Abstract
We have examined the molecular basis for the selective incorporation of the adhesion molecule ICAM-1 within human immunodeficiency virus type 1 (HIV-1). The process of ICAM-1 incorporation was investigated by using different ICAM-1 constructs in combination with virus capture and immunoprecipitation studies, Western blot and confocal microscopy analyses, and infectivity assays. Experiments conducted with viruses bearing a truncated version of ICAM-1 revealed that the cytoplasmic domain of ICAM-1 governs insertion of this adhesion molecule into HIV-1. Further experiments suggested that there is an association between ICAM-1 and the virus-encoded Pr55Gag polyprotein. This study represents the first demonstration that structural Gag polyproteins play a key role in the uptake of a host-derived cell surface by the virus entity. Taken together, our results indicate that interactions between viral and cellular proteins are responsible for the selective uptake of host ICAM-1 by HIV-1. This observation describes a new strategy by which HIV-1 can modulate its replicative cycle, considering that insertion of ICAM-1 within nascent virions has been shown to increase virus infectivity.
During the replicative cycle of enveloped viruses, the assembly of new virions involves multiple interactions among viral proteins, nucleic acids, and lipids, and to some extent with a number of proteins of cellular origin. The process during which the viral membrane is formed is thought to be restrictive, since viral envelope (Env) proteins displace the majority of proteins of cellular origin. This is convincingly illustrated by the observation that highly purified preparations of Sindbis virions are virtually devoid of host contaminants (64). The strong and specific interactions between the virus-encoded Env proteins and the nucleocapsid are most likely responsible for the efficient exclusion of host proteins (17). However, a less stringent protein-sorting process seems to operate in some viruses and more particularly in retroviruses. Indeed, it has been demonstrated in a large number of studies that retroviruses not only tolerate but also benefit from the incorporation of several plasma membrane proteins. Studies aimed at identifying the nature of foreign constituents embedded within human immunodeficiency virus type 1 (HIV-1) have revealed a high number of host plasma membrane proteins associated with mature virions, including significant amounts of HLA-DR and certain adhesion molecules such as CD44, intercellular adhesion molecule type 1 (ICAM-1), ICAM-2, ICAM-3, lymphocyte function-associated antigen-1 (LFA-1), VCAM-1, and VLA-4 (2, 10, 11, 33, 40, 47). The reported neutralization of retrovirus replication with antibodies specific for host proteins, in both in vitro and in vivo studies, represents strong evidence that such foreign constituents remain functional (2, 3, 7, 15, 23, 25, 26, 56).
Although the process by which host-encoded plasma membrane proteins are acquired by HIV-1 is still unclear, previous observations have shed light on some possible molecular mechanisms. For example, it has been proposed that interactions between host membrane proteins and certain cellular constituents might result in exclusion from mature HIV-1 virions due to a steric hindrance phenomenon and/or sequestration to some specific domains other than the viral assembly sites (37). This concept is supported by the demonstration that the normal association between CD4 and the src-family kinase p56lck prevents incorporation of CD4 into HIV-1 due to a phenomenon of steric inhibition (36). The known interactions of adhesion molecules with cytoskeletal components (13, 35), coupled with the incorporation of elements of the actin cytoskeleton into budding viruses (50, 51), represent another putative mechanism through which HIV-1 could selectively acquire cellular proteins (reviewed in reference 66). Moreover, it has also been proposed that the natural sorting of HIV-1 Gag proteins in lipid rafts leads to a preferential incorporation of host proteins known to be concentrated in such specialized cell surface microdomains (e.g., sphingolipids and glycosylphosphatidylinositol [GPI]-anchored proteins) (45).
The viral gene gag is composed of four major domains coding for proteins that all appear to have essential functions in virus assembly and egress. The N-myristoylated matrix domain (p17MA) targets Gag to the inner leaflet of the lipid bilayer; the capsid domain (p24CA) is required for particle assembly through its ability to effect protein-protein interactions; the primary role of the nucleocapsid domain (p7NC) is to encapsulate and protect the viral RNA genome; and the C-terminal p6 domain is necessary for the pinching off of the newly assembled virion from the host membrane (reviewed in reference 57). The gag gene is first translated in the cytosol as a large precursor polyprotein, Pr55Gag, that will migrate under the plasma membrane surface, where it will multimerize (43). Thereafter, each structural domain is produced by cleavage of the Gag polyprotein by the pol-encoded protease during the maturation process.
Until now, only one host cell surface constituent, HLA-DR, has been reported to be specifically acquired by mature HIV-1 particles through an interaction with a viral protein, namely, the Env glycoprotein (54). However, there are several lines of evidence indicating that viral Env glycoproteins themselves are incorporated into budding viral entities by direct or indirect interaction with Gag polyproteins (18, 21, 29, 30, 44, 68). As HIV-1 assembly and release are tightly regulated by Pr55Gag, it is legitimate to propose that the viral Gag precursor proteins might contribute to the phenomenon of insertion of certain host cell membrane proteins within virions.
ICAM-1 is a cell surface glycoprotein composed of five extracellular immunoglobulin-like domains, a hydrophobic transmembrane domain, and a short cytoplasmic tail. ICAM-1 is an inducible ligand for at least two members of the β2 family of leukocyte integrins, LFA-1 (αLβ2) and Mac-1 (αMβ2) (reviewed in reference 62). ICAM-1 is important for granulocyte extravasation, lymphocyte-mediated cytotoxicity, and the development of specific immunologic responses involving cell-cell interactions (reviewed in reference 53). A few years ago, it was shown that ICAM-1 interacts with the actin cytoskeleton in spite of its short intracytoplasmic tail, using ezrin as an intermediate (4, 13). It has been proposed that these ICAM-1/ezrin/actin partnership interactions could concentrate ICAM-1 on uropod-like structures and thus increase its accessibility for an efficient interaction with LFA-1 (34). More relevant to this study, it has been shown that ICAM-1 is acquired by clinical strains of HIV-1 bearing different tropisms that were produced in primary human cells (5, 8, 10, 12, 27). The presence of this adhesion molecule in HIV-1 increases virus infectivity and reduces sensitivity to antibody-mediated neutralization (14, 24, 25, 56). We recently provided evidence that Env glycoproteins are not a requisite for ICAM-1 incorporation (6). Therefore, the process directing incorporation of host ICAM-1 seems to operate differently from that for foreign HLA-DR (54).
In this study, we evaluated the importance of the cytoplasmic domain of ICAM-1 in the phenomenon of ICAM-1 incorporation. This goal was achieved using vectors coding for a truncated ICAM-1 form (ICAM-1/ΔCyt) or an ICAM-1 version that localizes within lipid rafts (ICAM-1/GPI) (13, 63). We report that the cytoplasmic region of this adhesion molecule plays a dominant role in its selective incorporation into HIV-1. We also demonstrate that ICAM-1 interacts either directly or indirectly with the viral Gag precursor polyprotein Pr55Gag in newly formed virions.
(This work was performed by Y.B. in partial fulfillment of the requirements for a Ph.D. at the Faculty of Medicine, Laval University, Quebec, Canada, 2004.)
MATERIALS AND METHODS
Cells.
The human embryonic kidney cell line 293T was used to produce virus stocks. This cell line was maintained in Dulbecco's modified Eagle medium (Gibco-BRL, Burlington, Ontario, Canada) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Norcross, Ga.), glutamine (2 mM), penicillin G (100 U/ml), and streptomycin (100 mg/ml). Primary peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by Ficoll-Hypaque density gradient centrifugation and cultured in the presence of 3 μg of phytohemagglutinin-P (PHA-P; Sigma, St. Louis, Mo.)/ml and 30 U of recombinant human interleukin-2/ml for 3 days at 37°C under a 5% CO2 atmosphere prior to virus infection.
Antibodies.
