Golgi complex by either electron or confocal microscopy using an anti-p23 Ab specific for the transmembrane protein of Golgi complex and the early secretory pathway (39) (data not shown).
FIG. 3.
Intracellular localization of MV N. (A and B) Representative examples of ultrathin sections of P815-N cells, analyzed by electron microscopy. Immunogold labeling revealed N in the cytoplasm, plasma membrane, and cytoplasmic vesicles (arrows). v, vesicle; m, cell membrane. Bars, 200 nm. (C) P815-N or P815-M cells or infected (G954; MOI, 0.1) human B lymphocytes or T lymphocytes were permeabilized and analyzed by confocal microscopy for intracellular expression of N (red), FcγR (green), or cathepsin B (blue). Yellow in merged panels, colocalization of N and FcγR; purple, colocalization of N and cathepsin B; white, all three markers.
Because transport of N to the cell surface was BFA sensitive (Fig. 1B) and BFA has been shown to affect endosomes and lysosomes in addition to the Golgi system (30), we analyzed the localization of N in the endolysosomal compartment (Fig. 3C). N colocalized with cathepsin B, an enzyme present in lysosomes and late endosomes. Interestingly, FcγR staining also colocalized with N and cathepsin B, demonstrating the association of N and FcγR in the late endocytic compartment. Moreover, while in P815-M cells, the majority of FcγR was expressed on the cell surface, rarely colocalizing with cathepsin B, in P815-N cells and infected B cells, most of the FcγR staining colocalized with cathepsin B, forming large clusters, illustrating the capacity of N to drive FcγR into the endolysosomal compartment. Furthermore, analyses of N distribution in MV-infected T lymphocytes, the majority of which did not express FcγR, also revealed the colocalization of N and cathepsin B in similar large clusters. These results were confirmed by using MV-infected FcγR-negative Jurkat cells, and localization of N in lysosomes in MV-infected T and B lymphocytes, as well as in the Raji, Daudi, and THP-1 cell lines, was also observed by using the lysosomal marker Lamp-1 (data not shown). Altogether, these results show that N, independently of FcγR, is able to enter into the endolysosomal pathway and there induce the recruitment of its receptor, required for N expression on cell membranes.
MV N is secreted and binds to neighboring cells.
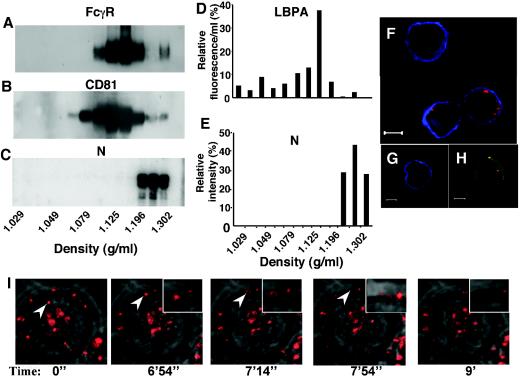
We next studied whether N can be secreted from viable cells, and we analyzed its presence in different fractions obtained by differential centrifugation of cell culture supernatants. While FcγR was found enriched in fractions corresponding to exosomes (Fig. 4A) and immunostained with either the exosome-specific tetraspan protein CD81 (Fig. 4B) or anti-exosomal polyclonal antibodies (data not shown), the majority of N was found in subsequent fractions with higher density (Fig. 4C). This dissociation of N from FcγR may be favored by decreasing concentrations of N, once it has reached an extracellular environment. Antibodies specific to either the N-terminal or the C-terminal region of N recognized secreted N, suggesting that both domains were preserved after secretion (data not shown). The fractions containing N were not associated with LBPA, a lipid linked to late endosome-derived microvesicles and exosomes (27) (Fig. 4D and E), demonstrating that secreted N is not associated with microvesicles and FcγR.
FIG. 4.
MV N is secreted and binds to neighboring cells. P815-N supernatants were subjected to CsCl gradients. After centrifugation, fractions were collected and analyzed by Western blotting for (A) FcγR, (B) CD81, and (C) N. (D) LBPA content in different fractions was determined by ELISA. (E) N content in different fractions was evaluated by phosphorimager. (F-H) P815-M cells, cultured 48 h with P815-N cells in transwell cultures (F and H) or alone (G), were analyzed for expression of FcγR, MV N, and MV M protein by confocal microscopy. N and FcγR expression is visualized by red and green, respectively. (F, G) M expression was analyzed after cell permeabilization and is visualized in blue. (H) Yellow in merged panels shows colocalization of N and FcγR. (I) Dynamics of cellular transit of N. Images are extracted from time-lapse video analysis of P815-N cells stained by an anti-N MAb, merged with a transmitted light image. Arrowheads follow a representative N-labeled spot up to its release from the cell surface and dispersion in the extracellular milieu.
