Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is the retrovirus responsible for adult T-cell leukemia and HTLV-1-associated myelopathy. Adult T-cell leukemia development is mainly due to the ability of the viral oncoprotein Tax to promote T-cell proliferation, whereas the appearance of HTLV-1-associated myelopathy involves the antigenic properties of Tax. Understanding the events regulating the intracellular level of Tax is therefore an important issue. How Tax is degraded has not been determined, but it is known that Tax binds to proteasomes, the major sites for degradation of intracellular proteins, generally tagged through polyubiquitin conjugation. In this study, we investigated the relationship between Tax, ubiquitin, and proteasomes. We report that mono- and polyubiquitinated Tax proteins can be recovered from both transfected 293T cells and T lymphocytes. We also show that lysine residues located in the carboxy-terminal domain of Tax are the principal targets of this process. Remarkably, we further demonstrate that mutation of lysine residues in the C-terminal part of Tax, which massively reduces Tax ubiquitination, impairs proteasome binding, and conversely, that a Tax mutant that binds poorly to this particle (M22) is faintly ubiquitinated, suggesting that Tax ubiquitination is required for association with cellular proteasomes. Finally, we document that comparable amounts of ubiquitinated species were found whether proteasome activities were inhibited or not, providing evidence that they are not directly addressed to proteasomes for degradation. These findings indicate that although it is ubiquitinated and binds to proteasomes, Tax is not massively degraded via the ubiquitin-proteasome pathway and therefore reveal that Tax conjugation to ubiquitin mediates a nonproteolytic function.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia, a malignant monoclonal proliferation of CD4+ T lymphocytes and of a chronic myelopathy called HTLV-1-associated myelopathy/tropical spastic paraparesis (36). Although these two diseases are definitely divergent in term of pathogenic mechanisms, the HTLV-1 Tax regulatory protein can be considered a key actor in both cases. First, via its ability to activate the viral promoter (31, 34), chronic Tax production is required to sustain viral replication. Second, HTLV-1-mediated immortalization of T lymphocytes, a fundamental event for subsequent cell transformation, results mainly from the ability of Tax to trigger T-cell proliferation through various mechanisms, including transcriptional transactivation of cellular genes (reviewed in reference 21) and promotion of cell cycle and deregulation of apoptosis (reviewed in reference 13).
HTLV-1-associated myelopathy/tropical spastic paraparesis is not related to T-cell transformation and is considered as an immune-mediated pathology (reviewed in reference 15). Complex mechanisms are involved, among which exacerbation of the antiviral cytotoxic T-cell response (7, 23) and cross recognition of cellular proteins by anti-HTLV-1 antibodies are of the utmost importance (25). Since Tax is chronically produced in vivo (16), is the highly immunodominant target of anti-HTLV-1 cytotoxic T cells (22), and the primary target of cross-reacting antibodies (25), it also plays a major role in the pathogenesis of HTLV-1-associated myelopathy/tropical spastic paraparesis. Exploring the mechanisms underlying the regulation of Tax protein turnover is therefore a central issue for the understanding of persistent HTLV-1 infection and associated pathologies.
The cellular mechanisms that regulate Tax production and stability have not been fully characterized. Tax is synthesized in the cytosol and then transported to the nucleus via an unknown mechanism requiring the integrity of the N-terminal amino acid sequence (32). Tax also possesses a nuclear export signal and can therefore shuttle between the nucleus and the cytosol (1). Tax is posttranslationally modified by phosphorylation on two adjacent serine residues at positions 300 and 301, a modification that is critically required for its transactivation properties (5). Although the mechanisms of Tax degradation are unknown, it has been shown that Tax interacts with the proteasome (3, 17, 26, 30), the major intracellular site for the degradation of cytosolic and nuclear proteins, including transcription factors.
Proteasomes are multisubunit proteases present in both the nucleus and the cytoplasm of eukaryotic cells (9). They are composed of a central core (20S) surrounded by various regulatory caps (19S) (reviewed in reference 37). The 20S cylinder, which accommodates the proteolytic compartment, is composed of two outer rings of seven α-subunits and two inner rings of seven β-subunits. Attached to both ends of the 20S cylinder to constitute the 26S proteasome, 19S particles are regulatory subunits, responsible for the recognition and unfolding of substrates and their subsequent gating into the core. Besides their role in the degradation of intracellular proteins, proteasomes are responsible for the generation of the majority of peptides presented by major histocompatibility complex class I molecules (29). Furthermore, a nonproteolytic role in excision repair and transcription elongation was recently identified for the Saccharomyces cerevisiae 19S proteasome regulatory particle (12, 14).
Tax has been shown to physically interact in vitro with the proteasome core and especially with the HC9 (α3) and HsN3 (β7) subunits (3, 30). Some Tax mutants were found to display an increased (M47, L319R/L320S) or a reduced (M22, T130A/L131S) affinity to these subunits (30). Recent studies further showed that Tax also binds to assembled proteasomes in both human and murine cells (17, 26).
One of the signals targeting proteins toward the proteasome is conjugation to ubiquitin (reviewed in reference 19). Ubiquitin is a small, abundant, highly conserved 76-amino-acid polypeptide found in all eukaryotic cells. Proteins can be modified by covalent attachment of ubiquitin molecules to either lysine residues or their N termini (10) via an ATP-dependent process. The ubiquitination reaction first involves an activating enzyme (E1) that activates the ubiquitin, and then the ligation of ubiquitin to the substrate is carried out by a complex composed of a ubiquitin conjugating enzyme (E2) and a ubiquitin-protein ligase (E3), in charge of substrate specificity. Conjugation to a chain containing at least four ubiquitin monomers is required to target a protein to the proteasome for destruction (35).
Tax is the immunodominant target of cytotoxic T lymphocytes directed to HTLV-1 and interacts with the proteasome. This prompted us to study the relationship between Tax, ubiquitin, and the proteasome. In this study, we demonstrate that Tax is ubiquitinated in both transfected cells and T lymphocytes. Moreover, we report that Tax ubiquitination is needed for proteasome binding but is not associated with rapid degradation.
MATERIALS AND METHODS
Plasmids and mutagenesis.
Tax protein was produced from the pSG5-Tax plasmid (provided by A. Bazarbachi, American University of Beirut, Beirut, Lebanon) (27) or from the pJFE-Tax plasmid (17), in which the Tax coding sequence was fused in frame at its C terminus with the sequence coding for the influenza virus hemagglutinin epitope (HA) followed by six histidine codons (Tax-HA-6His).
Tax mutants in which all (Tax K1-10R) or some (Tax K1-3R, Tax K4-10R, and Tax K6-8R) of the 10 lysine codons of Tax were substituted for arginine codons (see Fig. 3A) were generated from the pSG5-Tax plasmid by successive PCR-based site-directed mutagenesis (QuikChange kit, Stratagene) according to the manufacturer's instructions. All constructs were completely sequenced before use in transfections. The M22 (T130A/L131S) and M47 (L319R/L320S) mutants were described (33). All these Tax mutants were subcloned in pSG5-Tax with or without an additional sequence encoding the 6His tag (Tax-6His).
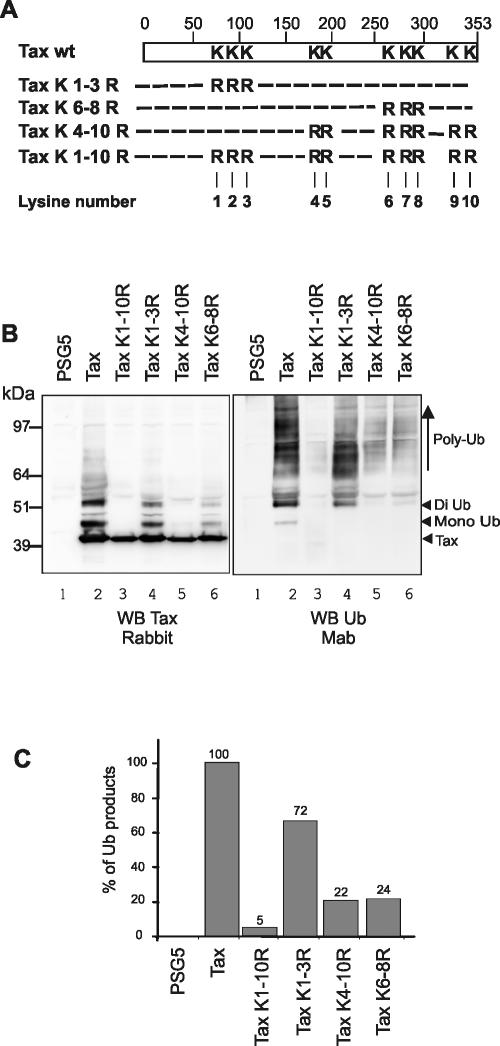
FIG. 3.