The anti-ICAM-1 antibody RR1/1.1.1 was kindly provided by R. Rothlein (Boehringer Ingelheim, Ridgefield, Conn.), while the anti-ICAM-1 antibody R6.5 and the anti-major histocompatibility complex class I (anti-MHC-I) antibody W6/32 were obtained from the American Type Culture Collection (Manassas, Va.). Pooled human sera were obtained from HIV-1-positive individuals attending the HIV/AIDS Clinic of the Centre Hospitalier de l'Université Laval (Quebec, Quebec, Canada). Informed consent was obtained from each individual before collection of blood. A fluorescein isothiocyanate-conjugated goat anti-mouse antibody was purchased from Jackson ImmunoResearch Laboratories (West Grove, Pa.), whereas a goat anti-human immunoglobulin secondary antibody conjugated to Alexa 488, streptavidin conjugated to Alexa 546, and rhodamine-conjugated phalloidin were obtained from Molecular Probes (Eugene, Oreg.). A commercial anti-ICAM-1 antibody (clone G-5) was used for Western blot studies (Santa Cruz Biotechnology, Santa Cruz, Calif.). An antibody specific for the major viral core protein p24 was purified from the 183-H12-5C hybridoma (AIDS Repository Reagent Program, Rockville, Md.).
Molecular constructs.
pNL4-3 is an X4-tropic full-length infectious molecular clone of HIV-1 (1). pHXB-Luc is derived from pHXB-2D in which the nef gene was replaced by the luciferase reporter gene (kindly provided by D. Baltimore, Massachusetts Institute of Technology, Cambridge) (16). pCD1.8 is a eukaryotic expression vector containing the complete human ICAM-1 cDNA and was obtained from T. A. Springer (The Center for Blood Research, Boston, Mass.). We also used two constructs of ICAM-1 that were modified at the C-terminal end, ICAM-1/ΔCyt and ICAM-1/GPI. The ICAM-1/ΔCyt vector was constructed by replacing the last residue of the transmembrane domain, Y503, with a stop codon, whereas the ICAM-1/GPI construct was generated by replacing the cytoplasmic and transmembrane domains of ICAM-1 with a GPI anchor (supplied by T. A. Springer) (13).
Virus preparations.
Isogenic virus particles differing only by the absence or presence of host-derived ICAM-1 proteins on their outer membranes were produced by calcium phosphate transfection in 293T cells, as described previously (9, 25). A commercial calcium phosphate coprecipitation kit was used according to the manufacturer's instructions (CalPhos Mammalian Transfection kit; Clontech Laboratories Inc., Palo Alto, Calif.). The contribution of the cytoplasmic region of ICAM-1 to the acquisition of this adhesion molecule by nascent HIV-1 was monitored by cotransfecting 293T cells with either pNL4-3 or pHXB-Luc and ICAM-1 constructs (wild-type ICAM-1 [ICAM-1/WT], ICAM-1/ΔCyt, or ICAM-1/GPI). Immature HIV-1 particles were produced by treating the virus producer cell line with indinavir (20 μM) at 16 h posttransfection. Virus stocks were normalized for virion content by using a sensitive in-house sandwich enzyme-linked immunosorbent assay that can detect both p24 and the Pr55Gag precursor (8). All virus preparations underwent only one freeze-thaw cycle before initiation of infection studies. For virus capture and immunoprecipitation assays, the virus-containing supernatants were first filtered through a 0.45-μM-pore-size cellulose acetate membrane (Millipore, Bedford, Mass.) and were next passed through a Centricon Plus-20 Biomax-100 filter. Finally, samples were aliquoted before storage at −85°C. For Western blot analyses of whole-virus lysates, the virus-containing supernatants were centrifuged twice at 16,000 × g for 90 min at 4°C through a 20% sucrose cushion, and the pellet was resuspended in phosphate-buffered saline (PBS).
Flow cytometry.
For staining, cells were washed twice with PBS and centrifuged at 1,000 × g for 5 min at 4°C. Pelleted cells were incubated for 30 min on ice with a saturated concentration of the biotinylated anti-ICAM-1 antibody R6.5 (1 μg/106 cells). The cells were washed twice in PBS and incubated for 30 min on ice with a saturated concentration of an R-phycoerythrin-conjugated goat anti-mouse antibody. Finally, cells were washed twice in PBS and resuspended in 500 μl of PBS containing 1% paraformaldehyde prior to flow cytometry analysis (EPICS XL; Coulter Corporation, Miami, Fla.).
Virus precipitation test.
The presence of host-derived ICAM-1 within HIV-1 was monitored by capturing viruses with immunomagnetic beads using a previously reported procedure with slight modifications (11). In brief, magnetic beads (6.25 × 105) (streptavidin-coated BioMag; PerSeptive Diagnostics Inc., Cambridge, Mass.) were washed four times with PBS supplemented with 0.1% bovine serum albumin (BSA) (binding medium) by using a vertical magnetic plate. The beads were incubated with biotinylated antibodies (R6.5 or W6/32) for 1 h at room temperature and then washed again with PBS before use. The immunomagnetic beads were next incubated for 16 h at 4°C with virus preparations (1 ng of p24) under gentle agitation. The beads were washed five times with 1 volume of binding medium. Finally, the amount of immunocaptured HIV-1 particles was determined by measuring the viral p24 protein content found associated with such immunomagnetic beads. Beads coated with biotinylated isotype-matched irrelevant antibodies (immunoglobulin G2a [IgG2a] for R6.5 and IgG1 for W6/32) were used as negative controls.
Monitoring ICAM-1-Gag interactions.
The putative association between ICAM-1 and HIV-1 Gag was assessed by an immunoprecipitation-based protocol. Briefly, flat-bottom 96-well plates (BD Bioscience) were coated with R6.5 or an isotype-matched irrelevant control antibody (IgG2a) for 1 h at 37°C and then washed three times with PBS supplemented with 0.5% Tween 20 (PBST). The coated plates were blocked with PBST containing 0.1% BSA (Sigma) for 30 min at 37°C and then washed again three times with PBST. The plates were next incubated for 1 h at 4°C with increasing amounts of virus preparations (3.1, 6.3, 12.5, 25, and 50 ng of p24). Each well was washed five times with PBS. Half of the wells were lysed and directly quantified for their p24 content to estimate the efficiency of HIV-1 capture. The other half were analyzed for p24 content and also analyzed by Western blotting to define viral proteins complexed with host ICAM-1. The latter goal was achieved by treatment of captured viruses with a hypertonic detergent solution (1.5% NP-40, 20 mM Tris-HCl [pH 7.5], 400 mM NaCl, 1 mM EDTA, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml, and 2 mM phenylmethylsulfonyl fluoride) for 20 min at 4°C under weak agitation. Wells were washed three times with the hypertonic detergent solution before analysis. The hypertonic detergent solution is a buffer that destroys HIV-1 particles without affecting protein-protein interactions (31, 68). The immunoprecipitated complexes were next treated either with a disruption buffer for p24 testing or with a loading buffer for Western blotting. Finally, samples were heated at 100°C for 5 min before analysis.
Western blot analyses.
Whole-virus extracts from ICAM-1-bearing virions were lysed in a loading buffer (125 mM Tris-HCl [pH 6.8], 4% sodium dodecyl sulfate [SDS], 10% β-mercaptoethanol, 17.5% glycerol, and 0.0025% bromophenol blue), and 10 ng of p24 was loaded per lane prior to electrophoresis on an SDS-10% polyacrylamide gel electrophoresis (PAGE) gel. Proteins were transferred onto Immobilon-P membranes by a standard blotting technique (Millipore). Proteins were revealed by use of an anti-p24 (183-H12-5C) or anti-ICAM-1 (G-5) antibody. Primary antibodies were detected with horseradish peroxidase-conjugated, species-specific goat secondary antibodies.