A potential consequence of N dissociation from FcγR would be the ability of secreted N to reach neighboring cells and bind their cell surface FcγR. P815-N and P185-M cells were therefore cultured in adjacent compartments separated by a membrane and then analyzed by confocal microscopy. After coculture with P815-N cells, some P815-M cells stained for N (Fig. 4F), and N colocalized with FcγR (Fig. 4H), demonstrating that N can be secreted and bind to the FcγR of neighboring cells. In addition to cell surface binding, N could also be internalized in P815-M cells, as revealed by confocal microscopy (Fig. 4). Finally, secretion of N was monitored by time-lapse imaging of P815-N cells labeled for N, showing the transit of N from the cytosol to the membrane and its release outside the cell (Fig. 4I; see also Movie S1 in the supplemental material). Similarly to the observation with immunofluorescence staining in Fig. 1D, the majority of membrane N staining is localized to one pole of the cell. One particular labeled N molecule was monitored for 9 min, from its appearance in the cytoplasm to its release in the extracellular environment. Thus, in addition to its cell membrane localization, N can be also liberated into the extracellular compartment and bind neighboring cells.
Cell-derived N inhibits IL-12 p40 secretion and the hypersensitivity reaction.
Finally, we analyzed whether cell-derived N could affect the secretion of IL-12, the central cytokine in the development of cell-mediated immunity. Cells of the human monocyte line THP-1 were stimulated with IFN-γ and LPS in order to induce IL-12 secretion and were then incubated with P815-N or P815-M cells. Coculture with P815-N cells, but not with P815-M cells, decreased the production of IL-12 p40 from monocytes (Fig. 5A). Further, inhibition of IL-12 p40 secretion was observed when recombinant MV N was incubated for 24 h with either monocytes (Fig. 5B) or murine macrophages (RAW 264.7) (Fig. 5C). These results demonstrate that cell-derived N, as well as exogenous recombinant N, inhibits IL-12 secretion.
FIG. 5.
MV N inhibits IL-12 secretion and the contact hypersensitivity reaction. (A and B) THP-1 or (C) RAW 264.7 cells were activated by IFN-γ and then stimulated with LPS in the presence of different numbers of P815-N or P815-M cells (A) or soluble recombinant N (rN) (B and C). IL-12 p40 production was analyzed by ELISA. Error bars correspond to standard deviations from duplicate samples. (D) Groups of five DBA-2 mice were injected intraperitoneally with either 2 × 106 P815-N cells or 2 × 106 P815-M cells, or with 100 μg of rN or PBS. Mice were sensitized 5 days later (P815-N and P815-M groups) or 6 h later (N and PBS groups) with DNFB, or were left unsensitized, and were challenged after 5 days with DNFB. Results are expressed for each mouse at 24 h after challenge, and the mean for each group is illustrated by a horizontal line. *, P < 0.05 by Student's t test; **, P < 0.01 by Student's t test.
Since inhibition of IL-12 secretion was observed with both human and murine cells, we analyzed the immunopathological importance of cell-derived N in vivo in mice. We used the IL-12-dependent hypersensitivity reaction to an allergen, DNFB. P815-N or P815-M cells were injected into syngeneic mice, and mice were sensitized to DNFB. Mice were then challenged by DNFB applied to the ear, and the inflammatory reaction, in the form of ear swelling, was measured 24 h later (Fig. 5D). Injection of P815-N cells, like injection of recombinant N, inhibited the inflammation. These results demonstrate that cell-derived N induces immunosuppression in vivo, revealing a novel mechanism for immune abnormalities observed during measles.
