Abstract
APOBEC3G is promiscuous with respect to its antiretroviral effect, requiring that it be packaged into diverse retrovirus particles. Here, we show that most virally encoded human immunodeficiency virus type 1 particle components are dispensable for APOPEC3G incorporation. However, replacement of the nucleocapsid (NC) Gag domain with a leucine zipper abolished APOBEC3G incorporation. Moreover, coprecipitation analysis showed that APOBEC3G-Gag interaction requires NC and nonspecific RNA. These observations suggest that APOBEC3G exploits an essential property of retroviruses, namely, RNA packaging, to infiltrate particles. Because it is, therefore, difficult to evolve specific sequences that confer escape from APOBEC3G, these findings may explain why lentiviruses evolved an activity that induces its destruction.
APOBEC3G is a cytidine deaminase that modifies nascent single-stranded retroviral DNA during reverse transcription (19), resulting in its degradation or hypermutation (6, 10, 12, 24). Although APOBEC3G acts during the early phases of the retrovirus life cycle, antiviral activity is observed only if APOBEC3G is expressed in the cell from which the virion is derived (6, 12, 13, 24). These findings suggest that a sequestered environment for reverse transcription that incorporates APOBEC3G is defined in virus-producing cells and is at least temporarily preserved upon subsequent infection of target cells. Accordingly, APOBEC3G incorporation into retroviral particles has been demonstrated (6, 13, 19). Most lentiviruses counteract this antiretroviral activity by expressing Vif proteins that prevent APOBEC3G incorporation into virions, primarily by inducing its degradation by proteasomes (4, 9, 11, 14, 17, 20, 21, 23) and perhaps by additional mechanisms (13, 21).
A feature of the antiretroviral activity exhibited by APOBEC3G is its broad specificity. Human immunodeficiency virus type 1 (HIV-1), a variety of simian immunodeficiency viruses, and more distantly related retroviruses, such as equine infectious anemia virus and murine leukemia virus, are inhibited by human APOBEC3G (6, 12, 13). Therefore, APOBEC3G must have the remarkable ability to be packaged into diverse retroviral particles whose protein and nucleic acid components exhibit little sequence homology. Here, we set out to determine what viral components mediate the packaging of APOBEC3G into HIV-1 particles that might explain this promiscuity.
Viral genomic RNA and virion proteins other than Gag are not required for APOBEC3G packaging.
APOBEC3G is active in experimental contexts that employ retroviral vector systems to deliver heterologous genes (6, 12). Typically, vector systems package versions of the cognate viral genome, where the majority of viral RNA sequences have been removed. Nonetheless, vector RNAs must retain certain cis-acting signals, such as those required for packaging (2), that could, in principle, be structurally conserved and responsible for recruitment and packaging of APOBEC3G into retroviral particles. Therefore, we first tested whether particles generated by expression of HIV-1 Gag-Pol proteins alone or in the presence of a packageable vector RNA genome differed in their ability to incorporate APOBEC3G. In this and other experiments, 293T cells in 35-mm-diameter dishes were transfected with plasmids expressing HIV-1 Gag-Pol proteins (7) and an amino-terminally myc-tagged APOBEC3G protein in the presence or absence of an HIV-1 vector genome expression plasmid, CSGW (5). As a control for nonspecific protein packaging and cellular debris contamination of particle preparations, an irrelevant myc-tagged cytoplasmic protein (green fluorescent protein [GFP]) was expressed in place of myc-APOBEC3G. Forty-eight hours after transfection, culture supernatants were clarified by low-speed centrifugation and passed through a 0.22-μm-pore-size filter. Particles were recovered by ultracentrifugation through a 20% sucrose cushion. Thereafter, cell and viral particle lysates were analyzed by Western blotting with anti-CA and anti-myc monoclonal antibodies, as previously described (16). As can be seen in Fig. 1A, the levels of APOBEC3G in cell and virion lysates were unaffected by the presence or absence of a packageable genome. Thus, Gag-Pol proteins are sufficient to mediate APOBEC3G incorporation into HIV-1 particles.
FIG. 1.