Virus infection.
The infectivity of recombinant luciferase-encoding viruses (HXB-Luc) either lacking or bearing the various ICAM-1 constructs (ICAM-1/WT, ICAM-1/ΔCyt, or ICAM-1/GPI) was tested by using PBMCs as targets. Briefly, PBMCs (105) were incubated for 72 h at 37°C with similar concentrations of isogenic virus stocks (10 ng of p24 in a final volume of 200 μl). Next, 100 μl of cell-free supernatants was withdrawn from each well before the addition of a cell culture lysis buffer (25 mM Tris phosphate [pH 7.8], 2.0 mM dithiothreitol, 1% Triton X-100, and 10% glycerol). Cells were next incubated under gentle agitation for 30 min at room temperature, followed by a freeze-thaw cycle to increase the efficiency of lysis. An aliquot of 20 μl was mixed with 100 μl of luciferase assay buffer. The photon emission produced by the reaction was measured with a microplate luminometer device (MLX; Dynex Technologies, Chantilly, Va.). Luciferase activity is expressed in relative light units (RLU).
Confocal microscopy.
For tracking of ICAM-1 and budding HIV-1 particles, 293T cells that were transiently transfected with ICAM-1 constructs and the NL4-3 vector were first washed with PBS-BSA and stained for 30 min on ice with pooled HIV-1-positive human sera. Next, cells were incubated with the goat anti-human immunoglobulin secondary antibody conjugated to Alexa 488. Cells were washed three times with PBS-BSA, fixed in 2% paraformaldehyde for 20 min on ice, and then incubated for 30 min on ice with the biotin-conjugated anti-ICAM-1 antibody RR1/1.1.1. Finally, cells were washed with PBS-BSA before incubation with Alexa 546-conjugated streptavidin. After several washes, slides were mounted in 90% glycerol in PBS. The samples were visualized by using confocal laser scanning microscopy (Olympus FluoView 300) at a magnification of ×600, and digital images were processed with Adobe Photoshop. A total of 100 cells were visualized to determine the frequency of colocalization events.
Statistical analysis.
Data shown are expressed as means ± standard deviations (SD) for triplicate samples and are representative of three independent experiments. Statistically significant differences between groups were computed by analysis of variance. P value calculations were done with the Student t test in Microsoft Excel, and P values of <0.05 were considered statistically significant.
RESULTS
The cytoplasmic domain of ICAM-1 is required for acquisition of host ICAM-1 by HIV-1.
In an attempt to assess the contribution of the cytoplasmic region of ICAM-1 to the insertion of this adhesion molecule within HIV-1, 293T cells were experimentally manipulated to transiently express wild-type ICAM-1 (ICAM-1/WT), a truncated version of ICAM-1 that lacks the entire cytoplasmic domain (ICAM-1/ΔCyt), or an ICAM-1 construct in which the transmembrane domain and cytoplasmic tail were removed and replaced with a GPI anchor (ICAM-1/GPI) (Fig. 1A). Addition of a GPI domain to ICAM-1 specifically concentrates the adhesion molecule into lipid rafts where HIV-1 Gag precursors are assembled (38, 45). Cotransfection of 293T cells with molecular clones of HIV-1 and the ICAM-1 coding vector studied resulted in surface expression of significant amounts of ICAM-1. The results of representative transient transfection experiments are shown in Fig. 1B and indicate that comparable surface expression levels of wild-type, truncated, and GPI-anchored ICAM-1 were obtained following cotransfection with either pNL4-3 or pHXB-Luc.
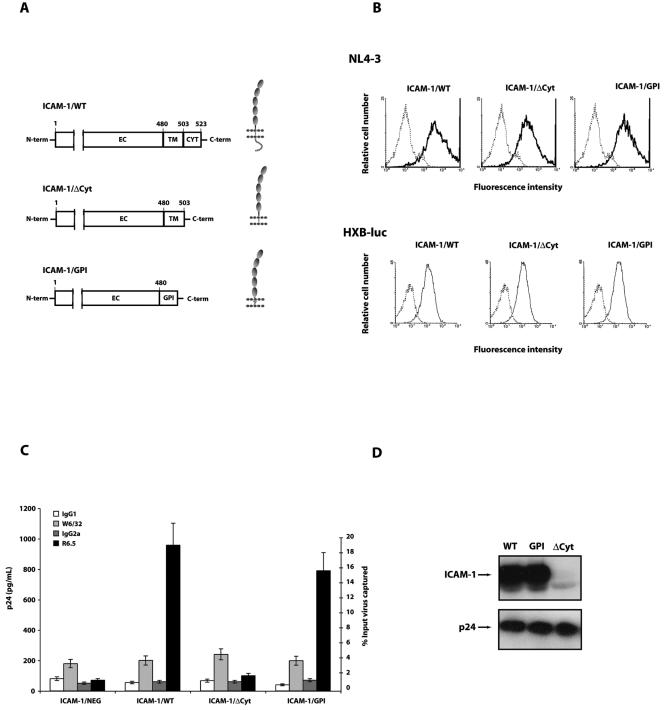
FIG. 1.
Immunocapture and Western blot analyses of viruses produced by cells expressing the ICAM-1 constructs studied. (A) Schematic representation of ICAM-1 molecular constructs used in this work. The C-terminal end of the wild-type ICAM-1 molecule was modified either by deletion of the entire cytoplasmic tail (ICAM-1/ΔCyt) or by replacement of the transmembrane and cytoplasmic domains by a GPI anchor (ICAM-1/GPI). EC, extracellular domain; TM, transmembrane domain; CYT, cytoplasmic domain. (B) Flow cytometric analyses of ICAM-1 expression were performed on 293T virus producer cells. Cells were transiently cotransfected with the ICAM-1/WT, ICAM-1/ΔCyt, or ICAM-1/GPI vector and HIV-1 molecular clone pNL4-3 or pHXB-Luc. Cells were first incubated with the anti-ICAM-1 antibody R6.5 or an isotype-matched irrelevant control antibody before addition of a fluorescein isothiocyanate-labeled secondary antibody. Controls are presented as dotted lines. (C) Viruses prepared from transiently transfected 293T cells were captured by using magnetic beads coated with an anti-ICAM-1 (R6.5) or anti-MHC-I (W6/32) antibody. Controls consisted of an appropriate isotype-matched irrelevant antibody. Precipitated virus entities were quantified by using an enzymatic p24 test. Data shown represent the means ± SD of triplicate samples and are representative of three independent experiments. (D) Whole-virus preparations from transfected 293T cells were purified by two sequential ultracentrifugation steps. Samples were lysed and loaded onto an SDS-10% PAGE gel. Viral proteins were next transferred onto a membrane and visualized with an anti-ICAM-1 (clone G-5) or anti-p24 (clone 183-H12-5C) antibody.
Virus stocks produced from such cells were next subjected to a virus capture assay. The process of ICAM-1 incorporation was totally abolished upon deletion of the cytoplasmic domain of ICAM-1, as illustrated by a comparable capture efficiency of HIV-1 particles produced in ICAM-1-negative parental 293T cells (ICAM-1/NEG) or cells expressing ICAM-1/ΔCyt (Fig. 1C). However, very efficient capture was seen for progeny virus produced by cells expressing a GPI-anchored ICAM-1 form. The validity of the virus capture test is supported by the observation that the capture efficiency of the tested virus preparations was similar when beads coated with the anti-MHC-I antibody W6/32 were used. Western blot analyses of lysates of whole viruses produced by cells expressing ICAM-1/WT, ICAM-1/ΔCyt, or ICAM-1/GPI confirmed that deletion of the cytoplasmic region of ICAM-1 results in exclusion of ICAM-1 from progeny virus, whereas the GPI-linked ICAM-1 is efficiently incorporated into HIV-1 (Fig. 1D).
