Abstract
Herpes simplex virus (HSV) and other alphaherpesviruses assemble enveloped virions in the trans-Golgi network (TGN) or endosomes. Enveloped particles are formed when capsids bud into TGN/endosomes and virus particles are subsequently ferried to the plasma membrane in TGN-derived vesicles. Little is known about the last stages of virus egress from the TGN/endosomes to cell surfaces except that the HSV directs transport of nascent virions to specific cell surface domains, i.e., epithelial cell junctions. Previously, we showed that HSV glycoprotein gE/gI accumulates extensively in the TGN at early times after infection and also when expressed without other viral proteins. At late times of infection, gE/gI and a cellular membrane protein, TGN46, were redistributed from the TGN to epithelial cell junctions. We show here that gE/gI and a second glycoprotein, gB, TGN46, and another cellular protein, carboxypeptidase D, all moved to cell junctions after infection with an HSV mutant unable to produce cytoplasmic capsids. This redistribution did not involve L particles. In contrast to TGN membrane proteins, several cellular proteins that normally adhere to the cytoplasmic face of TGN, Golgi, and endosomal membranes remained primarily dispersed throughout the cytoplasm. Therefore, cellular and viral membrane TGN proteins move to cell junctions at late times of HSV infection when the production of enveloped particles is blocked. This is consistent with the hypothesis that there are late HSV proteins that reorganize or redistribute TGN/endosomal compartments to promote virus egress and cell-to-cell spread.
Herpes simplex virus (HSV) and other alphaherpesviruses dramatically alter the ultrastructure of host cells, markedly reorganizing and enlarging the nucleus, rearranging the cytoskeleton, and dispersing and redistributing cytoplasmic membrane organelles. These changes may, in part, result from high-level production of viral DNA, RNA, and proteins and their concentration and assembly in various subcompartments of the cell. However, there is also evidence that herpesviruses specifically transform various cellular compartments, not only to replicate and assemble, but also to promote egress from cells. Alphaherpesvirus capsids are initially enveloped as particles bud into the space between the inner and outer nuclear membranes providing exit from the nucleus (12). During this process, the nuclear lamina is disrupted and the inner nuclear envelope is extensively modified, in part by the HSV UL31 and UL34 proteins (35, 41; J. Baines, unpublished data). To exit the perinuclear space, herpesviruses induce fusion between the virion envelope and the outer nuclear envelope delivering unenveloped capsids into the cytoplasm.
Tegument-coated capsids acquire a second envelope by budding into cytoplasmic membrane vesicles derived from the trans-Golgi network (TGN) or endosomes (3, 7, 15, 44, 49, 52). HSV infection promotes dispersal of the Golgi apparatus and disruption of cytoplasmic microtubules (1, 8). These processes occur in some, but not all cells, and may promote virus egress or may reflect high level traffic through the Golgi apparatus. Alphaherpesviruses also modify the TGN and endosomes. Viral membrane glycoproteins are targeted and concentrated in the TGN/endosomes and tegument proteins bind onto the cytoplasmic surfaces of the TGN, and then mature alphaherpesvirus particles bud into TGN/endosome vesicles and are subsequently transported in vesicles to the cell surface (3, 4, 9, 13, 15, 21, 26, 54). Little is known about the transport of virions from the TGN to cell surfaces. It is assumed that TGN-derived vesicles ferry nascent virions to the plasma membrane where fusion between the vesicle and the plasma membrane delivers virus particles onto the cell surface (reviewed in reference 24). Whether this is a passive process or one orchestrated by HSV is not clear.
In uninfected cells, membrane vesicles pinch off the trans-Golgi apparatus and are simultaneously coated with clathrin or at least two other morphologically distinct protein coats (29), and these transport vesicles are delivered to early or late endosomes or directly to lysosomes (18). The contents of these vesicles and where they are sorted, e.g., in polarized cells, depends upon a system of coat proteins that interact with the cytoplasmic tails of cargo proteins within each vesicle (reviewed in reference 30). Recycling loops are created between the TGN and endosomes and between endosomes and the plasma membrane, so that cellular proteins are transported and concentrated into specific compartments by virtue of interactions with different cargo sorting proteins. It is likely that HSV modifies TGN exocytosis or sorting machinery in order to facilitate and speed movement to the cell surface. This hypothesis is supported by observations that accumulation of enveloped HSV particles in the cytoplasm is often short-lived, e.g., in epithelial cells fewer than 10% of enveloped particles were observed in the cytoplasm and 90% were on the cell surface (15, 26).
Several previous observations from our laboratory are consistent with the hypothesis that HSV transforms specialized regions of the TGN in order to promote specific and directed virus egress. First, the HSV heterodimeric glycoprotein gE/gI accumulated extensively in the TGN early after infection (6 h) or when expressed without other HSV proteins (13, 33, 53). At late times of HSV infection (11 to 12 h), gE/gI moved to cell surfaces and specifically to cell junctions. Second, a cellular component of the TGN, TGN46, was also redistributed to the cell junctions at late times (33). Third, HSV particles were preferentially directed to epithelial cell junctions, and not apical surfaces, and this required the cytoplasmic domain (CT domain) of gE (26). The gE CT domain was also required for TGN localization, movement of gE/gI to cell junctions, and cell-to-cell spread of HSV between polarized epithelial cells (26, 33, 53). The gE and gI CT domains contain numerous motifs that interact with TGN sorting machinery (reviewed in reference 24), including the AP-1 clathrin adaptor, μ1B, which was implicated in the directed sorting of virions (26). Fourth, gE/gI in conjunction with gD, is required for envelopment in the TGN; without these glycoproteins, unenveloped capsids accumulated in the cytoplasm (15). These observations suggested a model in which gE/gI accumulates in specific domains of the TGN, subcompartments that are ultimately sorted to basolateral surfaces or cell junctions (24, 26). By promoting HSV envelopment into these specific TGN subcompartments, gE/gI causes preferential delivery of virus particles to cell junctions, a process that would promote more rapid cell-to-cell spread.
Observations that gE/gI and TGN46 were redistributed to cell junctions suggested that newly enveloped virions containing gE/gI move from the TGN to cell junctions in vesicles that also convey TGN46. Here, we showed that gE/gI and TGN46 can move to cell junctions in the absence of cytosolic nucleocapsids and the production of enveloped virions. A second TGN integral membrane protein, carboxypeptidase D (CPD), relocalized to the cell junctions, but several other peripheral proteins of the TGN, Golgi, endosomes, and lysosomes were dispersed in the cytoplasm and not redistributed to the cell junctions. A second HSV glycoprotein, gB, also moved from the TGN to cell junctions at late times. We concluded that HSV infection causes redistribution of TGN membrane proteins to epithelial cell junctions, and this process is not a function of virion envelopment but instead appears to be related to expression of HSV late gene products.
MATERIALS AND METHODS
Cells and viruses.
All tissue culture media were from BioWhittaker, Inc., (Walkersville, Md.) and fetal bovine sera (FBS) was from HyClone. HEC-1A human endometrial epithelial cells were grown on RPMI medium supplemented with 10% FBS. HaCaT (human keratinocyte) and Vero cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10 and 7% FBS, respectively. BMS-MG22 cells that express the HSV-1 protease, derived from Vero cells as described previously (16) and maintained in DMEM containing 7% FBS and 100 to 200 μg of G418/ml, were kindly provided by Min Gao and Richard J. Colonno (Bristol-Myers Squibb, Princeton, N.J.). HSV type 1 (HSV-1) wild-type strains F and KOS were propagated, and titers were determined on Vero cells. TsProt.A was derived from KOS, expresses a temperature-sensitive protease, and was grown on BMS-MF22 cells at 31°C as described previously (16).
Antibodies.
