Abstract
Although the effects of ethanol on protein receptors and lipid membranes have been studied extensively, ethanol’s effect on vesicles fusing to lipid bilayers is not known. To determine the effect of alcohols on fusion rates, we utilized the nystatin/ergosterol fusion assay to measure fusion of liposomes to a planar lipid bilayer (BLM). The addition of ethanol excited fusion when applied on the cis (vesicle) side, and inhibited fusion on the trans side. Other short-chain alcohols followed a similar pattern. In general, the inhibitory effect of alcohols (trans) occurs at lower doses than the excitatory (cis) effect, with a decrease of 29% in fusion rates at the legal driving limit of 0.08% (w/v) ethanol (IC50 = 0.2% v/v, 34 mM). Similar inhibitory effects were observed with methanol, propanol, and butanol, with ethanol being the most potent. Significant variability was observed with different alcohols when applied to the cis side. Ethanol and propanol enhanced fusion, butanol also enhanced fusion but was less potent, and low doses of methanol mildly inhibited fusion. The inhibition by trans addition of alcohols implies that they alter the planar membrane structure and thereby increase the activation energy required for fusion, likely through an increase in membrane fluidity. The cis data are likely a combination of the above effect and a proportionally greater lowering of the vesicle lysis tension and hydration repulsive pressure that combine to enhance fusion. Alternate hypotheses are also discussed. The inhibitory effect of ethanol on liposome-membrane fusion is large enough to provide a possible biophysical explanation of compromised neuronal behavior.
Introduction
Since prehistoric times, ethanol has been used both as a disinfectant and as an intoxicating and rewarding beverage (1). Ethanol is typically produced through anaerobic, and sometimes aerobic, fermentation of sugars by yeast. Fermentation is typically limited to 15% ethanol, as metabolism is slowed considerably in yeast at this level. Toxicity in yeast likely occurs through several pathways, including an excessive increase in fluidity of the yeast cell membrane by ethanol (2). Elucidating the molecular targets of alcohol’s action in brain and linking these sites to specific behavioral actions of ethanol is critical for understanding alcohol use, abuse, and dependence. The exact pathways whereby ethanol exerts its effect on the brain are controversial, but likely involve a constellation of effects on membrane proteins (3, 4, 5), electrical and chemical synaptic transmission, and fluidizing of the lipid membrane. Being a simple molecule (C2H6O) it would be expected that ethanol acts through a general mechanism rather than specific interactions such as antigen-antibody binding. Nevertheless, there is mounting evidence that ethanol may act directly on specific proteins at physiologically relevant levels as indicated below.
Ethanol’s main effects are on the central nervous system (CNS). Ethanol acts as a weak anesthetic following the Meyer-Overton rule, which simply states that the potency of an anesthetic is proportional to its lipid solubility. This fact alone has pointed to the nerve cell membrane as a target for ethanol’s effect on the brain. However, the dogma is that physiologically relevant levels of ethanol (i.e., concentrations below 100 mM) must be mediated by ligand-gated ion channels and voltage-gated calcium channels because the membrane lipid disordering properties of ethanol appear above 100 mM and are not physiologically relevant. But despite decades of investigation, the evidence is inconclusive for any specific molecular target for ethanol on any protein (for reviews see (6, 7)). Many potential protein targets have been identified (4, 8) including voltage-gated Na+ channels (9), adenylyl cyclase (10, 11), guanine nucleotide binding protein Gs (12), the GLT1 glutamate transporter (13), gap junctions (14), γ-aminobutyric acid (GABA)A receptors (6), n-methyl-d-aspartate (NMDA) receptors (15), nicotinic acetylcholine receptor (nAChR) channels (16, 17), and the cannabinoid receptor 1 (18). One system within the brain that appears to be sensitive to alcohol dependence is the endocannabinoid system (for review see (19)). All of these targets most likely act as an acute effect on the brain and recovery may be linked to “hangovers” but are likely distinct from “withdrawal syndrome” and other effects observed after chronic exposure to ethanol such as alcoholic neuropathies and delirium.
In addition to the brain, other organs are affected by ethanol, particularly the liver, which is responsible for the metabolism of ethanol. Pathologies associated with the liver and chronic alcohol exposure to the brain are not the topic of this study and will not be discussed further, although it is worth noting that ethanol-induced changes in the membrane properties of hepatocytes may be central to liver disease (see review (20)).
Alcohol-induced changes in membrane properties could alter cellular function in two principle ways: indirectly through membrane-induced changes in protein structure, or directly through changes in membrane structure. For example, an increase in Na+ conductance through the membrane could lead to membrane depolarization and firing of action potentials. Such conductance increases could occur by the membrane acting on Na+ channels to stabilize the open state, or by the lipid membrane directly becoming leaky to Na+ ions. In support of the indirect pathway, it has been proposed (21), and supported by statistical thermodynamics (3, 22) and coarse-grain modeling (23), that alcohol changes the lateral pressure within the membrane and that this increase can bias ion channels toward the closed state. In support of the direct pathway it has be shown that both ethanol (24) and other anesthetics (25) can change the permeability of lipid membranes.
One critical neuronal function that requires direct interaction from both proteins and membranes is the process of neurotransmitter release through exocytosis. Although SNARE proteins have been shown to directly drive this process (26, 27, 28, 29, 30), fundamentally it requires the fusion of two membranes: the synaptic vesicle membrane and the cell membrane. Alcohols could modify exocytosis and neurotransmitter release by either pathway described above or by two alternative pathways. Alcohols could alter the cell membrane such that SNARE proteins function more effectively (e.g., create lipid domains that cluster SNARE proteins enhancing their function (31)), or they could alter the energy required to fuse membranes (thus changing the timing or extent of neurotransmitter release). All of these pathways could lead to altered brain function leading to intoxication. Currently, there is little direct evidence of enhanced or reduced transmitter release from affected regions of the brain or whether excitatory or inhibitory neurons are involved. Multiple studies have measured changes in synaptic transmission between neurons following addition of alcohol, but most propose a postsynaptic target (17, 32, 33, 34). Additionally, the vast majority of studies are done in whole animal or tissue slice preparations where observation of an ethanol effect depends on a sequence of both pre- and postsynaptic events involving many proteins. This makes it virtually impossible to detect direct effects of alcohol on the membrane fusion step of exocytosis.
Molecular dynamics simulations (35, 36, 37, 38, 39) are one approach to look directly at the effects of alcohol on membranes. However as yet, these studies have only modeled the effects of alcohols on a single membrane and not their effect on the process of membrane fusion. Using coarse-grained molecular dynamics simulations, the process of membrane fusion has been studied (40, 41), but not in the presence of alcohols. Adding to the difficulty of understanding how alcohol alters membrane fusion, few techniques are available to study the direct effect of alcohol on neuronal processes such as exocytosis in the absence of proteins. One exception is the work by Chanturiya et al. (42) where short-chain alcohols were shown to promote hemifusion between two protein-free membranes. This and related methods model exocytosis in a protein-free system by using an osmotic gradient to fuse liposomes to a planar lipid bilayer (BLM) (43, 44, 45, 46). A variation of this, the nystatin/ergosterol (nys/erg) fusion assay, was originally developed in our lab (47) to aid in the reconstitution and study of a chloride channel, but now has been used as a model system to study protein (48, 49) and lipid (50, 51) factors that alter vesicle fusion. With such model systems, it may be possible to bridge the gap between molecular models and cellular studies of how alcohol modulates membrane function during neuronal synaptic transmission.
In this study we use the nys/erg assay as a model system of exocytosis to examine what direct effect, if any, short-chain alcohols have on membranes when two membranes fuse together. We hypothesized that the direct action of alcohol on membranes would alter the energy barrier for fusion and be detected as a change in fusion rates. Consistent with this hypothesis, we observe large enhancements and significant reductions of fusion rates depending on the type of alcohol and the side of the membrane exposed. These results suggest possible additional pathways whereby ethanol may alter neurotransmitter release in neurons and hence brain function in humans.
Materials and Methods
Materials
All solutions contained 150 mM KCl 8 mM HEPES, pH 7.1 (315 ± 3 mOsM), except where noted (alcohol and salt additions). The osmolarity of all solutions was measured with a μ-Osmette Osmometer (Precisions Systems, Natick, MA). Osmolarity measurements of alcohol-containing solutions were within 7.5% of values calculated from standard tables (52). All lipids were obtained from Avanti Polar Lipids (Alabaster, AL), other reagents were from Fisher Scientific (Waltham, MA).