Lysines located in the C-terminal half of Tax are the principal targets of ubiquitin. (A) Schematic representation of the lysine-to-arginine Tax mutants. (B) 293T cells were transfected with the control plasmid pSG5 (lane 1) or with Tax-6His (lane 2), Tax-6His K1-10R (lane 3), Tax-6His K1-3R (lane 4), Tax-6His K4-10R (lane 5), or Tax-6His K6-8R (lane 6). Proteins eluted after Ni-NTA pulldown were subjected to Western blot analysis with a rabbit polyclonal serum to Tax (left panel) or the anti-ubiquitin Fk2 monoclonal antibody (right panel). (C) Quantitation performed on the anti-ubiquitin blot after subtraction of the background in lane 1 and normalization to an equal amount of the unmodified 40-kDa species.
The Saccharomyces cerevisiae ubiquitin gene (ub) present in plasmid Yep96 (a gift from M. Hochstrasser, University of Chicago) was extracted by PCR with a 3′ primer containing an EcoRI site and corresponding to the first nucleotide of ub, including the initiation codon, and a 5′ primer containing the ub/tax fusion region, including the terminal sequence of ub followed by the Tax sequence from the initiation codon to the Tth111.1 restriction site. The PCR product was then ligated into the pSG5-Tax construct digested with EcoRI and Tth111.1. A mutation was next introduced into the ub gene to change the final glycine codon to a valine codon in order to prevent rapid cleavage of the ubiquitin part (11).
Antibodies.
The hybridoma producing the MCP 21 monoclonal antibody that recognizes the αHC3 subunit of the human 20S proteasome (18) was purchased from the European Collection of Cell Cultures (no. 96030418) and the anti-β4 subunit from Affinity Research (Tebu). HA-ubiquitinated proteins were revealed with the 12CA5 (Roche) or Y-11 (Tebu) monoclonal antibodies, polyubiquitinated products with Fk2 (Tebu), and actin with the 1501R monoclonal antibody (Euromedex).
Tax proteins were revealed with the anti-Tax monoclonal antibody from hybridoma 168-A51 (AIDS Research and Reagent Program, National Institutes of Health), a polyclonal serum obtained by immunizing rabbits with a purified maltose binding protein-Tax fusion protein (rabbit serum) or a pool of sera from HTLV-1-infected patients (patient sera).
Cells and transfections.
293T cells were maintained in Dulbecco's modified Eagle's medium supplemented with 2 mM l-glutamine, 10% fetal calf serum, and antibiotics. C8166 (6), a T-cell line with a deleted HTLV-1 provirus encoding for Tax and uninfected Jurkat and CEM T cells were maintained in RPMI medium supplemented with 2 mM l-glutamine, 10% fetal calf serum, and antibiotics. Transfections were carried out with the calcium phosphate precipitation procedure with either 1 μg of plasmid in a six-well or 5 μg in a 100-mm dish. T cells (2 × 107) were electroporated at 960 μF, 0. 25 kV, in the presence of 20 μg of plasmid.
Ni-NTA pull down.
At 24 h posttransfection, 293T cells were lysed in reducing and highly denaturing conditions with buffer A (6 M guanidinium-HCl, 0.1 M Na2HPO4/NaH2PO4, 0.01 M Tris-Cl, pH 8.0, 5 mM imidazole, 10 mM β-mercaptoethanol) and incubated with Ni2+ nitrilotriacetic acid (NTA) beads for 4 h at room temperature. The beads were washed with buffers A, B (8 M urea, 0.1 M Na2HPO4/NaH2PO4, 0.01 M Tris-Cl, pH 8.0, 10 mM imidazole, 10 mM β-mercaptoethanol), and C (8 M urea, 0.1 M Na2HPO4/NaH2PO4, 0.01 M Tris-Cl, pH 6.3, 10 mM imidazole, 10 mM β-mercaptoethanol), and the bound proteins were eluted with buffer D (300 mM imidazole, 0.15 M Tris-Cl, pH 6.7, 30% glycerol, 0.72 M β-mercaptoethanol, 5% sodium dodecyl sulfate).
Transactivation of the HTLV-1 long terminal repeat.
Tax-mediated transactivation of the HTLV-1 promoter via the CREB pathway was assayed by cotransfecting 293T cells with 500 ng of an HTLV long terminal repeat-β-galactosidase reporter plasmid (2) and with 500 ng of the constructs encoding wild-type Tax or the lysine mutants. Whole-cell extracts were prepared and analyzed by β-galactosidase reporter gene assay according to the manufacturer's instructions (Roche).
Western blot and immunoprecipitation.
Twenty-four hours posttransfection, 293T cells were lysed (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% IGEPAL CA-630, 0.5% Triton X-100, 1× protease inhibitor cocktail [Roche]) on ice for 30 min. To preclude deubiquitination during lysis, various inhibitors were added, including MG-132 (50 μM), ubiquitin aldehyde (1 μg/ml), and N-ethylmaleimide (10 mM) (Sigma). C8166 and Jurkat (5 × 106) cells were washed and lysed in the same conditions. Protein concentration was determined by the Bradford assay, and 50 μg of proteins were loaded and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis before transfer to nitrocellulose (Optitran, Merck).
Immunoprecipitation of assembled proteasomes was performed with 3 μl of the MCP21 antibody, and cell lysates were incubated overnight at 4°C. Antibody complexes were then captured on protein G-Sepharose (Amersham, France) for another 1 h at 4°C, and Sepharose beads were washed five times in lysis buffer before elution in Laemmli buffer.
For immunoblots, Tax proteins were revealed with a pool of sera from HTLV-1-infected patients (1/3,000), the anti-Tax monoclonal antibody (1/1,000), or the rabbit serum to Tax (1/100) and corresponding secondary antibodies and visualized by chemiluminescence with ECL+ (Amersham, France) or luminol (Tebu). Quantitation of chemiluminescent signals was performed with the Chemi-Smart 5000 and Bio-1D software (Vilber Lourmat).
Proteasome inhibition.
At 24 h after transfection, 293T cells were incubated in complete Dulbecco's modified Eagle's medium containing either 10 μM of MG-132 for 5 h or 1 μM of lactacystin (VWR) for 20 h. Cells were then directly lysed in 2× boiling Laemmli buffer, and total proteins were loaded onto a sodium dodecyl sulfate-10% polyacrylamide gel, subjected to electrophoresis, and transferred for immunoblotting.
RESULTS
High-molecular-weight Tax products exist in transfected cells and Tax-producing T lymphocytes.
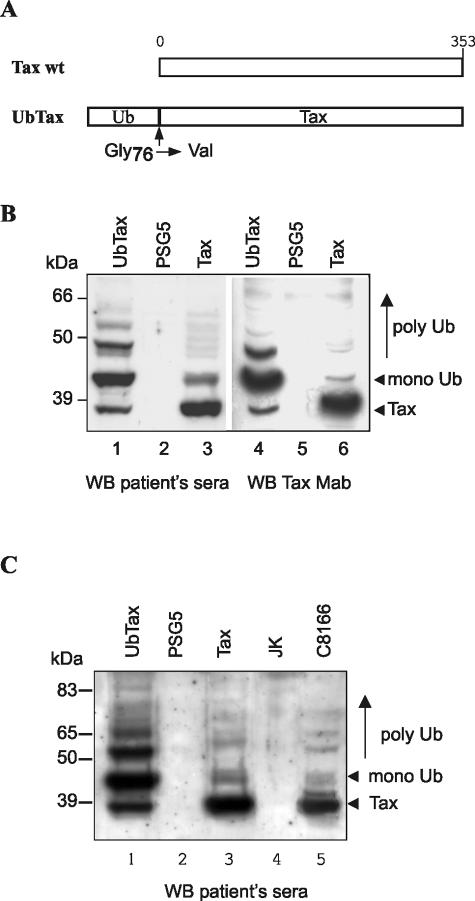
We first investigated whether modified Tax proteins are produced by using transiently transfected cells. As a positive control for the specific detection of ubiquitinated Tax products, we produced a ubiquitin-Tax fusion in which the tax sequence was fused in frame with a synthetic ub gene (Fig. 1A). In this construct, the last Gly codon of the ub gene was substituted for a Val codon to preclude rapid ubiquitin removal by isopeptidases acting as deubiquitinating enzymes (11).
