Abstract
The cytoplasmic tail (CT) of hemagglutinin (HA) of influenza B virus (BHA) contains at positions 578 and 581 two highly conserved cysteine residues (Cys578 and Cys581) that are modified with palmitic acid (PA) through a thioester linkage. To investigate the role of PA in the fusion activity of BHA, site-specific mutagenesis was performed with influenza B virus B/Kanagawa/73 HA cDNA. All of the HA mutants were expressed on Cos cells by an expression vector. The membrane fusion ability of the HA mutants at a low pH was quantitatively examined with lipid (octadecyl rhodamine B chloride) and aqueous (calcein) dye transfer assays and with the syncytium formation assay. Two deacylation mutants lacking a CT or carrying serine residues substituting for Cys578 and Cys581 promoted full fusion. However, one of the single-acylation-site mutants, C6, in which Cys581 is replaced with serine, promoted hemifusion but not pore formation. In contrast, four other single-acylation-site mutants that have a sole cysteine residue in the CT at position 575, 577, 579, or 581 promoted full fusion. The impaired pore-forming ability of C6 was improved by amino acid substitution between residues 578 and 582 or by deletion of the carboxy-terminal leucine at position 582. Syncytium-forming ability, however, was not adequately restored by these mutations. These facts indicated that the acylation was not significant in membrane fusion by BHA but that pore formation and pore dilation were appreciably affected by the particular amino acid sequence of the CT and the existence of a single acylation site in CT residue 578.
Membrane fusion is an important event for enveloped viruses in the early stage of infection. Enveloped viruses inject their genomes into host cells by membrane fusion, which is mediated by particular viral proteins. Among these, hemagglutinin (HA) of influenza A virus (AHA) is the best-characterized viral protein and has been shown to induce low-pH-dependent membrane fusion (30). HA is a type I homotrimeric glycoprotein, and the precursor of each of its monomers is synthesized as a single polypeptide chain. After virus budding or during intracellular transport, the HA precursor is processed by proteolytic enzymes to generate two disulfide-linked subunits, HA1 and HA2. This processing creates a new amino terminus on HA2, referred to as the fusion peptide. In addition to fusion activity, HA is also responsible for binding of the virus to cell surface receptors (14). Since determination of the three-dimensional structure of H3 HA by X-ray analysis, the functional regions, i.e., the receptor-binding site and the fusion peptide, have been located on three-dimensional models (36).
Following the entry of influenza viruses into host cells through receptor-mediated endocytosis, an acidic environment in the endosome triggers a conformational change in HA. Details of the conformational change were recently demonstrated by X-ray analysis of a fragment of low-pH-treated HA and by studies of recombinant, bacterially expressed HA2 (2, 3, 4). These studies showed that the conformational change induces the extension of two helical segments in the stem region of HA2 and the transposition of the fusion peptide from the lower portion toward the receptor-binding domain of HA1. The subsequent fusion process mediated by HA comprises three steps, (i) lipid mixing between outer leaflets of the viral and target membranes (hemifusion), (ii) fusion pore formation by merging of the inner lipid leaflets, which would bring about an aqueous connection between the virus and the target membrane interior, and (iii) pore dilation (30). A low-pH-dependent conformational change in the HA ectodomain and penetration of the exposed fusion peptide into the target membrane are triggers for hemifusion (32, 35). Recently, transmembrane and cytoplasmic domains of HA were shown to play an important role in fusion pore formation and pore dilation (1, 10, 11, 12, 16, 17, 18, 19, 26).
An evaluation of the amino acid sequence of the carboxy terminus of HA2 shows that the cytoplasmic tail (CT), including the cysteine residues, is well conserved among 15 HA subtypes of influenza A viruses (9, 23, 27). The cysteine residues, one (residue 555) in the transmembrane (TM) domain and two (residues 562 and 565) in the CT, are posttranslationally modified with palmitic acid (PA) through thioester linkages (21, 22, 29, 34). To examine the significance of this palmitylation in the fusion activity of HA, several studies were performed with cysteine substitution mutants of four HA subtypes (5, 17, 21, 22, 28, 29, 31). Analysis of the mutants initially showed that the palmitylation of H2 HA was important for fusion ability (21); however, subsequent experiments did not support this finding (22). In the earlier study, amino acid substitutions of cysteine with alanine in the CT severely inhibited syncytium formation (21), whereas in the later study, no effect on membrane fusion was observed with cysteine-to-serine changes (22). On the other hand, other deacylation mutants of H1 HA drastically restricted fusion pore formation (28), and analogous mutants of H7 HA impaired syncytium formation (5), while similar mutants of H3 HA promoted full fusion (29, 31). These studies showed that the effects of acylation on fusion ability were different among different subtypes of HA.
In contrast to that of AHA, the three-dimensional structure of HA of influenza B virus (BHA) has not been determined yet. BHA is thought to comprise an ectodomain (545 residues), a TM domain (28 residues), and a CT (10 residues) on the basis of a comparison of amino acid sequences of type A HA and type B HA. Although the deduced amino acid sequence of B/Lee/40 has 24% identity to that of HA1 of A/PR/8/34 and 39% identity to HA2 of A/PR/8/34, the 10 N-terminal residues of HA2 (fusion peptide) show high identity between both types of HAs. Furthermore, two cysteine residues in the CT are well conserved in AHA and BHA, although amino acid sequences other than cysteines are not conserved between them (13). In contrast to the TM domain of AHA, there is no cysteine residue in the TM domain of BHA. Palmitylation in the CT of BHA was previously reported (33); however, the role of PA in membrane fusion has not been clarified.
