Abstract
Human T-cell leukemia virus type 1 (HTLV-1) encodes a 40-kDa Tax phosphoprotein. Tax is a transcriptional activator which modulates expression of the viral long terminal repeat and transcription of many cellular genes. Because Tax is a critical HTLV-1 factor which mediates viral transformation of T cells during the genesis of adult T-cell leukemia, it is important to understand the processes which can activate or inactivate Tax function. Here, we report that ubiquitination of Tax is a posttranscriptional mechanism which regulates Tax function. We show that ubiquitination does not target Tax for degradation by the proteasome. Rather, ubiquitin addition modifies Tax in a proteasome-independent manner from an active to a less-active transcriptional form.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia (8, 22, 53, 67). This human retrovirus encodes a 40-kDa phosphoprotein, Tax (16, 25, 43). Evidence suggests that Tax is the HTLV-1-encoded viral factor which transforms T cells (6, 14, 15, 21, 44, 45, 48, 54, 59, 62-64, 66). Currently, the exact mechanism of Tax transformation remains incompletely understood. However, it has been suggested that the ability of Tax to transform human T cells is linked to its capacity to activate the expression of pro-proliferation genes (11, 50) and its interaction with cellular factors that regulate cell cycle control (1, 9, 17, 24, 31, 34, 36, 38-40, 43, 49, 56, 57, 61).
Recently, findings have emerged that posttranscriptional modifications such as phosphorylation, acetylation, ubiquitination, and sumoylation are prominently involved in regulating the activity of many transcription factors (10, 20, 29, 30, 37, 46, 47, 55, 60, 63). Several mechanistic roles have been proposed for ubiquitination. Ubiquitin regulates the degradation of proteins like p53 with short half-lives (19, 32, 46). Hence, polyubiquitinated p53 has been found to be rapidly degraded by a multisubunit ATP-dependent protease called the 26S proteasome (19). Interestingly, a second role for ubiquitination has been proposed recently (2, 55) in which ubiquitination does not serve to target proteins for proteasomal degradation. Salghetti and colleagues suggested that the polyconjugation of ubiquitin to some transcription factors positively regulates their transcriptional activity (55). Thus, for human immunodeficiency virus type 1 Tat protein, it was found that the addition of a single ubiquitin molecule to its C terminus serves to increase transcriptional function (2).
The finding that ubiquitination of some factors can increase their transcriptional activity in a degradation-independent manner suggests the possibility that the same signal might also decrease the activity of other transcription factors. For HTLV-1, a provocative observation is that the viral oncoprotein Tax serves an important role for the initial transformation of T cells (54, 59, 67), but long-term adult T-cell leukemia cells are paradoxically lost for Tax expression (35, 45, 58). Accordingly, one thought is that constitutive and permanent expression of active Tax may not be propitious for maintaining cellular viability and/or cellular transformation. Indeed, findings from ex vivo tissue culture illustrate that Tax expression can rapidly engender severe cellular DNA damage, and continuous Tax function can be toxic to cells (3, 12, 18, 33, 39, 41-43, 51, 52). Considered thus, it would seem advantageous for the cell to evolve a mechanism which can rapidly and perhaps reversibly regulate excessive cellular protein function which may no longer be needed. Such a regulatory mechanism might have been adapted to modulate the Tax function of an invading virus.
We examined here whether HTLV-1 Tax is ubiquitinated and whether such modification regulates its function. We found that Tax is predominantly monoubiquitinated and that monoubiquitination of Tax downregulates its transcriptional function through a proteasome-independent mechanism. Our results are consistent with similar observations of Tax ubiquitination from Pique and colleagues (4).
MATERIALS AND METHODS
Antibodies and plasmids.
pHTLV-1 LTR-luc, NF-κB-luc, pHA-ubiquitin, pMyc-ubiquitin, and pHis-ubiquitin were described previously (2, 23). HA-SUMO was kindly provided by Anne Dejean and Ronald A. DePinho. The Tax protein was detected with an anti-Tax rabbit antibody (26) or mouse monoclonal anti-Tax antibody (NIH AIDS Research and Reference Reagent Program). Tubulin was detected with mouse antitubulin from Sigma (clone DM 1A, T-9026).
Plasmid constructs.
The pCMV Tax-ubiquitin fusion protein was constructed as follows. First, Tax sequence was PCR amplified with primer 1 (5′) (5′-GAATTCAAGCTTGCCACCATGGCCCACTT CCCAGGGTTTGG-3′), primer 1a (3′) (5′-GGGGAATTCGACTTCTGTTTCGCGGAAATGTTTTTC-3′), and primer 1b (3′) (5′-CCAGTCGAATTCCTACTTGTCATCGTCGTCCTTGTAGTCGACTTCTGTTTCGCGGAAATGTTTTTCTCT-3′). PCR fragments were cloned into the HindIII and EcoRI sites of the pcDNA3.0 expression vector. Two products were obtained, pCMV-Tax-Flag and pCMV-Tax-noTer (which contained an EcoRI site instead of a termination codon). The ubiquitin sequence containing a Flag sequence (italic in 3′ primer 2) was PCR amplified with the human ubiquitin cDNA template (Sigma U-5007) and a 5′ primer 2 containing the EcoRI restriction site and a 3′ primer 2 in which the two carboxy-terminal glycine residues of ubiquitin were changed to alanine to prevent removal of the ubiquitin moiety by isopeptidases (32). Primer 2 (5′) was 5′-GGGGAATTCCAAATCTTCGTGAAAACCCTTACT-3′, and primer 2 (3′) was 5′-CCCTCTAGATTACTACTTGTCATCGTCGTCCTTGTAGTCCTTGTCATCGTC GTCCTTGTAGTCAGCAGCTCTCAGACGCAGGACCAAGTGCAGAGTGG-3′. After restriction with EcoRI and XbaI, the ubiquitin fragment was ligated to the C-terminal extremity of pCMV-Tax-noTer to create pCMV-Tax-ubiquitin.
The antisense ubiquitin vector antisense-Ha-ubiquitin was constructed by PCR amplification of a ubiquitin sequence (Sigma U-5007) with primer 2 (5′) (5′-GGGGAATTCCAAATCTTCGTGAAAACCCTTACT-3′) and primer 3 (3′) (5′-TTTTCTAGATCAAGCGTAATCTGGAACATCGTATGGGTAAGCGTAATCTGGA ACATCGTATGGGTAAGCAGCTCTCAGACGCAGGACCAAGTGCGAGTGG-3′). PCR fragment was cloned into the XbaI and EcoRI sites of the pcDNA 3.1(−) expression vector. All clones were verified by sequencing.
Transfection and reporter assays.
As previously described (24), 293 and HeLa cells were propagated in Dulbecco's modified Eagle's medium with 10% fetal bovine serum and transfected according to the manufacturer's protocol with TransIT LT1 transfection reagent (Mirus). Jurkat cells were propagated in RPMI 1640 with 10% fetal bovine serum and transfected with DMRIE-C reagent (Invitrogen) according to the manufacturer's protocol. To assay luciferase activity, cells were transfected with a plasmid DNA mixture containing reporter plasmids, 500 ng of HTLV-1-Luc, and 500 ng of Rous sarcoma virus β-galactosidase. Total amounts of plasmid DNA were normalized by addition of pcDNA3. Luciferase activity was measured 48 h after transfection. Cells were washed twice with 1× phosphate-buffered saline and then lysed in 1× luciferase lysis buffer (Promega). Cell extracts used for the luciferase assay were first quantified for protein concentration and then normalized. Luciferase assay substrate (Promega) was used according to the manufacturer's protocol, and activity was measured in an Opticom II luminometer (MGM Instruments). β-Galactosidase activity was measured with Galacto-Star (Tropix) as described by the manufacturer. Luciferase activities were normalized for transfection efficiency based on galactosidase readings. All transfections were performed at least three times. Error bars represent standard deviations.