Effect of HIV-1 virion protein and RNA composition on APOBEC3G incorporation into viral particles. (A) Western blot (WB) analyses of cell lysates (left panels) and extracellular VLPs (right panels) generated following transfection of 293T cells with plasmids expressing HIV-1 Gag-Pol and either myc-tagged APOBEC3G or myc-tagged GFP. A plasmid expressing a packageable RNA genome was included where indicated. The upper panels show Western blots probed with an anti-HIV-1 CA antibody, and the positions of the Gag precursor (Pr55 Gag) and processed p24 capsid (p24 CA) are indicated. The blots in the lower panels were probed with an anti-myc epitope antibody. Note that the VLP blots probed with the anti-myc antibody were exposed approximately 10-fold longer than the cell lysate blots. (B) Schematic representation of plasmids used to express HXB Gag from viral RNA-derived sequences (pCR/V1/HXB Gag; upper diagram) or a synthetic codon-optimized RNA (pCR3.1/synGag; lower diagram). (C) Western blot analyses of cell lysates (left panels) and extracellular VLPs (right panels) generated following transfection of 293T cells with plasmids expressing synGag or HXB Gag and myc-tagged APOBEC3G. α, anti; BGH, bovine growth hormone; CMV, cytomegalovirus.
We next tested whether Gag alone was sufficient to generate virus-like particles (VLPs) that incorporated APOBEC3G. An HXB-derived HIV-1 Gag protein, expressed by using a hybrid expression plasmid, pCR/V1 (shown schematically in Fig. 1B), generated VLPs that incorporated APOBEC3G as efficiently as Gag-Pol VLPs (Fig. 1C). However, because the plasmid (pCR/V1) used to generate Gag and Gag-Pol VLPs in Fig. 1A and C and in Fig. 2 included elements of the HIV-1 genomic RNA, including the 5′ untranslated nucleotides 1 through 330, it is possible that low levels of deleted viral RNA were incorporated into VLPs and that this RNA was responsible for APOBEC3G incorporation. Therefore, we also generated Gag VLPs, using an entirely synthetic, codon-optimized Gag(synGag) expression plasmid. This pCR3.1 (Invitrogen)-based construct expresses a Gag protein with the same amino acid sequence as that of HXB Gag but completely lacks flanking viral RNA sequences. Moreover, the RNA encoding the Gag protein is quite different from the viral RNA that normally encodes HXB Gag. Thus, it is impossible for these VLPs to contain any viral RNA, because none is present in the expression system. As shown in Fig. 1C, these VLPs incorporated APOBEC3G at least as efficiently as those generated using pCR/V1-based Gag or Gag-Pol expression vectors. We conclude that Gag protein is the only virally encoded component of virions that is essential for APOBEC3G incorporation.
FIG. 2.
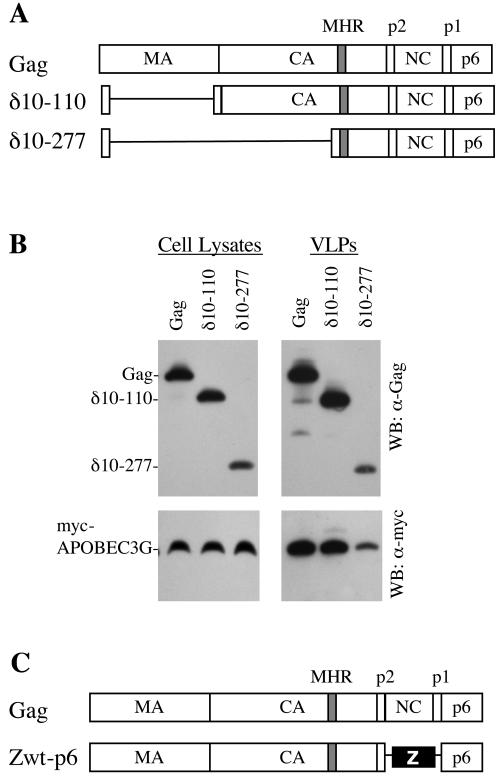
NC, but not the majority of Gag, is required for APOBEC3G incorporation into HIV-1 VLPs. (A) Schematic representation of the intact HIV-1 Gag precursor and the deletion mutants used in this study. The positions of the major homology region (MHR) and the various Gag cleavage sites are shown. (B) Western blot (WB) analyses of cell lysates (left panels) and extracellular VLPs (right panels) generated following transfection of 293T cells with plasmids expressing HIV-1 Gag or the deletion mutants shown in panel A and myc-tagged APOBEC3G. Blots were probed with anti-HIV-1 CA or anti-myc antibodies, as described in the legend to Fig. 1. (C) Schematic representation of the intact HIV-1 Gag precursor and its derivative, Zwt-p6. (D) Western blot analyses of cell lysates (left panels) and extracellular VLPs (right panels) generated following transfection of 293T cells with plasmids expressing HIV-1 Gag or Zwt-p6 and either myc-tagged APOBEC3G or myc-tagged GFP. The upper panels show Western blots probed with an anti-HIV-1 CA antibody, and the positions of the Gag and Zwt-p6 proteins are indicated. The blots in the lower panels were probed with an anti-myc epitope antibody. (E) Quantitation of RNA in wild-type Gag and Zwt-p6 VLPs, as determined by Ribogreen analysis of one-fifth of the VLPs pelleted from 30 ml of 293T cell supernatants. Pelletable material released from control-vector-transfected 293T cells was also analyzed as a control. A fourfold dilution series of the same wild-type Gag and Zwt-p6 VLPs was analyzed by Western blotting (right) to verify that equivalent amounts of VLPs were included in the Ribogreen analyses. α, anti.