The possible consequence of these observations for HIV-1 infectivity was next investigated by using primary human cells as targets. As expected, virus infection studies conducted with mitogen-stimulated PBMCs revealed that HIV-1 infectivity is augmented upon acquisition of host ICAM-1 (Table 1). Lower and comparable infectivities were obtained for a virus lacking ICAM-1 and for a virus produced in cells expressing ICAM-1/ΔCyt, an observation that is in line with previous results obtained by virus capture and Western blot assays. Finally, the infectivities of viruses bearing wild type or GPI-anchored ICAM-1 were found to be similar, suggesting that both molecules are still functional.
TABLE 1.
Infection of primary human cells with isogenic HIV-1 particles produced in cells either lacking or expressing ICAM-1 constructsa
| PBMC donor | Luciferase activity (mean RLU ± SD) in PBMCs infected with viruses from cells expressing:
|
ΔRLU (fold)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No ICAM-1
|
ICAM-1/WT
|
ICAM-1ΔCyt
|
ICAM-1/GPI
|
WT/ NEG | GPI/ NEG | WT/ ΔCYT | ΔCyt/ NEG | |||||
| w/o AZT | With AZT | w/o AZT | With AZT | w/o AZT | With AZT | w/o AZT | With AZT | |||||
| A | 35.0 ± 3.9 | 5.0 ± 0.6 | 130.0 ± 14.3 | 9.0 ± 1.0 | 45.0 ± 5.0 | 7.0 ± 0.8 | 118.7 ± 8.9 | 4.2 ± 0.9 | 3.7 | 3.4 | 2.9 | 1.3 |
| B | 43.0 ± 4.7 | 6.0 ± 0.7 | 180.0 ± 19.8 | 10.0 ± 1.1 | 65.0 ± 7.2 | 7.2 ± 0.8 | 180.3 ± 13.5 | 3.6 ± 0.3 | 4.2 | 4.2 | 2.8 | 1.5 |
| C | 45.0 ± 5.0 | 4.3 ± 0.5 | 168.0 ± 18.5 | 9.8 ± 1.1 | 55.0 ± 6.1 | 7.9 ± 0.9 | 150.6 ± 11.3 | 5.9 ± 0.4 | 3.7 | 3.3 | 3.1 | 1.2 |
| Mean | 41.0 ± 4.5 | 5.1 ± 0.6 | 159.3 ± 17.5 | 9.6 ± 1.1 | 55.0 ± 6.1 | 7.4 ± 0.8 | 149.9 ± 11.2 | 4.6 ± 0.5 | 3.9 | 3.6 | 2.9 | 1.3 |
| P value | NA | NA | NA | NA | NA | NA | NA | NA | 0.0107 | 0.0198 | 0.0085 | 0.0728 |
PBMCs from three different healthy donors were first stimulated for 3 days with PHA-P before being either left untreated or treated with azidothymidine (AZT; final concentration, 10 μM). PBMCs were next infected with HXB-Luc viruses harvested from parental 293T cells (ICAM-1/NEG; expressing no ICAM-1) or cells expressing ICAM-1/WT, ICAM- 1/ΔCyt, or ICAM-1/GPI. Virus infection was allowed to proceed for 72 h before luciferase activity was measured. Results are means ± SD from triplicate samples and are representative of three independent experiments. NA, not applicable; w/o, without.
Wild type ICAM-1, but not ICAM-1/ΔCyt, is concentrated at HIV-1 budding sites on virus producer cells.
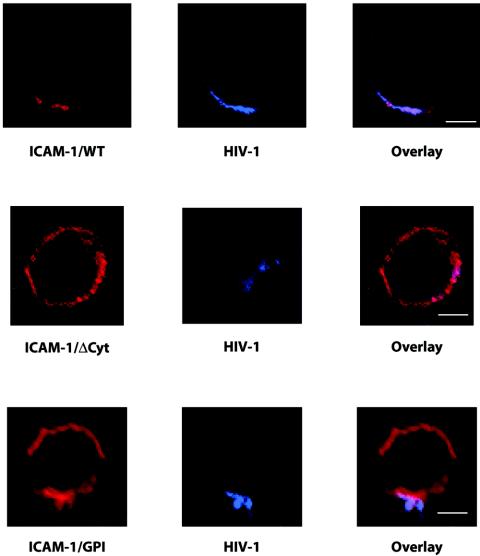
Confocal microscopy was next utilized to determine whether the absence of host ICAM-1/ΔCyt from HIV-1 particles could be linked with the location of this truncated version of ICAM-1 on the cell surface. HIV-1 budding sites were visualized by labeling transiently transfected 293T cells with purified polyclonal anti-HIV-1 antibodies from multiple pooled human sera. Wild type ICAM-1 was found to concentrate in one cell surface region in the majority of cotransfected cells (Fig. 2). Wild type ICAM-1 was found to colocalize with newly formed virions in ≈75% of cotransfected cells. In contrast, ICAM-1/ΔCyt is dispersed on the entire cell surface, and the frequency of HIV-1 colocalization is thus very low (less than 20%). ICAM-1/GPI was used as a control to visualize colocalization with the budding site. The distribution of this ICAM-1 construct seems to be much more diffuse than that of ICAM-1/WT, but it colocalizes strongly with the budding site in ≈90% of cotransfected cells. Thus, one can speculate from this last series of investigations that there might be a physical interaction at the virus assembly site between the cytoplasmic domain of ICAM-1 and some HIV-1 protein(s).
FIG. 2.
Cell surface localization of ICAM-1 constructs and HIV-1. Cells were cotransfected with the ICAM-1/WT, ICAM-1/ΔCyt, or ICAM-1/GPI vector and the molecular clone NL4-3. Next, cells were stained with pooled human sera from HIV-1-positive patients, followed by a goat anti-human immunoglobulin secondary antibody conjugated to Alexa 488. Cells were also incubated with biotin-conjugated RR1/1.1.1 in combination with Alexa 546-conjugated streptavidin. After a final wash, cells were imaged with a scanning confocal microscope. Data shown are representative of three independent experiments. Bars, 5 μm.
Selective acquisition of host ICAM-1 by HIV-1 occurs through an association between the cytoplasmic tail of ICAM-1 and Pr55Gag.
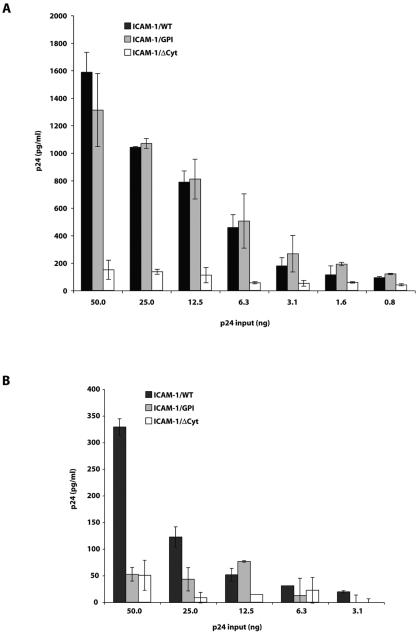
To shed light on a possible interaction between the cytoplasmic domain of ICAM-1 and the structural precursor polyprotein Pr55Gag, plates were coated with anti-ICAM-1 antibodies to capture viruses produced by cells expressing ICAM-1/WT, ICAM-1/GPI, or ICAM-1/ΔCyt. Next, the amounts of captured viruses were quantified by estimating p24 contents. In some instances, viruses that were fixed with anti-ICAM-1 were also subjected to a hypertonic detergent-based solution to lyse viral entities without destroying protein-protein interactions. As expected and in agreement with data from the virus capture assay, viruses harvested from 293T cells expressing either ICAM-1/WT or ICAM-1/GPI were efficiently captured, in contrast to those produced in cells expressing ICAM-1/ΔCyt (Fig. 3A). These findings provide additional evidence that the cytoplasmic tail of ICAM-1 indeed plays a cardinal role in the insertion of host ICAM-1 in budding viruses. As previously presented, the preferential sorting of HIV-1 particles in regions known to be enriched in GPI-linked cell surface proteins (i.e., lipid rafts) (22, 38, 45) is most likely responsible for the comparable capture efficiencies of viruses produced from either ICAM-1/WT- or ICAM-1/GPI-expressing cells.