In this study, we generated acylation mutants of BHA and examined their fusion ability. Our results showed that acylation was dispensable for membrane fusion by BHA; however, the carboxy-terminal amino acid sequence Cys-Ser-Iso-Ser-Leu (CSISL) between residues 578 and 582 significantly impaired pore formation, and the existence in the CT of a single acylation site at cysteine residue 578 had a deleterious effect on pore dilation.
MATERIALS AND METHODS
Cells and virus.
Mardin-Darby canine kidney (MDCK) cells and Cos cells were maintained in Dulbecco's modified minimum essential medium (DMEM) supplemented with 7.5% fetal bovine serum (FBS). B/Kanagawa/73 virus was propagated in the MDCK cells.
Site-specific mutagenesis of B/Kanagawa/73 HA cDNA.
cDNA of the HA of B/Kanagawa/73 (BKHA) was subcloned between the EcoRI and XbaI sites in plasmid DNA of the pME18s expression vector (pME18s/BKHA) as previously described (15). Site-specific mutagenesis was performed by standard PCR protocols with the overlap extension technique (7). Nine HA mutants (Fig. 1) were generated as follows. To construct single-acylation-site mutants, which we termed C6 and C9, plasmid DNA of pME18s/BKHA was used as a template. No-acylation-site mutant C0 and the C6 derivatives C6-d10 and C6-NIAI were constructed with DNA of C6 as a template. C6-d10 was generated by introducing a stop codon at residue 582 of C6. DNA of C0 was used as a template to generate single-acylation-site mutants C3, C5, and C7. No-acylation-site mutant CT− was generated by introducing a stop codon at residue 573 of C0. cDNA of HA mutants was sequenced to confirm that the respective mutants had the desired mutations and no additional ones.
FIG. 1.
Schematic diagram of HA-wt (WT) and HA mutants. The expanded region shows amino acid sequences of the CT. The nomenclature for the mutants was derived by sequentially assigning the numbers 1 to 10 to residues 573 to 582, respectively. Amino acid substitutions or deletions in the HA mutants are summarized. The asterisk indicates the introduction of translational stop codons.
Expression of mutant HAs.
Cos cells were transiently transfected with plasmid vector DNA of wild-type BKHA (HA-wt) and HA mutants by using Lipofectamine 2000 (Invitrogen Japan K.K., Tokyo, Japan) as described by the manufacturer with slight modifications. In brief, Cos cells (1.6 × 105 cells) were prepared 1 day before use. The growth medium was replaced with 0.33 ml of DMEM containing 240 ng of DNA and 0.9 μl of Lipofectamine. At 6 h posttransfection, the transfection medium was replaced with 7.5% FBS-DMEM. The characteristics of the respective HA proteins expressed on the Cos cells were analyzed at 48 h posttransfection.
Flow cytometric analysis.
For detection of the cell surface expression of HA, we performed fluorescence analysis as described previously with a slight modification (25). In brief, Cos cells expressing HA were detached from dishes with 0.02% EDTA and 0.05% trypsin and washed with phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin. The cells were stained with anti-B/Kanagawa/73 hyperimmune rabbit serum and fluorescein-conjugated goat anti-rabbit immunoglobulin G antibody. After suspension in 1 ml of PBS, the cells were subjected to fluorescence-activated cell sorting (Becton Dickinson, Rutherford, N.J.) analysis.
Metabolic labeling and immunoprecipitation.
At 40 h posttransfection, Cos cells were incubated in methionine- and cysteine-deficient DMEM (DMEM Met−/Cys− for 1 h, metabolically labeled with Tran-35S label (2.5 μCi) in 500 μl of DMEM Met−/Cys− for 3 h at 37°C, and chased for 4 h. At 29 h posttransfection, Cos cells were incubated in labeling medium (DMEM supplemented with 5 mM sodium pyruvate and 7.5% FBS) for 1 h. Next, metabolic labeling of the cells with [3H]PA (500 μCi/ml) in 500 μl of labeling medium was performed for 18 h at 37°C. The cells were washed with cold PBS, lysed with radioimmunoprecipitation assay buffer (0.15 M NaCl, 50 mM Tris-Cl [pH 7.4], 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) on ice for 20 min, and centrifuged at 15,000 rpm (Hitachi RT15A8 rotor) for 30 min to remove cell debris. The supernatant was incubated with rabbit serum against B/Kanagawa/73 virus and protein G-Sepharose beads overnight at 4°C. After centrifugation, the Sepharose pellets were washed four times with radioimmunoprecipitation assay buffer. To release the immunocomplexes from the Sepharose beads, the immunoprecipitates from Tran-35S-labeled cells were boiled for 5 min in sample buffer (50 mM Tris, 2% SDS, 0.1% bromophenol blue, 10% glycerol, 1% 2-mercaptoethanol), and those from [3H]PA-labeled cells were incubated for 3 min at 80°C in the same sample buffer but containing 20 mM dithiothreitol instead of 2-mercaptoethanol. After centrifugation, the supernatant was analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) in a 10% acrylamide gel and visualized by autoradiography of the dried gel with Kodak X-OMAT AR film. Quantification of the fluorograms was performed by using a Fluor-S Multi-Image system and Multi-Analyst software or by using a FUJIX BAS 2500 system. When necessary, cleavage of HA0 to HA1 and HA2 was performed after metabolic labeling by treatment of HA-expressing cells with 10 μg of (l-tosylamido-2-phenyl)ethyl chloromethyl ketone-treated trypsin (TPCK-trypsin)/ml for 15 min at 37°C.