DISCUSSION
One of the major difficulties in understanding the mechanism of MV-induced immunosuppression is to reconcile the small number of MV-infected cells with the development of a profound immunosuppression persisting for several weeks after the peak of infection. This study reveals the important role of MV nucleoprotein in the pathogenesis of this phenomenon. Recombinant MV N has been shown previously to bind both human and murine cell surface FcγRII and to inhibit antibody synthesis (36) and dendritic cell activity (31). However, the mechanism used by this cytosolic protein to reach the extracellular environment and bind FcγR remained elusive. Although release of N after the death of infected cells is one possibility, this study demonstrates another, complementary mechanism, whereby N translocates to the plasma membranes of infected cells, is secreted into the extracellular milieu, and therefore becomes directly available to interact with uninfected cells and induce immunosuppression.
N has been reported to associate with MV phosphoprotein (24) as well as with cellular heat shock protein (34) and the interferon regulatory factor IRF-3 (47). However, delivery of N to the cell surface has not been reported before. This study demonstrates the requirement of FcγR for the plasma membrane expression of N; FcγR is expressed principally on B lymphocytes, monocytes, mastocytes, and dendritic cells (1), which are not the common cell types used to propagate MV in the laboratory, making the observation of the cell surface expression of N less evident.
The capacity of the cytosolic protein N to translocate to the plasma membrane raised many intriguing questions concerning its intracellular transport mechanism. In contrast to the MV matrix protein, which targets the inner cell membrane only in the presence of some component(s) provided by the MV-infected cell (38), our results demonstrate that N does not require any other viral protein to reach the cell surface, suggesting some other mechanism of intracellular transport. It is tempting to speculate that MV N translocates to the plasma membrane using a nonclassical transport mechanism, as has been reported for some other virus proteins (35). This study revealed that recruitment of N into the late endocytic compartment is associated with the formation of large vesicular structures, strongly suggesting the capacity of N to accumulate in late endosomes and lysosomes, as has been shown for the HIV protein Nef (40). Furthermore, the strong increase in FcγR localization in the endolysosomal compartment in infected B lymphocytes and P815-N cells demonstrates that N is able to drive FcγR into the endocytic pathway. This is particularly surprising in the case of FcγRIIb1, the major FcγR isoform expressed in B cells, known to be inefficient at internalization even after the binding of a polyvalent ligand (1), and suggests the remarkable difference between N and other FcγR ligands. Finally, colocalization of N with cathepsin B and Lamp-1 in T lymphocytes, but lack of N expression on the membranes of these FcγR-negative cells, confirms the translocation of N after its cytosolic synthesis into the late endocytic compartment and consequent FcγR-mediated binding to the plasma membrane (Fig. 6). Interaction of N with FcγR in endolysosomes may protect N from proteolytic cleavage in this compartment and allow its expression on the cell surface.
FIG. 6.
Proposed model for delivery of MV N to cell membranes. N is synthesized in the cytosol of MV-infected cells and participates in the formation of new virus particles (step 1). In addition, excess N translocates directly into the endolysosomal compartment, where it interacts with FcγR, forming large clusters (step 2); then N is exported to the cell surface and/or released extracellularly (step 3). Finally, due to its oligomeric structure, cell surface-expressed N could induce the capping of free FcγR and facilitate its endocytosis and further localization in the late endocytic compartment (step 4). Cell surface-bound N may interact with neighboring cells expressing FcγR and induce immunosuppression, while secreted N could, in addition, modulate the immune response in distant targets.
The secretion of N, revealed by this study, was not associated with the exosomal microvesicle fraction, in contrast to the immunosuppressive LMP1 protein, shown to be secreted in the form of exosomes in Epstein-Barr virus-infected B cells (8). Since lysosomal exocytosis has been demonstrated to be important for removing excessive undigested material from the cell (2), our results suggest that this pathway may be a part of a process of export of excess N from MV-infected cells. Altogether, these results open new avenues toward further analysis of the intracellular fate of N and the modulation of cellular pathways induced by this protein.