FIG. 3.
Capture and immunoprecipitation studies on virus stocks produced in cells expressing ICAM-1/WT, ICAM-1/GPI, or ICAM-1/ΔCyt. Virus preparations harvested from 293T cells transiently expressing ICAM-1/WT, ICAM-1/GPI, or ICAM-1/ΔCyt were subjected to capture and immunoprecipitation tests as described in Materials and Methods. (A) Viruses were captured by the anti-ICAM-1 antibody (clone R6.5) or a control antibody (data not shown) and lysed, and p24 contents were estimated. (B) Levels of ICAM-1-associated HIV-1 Gag were measured by subjecting viruses captured at the bottoms of anti-ICAM-1-coated plates to a hypertonic detergent-based solution prior to p24 quantitation. Results shown are means ± SD for triplicate samples and are representative of three independent experiments.
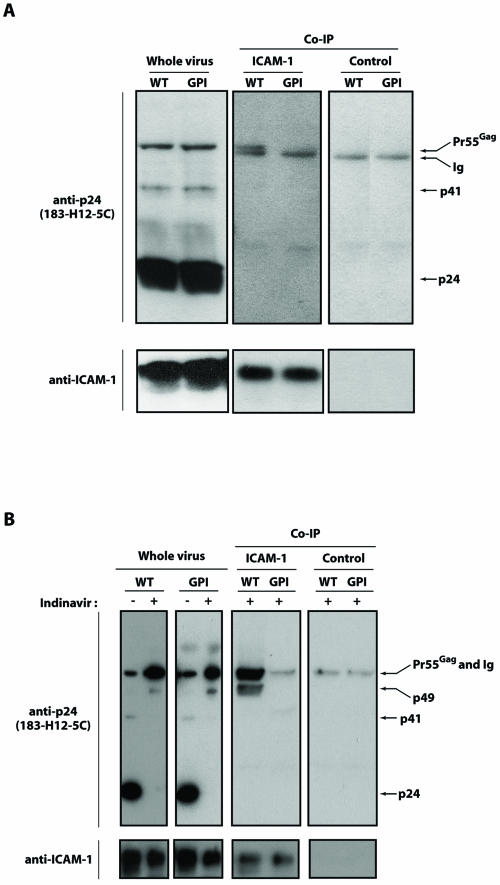
Data from experiments in which captured viruses were treated with the hypertonic detergent-based buffer suggest that wild-type ICAM-1 is associated with the HIV-1 structural precursor polyprotein Pr55Gag and/or the mature Gag cleavage product p24CA (Fig. 3B). In contrast, no such association with HIV-1 Gag could be seen when the GPI-linked form of ICAM-1 was used. The marked difference in p24 counts between the two assays (compare Fig. 3A and B) is related to the fact that a significant proportion of mature Gag p24 protein is eliminated by the detergent-based treatment, as previously reported (32). The composition of the protein complexes that were captured by anti-ICAM-1 at the bottoms of the plates was next analyzed by Western blot assays. Whole-virus extracts were also loaded in parallel to facilitate the identification of HIV-1 precursor and mature Gag proteins. Virion-anchored wild-type ICAM-1 was found to interact with the viral Gag precursor protein Pr55Gag but not with mature p24 capsid protein (Fig. 4A). Such an ICAM-1-Pr55Gag interaction was not seen for viruses bearing host-encoded ICAM-1/GPI. Virus producer cells were also treated with the protease inhibitor indinavir to increase the levels of uncleaved Gag products and ICAM-1-Pr55Gag complexes in newly formed viruses. The intensity of ICAM-1-Pr55Gag complexes was increased significantly upon indinavir treatment (Fig. 4B). The minor band of low molecular weight migrating at 49 kDa most likely reflects residual protease activity. The ICAM-1-Pr55Gag complexes were not present in viruses bearing host-encoded ICAM-1/GPI.
FIG. 4.
Western blot analyses of whole or immunoprecipitated virus lysates for mature and immature HIV-1. Viruses were produced in 293T cells transiently expressing ICAM-1/WT, ICAM-1/GPI, or ICAM-1/ΔCyt that were either left untreated (mature virus) (A) or treated with indinavir (immature virus) (B). Thereafter, whole viral lysates and lysed protein complexes that were captured at the bottoms of plates coated with an anti-ICAM-1 antibody (clone R6.5) or a control antibody (IgG2a) were analyzed by Western blotting. Lysates were revealed by use of an anti-p24 (183-H12-5C) or anti-ICAM-1 (G-5) antibody. Results shown are representative of three independent experiments. Co-IP, coimmunoprecipitation.
DISCUSSION
Following the entry of HIV-1 into target cells and the subsequent synthesis of viral products, genomic RNA and virus-encoded proteins will assemble on the inner leaflet of the plasma membrane to form spherical, enveloped particles that pinch off from the host cell. Virus release occurs by budding, a process allowing the virus to gain a host-derived lipid envelope. However, previous studies have shown that the composition of the retrovirus envelope and that of the plasma membrane are distinct, thus suggesting that virus assembly may occur in some specialized areas or subdomains of cellular membranes. This feature of retrovirus assembly is probably responsible for the selective acquisition of some specific constituents of host origin. Although it is now clear that several host proteins become incorporated into retroviruses and that some of them can affect several steps of the virus life cycle, only a few studies have demonstrated that host proteins are recruited into mature viruses through specific interactions with viral proteins. For example, the cytosolic protein cyclophilin A, the microtubule-based motor protein KIF4, the RNase L inhibitor HP68, and HLA-DR have been reported to associate with HIV-1 p24CA, murine leukemia virus Gag polyproteins, HIV-1 Gag, and HIV-1 gp41, respectively (28, 39, 54, 69). Given that the adhesion molecule ICAM-1 is inserted in all laboratory and clinical HIV-1 variants tested and virus infectivity is augmented severalfold upon acquisition of this host cell membrane protein, it is of the utmost importance to determine whether the uptake of host ICAM-1 is due to interactions with some specific HIV-1 proteins. Recently, we established that Env glycoproteins are not essential for achieving ICAM-1 incorporation (6), an observation that is in sharp contrast to the situation prevailing with HLA-DR (54).
Enveloped viruses are known to interact tightly with the cytoskeleton during the processes of assembly and budding (20), and data from several studies have led to the proposal that there is a link between retroviral proteins and actin filaments (reviewed in references 19, 42, and 61). More relevant to our study, it has been demonstrated that ICAM-1 interacts with members of the ezrin/radixin/moesin (ERM) family of proteins, which are considered cytoplasmic linker molecules that mediate interactions between plasma membrane components and the actin-containing cytoskeleton (4, 13, 34). Interestingly, these cytoplasmic linkers have also been found incorporated into HIV-1 virions (32, 50, 51). Among the various host proteins known to be linked with the cytoskeleton that have been shown to be inserted within mature HIV-1, several interact with ezrin (48, 49, 66). Given the demonstrated connection between ICAM-1 and the cytoskeleton and the importance of the cytoplasmic region of ICAM-1 in this process, we postulated that this domain might contribute to the insertion of this host cell membrane component into HIV-1. This working hypothesis was tested by using ICAM-1 molecules that either lack the cytoplasmic domain (ICAM-1/ΔCyt) or carry a GPI anchor (ICAM-1/GPI). Results from virus capture and infectivity assays revealed that the cytoplasmic domain of ICAM-1 plays a central role in the process of ICAM-1 incorporation. We demonstrate that the cleavage of the ICAM-1 intracytoplasmic tail can be compensated for (with respect to efficiency of incorporation and virus infectivity) by the introduction of a GPI-linked ICAM-1. Confocal microscopy provided evidence that incorporation of this adhesion molecule into newly formed viral entities is linked to its colocalization with budding HIV-1 particles. The observation of an efficient incorporation of the truncated GPI-linked ICAM-1 molecule into HIV-1 was not surprising, considering that ICAM-1/GPI is sequestered in glycolipid-enriched membrane domains or lipid rafts, which are specialized membrane subdomains that function as platforms for signal transduction and cytoskeletal reorganization (reviewed in reference 58) but also as preferential routes of HIV-1 sorting (45, 46).
