Abstract
Vertical transmission of human immunodeficiency virus type 1 (HIV-1) is the primary cause of infection by this retrovirus in infants. In this study, we report for the first time that there is a correlation between endocytic uptake of HIV-1 and virus gene expression in polarized trophoblasts. To shed light on the relationship between endocytosis and the fate of HIV-1 in polarized trophoblasts, the step-by-step movements of HIV-1 within the endocytic compartments were tracked by confocal imaging. Incoming virions were initially located in early endosomes. As time progressed, virions accumulated in late endosomes. HIV-1 was also found in apical recycling endosomes and at the basolateral pole. Experiments performed with indicator cells revealed that HIV-1 is recycled and transcytosed. These data indicate that the intracellular trafficking of HIV-1 upon entry into polarized human trophoblasts is a complex process which requires the active participation of the endocytic host cell machinery.
Worldwide, 3.2 million children under the age of 15 years are infected with human immunodeficiency virus type 1 (HIV-1) (36). Mother-to-child transmission of HIV-1 is the primary cause of infection with this retrovirus in children (36). Antiretroviral therapies significantly reduce the incidence of vertical transmission (25). However, access to the treatments currently available is extremely limited in developing countries. As a result, initiatives to prevent mother-to-child transmission of HIV-1 are now aimed at developing strategies that are readily available to women. In this context, it is well recognized that a better understanding of how and when vertical transmission occurs is crucial.
The mechanism of in utero transmission of HIV-1 is poorly understood, but several lines of evidence support direct implication of the placenta, which is composed of a double layer of cytotrophoblasts and syncytiotrophoblasts. These cells separate the maternal and fetal blood circulations and control fluxes between them. HIV-1 undergoes productive replication in the placenta both in vitro and in vivo (2, 20, 39), and the permissiveness of trophoblasts to infection by HIV-1 is an order of magnitude lower than that of CD4+ T lymphocytes, the primary target of HIV-1 (26). It has been shown that HIV-1 may also transcytose across the trophoblastic cell layer (15). Transcytosis is a process whereby virions (or molecules such as immunoglobulin G [IgG]) are internalized by endocytosis at one pole of the cells, then transported in a vesicular system, and released intact at the opposite pole. This process occurs independently of virus replication but may take place simultaneously. It has been postulated that following infection of trophoblasts and/or transcytosis across these cells, HIV-1 is released from the basolateral pole of the trophoblasts (facing the fetal circulation), leading to productive infection of the underlying fetal cells.
HIV-1 enters CD4+ T lymphocytes by fusion at the cell surface upon interaction between the viral envelope glycoprotein gp120 and the primary cellular receptor CD4 and coreceptor CXCR4 or CCR5 (33). The question of viral entry in trophoblasts is still a matter of debate, since these cells express no or very little CD4, while expression of the HIV-1 coreceptors, CXCR4 and CCR5, may decline from the first to the third trimester of pregnancy. Contrary to what was assumed, evidence that HIV-1 enters massively into trophoblasts, predominantly via endocytosis, has been published. These cells were also found to sustain virus production (37). Whether a functional correlation exists between endocytic uptake and infection in trophoblasts is an important question that remains to be answered.
Interestingly, trophoblasts form a polarized epithelium-like monolayer in vivo. One of the consequences of cell polarity is the presence of polarized (apical and basolateral) endocytic pathways, each of which uses a complex succession of intracellular compartments (which include the early, late, and recycling endosomes). These pathways lead to recycling, degradation, and transcytosis of internalized molecules on an ongoing basis. Given that HIV-1 is thought to enter polarized trophoblasts by endocytosis, upon viral entry, incoming virions will be trapped within endocytic compartments. Therefore, if HIV-1 takes different endocytic pathways, as is the case for internalized molecules, the incoming viral particles may have different fates. We hypothesize that these fates would be as follows. First, part of the internalized virions is degraded in the lysosomal machinery. Second, infection may be associated with early transit through the endosomes. Two other endocytic processes, which are independent of virus replication, are also likely to occur: transcytosis of virions to the basolateral pole and recycling to the apical pole. As discussed previously (15), transcytosis has already been suggested. However, recycling of HIV-1 has never been described previously. Recycling of HIV-1 would be the process whereby, upon internalizing HIV-1, the cells would rapidly return part of these internalized virions to the place from which they initially came, the apical pole. This is an interesting concept, because recycling may be a mechanism through which vertical transmission of HIV-1 may be avoided or reduced by the cells. Based on these avenues, knowing where HIV-1 particles are located within the intracellular vesicles upon internalization becomes crucial for (i) defining the pathway associated with the infectious cycle of HIV-1 in trophoblasts and (ii) unveiling the early steps of the HIV-1 life cycle associated with transcytosis and recycling.
In this paper, we provide the first evidence that there is a functional link between the presence of HIV-1 within the endosomes and infection of trophoblasts. In addition, we demonstrate that internalized HIV-1 particles are both transcytosed to the basolateral pole and recycled to the apical pole upon entry into these cells. By use of confocal imaging, incoming virions were found broadly distributed within the different endocytic compartments in polarized trophoblasts. The endocytic host cell machinery thus participates actively in HIV-1 replication in polarized human trophoblasts, fully supporting the notion that HIV-1, upon internalization by endocytosis, has several possible fates, including degradation, infection, recycling, and transcytosis in these cells. The clinical relevance of the data presented is high, since trophoblasts are believed to play a pivotal role in mother-to-infant transmission of this deadly retrovirus.
(This work was performed by G. Vidricaire in partial fulfillment of the requirements for a Ph.D. in the Program of Microbiology-Immunology, Faculty of Medicine, Laval University, Quebec, Canada, 2005.)
MATERIALS AND METHODS
Cells.
The JAR cell line was obtained from the American Type Culture Collection (Manassas, Va.). 293T cells were provided by W. C. Greene (J. Gladstone Institutes, San Francisco, Calif.). The LuSIV reporter cell line, kindly provided by J. E. Clements (The John Hopkins University School of Medicine, Baltimore, Md.), was derived from the CEMx174 parental cell line and carries the luciferase reporter gene under the control of the SIVmac239 long terminal repeat. All three cell lines were maintained as previously described (37).
Molecular constructs and preparation of virus stocks.
pNL4-3 is a full-length infectious molecular clone of HIV-1. The NL4-3-Luc E−R+ vector was constructed by inserting a frameshift mutation near the env gene and inserting the firefly luciferase reporter gene into the nef gene (4, 12). Both constructs were obtained through the AIDS Repository Reagent Program (Rockville, Md.). The pcDNA-1/Amp-based expression vector coding for the HIV-1 Ada-M (M-tropic) full-length envelope protein was generously provided by N. R. Landau (The Salk Institute for Biological Studies, La Jolla, Calif.). Viruses were produced by calcium phosphate transfection of 293T cells, as described previously (8, 10, 37).
Infection assays.