Liposomes
Artificial vesicles (liposomes) that contain nystatin and ergosterol were made as previously described (47, 51, 53). Briefly, liposomes (10 mg/ml) were made of a 4:1:1:2 mol ratio containing palmitoyloleoylphosphatidylethanolamine (PE), palmitoyloleoylphosphatidylcholine (PC), phosphatidylserine from porcine brain (PS), and ergosterol. The liposomes were in standard solution to which 50 μM nystatin was added. Liposome size was measured by a Brookhaven 90Plus particle sizer (Brookhaven Instruments, Holtsville, NY). Liposomes were bath sonicated to a final size of 150–300 nm in diameter. The bilayer chambers were filled (1.0 ml in cis and 0.8 ml in trans chamber) with standard buffer.
Planar bilayers
BLM were made as previously described except cholesterol was added to 23 mol% to a 7:3 mixture of PE:PC. Briefly, the lipid solution (20 mg/ml decane) was brushed onto the hole using a bubble formed with a pipette tip dipped in the same lipids as the pretreatment. Membrane capacitance (Cm) and conductance were measured and the membrane was repainted if Cm < 50% of maximum expected value based on hole diameter or if Gm was irregular or greater than 10 pA leak current.
Fusion assay
Fusion was measured with the nys/erg assay developed in our lab (47, 53). This assay has been used continuously in a variety of labs for fusion/reconstruction studies (48, 49, 50, 51, 54, 55, 56, 57, 58, 59, 60). Liposome fusion to planar bilayers was measured using standard electrophysiological equipment (Bilayer Clamp Amplifier model BC-535 or BC-525C, Warner Instruments, Hamden CT). Unless otherwise noted, the membrane-holding potential was –60 mV (trans chamber grounded). Stirring was maintained at 150–300 rpm to ensure constant delivery of liposomes to the planar bilayer. The Bilayer Clamp Amplifier was under the control of custom software using the DasyLab platform (DasyTec, Amherst, NH). Membrane current, voltage, and capacitance were low-pass filtered and saved to disk at 25 Hz. Fusion was initiated by adding 3–10 μl of liposomes to the cis chamber to which KCl had been added to create a transmembrane osmotic gradient. The osmotic gradient was made by adding 100 μl of 3 M KCl to the cis chamber (and withdrawing 100 μl) to give a final (KCl) of 409 mM (772 ± 7 mOsM). This creates a transmembrane gradient of ∼460 mOsM. Fusions were counted manually by the appearance of abrupt rises (<0.1 s) in membrane conductance of at least four times the RMS noise level, followed by a slower (>0.8 s) fall to baseline. To control for human bias, some experiments were also counted by a custom computer program that detected fusions based on the derivative of the current trace. Although human counting always detected more fusions, the ratio of fusion rates before and after addition of alcohol was the same for human and computer-based count rates. Fusions were recorded on a minute-by-minute basis and 6 min intervals were averaged for each condition. Typically, two consecutive 6 min intervals were recorded before addition of alcohol to the cis or trans compartment. Full experiments usually lasted 25–40 min with brief interruptions of current measurements every 6–18 min to monitor membrane capacitance. Fusion rates were corrected for changes in capacitance. Typically, this was a relative small correction as experiments with a >25% change in capacitance were discarded.
As previously demonstrated (44, 45, 61), liposomes will fuse when three conditions are met: 1) the vesicles have an ion channel (e.g., the nystatin channels) in their membrane, 2) the vesicles adhere or are docked to the bilayer, and 3) there is an osmotic gradient across the bilayer. Under these three conditions, there will be a net entry of water from the trans compartment through the bilayer membrane and into the docked vesicle. This water flux drives the compensatory entry of osmotically active ions (through nystatin channels) moving down their concentration gradient into the vesicle. This results in increased vesicular pressure, which increases surface tension according to the law of Laplace. The increase in tension is relieved through fusion of the vesicular membrane with the planar membrane, lowering the system free energy (45, 46).
Fluorescence
Sequential additions giving a final concentration of 0.08, 0.2, 0.4, and 4.0% (v/v) ethanol (EtOH) were made to a solution of nys/erg vesicles (∼250 nm diameter; nystatin = 9.4 μM, ergosterol = 23 mol% in 150 mM KCl 8 mM HEPES buffer, pH 7.1). Steady-state fluorescence emission was monitored with a Fluoromax (Jobin Yvon, Edison, NJ) photon-counting spectrofluorometer. Sample homogeneity was maintained by magnetic stirring. Samples were equilibrated in the fluorometer at 25°C. Emission spectra were collected at wavelengths appropriate for nystatin: excitation 315 nm, peak emission 398 nm. Band pass was set at 4 nm. Under the conditions used, interference from scattered light was negligible.
Results
Difference in fusion rates between cis and trans added ethanol
Using the nys/erg fusion assay, we were able to assess the effect of various reagents on fusion of artificial vesicles (liposomes) to a planar lipid bilayer (BLM). Initial investigation of ethanol’s effect on vesicle fusion rates was conducted by adding 3.85% v/v ethanol (660 mM) to either the cis or trans side of a BLM. The cis side was defined as the side of the planar membrane to which the vesicles were added. Ethanol exhibited large and opposite effects when added to the cis or trans chambers. Ethanol (cis) produced a three- to fourfold increase in fusion rates compared with controls (see Fig. 1 A). This excitation occurred within seconds of addition and decreased only slightly over the 40 min duration of our experiments, likely due to the reduction of the vesicle population over time. However, addition of an equal dose of ethanol to the trans side quickly decreased fusion rates to near control values (data not shown).
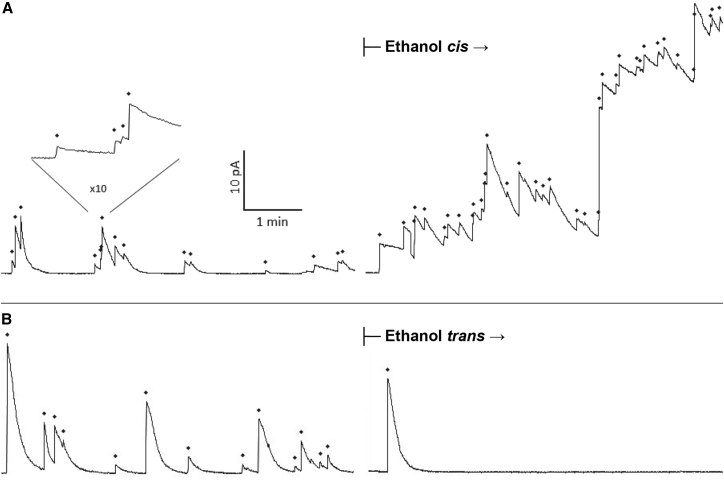
Figure 1.
Ethanol-induced changes in liposome fusion rates. Both panels show the time course of the membrane current in a typical experiment where fusions from control (no ethanol) conditions were followed by fusions after the addition of 3.85% v/v ethanol. Each sharp upward spike in current represents the fusion of one liposome to the bilayer and is marked above with a diamond. (A) The addition of ethanol to the cis chamber more than doubled the fusion rate (from 15/6 min to 37/6 min). Insert: expanded time trace shows the clear resolution of four fusion events. (B) The addition of ethanol to the trans chamber suppressed fusion (control: 13/6 min; after trans addition: 1/6 min). The kinetics of current decay remain unchanged following ethanol addition in both cases. Membrane holding potential Em = –60 mV (current scale is inverted so that upward deflection shows an increase in membrane conductance).
In sharp contrast, when ethanol was first added to the trans side of the chamber again at 3.85% (v/v), there was a dramatic decrease in fusion rates as compared with preaddition rates. This inhibition resulted in fusion rates decreasing ∼7-fold from that observed during control conditions (Fig. 1 B).