Hemadsorption test.
HA-expressing cells were washed once with DMEM and incubated with Vibrio cholerae neuraminidase (VCNA) (Roche Diagnostics K.K., Tokyo, Japan) (25 mU/ml) at 37°C for 2 h, because BHA requires VCNA pretreatment for its hemadsorption activity (15). After VCNA treatment, the cells were washed with DMEM and overlaid with freshly prepared 1% human red blood cells (HRBCs). After 20 min of adsorption at 4°C, unbound erythrocytes were removed with DMEM. Hemadsorption was quantified by measuring the optical density at 575 nm after hemolysis with distilled water as described previously (28).
Labeling of HRBCs with R18 and calcein-AM.
HRBCs were colabeled with the membrane probe octadecyl rhodamine B chloride (R18) and the aqueous dye calcein-AM (Molecular Probes, Inc., Eugene, Oreg.) as described previously (11, 20). Five milliliters of freshly prepared HRBCs (1% in PBS) was mixed with 5.0 μl of R18 (2 mM in ethanol)with vigorous shaking. The mixture was incubated in the dark for 30 min at room temperature and then incubated with 7.5% FBS-DMEM for 20 min at room temperature to remove unbound R18. The R18-labeled HRBCs were washed three times, resuspended in 1.25 ml of PBS (4% R18-labeled HRBCs), and kept at 4°C until use. A 5-μl aliquot of 4 mM calcein-AM in dimethyl sulfoxide was incubated with 500 μl of 4% R18-labeled HRBCs in the dark for 1 h at 37°C. The remaining procedure was the same as for R18 labeling. The R18- and calcein-labeled HRBCs were suspended in 5 ml of DMEM.
Fusion assay.
To analyze hemifusion and fusion pore formation by HA-wt and HA mutants, an R18 and calcein transfer assay was performed. Cos cells expressing HA were pretreated with VCNA and TPCK-trypsin as described above. At 48 h posttransfection, the cells were incubated with R18- and calcein-labeled HRBCs for 20 min on ice for hemadsorption. After unbound HRBCs were removed by three washes, the cells were incubated in acidic fusion medium (20 mM sodium citrate [pH 5.0], 125 mM NaCl) for 5 min at 37°C. The medium was replaced with PBS containing 1 mM CaCl2, 1 mM MgCl2, and 20 mM raffinose to prevent colloidal-osmotic swelling of the RBCs that could be induced by HA-mediated leakage (18). After incubation for 20 min at 37°C, hemadsorption and the transfer of fluorescence were observed with a phase-contrast microscope and a fluorescence microscope, respectively. Photographs of three microscopic fields selected at random were taken. On average, 500 cells were screened per culture dish.
Syncytium formation assay.
The syncytium formation assay was performed as described previously with modifications (24). At 48 h posttransfection, HA-expressing Cos cells were incubated with 10 μg of TPCK-trypsin/ml for 15 min at 37°C to cleave HA0 into HA1 and HA2. Syncytium formation was induced by exposure of the cells to prewarmed acidic fusion medium for 5 min at 37°C, and then the medium was replaced with 7.5% FBS-DMEM. After 6 h of incubation, the cells were fixed with ethanol-acetone (1:1) and stained with 10-fold-diluted Giemsa solution. Syncytium formation was observed with an inverted microscope. For quantitative analysis, nuclei in syncytia (>2 nuclei) were counted in four randomly chosen microscopic fields of a culture dish. Syncytium formation activity was calculated from the numbers of nuclei in syncytia divided by the number of cells expressing HA. The number of cells expressing HA was estimated from the results of flow cytometric analysis.
RESULTS
Expression of HA-wt and HA mutants.
We introduced mutations into HA cDNA of B/Kanagawa/73 virus by using site-specific mutagenesis to change acylation sites in the CT at cysteine residues 578 and 581 (Cys578 and Cys581). Designations and amino acid changes for HA mutants are shown in Fig. 1. Nine mutants were categorized into three groups, single-acylation-site mutants, C6 derivative mutants, and no-acylation-site mutants, on the basis of cysteine residue positions in the CT. HA-wt and HA mutants were expressed on Cos cells by using the pME18s expression vector. To examine the cellular localizations of HA proteins, HA mutants expressed in Cos cells were immunostained. The staining patterns of all HA mutants both within the cell and on the cell surface were indistinguishable from that of HA-wt (data not shown).
The levels of expression of HA-wt and each of the HA mutants on the Cos cell surface were examined by flow cytometric analysis and immunoprecipitation followed by SDS-PAGE. As shown in Table 1, the mean fluorescence intensity and the percentage of cells expressing HA were virtually identical among cells expressing HA-wt and the HA mutants, suggesting that the HA mutants were efficiently transported to the cell surface like HA-wt. In addition, metabolic labeling with [35S]methionine showed that the total synthesis of mutant HA proteins was not considerably different from that of HA-wt protein (Fig. 2). The amount of PA incorporation generally corresponded to the number of acylation sites, although the amounts of PA attached to C9, C6-NIAI, and C6-d10 were slightly smaller than the amounts attached to other single-acylation-site mutants. Two HA mutants, C0 and CT−, which have no cysteine residues in the CT, were not labeled with [3H]PA (Fig. 2).