The majority of Gag is dispensable for APOBEC3G packaging.
We next used deletion mutants to determine whether parts of Gag were dispensable for APOBEC3G packaging into virions. Two Gag deletion mutants, lacking residues 10 through 110 (the δ10-110 mutant) or residues 10 through 277 (the δ10-277 mutant) (Fig. 2A), were tested. In both cases, myc-APOBEC3G, but not myc-GFP, was efficiently incorporated into VLPs (Fig. 2B and data not shown). Thus, almost all matrix and the amino-terminal two-thirds of capsid are dispensable for APOBEC3G packaging.
The HIV-1 Gag NC domain is required for APOBEC3G packaging.
The aforementioned δ10-277 HIV-1 Gag protein has undergone what is nearly the largest deletion that can be tolerated by HIV-1 Gag while retaining the ability to form extracellular particles (1, 3). However, the interaction, or I, domain, encoded within NC, as well as the late budding, or L, domain, encoded within p6 Gag, can be replaced by heterologous sequences, yielding chimeric Gag proteins that retain particle-forming activity (1, 15, 22). Therefore, we tested whether an HIV-1 Gag protein termed Zwt-p6 (1), in which NC is replaced by a leucine zipper from GCN4 (Fig. 2C), could package APOBEC3G. An otherwise identical HXB-derived Gag protein was used as a control. Particles formed by the wild-type HXB Gag protein incorporated myc-APOBEC3G but not myc-GFP (Fig. 2D), but those formed by Zwt-p6 did not incorporate either protein. Thus, the NC Gag domain is required for incorporation of APOBEC3G into HIV-1 VLPs.
Some conserved features of retroviral NC proteins are the zinc fingers and basic residues that are required for the incorporation of RNA (2). Although we had excluded the possibility that specific HIV-1 RNA sequences mediated APOBEC3G incorporation (Fig. 1), retroviral Gag proteins can package nonspecific RNA into particles in the absence of viral genomes (2). Indeed, RNA-induced multimerization of Gag monomers may be required for particle assembly when Gag contains an authentic NC domain (18). Potentially, nonspecific RNA might be responsible for recruiting APOBEC3G into HIV-1 VLPs. We therefore measured the levels of RNA associated with VLPs generated by the HXB Gag and Zwt-p6 proteins, using a strategy similar to that described previously (18). VLPs were harvested from 30 ml of DNase I-treated 293T cell supernatant by ultracentrifugation through 20% sucrose, resuspended in 100 μl of phosphate-buffered saline-1% Triton X-100, and heated at 56°C. The RNA content in the pelleted material was measured with Ribogreen molecular probes. As can be seen in Fig. 2E, wild-type Gag VLPs contained four- to eightfold-higher levels of RNA than did Zwt-p6 VLPs. In fact, the levels of RNA in Zwt-p6 VLP pellets were only slightly higher than the background levels of pelletable RNA in the supernatant of cells transfected with a control plasmid vector (Fig. 2E). Thus, this result indicated that NC protein, nonspecific RNA, or both could be responsible for recruiting APOBEC3G into VLPs.
APOBEC3G physically associates with NC in an RNA-dependent manner.
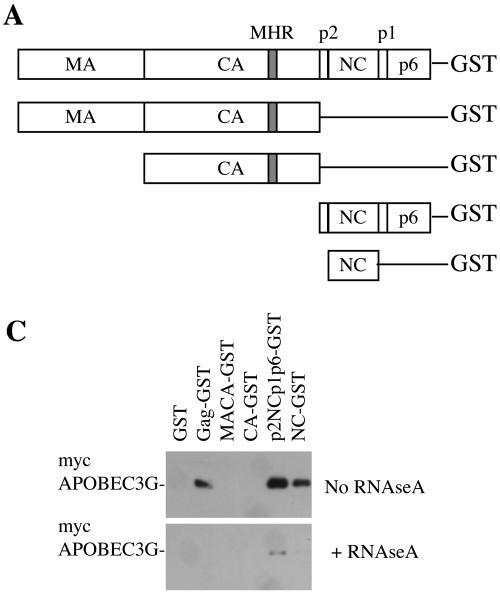
We next determined whether APOBEC3G physically associated with Gag or fragments of Gag in mammalian cells, by use of a previously described glutathione S-transferase (GST) fusion protein coprecipitation assay (15). myc-tagged APOBEC3G and GFP proteins were coexpressed in 293T cells with GST-fused HIV-1 synGag (or fragments thereof) (Fig. 3A). Thereafter, cell lysates were incubated with glutathione-Sepharose beads that were subsequently washed, and bound proteins were analyzed by Western blotting with anti-myc antibodies. As can be seen in Fig. 3B, intact HIV-1 Gag-GST or fragments (p2NCp1p6-GST and NC-GST) containing the NC Gag domain bound to APOBEC3G, while other Gag-GST fragments did not. Taken together, the results in Fig. 2 and 3B indicate that some form of interaction between NC and APOBEC3G is responsible for APOBEC3G incorporation into HIV-1 particles.