FIG. 1.
High-molecular-weight Tax products exist in transfected cells and Tax-producing T lymphocytes. (A) Schematic representation of the Tax and ubiquitin (Ub)-Tax proteins. (B) Detection of Tax products by Western blot in 293T cells transfected with the pSG5 control plasmid (lanes 2 and 5) or plasmids encoding ubiquitin-Tax (lanes 1 and 4) or wild-type (wt) Tax (lanes 3 and 6). Proteins were revealed with patient sera (lanes 1 to 3) or an anti-Tax monoclonal antibody (lanes 4 to 6). (C) Western blot performed on equal amounts of uninfected Jurkat (lane 4) or infected C8166 (lane 5) T cells with patient sera. 293T cells transfected with the ubiquitin-Tax (lane 1), Tax (lane 3), or pSG5 (lane 2) plasmids are also shown.
To favor the detection of ubiquitinated Tax proteins, 293T cells transfected with the wild-type Tax or the ubiquitin-Tax plasmid were lysed in a buffer containing both proteasome and isopeptidase inhibitors, which prevent rapid deubiquitination. Then, Tax proteins were revealed by Western blot with a pool of patient sera (Fig. 1B, lanes 1 to 3). As expected, a ladder of ubiquitinated products was seen in 293T cells transfected with the ubiquitin-Tax construct (Fig. 1B, lane 1), whereas no band was detected in the control lysate (Fig. 1B, lane 2). A band corresponding to the Tax moiety was also visible, which probably corresponds to internal initiation of translation from the ATG of tax which was conserved within the ub-tax gene. A band migrating at the same position as the ubiquitin-Tax protein as well as high-molecular-weight Tax products were visible in cells expressing wild-type Tax (Fig. 1B, lane 3). Blotting with an anti-Tax monoclonal antibody (Fig. 1B, lanes 4 to 6) allowed the detection of higher Tax species for both ubiquitin-Tax (Fig. 1B, lane 4) and wild-type Tax (Fig. 1B, lane 6), confirming that they indeed correspond to Tax. For the ubiquitin-Tax protein, the monoubiquitin is linearly attached and polyubiquitination can take place on both the ubiquitin and the Tax moieties. This could explain the difference observed in the migration profile of polyubiquitin products in Tax and ubiquitin-Tax-producing cells.
In parallel, a Western blot with patient sera was performed with the chronically infected C8166 T-cell line harboring a deleted provirus encoding Tax (6) and the HTLV-1-negative Jurkat T cells as a control (Fig. 1C). Bands migrating at the same positions as the mono- and polyubiquitinated Tax proteins found in cells transfected with the ubiquitin-Tax protein were visible for both Tax-transfected 293T cells (Fig. 1C, lane 3) and C8166 (Fig. 1C, lane 5), whereas no band was found in mock-transfected (lane 2) and Jurkat (Fig. 1C, lane 4) cells.
These results show that modified Tax proteins with sizes compatible with those of mono- and polyubiquitinated Tax proteins exist in both transfected 293T cells and chronically infected T lymphocytes.
Some high-molecular-weight Tax products are ubiquitinated Tax species.
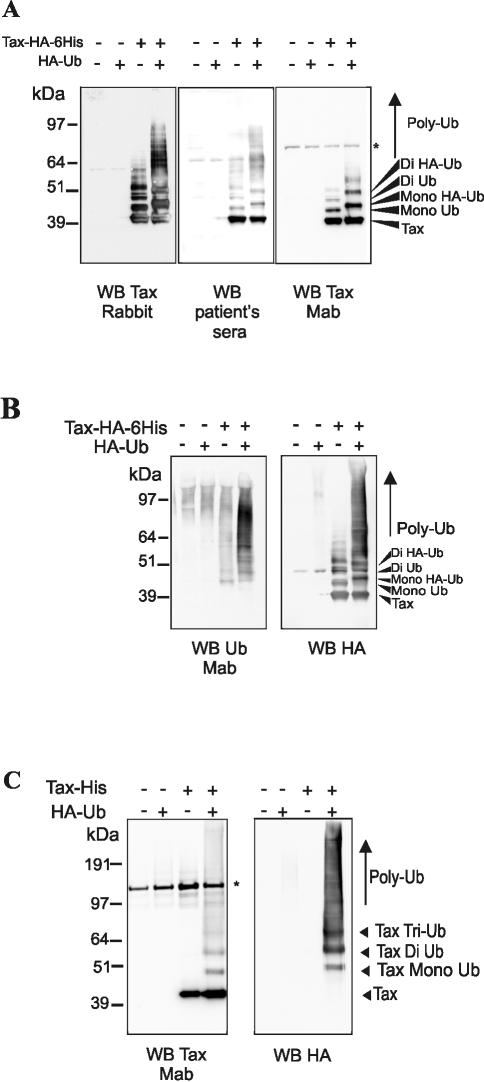
To further characterize the potential modification of Tax, we used the pJFE-Tax plasmid encoding a Tax protein fused to a C-terminal HA tag followed by a six-histidine tag (Tax-HA-6His), which allows protein purification by Ni-NTA pulldown. We used highly denaturing conditions for cell lysis and protein washes (guanidinium chloride and urea) to ensure both inactivation of deubiquitination enzymes and removal of contaminating proteins, except proteins covalently associated to Tax. Purified Tax proteins were then revealed with various antibodies (Fig. 2A). Confirming the above results, a ladder of high-molecular-weight Tax products was detected with either a rabbit polyclonal serum directed to Tax (Fig. 2A, left panel), patient sera (Fig. 2A, middle panel), or a monoclonal antibody directed to Tax (Fig. 2A, right panel). Coexpression of HA-ubiquitin with Tax led to an increase in both the size and the amount of high-molecular-weight Tax products. These species were specific for Tax because they were not observed in mock-transfected cells (Fig. 2A, lanes 1 and 2). Quantitation of anti-Tax reactive species was performed to evaluate the fraction of higher-molecular-weight products relative to the amount of unconjugated Tax (Fig. 2A, left panel). These fractions were 54 and 46%, respectively, in the absence of cotransfected HA-ubiquitin and 72 and 28%, respectively, in the presence of overexpressed HA-ubiquitin.
FIG. 2.
High-molecular-weight Tax products contain ubiquitinated species. 293T cells were transfected with the Tax-HA-6His construct in the presence of absence of an HA-ubiquitin (Ub) plasmid. (A) Proteins purified by Ni-NTA pulldown in highly denaturing conditions were subjected to immunoblotting (WB) with a rabbit serum to Tax (left panel), a pool of patient sera (middle panel), or an anti-Tax monoclonal antibody (right panel). (B) Stripped membranes were blotted with the anti-polyubiquitin chain Fk2 (left panel) or an anti-HA (right panel) monoclonal antibody. (C) CEM T cells were transfected with a Tax-6His plasmid with or without the HA-ubiquitin construct, and proteins purified by Ni-NTA pulldown were blotted with the Tax monoclonal antibody (left panel) or the anti-HA antibody (right panel). *, nonspecific species detected by the anti-Tax monoclonal antibody.