JAR cells were seeded in 24-well cell culture inserts containing a 0.4-μM-pore-size polyethylene terephthalate track-etched membrane (Becton Dickinson Labware, Franklin Lakes, N.J.). The cells were grown to complete confluence and polarized for 4 days. Transepithelial electric resistance (TEER) was measured by using a Millipore (Billerica, Mass.) instrument. The polarized JAR cells were pretreated at 37°C for 30 min with bafilomycin A1 (0.1 μM) or NH4Cl (10 mM). HIV-1 particles pseudotyped with Ada-M envelope (250 ng of p24) were then added, and the cells were incubated at 37°C for 24 h. At this point, tumor necrosis factor alpha (10 ng/ml) (generously provided by P. Naccache, CHUL Research Center, Quebec, Quebec, Canada) was added for 24 h to induce virus gene expression (37). Finally, luciferase activity was monitored in cell lysates as described previously (5).
Viral entry and fluorescence labeling.
JAR cells were seeded in 24-well cell culture inserts as described above. HIV-1 particles pseudotyped with Ada-M envelope (250 ng of p24) were added in the upper chamber for 5 min to 24 h at 37°C. To remove all noninternalized viral particles, the cells were then acid treated for 1 min (0.5 M NaCl, 1% acetic acid) and rapidly washed three times with cold phosphate-buffered saline (PBS). Cells were fixed with 2% paraformaldehyde for 20 min, permeabilized for 4 min with 0.3% Triton X-100, and blocked with 1% bovine serum albumin for 10 min. At this point, JAR cells were incubated for 1 h on ice with primary antibodies (diluted in PBS containing 1% bovine serum albumin and 0.03% Triton X-100) and subsequently with fluorescently conjugated secondary antibodies (diluted in the same way as the primary antibody). The membranes were peeled off from the transwells and placed on a slide, with the apical side facing up. A drop of 90% glycerol was added on the membrane, and a glass coverslip was placed on top.
Entry assay with bafilomycin A1.
JAR cells were seeded in 24-well cell culture inserts (as described above). The polarized JAR cells were pretreated at 37°C for 30 min with 95% ethanol (vehicle) or bafilomycin A1 (0.1 μM). HIV-1 particles pseudotyped with Ada-M envelope (250 ng of p24) were then added, and the cells were incubated at 37°C for 4 h. The cells were washed, fixed, and stained as described in the preceding section.
Recycling and transcytosis assays.
JAR cells were seeded in 24-well cell culture inserts as described above. NL4-3 (400 ng of p24) was added in the upper chamber for 5 min to 4 h at 37°C or for 1 h at 4°C. Each time point was done in triplicate. The medium was removed from the cells, and trypsin was added for 8 min at 4°C (this treatment did not affect the integrity of the cell barrier). The cells were washed with cold PBS three times on ice with mild agitation. The transwells were placed in new 24-well plates, fresh Dulbecco's modified Eagle medium was added to the lower and upper chambers, and the cells were incubated at 37°C for 3.5 h to allow recycling or transcytosis of internalized virions. At this point, the media in the upper and lower chambers were collected. LuSIV cells were added to the collected media and grown for 6 days. Luciferase activity was monitored in the LuSIV cell lysates as described previously (5).
Antibodies.
Purified human anti-HIV-1 (from pooled sera of HIV-1-infected patients) was prepared in our laboratory. Pooled sera from healthy individuals were used as controls (generously provided by P. Naccache). The following reagents were also used in this work: rhodamine-phalloidin, mouse anti-golgin-97 (Molecular Probes, Eugene, Oreg.), mouse anti-ZO-1, mouse anti-EEA1 (BD Biosciences, Mississauga, Ontario, Canada), mouse anti-CD63/tetramethyl rhodamine isothiocyanate (TRITC)-conjugated Lamp3 (Santa Cruz Biotechnologies, Santa Cruz, Calif.), rabbit anti-Rab11 (Zymed Laboratories Inc, South San Francisco, Calif.), and mouse anti-PDI (Stressgen Biotechnologies, Victoria, British Columbia, Canada). The secondary antibodies were an Alexa 488-conjugated goat anti-human antibody, Texas Red goat-anti mouse IgG (H+L), Alexa Fluor 633-conjugated goat anti-rabbit IgG (Molecular Probes), and Cy5-conjugated goat anti-mouse IgG (BioCan Scientific Inc., Mississauga, Ontario, Canada).
Confocal laser scanning, colocalization analysis, and digital image preparation.
Samples were analyzed by using a Fluoview FV300 confocal laser scanning biological microscope (Olympus America, Melville, N.Y.). Data were captured by using a 60× oil objective. Slides were scanned at 1-μm intervals and at a dimension of 512 by 512 pixels with a ×3 zoom magnification. For a given time course, all the settings on the microscope were kept constant for all the samples. Each experiment was repeated five times, and for each individual experiment, all the samples were scanned at three different locations. Colocalization analyses and statistics were generated using the MetaMorph Offline software (version 6.1; Universal Imaging Corp., West Chester, Pa.). Digital images were produced using the MetaMorph Offline software (version 6.1), Adobe Photoshop (version 8), and Adobe Illustrator (version 9; Adobe Systems Inc., Ottawa, Ontario, Canada).
To calculate colocalization, the following sequential operations were performed by the Metamorph software. The threshold was defined for each dye individually and applied to each cell layer and at all time points of a time course. The mock-infected sample (also labeled with anti-HIV-1) was used to define the background signal for HIV-1. Next, the area of signals and the integration were calculated for both HIV-1 and the marker of interest (EEA-1, CD63, or Rab11) sequentially for all the planes of a given stack (i.e., a time point). The area of a signal corresponds to the total number of pixels present on a given plane (cell layer). The integration corresponds to the total number of pixels multiplied by the average of the signal on a particular plane. Finally, colocalization events were calculated based on algorithms defined in the Metamorph software. Colocalization events are expressed as the ratio of the integration of Ada-M to the integration of the marker of interest and vice versa. The area of the signal, the integration, and the colocalization were calculated individually for Ada-M and the marker of interest, sequentially for all the planes of a given stack over the studied time course. Excel log files were generated to compile the calculations, and graphs were plotted. The overlay panel is a superimposition of both labels. The colocalization panel is a binary image corresponding to the simultaneous presence of both signals at the same location.
RESULTS
HIV-1 rapidly enters polarized trophoblasts.
Experimental models for studying virus entry into, and infection of, epithelial cells include primary cells. However, purification of primary trophoblasts is extremely difficult and results in the loss of cell polarity (7). The human trophoblastic cell lines BeWo, JAR, and JEG-3 offer an attractive alternative, since they exhibit many characteristics of the early placenta (29). Moreover, they can form tight polarized barriers when cultured on permeable supports (16). To more closely mimic the trophoblastic barrier in vivo, we used the cell line JAR, which was grown for 4 days on permeable supports, leading to formation of a polarized epithelium-like monolayer. Indeed, as monitored by confocal microscopy, they formed a compact cell monolayer that expressed the tight junction marker ZO-1. Moreover, they developed a TEER of 45 Ω per cm2. JAR cells were chosen over BeWo and JEG-3 cells because HIV-1 expression was higher in the former (37).