Ex vivo ubiquitination assays.
To assess ubiquitination ex vivo, cells were transfected with LT1 reagent as above. Cells were lysed under denaturing conditions. Briefly, cells were washed twice in phosphate-buffered saline, resuspended in 500 μl of either mild denaturing radioimmunoprecipitation assay (RIPA) buffer (50 mM HEPES, pH 7.3, 2 mM EDTA, 0.5% NP-40, 0.5 mM dithiothreitol, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 1 mM NaF, 1 mM Na3VO4, 20 mM β-glycerophosphate, and complete protease inhibitors) or strong denaturing RIPA buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% deoxycholate, 1% Triton X-100, 0.1% sodium dodecyl sulfate, and complete protease inhibitors). Proteins were separated on 10% polyacrylamide gels, transferred to polyvinylidene difluoride membranes, and developed with the relevant antibodies.
Nickel-agarose chromatography.
We transfected 106 293 cells with Tax, pHA-Ub, or pHis-Ub expression vector and lysed in Ni-agarose lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 5 mM imidazole, 0.05% Tween 20, 100 μg of N-ethylmaleimide per ml, and complete protease inhibitor). His-ubiquitin-conjugated proteins were purified by nickel chromatography (Ni-NTA-agarose, Qiagen) from cells cotransfected with Tax and His-ubiquitin. The beads were washed successively 10 times with Ni-agarose wash buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, 0.05% Tween 20). In order to reduce nonspecific binding to beads as much as possible, nickel binding proteins were resuspended in 2× sample buffer supplemented with 200 mM imidazole and heated for 10 min at 95°C before being subjected to Western blotting with anti-Tax antibody.
RESULTS
HTLV-1 Tax is predominantly monoubiquitinated.
Sumoylated and monoubiquitinated proteins are difficult to detect with standard cell lysis techniques. The reason for this difficulty is that upon cell disruption, endogenous isopeptidases efficiently cleave monoconjugated ubiquitin or conjugated SUMO from target proteins. However, the conjugated forms of protein can be preserved by including isopeptidase inhibitors such as N-ethylmaleimide in the cell lysis buffer. To check whether Tax is modified by ubiquitination, we examined cell lysates from 293 cells transfected with Tax expression plasmid alone (Tax) or Tax with a second plasmid expressing ubiquitin tagged with a Myc epitope (Myc-ubiquitin). Lysates were prepared with or without different isopeptidase inhibitors and analyzed in parallel by immunoblotting with a Tax-specific antibody.
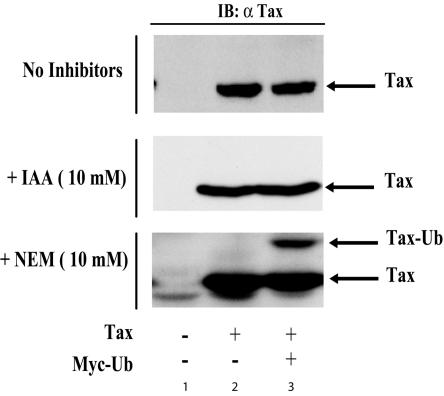
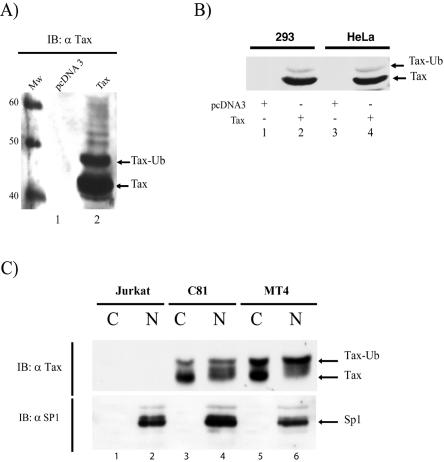
No ubiquitin-conjugated Tax was observed when cells were lysed in the absence of N-ethylmaleimide (Fig. 1, upper panel) or in the presence of iodoacetamide (Fig. 1, middle panel). However, in the presence of N-ethylmaleimide (Fig. 1, lower panel), we detected both a prominent anti-Tax reactive band at 40 kDa, as expected for unmodified Tax (Fig. 1, lane 2), and a slower migrating form consistent with monoubiquitinated Tax (Fig. 1, lane 3). (Even upon prolonged overexposure of our immunoblot), whereupon a progressive ladder of Tax bands could be seen (Fig. 2A, lane 2), monoubiquitination was by far the predominant Tax modification. Direct quantification of the blot showed that approximately 25% of the total amount of Tax was monoubiquitinated in this assay (Fig. 2A, lane 2). We further noted that monoubiquitinated Tax species can also be observed (albeit with lesser efficiency) without N-ethylmaleimide if Tax-transfected 293 or HeLa cells were directly lysed with hot boiling sodium dodecyl sulfate sample buffer (Fig. 2B, lanes 2 and 4).
FIG. 1.
Isopeptidase inhibitors are required to detect ubiquitinated Tax. 293 cells were transfected with 2 μg of a plasmid expressing empty vector (lane 1), Tax alone (lane 2), or Tax with a second plasmid expressing Myc-ubiquitin (Ub) (4 μg) (lane 3). Whole-cell extracts were prepared by lysis in RIPA buffer (see Materials and Methods) and subjected to immunoblotting (IB) with anti-Tax antibody. Where indicated, N-ethylmaleimide (NEM) or iodoacetamide (IAA) was included at 10 mM.
FIG. 2.
Detection of ubiquitinated Tax in transfected cells and in HTLV-1-transformed T cells. 293 or HeLa cells were transfected with 2.5 μg of control pcDNA3 vector (lane 1, panel A; lanes 1, 3, panel B) or a Tax-expressing plasmid (lane 2 panel A; lanes 2, 4, panel B). Whole-cell extracts were prepared either by lysis in RIPA buffer supplemented with N-ethylmaleimide (panel A) or in hot 2× sodium dodecyl sulfate sample buffer (panel B) and subjected to immunoblotting with anti-Tax. Tax-ubiquitin (Ub) indicates the position of monoubiquitinated Tax. In panel C, Jurkat, C8166-45 (C81), and MT4 cells were fractionated into cytoplasmic (C) and nuclear (N) portions with the NE-PER kit (Pierce). Immunoblotting was performed with polyclonal anti-Tax. Blotting of the same samples with anti-Sp1 verified the proper separation of nuclear and cytoplasmic fractions. The isopeptidase inhibitor N-ethylmaleimide (NEM) was included at 10 mM. Lane MW, size standards (sizes shown in kilodaltons).