To find out whether such modified proteins corresponded to ubiquitinated Tax products, the same membranes were probed with the Fk2 antibody specific for polyubiquitin chains (Fig. 2B, left panel) or with the anti-HA antibody (Fig. 2B, right panel). Fk2-reacting Tax products were found even in the absence of overexpressed ubiquitin (Fig. 2B, lanes 3). In cells coexpressing HA-ubiquitin, both the size and amount of Fk2-reacting products increased (Fig. 2B, left panel, lane 4), and they were also recognized by the anti-HA antibody (Fig. 2B, right panel, lane 4). Only few products were seen in the control lysates (Fig. 2B, left and right panels, lanes 1 and 2). This demonstrated that the high-molecular-weight Tax products contain ubiquitinated proteins.
We then investigated whether Tax ubiquitination can be detected in transfected T lymphocytes. To do so, we used a Tax-6His plasmid without the HA tag in order to allow the specific detection of HA-ubiquitin and transfected it into the CEM T-cell line with or without the HA-ubiquitin construct (Fig. 2C). Probably due to low transfection efficiency and/or a limiting pool of endogenous ubiquitin, ubiquitinated Tax products were not detected in cells not expressing HA-ubiquitin (Fig. 2C, lanes 3). However, such products were readily found in HA-ubiquitin cotransfected cells with either the anti-Tax monoclonal antibody (Fig. 2C, left panel, lane 4) or the anti-HA antibody (Fig. 2C, right panel, lane 4).
These results provide definitive evidence that Tax exists as both mono-and polyubiquitinated species in transfected 293T cells and that ubiquitination of Tax can also occur in the context of T lymphocytes, which represent the major target of HTLV-1 in vivo.
Lysine residues located in the carboxy-terminal half of Tax are the principal targets of ubiquitin.
We next investigated whether ubiquitin conjugation takes place on specific lysines of Tax (Fig. 3). Tax possesses 10 lysines, three (K1 to K3) located in the N-terminal half of the protein (residues 85, 88, and 111) and seven (K4 to K10) located in the C-terminal half (residues 189, 197, 263, 280, 284, 324, and 346) (Fig. 3A). Three new Tax-6His mutants were then produced in which all the lysine residues (Tax K1-10R) or the lysines of the N-terminal (Tax K1-3R) or C-terminal (Tax K4-10R) parts of the protein were replaced with arginines. We also tested an intermediate construct in which only lysines 263, 280 and 284 were mutated (Tax K6-8R) (Fig. 3A).
These plasmids were transfected into 293T cells, and proteins recovered by Ni-NTA pulldown in highly denaturing conditions were revealed with either the anti-Tax rabbit serum (Fig. 3B, left panel) or the anti-ubiquitin antibody (Fig. 3B, right panel). No reacting products were found in the absence of Tax (Fig. 3B, lanes 1), showing the high specificity of the Ni-NTA pulldown in this experiment. High-molecular-weight Tax products were still observed in cells expressing the Tax-6His K1-3R (Fig. 3B, left panel, lane 4), although their level was slightly reduced compared to the wild-type protein (Fig. 3B, lane 2). By contrast, modified Tax species were almost absent in cells producing the lysineless Tax-6His K1-10R (Fig. 3B, lane 3) or the Tax-6His K4-10R (Fig. 3B, lane 5) mutant, and a significant reduction was found in cells expressing Tax-6His K6-8R (Fig. 3B, lane 6).
To precisely characterize the degree of ubiquitination of each protein, same lysates were blotted with the anti-ubiquitin antibody, and band quantitation was performed after subtraction of the background (Fig. 3C, lane 1) and normalization to equal amount of the unmodified 40 kDa species (Fig. 3C). The level of modified ubiquitinated Tax species found for the wild type was arbitrarily defined as 100% (Fig. 3C). This procedure revealed that the amount of ubiquitinated Tax products was reduced by 95% for the lysineless Tax protein (K1-10R). Mutation of the first three lysines led to a minor reduction of the amount of ubiquitinated Tax products (28%), whereas mutation of the seven other lysines (K4-10R) reduced Tax ubiquitination by 78%. Remarkably, a comparable reduction (76%) was found when mutation of only lysines 6, 7, and 8 was performed. That the levels of ubiquitinated products (Fig. 3B, right panel) but not the amounts of high-molecular-weight Tax species (Fig. 3B, left panel) were comparable between Tax K4-10R and Tax K6-8R might signify that lysines other than K6 to K8 are the targets of additional posttranslational modifications.
Collectively, these experiments indicate that Tax ubiquitination occurs principally on the C-terminal half of Tax, even though the N-terminal part is also involved to a lesser extent. Moreover, they strongly suggest that lysines 263, 280, and/or 284 are the main target of ubiquitin within the C-terminal half of Tax.
Integrity of lysine residues at position 263, 280, and/or 284 is required for interaction with assembled proteasomes.
Tax has been shown to interact with subunits of the proteasome in vitro and with assembled proteasomes in cells (3, 17, 30). Since polyubiquitination is a major signal to address proteins towards the proteasome (35), we wondered whether Tax ubiquitination participates to this interaction. We then investigated the ability to associate with proteasomes of the wild-type protein and the mutants that are almost not (Tax K1-10R) or poorly (Tax K4-10R, Tax K6-8R) ubiquitinated. To avoid any nonspecific binding due to polyhistidine sequences, these experiments were performed with untagged Tax proteins.
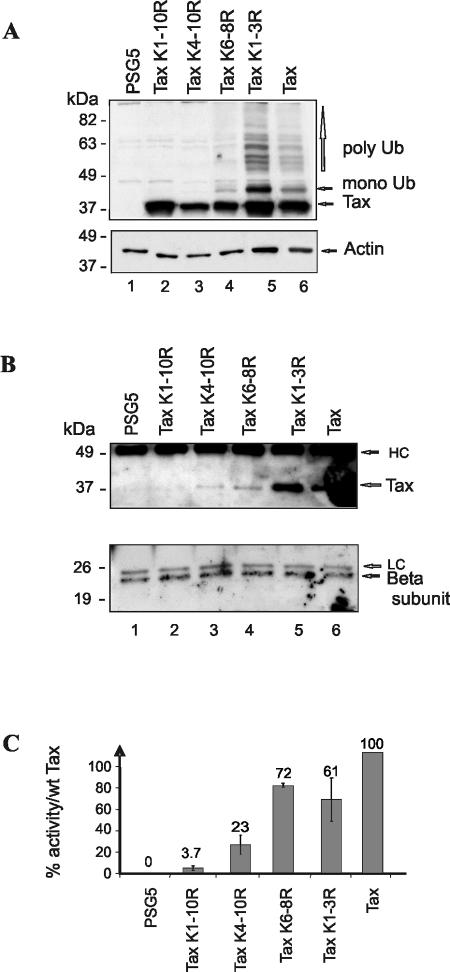
Consistent with our above results, blotting with patient sera showed various amounts of high-molecular-weight Tax products in cells transfected with the wild-type Tax plasmid and in those producing the lysine mutants (Fig. 4A). Indeed, compared to wild-type Tax (Fig. 4A, line 6) only background was detected for the Tax K1-10R and K4-10R proteins (Fig. 4A, lanes 2 and 3, upper panel) and fewer modified products were seen for the Tax K6-8R protein (Fig. 4A, lane 4). When adjusted to the amounts of loaded proteins (Fig. 4A, actin levels in lower panel), the level of high-molecular-weight Tax products seemed comparable between the Tax K1-3R mutant (Fig. 4A, lane 5) and wild-type Tax (Fig. 4A, lane 6).
FIG. 4.
Lysines 263, 280, and/or 284 are required for interaction with assembled proteasomes. 293T cells were transfected with the control pSG5 plasmid (lane 1) or with plasmids encoding Tax K1-10R (lane 2), Tax K4-10R (lane 3), Tax K6-8R (lane 4), Tax K1-3R (lane 5), and wild-type (wt) Tax (lane 6). (A) An aliquot of cell extract was subjected to direct immunoblot with patient sera (upper panel) and antiactin (lower panel). (B) Equal amounts of total proteins were immunoprecipitated with the antiproteasome antibody MCP21 and revealed by blotting with patient sera (upper panel) or an antibody specific for a proteasome β-subunit (lower panel). The positions of heavy chains (HC) and light chains (LC) revealed by the secondary antibody are indicated. (C) 293T cells were cotransfected with an HTLV-1 long terminal repeat-β-galactosidase reporter plasmid and a plasmid encoding wild-type or mutated Tax protein. The activity of the wild-type protein was arbitrarily set to 100% (wild-type induction = 18-fold).