HSV gE-specific monoclonal antibody (MAb) 3114 was a gift from Anne Cross and Nigel Stow (Institute of Virology, Glasgow, United Kingdom). Rat antiserum specific for gE and gI was produced by immunizing rats with a soluble form of gE/gI (10) and has been described elsewhere (33). A rabbit serum specific for HSV-1 gE and gI was produced by infecting rabbits with replicating (E1+) adenovirus vectors expressing these proteins (19); this serum also contains antibodies to adenovirus proteins not found in HSV-infected cells. Anti-gB MAb 15βB2 was described previously (20) and was combined with anti-gB MAb I-59 (37), a gift from Pat Spear, Northwestern University. Rabbit anti-β-catenin polyclonal serum was obtained from Sigma. Mouse MAbs specific for golgin p230 were obtained from BD Transduction (San Diego, Calif.), MAbs specific for AP-1 were obtained from Sigma (St. Louis, Mo.), and MAbs specific for LAMP1 were obtained from BD Pharmingen (San Diego, Calif.). Sheep anti-TGN46 antibodies were obtained from Serotec (Raleigh, N.C.). A rabbit polyclonal antiserum specific for CPD (45) was kindly provided by Lloyd Fricker, Albert Einstein College of Medicine. A rabbit polyclonal sera specific for 275-kDa cation-independent mannose-6-phosphate receptor (M6PR) (31) was kindly provided by Bernard Hoflack. Secondary antibodies—Alexa 594-conjugated goat anti-rat immunoglobulin G (IgG), Alexa 488- and Alexa 594-conjugated goat anti-mouse IgG, Alexa 488-conjugated donkey anti-sheep IgG, and Alexa 594-conjugated goat anti-rabbit IgG—were all obtained from Molecular Probes (Eugene, Oreg.). Cy5-coupled goat anti-mouse IgG was obtained from Jackson Immunoresearch Labs, Inc. (West Grove, Pa.).
Immunostaining cells and confocal microscopy.
HEC-1A or HaCaT cells were plated in 6- or 12-well plastic multiwell dishes containing glass coverslips for 3 to 6 days until the cells became largely confluent and formed extensive cell junctions. The cells were infected with HSV-1 by using 20 PFU/cell for HEC-1A cells and 10 PFU/cell for HaCaT cells for 5 to 12 h at 37°C in medium containing 5% FBS. In experiments involving TsProt.A, cells were incubated at 31 or 39°C for 5 to 12 h. The cells were washed once in phosphate-buffered saline (PBS) containing 1 mM MgCl2 and 1 mM CaCl2 (PBS++) and then fixed with PBS containing 4% paraformaldehyde. Samples were permeabilized by incubation with PBS containing 0.2% Triton X-100 for 15 to 20 min at room temperature, followed by incubation for 2 to 12 h with PBS containing 0.1% Tween (PBS-T) and 2% goat serum. The cells were incubated with primary antibodies diluted in PBS-T for 1 to 2 h at room temperature, washed, incubated with secondary antibodies diluted in PBS-T for 1 to 2 h, and then washed again. Samples were mounted on glass microscope slides coated with Prolong antifade reagent (Molecular Probes) and visualized on a Bio-Rad (Hercules, Calif.) 1024 ES laser scanning confocal system attached to a Nikon Eclipse TE300 fluorescence microscope, followed by the use of deconvolution software.
Biotinylation of cell surface proteins.
HEC-1A cells were infected with HSV-1 strain F, strain KOS, or TsProt.A for 18 h at either 31°C or 39°C, and then the cells were washed with PBS, followed by incubation with 2 mM EGTA for 15 min at 4°C in order to disrupt cell junctions. The cells were then incubated twice, each time for 20 min, at room temperature with EZ-link sulfo-NHS-SS-biotin (0.25 mg/ml; Pierce Chemicals) in 10 mM triethanolamine (pH 8.0)-150 mM NaCl. The cells were washed with PBS++ containing 20 mM glycine, incubated with this buffer for 20 min at 4°C to quench unreacted biotin reagent, and then lysed in 50 mM Tris-HCl (pH 7.5)-150 mM NaCl-5 mM EDTA containing 1% Triton X-100, 2 mg of bovine serum albumin/ml, of 0.5 mM phenylmethylsulfonyl fluoride. gE/gI was immunoprecipitated with mouse anti-gE MAb or gD precipitated by using a mixture of MAb DL6 and rabbit anti-gD polyclonal antibodies (both kind gifts of Roselyn Eisenberg and Gary Cohen) and protein A- and G-Sepharose (1:1) and then subjected to electrophoresis on 10% polyacrylamide gels as described previously (53). Proteins were transferred to polyvinylidene fluoride membranes (Immobilon-P; Millipore), and the membranes were blocked overnight by incubation with 0.1% Tween 20, 2% nonfat milk, 1% fish gelatin, 0.5% polyvinylpyrrolidone, and 1% goat serum in PBS. Biotinylated proteins were detected by incubation of the blots with horseradish peroxidase-conjugated streptavidin (1:2,500; Amersham) and then with Renaissance chemiluminescence reagent (New England Nuclear) and exposed to X-ray film.
Electron microscopy.
HEC-1A cells were infected with wild-type KOS or TsProt.A for 18 h, washed with PBS++ and then with 0.1 M sodium cacodylate buffer (pH 7.2), and then fixed in Ito and Karnovsky fixative (1.6% paraformaldehyde, 2.5% glutaraldehyde, and 0.5% picric acid, all buffered in 0.1 M sodium cacodylate [pH 7.2]). Samples were postfixed in 1.5% osmium tetroxide, rinsed, and then postfixed in 4% paraformaldehyde. Samples were then dehydrated in a graded acetone series and embedded in epoxy resin, and ultrathin sections were double stained in uranyl acetate-lead citrate and viewed with a Philips EM 300 electron microscope.
RESULTS
gE/gI and TGN46 redistribute to cell junctions in the absence of cytoplasmic nucleocapsids.
Previously, we showed that enveloped virions are transported specifically to epithelial cell junctions (26). gE/gI, other viral glycoproteins, and tegument proteins accumulate in the TGN or endosomes and are incorporated into enveloped virions there (4, 13, 15, 33). At late times of infection gE/gI and TGN46, a cellular component of the TGN, were both redistributed to cell junctions (33). One potential mechanism for this might involve the assembly of enveloped virions which then ferry viral glycoproteins and components of the TGN to cell surfaces. To determine whether enveloped virions are involved in this redistribution, we characterized an HSV-1 mutant, TsProt.A, that is unable to produce cytoplasmic capsids and thus does not produce significant amounts of enveloped virions. TsProt.A expresses a thermolabile protease encoded by the UL26 gene (16) and is similar to HSV-1 ts1201, which fails to cleave ICP35 (39). In TsProt.A-infected cells at the nonpermissive temperature, viral nucleocapsids accumulate in the nucleus, and there are few or no enveloped particles produced. Thus, if enveloped virions are necessary for this redistribution to cell junctions, then viral glycoproteins and cellular proteins might accumulate in the TGN with this mutant.
