Abstract
A variety of host factors, including membrane proteins acquired by human immunodeficiency virus type 1 (HIV-1), play a dominant role in HIV-1 adsorption onto host cells. Examples include the integrin intercellular adhesion molecule 1 (ICAM-1), which, once acquired by HIV-1, promotes virus infectivity via ligation to LFA-1. We tested the ability of statins to diminish HIV-1 replication, based on the idea that these compounds have been shown to block ICAM-1-LFA-1 interactions. Our data indicate that statins diminish HIV-1 attachment to target cells by suppressing ICAM-1-LFA-1 interactions. The capacity of statins to limit the initial steps in virus replication could represent an interesting approach for the treatment of HIV-1 infection.
Previous studies have provided evidence that intercellular adhesion molecule 1 (ICAM-1), when present on the surface of human immunodeficiency virus type 1 (HIV-1) particles, can enhance virus attachment through an association with its physiological counterreceptor LFA-1. This virus-cell interaction augments virus infectivity severalfold by facilitating binding and entry events (9), notably in human lymphoid tissue cultured ex vivo (3). Therefore, a strategy aimed at blocking this virus-cell interaction could represent a new therapy for the treatment of HIV-1 infection. This idea is supported by the observation that host-encoded ICAM-1 is acquired by all tested clinical strains of HIV-1 following production in primary human cells (2, 3, 5, 6, 11).
Statin compounds, the primary drugs used in the treatment of hypercholesterolemia, act as inhibitors of the rate-limiting enzyme of the cholesterol synthesis pathway, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (7). Interestingly, two members of this family, lovastatin and simvastatin, were recently shown to bind to LFA-1 and to inhibit its normal interaction with ICAM-1 (12, 18). The aim of the present study was to evaluate whether these two compounds can modulate HIV-1 production by affecting the earliest steps in the virus life cycle.
(This work was performed by J.-F.G. as partial fulfillment of a Ph.D. degree in the Microbiology-Immunology Program, Faculty of Medicine, Laval University.)
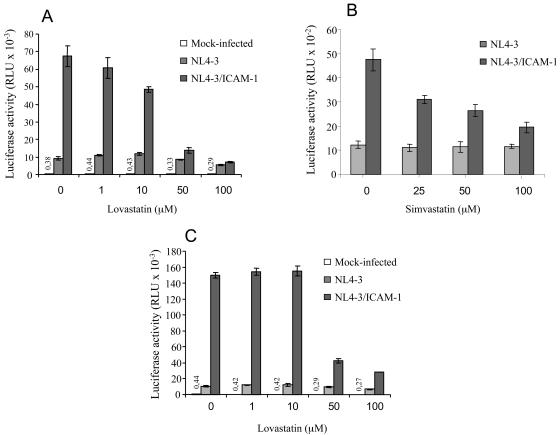
When an indicator cell line is used as the target, statin compounds reduce infection with ICAM-1-bearing viruses but not with virions lacking the host molecule.
To evaluate the possible anti-HIV-1 efficacy of lovastatin, the LFA-1+ reporter cell line LuSIV (17) was first pretreated with concentrations of 1 to 100 μM lovastatin (Mevinolin or MK-803; A. G. Scientific, Inc., San Diego, Calif.). The cells were next exposed to isogenic NL4-3 virus stocks, either bearing or lacking host ICAM-1, that were produced on 293T cells as described previously (9, 10). As expected, virus infectivity increased upon insertion of ICAM-1 within mature virions (6.5-fold) when infection was allowed to proceed in the absence of lovastatin (Fig. 1A). Interestingly, infection with ICAM-1-bearing viruses was reduced in a dose-dependent manner by lovastatin. The anti-HIV-1 activity of lovastatin seems to be linked to its capacity to inhibit LFA-1-ICAM-1 interactions, since replication of HIV-1 particles lacking ICAM-1 is affected much less by lovastatin. Simvastatin, another statin reported to block LFA-1-ICAM-1 interactions (18), was also tested by the same experimental procedure (simvastatin or MK733; Calbiochem, La Jolla, Calif.). Like lovastatin, simvastatin specifically blocks the infectivity of ICAM-1-bearing viral particles at similar concentrations (i.e., 50 and 100 μM) without affecting replication of NL4-3 devoid of ICAM-1 (Fig. 1B).
FIG. 1.