Lysates were then subjected to immunoprecipitation with a monoclonal antibody recognizing the α2 subunit of the proteasome, known to immunoprecipitate both free subunits and assembled proteasomes (28). Recovered proteins reacted with an antibody directed to a β subunit of proteasomes (Fig. 4B, lower panel), showing, first, that not only single α subunits but assembled α/β complexes have been indeed precipitated and, second, that their amounts were comparable in each condition. Blotting with patient sera showed that both the wild-type Tax (Fig. 4B, upper panel, lane 6) and the Tax K1-3R (Fig. 4B, upper panel, lane 5) proteins were present in assembled proteasome immunoprecipitates. By contrast, the lysineless Tax K1-10R protein was not visible (Fig. 4B, upper panel, lane 2), and weak signals were detected for the C-terminal Tax K4-10R (Fig. 4B, upper panel, lane 3) and Tax K6-8R (Fig. 4B, upper panel, lane 4) mutants, which could be related to their residual ubiquitination (20%) (Fig. 3C).
Decreased association with proteasomes could have reflected a global defect in protein folding due to lysine mutations. To address this point, we evaluated the ability of the Tax mutants to activate the HTLV-1 promoter in cells, since this pathway necessitates the conservation of multiple properties of Tax, including the ability to enter nuclei, to dimerize, and to recruit transcriptional cellular cofactors (4). As shown in Fig. 4C, Tax K1-10R was unable to activate the HTLV-1 promoter. By contrast, the Tax K4-10R and Tax K6-8R proteins retained 23 and 72% of the activity of the wild-type protein, respectively. Hence, even though conservative substitution of lysine residues was performed, the possibility that Tax K1-10R and, to a lesser extent, Tax K4-10R exhibit a structural defect cannot be ruled out. By contrast, for at least the Tax K6-8R protein, the strong decrease in proteasome binding cannot be attributed to a global defect in folding or function. Therefore, we concluded that a correlation exists between ubiquitination of Tax on lysines 263, 280, and/or 284 and proteasome binding.
Level of Tax ubiquitination correlates with binding to assembled proteasomes.
That some lysines of Tax targeted by ubiquitin were also required for association with proteasomes suggested that Tax ubiquitination is related to proteasome binding.
To explore this hypothesis, we first investigated whether the ubiquitin-Tax fusion protein associates with proteasomes. Following proteasome immunoprecipitation as above, the ubiquitin-Tax protein was indeed detected (Fig. 5A, lane 1, upper panel). Moreover, in this experiment, in which the amount of associated Tax proteins was particularly high, we also detected the mono-ubiquitinated form of wild-type Tax associated to proteasomes (Fig. 5A, lane 3). However, we noticed that the unconjugated form of Tax was also bound to proteasomes. Nevertheless, these results demonstrate that the monoubiquitinated species of Tax is stably attached to assembled proteasomes in cells.
FIG. 5.
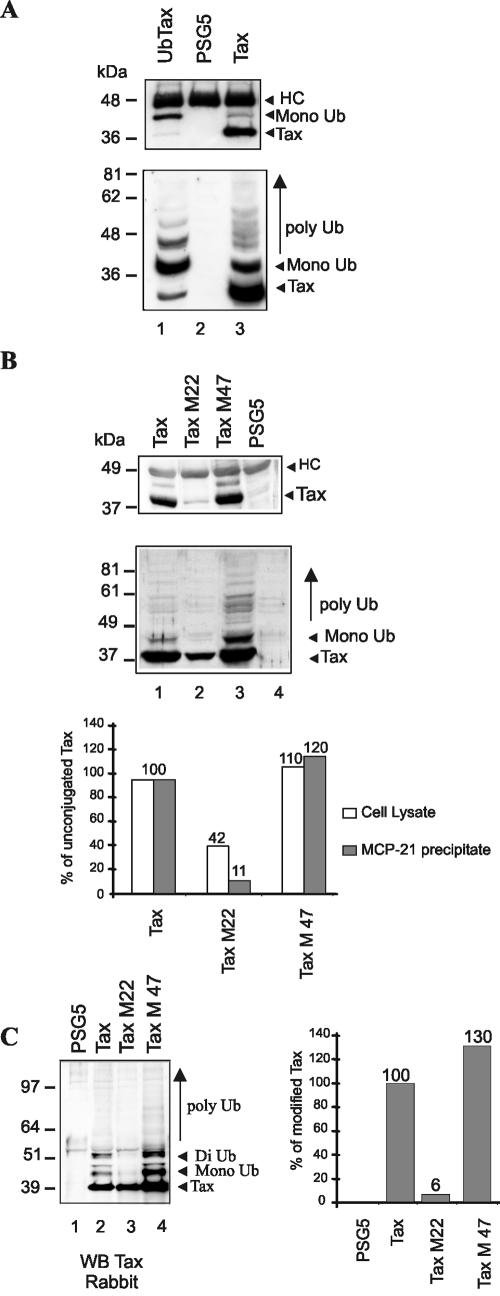
Level of Tax ubiquitination correlates with binding to assembled proteasomes. (A) 293T cells were transfected with the ubiquitin-Tax expression plasmid (lane 1), the control pSG5 plasmid (lane 2), or the plasmid encoding wild-type Tax (lane 3). Equal amounts of total proteins immunoprecipitated with the antiproteasome antibody MCP21 were revealed with patient sera (upper panel), and an aliquot of cell extract was subjected to direct immunoblot with patient sera (lower panel). (B) 293T cells were transfected with plasmids encoding wild-type Tax (lane 1), M22 (lane 2), or M47 (lane 3) protein or the control pSG5 plasmid (lane 4). Proteins from MCP21 precipitates (upper panel) or from cell extracts (middle panel) were revealed with patient sera, and the amount of unconjugated Tax proteins found in each blot was quantified, the level found for the wild-type protein being defined as 100% (lower panel). (C) 293T cells were transfected with control plasmid pSG5 (lane 1) or with plasmids encoding Tax-6His (lane 2), Tax-6His-M22 (lane 3), or Tax-6His-M47 (lane 4). Proteins purified by Ni-NTA pulldown were subjected to Western blot with a rabbit serum to Tax (left panel), and band quantitation was performed to evaluate the fraction of high-molecular-weight Tax products relative to the unconjugated form after subtraction of the background in lane 1 (right panel).
Tax mutants for which the in vitro interaction with subunits α3 and/or β7 of the proteasome core is impaired (M22, T131A/L131S) or strengthened (M47, L319R/L320S) (Fig. 5B) were described previously (30). These two proteins possess wild-type lysine residues, allowing us to explore the relationship between ubiquitination and proteasome binding in a context in which ubiquitin conjugation is still possible. Coimmunoprecipitations were performed as above. Blotting of MCP21-precipitated products with patient sera (Fig. 5B, upper panel) allowed the detection of Tax (lane 1) and M47 (lane 3), whereas only a weak signal was found for M22 (lane 2). Since the amount of Tax proteins in cell lysates seemed lower for M22 than for wild-type Tax and M47 (Fig. 5B, middle panel), we quantified the amount of the 40-kDa proteins in both experiments to calculate the percentage of proteins bound to the proteasome (lower panel). The level of 40-kDa Tax species found in cell lysates and proteasome precipitates was comparable between wild-type Tax and M47. The level of M22 was 42% of the wild-type protein in cell lysate for 11% in proteasome precipitate. This shows that the binding of M22 to the proteasome was reduced by more than 70% compared to the wild-type Tax and M47 proteins.