Because of the simplicity of our model system, there are only two obvious locations for the action of ethanol on fusion rates. Ethanol added to the cis chamber can interact with the planar membrane and the vesicle membrane. Ethanol added to the trans chamber can only interact with the planar membrane. Because of the differing results with addition of ethanol to different sides of the membrane, two different mechanisms appear to be responsible. We hypothesize that the inhibition results from changing membrane properties of the planar membrane and that excitation is attributable predominantly to effect of ethanol on the vesicular membrane. If correct, the excitatory effect on the vesicle membrane must surpass the effect of ethanol on the planar bilayer. This is consistent with our observation that following increased fusion rates from addition of ethanol cis, replacing the cis solution with identical but ethanol-free solution produced a significant decrease in fusion rates. This decrease was overcome by again adding ethanol cis, which increased fusion rates above controls.
It is also noteworthy that that is all cases the average fusion spike height and turnoff time are unchanged after alcohol addition. Our fusion assay uses the conductance of many open nystatin channels to detect fusion. Nystatin channels are stabilized at the boundary of ergosterol-containing superlattices in the vesicle membrane. Following fusion with an ergosterol-free membrane, ergosterol is lost and nystatin channels close. We have previously shown that the time course of nystatin turnoff is correlated with diffusion of ergosterol out of a lipid superlattice following fusion (57). The lack of change in turnoff kinetics (Fig. 1) suggests that alcohol neither speeds up nor slows down ergosterol diffusion in the fusing vesicle.
cis addition of alcohols
To further characterize the inhibitory and excitatory effects of alcohols on membrane fusion, we extended our study to lower concentrations of ethanol and to alcohols of different chain lengths. It is well known that alcohol chain length is directly correlated with its bilayer modifying potency (3, 62, 63). We hypothesized that if the observed effects on vesicle fusion from ethanol were the result of changing of some membrane properties, then changing alcohol chain length may influence the magnitude of the excitation and inhibition of fusion that we observe.
To test this hypothesis, we measured vesicle fusion rates in the presence of methanol, ethanol, propanol, and butanol at several different concentrations. Addition of alcohol was done in the same manner as described above, with the alcohol being added either to the cis or trans chambers after a control period with no alcohol.
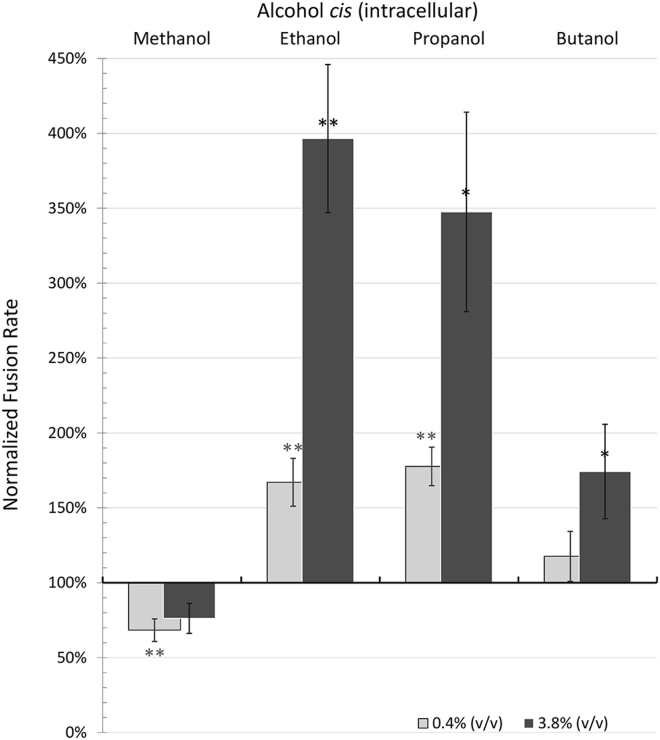
cis addition of alcohol models exocytosis with the alcohol present intracellularly. Using the same quantitative metric described above to measure the cis ethanol effect, we measured the percentage increase in fusion rates for all four short-chain alcohols: methanol, ethanol, propanol, and butanol at 0.4 and 3.85% v/v (Fig. 2). Control fusion rates during several 6 min periods in the absence of alcohol were compared with fusion rates after addition of alcohol to the cis chamber. Addition of alcohols to the cis chamber resulted in excitation in fusion rates by ethanol, propanol, and butanol, but not methanol, which gave variable rates with no significant change in fusion rates at 3.85% and a statistically significant decrease in vesicle fusion rates to 68% of control values at 0.4% (Table 1; Fig. 2). However, 8% v/v methanol did enhance vesicle fusions (data not shown). Ethanol and propanol caused similar increases in vesicle fusion rates with a ∼70% increase at 0.4% v/v and a three- to fourfold increase at 3.85% v/v. The largest alcohol, butanol, was much less effective at increasing fusion rates. We anticipated that with butanol, the excitation would increase due to increasing alcohol chain length and lipid solubility. Surprisingly, this was not the case. Effectiveness and significance of the change in fusion rates for all alcohols at both doses are given in Table 1.
Figure 2.
Effect of methanol, ethanol, propanol, and butanol on vesicle fusion rates when added to the cis chamber. Fusion rates are presented as a percentage of the control experiment (before addition of alcohol). Light gray bars are for 0.4% v/v alcohol and dark gray bars are for 3.8% v/v alcohol. Ethanol and propanol similarly enhanced fusion rates at each dose. Butanol slightly enhanced fusion rates at the higher dose. In contrast, methanol did not significantly enhance fusions, but actually reduced fusion rates at 0.4% v/v. Error bars are mean ± SE; additional details are given in Table 1. P-values (compared with control): ∗ < 0.05, ∗∗ < 0.01.
Table 1.
cis Addition of Alcohols
| Alcohol (% v/v) | Molarity (mM) | Fusion Rates Mean ± SE | n | |
|---|---|---|---|---|
| Methanol | 0.40% | 100 | 68% ± 8% | 6 |
| 3.85% | 950 | 76% ± 10% | 5 | |
| Ethanol | 0.40% | 70 | 167% ± 16% | 7 |
| 3.85% | 660 | 396% ± 50% | 12 | |
| Propanol | 0.40% | 55 | 178% ± 13% | 4 |
| 3.85% | 515 | 348% ± 67% | 4 | |
| Butanol | 0.40% | 45 | 118% ± 17% | 7 |
| 3.85% | 420 | 174% ± 31% | 14 |
Bolding indicates higher dose of alcohol.
trans addition of alcohol
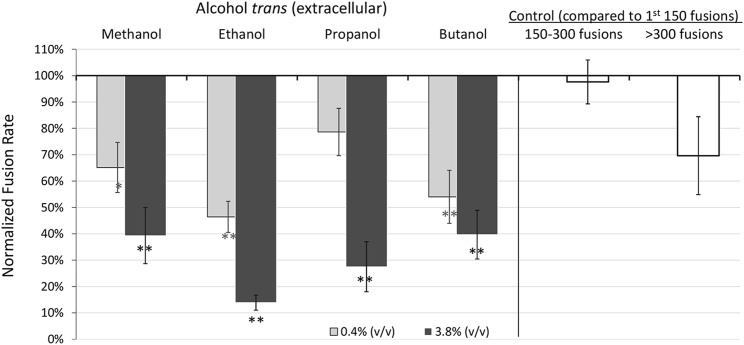
Adding alcohol to the trans chamber models exposure as would normally occur in humans, with the alcohol initially present extracellularly. In contrast to the enhancement seen with cis addition, trans addition reduces vesicle fusion rates. As shown in Fig. 3, at 3.85% (v/v) alcohol fusion rates were decreased to 14%–40% of controls rates, depending on the alcohol. The extent of inhibition between alcohols was not significant. However, with the exception of the low dose of methanol and propanol, all were significantly different from controls. A decrease in fusion rates was also seen in extended experiments without addition of alcohol, presumably because of depletion of the vesicle pool. The white bars in Fig. 3 show that this decrease only became significant after 300 fusions. Therefore, we excluded data with greater than ∼300 fusion. See Table 2 for complete summary of experimental values with trans alcohol.
Figure 3.
Same as Fig. 2 except showing the decrease in fusion rates when alcohols are added to the trans chamber. trans addition models initial extracellular exposure of cells to alcohol. The level of inhibition increased with dose. White bars show fusion rates in extended control experiments. Rates started to fall off after ∼300 fusion events. Error bars show mean ± SE; additional details are given in Table 2.