Henriksson and colleagues have proposed that surface glycoproteins interacting with cellular partners may not be incorporated into nascent retroviruses due to a sterically mediated inhibition phenomenon or sequestration to subcellular regions distinct from the viral assembly site (37). It was further proposed that surface glycoproteins lacking cellular interaction partners would be incorporated due to passive diffusion to the viral assembly site (52). It seems that these concepts cannot be applied to all host cell membrane proteins that are found embedded within HIV-1, since ICAM-1 is efficiently incorporated into newly formed viral entities despite its tight interaction with cellular proteins such as ezrin and moesin that link the actin-containing cytoskeleton to the plasma membrane.
Over the past few years, it was demonstrated that both ICAM-1 and Pr55Gag interact with the cytoskeleton (13, 41, 55, 67). Treatment of host cells with cytochalasin D, a drug known to depolymerize actin filaments, results in a marked reduction in HIV-1 release from infected cells, while wortmannin, an inhibitor of myosin light chain kinase, completely abolishes virus egress (reviewed in reference 19). These findings incited us to concentrate our efforts on a possible connection between HIV-1 Gag and virus-anchored host ICAM-1. Our results suggest that ICAM-1 can associate with Pr55Gag and with the Gag precursor p49. These data suggest that Pr55Gag and p49 share the domain responsible for the interaction with the intracytoplasmic tail of ICAM-1. This interaction was found to be stable, since it persisted despite treatment with a strong detergent-based buffer known to be sufficient to remove raft-associated proteins (68). However, our findings do not permit us to completely exclude the possibility that a lipid moiety might be involved in the interaction between ICAM-1 and viral Gag precursors. Further studies are needed to shed light on this issue.
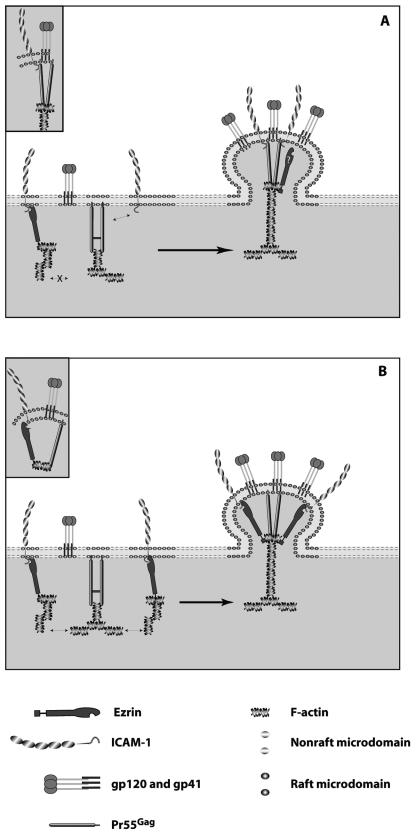
It seems clear that the adhesion molecule ICAM-1 interacts with Pr55Gag once inserted within immature HIV-1 virions and that this adhesion molecule remains associated with the mature virion. However, it is still unclear whether the process of ICAM-1 incorporation is due to a direct or an indirect link between these proteins. Thus, we propose two models that could explain the selective uptake of ICAM-1 based on the present study and already published data. A schematic representation of these hypothetical models is depicted in Fig. 5. The first model proposes that the selective acquisition of host-derived ICAM-1 by newly formed HIV-1 particles results from a direct physical link between ICAM-1 and Pr55Gag (Fig. 5A). The second scenario involves the participation of indirect complex interactions. In this case, the cytoplasmic tail of ICAM-1 would be connected with ezrin. The plasma membrane-actin linker ezrin would then mediate a tight link with the cortical actin. This multimolecular complex would be incorporated within nascent virions, based on observations indicating an interaction between HIV-1 Gag and target cell filamentous actin. Therefore, it can be proposed that HIV-1 assembly sites at the plasma membrane would be enriched in protein complexes made of host ICAM-1, ezrin, F-actin, and HIV-1 Gag precursors (Fig. 5B). It should be specified that human cytoskeletal proteins such as actin have been shown to be cleaved by HIV-1 through a nonrandom process (42, 59, 60, 65, 67). However, the observation that F-actin is protected from cleavage upon interaction with the viral p7NC (67) is in support of our findings. Experiments are currently under way to determine whether there is intimate contact between ICAM-1 and some specific processed Gag products such as p7NC.
FIG. 5.
Proposed working models for the selective incorporation of host ICAM-1 within newly formed HIV-1 virions. (A) A direct association between the cytoplasmic domain of ICAM-1 and viral Gag precursor polyproteins controls insertion of the adhesion molecule ICAM-1 into budding HIV-1. (B) The process of ICAM-1 incorporation is driven by interactions between ICAM-1 and ezrin, which is a member of the ERM family that connects membrane adhesion receptors to the actin-based cytoskeleton. The connection with Pr55Gag is due to the natural tendency of the retrovirus Gag proteins to interact closely with the actin cytoskeleton at the level of the nucleocapsid domain of the Gag precursors. The proposed protein-protein interactions are illustrated in the insets.
In summary, the data presented in this work provide new information on the molecular mechanism governing the acquisition of the adhesion molecule ICAM-1 by HIV-1. Our findings point toward an interaction between ICAM-1 and viral Gag precursors. Additional experiments are required to determine whether incorporation of some other host cell membrane proteins into HIV-1 follows a similar pattern.
Acknowledgments
We thank M. Dufour for technical assistance in flow cytometry studies and S. Méthot for critical reading of the manuscript.