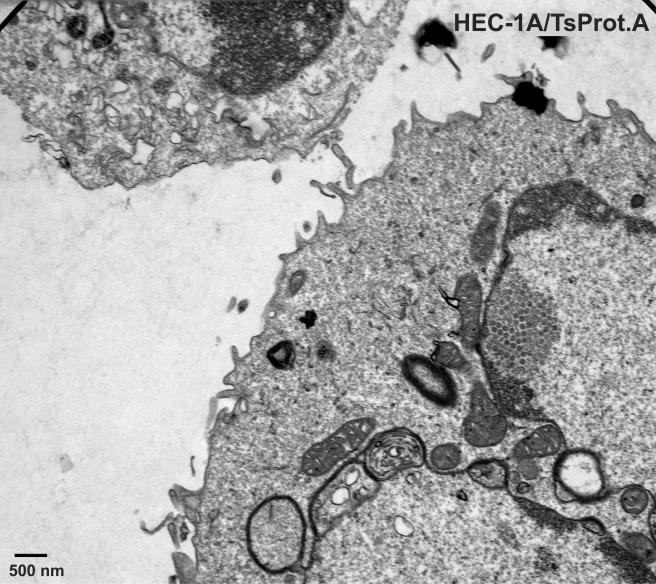
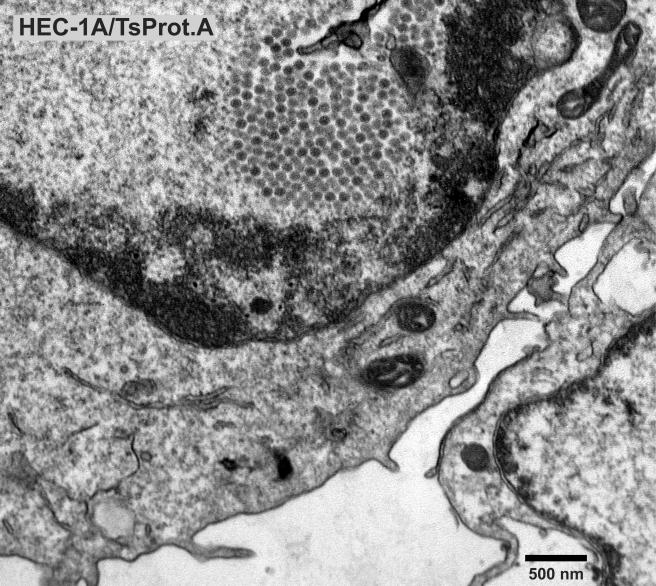
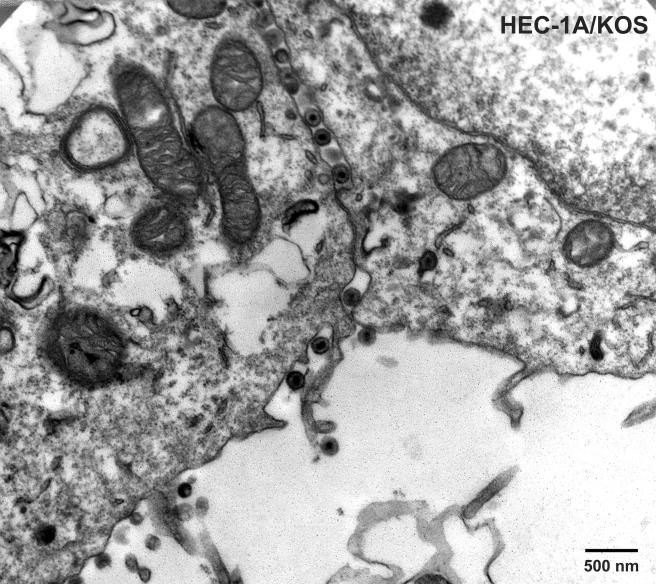
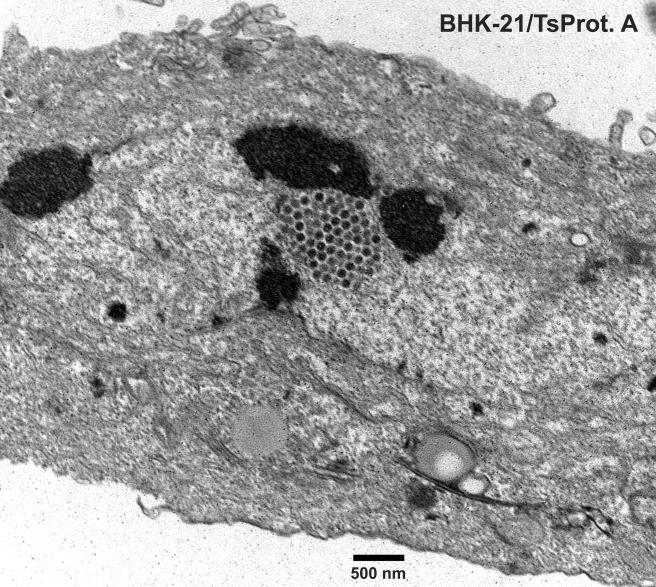
To characterize TsProt.A in HEC-1A human epithelial cells, the cells were infected with TsProt.A or wild-type HSV-1 strains F or (parental) KOS at 39°C, and infectious virus was determined by using complementing cells. Table 1 shows that TsProt.A produced ca. 1% the infectious virus compared to wild-type HSV-1 strains KOS and F at 39°C. At the permissive temperature of 31°C, TsProt.A produced only slightly less infectious virus than wild-type KOS at 31°C (not shown). Electron microscopic examination of HEC-1A cells infected with TsProt.A at 39°C revealed that there were numerous capsids that accumulated in large masses frequently at one margin of the nucleus (Fig. 1 and 2). These masses frequently included more than 150 particles just in the plane of the section. Most cells contained no enveloped capsids (Fig. 1 and 2), although rare cells displayed one or two enveloped particles in the cytoplasm or on the cell surface (not shown). In contrast, numerous cell surface and cytoplasmic enveloped particles were observed in KOS-infected HEC-1A cells at 39°C (Fig. 3 and Table 2). Although KOS-infected cells contained nuclear capsids, there were seldom large arrays as observed with TsProt.A-infected HEC-1A cells. The numbers of enveloped particles at the cell surfaces of TsProt.A-infected HEC-1A cells was reduced to 0.6% of that observed with KOS-infected HEC-1A cells (Table 2).
TABLE 1.
Yields of infectious virus produced by wild-type HSV-1 strains KOS and F and by TsProt.A
| Virus | Temp (°C) | Infectivity produced (PFU/dish)a |
|---|---|---|
| F | 39 | 1.6 × 107 |
| KOS | 39 | 1.6 × 107 |
| TsProt.A | 39 | 1.7 × 105 |
HEC-1 cells growing in 35-mm dishes were infected for 18 h, and then cells and media were harvested together in a volume of 1 ml and sonicated. Plaque assays were then performed with MG22 cells.
FIG. 1 to 3.
Electron microscopic examination of HEC-1A cells infected with TsProt.A and wild-type HSV strain KOS. HEC-1A cells were infected with TsProt.A (Fig. 1 and 2) or KOS (Fig. 3) at 39°C for 18 to 19 h. The cells were washed once with PBS+/+, fixed with glutaraldehyde for 30 min while still affixed to plastic dishes, washed twice with sodium cacodylate buffer, scraped, and collected by centrifugation at 1,000 × g for 5 min. The cell pellet was postfixed and embedded in epoxy, and ultrathin sections were stained with uranyl acetate and lead citrate and then viewed in an electron microscope. Bar, 500 nm.
TABLE 2.
Virus particles produced in HEC-1A and BHK-21 cells infected with TsProt.A
| Cell line | Virus | No. of nuclear capsidsa | No. of cell surface virions or L particles |
|---|---|---|---|
| HEC-1A | KOS | 516 | 853 |
| HEC-1A | TsProt.A | 1,066 | 5 |
| BHK-21 | TsProt.A | 650 | 84 |
HEC-1 or BHK-21 cells were infected with wild-type HSV-1 KOS or TsProt.A for 19 h at 39°C, and then the cells processed for electron microscopy. The number of capsids in the nucleus or cell surface-enveloped virions or the number of L particles in 10 to 15 cells was determined.
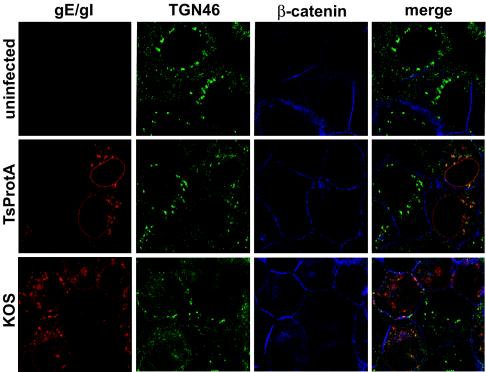
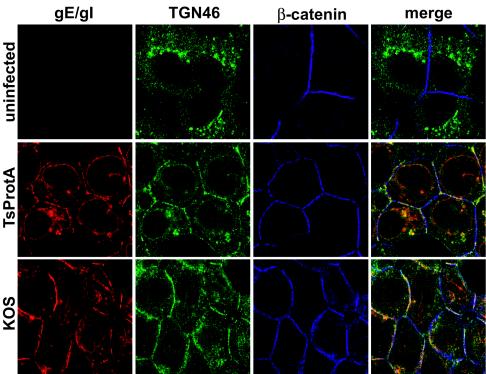
To determine whether gE/gI and TGN46 were redistributed to epithelial cell junctions in the absence of cytosolic capsids, HEC-1A cells were infected with TsProt.A or wild-type HSV-1 KOS at 39°C, and then confocal microscopy was performed at early (6 h) and late (11.5 h) times. Cells were stained simultaneously with rat anti-gE/gI polyclonal antibodies (red), sheep anti-TGN46 antibodies (green), and rabbit anti-β-catenin (blue) antibodies. After 6 h of infection with both KOS- and TsProt.A-infected cells, gE/gI was found in perinuclear vesicles that also stained with anti-TGN46 antibodies (Fig. 4). There was also a significant fraction of gE/gI in smaller vesicles distributed more uniformly within the cytoplasm, as well as in the nuclear envelope. After 11.5 h of infection with wild-type HSV-1 strain KOS, a substantial fraction of both gE/gI and TGN46 were at cell junctions that costained with β-catenin antibodies (Fig. 5), as had previously been observed with wild-type HSV-1 strain F (33). A similar pattern was observed in cells infected for 11.5 h with TsProt.A at 39°C: TGN46 and gE/gI were at the cell junctions. Interestingly, gE/gI was found extensively in the nuclear envelope of TsProt-infected cells. This observation was also made with other gE/gI antibodies (not shown). Accumulation of gE/gI in the nuclear envelope may reflect inhibition of virus egress across the nuclear envelope, leaving larger amounts of viral glycoproteins in nuclear membranes. We are studying this further.
FIG. 4 and 5.