Table 2.
trans Addition of Alcohols
| Alcohol (% v/v) | Molarity (mM) | Fusion Rates Mean ± SE | n | |
|---|---|---|---|---|
| Methanol | 0.40% | 100 | 65% ± 9% | 3 |
| 3.85% | 950 | 39% ± 11% | 5 | |
| Ethanol | 0.44% | 75 | 46% ± 6% | 5 |
| 3.85% | 660 | 14% ± 3% | 4 | |
| Propanol | 0.40% | 55 | 79% ± 9% | 3 |
| 3.85% | 515 | 28% ± 9% | 4 | |
| Butanol | 0.36% | 40 | 54% ± 10% | 7 |
| 3.81% | 415 | 40% ± 9% | 8 |
Bolding indicates higher dose of alcohol.
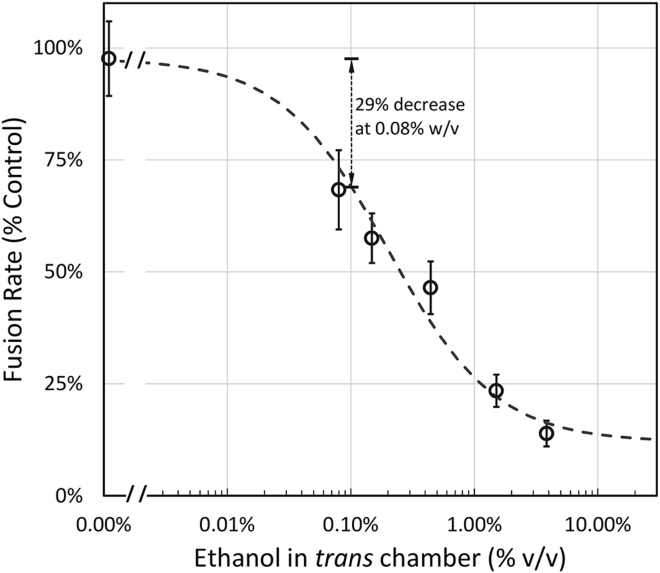
Ethanol dose response curve for fusion
To further determine the effectiveness of ethanol in inhibiting fusion at physiologically relevant concentrations and the IC50 of ethanol added to the trans chamber, we expanded the range of doses. Fig. 4 shows the ethanol dose response curve. The IC50 was determined from the least squares best-fit dose curve through the data and was 0.20% (v/v) or 34 mM. At 0.08% w/v (17 mM), the established legal driving limit in the United States for blood alcohol concentration, there was a 29% decrease in fusion rates as compared with control fusion values. Thus, when ethanol is applied to the extracellular side of a model membrane at physiologically relevant concentrations, a significant decrease in fusion rates is observed.
Figure 4.
Ethanol added to the trans chamber inhibits fusion rates with an IC50 of 0.20% v/v (34 mM). At 0.08% w/v ethanol, the legal driving limit, there was a 29% decrease in fusion rates. Error bars are mean ± SE; N = 3–5.
Ethanol changes membrane fluidity
We considered the possibility that the changes in fusion rates may be due to fluidity changes in either the vesicle or bilayer membrane. To independently measure fluidity changes in membranes due to ethanol, we took advantage of the fact that nystatin, used for the fusion assay above, also fluoresces in membranes (64). The fluorescence of nystatin has been shown to reflect membrane fluidity (65). When a membrane is more rigid, nystatin partitions deeper into the bilayer and its fluorescence increases. On the other hand, when nystatin is in bulk solution or not partitioned as deeply, its fluorescence is somewhat quenched. Thus, a decrease in fluorescence emission corresponds to an increase in membrane fluidity.
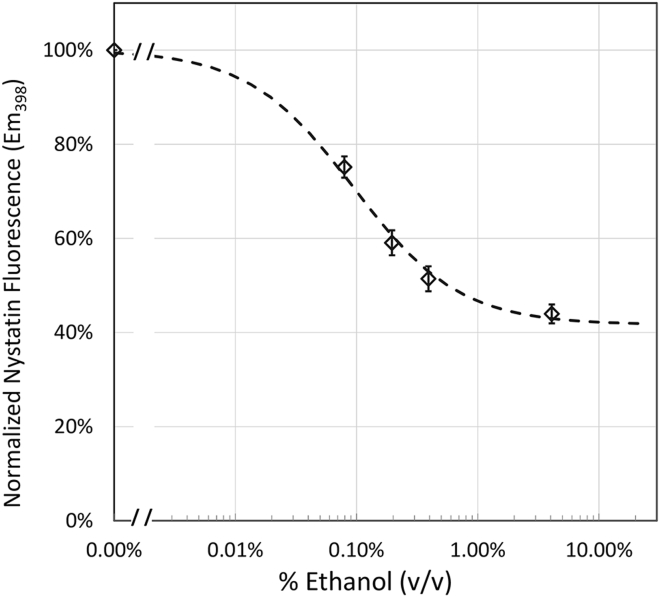
As shown in Fig. 5, ethanol decreases nystatin fluorescence, and hence increases membrane fluidity with a dose curve very similar to that shown for fusion in Fig. 4. The IC50 was ∼0.1% v/v ethanol, which compares well with 0.2% v/v ethanol obtained with the fusion assay for trans addition. At the legal limit of alcohol (0.08% w/v), there was a 30% decrease in fluorescence emission. Decreases in fluorescence emission (increase in membrane fluidity) was also seen with methanol, propanol, and butanol (data not shown). Although fluorescence spectroscopy cannot distinguish whether or not alcohol is being added to the cis or trans side of the bilayer membrane, what is clear is that physiologically relevant concentrations of ethanol change membrane properties in ways that correlate well with decreases in fusion rates due to ethanol in the planar membrane, but do not correlate with increases in fusion rates with ethanol in the vesicle membrane (cis addition).
Figure 5.
Ethanol decreases membrane fluidity as reflected by the decrease in nystatin fluorescence. Standard nystatin-containing vesicles were exposed to the indicated percentage (v/v) of ethanol and fluorescence emission at 398 nm was monitored. The decrease in fluorescence with increasing ethanol concentration has an IC50 of 0.095% (16 mM). Error bars are mean ± SE; N = 7.
Alcohols do not act by an osmotic mechanism
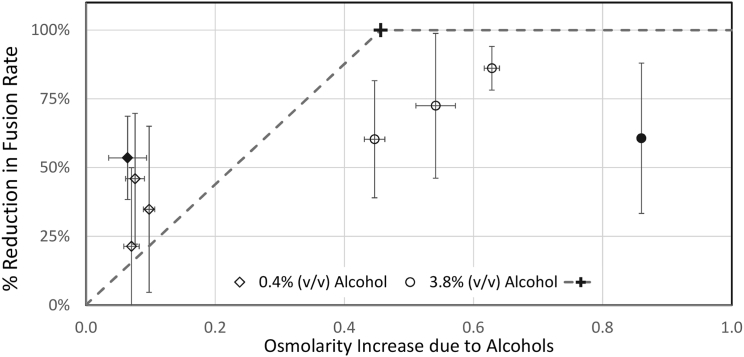
A mechanism by which trans alcohol could reduce fusion rates is through the depletion of the osmotic driving force. Because our system is a protein-free system where fusion is driven by an osmotic gradient, any increase in the osmolarity of the trans chamber could reduce the tonicity across the bilayer membrane and thereby reduce fusion rates. The transmembrane osmotic gradient initially was ∼460 mOsM (cis side high, see Materials and Methods). Raising the trans side by adding enough alcohol to cancel this gradient would be expected to stop fusions. Fig. 6 shows that this is clearly not the case. The dashed line in Fig. 6 shows the predicted reduction in fusion rate if alcohols added to the trans side reduced fusion through an osmotic mechanism. The “+” at 100% reduction in fusion rates shows the point where the osmolarity of the alcohol addition would exactly cancel out the higher osmolarity of the cis side. No fusions would be expected at and above this osmolarity. At lower osmolarities, a linear relationship between osmolarity and fusion (actually surface tension in the vesicle membrane) is assumed based on Eq. 15 of (45). Note that most of the alcohols at 3.8% (v/v) would be predicted to totally block fusion, which was not observed. Additionally, ethanol at 0.4% (solid diamond) and methanol at 3.8% (solid circle) both reduce fusion by ∼60%, but produce vastly different increases in osmolarity (50 and 850 mOsM, respectively).