TABLE 1.
Cell surface expression and receptor-binding abilities of HA-wt and HA mutants
| HA | % of cells expressing HA (mean ± SD) | Fluorescence/cella (mean ± SD) | Hemadsorption abilityb (% of wt HA, mean ± SD) |
|---|---|---|---|
| HA-wt | 51.3 ± 0.5 | 100 | 100 |
| C3 | 50.0 ± 1.2 | 93.6 ± 2.5 | 120.0 ± 11.0 |
| C5 | 48.7 ± 2.3 | 102.9 ± 0.2 | 108.5 ± 5.0 |
| C6 | 50.6 ± 2.6 | 95.3 ± 2.4 | 108.4 ± 6.9 |
| C7 | 51.9 ± 2.2 | 100.9 ± 0.7 | 101.2 ± 3.4 |
| C9 | 53.9 ± 1.4 | 94.0 ± 2.0 | 103.2 ± 6.2 |
| C6-NIAI | 65.8 ± 2.8 | 97.8 ± 3.5 | 130.9 ± 16.5 |
| C6-d10 | 44.4 ± 3.3 | 104.7 ± 4.0 | 116.3 ± 11.1 |
| CT− | 50.6 ± 4.5 | 90.3 ± 3.4 | 104.6 ± 7.4 |
| C0 | 51.3 ± 2.6 | 103.7 ± 7.8 | 126.2 ± 10.8 |
Arbitrary units normalized to HA-wt.
Calculated from the absorbance of the hemoglobin released at 575 nm as described in the text.
FIG. 2.
Expression and rate of acylation of HA-wt (WT) and HA mutants. Expressed HA mutants and HA-wt were metabolically labeled with [35S]methionine-cysteine (A) and with [3H]PA (B) and treated with TPCK-trypsin to cleave HA0 into HA1 and HA2. The HA proteins were immunoprecipitated and subjected to SDS-PAGE followed by fluorography.
Receptor-binding abilities of HA mutants.
To examine whether the receptor-binding abilities of the HA mutants were comparable to that of HA-wt, we performed a hemadsorption test to assess each HA mutant. HRBCs were applied to Cos cells expressing the respective HA proteins, and the bound HRBCs were quantified. As shown in Table 1, the amounts of erythrocytes bound to Cos cells were almost identical among cells expressing the HA mutants and HA-wt.
Hemifusion abilities of HA mutants.
HA-mediated membrane fusion is initiated by merging of the outer monolayers of the fusing membranes (hemifusion) (10). To determine the effect of the mutations on the first step of the fusion process, we examined the hemifusion abilities of the HA mutants by using a transfer assay with fluorescent dye R18. R18- and calcein-labeled HRBCs were bound to Cos cells expressing the HA mutants. Membrane fusion was initiated by exposure of the Cos cells to a low pH, and then R18 redistribution from HRBCs to Cos cells was observed. All of the HA mutants induced the transfer of R18 from HRBCs to hemadsorbing Cos cells at levels similar to that of HA-wt (Fig. 3). These results showed that mutations in the acylation sites of the CT did not affect the hemifusion ability of BHA.
FIG. 3.
Hemifusion and fusion pore formation by HA-wt and HA mutants. After hemadsorption with R18- and calcein-labeled HRBCs, Cos cells expressing HA-wt and HA mutants were exposed to acidic fusion medium (pH 5.0) to induce membrane fusion. wt uncleaved, HA-wt without trypsin treatment. Magnification, ×144.
Pore formation abilities of HA mutants.
To find out whether deacylation in the CT affected the ability of cells to form aqueous fusion pores, we examined the transfer of the small soluble fluorescent dye calcein from erythrocytes to Cos cells. R18- and calcein-labeled HRBCs were bound to Cos cells expressing HA. After low-pH treatment, the cells were neutralized and observed with a fluorescence microscope. No transfer was observed with cells expressing uncleaved HA (Fig. 3).
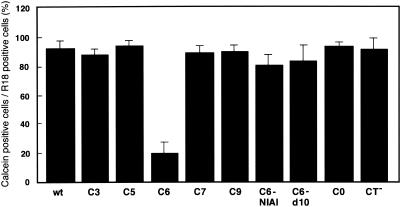
The ratio of calcein-transferred cells to R18-transferred cells was used as a measure of fusion pore formation ability (Fig. 4). First, we analyzed the fusion ability of no-acylation-site mutants. C0 and CT−, which do not have acylation sites because of substitution of amino acid residues and elimination of the CT, respectively, were able to induce pore formation equally as well as HA-wt (Fig. 3 and 4). This fact suggested that PA in the CT was not needed for BHA to form fusion pores. Among the single-acylation-site mutants, C6 and C9, which contain conserved acylation sites at residues 578 and 581, respectively, exhibited dissimilarity in their ability to form pores. The efficiency of pore formation by C9 (90%) was similar to that of HA-wt (92%); however, that of C6 was appreciably lower (20%). To examine whether the decreased ability of C6 to form pores was due to the substituted amino acid at position 581, we created another mutant, C6/Ala, which has an alanine at residue 581. C6/Ala showed the same impaired pore formation (18%) as C6 (data not shown). Therefore, we ascribed the restricted pore formation ability of C6 to the substitution of other amino acids for cysteine at position 581. Properly folded C6 was expressed on Cos cells as efficiently as HA-wt and bound HRBCs efficiently (Table 1 and Fig. 2) but was unable to induce efficient fusion pore formation. We then searched for the reason for the difference in the effects of deacylation observed with the single-acylation-site mutants C6 and C9.