FIG. 3.
NC binds to APOBEC3G in an RNA-dependent manner. (A) Schematic representation of intact and fragmented Gag-GST fusion proteins. MHR, major homology region. (B) Gag-GST fusion proteins were coexpressed with myc-APOBEC3G (left panels) or myc-GFP (right panels). The upper and middle panels show Western blot (WB) analyses of total cell lysates. The blots in the upper panels were probed with an anti-GST antibody, and the blots in the middle panels were probed with an anti-myc antibody. The lower panels show a Western analysis (blots probed with an anti-myc antibody) of proteins precipitated from each of the cell lysates by glutathione-Sepharose beads. (C) The same experiment illustrated in the left panels of panel B was done, and Western blot analyses of the glutathione-bound proteins are shown. myc-APOBEC3G was precipitated by Gag-GST fusion proteins from duplicate aliquots of cell lysates that were left untreated (upper panel) or treated with RNase A (lower panel).
We therefore tested whether the interaction that we observed between HIV-1 Gag and APOBEC3G was dependent on the presence of RNA by repeating the coprecipitation experiment illustrated in Fig. 3B, but in the presence or absence of RNase A. As can be seen in Fig. 3C, the ability of the Gag-GST fusion proteins to coprecipitate APOBEC3G was inhibited when 50 μg of RNase A per ml was added to the cell lysate. Thus, RNA is required for the interaction between HIV-1 Gag and APOBEC3G.
Conclusions.
Based on the ability of APOBEC3G to be incorporated into HIV-1 VLPs in an NC-dependent manner and to physically associate with HIV-1 NC and Gag only in the presence of RNA, we conclude that NC recruits APOBEC3G into HIV-1 virions via interactions with RNA. While we cannot discount the possibility that specific protein-protein contacts exist between APOBEC3G and Gag, if these do occur, they appear insufficient for stable interaction. At a minimum, RNA strongly facilitates APOBEC3G-Gag interaction, and it may be that RNA is only virion molecule bound by APOBEC3G. In fact, APOBEC3G has previously been shown to bind AU-rich RNA (8). These observations suggest that APOBEC3G exploits the obligate RNA-packaging activity of Gag proteins to infiltrate virus particles. Because it would therefore be unlikely for retroviruses to evolve specific APOBEC3G “escape mutants,” these findings may explain why lentiviruses have taken the extraordinary evolutionary step of acquiring an additional gene whose sole function is to remove APOBEC3G from the cell (6, 12, 13).
Acknowledgments
We thank Cameron Bess for assistance and Theodora Hatziioannou and Juan Martin-Serrano for helpful advice.
This work was supported by NIH grants R01AI50111 (to P.D.B.) and R01 50466 (to H.G.). P.D.B. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation.