Detection of Tax proteins in cell lysates (Fig. 5B, middle panel) also suggested that compared to wild-type Tax (lane 2), the level of modified Tax products was lower for M22 (lane 3) and comparable or even increased for M47 (lane 4). To precisely assess the ubiquitination status of the M22 and M47, the corresponding mutations were introduced into the Tax-6His plasmid. Blotting of Ni-NTA-purified proteins with the anti-Tax rabbit antibody (Fig. 5C, left panel) showed that high-molecular-weight Tax products were found in both Tax- (lane 2) and M47- (lane 4) transfected cells. No such products were found for M22 (lane 3), although the level of the nonconjugated band was equivalent to that of the wild-type protein. Quantitation of higher Tax products (Fig. 5C, right panel) indeed evidenced a 94% reduction for M22 and a 30% increase for M47. This strengthened the correlation between the amount of Tax found in association with the proteasome and the level of Tax ubiquitination.
Proteasome inhibition does not result in Tax stabilization.
Since polyubiquitination is a signal for rapid degradation by proteasomes, ubiquitinated substrates are generally detected in cells only if proteasome activities are inhibited. In contrast, our data clearly show that ubiquitinated Tax products can be recovered from cells not treated with proteasome inhibitors. This suggested that ubiquitinated Tax products were stable in cells and therefore that they were not rapidly degraded by proteasomes.
To test this model, we studied the effect of proteasome inhibition on the intracellular level of wild-type Tax by treating transfected cells with either MG-132 or lactacystin, two potent proteasome inhibitors.
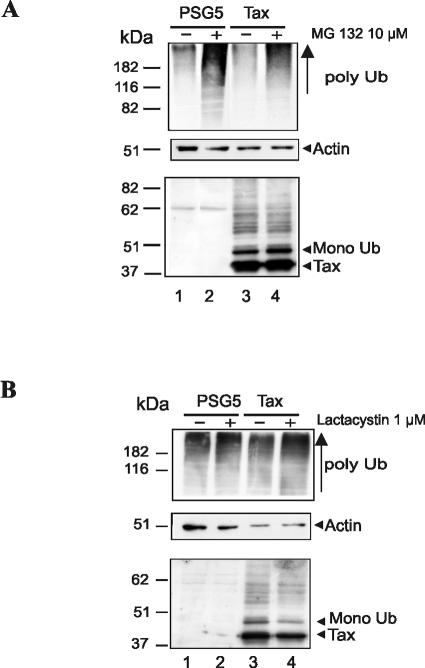
Detection of the pool of polyubiquitinated cellular proteins that accumulated as the result of free ubiquitin depletion is a common way to document proteasome inhibition (20, 24). Blots performed with the specific anti-polyubiquitin antibody FK2 (Fig. 6A and B, upper panels) showed that polyubiquitinated proteins were significantly increased in both MG-132- and lactacystin-treated cells, proving that proteasomes were efficiently inhibited in each case. Actin detection showed that the quantity of protein loaded was comparable in Tax-producing cells treated or not with either inhibitor (Fig. 6A and B, middle panels). Blotting with patient sera further evidenced that the global amount of Tax proteins was unaffected by treatment with either MG-132 (Fig. 6A, lower panel) or lactacystin (Fig. 6B, lower panel). Indeed, no stabilization of either the unconjugated form or ubiquitinated products was found. This reveals that ubiquitinated Tax products are not degraded by the proteasomes.
FIG. 6.
Proteasome inhibition does not result in Tax stabilization. 293T cells were transfected with control plasmid pSG5 (lanes 1 and 2) or a plasmid encoding Tax (lanes 3 and 4). (A) At 12 h posttransfection, cells were treated with dimethyl sulfoxide (−) or 10 μM MG-132 (+) for 5 h and then directly lysed in boiling Laemmli buffer. Equal amounts of total proteins were blotted with the anti-polyubiquitinated protein monoclonal antibody FK2 (upper panel), an antiactin monoclonal antibody (middle panel), or patient sera (lower panel). (B) A similar experiment was performed by treating cells with dimethyl sulfoxide (−) or 1 μM lactacystin for 20 h (+).
DISCUSSION
The ubiquitin-proteasome system is a central pathway for regulating protein stability in eukaryotic cells (19). Here we show that the Tax oncoprotein of HTLV-1 is ubiquitinated and that this process involves principally lysine residues located in the C-terminal part of the protein. We also provide evidence that Tax ubiquitination is related to proteasome binding but not to rapid degradation. This reveals a novel posttranslational modification undergone by Tax and opens new issues concerning the intracellular maturation and regulation of this protein.
Since Tax is the major target of the cytotoxic T-cell response to HTLV-1 and interacts with the proteasome, we wondered whether it was ubiquitinated. Ubiquitinated proteins are generally difficult to detect in cells, either because they are rapidly degraded by proteasomes or because conjugated ubiquitin is rapidly removed from substrates. To overcome this difficulty, we first used lysis buffers containing both proteasome inhibitors and inhibitors of deubiquitination. This allowed us to detect high-molecular-weight Tax products in transfected cells and, more importantly, in Tax-producing T lymphocytes.
Using purified Tax-6His proteins and Ni-NTA purification in highly denaturing conditions, we further demonstrated that purified Tax products reacted with anti-ubiquitin antibodies. Products ranging from 48 to more than 80 kDa were detected, consistent with conjugation of 1 to more than 5 ubiquitin molecules. Moreover, ubiquitinated forms were quantitatively important, since in our experiments, both mono- and polyubiquitinated forms represented 54% of the amount of total Tax species and increased to 72% in the presence of overexpressed ubiquitin. From these data, we concluded that Tax is both mono- and polyubiquitinated in transfected cells as well as in T lymphocytes, which represent the major target cells of HTLV-1.
Also in this issue, Peloponese and colleagues (27a) show data also demonstrating that Tax is ubiquitinated, although they mainly detected the monoubiquitinated form. That we found a larger amount of polyubiquitinated Tax species is probably due to the maximal denaturing conditions that we used in the Ni-NTA pulldown, which completely inhibited deubiquitination of substrates. However, these complementary data conclusively demonstrated that Tax is mainly present in cells as ubiquitinated species, although it remains possible that Tax proteins bearing additional posttranslational modifications also exist in cells.
Although ubiquitination occurs principally on lysine residues, recent studies demonstrated that lysineless substrates can be ubiquitinated on their N-terminal amino acid (10). Quantitation of anti-ubiquitin-reactive Tax products showed that mutation of all the lysine residues of Tax reduced the amount of total ubiquitination to 5%. Hence, classical conjugation on lysine residues seems to account for Tax ubiquitination.
With various Tax-6His mutants and direct detection of ubiquitin on Ni-NTA-purified Tax proteins, we further documented that Tax ubiquitination occurs mainly on its C-terminal half. Indeed, mutation of N-terminal lysines K1 to K3 reduced ubiquitination by 28% whereas mutation of the C-terminal lysines K4 to K10 reduced the amount of ubiquitinated Tax species by 80%. Remarkably, the restricted mutation of lysines K6 to K8 (residues 263, 280, and 284) reduced total Tax ubiquitination to a similar extent, indicating that they are the principal targets of ubiquitin within the C-terminal part of Tax. Interestingly, we noticed that although their levels of ubiquitination were comparable, the amount of high-molecular-weight Tax products was higher for Tax K4-10R than for Tax K6-8R. This might indicate that additional posttranslational modifications occur on lysine residues other than K6 to K8 within the C-terminal part of Tax. Otherwise, since the interaction site between the substrate and E3 is suspected to be located near the lysines targeted by ubiquitin (39), it might be assumed that the 260 to 290 region of Tax represents the binding site for the putative E3. However, the Tax M22 mutant (Thr130-Leu131 → Ala-Ser), which has wild-type lysines, is only poorly ubiquitinated, suggesting that the central domain of Tax might also be involved in such an interaction. Further studies are needed to identify this ubiquitin ligase and the molecular mechanisms involved in this reaction.