To check that monoubiquitination of Tax is a physiologically relevant finding, we also investigated Tax modification in two HTLV-1-transformed T cell lines, C8166-45 (C81) and MT4 (Fig. 2C). Both C81 and MT4 are Tax-expressing cell lines. In settings where neither Tax nor ubiquitin was overexpressed from transfected plasmids, we fractionated C81 and MT4 cells with nonionic detergent buffer containing N-ethylmaleimide into nuclear and cytoplasmic components. With antibody directed against nuclear transcription factor Sp1 (Fig. 2C, bottom), we verified the separation of cytoplasmic (Fig. 2C, lanes 1, 3, and 5) from nuclear fractions (Fig. 2C, lanes 2, 4, and 6). We next probed our samples with anti-Tax (Fig. 2C, top). Consistent with the results from transfected HeLa and 293 cells (Fig. 1 and 2B), in the presence of isopeptidase inhibitor, both Tax and Tax-ubiquitin species were clearly seen in C81 and MT4 cells (Fig. 2C). Depending on whether we assessed the cytoplasmic or nuclear fractions from these two T-cell lines, 50% or more of Tax was found to be modified by monoubiquitination. These results show that significant levels of Tax-ubiquitin exist in physiological settings without transfection-mediated overexpression.
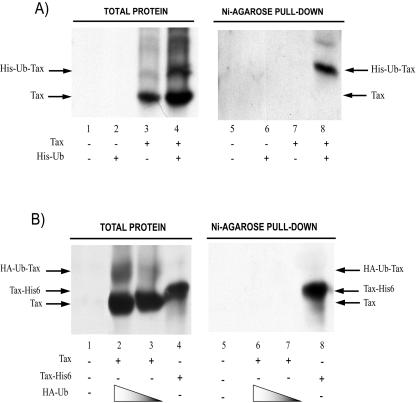
Next, to verify that the observed modification is ubiquitination, we transfected 293 cells with a Tax-expressing vector with or without a second plasmid expressing histidine (His)-tagged ubiquitin (His-ubiquitin). His-tagged protein can be captured with nickel-agarose. We reasoned that if Tax were ubiquitinated with His-ubiquitin, then His-ubiquitin-Tax would be retained by Ni beads. Accordingly, transfected cell lysates were chromatographed over nickel-agarose (Ni-agarose pull down), and bound proteins were queried for Tax moieties. We indeed observed, in Tax- and His-ubiquitin-cotransfected samples (Fig. 3A, lanes 4 and 8), an enriched pulldown of a ≈47-kDa anti-Tax reactive band consistent with mono-His-Tax-ubiquitin (Fig. 3A, lane 8).
FIG. 3.
Identification of His-ubiquitin-conjugated Tax by Ni-agarose chromatography. His-ubiquitin-Tax but not HA-ubiquitin-Tax is bound by nickel-agarose beads. (A) 293 cells were transfected with a plasmid expressing the control vector (lanes 1 and 5), His-ubiquitin (Ub) (lanes 2 and 6), Tax (4 μg) (lanes 3 and 7), or Tax (4 μg) plus His-ubiquitin (4 μg; lanes 4 and 8). His-ubiquitin-conjugated proteins were captured by nickel-agarose chromatography (lanes 5 to 8; see Materials and Methods). Proteins bound to nickel-agarose beads were detected by Western blotting with anti-Tax (lanes 5 to 8). The starting cell extracts were also immunoblotted with anti-Tax (lanes 1 to 4). Arrows point to the migration positions expected for Tax and His-ubiquitin-Tax. (B) 293 cells were transfected with the control plasmid (lanes 1 and 5) or Tax with decreasing amounts (8 μ and 4 μg) of a plasmid expressing HA-ubiquitin (HA-Ub) (lanes 2, 3 and 6, 7). Tax-His6, a Tax protein containing six histidines at its C terminus, was used as a positive control (lanes 7 and 14). Proteins were chromatographed over nickel-agarose. Proteins bound to nickel-agarose beads were subjected to Western blotting with anti-Tax (lanes 8 to 14). The starting cell extracts were also immunoblotted with anti-Tax antibody (lanes 1 to 7). The arrow labeled Tax indicates the migration position of unmodified protein; the arrow labeled HA-ubiquitin-Tax indicates the migration position of HA-monoubiquitinated Tax; and the arrow labeled Tax-His6 indicates the migration position of input and bead-retained Tax-His6 protein.
To verify the specificity of His-Tax-ubiquitin capture by Ni-agarose, we performed an additional control. We transfected 293 cells with Tax, and in place of His-ubiquitin we cotransfected a hemagglutinin (HA)-tagged ubiquitin vector (HA-ubiquitin). In this setting, some Tax molecules are expected to be converted to mono-HA-Tax-ubiquitin (Fig. 2B, lanes 2 and 3), which, unlike mono-His-Tax, cannot be chelated by Ni-agarose. In parallel, we also transfected 293 cells with Tax-His6, a Tax protein covalently fused with six histidine repeats at its C terminus (Fig. 2B, lane 4). Tax-His6 was used as a positive control because it was expected to be bound efficiently by Ni-agarose. Cell lysates from the transfected cells were then separately chromatographed over Ni-agarose, and bound proteins were assayed by immunoblotting with anti-Tax. Whereas Tax-His6 was captured by Ni-agarose (Fig. 3B, lane 8), mono-HA-Tax-ubiquitin was not (Fig. 2B, lanes 6 and 7). Collectively, the His- and HA-ubiquitin results in Fig. 2A and B are consistent with a portion of intracellular Tax's being monoubiquitinated.
Ubiquitination of Tax reduces its transcriptional activity.
An increasing number of ubiquitin-conjugated proteins have been reported (2, 10, 20, 46, 47, 55, 60, 63). The functional impact of monoubiquitination remains complex and not well understood (20). We wondered how monoubiquitination of Tax might influence its function.
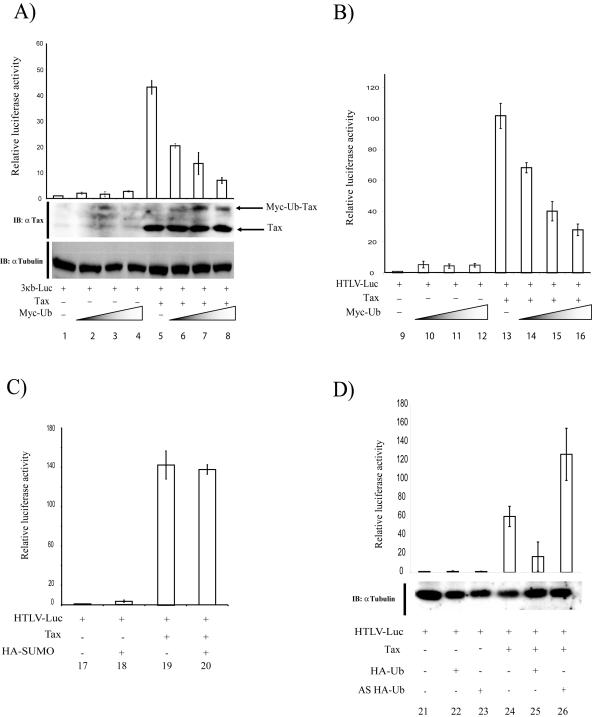
To examine whether Tax's transcriptional activity was affected by ubiquitination, we measured luciferase activity in 293 cells transfected with a luciferase reporter under the control of either an NF-κB-responsive promoter (3κB-Luc; Fig. 4A) or the HTLV-1 long terminal repeat (HTLV-Luc; Fig. 4B). The reporter plasmids (HTLV-Luc and 3κB-Luc) were either transfected individually into cells or cotransfected with Tax and increasing amounts of Myc-ubiquitin. We found that increasing the amount of Myc-ubiquitin plasmid enhanced the formation of Tax-ubiquitin (Fig. 4A, αTax; lanes 6, 7, and 8) and correlated with up to a fourfold decrease in Tax's transcriptional activity on either the NF-κB (Fig. 4A) or the HTLV-1 long terminal repeat (Fig. 4B) promoter. As a control, transfection of a SUMO-1-expressing vector (HA-SUMO) did not affect Tax activity (Fig. 4C).