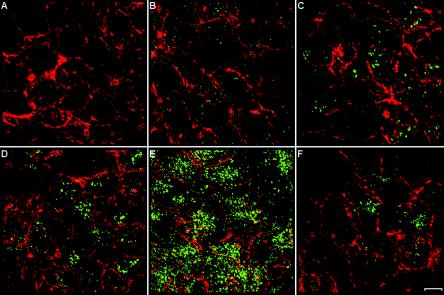
Following exposure to the macrophage-tropic HIV-1 strain Ada-M for 5 min to 12 h, the intracellular behavior of HIV-1 in polarized human trophoblasts was visualized by confocal microscopy. Purified polyclonal antibodies isolated from pooled human sera from HIV-1-positive patients were used (34), followed by an Alexa 488-conjugated goat anti-human antibody. The specificity of the signal was tested by incubating mock- and virus-infected polarized JAR cells with pooled sera from uninfected healthy donors (data not shown). Via actin staining (rhodamine-phalloidin), we clearly see that the cells are confluent and cover the entire field of view (Fig. 1), confirming that the cells form a tight layer. Interestingly, HIV-1 was found to enter the cells rapidly, since viruses were already detected 5 min after exposure. The amount of viruses gradually increased to reach a peak by 4 h after entry. At 4 h, the amount of viral internalization was truly impressive and HIV-1 had penetrated into virtually 100% of the cells. These data are perfectly in line with a previous study evaluating the kinetics of HIV-1 entry by use of a p24 assay (37). We estimated that the number of viral particles internalized by polarized trophoblasts corresponds to approximately 25% of the initial viral inoculum added to these cells. Of note, similar results were obtained with a T-tropic variant of HIV-1, NL4-3 (data not shown). Studies were also conducted with HIV-1 virions loaded with an enhanced green fluorescent protein-Vpr fusion protein. The intensity of the HIV-1-specific signal was much lower when this technical strategy was used, probably because of the low number of Vpr molecules known to be inserted per single mature virus (an average of 100 to 200). Moreover, different HIV-1 proteins are most likely recognized by polyclonal anti-HIV-1 antibodies, a factor resulting in enhancement of HIV-1-specific signal intensity. Therefore, the intracellular behavior of HIV-1 in polarized human trophoblasts was visualized by using purified polyclonal antibodies isolated from pooled human sera from HIV-1-positive patients.
FIG. 1.
Time course of HIV-1 entry in polarized trophoblasts. Polarized JAR cells were either mock infected (A) or exposed to Ada-M for 5 min (B), 30 min (C), 2 h (D), 4 h (E), or 12 h (F). Cells were colabeled with rhodamine-phalloidin (red) and a purified human anti-HIV-1 primary antibody, followed by an Alexa 488-conjugated goat anti-human secondary antibody (green). The samples were scanned through a confocal laser microscope, and the images shown are the arithmetic sum of entire stacks of the cell monolayer. Bar, 10 μm.
Correlation between endocytic uptake and subsequent infection in trophoblasts.
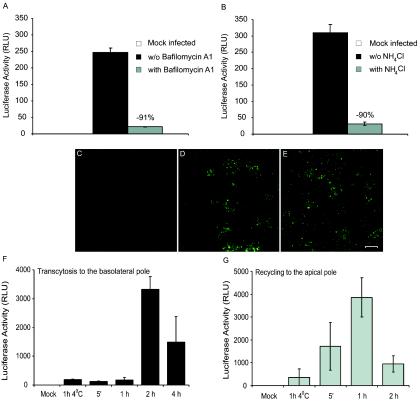
If a correlation exists between HIV-1 endocytosis and infection of trophoblasts, then the endosomes must play a pivotal role in this process. Therefore, blocking the function of the endosomes would translate into a reduction of virus gene expression. To verify this hypothesis, endosome inhibitors of two classes were tested: the inhibitor of vacuolar proton ATPase bafilomycin A1 and the lysosomotropic weak base NH4Cl. Both drugs are known to block vesicle acidification as well as endosomal and lysosomal degradation systems (1, 6). Polarized JAR cells were inoculated with single-cycle reporter virus pseudotyped with the Ada-M envelope. Of note, tumor necrosis factor alpha was added in our infection assays because its presence is required to promote viral gene expression in trophoblasts (37). When cells were pretreated with either bafilomycin A1 or NH4Cl, the HIV-1 promoter-driven gene activity was nearly completely abolished (Fig. 2A and B). Moreover, both inhibitors acted within a few hours of HIV-1 exposure to trophoblasts (data not shown). This implies that for viral expression to ensue in trophoblasts, HIV-1 must first transit through the endosomes. Therefore, a strict association between the presence of HIV-1 within the endocytic compartments upon internalization and viral replication exists in polarized trophoblasts. To ensure that the drugs did not act on the cells at the level of viral entry, JAR cells were pretreated with bafilomycin A1 and then exposed to Ada-M particles for 4 h. The samples were analyzed by confocal microscopy. We observed comparable amounts of staining for HIV-1 particles in cells exposed or not to bafilomycin A1, implying that the drug does not impact viral entry (Fig. 2C to E)
FIG. 2.
HIV-1 expression is suppressed by endosomal acidification inhibitors, and viruses are recycled and transcytosed in polarized trophoblasts. Polarized JAR cells were pretreated with bafilomycin A1 (A) or NH4Cl (B). Viral expression was measured (luciferase activity) in these cells subsequent to a 24-h exposure to Ada-M followed by a 24-h treatment with TNF-α. Polarized JAR cells were pretreated with ethanol (vehicle) (D) or bafilomycin A1 (E). The cells were next exposed to Ada-M for 4 h at 37°C and then colabeled with a purified human anti-HIV-1 antibody followed by an Alexa 488-conjugated goat anti-human antibody. As a control for the staining, cells were mock infected (C). The samples were scanned through a confocal laser microscope, and the images shown are the arithmetic sum of entire stacks of the cell monolayer. Bar, 10 μm. Polarized JAR cells were also exposed to NL4-3 at 4°C for 1 h or at 37°C for 5 min to 2 h. After extensive washes, fresh medium was added to the upper and lower chambers, and the cells were incubated for 3.5 h at 37°C. Media from the upper chamber (recycling) (F) and lower chamber (transcytosis) (G) were collected, and LuSIV indicator cells were added and grown for 6 days. Luciferase activity was monitored in each cell lysate. Values from the luminometer are expressed as relative light units (RLU). Data shown are means ± standard deviations from triplicate samples and are representative of three independent experiments.
HIV-1 particles recycle to the apical pole and transcytose to the basolateral pole.