Polyubiquitination of proteins is a major signal for interaction with proteasomes (35). Since Tax has been shown to interact with proteasomes both in vitro and in cells, we investigated whether Tax ubiquitination influences this phenomenon. We first found that the ubiquitin-Tax fusion protein and the monoubiquitinated form of wild-type Tax were stably attached to proteasomes. Furthermore, we obtained data revealing a reciprocal correlation between Tax ubiquitination and proteasome binding. On the one hand, we found that all the Tax mutants that are not or very poorly ubiquitinated (Tax K1-10R, Tax K4-10R, and Tax K6-8R) display a complete or strong reduction in proteasome binding. Importantly, at least for the Tax K6-8R protein, such a reduction cannot be attributed to a global structural defect because this mutant was still functional for transactivation of the HTLV-1 promoter. Conversely, mutation of the lysine residues located in the N-terminal part of Tax, which minimally contribute to Tax ubiquitination (28%), did not affect proteasome binding. On the other hand, we showed that the M22 mutant, known to be impaired for proteasomes binding (30), was also poorly ubiquitinated, whereas the M47 protein, which still interacts with the proteasome, was properly and even slightly overubiquitinated compared to the wild-type protein. These findings suggest either that Tax binding to proteasomes is required for its ubiquitination or that prior ubiquitination of Tax is necessary for stable attachment to assembled proteasomes. We favor the second hypothesis because Tax mutants defective for ubiquitination were unable to bind to proteasomes.
However, we noticed that unconjugated Tax was the major form attached to proteasomes. Ubiquitinated products are very unstable, and because coimmunoprecipitation cannot be performed in sufficiently denaturing conditions, partial deubiquitination during the immunoprecipitation procedure is likely to occur. Alternatively, it can be envisaged that Tax is partially deubiquitinated within cells upon proteasome binding. Both hypotheses could explain why multiubiquitinated proteins were not found in proteasome precipitates (not show). Another explanation is that ubiquitination is involved in a chain of events preceding binding to α/β subunits and then contributes indirectly to the stable attachment of Tax on proteasome cores.
That Tax ubiquitination is required for proteasome binding could also signify that Tax interacts specifically with the polyubiquitin chain receptors included in the regulatory caps of proteasomes (38, 40). Our findings also suggest that the respective affinities for proteasomes of M22 and M47 proteins might be related to their ubiquitination status. However, the relative binding abilities of these mutants were evidenced in a yeast two-hybrid system with isolated subunits of the proteasome core and not with components of the regulatory caps (30). One explanation could be that Tax associates both with the regulatory cap of the proteasome and with subunits of the core and that both interactions contribute to stable Tax binding to assembled proteasomes.
Polyubiquitinated proteins are generally rapidly degraded and, in consequence, are difficult to detect in cells containing active proteasomes. In contrast, we found that ubiquitinated Tax products were readily detectable in cells not treated with proteasome inhibitors. Furthermore, we found that neither the steady-state level of unconjugated Tax nor the amount of ubiquitinated products was affected by proteasome inhibitor treatment. Similar results are presented in the accompanying paper from Jeang's group (27a). This strongly supports the view that ubiquitinated Tax molecules are not major targets for proteasome degradation, although it remains possible that a minor fraction of Tax can be degraded via this pathway. Hence, Tax ubiquitination does not seem to mediate a direct proteolytic function. Since, as we showed here, Tax ubiquitination contributes to proteasome binding, it might be postulated that Tax attachment to the proteasome is responsible for protein stabilization. Therefore, we propose a model in which the stable attachment of Tax to subunits of the proteasome core precludes its entry into the catalytic cylinder, thereby preventing its degradation. Tax transfer from the cap to the core implies that Tax is not degraded via the proteasome or that the degradation is only delayed. This second hypothesis could explain the apparent discrepancy between the fact that Tax does not seem to be massively degraded by the proteasome and its immunodominance in terms of cytotoxic T-lymphocyte recognition.
In conclusion, we identified a new posttranslational modification of Tax that contributes to its ability to bind to proteasomes. The impact of this mechanism on the numerous functions of Tax remains to be clarified. In this regard, Jeang's group (27a) reports that Tax ubiquitination is associated with reduced transactivation. Interestingly, a recent study showed that the human immunodeficiency virus transactivator Tat protein is also ubiquitinated and that this conjugation mediates a nonproteolytic function (8). Hence, ubiquitination could constitute a novel general mechanism to regulate the intracellular fate and/or function of various retroviral transcriptional transactivators.
Acknowledgments
We thank R. Mahieux, A. Saïb, and V. Blot for helpful discussions and B. Papp and D. Ghez for critical reading of the manuscript. We also thank A. M. Poorter for plasmid preparation, H. Bui (CEPH) for plasmid sequencing, and E. Savariau (LPH) for the design of figures.
This work was supported by the Association de Recherche contre le Cancer (ARC) and the Fondation contre la Leucémie (Fondation de France), the Belgian Fonds National de la Recherche Scientifique, Télévie and the International Brachet Stiftung. E.C. is the recipient of a Fellowship from the French Ministry of Research (MENRT), and I.L. is the recipient of a grant from the Belgian Foundation for Research in Industry and Agriculture (FRIA).
REFERENCES
- 1.Alefantis, T., K. Barmak, E. W. Harhaj, C. Grant, and B. Wigdahl. 2003. Characterization of a nuclear export signal within the human T-cell leukemia virus type I transactivator protein Tax. J. Biol. Chem. 278:21814-21822. [DOI] [PubMed] [Google Scholar]
- 2.Astier-Gin, T., J. P. Portail, F. Lafond, and B. Guillemain. 1995. Identification of HTLV-1- or HTLV-1I-producing cells by cocultivation with BHK-21 cells stably transfected with a long terminal repeat-lacZ gene construct. J. Virol. Methods 51:19-29. [DOI] [PubMed] [Google Scholar]
- 3.Beraud, C., and W. C. Greene. 1996. Interaction of HTLV-1 Tax with the human proteasome: implications for NF-kappa B induction. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13(Suppl. 1):S76-S84. [DOI] [PubMed] [Google Scholar]
- 4.Bex, F., and R. B. Gaynor. 1998. Regulation of gene expression by HTLV-1 Tax protein. Methods 16:83-94. [DOI] [PubMed] [Google Scholar]
- 5.Bex, F., K. Murphy, R. Wattiez, A. Burny, and R. B. Gaynor. 1999. Phosphorylation of the human T-cell leukemia virus type 1 transactivator tax on adjacent serine residues is critical for tax activation. J. Virol. 73:738-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhat, N. K., Y. Adachi, K. P. Samuel, and D. Derse. 1993. HTLV-1 gene expression by defective proviruses in an infected T-cell line. Virology 196:15-24. [DOI] [PubMed] [Google Scholar]
- 7.Bieganowska, K., P. Hollsberg, G. J. Buckle, D. G. Lim, T. F. Greten, J. Schneck, J. D. Altman, S. Jacobson, S. L. Ledis, B. Hanchard, J. Chin, O. Morgan, P. A. Roth, and D. A. Hafler. 1999. Direct analysis of viral-specific CD8+ T cells with soluble HLA-A2/Tax11-19 tetramer complexes in patients with human T-cell lymphotropic virus-associated myelopathy. J. Immunol. 162:1765-1771. [PubMed] [Google Scholar]