FIG. 4.
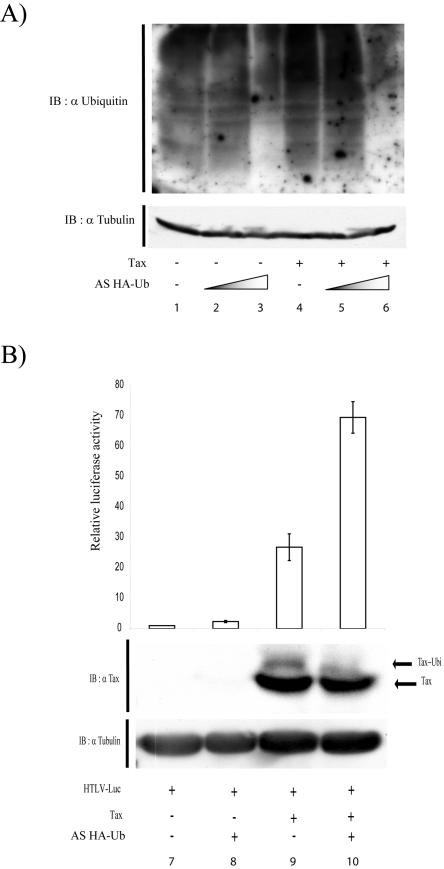
Ubiquitin but not SUMO modification decreased Tax-mediated transcription. (A) 293 cells were cotransfected with the 3κB-Luc plasmid (NF-κB-dependent luciferase reporter) and control vector (lane 1) or 3κB-Luc with the Myc-ubiquitin (Ub) vector in increasing concentrations (2 μg, lanes 2 and 6; 4 μg, lanes 3 and 7; and 8 μg, lanes 4 and 8), or 3κB-Luc with the Tax-expressing vector alone (2 μg; lane 5) or with increasing amounts of the Myc-ubiquitin vector (2, 4, and 8 μg; lanes 6, 7, and 8). The same amounts of cellular extract from each transfected sample were subjected to the luciferase assay and then analyzed by Western blotting with anti-Tax. To normalize for protein loading, membranes were stripped in 1% sodium dodecyl sulfate-62.5 mM Tris-HCl-100 mM β-mercaptoethanol for 30 min at 55°C and reblotted with an antitubulin antibody. The isopeptidase inhibitor N-ethylmaleimide (NEM) was included at 10 mM in the lysis buffer. (B) 293 cells were cotransfected with HTLV-Luc and control vector (lane 9),or HTLV-Luc- and Myc-ubiquitin-expressing vector in increasing concentrations: 2 μg (lanes 10 and 14), 4 μg (lanes 11 and 15), and 8 μg (lanes 12 and 16), or HTLV-Luc- and Tax-expressing vector (2 μg) (lane 13), with increasing amounts of Myc-ubiquitin-expressing vector (2, 4, and 8 μg; lanes 14, 15, and 16). The same amounts of cellular extract from each sample were subjected to the luciferase assay (see Materials and Methods). (C) 293 cells were transfected with HTLV-Luc and the control vector (lane 17), HTLV-Luc plus the HA-SUMO vector (8 μg; lane 18), HTLV-Luc and Tax (2 μg; lane 19), and HTLV-Luc and Tax (2 μg) plus HA-SUMO (8 μg) (lane 20). The same amounts of cellular extract from each transfected sample were analyzed by the luciferase assay. Values are averages ± standard deviation from three independent experiments. (D) Jurkat cells were cotransfected with HTLV-Luc and the control vector (lane 21), HTLV-Luc plus HA-ubiquitin (2 μg) vector (lane 22), HTLV-Luc plus antisense HA-ubiquitin (AS HA-Ub; 2 μg) (lane 23), HTLV-Luc plus Tax (2 μg; lane 24), HTLV-Luc plus Tax plus HA-ubiquitin (2 μg; lane 25), or HTLV-Luc plus Tax plus antisense HA-ubiquitin (2 μg, lane 26). Identical amounts of cellular extracts prepared from each of the transfections were used in luciferase assays. Western blotting with antitubulin verified that equal amounts of extract were used in the assays.
We next asked whether we could replicate the ubiquitination effect seen with 293 cells with suspended Jurkat T cells. To verify this, we transfected Jurkat cells with HTLV-Luc and Tax without (Fig. 4D, lane 24) or with (Fig. 4D, lane 25) overexpressed HA-ubiquitin. Consistent with the findings in 293 cells (Fig. 4B), HA-ubiquitin overexpression repressed Tax activation of HTLV-Luc by more than threefold, while expression of an antisense HA-ubiquitin vector moderately increased HTLV-Luc activation by Tax (Fig. 4D, lane 26).
The findings established with transfection-mediated overexpression of ubiquitin do not comment directly on the physiological effect of endogenous ubiquitin on Tax function. To ask systematically if intracellular ubiquitin plays a role in moderating the transcriptional activity of Tax, we attempted to knockdown endogenous ubiquitin expression (Fig. 5). We were unable to construct an anti-ubiquitin short interfering RNA that could reduce cellular ubiquitination activity. However, we did create and test successfully an antisense ubiquitin vector (HA-ubiquitin) which significantly, albeit not completely, reduced endogenous ubiquitin activity (Fig. 5A, compare lanes 1 and 4 to lanes 3 and 6). Thus, at the highest level of antisense HA-ubiquitin overexpression (Fig. 5A, lanes 3 and 6), the normal ambient levels of endogenous ubiquitination of cellular proteins (Fig. 5A, lanes 1 and 4), as measured by Western blotting with anti-ubiquitin antiserum, were clearly reduced.
FIG. 5.
Knockdown of endogenous ubiquitin with anti-sense ubiquitin increased Tax activity. 293 cells were cotransfected with HTLV-Luc-expressing plasmid in the presence or absence of an antisense (AS) HA-ubiquitin (Ub) vector. Lane 1 and 4 were transfected with an empty vector and Tax, respectively. Antisense HA-ubiquitin was introduced in increasing concentrations (2 μg, lanes 2 and 5; 4 μg, lanes 3 and 6). (A) Half of the transfected cells were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by immunoblotting (IB) with anti-ubiquitin polyclonal serum. Normalization of sample loading was based on probing for α-tubulin (lower panel). (B) The second half of the transfected cells were subjected, as indicated, to the luciferase assay. Values are averages ± standard deviation from three independent experiments. The amount of Tax expression was also quantified by Western blotting (bottom, α-Tax). To ensure equivalence of protein loading, membranes were stripped in 1% sodium dodecyl sulfate-62.5 mM Tris-HCl-100 mM β-mercaptoethanol for 30 min at 55°C and reblotted with antitubulin antibody (bottom, α-tubulin).