Redistribution of gE/gI and TGN46 to cell junctions in the absence of cytoplasmic capsids. HEC-1A cells were left uninfected or infected with wild-type HSV-1 strain KOS or TsProt.A for 6 h (Fig. 4) or 11.5 h (Fig. 5). The cells were fixed with paraformaldehyde, permeabilized, and then stained with rat anti-gE/gI polyclonal antibodies (red) and simultaneously with sheep anti-TGN46 (green) and rabbit anti-β-catenin (blue) antibodies. The cells were washed and stained with secondary antibodies: Alexa 594-conjugated goat anti-rat IgG, Alexa 488-conjugated donkey anti-sheep IgG, and Cy5-conjugated goat anti-rabbit IgG.
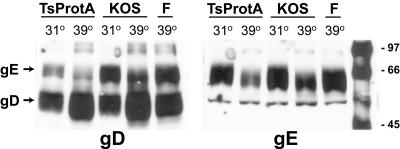
To quantify the cell surface appearance of gE/gI in TsProt.A-infected cells, biotinylation of surface proteins was performed. HEC-1A cells were infected with HSV-1 strain F, strain KOS, or TsProt.A for 18 h at either 31 or 39°C, and then the cells were incubated with 2 mM EGTA for 15 min at 4°C to disrupt tight and adherens junctions. The cells were then incubated with sulfo-NHS-SS-biotin (a reagent that does not cross membranes) to biotinylate cell surface proteins. Two HSV-1 glycoproteins, gD and gE, were immunoprecipitated from extracts of the cells and subjected to electrophoresis, and biotinylated proteins were detected by using peroxidase-conjugated streptavidin. gD was chosen as a positive control because this glycoprotein normally resides on both the apical and the lateral cell surfaces of these cells and is not initially concentrated in the TGN as with gE/gI (33). As expected, gD was labeled on the surfaces of TsProt.A- and KOS-infected cells at 39°C (Fig. 6, left panel). Only the mature form of gD was detected, but some gE (upper band) was also precipitated by using the rabbit antibodies that are bound through the IgG Fc receptor activity of gE. Since we did not observe immature gD, it is unlikely that the biotinylation reagent entered into cells. The amount of cell surface gE immunoprecipitated with an anti-gE antibody from cells infected with TsProt.A was similar to that in KOS-infected cells at 39°C (Fig. 6, right panel). Again, only the mature form of gE was detected, although a smaller fraction of gI coprecipitated with the anti-gE MAb (Fig. 6, right panel, lower band). There was more gE on the surfaces of both KOS- and TsProt.A-infected cells at 31°C than at 39°C; this is perhaps related to the observation that radiolabeled gE was also more rapidly turned over at 39°C (results not shown). In summary, both confocal and biotinylation experiments demonstrated that gE/gI and TGN46 moved to cell junctions in cells infected with TsProt.A that produced fewer than 0.6% the number of cell surface enveloped particles compared to wild-type HSV. The marked redistribution of gE/gI and TGN46 observed in every cell cannot be explained by such a small number of cell surface virus particles observed in a minority of cells.
FIG. 6.
Cell surface expression of gE and gD in cells infected with TsProt.A. HEC-1A cells were infected with wild-type HSV strains KOS or F or with TsProt.A for 18 h at either 31 or 39°C. The cells were washed with cold PBS, cell junctions were disrupted by incubation with 2 mM EGTA for 25 min at 4°C, and then cells incubated with NHS-SS-biotin to biotinylate cell surface proteins. Unreacted biotinylation reagent was quenched, cells were lysed, and gD (left panel) or gE (right panel) was immunoprecipitated with rabbit anti-gD polyclonal sera or mouse anti-gE MAb 3114, respectively. The immunoprecipitated proteins were subjected to electrophoresis on sodium dodecyl sulfate-polyacrylamide gels, and then the proteins were transferred to membranes and incubated with streptavidin-conjugated peroxidase and chemiluminescence reagent. Images were captured on X-ray film. The positions of molecular weight markers and of gD and gE are indicated.
L particles do not account for the redistribution of viral and cellular proteins to cell junctions.
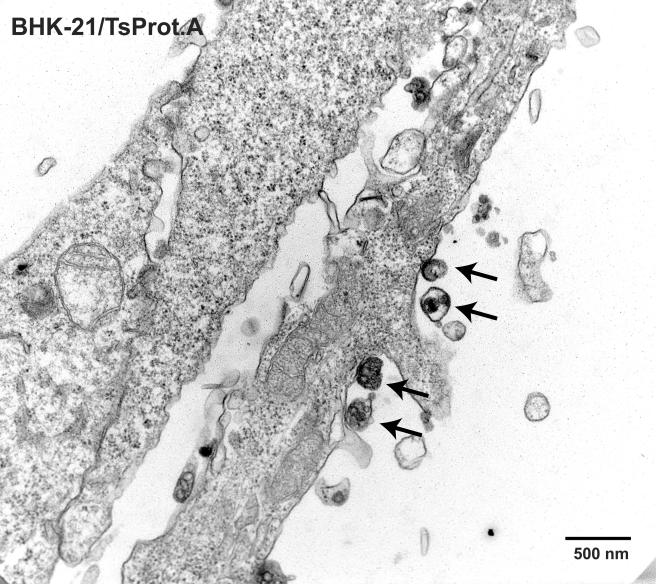
Previously, baby hamster kidney BHK-21 cells infected with wild-type HSV-1 strain 17syn+ were found to contain light or L particles composed of viral tegument proteins and an envelope but lacking nucleocapsids (42). L particles were detected on the surfaces of BHK-21 cells infected with an HSV-1 mutant ts1201, which also produces a temperature-sensitive protease similar to that for TsProt.A (47). L particles could potentially account for our observed redistribution of gE/gI and TGN proteins to the cell surface. BHK-21 cells infected with TsProt.A exhibited both large-scale accumulation of nuclear capsids and a moderate number of cell surface virus particles that lacked capsids (Fig. 7 and 8 and Table 2). These cell surface virus particles (see arrows in Fig. 8) were considered L particles because they were of the size and shape of HSV particles, although with irregular membranes as described previously (42). BHK-21 L particles were much less numerous compared to capsids that accumulated in large arrays in the nucleus and compared to the numbers of enveloped virions observed on the surfaces of HEC-1A cells infected with wild-type HSV (Table 2). Whether or not BHK-21 or HEC-1A cells contained cytoplasmic L particles, e.g., as assessed by using gradient centrifugation, was not addressed here because cytoplasmic L particles cannot account for the redistribution of glycoproteins to the cell surface. More germane for our studies, the numbers of virions or L particles on the surfaces of TsProt.A-infected HEC-1A cells was very low, <0.6% the number of enveloped particles observed with KOS-infected HEC-1A cells (Table 2). In fact, most or all of the cell surface particles observed on TsProt.A-infected HEC-1A cells contained capsids. These results exclude the possibility that L particles account for the cell surface redistribution of gE/gI and TGN46 in TsProt.A-infected cells.
FIG. 7 and 8.
Nuclear capsids and cell surface L particles in TsProt.A-infected BHK-21 cells. BHK-21 cells were infected with TsProt.A at 39°C for 18 to 19 h. The cells were washed, fixed, and processed for electron microscopy as described in Fig. 1 to 3. Bar, 500 nm. Arrows in Fig. 8 indicate cell surface L particles.
Redistribution of other viral glycoproteins and cellular markers to cell junctions.