The relative importance of different MV proteins in the induction of immunosuppression has been suggested in several different studies. The interaction of the MV glycoproteins H and F expressed on the cell membranes of infected PBLs with an unknown receptor expressed on the surfaces of different types of hematopoietic cells has been shown to inhibit cell-proliferation (41) and disrupt Akt kinase activation in T lymphocytes (4), and MV envelope proteins have been demonstrated to limit the capacity of mouse B cells to produce MV-neutralizing antibodies (12). Further, cell membrane-associated MV components have been reported to inhibit antigen processing of mononuclear cells to antigen-specific T cells (32). The existence of a soluble factor, capable of inhibiting lymphocyte proliferation, produced by infected PBLs or B-cell lines has been demonstrated (14, 19). Whether N or its fragments may have some of the immunosuppressive effects seen in these studies remains to be analyzed. In contrast to MV membrane glycoproteins, which could express their immunosuppressive action only in the context of the infected cell membrane, N can in addition be secreted and contribute to the immunosuppressive effect of MV glycoproteins. In this manner, N can increase its ability to bind distant, uninfected FcγR-expressing cells essential for the immune response, such as macrophages or dendritic cells, and consequently to perturb their function. In addition, secreted N may carry out its immunosuppressive activity via a recently identified, widely expressed receptor distinct from FcγRII (29). Furthermore, the circulation of the few MV-infected blood cells could be a way to deliver N to a wide number of uninfected cells and affect their function.
In children infected with MV, production of IL-12 is decreased (3). Inhibition of IL-12 secretion has been suggested to be an important mechanism of suppression of cellular immunity during measles, induced by interaction of MV H (vaccine strain) with the MV receptor CD46 (26). However, the recent identification of CD150 (SLAM) as a receptor for wild-type MV made this hypothesis less likely, since the majority of wild type MV strains do not seem to use CD46 (46). Regulation of IL-12 production is FcγR dependent (19), and inactivated MV was unable to inhibit IL-12 production by dendritic cells in FcγR-deficient mice (31). The results presented in this study demonstrate that N inhibits IL-12 secretion. Thus, the N-FcγR interaction might play a double role in MV-induced immunosuppression, allowing (i) expression of N on the cell membrane after the translocation of N from the endocytic compartment in the infected cell and (ii) a direct immunosuppressive effect after binding of either the membrane or secreted N to FcγR on uninfected cells. Because infection with both wild-type and vaccine strains of MV produces N capable of interacting with FcγR, this mechanism could play an important role in the immunological alterations observed during natural measles as well as after anti-measles vaccination.
Surprisingly, N induces a good anti-N immune response, and the most abundant and the most rapidly produced antibodies in measles patients are those specific to N (18). It has been demonstrated that buccal application of recombinant N without any adjuvant induces dendritic cell migration and cytotoxic responses in mice (11). Thus, immunosuppression during measles is accompanied by a strong anti-measles immune response, particularly against N, presenting an “immunological paradox” (20, 22). Interestingly, the FcγR-mediated internalization of immune complexes by dendritic cells has been shown to be associated with efficient cross-priming (37), as well as the enhancement of MHC class II antigen presentation (1). Therefore, the internalization of the FcγR-N complex shown in this study may play a role in the generation of a good anti-N immune response during measles infection (Fig. 6). The molecular mechanism of the apparent paradox of measles is not yet understood, but the results presented in this work offer some clues for future studies of the intracellular processing of N, which may be important for its immunosuppressive action as well as for immunostimulatory action.
Altogether, this study provides compelling evidence that a small number of MV-infected cells are able to induce immunosuppression via cell-derived MV N, suggesting a major role of this protein in the perturbation of the immune response during measles and revealing a novel viral strategy in measles pathogenesis. Future studies should define the sites of interaction between N and FcγR and allow the design of specific inhibitors of this interaction, providing a framework for intervention during measles. Finally, N or its fragments may have potential therapeutic application in immunomodulatory protocols aimed at treating IL-12-mediated inflammatory diseases or preventing graft rejection in transplanted patients.
Supplementary Material
Acknowledgments
We are grateful to F. Letourneur for suggestions and critical reading of the manuscript and to S. Peyrole for help with electron microscopy studies. The expert technical assistance of C. Bella, M. Casamayor-Palleja, Y. Kerdiles, N. Pelletier, L. Perrin-Cocon, R. Ruigrok, and K. Rodet is acknowledged.
This work was supported by institutional grants from INSERM, FITT-Region Rhône-Alpes, and grant 4450 from ARC (to B.H.).