FIG. 4.
Efficiency of pore formation by HA-wt and HA mutants. The efficiency of pore formation was estimated from the ratio of the number of calcein-transferred cells to the number of R18-transferred cells. The mean and standard deviation determined from three independent experiments are shown.
Amino acid sequences which impaired fusion pore formation.
To examine the effect on fusion pore formation of the position of a single acylation site in the CT, we analyzed the fusion abilities of three other single-acylation-site mutants, C3, C5, and C7, which have cysteine at residues 575, 577, and 579, respectively, in addition to substitutions of serine for cysteine at residues 578 and 581 (Fig. 1). As shown in Fig. 4, these mutants had pore formation activities as efficient as those of C9. These results suggested that PA at amino acid residue 578 (PA578) in C6 had a negative effect on fusion pore formation. HA-wt also had PA578 but promoted full fusion. This fact indicated that PA at residue 581 (PA581) of HA-wt suppressed the negative effect of PA578.
To determine the reason for the impaired pore formation ability of C6, we examined the effects of the number and sequence of amino acid residues downstream of Cys578. For this purpose, we created two other mutants, C6-d10 and C6-NIAI. C6-d10, which contains a translational stop codon at residue 582, has three amino acid residues after Cys578. Using reverse genetics techniques, Jin and colleagues rescued a virus with deacylation mutant H3 HA with the amino acid sequence CNIAI in the region corresponding to residues 578 to 582 of BHA (8). Therefore, in C6-NIAI, the amino acid sequence SISL between residues 579 and 582 was replaced with the sequence NIAI in anticipation of recovering the fusion pore formation ability of C6. As shown in Fig. 4, the calcein transfer assay indicated that the efficiencies of fusion pore formation by C6-d10 and C6-NIAI were 84 and 81%, respectively. These results suggested that the decreased efficiency of C6 fusion pore formation could be ascribed to the amino acid sequence CSISL between residues 578 and 582. To determine whether only CSISL or amino acid positions 578 to 582 or both affected the pore formation ability of C6, we constructed another mutant, C5:SISL, that has the amino acid sequence CSISL between residues 577 and 581 and a deletion of one amino acid at residue 582. Mutant C5:SISL incorporated PA to an extent similar to that of C5 and also resembled C5 in fusion ability (data not shown). These results indicated that the amino acid sequence CSISL, which is located between residues 578 and 582 and includes a single acylation site at Cys578, was responsible for the impaired pore formation ability of C6.
An acylation site which affects syncytium formation by BHA.
To analyze the effect of CT acylation on pore dilation, we examined the abilities of the respective HA mutants to form syncytia. In contrast to the situation in AHA, the efficiencies of syncytium formation differed considerably among the HA mutants of BHA (Fig. 5 and 6). The no-acylation-site mutant C0 induced syncytia to an extent similar to that of HA-wt, indicating that acylation did not play an important role in pore dilation by BHA. On the other hand, C6, C6-d10, and C6-NIAI induced syncytia at an efficiency less than 17% that of HA-wt. However, the activities of other single-acylation-site mutants were between 44% (C7) and 103% (C3) that of HA-wt, although the significance of these variations was not clear. CT− exhibited slightly less syncytium-forming ability (61.6%) than HA-wt, suggesting that the CT was not indispensable for pore dilation but presumably acted to enhance HA mobility during pore enlargement. In contrast to their superior pore-forming abilities, C6-NIAI and C6-d10 did not exhibit similar capacities for forming syncytia. The amount of PA incorporated into Cys578 in C6-NIAI and C6-d10 was lower than that in C6.
FIG. 5.
Syncytium formation by HA-wt and HA mutants. Cos cells expressing HA-wt and HA mutants were exposed to acidic fusion medium (pH 5.0) to induce membrane fusion. wt (uncleaved), HA-wt without trypsin treatment. Magnification, ×85.
FIG. 6.
Efficiency of syncytium formation by HA-wt and HA mutants. The efficiency of syncytium formation by wt-HA was estimated from the total number of nuclei in a syncytium as described in Materials and Methods. The efficiency of syncytium formation by HA mutants was calculated relative to that of HA-wt (100%). The mean and standard deviation determined from five independent experiments are shown.
To examine whether the nonpalmitylated HA molecules of C6-NIAI and C6-d10 were responsible for the decreased syncytium formation, we introduced an amino acid substitution of serine for cysteine at residue 578 in both HA mutants and analyzed their fusion abilities. The activities were restored up to 70%, implying that the decreased syncytium formation by C6-NIAI and C6-d10 could be ascribed to PA578. Even with C6, the average pore-forming ability was 22% that of HA-wt, whereas its average syncytium-forming ability was 3% that of HA-wt. These results implied that the decreased syncytium formation by C6 was due not only to the impaired pore formation ability but also to the direct effect of the existence of a sole PA at Cys578 on the pore dilation step.