During transcytosis, it is presumed that fully infectious viruses are endocytosed by the cells at the apical pole, transported within vesicles across the cells, and released intact from the basolateral pole into the underlying medium. This process is independent of virus replication. We reasoned that if such a process occurs, perhaps HIV-1 can also be released from the apical end. More specifically, upon internalization of HIV-1, the cells could rapidly not only send virions to the basolateral pole but also return a fraction of the internalized viruses to the place from which they came initially—the apical pole. We called this process recycling of HIV-1. To verify whether HIV-1 was indeed recycled and/or transcytosed, polarized JAR cells were exposed to NL4-3 for 5 min to 2 h (for recycling) or 4 h (for transcytosis). As a control, the cells were exposed to HIV-1 for 1 h at 4°C. Endocytosis is inhibited at this temperature such that viral internalization and fusion should also be repressed. At this point, cells were treated with trypsin and extensively washed to remove all noninternalized viral particles. This step is crucial to assess the recycling process. The cells were incubated for another 3.5 h at 37°C to allow internalized virions either to be returned to the apical pole (recycling) or to be transported across the cell layer and released in the lower chamber (transcytosis). Since the total incubation period of JAR cells with HIV-1 was short, a replication cycle of HIV-1 could not have been completed yet. Therefore, virions that were collected in the upper or lower chambers could only be the result of recycling or transcytosis, respectively. The amounts of virions released by the cells were too small to be readily detected by a p24 test (detection limit, 50 pg/ml). Consequently, the medium from the upper and lower chambers were collected and put in contact with LuSIV indicator cells. These cells are susceptible to infection with very low levels of HIV-1 (unpublished data). Fully competent viruses were released in the lower chamber by the cells, implying transcytosis (Fig. 2F). Interestingly, we also observed that fully infectious virus particles were returned to the upper chamber, i.e., the apical pole of the cells, thus suggesting recycling of HIV-1 (Fig. 2G).
Monitoring the movements of HIV-1 within the endocytic compartments.
Defining exactly where HIV-1 is located within the endocytosis complex is crucial for unveiling the early steps of HIV-1 replication in trophoblasts. The step-by-step movements of HIV-1 within the endocytic compartments in polarized JAR cells were tracked by confocal analyses. EEA-1 served as a marker of early endosomes, CD63 was used for late endosomes, and Rab11 was used for recycling endosomes. To estimate the colocalization events, a series of operations were performed with Metamorph software as described in Materials and Methods.
Incoming HIV-1 partly colocalizes with early endosomes.
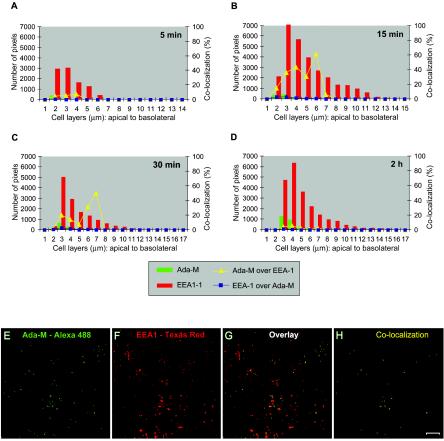
On the basis of the calculations described in Materials and Methods, the relationship between Ada-M and EEA-1 is presented in Fig. 3. Following a contact period of 5 min, 5 to 7% of the HIV-1-specific signal colocalized with EEA-1 at 2 to 4 μm (Fig. 3A). Within 15 min, the majority of the virions were located on the second and third cell layers, with 15 and 36%, respectively, of the internalized virions present on these cell layers colocalizing with EEA-1. Of note, few virions migrated into the cells to reach 6 μm, where 60% of the signal detected colocalized with EEA-1, but this may not be significant given the small amount of HIV-1 that reached this layer at this time point. Moreover, between 4 and 7 μm, the signal for Ada-M is not apparent on the graph because it is very weak and thus below the scale's units. The virions then continued their path as the percentage of colocalization declined by 30 min following incubation with HIV-1. A small percentage of colocalization remained because virions are continuously entering the cells. The distribution of EEA-1 was fairly broad over the entire depth of the cell monolayer. However, its peak level was located at the apex of the cells (3 to 4 μm). Where EEA-1 was most present (3 μm), the percentages of colocalization of EEA-1 with Ada-M were between 0.5 and 3%. Digital images of the cells at 3 μm following a 15-min exposure to HIV-1 are shown to provide a clear representation of the colocalization events (Fig. 3E to H).
FIG. 3.
HIV-1 colocalizes with early endosomes. (A to D) Polarized JAR cells were colabeled with purified human anti-HIV-1 followed by an Alexa 488-conjugated goat anti-human antibody and mouse anti-EEA-1 followed by Texas Red-conjugated goat-anti mouse IgG. The x axis corresponds to the cell layers from the apex to the bottom. The left y axis illustrates the number of pixels (HIV-1 in green bars and EEA-1 in red bars) present over the entire depth of the cells 5 min, 15 min, 30 min, or 2 h following exposure to HIV-1. The right y axis refers to the percentage of colocalization of Ada-M versus EEA-1 (yellow triangles) or EEA-1 versus Ada-M (blue squares) over the entire depth of the cells. (E to H) Confocal images of the 3-μm cell layer following a 15-min exposure to HIV-1. (E) Ada-M is shown in green. (F) EEA-1 is depicted in red. (G) Overlay of signals specific for HIV-1 and EEA-1. (H) Binary image showing the regions of colocalization between Ada-M and EEA-1 on this cell layer (yellow dots). Bar, 10 μm.
HIV-1 strongly colocalizes with late endosomes.
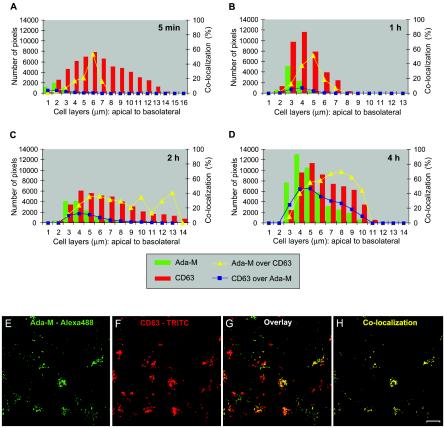
Next, by determining the relationship between Ada-M and CD63, we investigated whether HIV-1 can migrate to late endosomes. The percentages of colocalization between HIV-1 and CD63 progressively increased over the entire time course such that, as entry evolved, HIV-1 steadily reached late endosomes and accumulated in these organelles (Fig. 4). By 4 h, most of the virions were located in CD63-expressing organelles: from 4 to 9 μm of the cells' depth, 40, 55, 57, 66, 69, and 62% of the signal detected for Ada-M colocalized with CD63, respectively. The frequency of colocalization events declined on deeper cell layers. On the other hand, CD63 showed a fairly constant signal and broad distribution over the entire depth of the cells. This being said, the highest signal value could be found at the apex of the cells (4 to 6 μm). The highest percentages of colocalization of CD63 with Ada-M were 4, 8, 13, and 47% upon exposure of polarized trophoblasts to HIV-1 for 5 min and 1, 2, and 4 h, respectively. This indicates that the late endosomes gradually became occupied by incoming HIV-1.
FIG. 4.