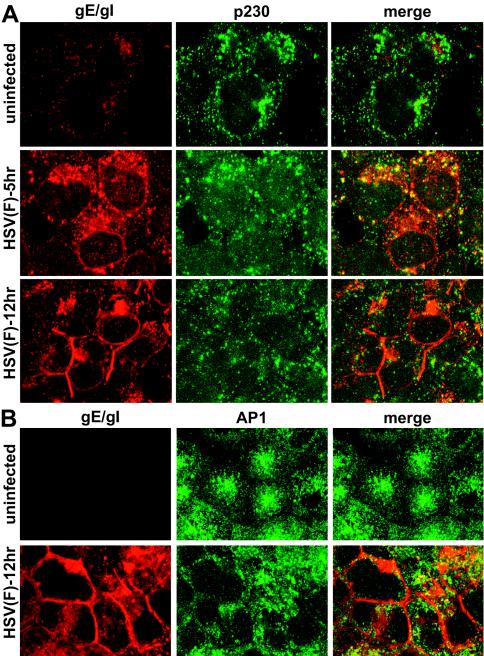
It was of interest to determine whether TGN proteins other than TGN46 were also relocalized to epithelial cell junctions or whether the proteins were dispersed into smaller, less perinuclear vesicles, as was the case with Golgi markers (1, 8). Golgin p230 is a hydrophilic cellular protein of unknown function that attaches onto the cytoplasmic face of Golgi and TGN membranes (6). p230 is a member of a family of proteins that utilize conserved C-terminal GRIP domains to localize to the TGN, specifically to a population of non-clathrin-coated vesicles and tubulovesicular structures. HEC-1A human endometrial epithelial cells were left uninfected or infected with HSV-1 and then fixed, permeabilized, and stained with rabbit anti-gE/gI polyclonal antibodies and simultaneously with mouse anti-p230 antibodies. In uninfected cells, p230 was found in a network of vesicles radiating from a perinuclear position (Fig. 9A). After 5 h of infection with wild-type HSV-1, there was dispersal of p230 to smaller vesicles so that the protein was redistributed to a more diffuse pattern that partially overlapped with gE/gI. After 12 h of HSV infection, p230 was more uniformly dispersed throughout the cytoplasm into smaller membrane vesicles (Fig. 9A). As before, a substantial fraction of gE/gI at 12 h was found at cell junctions. There was little colocalization of p230 with gE/gI at 12 h and no obvious p230 at the cell junctions.
FIG. 9.
Subcellular distribution of TGN-resident cellular proteins p230 and AP-1 in HSV-infected epithelial cells. HEC-1A cells were left uninfected or infected with HSV-1 (strain F) for 6 or 12 h; the cells were then fixed with paraformaldehyde, permeabilized, blocked, and stained with mouse anti-p230 and simultaneously with rabbit anti-gE/gI polyclonal antibodies (A) or mouse anti-AP-1 and simultaneously with rabbit anti-gE/gI antibodies (B). The cells were washed and incubated with Alexa 488-conjugated goat anti-mouse IgG and Alexa 594-conjugated goat anti-rabbit IgG. The images show gE/gI as red (Alexa 594) and AP-1 or p230 as green (Alexa 488).
AP-1 is an adaptor protein that bridges transmembrane receptors onto clathrin coats in the TGN and endosomes (reviewed in references 5, 18, and 32). By binding onto the CT domains of cellular proteins, AP-1 promotes their incorporation into clathrin-coated vesicles that are subsequently sorted from the TGN to endosomes and from endosomes to the TGN. Other clathrin adaptors, AP-2 and AP-3, function primarily at the cell surface and in transport from the TGN to lysosomes, respectively. In uninfected HEC-1A cells, AP-1 was found not only in a perinuclear region of the cell but also in small vesicles distributed more extensively throughout the cytoplasm (Fig. 9B). After infection with HSV-1 for 12 h, AP-1 was less frequently perinuclear and more uniformly dispersed throughout the cytoplasm. In cells that were more extensively infected (with larger quantities of gE/gI) there was a fraction of AP-1 at the cell junctions, but in other cells (with less gE/gI) AP-1 was more substantially in the cytoplasm (Fig. 9B).
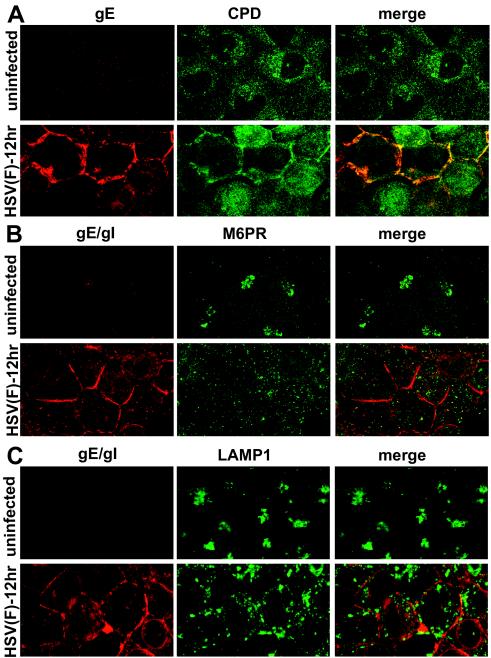
AP-1 and p230 are both cytoplasmic proteins, although a substantial fraction of these proteins are bound onto the cytosolic surfaces of TGN and endosomal vesicles. In contrast, TGN46 is a type I membrane protein. Thus, we studied a second integral membrane protein, CPD. CPD is a metalloprotease that functions in posttranslational processing of peptides and proteins that transit secretory pathways (50). CPD is found primarily in the TGN but also in endosomes and on the cell surface and is rapidly recycled from endosomes and the cell surface back to the TGN. In uninfected HEC-1A cells, CPD was present in numerous small vesicles radiating out from the perinuclear region of the cytoplasm (Fig. 10A). After HSV infection for 12 h, CPD was found distributed more uniformly throughout the cytoplasm and less frequently in discrete vesicles. A substantial fraction of CPD was found on the cell surfaces, at cell junctions, and this CPD largely colocalized with gE (Fig. 10A). Therefore, CPD relocalizes to cell junctions late in HSV replication.
FIG. 10.
Subcellular distribution of cellular proteins CPD, M6PR, and LAMP1 in HSV-infected HEC-1A cells. HEC-1A cells were infected with wild-type HSV-1 strain F then fixed and processed as described Fig. 4. The cells were then stained with the following antibodies: rabbit anti-CPD and anti-gE mouse MAb 3114, followed by Alexa 594-labeled goat anti-rabbit IgG and Alexa 488-labeled goat anti-mouse IgG (A); mouse anti-M6PR and rabbit anti-gE/gI polyclonal antibodies, followed by Alexa 488-labeled goat anti-mouse IgG and Alexa 594-labeled goat anti-rabbit IgG (B); or mouse anti-LAMP1 and rabbit anti-gE/gI, followed by Alexa 594-labeled goat anti-rabbit IgG and CY5 goat anti-mouse IgG (C). For clarity, in each case the gE or gE/gI is shown in red, and CPD, M6PR, or LAMP1 is shown in green. Thus, in panel A the red and green channels were switched, and in panel C the blue (CY5) channel was converted to green.
The 275-kDa M6PR is another membrane protein that binds lysosomal enzymes in the Golgi and TGN and moves these proteins to late endosomes (reviewed in reference 27). The M6PR is localized in the TGN and Golgi apparatus and also in early and late endosomes and, to a limited extent, in coated pits on cell surfaces. In uninfected HEC-1A cells, the M6PR was found in large, more-dense vesicles in a perinuclear location, as well as in smaller, more uniformly distributed cytoplasmic vesicles (Fig. 10B). After HSV infection for 12 h, the M6PR was found in smaller vesicles distributed more uniformly throughout the cytoplasm and did not colocalize with gE/gI or on the cell surface.
LAMP1 is a type I transmembrane protein that is concentrated in lysosomes but also found in lesser amounts in late endosomes (22, 43). In uninfected HEC-1 cells, LAMP1 was found in clusters of larger vesicles in a tight perinuclear location (Fig. 10C). After HSV-1 infection for 12 h, LAMP1 was dispersed into smaller and medium-sized vesicles and was less perinuclear. LAMP1-positive vesicles in HSV-infected cells tended to be larger than those containing Golgi or TGN markers. There was no obvious LAMP1 at cell junctions or colocalization with gE/gI.
In summary, there was dispersal of TGN, endosomal, and, to a lesser degree, lysosomal proteins in the late stages of HSV infection. Generally, larger vesicles containing TGN, endosomal, or lysosomal proteins that were concentrated in a perinuclear region in uninfected cells became distributed more uniformly throughout the cytoplasm, increased in number, and were reduced in size. CPD and TGN46, which are both integral membrane proteins of the TGN, were redistributed to cell junctions, colocalizing with gE/gI. However, M6PR and LAMP1, which are also integral membrane proteins but not strictly TGN proteins, did not reach the cell surface. Peripheral proteins such as p230 and AP-1 were largely dispersed throughout the cytoplasm.
Subcellular distribution of HSV gB in HEC-1A cells.