Statins decrease infection of LuSIV cells with ICAM-1-bearing virions but not with viruses lacking host ICAM-1. LuSIV reporter cells were first treated with lovastatin (A) or simvastatin (B) at the indicated concentrations at 37°C for 20 min. Next, target cells were inoculated with similar amounts of isogenic NL4-3 virus stocks either lacking (NL4-3) or bearing (NL4-3/ICAM-1) host-encoded ICAM-1 (10 ng of p24 for 105 cells). (C) An experimental strategy similar to that for panels A and B was used, except that cells were initially pretreated with the anti-LFA-1-activating antibody NKI-L16 for 30 min at 37°C (final concentration of 3 μg/ml) before the addition of lovastatin. Infection was allowed to proceed for 24 h before cells were lysed to monitor luciferase activity (MLX; Dynex Technologies, Chantilly, Va.). Luciferase activity is expressed in relative light units (RLU). Data shown represent the means ± standard deviations of results from triplicate samples, and these results are representative of three independent experiments. Luciferase activity values for mock-infected samples are shown on top of relevant bars.
LFA-1 can be found in two distinct conformational states, i.e., with low or high affinity for ICAM-1. The high-affinity state has been shown to further enhance the susceptibility of human cells to infection by ICAM-1-bearing HIV-1 particles (8, 10). We thus investigated whether the activation state of LFA-1 on the target cell surface could affect the anti-HIV-1 potency of lovastatin. To this end, LuSIV target cells were pretreated with an LFA-1-activating antibody (i.e., NKI-L16) before the addition of lovastatin and the studied virus stocks. As depicted in Fig. 1C, in spite of the higher level of infection with ICAM-1-bearing virions in cells expressing LFA-1 under an activated state, lovastatin preserves its capacity to decrease the infectivity of virions bearing host ICAM-1 on their surfaces.
HIV-1 replication and attachment to primary human cells are diminished by lovastatin via inhibition of ICAM-1-LFA-1 interactions.
The next step was to define whether lovastatin can modulate HIV-1 replication in a more natural cellular reservoir, i.e., primary human cells, such as peripheral blood mononuclear cells (PBMCs). The results show that lovastatin and MEM30, an anti-LFA-1 antibody known to block the interaction between LFA-1 and ICAM-1, decrease the replication of ICAM-1-bearing virions to similar levels (Fig. 2A). Replication of isogenic HIV-1 particles lacking host-encoded ICAM-1 was affected neither by lovastatin nor by MEM30. In order to shed light on the possible effect of lovastatin on the early events in the HIV-1 life cycle, the levels of cell-associated viruses were assessed after 2 h following incubation of PBMCs with the studied NL4-3-based virus preparations. Lovastatin is as efficient as MEM30 in inhibiting attachment of ICAM-1-bearing virions to the surfaces of primary human cells, while neither agent has an effect on the adsorption of viruses lacking ICAM-1 to PBMCs (Fig. 2B). Similar findings were obtained when PBMCs were pretreated with the LFA-1-activating antibody NKI-L16 (data not shown), thus confirming that the ability of lovastatin to block LFA-1-ICAM-1 interactions is not influenced by the conformational state of LFA-1.
FIG. 2.
Lovastatin efficiently abolishes replication and attachment of ICAM-1-bearing virions in primary human cells. (A) PBMCs were treated with lovastatin (50 μM) or the blocking anti-LFA-1 antibody MEM30 (1 μg/ml) for 20 min at 37°C before incubation for 2 h with viruses either lacking (NL4-3) or bearing (NL4-3/ICAM-1) host-encoded ICAM-1. Cells were extensively washed and resuspended in complete culture medium free of lovastatin or anti-LFA-1 antibody. Virus production was assessed with an in-house double-antibody sandwich enzyme-linked immunosorbent assay specific for the major viral p24 protein (3). (B) Pelleted cells were resuspended in lysis buffer (0.5% Triton X-100 in phosphate-buffered saline) to evaluate the amount of cell-associated viruses through p24 measurements. Data shown represent the means ± standard deviations of results from triplicate samples, and these results are representative of three independent experiments.
Replication of clinical isolates of HIV-1 in primary human cells is affected by treatment with lovastatin.
Previous studies demonstrated that clinical strains of HIV-1 produced in primary human cells do acquire host ICAM-1 (2, 3, 5, 6, 11). Therefore, to provide physiological relevance to the present data with statins, two dual-tropic field isolates of HIV-1 were expanded in PBMCs and used for infectivity studies of primary human cells. The kinetics of virus production was monitored by measuring p24 levels in cell-free culture supernatants at several time points following the initial infection. Replication of the two HIV-1 clinical variants decreased upon treatment with lovastatin (Fig. 3). The observed diminution of 92US151-virion production is comparable for lovastatin and MEM30 (Fig. 3A), whereas replication of 92RW009 was more severely diminished by lovastatin than by MEM30 (Fig. 3B).