HIV-1 colocalizes with late endosomes. Polarized JAR cells were colabeled with purified human anti-HIV-1 followed by an Alexa 488-conjugated goat anti-human antibody and TRITC-conjugated mouse anti-CD63. (A to D) Bars indicate the numbers of HIV-1-specific (green) and CD63-specific (red) pixels over the entire depth of the cells 5 min, 1 h, 2 h, or 4 h following virus exposure. Curves represent the percentage of colocalization of HIV-1 versus CD63 (yellow triangles) or CD63 versus HIV-1 (blue squares) over the entire depth of the cell monolayer. (E to H) Confocal images of the 5-μm cell layer following a 4-h exposure to HIV-1. (E) Ada-M is shown in green. (F) CD63 is depicted in red. (G) Overlay of signals specific for HIV-1 and CD63. (H) Binary image showing the regions of colocalization between HIV-1 and CD63 on this cell layer (yellow dots). Bar, 10 μm.
HIV-1 colocalizes with the apical recycling endosomes.
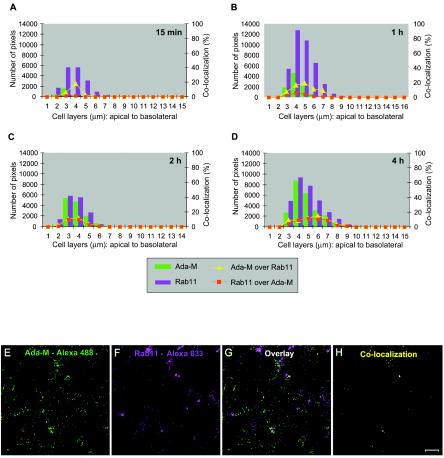
The possible location of HIV-1 in the apical recycling endosomes was also studied, by use of Rab11 (Fig. 5). A small fraction of incoming virions reached the Rab11+ recycling endosomes. The peak was seen after 1 h of exposure to HIV-1, and colocalization events after that time point remained at 9 to 11% at the cell layers where most of the signal associated with HIV-1 was present. The distribution of the signal for Rab11 was concentrated over the first half of cells' depth, and its maximum was located at the apex of the cells (4 to 5 μm). Where HIV-1 was most present (3 to 5 μm), the percentages of colocalization of Rab11 with Ada-M were low at first: 1% after 5 min and 2 to 6% after 1 h of incubation with HIV-1. However, these percentages reached 10% by 2 h and 11% by 4 h, suggesting that recycling endosomes are also involved in the transit of HIV-1 in polarized trophoblasts.
FIG. 5.
HIV-1 colocalizes weakly with recycling endosomes. Polarized JAR cells were colabeled with purified human anti-HIV-1 followed by an Alexa 488-conjugated goat anti-human antibody and rabbit anti-Rab11 followed by Alexa Fluor 633-conjugated goat anti-rabbit IgG. (A to D) Bars indicate the numbers of HIV-1-specific (green) and Rab11-specific (magenta) pixels over the entire depth of the cells 15 min, 1 h, 2 h, or 4 h following exposure to HIV-1. Curves represent the percentage of colocalization of Ada-M versus Rab11 (yellow triangles) or Rab11 versus Ada-M (red squares) over the entire depth of the cells. (E to H) Digital images of the 4-μm cell layer following a 1-h exposure to HIV-1. (E) Ada-M is shown in green. (F) Rab11 is depicted in magenta. (G) Overlay of signals specific for HIV-1 and Rab11. (H) Binary image showing the regions of colocalization between HIV-1 and Rab11 on this cell layer (yellow dots). Bar, 10 μm.
HIV-1 is predominantly located at the apex but also migrates to the basolateral pole.
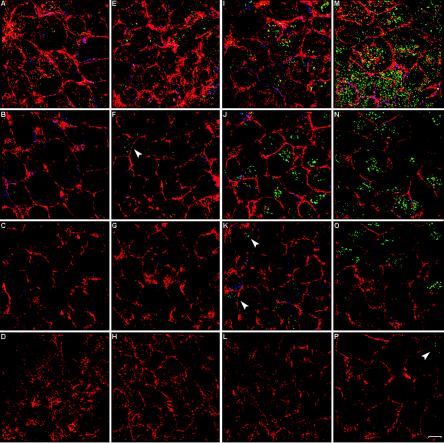
If HIV-1 is transcytosed across the trophoblastic cell barrier to reach the basolateral pole, then it can be argued that HIV-1 particles must be seen traveling from the apex of the cells to the bottom. To test this hypothesis, infected polarized JAR cells were fixed and triple labeled with anti-HIV-1, anti-ZO-1, and rhodamine-phalloidin. Confocal scanning was then performed, and the Z-stages were set to ensure that the entire depth of the cell monolayer was encompassed. The presence of HIV-1 was monitored over the entire stack of cell layers over a 4-h time course. Figure 6 presents images of the cell layers corresponding to 3 μm (Fig. 6A, E, I, and M), 4 μm (Fig. 6B, F, J, and N), the middle of the stack (Fig. 6C, G, K, and O), and the bottom of the stack (Fig. 6D, H, L, and P). We found that after 5 min, HIV-1 was located solely at the top of the cells (Fig. 6A to D). With time, viral entry progressively increased and virions gradually moved deeper into the cells. Thirty minutes after the initial contact, virions were present at the 4-μm layer (Fig. 6F), and after 2 h, some were present at the 6-μm layer (Fig. 6K). Interestingly, a few virions had reached the basolateral pole of the cells at 4 h following incubation with HIV-1 (Fig. 6P). The majority of the internalized virions accumulated at the apex of the cells (the 3- to 4-μm area).
FIG. 6.
Migration of HIV-1 in polarized trophoblasts. Polarized JAR cells were exposed to Ada-M for 5 min (A to D), 30 min (E to H), 2 h (I to L), or 4 h (M to P). Cells were triple labeled with rhodamine-phalloidin (red), mouse anti-ZO-1 followed by Cy5-conjugated goat anti-mouse IgG (blue) (to detect the tight junctions), and purified human anti-HIV-1 followed by an Alexa 488-conjugated goat anti-human antibody (green). Bar, 10 μm. The first row (A, E, I, and M) corresponds to 3 μm from the apex of the cells, the second row (B, F, J, and N) corresponds to 4 μm, the third row (C, G, K, and O) corresponds to the middle of the cells (7 to 9 μm), and the fourth row (D, H, L, and P) corresponds to the basolateral pole of the cells (14 to 18 μm).
Quantification of the migration of HIV-1 in trophoblasts.