We next queried the effect of antisense HA-ubiquitin on Tax activity. Antisense HA-ubiquitin had no effect on basal long terminal repeat-Luc expression (Fig. 5B, compare lane 8 to lane 7). However, increasing the amount of transfected antisense HA-ubiquitin enhanced Tax-activated long terminal repeat transcription (Fig. 5B, compare lane 10 to lane 9). These results suggest that endogenous cellular ubiquitin does play a physiological role in moderating the magnitude of Tax's transcriptional activity.
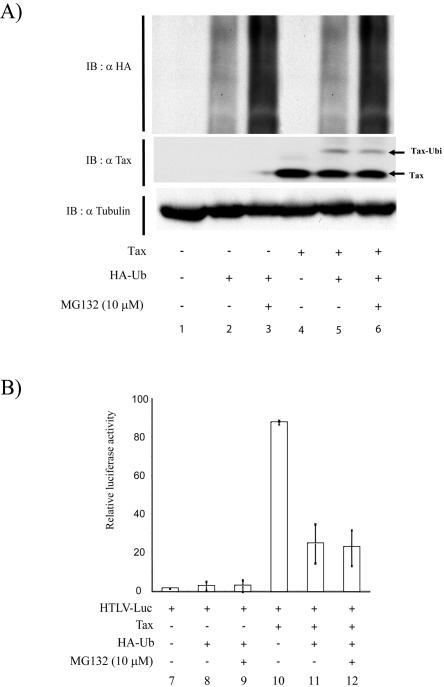
Tax uses separate domains to activate the HTLV-1 long terminal repeat and NF-κB (25). The observed decrease in both functions as a consequence of ubiquitination (Fig. 4) suggested that ubiquitin addition either promoted proteasomal degradation of Tax or altered Tax conformation globally. The former explanation seemed unlikely because we observed no significant change in steady-state amounts of Tax with increased transfection of Myc-ubiquitin (Fig. 4A, αTax, lanes 5 to 8). To further verify a lack of proteasomal involvement in Tax activity, we next treated 293 cells transfected with combinations of HTLV-Luc, Tax, and HA-ubiquitin with or without 10 μM MG132, a proteasome inhibitor, for 12 h (Fig. 6). Immunoblotting of total cell lysates with anti-HA revealed, as expected, the ubiquitination of many cellular proteins (Fig. 6A, αHA, top). The stability of these ubiquitinated proteins was enhanced by MG132 (Fig. 6A, compare lanes 2 and 5 to 3 lanes and 6), indicating that our treatment did successfully inhibit ubiquitination-dependent proteasomal activity. By contrast, MG132 treatment in the context of increased HA-ubiquitin transfection neither increased Tax's stability (Fig. 6A, αTax, lane 6) nor enhanced its transcriptional activity (Fig. 6B. lane 12). Both results support a lack of involvement of the proteasome in the functional activity of ubiquitin-modified Tax.
FIG. 6.
Proteasome inhibition by MG132 treatment does not affect monoubiquitin-mediated repression of Tax activity. 293 cells were cotransfected with HTLV-Luc and a control plasmid (lane 1), HTLV-Luc plus HA-ubiquitin (8 μg) (lane 2), or HTLV-Luc plus HA-ubiquitin plus MG132 (10 μM for 12 h; lane 3). Lanes 4, 5, and 6 are the same as as lanes 1, 2, and 3 but also included transfection with a Tax plasmid. (A) Half of the transfected samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by immunoblotting with anti-HA, anti-Tax, or antitubulin. (B) The other half of the transfected cells, as indicated, were subjected to the luciferase assay to quantify expression from the long terminal repeat promoter. Values are averages ± standard deviation from three independent experiments.
Monoubiquitin addition to Tax is sufficient to reduce transcriptional activity.
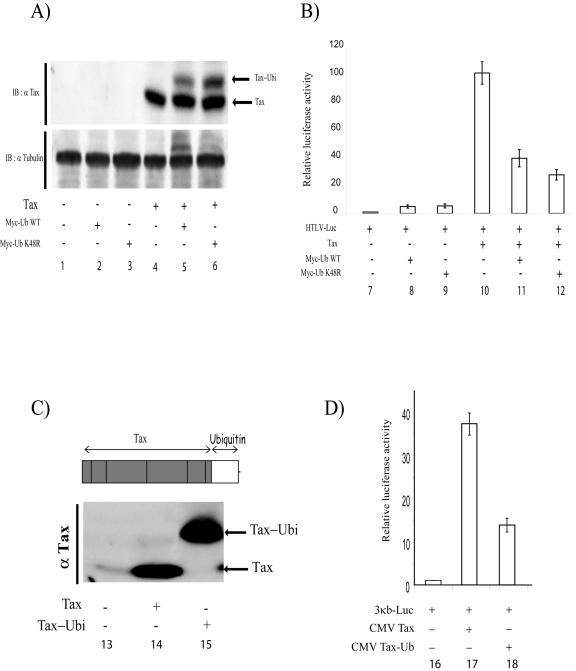
The lack of proteasomal involvement prompted us to consider if monoubiquitin addition to Tax is entirely sufficient to affect function. We addressed this issue in two ways. First, we used a Myc-tagged ubiquitin molecule changed at its normal lysine 48 to arginine (Myc-UbK48R). Because of this K to R mutation, Myc-UbK48R can mediate only monoubiquitination and not polyubiquitination of substrate proteins. We next verified that both Myc-wild-type ubiquitin (Fig. 7A, lane 5) and Myc-UbK48R (Fig. 7A, lane 6), upon transfection into cells, can be ligated equivalently to Tax. When we checked the ability of Myc-UbK48R to repress Tax activation of HTLV-Luc (Fig. 7B, lane 12), we observed that it worked as efficiently as Myc-wild-type ubiquitin (Fig. 7B, lane 11). This results shows that monoubiquitination is sufficient to repress Tax transcriptional function.
FIG. 7.
Monoubiquitin addition to Tax reduces transcriptional activity. 293 cells were transfected with HTLV-Luc and 2 μg of a control vector (lane 1) or a vector expressing Tax (2 μg; lane 4) alone or Tax plus a second plasmid expressing Myc-wild-type (WT) ubiquitin (Ub) (4 μg; lane 5), or Tax plus a second plasmid expressing Myc-ubiquitin K48R (4 μg; lane 6). Lanes 2 and 3 contain transfections with Myc-wild-type ubiquitin alone and Myc-ubiquitin K48R alone, respectively. (A) Half of the transfected cells were lysed in RIPA buffer (see Materials and Methods) and subjected to immunoblotting with anti-Tax (top). To assess the equivalence of protein loading, membranes were stripped and reblotted with an antitubulin antibody (bottom). (B) The other half of the transfected 293 cells were subjected to the luciferase assay (lanes 7 to 12). Graphed results represent the averages from three independent transfections. (C) Schematic representation of the construction of an in-frame Tax-ubiquitin fusion protein (top), with the immunoblotting analysis (bottom) of the expression of Tax (lane 14) and in-frame Tax-ubiquitin (lane 15). (D) 293 cells were cotransfected with 3κB-Luc (NF-κB-dependent promoter) and control vector (lane 16), 3κb-Luc plus Tax (lane 17), and 3κB-Luc plus in-frame Tax-ubiquitin (lane 18). Luciferase assays were performed 48 h later. Values are averages ± standard deviation from three independent experiments.