REFERENCES
- 1.Accola, M. A., B. Strack, and H. G. Gottlinger. 2000. Efficient particle production by minimal Gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain. J. Virol. 74:5395-5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkowitz, R., J. Fisher, and S. P. Goff. 1996. RNA packaging. Curr. Top. Microbiol. Immunol. 214:177-218. [DOI] [PubMed] [Google Scholar]
- 3.Borsetti, A., A. Ohagen, and H. G. Gottlinger. 1998. The C-terminal half of the human immunodeficiency virus type 1 Gag precursor is sufficient for efficient particle assembly. J. Virol. 72:9313-9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conticello, S. G., R. S. Harris, and M. S. Neuberger. 2003. The Vif protein of HIV triggers degradation of the human antiretroviral DNA deaminase APOBEC3G. Curr. Biol. 13:2009-2013. [DOI] [PubMed] [Google Scholar]
- 5.Demaison, C., K. Parsley, G. Brouns, M. Scherr, K. Battmer, C. Kinnon, M. Grez, and A. J. Thrasher. 2002. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum. Gene Ther. 13:803-813. [DOI] [PubMed] [Google Scholar]
- 6.Harris, R. S., K. N. Bishop, A. M. Sheehy, H. M. Craig, S. K. Petersen-Mahrt, I. N. Watt, M. S. Neuberger, and M. H. Malim. 2003. DNA deamination mediates innate immunity to retroviral infection. Cell 113:803-809. [DOI] [PubMed] [Google Scholar]
- 7.Hatziioannou, T., S. Cowan, and P. D. Bieniasz. 2004. Capsid-dependent and -independent postentry restriction of primate lentivirus tropism in rodent cells. J. Virol. 78:1006-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarmuz, A., A. Chester, J. Bayliss, J. Gisbourne, I. Dunham, J. Scott, and N. Navaratnam. 2002. An anthropoid-specific locus of orphan C to U RNA-editing enzymes on chromosome 22. Genomics 79:285-296. [DOI] [PubMed] [Google Scholar]
- 9.Kao, S., M. A. Khan, E. Miyagi, R. Plishka, A. Buckler-White, and K. Strebel. 2003. The human immunodeficiency virus type 1 Vif protein reduces intracellular expression and inhibits packaging of APOBEC3G (CEM15), a cellular inhibitor of virus infectivity. J. Virol. 77:11398-11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lecossier, D., F. Bouchonnet, F. Clavel, and A. J. Hance. 2003. Hypermutation of HIV-1 DNA in the absence of the Vif protein. Science 300:1112. [DOI] [PubMed] [Google Scholar]
- 11.Liu, B., X. Yu, K. Luo, Y. Yu, and X.-F. Yu. 2004. Influence of primate lentiviral Vif and proteasome inhibitors on human immunodeficiency virus type 1 virion packaging of APOBEC3G. J. Virol. 78:2072-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mangeat, B., P. Turelli, G. Caron, M. Friedli, L. Perrin, and D. Trono. 2003. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 424:99-103. [DOI] [PubMed] [Google Scholar]
- 13.Mariani, R., D. Chen, B. Schrofelbauer, F. Navarro, R. Konig, B. Bollman, C. Munk, H. Nymark-McMahon, and N. R. Landau. 2003. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell 114:21-31. [DOI] [PubMed] [Google Scholar]
- 14.Marin, M., K. M. Rose, S. L. Kozak, and D. Kabat. 2003. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat. Med. 9:1398-1403. [DOI] [PubMed] [Google Scholar]
- 15.Martin-Serrano, J., A. Yarovoy, D. Perez-Caballero, and P. D. Bieniasz. 2003. Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc. Natl. Acad. Sci. USA 100:12414-12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin-Serrano, J., T. Zang, and P. D. Bieniasz. 2001. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med. 7:1313-1319. [DOI] [PubMed] [Google Scholar]
- 17.Mehle, A., B. Strack, P. Ancuta, C. Zhang, M. McPike, and D. Gabuzda. 2004. Vif overcomes the innate antiviral activity of APOBEC3G by promoting its degradation in the ubiquitin-proteasome pathway. J. Biol. Chem. 279:7792-7798. [DOI] [PubMed] [Google Scholar]
- 18.Muriaux, D., J. Mirro, D. Harvin, and A. Rein. 2001. RNA is a structural element in retrovirus particles. Proc. Natl. Acad. Sci. USA 98:5246-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheehy, A. M., N. C. Gaddis, J. D. Choi, and M. H. Malim. 2002. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418:646-650. [DOI] [PubMed] [Google Scholar]
- 20.Sheehy, A. M., N. C. Gaddis, and M. H. Malim. 2003. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat. Med. 9:1404-1407. [DOI] [PubMed] [Google Scholar]
- 21.Stopak, K., C. de Noronha, W. Yonemoto, and W. C. Greene. 2003. HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Mol. Cell 12:591-601. [DOI] [PubMed] [Google Scholar]
- 22.Strack, B., A. Calistri, M. A. Accola, G. Palu, and H. G. Gottlinger. 2000. A role for ubiquitin ligase recruitment in retrovirus release. Proc. Natl. Acad. Sci. USA 97:13063-13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu, X., Y. Yu, B. Liu, K. Luo, W. Kong, P. Mao, and X. F. Yu. 2003. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science 302:1056-1060. [DOI] [PubMed] [Google Scholar]
- 24.Zhang, H., B. Yang, R. J. Pomerantz, C. Zhang, S. C. Arunachalam, and L. Gao. 2003. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature 424:94-98. [DOI] [PMC free article] [PubMed] [Google Scholar]