This study was supported by a grant to M.J.T. from the Canadian Institutes of Health Research (CIHR) HIV/AIDS Research Program (grant HOP-14438). Y.B. holds a CIHR Doctoral Award, and M.J.T. is the recipient of a Tier 1 Canada Research Chair in Human Immuno-Retrovirology.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur, L. O., J. W. Bess, Jr., R. C. Sowder II, R. E. Benveniste, D. L. Mann, J. C. Chermann, and L. E. Henderson. 1992. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science 258:1935-1938. [DOI] [PubMed] [Google Scholar]
- 3.Arthur, L. O., J. W. Bess, Jr., R. G. Urban, J. L. Strominger, W. R. Morton, D. L. Mann, L. E. Henderson, and R. E. Benveniste. 1995. Macaques immunized with HLA-DR are protected from challenge with simian immunodeficiency virus. J. Virol. 69:3117-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barreiro, O., M. Yanez-Mo, J. M. Serrador, M. C. Montoya, M. Vicente-Manzanares, R. Tejedor, H. Furthmayr, and F. Sanchez-Madrid. 2002. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J. Cell Biol. 157:1233-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastiani, L., S. Laal, M. Kim, and S. Zolla-Pazner. 1997. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J. Virol. 71:3444-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beausejour, Y., and M. J. Tremblay. 2004. Envelope glycoproteins are not required for insertion of host ICAM-1 into human immunodeficiency virus type 1 and ICAM-1-bearing viruses are still infectious despite a suboptimal level of trimeric envelope proteins. Virology 324:165-172. [DOI] [PubMed] [Google Scholar]
- 7.Bounou, S., N. Dumais, and M. J. Tremblay. 2001. Attachment of human immunodeficiency virus-1 (HIV-1) particles bearing host-encoded B7-2 proteins leads to nuclear factor-kappa B- and nuclear factor of activated T cells-dependent activation of HIV-1 long terminal repeat transcription. J. Biol. Chem. 276:6359-6369. [DOI] [PubMed] [Google Scholar]
- 8.Bounou, S., J. E. Leclerc, and M. J. Tremblay. 2002. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4+ T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J. Virol. 76:1004-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantin, R., J. F. Fortin, G. Lamontagne, and M. Tremblay. 1997. The presence of host-derived HLA-DR1 on human immunodeficiency virus type 1 increases viral infectivity. J. Virol. 71:1922-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantin, R., J. F. Fortin, and M. Tremblay. 1996. The amount of host HLA-DR proteins acquired by HIV-1 is virus strain- and cell type-specific. Virology 218:372-381. [DOI] [PubMed] [Google Scholar]
- 11.Cantin, R., G. Martin, and M. J. Tremblay. 2001. A novel virus capture assay reveals a differential acquisition of host HLA-DR by clinical isolates of human immunodeficiency virus type 1 expanded in primary human cells depending on the nature of producing cells and the donor source. J. Gen. Virol. 82:2979-2987. [DOI] [PubMed] [Google Scholar]
- 12.Capobianchi, M. R., S. Fais, C. Castilletti, M. Gentile, F. Ameglio, and F. Dianzani. 1994. A simple and reliable method to detect cell membrane proteins on infectious human immunodeficiency virus type 1 particles. J. Infect. Dis. 169:886-889. [DOI] [PubMed] [Google Scholar]
- 13.Carpen, O., P. Pallai, D. E. Staunton, and T. A. Springer. 1992. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and α-actinin. J. Cell Biol. 118:1223-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castilletti, C., M. R. Capobianchi, S. Fais, I. Abbate, B. Ficociello, F. Ameglio, P. Cordiali Fei, S. M. Santini, and F. Dianzani. 1995. HIV type 1 grown on interferon gamma-treated U937 cells shows selective increase in virion-associated intercellular adhesion molecule 1 and HLA-DR and enhanced infectivity for CD4-negative cells. AIDS Res. Hum. Retrovir. 11:547-553. [DOI] [PubMed] [Google Scholar]
- 15.Chan, W. L., A. Rodgers, C. Grief, N. Almond, S. Ellis, B. Flanagan, P. Silvera, J. Bootman, J. Stott, K. Kent, et al. 1995. Immunization with class I human histocompatibility leukocyte antigen can protect macaques against challenge infection with SIVmac-32H. AIDS 9:223-228. [PubMed] [Google Scholar]
- 16.Chen, B. K., K. Saksela, R. Andino, and D. Baltimore. 1994. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. J. Virol. 68:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng, R. H., R. J. Kuhn, N. H. Olson, M. G. Rossmann, H. K. Choi, T. J. Smith, and T. S. Baker. 1995. Nucleocapsid and glycoprotein organization in an enveloped virus. Cell 80:621-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosson, P. 1996. Direct interaction between the envelope and matrix proteins of HIV-1. EMBO J. 15:5783-5788. [PMC free article] [PubMed] [Google Scholar]
- 19.Cudmore, S., I. Reckmann, and M. Way. 1997. Viral manipulations of the actin cytoskeleton. Trends Microbiol. 5:142-148. [DOI] [PubMed] [Google Scholar]
- 20.Damsky, C. H., J. B. Sheffield, G. P. Tuszynski, and L. Warren. 1977. Is there a role for actin in virus budding? J. Cell Biol. 75:593-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorfman, T., F. Mammano, W. A. Haseltine, and H. G. Gottlinger. 1994. Role of the matrix protein in the virion association of the human immunodeficiency virus type 1 envelope glycoprotein. J. Virol. 68:1689-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esser, M. T., D. R. Graham, L. V. Coren, C. M. Trubey, J. W. Bess, Jr., L. O. Arthur, D. E. Ott, and J. D. Lifson. 2001. Differential incorporation of CD45, CD80 (B7-1), CD86 (B7-2), and major histocompatibility complex class I and II molecules into human immunodeficiency virus type 1 virions and microvesicles: implications for viral pathogenesis and immune regulation. J. Virol. 75:6173-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortin, J. F., B. Barbeau, H. Hedman, E. Lundgren, and M. J. Tremblay. 1999. Role of the leukocyte function antigen-1 conformational state in the process of human immunodeficiency virus type 1-mediated syncytium formation and virus infection. Virology 257:228-238. [DOI] [PubMed] [Google Scholar]
- 24.Fortin, J. F., R. Cantin, M. G. Bergeron, and M. J. Tremblay. 2000. Interaction between virion-bound host intercellular adhesion molecule-1 and the high-affinity state of lymphocyte function-associated antigen-1 on target cells renders R5 and X4 isolates of human immunodeficiency virus type 1 more refractory to neutralization. Virology 268:493-503. [DOI] [PubMed] [Google Scholar]
- 25.Fortin, J. F., R. Cantin, G. Lamontagne, and M. Tremblay. 1997. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 71:3588-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortin, J. F., R. Cantin, and M. J. Tremblay. 1998. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM-1. J. Virol. 72:2105-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank, I., H. Stoiber, S. Godar, H. Stockinger, F. Steindl, H. W. Katinger, and M. P. Dierich. 1996. Acquisition of host cell-surface-derived molecules by HIV-1. AIDS 10:1611-1620. [DOI] [PubMed] [Google Scholar]
- 28.Franke, E. K., H. E. Yuan, and J. Luban. 1994. Specific incorporation of cyclophilin A into HIV-1 virions. Nature 372:359-362. [DOI] [PubMed] [Google Scholar]
- 29.Freed, E. O., and M. A. Martin. 1996. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J. Virol. 70:341-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freed, E. O., and M. A. Martin. 1995. Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single amino acid substitutions in the human immunodeficiency virus type 1 matrix. J. Virol. 69:1984-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert, C., E. Rollet-Labelle, and P. H. Naccache. 2002. Preservation of the pattern of tyrosine phosphorylation in human neutrophil lysates. II. A sequential lysis protocol for the analysis of tyrosine phosphorylation-dependent signalling. J. Immunol. Methods 261:85-101. [DOI] [PubMed] [Google Scholar]