Elsewhere, a direct in-frame Tat-monoubiquitin fusion protein was shown to alter Tat structure and function (2). Following that line of reasoning, we asked whether a similar in-frame single monoaddition of Tax via direct fusion would recapitulate the effect otherwise produced from ubiquitin ligase-mediated modification. We thus constructed a chimeric Tax-ubiquitin fusion, joining in-frame a ubiquitin monomer to the C terminus of Tax. When characterized by immunoblotting, this chimeric protein was appropriately increased in size by ≈7 kDa with no evidence of decreased stability (Fig. 7C, lane 15). We next compared Tax to in-frame Tax-ubiquitin fusion for transcriptional activity. Interestingly, in-frame Tax-ubiquitin was <30% as active as its wild-type counterpart (Fig. 7D, compare lane 17 to 18), a result similar to that seen with Myc-ubiquitin plus Tax cotransfections (Fig. 4A). Other explanations not withstanding, these findings are compatible with the notion that addition of a single ubiquitin molecule to Tax perturbs protein structure sufficiently to reduce functional activity. We note that this interpretation is consistent with suggestions elsewhere that ubiquitin modification can change the conformation of substrates such as the TRAF6 protein (28).
DISCUSSION
HTLV-1 and human immunodeficiency virus type 1 have similarities and differences. Both viruses have a similar genomic organization, and both encode transcription factors, Tax and Tat, which play important roles in their life cycles (25, 27). Tax and Tat are important for activating the expression of viral genes from long terminal repeats. Both are also able to interact with and modulate the activity of several cellular factors (25, 27).
Here, we report that, like Tat (2), Tax is also modified by ubiquitin. However, the effect of ubiquitination on Tax is different from its effect on Tat. It was recently reported that polyubiquitin conjugation plays a nonproteolytic role in enhancing Tat's transcriptional activity (2). In contrast to human immunodeficiency virus type 1 Tat, we find that Tax is predominantly monoubiquitinated (Fig. 2A). Furthermore, ubiquitin modification affected the transcriptional activity of Tax differently. For Tat, overexpression of ubiquitin enhanced transcriptional activity; for Tax, overexpression of ubiquitin decreased transcriptional activity (Fig. 4). Interestingly, for neither Tat nor Tax was ubiquitin conjugation a proteasomal degradation signal (Fig. 4 and 6).
Whereas ubiquitin addition was proposed to facilitate the interaction of Tat with a positive transcription factor (i.e., the regulatory 19S subunit of the proteasome machinery) (2, 7, 13), we showed that a single ubiquitin addition led to a less active Tax protein.
Pending definitive physical structural characterization, we suggest provisionally that because monoubiquitination represses two discrete Tax functions (HTLV-1 long terminal repeat and NF-κB activation) without altering steady-state stability, this modification may promote a conformational change resulting in a less active form of Tax (Fig. 4, 6, and 7). We have observed that ubiquitin does not change Tax localization inside cells (V. R. K. Yedavalli, unpublished observations). Hence, mislocation of protein is unlikely to be an explanation for reduced Tax activity.
Monoubiquitination appears to be a physiologically relevant modification for HTLV-1 in that >50% or more of Tax was observed to have this conjugation in C8166-45 and MT4 cells treated with isopeptidase inhibitors (Fig. 2C). We did find that under different experimental conditions, various amounts of monoubiquitinated Tax were seen. Potentially, this can be explained by rapid intracellular recycling by deubiquitination of Tax-ubiquitin to its unmodified form. Indeed, it is known that the mammalian genome encodes more than 90 deubiquitinating enzymes (5). Proteins such as p53 and TRAF6 have now been demonstrated to be regulated by deubiquitination as well as ubiquitination (28, 65). Whether ubiquitinated Tax is reversibly regulated by deubiquitination remains to be fully clarified. Nevertheless, to our knowledge, Tax represents the first example of a transcriptional activator which is suppressed in function by monoubiquitination. Formally, we cannot rule out that some unseen polyubiquitination event may also contribute to our functional findings on Tax, although the genetic results obtained with UbK48R (Fig. 7A) disfavor this possibility.
Acknowledgments
We thank Ronald DePinho and Anne Dejean for the generous gift of plasmids, members of the Jeang laboratory for critical readings of the manuscript, and Ron Plishka and Alicia Buckler-White for assistance with DNA sequencing and oligonucleotide synthesis.
REFERENCES
- 1.Albrecht, B., and M. D. Lairmore. 2002. Critical role of human T-lymphotropic virus type 1 accessory proteins in viral replication and pathogenesis. Microbiol. Mol. Biol. Rev. 66:396-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bres, V., R. E. Kiernan, L. K. Linares, C. Chable-Bessia, O. Plechakova, C. Treand, S. Emiliani, J. M. Peloponese, K.-T. Jeang, O. Coux, M. Scheffner, and M. Benkirane. 2003. A non-proteolytic role for ubiquitin in Tat-mediated transactivation of the HIV-1 promoter. Nat. Cell Biol. 8:754-761. [DOI] [PubMed] [Google Scholar]
- 3.Cereseto, A., F. Diella, J. C. Mulloy, A. Cara, P. Michieli, R. Grassmann, G. Franchini, and M. E. Klotman. 1996. p53 functional impairment and high p21waf1/cip1 expression in human T-cell lymphotropic /leukemia virus type I-transformed T cells. Blood 88:1551-1560. [PubMed] [Google Scholar]
- 4.Chiari, E., I. Lamsoul, J. Lodewick, C. Chopin, F. Bex, and C. Pique. 2004. Stable ubiquitination of human T-cell leukemia virus type 1 Tax is required for proteasome binding. J. Virol. 78:•-•. [DOI] [PMC free article] [PubMed]
- 5.Chung, C. H., and S. H. Baek. 1999. Deubiquitinating enzymes: their diversity and emerging roles. Biochem. Biophys. Res. Commun. 266:633-640. [DOI] [PubMed] [Google Scholar]
- 6.Endo, K., A. Hirata, K. Iwai, M. Sakurai, M. Fukushi, M. Oie, M. Higuchi, W. W. Hall, F. Gejyo, and M. Fujii. 2002. Human T-cell leukemia virus type 2 (HTLV-2) Tax protein transforms a rat fibroblast cell line but less efficiently than HTLV-1 Tax. J. Virol. 76:2648-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferdous, A., F. Gonzalez, L. Sun, T. Kodadek, and S. A. Johnston. 2001. The 19S regulatory particle of the proteasome is required for efficient transcription elongation by RNA polymerase II. Mol. Cell 7:981-991. [DOI] [PubMed] [Google Scholar]