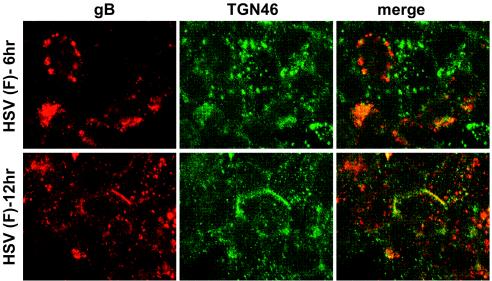
Previously, we characterized the distribution of HSV-1 gD in epithelial cells (13, 33). Unlike gE/gI that extensively localizes in the TGN when expressed by using adenovirus vectors, gD is found on apical and basolateral surfaces, as well as in cytoplasmic membranes, when expressed without other HSV proteins. However, in HSV-infected cells, gM apparently causes gD to more extensively accumulate in the TGN (C. Crump, H. Browne, and A. Minson, unpublished data). HSV-1 gB is a different glycoprotein that appears to be more like gE/gI. gB has obvious TGN sorting sequences, a relatively large CT domain (109 amino acids) that contains two putative tyrosine motifs (YXXØ, where Ø represents a hydrophobic residue), a dileucine motif, and acidic clusters. A 36-amino-acid CT domain of the related varicella-zoster virus (VZV) gB was important for localization to the TGN or Golgi apparatus and for movement to the cell surface (23). Moreover, transfer of the pseudorabies virus gB CT domain onto gD caused gD to localize more extensively to the TGN than wild-type gD that was found more extensively on cell surfaces (36). Thus, it was of interest to determine whether gB was initially found in the TGN and then later redistributed to cell junctions. In HEC-1A cells, 6 h after HSV infection, gB was found in large and medium-size perinuclear vesicles, some of which stained with anti-TGN46 antibodies while others did not (Fig. 11). The larger gB-containing vesicles were not observed in all cells. Later, after 12 h of infection, a fraction of the gB was still dispersed in the cytoplasm, but a substantial fraction of gB was also present at cell junctions, especially with more infected cells (Fig. 11).
FIG. 11.
Subcellular localization of gB in HSV-infected HEC-1A epithelial cells. HEC-1A cells were infected with wild-type HSV-1 (F) for 6 or 12 h, fixed, permeabilized, and stained as follows: mouse anti-gB MAb 15βB2 and I-59 (red) and simultaneously with sheep anti-TGN46 (green), followed by secondary Alexa 594-conjugated goat anti-mouse IgG and Alexa 488-conjugated donkey anti-sheep IgG.
Redistribution of gB, gE/gI and TGN46 in human keratinocytes.
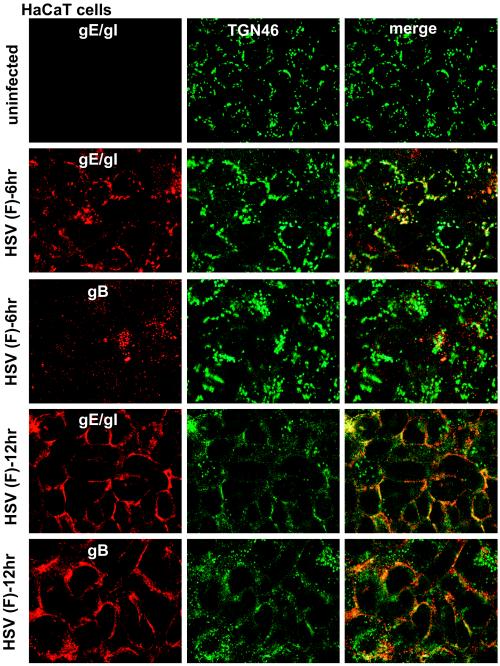
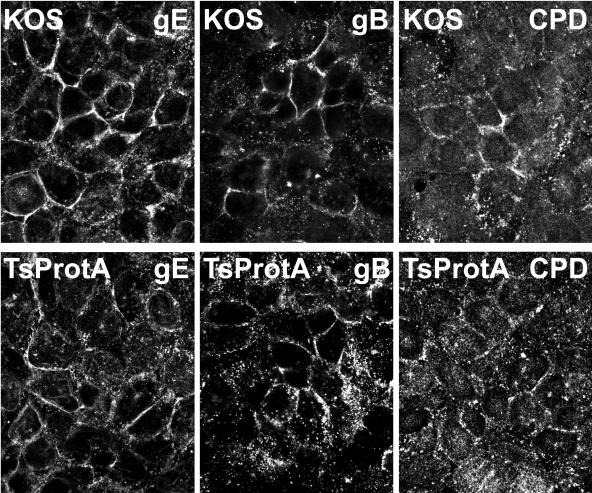
HaCaT cells are a human keratinocyte cell line that mimic the epithelial cells that HSV infects normally in vivo. These cells form extensive adherens junctions and replicate HSV well (producing more virus per cell than Vero cells). Moreover, HSV-1 gE-null mutants show profound defects in cell-to-cell spread on these cells (53), as is the case in vivo. After 6 h of HSV infection, gE/gI was found extensively in the TGN of HaCaT cells, colocalizing with TGN46 (Fig. 12). gB was present in larger cytoplasmic perinuclear vesicles, some that stained with TGN46-specific antibodies and some that did not, as well as in smaller vesicles. After 12 h of HSV infection, both gE/gI and gB moved extensively to HaCaT cell junctions, as did TGN46, so that there was extensive colocalization of the viral glycoproteins and TGN46 at cell junctions (Fig. 12). Moreover, gE/gI and gB were both observed at junctions of HaCaT cells infected with TsProt.A for 12 h (Fig. 13). Similarly, CPD was present at junctions of TsProt.A-infected HaCaT cells, albeit not as extensively as with TGN46 or gE/gI (Fig. 13). Therefore, the redistribution of viral and cellular TGN proteins to cell junctions extends to biologically relevant keratinocytes.
FIG. 12.
Redistribution of gE/gI, gB, and TGN46 to cell junction in HaCaT keratinocytes. HaCaT cells were infected with wild-type HSV (F) for 6 or 12 h, fixed, permeabilized, and stained with rabbit anti-gE/gI (red) and sheep anti-TGN46 (green) antibodies, followed by Alexa 594-conjugated goat anti-rabbit IgG and Alexa 488-conjugated donkey anti-sheep IgG. Alternatively, cells were stained with mouse anti-gB MAb 15βB2 and I-59 (red) and sheep anti-TGN46 (green), followed by secondary Alexa 594-conjugated goat anti-mouse IgG and Alexa 488-conjugated donkey anti-sheep IgG.
FIG. 13.
gE/gI, gB, and CPD are found at junctions of HaCaT cells infected with TsProt.A. HaCaT cells were infected with wild-type HSV strain KOS or TsProt.A at 39°C for 12 h. The cells were then stained for gE/gI (denoted gE) by using rabbit anti-gE/gI, for gB by using MAb 15βB2 and I-59, or for CPD by using rabbit anti-CPD and appropriate secondary fluorescent antibodies.
DISCUSSION
Many details of the egress pathway by which alphaherpesviruses move from the nucleus to the cell surface have recently been elucidated (reviewed in references 24, 34, and 44). The secondary envelopment of alphaherpesviruses that occurs on TGN/endosomes was initially controversial and largely based on electron microscopic observations of capsids being enveloped on the surfaces of cytoplasmic membranes or transiting through these membranes (17, 25, 46, 48, 51). The static nature of electron micrographs made these studies difficult to interpret; it was never clear whether capsids were acquiring or losing their envelope or whether partially enveloped became infectious. Subsequent biochemical and genetic studies substantiated the notion that the final virion envelope is acquired by wrapping tegument-coated capsids with membranes derived from the TGN or endosomes (3, 7, 15, 21, 44, 49). There is also evidence that the acidic pH of TGN/endosomal compartments may be important for the acquisition of infectivity (2, 21, 28). Electron microscopic and other studies have not, to date, elucidated how virions are transported from the TGN to the cell surface. This last stage of egress might occur by passive mechanisms, i.e., transport vesicles that constitute normal exocytic pathways might ferry virions to the cell surface. Alternatively, viral proteins might actively affect the exocytic machinery to promote egress and, in some cases, direct viral progeny to specific cell surfaces, e.g., epithelial cell junctions. In this case, one might expect that there would be more substantial effects on cellular components of the TGN/endosomes.
We previously observed that HSV gE/gI accumulates extensively in the TGN at early times of infection or when expressed without other HSV proteins (13, 33, 53). Late in infection, gE/gI and TGN46 move specifically to cell junctions. To extend these observations and determine whether this redistribution was related to late stages of virus egress from the TGN, we extended our analysis to other cellular components of the TGN and endosomes and a second HSV glycoprotein, gB. CPD, a type 1 membrane protein with some properties similar to those of TGN46, also extensively translocated to epithelial cell junctions, as did gB. These results extended to a HaCaT human keratinocytes, cells that are biologically relevant for HSV.