Figure 6.
Changes in the apparent osmotic gradient do not correlate with reduced fusion rates. Addition of alcohols to the trans chamber reduced fusion rates but did not correlation with respect to the corresponding osmolarity changes. The “+” at 0.46 OsM and 100% reduction in fusions shows the osmolarity increase needed to exactly match the osmotic gradient in the chamber, which should stop fusion. The dashed line is the expected correlation, assuming osmotic forces drive the reduction in fusions produced by alcohols. The fusion rates data are from Fig. 3; error bars are 95% confidence limits.
To further test for a possible osmotic effect, additional experiments were performed where equiosmolar amounts of 3M KCl were added to the cis chamber simultaneous with addition of alcohol to the trans chamber (data not shown). These simultaneous addition experiments produced inhibition of fusion that was not significantly different from those without equiosmolar KCl (Fig. 3). These data rule out a significant role of osmotic forces in the observed effect of alcohols on vesicle-membrane fusion. This is also consistent with short-chain alcohols having a fairly high permeability through the membrane (63), although not high enough (due to the large volume of the chambers) to significantly raise the alcohol concentration on cis side.
Discussion
As shown in Fig. 1, we observe a strong effect of ethanol on the process of membrane-membrane fusion, a core step in cellular exocytosis. Addition to the cis side increased fusion (Fig. 2) and trans addition inhibited fusion (Fig. 3) with an IC50 of 34 mM (Fig. 4). Because osmotic forces do not play a significant role in producing the effect (Fig. 6), we assume that alcohols are altering fusion by their direct action on membranes. Methanol has the shortest carbon tail and is consequently the least lipid-soluble of the alcohols. Hence, we anticipated that methanol would have the smallest effect on bilayer properties, interacting primarily at the interface between lipid heads and water. Conversely, of the alcohols tested, butanol has the longest carbon tail and is consequently the most lipid-soluble. We expected butanol to have the most dramatic effect on bilayer properties because it inserts deeper into the lipid tails and equilibrates quickly across the membrane. With trans addition, the variability in our data was too large to draw any direct conclusions about potency as a direct function of carbon chain length, but all alcohols produced a significant reduction compared with controls (Fig. 3). With cis addition (Fig. 2) the biphasic enhancement is significant, with fusion rates peaking with ethanol and propanol and falling off with methanol and butanol. Below we consider multiple mechanisms that may underlie these results. trans addition is considered first, as it is simpler.
Possible mechanisms for inhibition of fusion by trans addition of alcohol
A likely mechanism by which alcohol could inhibit fusion is through increased disordering and increased fluidity of the bilayer membrane. It is known that ethanol increases membrane fluidity and that cholesterol decreases fluidity by increasing the order and packing of the lipids (65). Furthermore, ethanol’s disordering effect is more potent in cholesterol-containing membranes (66, 67). If the suppression of fusion by trans addition is due to increased fluidity of the planar membrane then this same suppression would be expected with cis addition also, because in both cases the planar bilayer is exposed to alcohol. Consequently, the excitation due to cis addition would have to be due to a significantly stronger mechanism(s), to overcome the fluidity-induced inhibition in the planar membrane. However, if alcohol flip-flop across the membrane is slow, then the change in fluidity might only effect the exposed leaflet of the planar membrane. A membrane with different fluidity in each leaflet could enhance fusion of vesicles on one side and suppress fusion of vesicles on the other, thus directly explaining the key observation of this study.
If alcohols affect fusion by acting on only one leaflet of the bilayer membrane, then two things must be true: each leaflet of the membrane must be able to form a domain independent of the other side, and alcohol flip across the membrane must be slow on the timescale of our experiments. In support of the first point, Collins and Keller (68) have shown that different lipid domains can exist independently in each leaflet of a membrane. The issue of alcohol flip, however, requires further examination. Flip times for short-chain alcohols have not been directly measured; however, flip of ionized fatty acids has been reported to be no slower than seconds (69), and for unionized fatty acids on the millisecond timescale (70). Results from molecular modeling show flip of ethanol, propanol, and butanol on the submicrosecond timescale, depending on lipid composition (35, 37, 38). Additionally, butanol was observed to enhance fast phospholipid flip on the timescale of microseconds (38). These data are consistent with alcohol flip-flop occurring much faster than the time course of our experiments and hence imply that asymmetrically distributed alcohol is not the main factor altering vesicle fusion rates. This is also consistent with our conclusion that these alcohols have high membrane permeability and hence cannot change fusion rates through an osmotic (tonic) mechanism.
The suppression of fusion due to addition of alcohol to the trans side is most likely explained by the fluidizing effect of alcohol on both leaflets of the planar membrane. This is also consistent with data that cholesterol in the planar bilayer increases vesicle fusion rates, presumably through a decrease in membrane fluidity (51). The fluorescence data (Fig. 5) confirm that ethanol increases membrane fluidity as reflected by the decrease in nystatin fluorescence. However, if ethanol is altering fluidity in the vesicle, then it did not change the fluidity of the sections of the membrane superlattice containing ergosterol and nystatin channels, since channel turnoff time was not altered (see Fig. 1). Thus, our current data support the hypothesis presented previously that decreased fluidity of the planar membrane (due to addition of cholesterol) enhances fusions and increased fluidity (due to addition of alcohol) suppresses fusion.
Possible mechanisms for enhancement of fusion by cis addition of alcohol
It is more difficult to narrow down possible mechanisms for the enhancement of fusion rates following addition of alcohol. This is both because our results are biphasic and because alcohol may be acting at the planar membrane, the vesicle membrane, or the thin layer of water between them. One mechanism by which alcohol can enhance vesicle fusion is by decreasing vesicle lysis tension. Ly and Longo (63) showed that methanol, ethanol, and butanol (propanol was not included in their study) decrease vesicle lysis tension (see also (71)). A general decrease in lysis tension increases the probability that any docked vesicle will fuse with the BLM, under the same fixed driving force (45, 46) and hence increases fusion rates. We consider this the most likely mechanism and discuss it further below, after considering several alternatives.
Another potential mechanism by which alcohol can result in increased fusogenicity of vesicles is through increased interdigitation of tails within the membrane. Interdigitation happens when alcohol inserts at the head groups, expanding the membrane and leaving extra room for the lipid tails of opposing leaflets to overlap. During this process alcohol, which primarily resides adjacent to the phosphate group (39), decreases the extent of water hydration of the lipid heads and provides greater exposure of the lipid tail; processes that could increase the likelihood of fusion events (72). However, interdigitation is usually only observed in pure PC membranes and at alcohol concentrations higher than 5% v/v (36, 72, 73). Therefore, interdigitation does not explain the phenomena we observe at lower doses with membranes that contain PC, PE, and cholesterol lipids (see Materials and Methods). Interdigitation would also produce an increase in bilayer membrane capacitance, which we and others (42) did not observe.
A more plausible hypothesis is that alcohol can enhance the rate of fusion by promoting a hemifusion state between the vesicle and the BLM. Chanturiya et al. (42) showed that alcohols promote membrane hemifusion, a preliminary stage on the pathway to fusion. Specifically, methanol, ethanol, and butanol increased the prevalence of hemifusion by 5–50 times when compared with alcohol-free control conditions. They found that hemifusion occurs in the same range of concentrations that we utilize in our assay; however, the enhancement in hemifusion plateaus ∼2% v/v. We continue to observe increasing fusion rates with increasing alcohol concentration as high as 8% v/v (data not shown). Thus, it is likely that if alcohol enhances fusion through the promotion of hemifusion, then additional mechanisms of enhancement, such as the reduction of lysis tension or interdigitation, must be responsible when concentrations exceed 2% v/v.
Alcohol could also induce fusion by lowering the hydration repulsive pressure, either directly or indirectly. One of the major barriers inhibiting the fusion of two lipid bilayers is the layer of water between them creating a hydration repulsive pressure (74). Alcohol at the surface of the membrane can replace water thereby lowering the hydration repulsive pressure (75). Additionally, alcohol may indirectly influence the hydration repulsive force by increasing the surface area of the lipid interface through its insertion into the leaflets. Such changes depend on the exact composition of lipids (35). The increase in surface area will decreases hydration pressure (76). This suggests a plausible mechanism for the action of alcohol in increasing fusion as it has been shown that alcohol increases surface area per head group (63).