- 8.Feuer, G., and I. S. Chen. 1992. Mechanisms of human T-cell leukemia virus-induced leukemogenesis. Biochim. Biophys. Acta 1114:223-233. [DOI] [PubMed] [Google Scholar]
- 9.Franklin, A. A., and J. K. Nyborg. 1996. Mechanisms of Tax regulation of human T-cell leukemia virus I gene expression. J. Biomed. Sci. 2:17-29. [DOI] [PubMed] [Google Scholar]
- 10.Freiman, R. N., and R. Tjian. 2003. Regulating the regulators: lysine modifications make their mark. Cell 112:7-11. [DOI] [PubMed] [Google Scholar]
- 11.Fujii, M., T. Niki, T. Mori, T. Matsuda, M. Matsui, N. Nomura, and M. Seiki. 1991. HTLV-1 Tax induces expression of various immediate early serum responsive genes. Oncogene. 6:1023-1029. [PubMed] [Google Scholar]
- 12.Gabet, A. S., F. Mortreux, P. Charneau, P. Riou, M. Duc-Dodon, Y. Wu, K. T. Jeang, and E. Wattel. 2003. Inactivation of hTERT transcription by Tax. Oncogene 22:3734-3741. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez, F., A. Delahodde, T. Kodadek, and S. A. Johnston. 2002. Recruitment of a 19S proteasome subcomplex to an activated promoter. Science 296:548-550. [DOI] [PubMed] [Google Scholar]
- 14.Grassmann, R., C. Dengler, I. Muller-Fleckenstein, B. Fleckenstein, K. McGuire, M. C. Dokhelar, J. G. Sodroski, and W. A. Haseltine. 1989. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a herpesvirus saimiri vector. Proc. Natl. Acad. Sci. USA 86:3351-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grassmann, R., S. Berchtold, I. Radant, M. Alt, B. Fleckenstein, J. G. Sodroski, W. A. Haseltine, and U. Ramstedt. 1992. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J. Virol. 66:4570-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green, P. L., and I. S. Chen. 1990. Regulation of human T-cell leukemia virus expression. FASEB J. 4:169-175. [DOI] [PubMed] [Google Scholar]
- 17.Haller, K., Y. Wu, E. Derow, I. Schmitt, K. T. Jeang, and R. Grassmann. 2002. Physical interaction of human T-cell leukemia virus type 1 Tax with cyclin-dependent kinase 4 stimulates the phosphorylation of retinoblastoma protein. Mol. Cell. Biol. 10:3327-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haoudi, A., and O. J. Semmes. 2003. The HTLV-1 tax oncoprotein attenuates DNA damage induced G1 arrest and enhances apoptosis in p53 null cells. Virology 305:229-239. [DOI] [PubMed] [Google Scholar]
- 19.Haupt, Y., R. Maya, A. Kazaz, and M. Oren. 1997. Mdm2 promotes the rapid degradation of p53. Nature 387:296-299. [DOI] [PubMed] [Google Scholar]
- 20.Hicke, L. 2001. Protein regulation by monoubiquitin. Nature Rev. Mol. Cell. Biol. 2:195-201. [DOI] [PubMed] [Google Scholar]
- 21.Hinrichs, S. H., M. Nerenberg, R. K. Reynolds, G. Khoury, and G. Jay. 1987. A transgenic mouse model for human neurofibromatosis. Science 237:1340-1343. [DOI] [PubMed] [Google Scholar]
- 22.Hinuma, Y., K. Nagata, M. Hanaoka, M. Nakai, T. Matsumoto, K. I. Kinoshita, S. Shirakawa, and I. Miyoshi. 1981. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 10:6476-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iha, H., K. V. Kibler, R. K. V. Yedavalli, JM. Peloponese, K. Haller, A. Miyazato, T. Kasai, and K. T. Jeang. 2003. Segregation of NF-kB activation through NEMO/IKKg by Tax and TNFα: implications for stimulus-specific interruption of oncogenic signaling. Oncogene 22:8912-8923. [DOI] [PubMed] [Google Scholar]
- 24.Iha, H., T. Kasai, K. V. Kibler, Y. Iwanaga, K. Tsurugi, and K. T. Jeang. 2000. Pleiotropic effects of HTLV type 1 Tax protein on cellular metabolism: mitotic checkpoint abrogation and NF-κb activation. AIDS Res. Hum. Retroviruses 16:1633-1638. [DOI] [PubMed] [Google Scholar]
- 25.Jeang, K.-T. 2001. Functional activities of the human T-cell leukemia virus type I Tax oncoprotein: cellular signaling through NF-κB. Cytokine Growth Factor Rev. 12:207-217. [DOI] [PubMed] [Google Scholar]
- 26.Jeang, K.-T., S. G. Widen, O. J. Semmes 4th, and S. H. Wilson. 1990. HTLV-1 trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science 247:1082-1084. [DOI] [PubMed] [Google Scholar]
- 27.Jeang, K.-T., Xiao, H., and E. A. Rich. 1999. Multifaceted activities of the HIV-1 transactivator of transcription Tat. J. Biol. Chem. 274:28837-28840. [DOI] [PubMed] [Google Scholar]
- 28.Jensen, L. E., and A. S. Whitehead. 2003. Ubiquitin activated tumor necrosis factor receptor associated factor-6 (TRAF6) is recycled via deubiquitination. FEBS Lett. 553:190-194. [DOI] [PubMed] [Google Scholar]
- 29.Jentsch, S., W. Seufert, and H. P. Hauser. 1991. Genetic analysis of the ubiquitin system. Biochim. Biophys. Acta 1089:127-139. [DOI] [PubMed] [Google Scholar]
- 30.Jesenberg, V., and S. Jentsch. 2002. Deadly encounter: Ubiquitin meets apoptosis. Nat. Rev. Mol. Cell. Biol. 3:112-121. [DOI] [PubMed] [Google Scholar]
- 31.Jin, D. Y., F. Spencer, and K. T. Jeang. 1998. Human T-cell leukemia virus type I oncoprotein Tax targets the human mitotic checkpoint protein MADI. Cell 93:81-91. [DOI] [PubMed] [Google Scholar]
- 32.Johnson, E. S., P. C. Ma, I. M. Ota, and A. Varshavsky. 1995. A proteolytic pathway that recognizes ubiquitin as a degradation signal. J. Biol. Chem. 270:17442-17456. [DOI] [PubMed] [Google Scholar]
- 33.Kao, S. Y., and S. J. Marriot. 1999. Disruption of nucleotide excision repair by the human T-cell leukemia virus type 1 Tax protein. J. Virol. 73:4299-4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasai, T., Y. Iwanaga, H. Iha, and K. T. Jeang. 2002. Prevalent loss of mitotic spindle checkpoint in adult T-cell leukemia confers resistance to microtubule inhibitors. J. Biol. Chem. 277:5187-5193. [DOI] [PubMed] [Google Scholar]
- 35.Kibler, K. V., and K. T. Jeang. 1999. Taxing the cellular capacity for repair: human T-cell leukemia virus type 1, DNA damage, and adult T-cell leukemia. J. Natl. Cancer Inst. 11:903-904. [DOI] [PubMed] [Google Scholar]
- 36.Kibler, K. V., and K.-T. Jeang. 2001. CREB/ATF-dependent repression of cyclin a by human T-cell leukemia virus type 1 Tax protein. J. Virol. 5:2161-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiernan, R. E., C. Vanhulle, L. Schiltz, E. Adam, H. Xiao, F. Maudoux, C. Calomme, A. Burny, Y. Nakatani, K.-T. Jeang, M. Benkirane, and C. Van Lint. 1999. HIV-1 Tat transcriptional activity is regulated by acetylation. EMBO J. 18:6106-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lemoine, F. J., D. R. Wycuff, and S. J. Marriott. 2001. Transcriptional activity of HTLV-1 Tax influences the expression of marker genes associated with cellular transformation. Disease Marker 17:129-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu, B., M. H. Liang, Y. L. Kuo, W. Liao, I. Boros, T. Kleinberger, J. Blancato, and C. Z. Giam. 2003. Human T-lymphotropic virus type 1 oncoprotein tax promotes unscheduled degradation of Pds1p/securin and Clb2p/cyclin B1 and causes chromosomal instability. Mol. Cell. Biol. 15:5269-5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Low, K. G., L. F. Dorner, D. B. Fernando, J. Grossman, K. T. Jeang, and M. J. Comb. 1997. Human T-cell leukemia virus type 1 Tax releases cell cycle arrest induced by p16INK4a. J. Virol. 71:1956-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majone, F., O. J. Semmes, and K. T. Jeang. 1993. Induction of micronuclei by HTLV-1 Tax: a cellular assay for function. Virology 193:456-459. [DOI] [PubMed] [Google Scholar]