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Amigorena, S., and C. Bonnerot. 1999. Fc receptor signaling and trafficking: a connection for antigen processing. Immunol. Rev. 172:279-284. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, N. W. 2000. Regulated secretion of conventional lysosomes. Trends Cell Biol. 10:316-321. [DOI] [PubMed] [Google Scholar]
- 3.Atabani, S. F., A. A. Byrnes, A. Jaye, I. M. Kidd, A. F. Magnusen, H. Whittle, and C. L. Karp. 2001. Natural measles causes prolonged suppression of interleukin-12 production. J. Infect. Dis. 184:1-9. [DOI] [PubMed] [Google Scholar]
- 4.Avota, E., A. Avots, S. Niewiesk, L. P. Kane, U. Bommhardt, V. ter Meulen, and S. Schneider-Schaulies. 2001. Disruption of Akt kinase activation is important for immunosuppression induced by measles virus. Nat. Med. 7:725-731. [DOI] [PubMed] [Google Scholar]
- 5.Beauverger, P., R. Buckland, and T. F. Wild. 1993. Measles virus antigens induce both type-specific and canine distemper virus cross-reactive cytotoxic T lymphocytes in mice: localization of a common Ld-restricted nucleoprotein epitope. J. Gen. Virol. 74:2357-2363. [DOI] [PubMed] [Google Scholar]
- 6.Borrow, P., and M. B. Oldstone. 1995. Measles virus-mononuclear cell interactions. Curr. Top. Microbiol. Immunol. 191:85-100. [DOI] [PubMed] [Google Scholar]
- 7.Buckland, R., P. Giraudon, and F. Wild. 1989. Expression of measles virus nucleoprotein in Escherichia coli: use of deletion mutants to locate the antigenic sites. J. Gen. Virol. 70:435-441. [DOI] [PubMed] [Google Scholar]
- 8.Dukers, D. F., P. Meij, M. B. Vervoort, W. Vos, R. J. Scheper, C. J. Meijer, E. Bloemena, and J. M. Middeldorp. 2000. Direct immunosuppressive effects of EBV-encoded latent membrane protein 1. J. Immunol. 165:663-670. [DOI] [PubMed] [Google Scholar]
- 9.Escola, J. M., M. J. Kleijmeer, W. Stoorvogel, J. M. Griffith, O. Yoshie, and H. J. Geuze. 1998. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 273:20121-21027. [DOI] [PubMed] [Google Scholar]
- 10.Esolen, L. M., B. J. Ward, T. R. Moench, and D. E. Griffin. 1993. Infection of monocytes during measles. J. Infect. Dis. 168:47-52. [DOI] [PubMed] [Google Scholar]
- 11.Etchart, N., P. O. Desmoulins, K. Chemin, C. Maliszewski, B. Dubois, F. Wild, and D. Kaiserlian. 2001. Dendritic cells recruitment and in vivo priming of CD8+ CTL induced by a single topical or transepithelial immunization via the buccal mucosa with measles virus nucleoprotein. J. Immunol. 167:384-391. [DOI] [PubMed] [Google Scholar]
- 12.Fehr, T., H. Y. Naim, M. F. Bachmann, A. F. Ochsenbein, P. Spielhofer, E. Bucher, H. Hengartner, M. A. Billeter, and R. M. Zinkernagel. 1998. T-cell independent IgM and enduring protective IgG antibodies induced by chimeric measles viruses. Nat. Med. 4:945-948. [DOI] [PubMed] [Google Scholar]
- 13.Fugier-Vivier, I., C. Servet-Delprat, P. Rivailler, M. C. Rissoan, Y. J. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med. 186:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujinami, R. S., X. Sun, J. M. Howell, J. C. Jenkin, and J. B. Burns. 1998. Modulation of immune system function by measles virus infection: role of soluble factor and direct infection. J. Virol. 72:9421-9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galama, J. M. D., J. Ubels-Postma, A. Vos, and C. J. Lucas. 1980. Measles virus inhibits acquisition of lymphocyte functions but not established effector functions. Cell. Immunol. 50:401. [DOI] [PubMed] [Google Scholar]
- 16.Gately, M. K., R. Chizzonite, and D. H. Presky. 1995. Measurement of human and mouse interleukin 12, p. 6.16.1-6.16.15. In J. E. Coligan et al. (ed.), Current protocols in immunology, vol. 1. John Wiley & Sons Inc., New York, N.Y. [DOI] [PubMed] [Google Scholar]