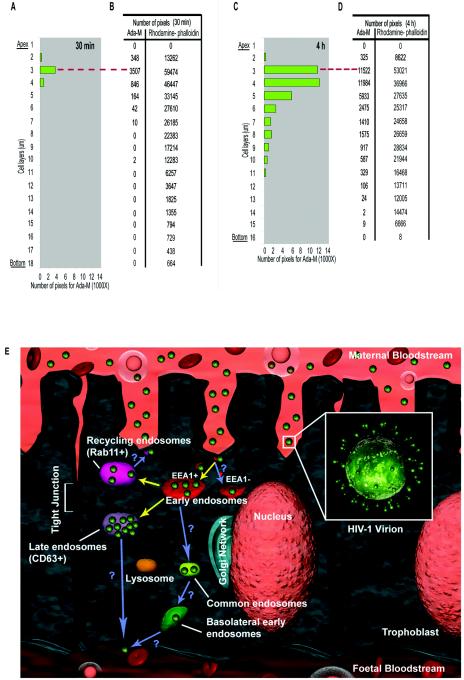
In order to quantify the intracellular distribution of HIV-1 in polarized trophoblasts, the signal intensities specific for HIV-1 and rhodamine-phalloidin were determined by Metamorph software. The bar graphs in Fig. 7 illustrate the number of pixels for Ada-M at 30 min (Fig. 7A) or 4 h (Fig. 7C) postinfection. The actual numbers of pixels are provided for both Ada-M and rhodamine-phalloidin (Fig. 7B and D). The data are vertically tiled in order to match the physical appearance of a cell monolayer. The first layer of the stack corresponds to the apex, while the last layer represents its bottom. The data indicate that HIV-1 is located within the first six layers as early as 30 min following the initial virus exposure. The majority of the internalized virions are present on the third layer. In comparison, the number of internalized viruses is significantly increased at a later time point (4 h). However, 85% of the virions remained within the first six layers of the cells, with the highest number of virions located on the fourth layer. Fifteen percent of the virions moved deeper, and a small fraction even reached the basolateral pole of the cells (Fig. 7D). The data underscore the distribution of HIV-1 in polarized trophoblasts and demonstrate for the first time the ability of HIV-1 to migrate across the entire depth of the cell layer.
FIG. 7.
HIV-1 migrates to the basolateral pole of trophoblasts. (A to D) Polarized JAR cells were exposed to Ada-M for 30 min (A and B) or 4 h (C and D). Cells were double labeled with rhodamine-phalloidin and purified human anti-HIV-1 followed by an Alexa 488-conjugated goat anti-human antibody. Bar charts illustrate the number of pixels (specific for HIV-1) present on each cell layer from the apex to the bottom after 30 min (A) or 4 h (C) of virus exposure. Tables give the number of pixels (specific for HIV-1 and actin cytoskeleton) present on each cell layer from the apex to the bottom after 30 min (B) or 4 h (D) of virus exposure. Dashed red lines indicate the correspondence between the bar charts and the tables. (E) The intracellular itinerary of incoming HIV-1 in trophoblasts is summarized. Upon endocytic entry in polarized trophoblasts, HIV-1 is associated with EEA-1-positive organelles and possibly with EEA1-negative organelles. The virions rapidly traffic to CD63+ late endosomes, where they accumulate. Infection and degradation may be associated with events occurring in these organelles. Also, HIV-1 may recycle to the apical pole via Rab11+ organelles. Finally, HIV-1 virions migrate to the basolateral pole. Transcytosis of HIV-1 may occur through a transit via the common endosome and basolateral endosome and/or concomitantly with the release of exosomes via CD63+ organelles. Demonstrated pathways are indicated by yellow arrows; postulated pathways are indicated by blue arrows.
DISCUSSION
In this study, treatment with endosome inhibitors resulted in a significant reduction of HIV-1 expression in polarized trophoblasts but did not affect viral entry. Several explanations can be suggested to account for these findings. First, HIV-1 may require an acid pH for fusion within the endosomes (as is the case, for instance, for vesicular stomatitis virus) in trophoblasts. Alternatively, endosomal hydrolases may be required for HIV-1 to be released into the cytoplasm. In either case, these data underscore that (i) a functional correlation between the endocytic uptake of HIV-1 and subsequent trafficking leads to infection in these cells and (ii) the mechanism through which HIV-1 escapes the endosomes to access the cytoplasm is completely different from what is known for other cell types. Indeed, when endosomal acidification is inhibited in epithelial HeLa CD4+ cells and T lymphocytes, viral replication is increased. Under such conditions, the incoming virions are believed to be spared from degradation (13, 14, 21, 30).
We do recognize that aside from infecting trophoblasts, part of the incoming virions might be degraded within the lysosomal machinery. Interestingly, we found that HIV-1 may not only be degraded but may also infect polarized trophoblasts and be transcytosed to the basolateral pole, a process also documented by other scientists. We also observed that HIV-1 was in part returned to the medium facing the apical pole upon its internalization, a process we called recycling of HIV-1. Since the risks of in utero transmission of HIV-1 are directly related to viremia, reducing the presence of HIV-1 within the placental cells would therefore be associated with smaller chances of vertical transmission of HIV-1. Both transcytosis and recycling of molecules involve the endocytic machinery, and as discussed, viral expression in trophoblasts requires the active participation of the endosomes. It would therefore appear that the endocytic machinery plays a critical role in the biology of HIV-1 in trophoblasts. The fact that incoming HIV-1 is present within several endocytic compartments fully supports this notion.
Dissection of the endocytic pathway associated with viral entry and infection is the focus of intense research. Previous reports have elegantly addressed the behavior of HIV-1 in CD4-expressing HeLa cells, lymphocytes, and dendritic cells in relation to CD4 and the coreceptors for HIV-1 (22, 23, 32, 35). However, data showing the progression of HIV-1 in epithelial cells were still completely lacking. In this study, we addressed this key question in polarized trophoblasts, a cell type thought to play a pivotal role in the vertical transmission of HIV-1. We provide for the first time an accurate and broad representation of the route taken by HIV-1 in polarized trophoblasts. In contrast to a previous study (24), we have successfully grown JAR cells as a polarized tight monolayer. Indeed, for the time JAR cells were grown in our assays, they (i) spread in a single plane, (ii) expressed the tight junction marker ZO-1, and (iii) developed a TEER of 45 Ω per cm2. The human trophoblastic cell line BeWo can also form a tight, polarized monolayer when cultured in a two-chamber culture system, an experimental strategy similar to that used in our experiments. As a comparison, BeWo gives a TEER of 65 Ω · cm2 (16). One reason that may explain the inability of Mitchell and coworkers (24) to grow JAR cells as a tight polarized monolayer could relate to the maintenance of such trophoblasts in culture flasks. When epithelial cells are cultured on glass or plastic, they are forced to feed from the apical surface, which faces the culture medium. Hence, the basolateral surface becomes isolated from the growth medium as the monolayer is sealed by the formation of tight junctions. This difficulty is overcome by growing the epithelial cells on permeable supports (31).