FIG. 3.
Replication of two clinical variants of HIV-1 in primary human cells is reduced by lovastatin. PBMCs were treated with lovastatin (50 μM) or the anti-LFA-1 antibody MEM30 (1 μg/ml) for 20 min at 37°C. Target cells were next exposed to the dual-tropic HIV-1 clinical isolates 92US151 (A) and 92RW009 (B). After several washes, cell pellets were resuspended in complete culture medium, and p24 levels were evaluated in the culture supernatant at days 2, 4, and 6 postinfection. Data shown represent the means ± standard deviations of results from triplicate samples, and these results are representative of three independent experiments.
Different types of inhibitors have been developed to suppress ICAM-1-LFA-1 interactions, and some of these molecules are currently parts of clinical trials (e.g., antibodies, peptides, and small molecules) (reviewed in reference 1). We focused our efforts on lovastatin, a fungal metabolite that binds to the I domain of LFA-1 at the I-domain allosteric site. Lovastatin inhibits ICAM-1-LFA-1 interactions by preventing the conformational change of LFA-1 to the activated form via allosteric control (13). We found that lovastatin diminishes HIV-1 replication by inhibiting the interaction of virus-associated host ICAM-1 with its normal counterreceptor LFA-1. We made these observations when using lovastatin at concentrations higher than 10 μM, different strains of HIV-1 (laboratory and primary variants), and LFA-1-expressing lymphoid cells (LuSIV) and primary cells (PBMCs). Our results are in line with a previous study performed by Weitz-Schmidt and colleagues, who reported 50% inhibitory concentrations of 25 and 22 μM for lovastatin and simvastatin, respectively, in an assay measuring the adhesion of LFA-1-positive HUT78 cells to immobilized ICAM-1 (18). The propensity of statins to modify cholesterol synthesis cannot account for the antiviral properties of statins seen in this study, since infectivity of HIV-1 virions lacking host ICAM-1 is only slightly affected upon treatment with these compounds. This is an important issue, considering the importance of cholesterol and lipid rafts in the virus life cycle (reviewed in reference 4). Finally, the observation that the attachment of ICAM-1-bearing viruses, but not that of isogenic virions devoid of host ICAM-1, is reduced upon addition of statins provides direct proof that the mechanism of action of statins is by specifically targeting ICAM-1-LFA-1 interactions. Interestingly, lovastatin still efficiently reduces HIV-1 infection when target cells express the activated form of LFA-1. To the best of our knowledge, this represents the first demonstration that lovastatin can inhibit the interaction of ICAM-1 with LFA-1 under an active state. This last finding might be clinically relevant, given that lymphoid tissues constitute preferred sites for HIV-1 replication and propagation (reviewed in references 14 to 16) and contain a high number of cells susceptible to virus infection-expressing LFA-1 under an activated form, since 25 to 50% of the CD4+ T cells found in lymph nodes of HIV-1-infected individuals are in an activated state (15).
Although HMG-CoA reductase inhibitors such as statins are efficient at reducing the risk of coronary events and are generally very well tolerated, clinical studies have demonstrated that these drugs show very low systemic bioavailability, due to an extensive first-pass effect at the intestinal and/or hepatic level. The use of lovastatin or simvastatin as an inhibitor of HIV-1 replication is thus very unlikely, given that potentially toxic doses would have to be administered to achieve a plasmatic concentration sufficient to affect virus production. However, it must be noted that Weitz-Schmidt and coworkers recently developed a derivative compound of lovastatin (with a 50% inhibitory concentration of 0.7 μM) that does not inhibit HMG-CoA reductase activity (18). This new compound seems to block ICAM-1-LFA-1 interactions under in vivo conditions, since the migration of neutrophils was abolished upon oral administration in an animal model. It is thus tempting to speculate that newly designed statin compounds might be administered at doses sufficient to reach plasmatic concentrations that could reduce HIV-1 load in infected individuals.
Acknowledgments
We thank M. Dufour for technical assistance in flow cytometry studies. We are grateful to S. Méthot for editorial assistance, and we address special thanks to Caroline Côté for technical support.
J.-F.G. is the recipient of a Ph.D. fellowship from the “Fonds de la Recherche en Santé du Québec” (Réseau FRSQ SIDA et Maladies Infectieuses), and M.J.T. holds the Canada Research Chair in Human Immuno-Retrovirology (tier 1 level). This study was supported by a grant to M.J.T. from the Canadian Institutes of Health Research HIV/AIDS Research Program (grant HOP-14438).