- 17.Gombart, A. F., A. Hirano, and T. C. Wong. 1993. Conformational maturation of measles virus nucleocapsid protein. J. Virol. 67:4133-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graves, M., D. E. Griffin, R. T. Johnson, R. L. Hirsch, I. L. de Soriano, S. Roedenbeck, and A. Vaisberg. 1984. Development of antibody to measles virus polypeptides during complicated and uncomplicated measles virus infections. J. Virol. 49:409-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grazia Cappiello, M., F. S. Sutterwala, G. Trinchieri, D. M. Mosser, and X. Ma. 2001. Suppression of IL-12 transcription in macrophages following Fcγ receptor ligation. J. Immunol. 166:4498-4506. [DOI] [PubMed] [Google Scholar]
- 20.Griffin, D. E., and W. J. Bellini. 1996. Measles virus, p. 1267-1308. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 21.Griffin, D. E., and B. J. Ward. 1993. Differential CD4 T cell activation in measles. J. Infect. Dis. 168:275-281. [DOI] [PubMed] [Google Scholar]
- 22.Griffin, D. E., B. J. Ward, and L. M. Esolen. 1994. Pathogenesis of measles virus infection: an hypothesis for altered immune responses. J. Infect. Dis. 170:S24-S31. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch, R. L., D. E. Griffin, R. T. Johnson, S. J. Cooper, I. Lindo de Soriano, S. Roedenbeck, and A. Vaisberg. 1984. Cellular immune responses during complicated and uncomplicated measles virus infections of man. Clin. Immunol. Immunopathol. 31:1-12. [DOI] [PubMed] [Google Scholar]
- 24.Huber, M., R. Cattaneo, P. Spielhofer, C. Orvell, E. Norrby, M. Messerli, J. C. Perriard, and M. A. Billeter. 1991. Measles virus phosphoprotein retains the nucleocapsid protein in the cytoplasm. Virology 185:299-308. [DOI] [PubMed] [Google Scholar]
- 25.Hussey, G. D., E. A. Goddard, J. Hughes, J. J. Ryon, M. Kerran, E. Carelse, P. M. Strebel, L. E. Markowitz, J. Moodie, P. Barron, Z. Latief, R. Sayed, D. Beatty, and D. E. Griffin. 1996. The effect of Edmonston-Zagreb and Schwarz measles vaccines on immune response in infants. J. Infect. Dis. 173:1320-1326. [DOI] [PubMed] [Google Scholar]
- 26.Karp, C. L., M. Wysocka, L. M. Wahl, J. M. Ahearn, P. J. Cuomo, B. Sherry, G. Trinchieri, and D. E. Griffin. 1996. Mechanism of suppression of cell-mediated immunity by measles virus. Science 273:228-231. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi, T., M. H. Beuchat, J. Chevallier, A. Makino, N. Mayran, J. M. Escola, C. Lebrand, P. Cosson, and J. Gruenberg. 2002. Separation and characterization of late endosomal membrane domains. J. Biol. Chem. 277:32157-32164. [DOI] [PubMed] [Google Scholar]
- 28.Kouomou, D. W., E. Nerrienet, J. Mfoupouendoun, G. Tene, H. Whittle, and T. F. Wild. 2002. Measles virus strains circulating in Central and West Africa: geographical distribution of two B3 genotypes. J. Med. Virol. 68:433-440. [DOI] [PubMed] [Google Scholar]
- 29.Laine, D., M. C. Trescol-Biemont, S. Longhi, G. Libeau, J. C. Marie, P. O. Vidalain, O. Azocar, A. Diallo, B. Canard, C. Rabourdin-Combe, and H. Valentin. 2003. Measles virus (MV) nucleoprotein binds to a novel cell surface receptor distinct from FcγRII via its C-terminal domain: role in MV-induced immunosuppression. J. Virol. 77:11332-11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lippincott-Schwartz, J., L. Yuan, C. Tipper, M. Amherdt, L. Orci, and R. D. Klausner. 1991. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell 67:601-616. [DOI] [PubMed] [Google Scholar]
- 31.Marie, J. C., J. Kehren, M. C. Trescol-Biemont, A. Evlashev, H. Valentin, T. Walzer, R. Tedone, B. Loveland, J. F. Nicolas, C. Rabourdin-Combe, and B. Horvat. 2001. Mechanism of measles virus-induced suppression of inflammatory immune responses. Immunity 14:69-79. [DOI] [PubMed] [Google Scholar]
- 32.Marttila, J., A. Hinkkanen, T. Ziegler, R. Vainionpaa, A. Salmi, and J. Ilonen. 2001. Cell membrane-associated measles virus components inhibit antigen processing. Virology 279:422-428. [DOI] [PubMed] [Google Scholar]
- 33.Murray, C. J., and A. D. Lopez. 1997. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 349:1269-1276. [DOI] [PubMed] [Google Scholar]