Taking these results together, we conclude that acylation at conserved cysteine residues in CT and CT itself of BHA are both dispensable for the syncytium-forming ability of this HA; however, acylation at Cys578 alone will suppress pore dilation regardless of the amino acid sequences downstream of residue 578.
DISCUSSION
The role of acylation in the membrane fusion of influenza A viruses has been investigated in many studies with deacylation mutants (5, 17, 21, 22, 28, 29, 31). It is noteworthy that the effects of deacylation on membrane fusion were different in various HA subtypes. However, the deacylation mutants derived from one HA subtype exhibited fusion phenotypes that were similar to each other, regardless of the numbers and locations of the acylation sites in the CT. In contrast to the situation in AHA, the role of acylation in BHA has not been investigated. In the present study, to examine the effects of deacylation on membrane fusion by BHA, we quantified the pore-forming and syncytium-forming abilities of HA mutants lacking an acylation site(s) or carrying a novel acylation site(s) in the CT. These site variations had no influence on the intracellular transportation or receptor-binding ability of any of the HA mutants. Hemifusion between Cos cells expressing HA and HRBCs was also induced by all BHA mutants to an extent similar to that induced by HA-wt. It was surmised that palmitylation within the CT did not play an important role in the hemifusion step. However, depending on the position(s) of the deleted acylation site(s), the effects on the fusion event were different in the various HA mutants. As for pore formation, only mutation Cys581Ser(Ala) in C6 suppressed pore-forming ability, whereas this activity was seen in all other single-acylation-site mutants and deacylation mutant C0. C6-d10 and C6-NIAI, which have amino acid changes downstream of Cys578 of C6, showed restoration of the pore-forming ability up to 84 and 81%, respectively. These results indicated that acylation at Cys578 in the amino acid sequence CSISL (between residues 578 and 582) arrested the transition from hemifusion to fusion pore formation.
Using electrophysiological analysis, Melikyan and colleagues recently found “flickering” immediately before the transition from hemifusion to stable pore formation (17). Based on their study with H3 HA mutants lacking acylation sites, they deduced that acylation is required for pore flickering. In the present study, we did not examine the frequency of flickering induced by C6. However, even granting the possibility that deacylation affected flickering induced by C6, pore formation by the no-acylation-site mutant C0 was not impaired. Therefore, it is less likely that the impaired pore formation seen with C6 was attributable to restricted flickering.
Sakai and colleagues recently proposed a role for acyl chains of H1 HA during the transition from hemifusion to pore formation; they suggested that the acyl chains orient the TM domain and facilitate the interaction between the fusion peptide and the TM domain in the hemifusion diaphragm (28). If the acyl chains of BHA have a role similar to those of H1 HA in pore formation, only the acyl chain of C6 would be unable to react with the hemifusion diaphragm. However, when one considers the efficient pore formation ability of C0, it is not likely that the acyl chains of BHA play a significant role in pore formation.
As to what hinders C6 from inducing pore formation and dilation, several possibilities can be considered. When one considers the amino acid sequences of the TM domain of viral fusion proteins, it is apparent that glycine residues are generally included in these regions. The requirement for these conserved glycine residues in membrane fusion has been identified with herpes simplex virus and vesicular stomatitis virus (4, 6). Cleverley and Lenard proposed a model in which a bend around a glycine hinge in the TM domain could destabilize the hemifusion diaphragm and catalyze fusion pore formation (4). All HA subtypes except for H4 of influenza A viruses include glycine residues in the TM domain (9, 23, 27). In H2 HA, an amino acid substitution for glycine at residue 520 in the TM domain arrested the process before the hemifusion step (18). In contrast, BHA has no glycine residues in the TM domain. It has been surmised that the participation of the TM domain in the fusion process in AHA is different from that in BHA. On the basis of the results obtained with single-acylation-site mutants, the inhibitory effect of C6 on pore formation appears to be a reflection of the specific amino acid sequence. If the CT and the TM domain of BHA are not independent functional entities, as in AHA (12, 18), then a particular amino acid sequence in the CT carboxy terminus must be required to destabilize the hemifusion diaphragm in cooperation with the glycine-less TM domain.
The role of acylation in pore dilation has been examined with AHA deacylation mutants (5, 29). Fischer and colleagues (5) proposed an orientational shift of the TM domain from a vertical to a tilted position within the membrane during the fusion process. They explained that the suppressed syncytium formation of deacylated H7 HA was due to a reduction of hydrophobicity in its CT, because the orientational shift requires partial immersion of the tail in the lipid bilayer. In BHA, the average hydrophobicities calculated from the hydrophobic values of the amino acid residues in the CT were not very different in C6 and C9. Nevertheless, only the pore dilation ability of C6 was significantly impaired. Furthermore, C0 could induce syncytia. These facts suggested that the hydrophobicity of the CT may not play an essential role in fusion pore dilation by BHA.