Alcohol could alter fusion by changing membrane curvature. Previously we and others showed that cholesterol, which can cause membranes to have negative curvature, enhances membrane fusion (51, 77, 78). We considered the possibility that alcohol might also alter fusion by changing membrane curvature. Because the bilayer membrane is planar, it seemed most likely that any change would act primarily on vesicle membranes and would depend on vesicle size. However, we cannot rule out an effect on bilayer membrane curvature due to asymmetric alcohol distribution across the membrane. With respect to vesicle size, we noted that ethanol had the same effect on vesicles that were twice as large or twice as small as control vesicles and there was no shift in the size of the fusing vesicles (estimated from conductance spike height) following addition of alcohol (data not shown).
Finally, we also considered the possibility of the increase in fusion rates observed is simply an artifact specific to the constraints of our system. Specifically, the observed enhancement might be due to alcohol increasing the sensitivity of the nys/erg fusion assay. The assay uses nystatin channels in vesicular membranes to detect when vesicles fuse to a BLM. Because alcohols influence various membrane properties, changing lipid behavior and domains, it is possible that alcohol’s impact on the bilayer influences the conductance, grouping, or structure of nystatin channels. Changes in nystatin conductance could increase the observed fusion rate through two mechanisms. 1) Increased nystatin conductance would make each fusion easier to observe. Hence fusion of vesicles with only a few nystatin channels, that would not be observed before alcohol addition, would be detected after addition. 2) Changes in nystatin conductance could alter the population of vesicles able to fuse under the specific osmotic conditions of the assay (45). To test these possibilities, we directly measured nystatin conductance in an ergosterol-containing planar bilayer as previously reported (47), and then added ethanol. At low doses (<0.5% v/v) ethanol had no measurable effect on nystatin conductance, but at higher doses nystatin conductance dropped by up to half of the alcohol-free controls (data not shown). This is consistent with the fluorescence data that show nystatin moving out of the membrane following ethanol addition (Fig. 5). This decrease would lead to an underestimate of fusion rates and therefore can be ruled out as the mechanism that is producing the observed enhancement. Furthermore, this mechanism cannot produce the inhibitory effect observed with alcohol in the trans chamber since the trans chamber does not come in contact with the vesicles or their nystatin channels until after fusion events.
Of the six possible mechanisms presented above for the enhancement of fusion by cis addition of alcohol, two seem most likely: decreasing vesicle lysis tension and lowering the hydration repulsive pressure. With both of these mechanisms alcohol would be expected to enhance fusion only if added to the cis side. If these are indeed the two major factors for enhancing fusion, then their combined effect must still overcome the reduction in fusion caused by the increased fluidity of the planar membrane by alcohol.
The extent of enhancement due to decreases in lysis tension and hydration pressure is dependent on both concentration and alcohol chain length. According to Ly and Longo (63) the lysis tension decreases with concentration and with chain length. This is mostly consistent with all of our data except for butanol, where it was unexpectedly less effective than propanol and ethanol at promoting fusion (Fig. 2). We also observe a mild suppression of fusion with low doses of methanol implying that with this short-chain alcohol the small decrease in lysis tension in vesicles and the mild lowering of the hydration repulsive pressure between vesicle and planar bilayer are not sufficient to overcome the inhibition to fusion caused by the increased fluidity of the planar membrane. For butanol, we speculate that the presence of sterol (cholesterol in the planar membrane and ergosterol in the liposomes) alters the membrane response to this longer alcohol. The alcohol-induced membrane changes reported by Ly and Longo (63) were for sterol-free membranes. They report that alcohol absorption into the membrane generally expanded the membrane heads. However, in the presence of sterol, the longer hydrocarbon chain of butanol would compete with the sterol and would not be expected to localize in the membrane in the same way. Indeed, Brahm (79) observed an unexpected increased in membrane permeability with butanol that may be attributable to cholesterol in the membrane. A clearer understanding of butanol’s interactions with sterol-containing membranes may provide further clues on the action of this alcohol on membrane fusion and exocytosis.
Conclusions
In many past studies of the direct effect on lipid membranes caused by ethanol and other short-chain alcohols, relatively high concentrations of ethanol (500–2000 mM) were required to observe a measurable effect (6, 7, 24, 71). The major finding of this research is that relatively low doses of ethanol (0.08% w/v or 17 mM) added to the membrane corresponding to the extracellular side are sufficient to cause a 29% inhibition in fusion rates of liposomes to BLMs. Multiple mechanisms are likely responsible for the effects of alcohol on liposome-membrane fusion and these effects will depend on the specific composition of the membranes (35). Application of these results to in vivo studies will require consideration of how additional components in cell membranes might modulate these results. For example, synaptic vesicles are known to contain not only the lipids PE, PC, PS, and sterol (used in this study), but also phosphatidylinositol (PI), sphingomyelin, and hexylceramide (80). It is likely that proteins associated with the fusing membranes will also modify the alcohol-induced effects.
Although these data do not prove that the pharmacological effects of ethanol on humans are due to a suppression of exocytosis and neurotransmitter release from neurons, it seems worth pointing out that the effect we observe in our simplified model of membrane-membrane fusion is large enough to provide a possible biophysical explanation of compromised neuronal behavior. For example, an ethanol-induced general decrease in neurotransmitter release may lead to a greater relative decrease from excitatory versus inhibitory synapses leading to a general depression of the CNS. This is consistent with fast-scan cyclic voltammetry results using mice brain slice preparations where ethanol causes inhibition of dopamine release at terminals in the striatum with an IC50 of 40–60 mM (17, 81).
Author Contributions
B.H. conceived the initial study. B.H., J.P., and S.R.Z ran bilayer experiments. D.H. ran fluorescence experiments. J.P. and D.J.W wrote most of the article. All authors helped analyze results and prepared figures. D.J.W. provided training for all protocols.
Acknowledgments
We thank Coulson Huntington, Ben Cutler, Colby Erickson, David Calderwood, and Mitchell Allphin for their help in running some of the bilayer experiments including osmolarity measurements, as well as Scott C. Steffensen, Jeff G. Edwards, and J. Walter Woodbury for their helpful comments and review of the manuscript.