EEA-1 is one of the most specific early endosomal markers; it is associated with both the sorting domain of the apical early endosome and the basolateral early endosome. Consistent with published results (38), the distribution of EEA-1 in polarized JAR cells was fairly broad over the cell layers' depth; however, its maximal expression was located at the apex of the cells. If we look at the entire time course, the overall percentage of colocalization between HIV-1 and EEA-1 was low. In parallel, EEA-1 weakly colocalized with HIV-1, indicating that few of these organelles were occupied by HIV-1. Two conclusions can be drawn from these data. First, the transit of HIV-1 via EEA-1+ organelles might occur so rapidly that the percentage of colocalization is underestimated. Alternatively, it is possible that only a fraction of the incoming virions transit through the EEA-1-positive organelles, while the majority use EEA-1-negative early endosomes.
The signal associated with CD63 appears as punctate dots evenly distributed over the entire cell, while an intense signal is also present in the perinuclear region (3). A similar pattern was observed in JAR cells, although the signal was strongest within the top layers of the cells. In contrast to the early and recycling endosomes, where incoming virions appeared to transit quickly, HIV-1 clearly accumulated within CD63+ organelles over time, as these organelles became increasingly occupied by HIV-1. The presence of molecules in late endosomes has typically been associated with a degradative pathway. However, the ultimate fate of molecules reaching late endosomes is a complex phenomenon. Molecules present on the limiting membranes of late endosomes are recycled, whereas constituents targeted to the inner membranes (i.e., inner vesicles) of late endosomes are destined for degradation. A selected set of proteins are also stable in the late endosome's internal vesicles (28). If we now consider the case of HIV-1, upon reaching late endosomes, this retrovirus as an enveloped virus will be among the inner vesicles present in these compartments. Therefore, it is possible that a yet undefined portion of HIV-1 is degraded in trophoblasts upon reaching CD63+ organelles. On the other hand, it is also conceivable that some HIV-1 particles fuse within the endocytic compartment, possibly in the late endosomes, to reach the cytoplasm as part of the virus's infectious cycle in trophoblasts. This model is in agreement with our finding that bafilomycin A1 and NH4Cl severely reduced virus gene expression in trophoblasts. Interestingly, bafilomycin A1 not only affects endosomal pH but also affects the transit from early to late endosomes (30). Thus, it is possible that the transit of HIV-1 to the late endosomes is required for viral infection to proceed. Further experiments are needed to confirm this hypothesis.
The small GTPase Rab11 is an established marker protein associated with recycling endosomes (11). In polarized MDCK cells, Rab11 was observed in a punctate vesicular pattern, concentrated to a single focus around the centrosome (9). Although we did not label the centrosome in this study, Rab11 in trophoblasts presented a similar pattern of expression. Interestingly, HIV-1 was seen to partly colocalize with Rab11 at a fairly constant rate. This indicates that there is a continuous transit of HIV-1 particles through these organelles as they migrate from the plasma membrane. This is the first report showing the presence of a retrovirus in Rab11+ organelles. A subpopulation of mouse polyomavirus was also recently found in perinuclear areas associated with Rab11 GTPase (19). The colocalization of HIV-1 with Rab11 has important consequences for the virus. Indeed, some molecules found in recycling endosomes are returned to the plasma membrane (9), while others are directed to the Golgi apparatus (18). In contrast to other types of virus (27), we found that HIV-1 did not colocalize with either the Golgi complex or the endoplasmic reticulum in polarized human trophoblasts (data not shown). On the other hand, we did observe that a fraction of incoming virions in trophoblasts is rapidly returned to the apical pole (i.e., the maternal circulation) upon internalization, a process we called recycling of HIV-1. It will be important to verify whether this process is Rab11 dependent.
Transcytosis of virions has been postulated previously, but HIV-1 had never been visualized migrating from one pole to the opposite pole before. In this study, although HIV-1 was predominantly concentrated at the apex of the cells, we showed that a fraction of the internalized viruses migrated below the tight junctions and reached the basolateral pole. These data are fully in support of the notion that HIV-1 is able to reach the basolateral pole in trophoblasts. From there, it is possible that HIV-1 is exocytosed from the basolateral side to reach the fetal circulation. Interestingly, it has been reported that only 1% of the virus inoculum crosses the blood-brain barrier in a similar fashion (17). Two pathways of transcytosis in trophoblasts are possible: through a transit via the common endosome and basolateral endosome and/or concomitantly with the release of exosomes via CD63-expressing organelles.
All in all, the data presented in this work provide crucial information on the intracellular trafficking and fates of HIV-1 in polarized human trophoblasts. A summary of the different pathways of HIV-1 within the endocytic compartments is depicted in Fig. 7E. The requirement for HIV-1 to transit through the endocytic compartments in order for virus gene expression to ensue stresses a completely novel mode of entry process associated with infection in these cells. Moreover, we provide evidence that at least two other fates are possible in these cells: recycling and transcytosis. These data underscore the complexity of the biology of this retrovirus, and the physiological significance of these results is high, since the human polarized trophoblast barrier is considered a primary target for maternal blood-borne HIV-1 infection. The data obtained also increase our understanding of the HIV-1 infection process in adults. Indeed, epithelial cells are the portal of entry for HIV-1 during primary infection in adults, and viral internalization is associated with endocytosis in these cells. Moreover, as discussed, endocytosis may be important for macrophages and dendritic cells, cell types that play key roles in the pathogenesis of HIV-1 infection.
Acknowledgments
We thank K. Vandal for technical assistance and J. Sims (Universal Imaging) for programming done in Metamorph.
This study was supported by grants to M. J. Tremblay from the Canadian Institutes of Health Research (CIHR) HIV/AIDS Research Program (HOP-67259). G. Vidricaire is the recipient of a CIHR Doctoral Research Award from the HIV/AIDS Research Program, and M. J. Tremblay holds a Tier 1 Canada Research Chair in Human Immuno-Retrovirology.