CPD is an exopeptidase that removes basic amino acids at the C terminus of bioactive peptides which are generated by the TGN endoprotease furin (18, 45, 50). TGN46 is an abundant, heavily glycosylated protein of unknown function, known as TGN38 in mice, and concentrated in the TGN, especially in tubules and budding structures (38). Both CPD and TGN46 possess CT domains that are rich in obvious TGN sorting sequences, including YXXØ-like motifs, acidic clusters, and phosphorylated serines and threonines. gE, gI, and gB also contains YXXØ and dileucine motifs, acidic clusters, and phosphorylated serines and threonines (reviewed in reference 24). All of these cellular and viral proteins recycle between the TGN and endosomes and between endosomes and the plasma membrane (40, 45, 50). Truncation of the CT domain of CPD causes the protein to largely remain on the cell surface (14), as with gE (53). In polarized cells, TGN46 traffics specifically to basolateral domains of the plasma membrane, and not onto apical surfaces, through sorting domains present in the CT domain (40), as with gE/gI (33). Therefore, TGN46, CPD, gE/gI, and gB are not static components of the TGN but are continually recycled there.
To account for our results, it appears likely that HSV blocks recycling back to the TGN from endosomes and/or the cell surface, causing viral and cellular proteins to remain largely on the cell surface. Similarly, inhibition of traffic from endosomes to the TGN, e.g., by dominant-negative PACS-1, causes redistribution of certain TGN proteins to endosomes (11). However, we previously reported that HSV infection does not inhibit endocytosis of the cell surface transferrin receptor (33). Thus, any effects on endocytosis may be more specific to TGN-resident proteins rather than to cell surface proteins.
Two other components of the TGN, p230 and AP-1, did not extensively redistribute to cell junctions. p230 and AP-1 are cytoplasmic proteins that adhere to the cytosolic surfaces of membrane components of the TGN. AP-1 interacts with YXXL or dileucine motifs in the CT domains of receptors bridging these to clathrin coats. p230 docks onto a subset of TGN membranes associated with dynamic tubular extensions (6). Our observations that p230 and AP-1 did not accumulate at cell junctions likely reflects their transient interactions with membranes. After HSV-induced redistribution of TGN membrane proteins, apparently AP-1 and p230 uncouple from their cargo and become dispersed in the cytoplasm, either as soluble proteins or on remnants of the Golgi or TGN. The 275-kDa M6PR was also dispersed in cells, found more uniformly throughout the cytoplasm, and did not substantially accumulate at cell junctions. The 275-kDa M6PR is an integral membrane protein that recycles between endosomes, the TGN, and the cell surface. To a much greater extent than with the other TGN proteins, the 275-kDa M6PR is found in the Golgi apparatus and in early and late endosomes. It is not clear why this membrane protein is not redistributed to the cell surface except that it is likely that recycling of the M6PR is substantially different from that of TGN46 and CPD. The lysosomal marker LAMP1 was also more dispersed in HSV-infected cells and not found at cell junctions.
The redistribution of gE/gI, gB, CPD, and TGN46 to cell junctions occurred in TsProt.A-infected HEC-1A cells at the nonpermissive temperature. Under these conditions the numbers of cell surface virus particles detected by electron microscopy was reduced to <0.6%, and infectivity was reduced to ∼1% compared to wild-type HSV-infected cells. Most of the cell surface particles in TsProt.A-infected HEC-1A cells contained capsids, and very few or, for the vast majority of cells, no cell surface L particles were detected. This was different from BHK-21 cells that produced moderate numbers of cell surface L particles. Our studies do not exclude the possibility that TsProt.A-infected HEC-1A cells produce L particles in the cytoplasm. L particles were originally detected by using density gradient centrifugation by extracting the particles from cells (42). However, cytosolic L particles would not cause redistribution of HSV glycoproteins and TGN proteins to cell surfaces. For this reason, we did not attempt to quantify cytoplasmic L particles in these cells. Instead, we focused on whether or not there were cell surface virus particles in TsProt.A-infected HEC-1A cells, and these were too rare (0.6%) to account for the observed redistribution of viral glycoproteins and cellular TGN proteins to cell junctions.
Our observation that cellular TGN proteins and viral glycoproteins move from the TGN to the plasma membrane has a number of important implications for our understanding of alphaherpesvirus egress pathways. First, both viral and cellular proteins were specifically delivered to cell junctions, as was the case with enveloped virions (26). This emphasizes once again that there is specific sorting from the TGN delivering virus components to sites of cell-cell contact, a process that we believe enhances cell-to-cell spread in epithelial tissues (24). Second, before these studies, it appeared that viral and cellular TGN proteins might be ferried to cell junctions in conjunction or simultaneously with enveloped virions. However, since TGN46, CPD, gB and gE/gI moved to cell junctions in cells lacking cytoplasmic nucleocapsids, it is not necessary for enveloped virions to be produced. HSV glycoproteins could potentially assemble into L particles or other subassemblies that are then transported to the plasma membrane but cell surface L particles were not observed. Whether or not viral glycoproteins assemble into larger structures, the redistribution of TGN proteins to the cell surface is consistent with the hypothesis that there are viral gene products that transform the TGN. This might relate to the inhibition of recycling loops and affect only a fraction of TGN proteins so that the TGN remains intact or, alternatively, the TGN may be largely remodeled and delivered to the cell surface. In either case, this would obviously hasten the movement of nascent virions to cell surfaces at late times of infection.
Acknowledgments
We thank Aurelie Snyder for her hard work and valuable expertise with the laser scanning confocal and deconvolution software. We thank Gary Thomas for advice and antibodies specific to TGN markers and Lloyd Fricker for CPD-specific antibodies. We are also grateful to Mike Webb for technical assistance with electron microscopy and Aaron Farnsworth for help with the EM samples and advice. We thank Jeff Vandehey for his assistance in submitting the manuscript online.
We acknowledge support from NIH grant AI 73996.
REFERENCES
- 1.Avitabile, E., S. Di Gaeta, M. R. Torrisi, P. L. Ward, B. Roizman, and G. Campadelli-Fiume. 1995. Redistribution of microtubules and Golgi apparatus in herpes simplex virus-infected cells and their role in viral exocytosis. J. Virol. 69:7472-7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banfield, W. J., and A. L. Kisch. 1973. The effect of chloroquine on herpesvirus infection in vitro and in vivo. Proc. Soc. Exp. Biol. Med. 142:1018-1022. [DOI] [PubMed] [Google Scholar]
- 3.Brack, A. R., B. G. Klupp, H. Granzow, R. Tirabassi, L. W. Enquist, and T. C. Mettenleiter. 2000. Role of the cytoplasmic tail of pseudorabies virus glycoprotein E in virion formation. J. Virol. 74:4004-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brignati, M. J., J. S. Loomis, J. W. Wills, and R. J. Courtney. 2003. Membrane association of VP22, a herpes simplex virus type 1 tegument protein. J. Virol. 77:4888-4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodsky, F. M., C. Y. Chen, C. Knuehl, M. C. Towler, and D. E. Wakeham. 2001. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17:517-568. [DOI] [PubMed] [Google Scholar]
- 6.Brown, D. L., K. Heimann, J. Lock, L. Kjer-Nielsen, C. van Vliet, J. L. Stow, and P. A. Gleeson. 2001. The GRIP domain is a specific targeting sequence for a population of trans-Golgi network derived tubulo-vesicular carriers. Traffic 2:336-344. [DOI] [PubMed] [Google Scholar]
- 7.Browne, H., S. Bell, T. Minson, and D. W. Wilson. 1996. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J. Virol. 70:4311-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campadelli-Fiume, G., R. Brandimarti, C. Di Lazzaro, P. L. Ward, B. Roizman, and M. R. Torrisi. 1993. Fragmentation and dispersal of Golgi proteins and redistribution of glycoproteins and glycolipids processed through the Golgi apparatus after infection with herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 90:2798-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Card, J. P., M. E. Whealy, A. K. Robbins, R. Y. Moore, and L. W. Enquist. 1991. Two alpha-herpesvirus strains are transported differentially in the rodent visual system. Neuron 6:957-969. [DOI] [PubMed] [Google Scholar]
- 10.Chapman, T. L., I. You, I. M. Joseph, P. J. Bjorkman, S. L. Morrison, and M. Raghavan. 1999. Characterization of the interaction between the herpes simplex virus type I Fc receptor and immunoglobulin G. J. Biol. Chem. 274:6911-6919. [DOI] [PubMed] [Google Scholar]