Editor: Edward Stuenkel
References
- 1.Vallee B.L. Alcohol in the western world. Sci. Am. 1998;278:80–85. doi: 10.1038/scientificamerican0698-80. [DOI] [PubMed] [Google Scholar]
- 2.You K.M., Rosenfield C.L., Knipple D.C. Ethanol tolerance in the yeast Saccharomyces cerevisiae is dependent on cellular oleic acid content. Appl. Environ. Microbiol. 2003;69:1499–1503. doi: 10.1128/AEM.69.3.1499-1503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantor R.S. Breaking the Meyer-Overton rule: predicted effects of varying stiffness and interfacial activity on the intrinsic potency of anesthetics. Biophys. J. 2001;80:2284–2297. doi: 10.1016/S0006-3495(01)76200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCool B.A. Ethanol modulation of synaptic plasticity. Neuropharmacology. 2011;61:1097–1108. doi: 10.1016/j.neuropharm.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckenhoff R.G. Do specific or nonspecific interactions with proteins underlie inhalational anesthetic action? Mol. Pharmacol. 1998;54:610–615. [PubMed] [Google Scholar]
- 6.Harris R.A., Trudell J.R., Mihic S.J. Ethanol’s molecular targets. Sci. Signal. 2008;1:re7. doi: 10.1126/scisignal.128re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun G.Y., Sun A.Y. Ethanol and membrane lipids. Alcohol. Clin. Exp. Res. 1985;9:164–180. doi: 10.1111/j.1530-0277.1985.tb05543.x. [DOI] [PubMed] [Google Scholar]
- 8.Krasowski M.D., Harrison N.L. General anaesthetic actions on ligand-gated ion channels. Cell. Mol. Life Sci. 1999;55:1278–1303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horishita T., Harris R.A. n-Alcohols inhibit voltage-gated Na+ channels expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 2008;326:270–277. doi: 10.1124/jpet.108.138370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandey S.C. Neuronal signaling systems and ethanol dependence. Mol. Neurobiol. 1998;17:1–15. doi: 10.1007/BF02802021. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura M., Pearson S., Gonzalez C.E. Identification of ethanol responsive domains of adenylyl cyclase. Alcohol. Clin. Exp. Res. 2006;30:1824–1832. doi: 10.1111/j.1530-0277.2006.00219.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman P.L., Tabakoff B. Ethanol and guanine nucleotide binding proteins: a selective interaction. FASEB J. 1990;4:2612–2622. doi: 10.1096/fasebj.4.9.2161371. [DOI] [PubMed] [Google Scholar]
- 13.Rao P.S.S., Sari Y. Glutamate transporter 1: target for the treatment of alcohol dependence. Curr. Med. Chem. 2012;19:5148–5156. doi: 10.2174/092986712803530511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steffensen S.C., Bradley K.D., Edwards J.G. The role of connexin-36 gap junctions in alcohol intoxication and consumption. Synapse. 2011;65:695–707. doi: 10.1002/syn.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stobbs S.H., Ohran A.J., Steffensen S.C. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves N-methyl-D-aspartate receptors. J. Pharmacol. Exp. Ther. 2004;311:282–289. doi: 10.1124/jpet.104.071860. [DOI] [PubMed] [Google Scholar]
- 16.Borghese C.M., Henderson L.A., Harris R.A. Sites of excitatory and inhibitory actions of alcohols on neuronal alpha2beta4 nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2003;307:42–52. doi: 10.1124/jpet.102.053710. [DOI] [PubMed] [Google Scholar]
- 17.Schilaty N.D., Hedges D.M., Steffensen S.C. Acute ethanol inhibits dopamine release in the nucleus accumbens via α6 nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2014;349:559–567. doi: 10.1124/jpet.113.211490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basavarajappa B.S., Hungund B.L. Role of the endocannabinoid system in the development of tolerance to alcohol. Alcohol Alcohol. 2005;40:15–24. doi: 10.1093/alcalc/agh111. [DOI] [PubMed] [Google Scholar]
- 19.Pava M.J., Woodward J.J. A review of the interactions between alcohol and the endocannabinoid system: implications for alcohol dependence and future directions for research. Alcohol. 2012;46:185–204. doi: 10.1016/j.alcohol.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sergent O., Fatiha D.-A., Lagadic-Gossmann D. Up-to-date insight about membrane remodeling as a mechanism of action for ethanol-induced liver toxicity. In: Shimizu I., editor. Trends in Alcoholic Liver Disease Research—Clinical and Scientific Aspects. InTech; Rijeka, Croatia: 2012. [Google Scholar]
- 21.Gruner S.M., Shyamsunder E. Is the mechanism of general anesthesia related to lipid membrane spontaneous curvature? Ann. N.Y. Acad. Sci. 1991;625:685–697. doi: 10.1111/j.1749-6632.1991.tb33902.x. [DOI] [PubMed] [Google Scholar]
- 22.Cantor R.S. The lateral pressure profile in membranes: a physical mechanism of general anesthesia. Biochemistry. 1997;36:2339–2344. doi: 10.1021/bi9627323. [DOI] [PubMed] [Google Scholar]
- 23.Frischknecht A.L., Frink L.J. Alcohols reduce lateral membrane pressures: predictions from molecular theory. Biophys. J. 2006;91:4081–4090. doi: 10.1529/biophysj.106.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komatsu H., Okada S. Effects of ethanol on permeability of phosphatidylcholine/cholesterol mixed liposomal membranes. Chem. Phys. Lipids. 1997;85:67–74. [Google Scholar]
- 25.Blicher A., Wodzinska K., Heimburg T. The temperature dependence of lipid membrane permeability, its quantized nature, and the influence of anesthetics. Biophys. J. 2009;96:4581–4591. doi: 10.1016/j.bpj.2009.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber T., Zemelman B.V., Rothman J.E. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 27.Söllner T., Whiteheart S.W., Rothman J.E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 28.Lin R.C., Scheller R.H. Mechanisms of synaptic vesicle exocytosis. Annu. Rev. Cell Dev. Biol. 2000;16:19–49. doi: 10.1146/annurev.cellbio.16.1.19. [DOI] [PubMed] [Google Scholar]
- 29.Woodbury D.J., Rognlien K. The t-SNARE syntaxin is sufficient for spontaneous fusion of synaptic vesicles to planar membranes. Cell Biol. Int. 2000;24:809–818. doi: 10.1006/cbir.2000.0631. [DOI] [PubMed] [Google Scholar]
- 30.Jahn R., Scheller R.H. SNAREs—engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 31.Bacia K., Schuette C.G., Schwille P. SNAREs prefer liquid-disordered over “raft” (liquid-ordered) domains when reconstituted into giant unilamellar vesicles. J. Biol. Chem. 2004;279:37951–37955. doi: 10.1074/jbc.M407020200. [DOI] [PubMed] [Google Scholar]
- 32.Maguire E.P., Mitchell E.A., Belelli D. Extrasynaptic glycine receptors of rodent dorsal raphe serotonergic neurons: a sensitive target for ethanol. Neuropsychopharmacology. 2014;39:1232–1244. doi: 10.1038/npp.2013.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariqueo T.A., Agurto A., Aguayo L.G. Effects of ethanol on glycinergic synaptic currents in mouse spinal cord neurons. J. Neurophysiol. 2014;111:1940–1948. doi: 10.1152/jn.00789.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bajo M., Herman M.A., Roberto M. Role of the IL-1 receptor antagonist in ethanol-induced regulation of GABAergic transmission in the central amygdala. Brain Behav. Immun. 2015;45:189–197. doi: 10.1016/j.bbi.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konas R.M., Daristotle J.L., Klauda J.B. Biophysical changes of lipid membranes in the presence of ethanol at varying concentrations. J. Phys. Chem. B. 2015;119:13134–13141. doi: 10.1021/acs.jpcb.5b06066. [DOI] [PubMed] [Google Scholar]
- 36.Dickey A.N., Yim W.-S., Faller R. Using ergosterol to mitigate the deleterious effects of ethanol on bilayer structure. J. Phys. Chem. B. 2009;113:2388–2397. doi: 10.1021/jp803092z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patra M., Salonen E., Karttunen M. Under the influence of alcohol: the effect of ethanol and methanol on lipid bilayers. Biophys. J. 2006;90:1121–1135. doi: 10.1529/biophysj.105.062364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickey A.N., Faller R. How alcohol chain-length and concentration modulate hydrogen bond formation in a lipid bilayer. Biophys. J. 2007;92:2366–2376. doi: 10.1529/biophysj.106.097022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feller S.E., Brown C.A., Gawrisch K. Nuclear Overhauser enhancement spectroscopy cross-relaxation rates and ethanol distribution across membranes. Biophys. J. 2002;82:1396–1404. doi: 10.1016/S0006-3495(02)75494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Risselada H.J., Bubnis G., Grubmüller H. Expansion of the fusion stalk and its implication for biological membrane fusion. Proc. Natl. Acad. Sci. USA. 2014;111:11043–11048. doi: 10.1073/pnas.1323221111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Risselada H.J., Grubmüller H. How SNARE molecules mediate membrane fusion: recent insights from molecular simulations. Curr. Opin. Struct. Biol. 2012;22:187–196. doi: 10.1016/j.sbi.