- 8.Bres, V., R. E. Kiernan, L. K. Linares, C. Chable-Bessia, O. Plechakova, C. Treand, S. Emiliani, J. M. Peloponese, K. T. Jeang, O. Coux, M. Scheffner, and M. Benkirane. 2003. A non-proteolytic role for ubiquitin in Tat-mediated transactivation of the HIV-1 promoter. Nat. Cell Biol. 5:754-761. [DOI] [PubMed] [Google Scholar]
- 9.Brooks, P., G. Fuertes, R. Z. Murray, S. Bose, E. Knecht, M. C. Rechsteiner, K. B. Hendil, K. Tanaka, J. Dyson, and J. Rivett. 2000. Subcellular localization of proteasomes and their regulatory complexes in mammalian cells. Biochem. J. 346:155-161. [PMC free article] [PubMed] [Google Scholar]
- 10.Ciechanover, A., and R. Ben-Saadon. 2004. N-terminal ubiquitination: more protein substrates join in. Trends Cell Biol. 14:103-106. [DOI] [PubMed] [Google Scholar]
- 11.Ecker, D. J., J. M. Stadel, T. R. Butt, J. A. Marsh, B. P. Monia, D. A. Powers, J. A. Gorman, P. E. Clark, F. Warren, A. Shatzman, et al. 1989. Increasing gene expression in yeast by fusion to ubiquitin. J. Biol. Chem. 264:7715-7719. [PubMed] [Google Scholar]
- 12.Ferdous, A., F. Gonzalez, L. Sun, T. Kodadek, and S. A. Johnston. 2001. The 19S regulatory particle of the proteasome is required for efficient transcription elongation by RNA polymerase II. Mol. Cell 7:981-991. [DOI] [PubMed] [Google Scholar]
- 13.Gatza, M. L., J. C. Watt, and S. J. Marriott. 2003. Cellular transformation by the HTLV-1 Tax protein, a jack-of-all-trades. Oncogene 22:5141-5149. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez, F., A. Delahodde, T. Kodadek, and S. A. Johnston. 2002. Recruitment of a 19S proteasome subcomplex to an activated promoter. Science 296:548-550. [DOI] [PubMed] [Google Scholar]
- 15.Grant, C., K. Barmak, T. Alefantis, J. Yao, S. Jacobson, and B. Wigdahl. 2002. Human T-cell leukemia virus type I and neurologic disease: events in bone marrow, peripheral blood, and central nervous system during normal immune surveillance and neuroinflammation. J. Cell Physiol. 190:133-159. [DOI] [PubMed] [Google Scholar]
- 16.Hanon, E., S. Hall, G. P. Taylor, M. Saito, R. Davis, Y. Tanaka, K. Usuku, M. Osame, J. N. Weber, and C. R. Bangham. 2000. Abundant tax protein expression in CD4+ T cells infected with human T-cell lymphotropic virus type I (HTLV-1) is prevented by cytotoxic T lymphocytes. Blood 95:1386-1392. [PubMed] [Google Scholar]
- 17.Hemelaar, J., F. Bex, B. Booth, V. Cerundolo, A. McMichael, and S. Daenke. 2001. Human T-cell leukemia virus type 1 Tax protein binds to assembled nuclear proteasomes and enhances their proteolytic activity. J. Virol. 75:11106-11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendil, K. B., P. Kristensen, and W. Uerkvitz. 1995. Hum. proteasomes analysed with monoclonal antibodies. Biochem. J. 305:245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425-479. [DOI] [PubMed] [Google Scholar]
- 20.Hu, Z., Z. Zhang, E. Doo, O. Coux, A. L. Goldberg, and T. J. Liang. 1999. Hepatitis B virus X protein is both a substrate and a potential inhibitor of the proteasome complex. J. Virol. 73:7231-7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeang, K. T. 2001. Functional activities of the human T-cell leukemia virus type I Tax oncoprotein: cellular signaling through NF-kappa B. Cytokine Growth Factor Rev. 12:207-217. [DOI] [PubMed] [Google Scholar]
- 22.Kannagi, M., S. Harada, I. Maruyama, H. Inoko, H. Igarashi, G. Kuwashima, S. Sato, M. Morita, M. Kidokoro, M. Sugimoto, and et al. 1991. Predominant recognition of human T-cell leukemia virus type I (HTLV-1) pX gene products by human CD8+ cytotoxic T cells directed against HTLV-1-infected cells. Int. Immunol. 3:761-767. [DOI] [PubMed] [Google Scholar]
- 23.Kubota, R., T. Kawanishi, H. Matsubara, A. Manns, and S. Jacobson. 2000. HTLV-1 specific IFN-gamma+ CD8+ lymphocytes correlate with the proviral load in peripheral blood of infected individuals. J. Neuroimmunol. 102:208-215. [DOI] [PubMed] [Google Scholar]
- 24.Leitch, V., P. Agre, and L. S. King. 2001. Altered ubiquitination and stability of aquaporin-1 in hypertonic stress. Proc. Natl. Acad. Sci. USA 98:2894-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin, M. C., S. M. Lee, F. Kalume, Y. Morcos, F. C. Dohan, Jr., K. A. Hasty, J. C. Callaway, J. Zunt, D. Desiderio, and J. M. Stuart. 2002. Autoimmunity due to molecular mimicry as a cause of neurological disease. Nat. Med. 8:509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lomas, M., E. Hanon, Y. Tanaka, C. R. Bangham, and K. G. Gould. 2002. Presentation of a new H-2D(k)-restricted epitope in the Tax protein of human T-lymphotropic virus type I is enhanced by the proteasome inhibitor lactacystin. J. Gen. Virol. 83:641-650. [DOI] [PubMed] [Google Scholar]
- 27.Nasr, R., A. Rosenwald, M. E. El-Sabban, B. Arnulf, P. Zalloua, Y. Lepelletier, F. Bex, O. Hermine, L. Staudt, H. de The, and A. Bazarbachi. 2003. Arsenic/interferon specifically reverses 2 distinct gene networks critical for the survival of HTLV-1-infected leukemic cells. Blood 101:4576-4582. [DOI] [PubMed] [Google Scholar]
- 27a.Peloponese, J. M., Jr., H. Iha, V. R. K. Yedavalli, Akiko Miyazato, Y. Li, K. Haller, M. Benkirane, and K.-T. Jeang. 2004. Ubiquitination of human T-cell leukemia virus type 1 Tax modulates its activity. J. Virol. 78:11686-11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reits, E. A., A. M. Benham, B. Plougastel, J. Neefjes, and J. Trowsdale. 1997. Dynamics of proteasome distribution in living cells. EMBO J. 16:6087-6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rock, K. L., C. Gramm, L. Rothstein, K. Clark, R. Stein, L. Dick, D. Hwang, and A. L. Goldberg. 1994. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78:761-771. [DOI] [PubMed] [Google Scholar]
- 30.Rousset, R., C. Desbois, F. Bantignies, and P. Jalinot. 1996. Effects on NF-kappa B1/p105 processing of the interaction between the HTLV-1 transactivator Tax and the proteasome. Nature 381:328-331. [DOI] [PubMed] [Google Scholar]
- 31.Slamon, D. J., K. Shimotohno, M. J. Cline, D. W. Golde, and I. S. Chen. 1984. Identification of the putative transforming protein of the human T-cell leukemia viruses HTLV-1 and HTLV-1I. Science 226:61-65. [DOI] [PubMed] [Google Scholar]
- 32.Smith, M. R., and W. C. Greene. 1992. Characterization of a novel nuclear localization signal in the HTLV-1 tax transactivator protein. Virology 187:316-320. [DOI] [PubMed] [Google Scholar]
- 33.Smith, M. R., and W. C. Greene. 1990. Identification of HTLV-1 tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 4:1875-1885. [DOI] [PubMed] [Google Scholar]
- 34.Sodroski, J., C. Rosen, W. C. Goh, and W. Haseltine. 1985. A transcriptional activator protein encoded by the x-lor region of the human T-cell leukemia virus. Science 228:1430-1434. [DOI] [PubMed] [Google Scholar]
- 35.Thrower, J. S., L. Hoffman, M. Rechsteiner, and C. M. Pickart. 2000. Recognition of the polyubiquitin proteolytic signal. EMBO J. 19:94-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uchiyama, T. 1997. Human T-cell leukemia virus type I (HTLV-1) and human diseases. Annu. Rev. Immunol. 15:15-37. [DOI] [PubMed] [Google Scholar]
- 37.Voges, D., P. Zwickl, and W. Baumeister. 1999. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu. Rev. Biochem. 68:1015-1068. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson, C. R., M. Seeger, R. Hartmann-Petersen, M. Stone, M. Wallace, C. Semple, and C. Gordon. 2001. Proteins containing the UBA domain are able to bind to multi-ubiquitin chains. Nat. Cell Biol. 3:939-943. [DOI] [PubMed] [Google Scholar]
- 39.Wu, G., G. Xu, B. A. Schulman, P. D. Jeffrey, J. W. Harper, and N. P. Pavletich. 2003. Structure of a beta-TrCP1-Skp1-beta-catenin complex: destruction motif binding and lysine specificity of the SCF(beta-TrCP1) ubiquitin ligase. Mol. Cell 11:1445-1456. [DOI] [PubMed] [Google Scholar]
- 40.Xie, Y., and A. Varshavsky. 2000. Physical association of ubiquitin ligases and the 26S proteasome. Proc. Natl. Acad. Sci. USA 97:2497-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]