- 34.Oglesbee, M., and S. Krakowka. 1993. Cellular stress response induces selective intranuclear trafficking and accumulation of morbillivirus major core protein. Lab. Investig. 68:109-117. [PubMed] [Google Scholar]
- 35.Prochiantz, A. 2000. Messenger proteins: homeoproteins, TAT and others. Curr. Opin. Cell Biol. 12:400-406. [DOI] [PubMed] [Google Scholar]
- 36.Ravanel, K., C. Castelle, T. Defrance, T. F. Wild, D. Charron, V. Lotteau, and C. Rabourdin-Combe. 1997. Measles virus nucleocapsid protein binds to FcγRII and inhibits human B cell antibody production. J. Exp. Med. 186:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regnault, A., D. Lankar, V. Lacabanne, A. Rodriguez, C. Thery, M. Rescigno, T. Saito, S. Verbeek, C. Bonnerot, P. Ricciardi-Castagnoli, and S. Amigorena. 1999. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189:371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riedl, P., M. Moll, H. D. Klenk, and A. Maisner. 2002. Measles virus matrix protein is not cotransported with the viral glycoproteins but requires virus infection for efficient surface targeting. Virus Res. 83:1-12. [DOI] [PubMed] [Google Scholar]
- 39.Rojo, M., R. Pepperkok, G. Emery, R. Kellner, E. Stang, R. G. Parton, and J. Gruenberg. 1997. Involvement of the transmembrane protein p23 in biosynthetic protein transport. J. Cell Biol. 139:1119-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanfridson, A., S. Hester, and C. Doyle. 1997. Nef proteins encoded by human and simian immunodeficiency viruses induce the accumulation of endosomes and lysosomes in human T cells. Proc. Natl. Acad. Sci. USA 94:873-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlender, J., J. J. Schnorr, P. Spielhoffer, T. Cathomen, R. Cattaneo, M. A. Billeter, V. ter Meulen, and S. Schneider-Schaulies. 1996. Interaction of measles virus glycoproteins with the surface of uninfected peripheral blood lymphocytes induces immunosuppression in vitro. Proc. Natl. Acad. Sci. USA 93:13194-13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shearer, G. M. 1998. HIV-induced immunopathogenesis. Immunity 9:587-593. [DOI] [PubMed] [Google Scholar]
- 43.Spehner, D., A. Kirn, and R. Drillien. 1991. Assembly of nucleocapsidlike structures in animal cells infected with a vaccinia virus recombinant encoding the measles virus nucleoprotein. J. Virol. 65:6296-6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spielhofer, P., T. Bachi, T. Fehr, G. Christiansen, R. Cattaneo, K. Kaelin, M. A. Billeter, and H. Y. Naim. 1998. Chimeric measles viruses with a foreign envelope. J. Virol. 72:2150-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamashiro, V. G., H. H. Perez, and D. E. Griffin. 1987. Prospective study of the magnitude and duration of changes in tuberculin reactivity during uncomplicated and complicated measles. Pediatr. Infect. Dis. J. 6:451-454. [DOI] [PubMed] [Google Scholar]
- 46.Tatsuo, H., N. Ono, K. Tanaka, and Y. Yanagi. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893-897. [DOI] [PubMed] [Google Scholar]
- 47.TenOever, B. R., M. J. Servant, N. Grandvaux, R. Lin, and J. Hiscott. 2002. Recognition of the measles virus nucleocapsid as a mechanism of IRF-3 activation. J. Virol. 76:3659-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Den Herik-Oudijk, I. E., N. A. Westerdaal, N. V. Henriquez, P. J. Capel, and J. G. Van De Winkel. 1994. Functional analysis of human FcγRII (CD32) isoforms expressed in B lymphocytes. J. Immunol. 152:574-585. [PubMed] [Google Scholar]
- 49.Von Pirquet, C. 1908. Das Verhalten der kutanen Tuberculinreaktion während der Mäsern. Dtsch. Med. Wochenschr. 30:1297-1300. [Google Scholar]
- 50.Wild, T. F., A. Bernard, D. Spehner, and R. Drillien. 1992. Construction of vaccinia virus recombinants expressing several measles virus proteins and analysis of their efficacy in vaccination of mice. J. Gen. Virol. 73:359-367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.