- 11.Crump, C. M., Y. Xiang, L. Thomas, F. Gu, C. Austin, S. A. Tooze, and G. Thomas. 2001. PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. EMBO J. 20:2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darlington, R. W., and L. H. Moss III. 1968. Herpesvirus envelopment. J. Virol. 2:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dingwell, K. S., and D. C. Johnson. 1998. Herpes simplex virus gE/gI facilitates cell-to-cell spread and binds to components of cell junctions. J. Virol. 72:8933-8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eng, F. J., O. Varlamov, and L. D. Fricker. 1999. Sequences within the cytoplasmic domain of gp180/carboxypeptidase D mediate localization to the trans-Golgi network. Mol. Biol. Cell 10:35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farnsworth, A., K. Goldsmith, and D. C. Johnson. 2003. Herpes simplex virus glycoproteins gD and gE/gI serve essential but redundant functions during acquisition of the virion envelope in the cytoplasm. J. Virol. 77:8481-8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao, M., L. Matusick-Kumar, W. Hurlburt, S. F. DiTusa, W. W. Newcomb, J. C. Brown, P. J. McCann III, I. Deckman, and R. J. Colonno. 1994. The protease of herpes simplex virus type 1 is essential for functional capsid formation and viral growth. J. Virol. 68:3702-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granzow, H., B. G. Klupp, W. Fuchs, J. Veits, N. Osterrieder, and T. C. Mettenleiter. 2001. Egress of alphaherpesviruses: comparative ultrastructural study. J. Virol. 75:3675-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu, F., C. M. Crump, and G. Thomas. 2001. Trans-Golgi network sorting. Cell Mol. Life Sci. 58:1067-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanke, T., F. L. Graham, V. Lulitanond, and D. C. Johnson. 1990. Herpes simplex virus IgG Fc receptors induced using recombinant adenovirus vectors expressing glycoproteins E and I. Virology 177:437-444. [DOI] [PubMed] [Google Scholar]
- 20.Hanke, T., F. L. Graham, K. L. Rosenthal, and D. C. Johnson. 1991. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J. Virol. 65:1177-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harley, C. A., A. Dasgupta, and D. W. Wilson. 2001. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: role for organelle acidification in assembly of infectious particles. J. Virol. 75:1236-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harter, C., and I. Mellman. 1992. Transport of the lysosomal membrane glycoprotein lgp120 (lgp-A) to lysosomes does not require appearance on the plasma membrane. J. Cell Biol. 117:311-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heineman, T. C., and S. L. Hall. 2002. Role of the varicella-zoster virus gB cytoplasmic domain in gB transport and viral egress. J. Virol. 76:591-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson, D. C., and M. T. Huber. 2002. Directed egress of animal viruses promotes cell-to-cell spread. J. Virol. 76:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, D. C., and P. G. Spear. 1982. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J. Virol. 43:1102-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, D. C., M. Webb, T. W. Wisner, and C. Brunetti. 2001. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J. Virol. 75:821-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kornfeld, S. 1992. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu. Rev. Biochem. 61:307-330. [DOI] [PubMed] [Google Scholar]
- 28.Kousoulas, K. G., S. Person, and T. C. Holland. 1982. Herpes simplex virus type 1 cell fusion occurs in the presence of ammonium chloride-inhibited glycoproteins. Virology 123:257-263. [DOI] [PubMed] [Google Scholar]
- 29.Ladinsky, M. S., D. N. Mastronarde, J. R. McIntosh, K. E. Howell, and L. A. Staehelin. 1999. Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J. Cell Biol. 144:1135-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lippincott-Schwartz, J., and W. Liu. 2003. Membrane trafficking: coat control by curvature. Nature 426:507-508. [DOI] [PubMed] [Google Scholar]
- 31.Ludwig, T., G. Griffiths, and B. Hoflack. 1991. Distribution of newly synthesized lysosomal enzymes in the endocytic pathway of normal rat kidney cells. J. Cell Biol. 115:1561-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallet, W. G., and F. M. Brodsky. 1996. A membrane-associated protein complex with selective binding to the clathrin coat adaptor AP1. J. Cell Sci. 109(Pt. 13):3059-3068. [DOI] [PubMed] [Google Scholar]
- 33.McMillan, T. N., and D. C. Johnson. 2001. Cytoplasmic domain of herpes simplex virus gE causes accumulation in the trans-Golgi network, a site of virus envelopment and sorting of virions to cell junctions. J. Virol. 75:1928-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muranyi, W., J. Haas, M. Wagner, G. Krohne, and U. H. Koszinowski. 2002. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science 297:854-857. [DOI] [PubMed] [Google Scholar]
- 36.Nixdorf, R., B. G. Klupp, and T. C. Mettenleiter. 2001. Role of the cytoplasmic tails of pseudorabies virus glycoproteins B, E and M in intracellular localization and virion incorporation. J. Gen. Virol. 82:215-226. [DOI] [PubMed] [Google Scholar]
- 37.Para, M. F., M. L. Parish, A. G. Noble, and P. G. Spear. 1985. Potent neutralizing activity associated with anti-glycoprotein D specificity among monoclonal antibodies selected for binding to herpes simplex virions. J. Virol. 55:483-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prescott, A. R., J. M. Lucocq, J. James, J. M. Lister, and S. Ponnambalam. 1997. Distinct compartmentalization of TGN46 and beta 1,4-galactosyltransferase in HeLa cells. Eur. J. Cell Biol. 72:238-246. [PubMed] [Google Scholar]
- 39.Preston, V. G., J. A. Coates, and F. J. Rixon. 1983. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J. Virol. 45:1056-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reaves, B. J., E. P. Roquemore, J. P. Luzio, and G. Banting. 1998. TGN38 cycles via the basolateral membrane of polarized Caco-2 cells. Mol. Membr. Biol. 15:133-139. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds, A. E., B. J. Ryckman, J. D. Baines, Y. Zhou, L. Liang, and R. J. Roller. 2001. U(L)31 and U(L)34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J. Virol. 75:8803-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rixon, F. J., C. Addison, and J. McLauchlan. 1992. Assembly of enveloped tegument structures (L particles) can occur independently of virion maturation in herpes simplex virus type 1-infected cells. J. Gen. Virol. 73(Pt. 2):277-284. [DOI] [PubMed] [Google Scholar]
- 43.Rohrer, J., A. Schweizer, D. Russell, and S. Kornfeld. 1996. The targeting of Lamp1 to lysosomes is dependent on the spacing of its cytoplasmic tail tyrosine sorting motif relative to the membrane. J. Cell Biol. 132:565-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skepper, J. N., A. Whiteley, H. Browne, and A. Minson. 2001. Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment → deenvelopment → reenvelopment pathway. J. Virol. 75:5697-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song, L., and L. D. Fricker. 1996. Tissue distribution and characterization of soluble and membrane-bound forms of metallocarboxypeptidase D. J. Biol. Chem. 271:28884-28889. [DOI] [PubMed] [Google Scholar]
- 46.Stackpole, C. W. 1969. Herpes-type virus of the frog renal adenocarcinoma. I. Virus development in tumour transplants maintained at low temperature. J. Virol. 4:75-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szilagyi, J. F., and C. Cunningham. 1991. Identification and characterization of a novel non-infectious herpes simplex virus-related particle. J. Gen. Virol. 72(Pt. 3):661-668. [DOI] [PubMed] [Google Scholar]
- 48.Torrisi, M. R., C. Di Lazzaro, A. Pavan, L. Pereira, and G. Campadelli-Fiume. 1992. Herpes simplex virus envelopment and maturation studied by fracture label. J. Virol. 66:554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Genderen, I. L., R. Brandimarti, M. R. Torrisi, G. Campadelli, and G. van Meer. 1994. The phospholipid composition of extracellular herpes simplex virions differs from that of host cell nuclei. Virology 200:831-836. [DOI] [PubMed] [Google Scholar]
- 50.Varlamov, O., and L. D. Fricker. 1998. Intracellular trafficking of metallocarboxypeptidase D in AtT-20 cells: localization to the trans-Golgi network and recycling from the cell surface. J. Cell Sci. 111(Pt. 7):877-885. [DOI] [PubMed] [Google Scholar]
- 51.Whealy, M. E., J. P. Card, R. P. Meade, A. K. Robbins, and L. W. Enquist. 1991. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J. Virol. 65:1066-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whiteley, A., B. Bruun, T. Minson, and H. Browne. 1999. Effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network. J. Virol. 73:9515-9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wisner, T., C. Brunetti, K. Dingwell, and D. C. Johnson. 2000. The extracellular domain of herpes simplex virus gE is sufficient for accumulation at cell junctions but not for cell-to-cell spread. J. Virol. 74:2278-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu, Z., M. D. Gershon, Y. Hao, R. T. Ambron, C. A. Gabel, and A. A. Gershon. 1995. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J. Virol. 69:7951-7959. [DOI] [PMC free article] [PubMed] [Google Scholar]