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Chanturiya A., Leikina E., Chernomordik L.V. Short-chain alcohols promote an early stage of membrane hemifusion. Biophys. J. 1999;77:2035–2045. doi: 10.1016/S0006-3495(99)77044-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodbury D.J., Hall J.E. Vesicle-membrane fusion. Observation of simultaneous membrane incorporation and content release. Biophys. J. 1988;54:345–349. doi: 10.1016/S0006-3495(88)82965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niles W.D., Cohen F.S., Finkelstein A. Hydrostatic pressures developed by osmotically swelling vesicles bound to planar membranes. J. Gen. Physiol. 1989;93:211–244. doi: 10.1085/jgp.93.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodbury D.J., Hall J.E. Role of channels in the fusion of vesicles with a planar bilayer. Biophys. J. 1988;54:1053–1063. doi: 10.1016/S0006-3495(88)83042-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen F.S., Niles W.D., Akabas M.H. Fusion of phospholipid vesicles with a planar membrane depends on the membrane permeability of the solute used to create the osmotic pressure. J. Gen. Physiol. 1989;93:201–210. doi: 10.1085/jgp.93.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodbury D.J., Miller C. Nystatin-induced liposome fusion. A versatile approach to ion channel reconstitution into planar bilayers. Biophys. J. 1990;58:833–839. doi: 10.1016/S0006-3495(90)82429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pohl P., Antonenko Y.N., Tonevitsky A.G. Membrane fusion mediated by ricin and viscumin. Biochim. Biophys. Acta. 1998;1371:11–16. doi: 10.1016/s0005-2736(98)00024-8. [DOI] [PubMed] [Google Scholar]
- 49.McNally J.M., Woodbury D.J., Lemos J.R. Syntaxin 1A drives fusion of large dense-core neurosecretory granules into a planar lipid bilayer. Cell Biochem. Biophys. 2004;41:11–24. doi: 10.1385/CBB:41:1:011. [DOI] [PubMed] [Google Scholar]
- 50.Anzai K., Ogawa K., Yamamoto H. Quantitative comparison of two types of planar lipid bilayers—folded and painted—with respect to fusion with vesicles. J. Biochem. Biophys. Methods. 2001;48:283–291. doi: 10.1016/s0165-022x(01)00160-9. [DOI] [PubMed] [Google Scholar]
- 51.Lee D.E., Lew M.G., Woodbury D.J. Vesicle fusion to planar membranes is enhanced by cholesterol and low temperature. Chem. Phys. Lipids. 2013;166:45–54. doi: 10.1016/j.chemphyslip.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Weast R.C. CRC Press; Boca Raton, FL: 1980. CRC Handbook of Chemistry and Physics. [Google Scholar]
- 53.Woodbury D.J. Nystatin/ergosterol method for reconstituting ion channels into planar lipid bilayers. Methods Enzymol. 1999;294:319–339. doi: 10.1016/s0076-6879(99)94020-x. [DOI] [PubMed] [Google Scholar]
- 54.Studer A., Demarche S., Tiefenauer L. Integration and recording of a reconstituted voltage-gated sodium channel in planar lipid bilayers. Biosens. Bioelectron. 2011;26:1924–1928. doi: 10.1016/j.bios.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Zagnoni M., Sandison M.E., Morgan H. Controlled delivery of proteins into bilayer lipid membranes on chip. Lab Chip. 2007;7:1176–1183. doi: 10.1039/b703818f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Planque M.R., de Planque M.R., Morgan H. Controlled delivery of membrane proteins to artificial lipid bilayers by nystatin-ergosterol modulated vesicle fusion. IEE Proc. Nanobiotechnol. 2006;153:21–30. doi: 10.1049/ip-nbt:20050039. [DOI] [PubMed] [Google Scholar]
- 57.Helrich C.S., Schmucker J.A., Woodbury D.J. Evidence that nystatin channels form at the boundaries, not the interiors of lipid domains. Biophys. J. 2006;91:1116–1127. doi: 10.1529/biophysj.105.076281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelly M.L., Woodbury D.J. Ion channels from synaptic vesicle membrane fragments reconstituted into lipid bilayers. Biophys. J. 1996;70:2593–2599. doi: 10.1016/S0006-3495(96)79830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woodbury D.J. Evaluation of the evidence for ion channels in synaptic vesicles. Mol. Membr. Biol. 1995;12:165–171. doi: 10.3109/09687689509027504. [DOI] [PubMed] [Google Scholar]
- 60.Bear C.E., Li C.H., Riordan J.R. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR) Cell. 1992;68:809–818. doi: 10.1016/0092-8674(92)90155-6. [DOI] [PubMed] [Google Scholar]
- 61.Kozlov M.M., Chernomordik L.V. Membrane tension and membrane fusion. Curr. Opin. Struct. Biol. 2015;33:61–67. doi: 10.1016/j.sbi.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ingolfsson H.I., Andersen O.S. Alcohol effects on lipid bilayer properties as measured using a gramicidin-based fluorescence assay. Biophys. J. 2011;100:35a. doi: 10.1016/j.bpj.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ly H.V., Longo M.L. The influence of short-chain alcohols on interfacial tension, mechanical properties, area/molecule, and permeability of fluid lipid bilayers. Biophys. J. 2004;87:1013–1033. doi: 10.1529/biophysj.103.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coutinho A., Prieto M. Cooperative partition model of nystatin interaction with phospholipid vesicles. Biophys. J. 2003;84:3061–3078. doi: 10.1016/S0006-3495(03)70032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson-Ashworth H.A., Bahm Q., Bell J.D. Differential detection of phospholipid fluidity, order, and spacing by fluorescence spectroscopy of bis-pyrene, prodan, nystatin, and merocyanine 540. Biophys. J. 2006;91:4091–4101. doi: 10.1529/biophysj.106.090860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J., Cao H., Regen S.L. Ethanol-induced reorganization of the liquid-ordered phase: enhancement of cholesterol-phospholipid association. J. Am. Chem. Soc. 2006;128:265–269. doi: 10.1021/ja056918d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han S. Effect of cholesterol on ethanol-induced disruption of a POPC bilayer: a molecular dynamics simulation study. Bull. Korean Chem. Soc. 2015;36:2569–2572. [Google Scholar]
- 68.Collins M.D., Keller S.L. Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers. Proc. Natl. Acad. Sci. USA. 2008;105:124–128. doi: 10.1073/pnas.0702970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alix S.N., Woodbury D.J. Phospholipase A2 action on planar lipid bilayers generates a small, transitory current that is voltage independent. Biophys. J. 1997;72:247–253. doi: 10.1016/S0006-3495(97)78663-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamp F., Zakim D., Hamilton J.A. Fatty acid flip-flop in phospholipid bilayers is extremely fast. Biochemistry. 1995;34:11928–11937. doi: 10.1021/bi00037a034. [DOI] [PubMed] [Google Scholar]
- 71.Ly H.V., Block D.E., Longo M.L. Interfacial tension effect of ethanol on lipid bilayer rigidity, stability, and area/molecule: a micropipet aspiration approach. Langmuir. 2002;18:8988–8995. [Google Scholar]
- 72.Komatsu H., Okada S. Ethanol-induced aggregation and fusion of small phosphatidylcholine liposome: participation of interdigitated membrane formation in their processes. Biochim. Biophys. Acta. 1995;1235:270–280. doi: 10.1016/0005-2736(95)80014-7. [DOI] [PubMed] [Google Scholar]
- 73.Simon S.A., McIntosh T.J. Interdigitated hydrocarbon chain packing causes the biphasic transition behavior in lipid/alcohol suspensions. Biochim. Biophys. Acta. 1984;773:169–172. doi: 10.1016/0005-2736(84)90562-5. [DOI] [PubMed] [Google Scholar]
- 74.Rand R.P. Interacting phospholipid bilayers: measured forces and induced structural changes. Annu. Rev. Biophys. Bioeng. 1981;10:277–314. doi: 10.1146/annurev.bb.10.060181.001425. [DOI] [PubMed] [Google Scholar]
- 75.Stetter F.W.S., Hugel T. The nanomechanical properties of lipid membranes are significantly influenced by the presence of ethanol. Biophys. J. 2013;104:1049–1055. doi: 10.1016/j.bpj.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Slater J.L., Huang C.H. Interdigitated bilayer membranes. Prog. Lipid Res. 1988;27:325–359. doi: 10.1016/0163-7827(88)90010-0. [DOI] [PubMed] [Google Scholar]
- 77.Siegel D.P. The modified stalk mechanism of lamellar/inverted phase transitions and its implications for membrane fusion. Biophys. J. 1999;76:291–313. doi: 10.1016/S0006-3495(99)77197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chernomordik L., Chanturiya A., Zimmerberg J. The hemifusion intermediate and its conversion to complete fusion: regulation by membrane composition. Biophys. J. 1995;69:922–929. doi: 10.1016/S0006-3495(95)79966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brahm J. Permeability of human red cells to a homologous series of aliphatic alcohols. Limitations of the continuous flow-tube method. J. Gen. Physiol. 1983;81:283–304. doi: 10.1085/jgp.81.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takamori S., Holt M., Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 81.Yorgason J.T., Ferris M.J., Jones S.R. Frequency-dependent effects of ethanol on dopamine release in the nucleus accumbens. Alcohol. Clin. Exp. Res. 2014;38:438–447. doi: 10.1111/acer.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]