- 32.Graham, D. R., E. Chertova, J. M. Hilburn, L. O. Arthur, and J. E. Hildreth. 2003. Cholesterol depletion of human immunodeficiency virus type 1 and simian immunodeficiency virus with beta-cyclodextrin inactivates and permeabilizes the virions: evidence for virion-associated lipid rafts. J. Virol. 77:8237-8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo, M. M., and J. E. Hildreth. 1995. HIV acquires functional adhesion receptors from host cells. AIDS Res. Hum. Retrovir. 11:1007-1013. [DOI] [PubMed] [Google Scholar]
- 34.Heiska, L., K. Alfthan, M. Gronholm, P. Vilja, A. Vaheri, and O. Carpen. 1998. Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 273:21893-21900. [DOI] [PubMed] [Google Scholar]
- 35.Heiska, L., C. Kantor, T. Parr, D. R. Critchley, P. Vilja, C. G. Gahmberg, and O. Carpen. 1996. Binding of the cytoplasmic domain of intercellular adhesion molecule-2 (ICAM-2) to α-actinin. J. Biol. Chem. 271:26214-26219. [DOI] [PubMed] [Google Scholar]
- 36.Henriksson, P., and V. Bosch. 1998. Inhibition of cellular glycoprotein incorporation into human immunodeficiency virus-like particles by coexpression of additional cellular interaction partner. Virology 251:16-21. [DOI] [PubMed] [Google Scholar]
- 37.Henriksson, P., T. Pfeiffer, H. Zentgraf, A. Alke, and V. Bosch. 1999. Incorporation of wild-type and C-terminally truncated human epidermal growth factor receptor into human immunodeficiency virus-like particles: insight into the processes governing glycoprotein incorporation into retroviral particles. J. Virol. 73:9294-9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hermida-Matsumoto, L., and M. D. Resh. 2000. Localization of human immunodeficiency virus type 1 Gag and Env at the plasma membrane by confocal imaging. J. Virol. 74:8670-8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim, W., Y. Tang, Y. Okada, T. A. Torrey, S. K. Chattopadhyay, M. Pfleiderer, F. G. Falkner, F. Dorner, W. Choi, N. Hirokawa, and H. C. Morse III. 1998. Binding of murine leukemia virus Gag polyproteins to KIF4, a microtubule-based motor protein. J. Virol. 72:6898-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao, Z., J. W. Roos, and J. E. Hildreth. 2000. Increased infectivity of HIV type 1 particles bound to cell surface and solid-phase ICAM-1 and VCAM-1 through acquired adhesion molecules LFA-1 and VLA-4. AIDS Res. Hum. Retrovir. 16:355-366. [DOI] [PubMed] [Google Scholar]
- 41.Liu, B., R. Dai, C. J. Tian, L. Dawson, R. Gorelick, and X. F. Yu. 1999. Interaction of the human immunodeficiency virus type 1 nucleocapsid with actin. J. Virol. 73:2901-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luftig, R. B., and L. D. Lupo. 1994. Viral interactions with the host-cell cytoskeleton: the role of retroviral proteases. Trends Microbiol. 2:178-182. [DOI] [PubMed] [Google Scholar]
- 43.Mervis, R. J., N. Ahmad, E. P. Lillehoj, M. G. Raum, F. H. Salazar, H. W. Chan, and S. Venkatesan. 1988. The gag gene products of human immunodeficiency virus type 1: alignment within the gag open reading frame, identification of posttranslational modifications, and evidence for alternative gag precursors. J. Virol. 62:3993-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murakami, T., and E. O. Freed. 2000. Genetic evidence for an interaction between human immunodeficiency virus type 1 matrix and alpha-helix 2 of the gp41 cytoplasmic tail. J. Virol. 74:3548-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ono, A., and E. O. Freed. 2001. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. USA 98:13925-13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orentas, R. J., and J. E. Hildreth. 1993. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res. Hum. Retrovir. 9:1157-1165. [DOI] [PubMed] [Google Scholar]
- 48.Ott, D. E. 1997. Cellular proteins in HIV virions. Rev. Med. Virol. 7:167-180. [DOI] [PubMed] [Google Scholar]
- 49.Ott, D. E. 2002. Potential roles of cellular proteins in HIV-1. Rev. Med. Virol. 12:359-374. [DOI] [PubMed] [Google Scholar]
- 50.Ott, D. E., L. V. Coren, D. G. Johnson, B. P. Kane, R. C. Sowder II, Y. D. Kim, R. J. Fisher, X. Z. Zhou, K. P. Lu, and L. E. Henderson. 2000. Actin-binding cellular proteins inside human immunodeficiency virus type 1. Virology 266:42-51. [DOI] [PubMed] [Google Scholar]
- 51.Ott, D. E., L. V. Coren, B. P. Kane, L. K. Busch, D. G. Johnson, R. C. Sowder II, E. N. Chertova, L. O. Arthur, and L. E. Henderson. 1996. Cytoskeletal proteins inside human immunodeficiency virus type 1 virions. J. Virol. 70:7734-7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paquette, J. S., J. F. Fortin, L. Blanchard, and M. J. Tremblay. 1998. Level of ICAM-1 surface expression on virus producer cells influences both the amount of virion-bound host ICAM-1 and human immunodeficiency virus type 1 infectivity. J. Virol. 72:9329-9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Plow, E. F., T. A. Haas, L. Zhang, J. Loftus, and J. W. Smith. 2000. Ligand binding to integrins. J. Biol. Chem. 275:21785-21788. [DOI] [PubMed] [Google Scholar]
- 54.Poon, D. T., L. V. Coren, and D. E. Ott. 2000. Efficient incorporation of HLA class II onto human immunodeficiency virus type 1 requires envelope glycoprotein packaging. J. Virol. 74:3918-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rey, O., J. Canon, and P. Krogstad. 1996. HIV-1 Gag protein associates with F-actin present in microfilaments. Virology 220:530-534. [DOI] [PubMed] [Google Scholar]
- 56.Rizzuto, C. D., and J. G. Sodroski. 1997. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J. Virol. 71:4847-4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scarlata, S., and C. Carter. 2003. Role of HIV-1 Gag domains in viral assembly. Biochim. Biophys. Acta 1614:62-72. [DOI] [PubMed] [Google Scholar]
- 58.Sedwick, C. E., and A. Altman. 2002. Ordered just so: lipid rafts and lymphocyte function. Sci. STKE 2002:RE2. [DOI] [PubMed] [Google Scholar]
- 59.Shoeman, R. L., C. Kesselmier, E. Mothes, B. Honer, and P. Traub. 1991. Non-viral cellular substrates for human immunodeficiency virus type 1 protease. FEBS Lett. 278:199-203. [DOI] [PubMed] [Google Scholar]
- 60.Shoeman, R. L., C. Sachse, B. Honer, E. Mothes, M. Kaufmann, and P. Traub. 1993. Cleavage of human and mouse cytoskeletal and sarcomeric proteins by human immunodeficiency virus type 1 protease. Actin, desmin, myosin, and tropomyosin. Am. J. Pathol. 142:221-230. [PMC free article] [PubMed] [Google Scholar]
- 61.Sodeik, B. 2002. Unchain my heart, baby let me go—the entry and intracellular transport of HIV. J. Cell Biol. 159:393-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Springer, T. A. 1990. Adhesion receptors of the immune system. Nature 346:425-434. [DOI] [PubMed] [Google Scholar]
- 63.Staunton, D. E., M. L. Dustin, H. P. Erickson, and T. A. Springer. 1990. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell 61:243-254. [DOI] [PubMed] [Google Scholar]
- 64.Strauss, E. G. 1978. Mutants of Sindbis virus. III. Host polypeptides present in purified HR and ts103 virus particles. J. Virol. 28:466-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomasselli, A. G., J. O. Hui, L. Adams, J. Chosay, D. Lowery, B. Greenberg, A. Yem, M. R. Deibel, H. Zurcher-Neely, and R. L. Heinrikson. 1991. Actin, troponin C, Alzheimer amyloid precursor protein and pro-interleukin 1 beta as substrates of the protease from human immunodeficiency virus. J. Biol. Chem. 266:14548-14553. [PubMed] [Google Scholar]
- 66.Tremblay, M. J., J. F. Fortin, and R. Cantin. 1998. The acquisition of host-encoded proteins by nascent HIV-1. Immunol. Today 19:346-351. [DOI] [PubMed] [Google Scholar]
- 67.Wilk, T., B. Gowen, and S. D. Fuller. 1999. Actin associates with the nucleocapsid domain of the human immunodeficiency virus Gag polyprotein. J. Virol. 73:1931-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wyma, D. J., A. Kotov, and C. Aiken. 2000. Evidence for a stable interaction of gp41 with Pr55gag in immature human immunodeficiency virus type 1 particles. J. Virol. 74:9381-9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zimmerman, C., K. C. Klein, P. K. Kiser, A. R. Singh, B. L. Firestein, S. C. Riba, and J. R. Lingappa. 2002. Identification of a host protein essential for assembly of immature HIV-1 capsids. Nature 415:88-92. [DOI] [PubMed] [Google Scholar]