In this study, we have demonstrated that the no-acylation-site mutants C0 and CT− could promote full fusion in vitro, although the pore dilation ability of CT− was slightly lower than that of HA-wt. Therefore, the acylation of the CT and even the CT itself were dispensable for membrane fusion by BHA in vitro; however, they might be significant in virus maturation and genome packaging, as has been observed with the AHA CT (37, 38). There is no reason to suppose that the roles of the CT in the membrane fusion process in AHA and BHA are identical. The distinctive effect of the Cys581Ser change in C6 on membrane fusion suggested that characteristic amino acid sequences and acylation at a particular position in the CT do indeed influence membrane fusion by BHA. One possible explanation for the inhibitory effect of PA578 of C6 is as follows. There are no data indicating whether PA in the CT of BHA is incorporated into the inner viral membrane surface at the hemifusion diaphragm or remains outside the diaphragm. However, if PA578 were incorporated into the inner lipid bilayer of the hemifusion diaphragm at a particular position at a certain distance from the TM domain, then it would presumably interrupt the opening of the fusion pore. Similarly, if PA578 were incorporated into the membrane outside the hemifusion diaphragm at a certain distance from the TM domain, then it would probably fix the TM domain in an unsuitable orientation for opening of the fusion pore. PA581 of HA-wt and amino acid sequence NIAI of C6-NIAI probably altered the viral inner membrane at the position at which PA578 was incorporated, thereby preventing the inhibitory effect of PA578 on the pore-forming abilities of both HAs. In the viral membrane lipid bilayer, there may be a region in which an interaction with the acyl chain of CT suppresses the transition from hemifusion to fusion pore formation. Further investigations are required to shed light on the fusion process of BHA.
Acknowledgments
We thank Kazuo Maruyama for generously providing the pME18s expression vector and Fumi Yamamoto-Goshima for providing rabbit anti-B/Kanagawa/73 serum.
This work was supported in part by a scientific research grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- 1.Armstrong, R. T., A. S. Kushnir, and J. M. White. 2000. The transmembrane domain of influenza hemagglutinin exhibits a stringent length requirement to support the hemifusion to fusion transition. J. Cell Biol. 151:425-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bullough, P. A., F. M. Hughson, J. J. Skehel, and D. C. Wiley. 1994. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature 371:37-43. [DOI] [PubMed] [Google Scholar]
- 3.Carr, C. M., and P. S. Kim. 1993. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell 73:823-832. [DOI] [PubMed] [Google Scholar]
- 4.Cleverley, D. Z., and J. Lenard. 1998. The transmembrane domain in viral fusion: essential role for a conserved glycine residue in vesicular stomatitis virus G protein. Proc. Natl. Acad. Sci. USA 31:3425-3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer, C., B. Schroth-Diez, A. Herrmann, W. Garten, and H. D. Klenk. 1998. Acylation of the influenza hemagglutinin modulates fusion activity. Virology 248:284-294. [DOI] [PubMed] [Google Scholar]
- 6.Harman, A., H. Browne, and T. Minson. 2002. The transmembrane domain and cytoplasmic tail of herpes simplex virus type 1 glycoprotein H play a role in membrane fusion. J. Virol. 76:10708-10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horton, M. R., and L. R. Pease. 1991. Recombination and mutagenesis of DNA-sequences using PCR, p. 217-247. In M. J. McPherson (ed.), Directed mutagenesis: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 8.Jin, H., K. Subbarao, S. Bagai, G. P. Leser, B. R. Murphy, and R. A. Lamb. 1996. Palmitylation of the influenza virus hemagglutinin (H3) is not essential for virus assembly or infectivity. J. Virol. 70:1406-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawaoka, Y., S. Yamnikova, T. M. Chambers, D. K. Lvov, and R. G. Webster. 1990. Molecular characterization of a new hemagglutinin, subtype H14, of influenza A virus. Virology 179:759-767. [DOI] [PubMed] [Google Scholar]
- 10.Kemble, G. W., T. Danieli, and J. M. White. 1994. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell 76:383-391. [DOI] [PubMed] [Google Scholar]
- 11.Kemble, G. W., Y. I. Henis, and J. M. White. 1993. GPI- and transmembrane-anchored influenza hemagglutinin differ in structure and receptor binding activity. J. Cell Biol. 122:1253-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozerski, C., E. Ponimaskin, B. Schroth-Diez, M. F. Schmidt, and A. Herrmann. 2000. Modification of the cytoplasmic domain of influenza virus hemagglutinin affects enlargement of the fusion pore. J. Virol. 74:7529-7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krystal, M., R. M. Elliott, E. W. Benz, Jr., J. F. Young, and P. Palese. 1982. Evolution of influenza A and B viruses: conservation of structural features in the hemagglutinin genes. Proc. Natl. Acad. Sci. USA 79:4800-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamb, R. A., and R. M. Krug. 2001. Orthomyxoviridae: the viruses and their replication, p. 1487-1531. In D. M. Knipe et al. (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 15.Luo, C., E. Nobusawa, and K. Nakajima. 1999. An analysis of the role of neuraminidase in the receptor-binding activity of influenza B virus: the inhibitory effect of Zanamivir on haemadsorption. J. Gen. Virol. 80:2969-2976. [DOI] [PubMed] [Google Scholar]