REFERENCES
- 1.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias, R. A., L. D. Munoz, and M. A. Munoz-Fernandez. 2003. Transmission of HIV-1 infection between trophoblast placental cells and T-cells takes place via an LFA-1-mediated cell to cell contact. Virology 307:266-277. [DOI] [PubMed] [Google Scholar]
- 3.Ayala, P., L. Lin, S. Hopper, M. Fukuda, and M. So. 1998. Infection of epithelial cells by pathogenic neisseriae reduces the levels of multiple lysosomal constituents. Infect. Immun. 66:5001-5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azocar, J., and M. Essex. 1979. Incorporation of HLA antigens into the envelope of RNA tumor viruses grown in human cells. Cancer Res. 39:3388-3391. [PubMed] [Google Scholar]
- 5.Barbeau, B., R. Bernier, N. Dumais, G. Briand, M. Olivier, R. Faure, B. I. Posner, and M. Tremblay. 1997. Activation of HIV-1 long terminal repeat transcription and virus replication via NF-κB-dependent and -independent pathways by potent phosphotyrosine phosphatase inhibitors, the peroxovanadium compounds. J. Biol. Chem. 272:12968-12977. [DOI] [PubMed] [Google Scholar]
- 6.Bayer, N., D. Schober, E. Prchla, R. F. Murphy, D. Blaas, and R. Fuchs. 1998. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J. Virol. 72:9645-9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bomsel, M., and A. Alfsen. 2003. Entry of viruses through the epithelial barrier: pathogenic trickery. Nat. Rev. Mol. Cell. Biol. 4:57-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantin, R., J. F. Fortin, G. Lamontagne, and M. Tremblay. 1997. The presence of host-derived HLA-DR1 on human immunodeficiency virus type 1 increases viral infectivity. J. Virol. 71:1922-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casanova, J. E., X. Wang, R. Kumar, S. G. Bhartur, J. Navarre, J. E. Woodrum, Y. Altschuler, G. S. Ray, and J. R. Goldenring. 1999. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol. Biol. Cell 10:47-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, B. K., K. Saksela, R. Andino, and D. Baltimore. 1994. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. J. Virol. 68:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clague, M. J. 1998. Molecular aspects of the endocytic pathway. Biochem. J. 336:271-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 13.Fackler, O. T., and B. M. Peterlin. 2000. Endocytic entry of HIV-1. Curr. Biol. 10:1005-1008. [DOI] [PubMed] [Google Scholar]
- 14.Fredericksen, B. L., B. L. Wei, J. Yao, T. Luo, and J. V. Garcia. 2002. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J. Virol. 76:11440-11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagaye, S., M. Derrien, E. Menu, C. Coito, E. Tresoldi, P. Mauclere, G. Scarlatti, G. Chaouat, F. Barre-Sinoussi, and M. Bomsel. 2001. Cell-to-cell contact results in a selective translocation of maternal human immunodeficiency virus type 1 quasispecies across a trophoblastic barrier by both transcytosis and infection. J. Virol. 75:4780-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, F., M. J. Soares, and K. L. Audus. 1997. Permeability properties of monolayers of the human trophoblast cell line BeWo. Am. J. Physiol. 273:C1596-C1604. [DOI] [PubMed] [Google Scholar]
- 17.Liu, N. Q., A. S. Lossinsky, W. Popik, X. Li, C. Gujuluva, B. Kriederman, J. Roberts, T. Pushkarsky, M. Bukrinsky, M. Witte, M. Weinand, and M. Fiala. 2002. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J. Virol. 76:6689-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallard, F., C. Antony, D. Tenza, J. Salamero, B. Goud, and L. Johannes. 1998. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of Shiga toxin B-fragment transport. J. Cell Biol. 143:973-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mannova, P., and J. Forstova. 2003. Mouse polyomavirus utilizes recycling endosomes for a traffic pathway independent of COPI vesicle transport. J. Virol. 77:1672-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mano, H., and J. C. Chermann. 1991. Fetal human immunodeficiency virus type 1 infection of different organs in the second trimester. AIDS Res. Hum. Retrovir. 7:83-88. [DOI] [PubMed] [Google Scholar]
- 21.Maréchal, V., M. C. Prevost, C. Petit, E. Perret, J. M. Heard, and O. Schwartz. 2001. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 75:11166-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald, D., M. A. Vodicka, G. Lucero, T. M. Svitkina, G. G. Borisy, M. Emerman, and T. J. Hope. 2002. Visualization of the intracellular behavior of HIV in living cells. J. Cell Biol. 159:441-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295-1297. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell, A. M., A. S. Yap, E. J. Payne, S. W. Manley, and R. H. Mortimer. 1995. Characterization of cell polarity and epithelial junctions in the choriocarcinoma cell line, JAR. Placenta 16:31-39. [DOI] [PubMed] [Google Scholar]
- 25.Mofenson, L. M., and P. Munderi. 2002. Safety of antiretroviral prophylaxis of perinatal transmission for HIV-infected pregnant women and their infants. J. Acquir. Immune Defic. Syndr. 30:200-215. [DOI] [PubMed] [Google Scholar]
- 26.Owens, R. J., J. W. Dubay, E. Hunter, and R. W. Compans. 1991. Human immunodeficiency virus envelope protein determines the site of virus release in polarized epithelial cells. Proc. Natl. Acad. Sci. USA 88:3987-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 28.Piper, R. C., and J. P. Luzio. 2001. Late endosomes: sorting and partitioning in multivesicular bodies. Traffic 2:612-621. [DOI] [PubMed] [Google Scholar]
- 29.Ringler, G. E., and J. F. Strauss III. 1990. In vitro systems for the study of human placental endocrine function. Endocr. Rev. 11:105-123. [DOI] [PubMed] [Google Scholar]
- 30.Schaeffer, E., V. B. Soros, and W. C. Greene. 2004. Compensatory link between fusion and endocytosis of human immunodeficiency virus type 1 in human CD4 T lymphocytes. J. Virol. 78:1375-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simons, K., and S. D. Fuller. 1985. Cell surface polarity in epithelia. Annu. Rev. Cell Biol. 1:243-288. [DOI] [PubMed] [Google Scholar]
- 32.Steffens, C. M., and T. J. Hope. 2003. Localization of CD4 and CCR5 in living cells. J. Virol. 77:4985-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein, B. S., S. D. Gowda, J. D. Lifson, R. C. Penhallow, K. G. Bensch, and E. G. Engleman. 1987. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell 49:659-668. [DOI] [PubMed] [Google Scholar]
- 34.Tardif, M. R., and M. J. Tremblay. 2003. Presence of host ICAM-1 in human immunodeficiency virus type 1 virions increases productive infection of CD4+ T lymphocytes by favoring cytosolic delivery of viral material. J. Virol. 77:12299-12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turelli, P., V. Doucas, E. Craig, B. Mangeat, N. Klages, R. Evans, G. Kalpana, and D. Trono. 2001. Cytoplasmic recruitment of INI1 and PML on incoming HIV preintegration complexes: interference with early steps of viral replication. Mol. Cell 7:1245-1254. [DOI] [PubMed] [Google Scholar]
- 36.UNAIDS. 2003. AIDS Epidemic Update 2003. [Online.] http://www.unaids.org/Unaids/EN/Resources/publications/corporate+publications/aids+epidemic+update+-+december+2003.asp.
- 37.Vidricaire, G., M. R. Tardif, and M. J. Tremblay. 2003. The low viral production in trophoblastic cells is due to a high endocytic internalization of the human immunodeficiency virus type 1 and can be overcome by the pro-inflammatory cytokines tumor necrosis factor-alpha and interleukin-1. J. Biol. Chem. 278:15832-15841. [DOI] [PubMed] [Google Scholar]
- 38.Wilson, J. M., M. de Hoop, N. Zorzi, B. H. Toh, C. G. Dotti, and R. G. Parton. 2000. EEA1, a tethering protein of the early sorting endosome, shows a polarized distribution in hippocampal neurons, epithelial cells, and fibroblasts. Mol. Biol. Cell 11:2657-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zachar, V., V. Zacharova, T. Fink, R. A. Thomas, B. R. King, P. Ebbesen, T. B. Jones, and A. S. Goustin. 1999. Genetic analysis reveals ongoing HIV type 1 evolution in infected human placental trophoblast. AIDS Res. Hum. Retrovir. 15:1673-1683. [DOI] [PubMed] [Google Scholar]