- 42.Majone, F., and K.-T. Jeang. 2000. Clastogenic effect of the human T-cell leukemia virus type I Tax onco-protein correlates with unstablized DNA breaks. J. Biol. Chem. 275:32906-32910. [DOI] [PubMed] [Google Scholar]
- 43.Marriott, S. J., F. J. Lemoine., and K. T. Jeang. 2002. Damaged DNA and miscounted chromosomes: human T-cell leukemia virus type I tax oncoprotein and genetic lesions in transformed cells. J. Biomed. Sci. 9:292-298. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto, K., H. Shibata, J. I. Fujisawa, H. Inoue, A. Hakura, T. Tsukahara, and M. Fujii. 1997. Human T-cell leukemia virus type 1 Tax protein transforms rat fibroblasts via two distinct pathways. J. Virol. 71:4445-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuoka, M. 2003. Human T-cell leukemia virus type I and adult T-cell leukemia. Oncogene 22:5131-5140. [DOI] [PubMed] [Google Scholar]
- 46.Molinari, E., M. Gilman, and S. Natesan. 1999. Proteasome mediated degradation of transcriptional activators correlates with activation domain potency in vivo. EMBO. J. 18:6439-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muratani, M., and W. P. Tansey. 2003. How the ubiquitin-proteasome system controls transcription. Nat. Rev. Mol. Cell. Biol. 4:192-201. [DOI] [PubMed] [Google Scholar]
- 48.Nerenberg, M., S. H. Hinrichs, R. K. Reynolds, G. Khoury, and G. Jay. 1987. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science 237:1324-1329. [DOI] [PubMed] [Google Scholar]
- 49.Neuveut, C., K. G. Low, F. Maldarelli, I. Schmitt, F. Majone, R. Grassmann, and K. T. Jeang. 1998. Human T-cell leukemia virus type 1 Tax and cell cycle progression: role of cyclin D-cdk and p110Rb. Mol. Cell. Biol. 18:3620-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ng, P. W., H. Iha, Y. Iwanaga, M. Bittner, Y. Chen, Y. Jiang, G. Gooden, JM. Trent, P. Meltzer, K. T. Jeang, and S. L. Zeichner. 2000. Genome-wide expression changes induced by HTLV-1 Tax: evidence for MLK-3 mixed lineage kinase involvement in Tax-mediated NF-κB activation. Oncogene 20:4484-4496. [DOI] [PubMed] [Google Scholar]
- 51.Philpott, S. M., and G. C. Buehring. 1999. Defective DNA repair in cells with human T-cell leukemia/bovine leukemia viruses: role of tax gene. J. Natl. Canc. Inst. 91:933-942. [DOI] [PubMed] [Google Scholar]
- 52.Pise-Masison, C. A., R. Mahieux, H. Jiang, M. Ashcroft, M. Radonovich, J. Duvall, C. Guillerm, and J. N. Brady. 2000. Inactivation of p53 by human T-cell lymphotropic virus type 1 Tax requires activation of the NF-κB pathway and is dependent on p53 phosphorylation. Mol. Cell. Biol. 20:3377-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 12:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pozzati, R., J. Vogel, and G. Jay. 1990. The human T lymphotropic virus I Tax gene can cooperate with Ras oncogene to induce neoplastic transformation of cells. Mol. Cell. Biol. 10:413-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salghetti, S. E., A. A. Caudy, J. G. Chenoweth, and W. P. Tansey. 2001. Regulation of transcriptional activation domain function by ubiquitin. Science 293:1651-1653. [DOI] [PubMed] [Google Scholar]
- 56.Schmitt, I., O. Rosin, P. Rohwer, M. Gossen, and R. Grassmann. 1998. Stimulation of cyclin-dependent kinase activity and G1- to S-phase transition in human lymphocytes by the human T-cell leukemia/lymphotropic virus type 1 Tax protein. J. Virol. 72:633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki, T., S. Kitao, H. Matsushime, and M. Yoshida. 1996. HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity towards CDK4. EMBO J. 15:1607-1614. [PMC free article] [PubMed] [Google Scholar]
- 58.Tamiya, S., M. Matsuoka, K. Etoh, T. Watanabe, S. Kamihira, K. Yamaguchi., and K. Takatsuki. 1996. Two types of defective human T-lymphotropic virus type I provirus in adult T-cell leukemia. Blood 88:3065-3073. [PubMed] [Google Scholar]
- 59.Tanaka, A., C. Takahashi, S. Yamaoka, T. Nosaka, M. Maki, and M. Hatanaka. 1990. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc. Natl. Acad. Sci. USA 87:1071-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas, D., and M. Tyers. 2000. Transcriptional regulation: kamikaze activators. Curr. Biol. 10:341-343. [DOI] [PubMed] [Google Scholar]
- 61.Torgeman, A., Z. Ben-Aroya, A. Grunspan, E. Zelin, E. Butovsky, M. Hallak, M. Lochelt, R. M. Flugel, E. Livneh, M. Wolfson, I. Kedar, and M. Aboud. 2001. Activation of HTLV-1 long terminal repeat by stress-inducing agents and protection of HTLV-1-infected T cells from apoptosis by the viral tax protein. Exp. Cell Res. 271:169-179. [DOI] [PubMed] [Google Scholar]
- 62.Tripp, A., Y. Liu, M. Sieburg, J. Montalbano, S. Wrzesinski, and G. Feuer. 2003. Human T-cell leukemia virus type 1 Tax oncoprotein suppression of multilineage hematopoiesis of CD34+ cells in vitro. J. Virol. 77:12152-12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilkinson, K. D. 2000. Ubiquitin and deubiquitination: Targeting of proteins for degradation by proteasome. Cell Dev. Biol. 11:141-148. [DOI] [PubMed] [Google Scholar]
- 64.Willems, L., C. Grimonpont, P. Kerkhofs, C. Capiau, D. Gheysen, K. Conrath, R. Roussef, R. Mamoun, D. Portetelle, A. Burny, E. Adam, L. Lefebvre, J. C. Twizere, H. Heremans, and R. Kettmann. 1998. Phosphorylation of bovine leukemia virus Tax protein is required for in vitro transformation but not for transactivation. Oncogene 16:2165-2176. [DOI] [PubMed] [Google Scholar]
- 65.Wing, S. S. 2003. Deubiquitinating enzymes-the importance of driving in reverse along the ubiquitin-proteasome pathway. The International J. Biochem. Cell. Biol. 35:590-605. [DOI] [PubMed] [Google Scholar]
- 66.Ye, J., L. Xie, and P. L. Green. 2003. tax and overlapping rex sequences do not confer the distinct transformation tropisms of human T-cell leukemia virus types 1 and 2. J. Virol. 77:7728-7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 79:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]