- 16.Melikyan, G. B., J. M. White, and F. S. Cohen. 1995. GPI-anchored influenza hemagglutinin induces hemifusion to both red blood cell and planar bilayer membranes. J. Cell Biol. 131:679-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melikyan, G. B., H. Jin, R. A. Lamb, and F. S. Cohen. 1997. The role of the cytoplasmic tail region of influenza virus hemagglutinin in formation and growth of fusion pores. Virology 235:118-128. [DOI] [PubMed] [Google Scholar]
- 18.Melikyan, G. B., S. Lin, M. G. Roth, and F. S. Cohen. 1999. Amino acid sequence requirements of the transmembrane and cytoplasmic domains of influenza virus hemagglutinin for viable membrane fusion. Mol. Biol. Cell 10:1821-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melikyan, G. B., R. M. Markosyan, M. G. Roth, and F. S. Cohen. 2000. A point mutation in the transmembrane domain of the hemagglutinin of influenza virus stabilizes a hemifusion intermediate that can transit to fusion. Mol. Biol. Cell 11:3765-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris, S. J., D. P. Sarkar, J. M. White, and R. Blumenthal. 1989. Kinetics of pH-dependent fusion between 3T3 fibroblasts expressing influenza hemagglutinin and red blood cells. J. Biol. Chem. 264:3972-3978. [PubMed] [Google Scholar]
- 21.Naeve, C. W., and D. Williams. 1990. Fatty acids on the A/Japan/305/57 influenza virus hemagglutinin have a role in membrane fusion. EMBO J. 9:3857-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naim, H. Y., B. Amarneh, N. T. Ktistakis, and M. G. Roth. 1992. Effects of altering palmitylation sites on biosynthesis and function of the influenza virus hemagglutinin. J. Virol. 66:7585-7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nobusawa, E., T. Aoyama, H. Kato, Y. Suzuki, Y. Tateno, and K. Nakajima. 1991. Comparison of complete amino acid sequences and receptor-binding properties among 13 serotypes of hemagglutinins of influenza A viruses. Virology 182:475-485. [DOI] [PubMed] [Google Scholar]
- 24.Nobusawa, E., R. Hishida, M. Murata, K. Kawasaki, S. Ohnishi, and K. Nakajima. 1995. The role of acidic residues in the ”fusion segment“ of influenza A virus hemagglutinin in low pH-dependent membrane fusion. Arch. Virol. 140:865-875. [DOI] [PubMed] [Google Scholar]
- 25.Nobusawa, E., H. Ishihara, T. Morishita, K. Sato, and K. Nakajima. 2000. Change in receptor-binding specificity of recent human influenza A viruses (H3N2): a single amino acid change in hemagglutinin altered its recognition of sialyloligosaccharides. Virology 278:587-596. [DOI] [PubMed] [Google Scholar]
- 26.Ohuchi, M., C. Fischer, R. Ohuchi, A. Herwig, and H. D. Klenk. 1998. Elongation of the cytoplasmic tail interferes with the fusion activity of influenza virus hemagglutinin. J. Virol. 72:3554-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohm, C., N. Zhou, J. Suss, J. Mackenzie, and R. G. Webster. 1996. Characterization of a novel influenza hemagglutinin, H15: criteria for determination of influenza A subtypes. Virology 217:508-516. [DOI] [PubMed] [Google Scholar]
- 28.Sakai, T., R. Ohuchi, and M. Ohuchi. 2002. Fatty acids on the A/USSR/77 influenza virus hemagglutinin facilitate the transition from hemifusion to fusion pore formation. J. Virol. 76:4603-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson, D. A., and R. A. Lamb. 1992. Alterations to influenza virus hemagglutinin cytoplasmic tail modulate virus infectivity. J. Virol. 66:790-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 31.Steinhauer, D. A., S. A. Wharton, D. C. Wiley, and J. J. Skehel. 1991. Deacylation of the hemagglutinin of influenza A/Aichi/2/68 has no effect on membrane fusion properties. Virology 184:445-448. [DOI] [PubMed] [Google Scholar]
- 32.Tsurudome, M., R. Gluck, R. Graf, R. Falchetto, U. Schaller, and J. Brunner. 1992. Lipid interactions of the hemagglutinin HA2 NH2-terminal segment during influenza virus-induced membrane fusion. J. Biol. Chem. 267:20225-20232. [PubMed] [Google Scholar]
- 33.Veit, M., G. Herrler, M. F. Schmidt, R. Rott, and H. D. Klenk. 1990. The hemagglutinating glycoproteins of influenza B and C viruses are acylated with different fatty acids. Virology 177:807-811. [DOI] [PubMed] [Google Scholar]
- 34.Veit, M., E. Kretzschmar, K. Kuroda, W. Garten, M. F. Schmidt, H. D. Klenk, and R. Rott. 1991. Site-specific mutagenesis identifies three cysteine residues in the cytoplasmic tail as acylation sites of influenza virus hemagglutinin. J. Virol. 65:2491-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber, T., G. Paesold, C. Galli, R. Mischler, G. Semenza, and J. Brunner. 1994. Evidence for H(+)-induced insertion of influenza hemagglutinin HA2 N-terminal segment into viral membrane. J. Biol. Chem. 269:18353-18358. [PubMed] [Google Scholar]
- 36.Wilson, I. A., J. J. Skehel, and D. C. Wiley. 1981. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature 289:366-373. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, J., G. P. Leser, A. Pekosz, and R. A. Lamb. 2000. The cytoplasmic tails of the influenza virus spike glycoproteins are required for normal genome packaging. Virology 269:325-334. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, J., A. Pekosz, and R. A. Lamb. 2000. Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins. J. Virol. 74:4634-4644. [DOI] [PMC free article] [PubMed] [Google Scholar]