REFERENCES
- 1.Anderson, M. E., and T. J. Siahaan. 2003. Targeting ICAM-1/LFA-1 interaction for controlling autoimmune diseases: designing peptide and small molecule inhibitors. Peptides 24:487-501. [DOI] [PubMed] [Google Scholar]
- 2.Bastiani, L., S. Laal, M. Kim, and S. Zolla-Pazner. 1997. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J. Virol. 71:3444-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bounou, S., J. E. Leclerc, and M. J. Tremblay. 2002. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4+-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J. Virol. 76:1004-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell, S. M., S. M. Crowe, and J. Mak. 2001. Lipid rafts and HIV-1: from viral entry to assembly of progeny virions. J. Clin. Virol. 22:217-227. [DOI] [PubMed] [Google Scholar]
- 5.Cantin, R., J.-F. Fortin, and M. J. Tremblay. 1996. The amount of host HLA-DR proteins acquired by HIV-1 is virus strain- and cell type-specific. Virology 218:372-381. [DOI] [PubMed] [Google Scholar]
- 6.Capobianchi, M. R., S. Fais, C. Castilletti, M. Gentile, F. Ameglio, and F. Dianzani. 1994. A simple and reliable method to detect cell membrane proteins on infectious human immunodeficiency virus type 1 particles. J. Infect. Dis. 169:886-889. [DOI] [PubMed] [Google Scholar]
- 7.Corsini, A., F. M. Maggi, and A. L. Catapano. 1995. Pharmacology of competitive inhibitors of HMG-CoA reductase. Pharmacol. Res. 31:9-27. [DOI] [PubMed] [Google Scholar]
- 8.Fortin, J. F., B. Barbeau, H. Hedman, E. Lundgren, and M. J. Tremblay. 1999. Role of the leukocyte function antigen-1 conformational state in the process of human immunodeficiency virus type 1-mediated syncytium formation and virus infection. Virology 257:228-238. [DOI] [PubMed] [Google Scholar]
- 9.Fortin, J.-F., R. Cantin, G. Lamontagne, and M. Tremblay. 1997. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 71:3588-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortin, J.-F., R. Cantin, and M. J. Tremblay. 1998. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM-1. J. Virol. 72:2105-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank, I., H. Stoiber, S. Godar, H. Stockinger, F. Steindl, H. W. Katinger, and M. P. Dierich. 1996. Acquisition of host cell-surface-derived molecules by HIV-1. AIDS 10:1611-1620. [DOI] [PubMed] [Google Scholar]
- 12.Kallen, J., K. Welzenbach, P. Ramage, D. Geyl, R. Kriwacki, G. Legge, S. Cottens, G. Weitz-Schmidt, and U. Hommel. 1999. Structural basis for LFA-1 inhibition upon lovastatin binding to the CD11a I-domain. J. Mol. Biol. 292:1-9. [DOI] [PubMed] [Google Scholar]
- 13.Liu, G., J. R. Huth, E. T. Olejniczak, R. Mendoza, P. DeVries, S. Leitza, E. B. Reilly, G. F. Okasinski, S. W. Fesik, and T. W. von Geldern. 2001. Novel p-arylthio cinnamides as antagonists of leukocyte function-associated antigen-1/intracellular adhesion molecule-1 interaction. 2. Mechanism of inhibition and structure-based improvement of pharmaceutical properties. J. Med. Chem. 44:1202-1210. [DOI] [PubMed] [Google Scholar]
- 14.Pantaleo, G., C. Graziosi, and A. S. Fauci. 1993. New concepts in the immunopathogenesis of human immunodeficiency virus infection. N. Engl. J. Med. 328:327-335. [DOI] [PubMed] [Google Scholar]
- 15.Pantaleo, G., C. Graziosi, and A. S. Fauci. 1993. The role of lymphoid organs in the immunopathogenesis of HIV infection. AIDS 7(Suppl. 1):S19-S23. [PubMed] [Google Scholar]
- 16.Pantaleo, G., C. Graziosi, and A. S. Fauci. 1993. The role of lymphoid organs in the pathogenesis of HIV infection. Semin. Immunol. 5:157-163. [DOI] [PubMed] [Google Scholar]
- 17.Roos, J. W., M. F. Maughan, Z. Liao, J. E. Hildreth, and J. E. Clements. 2000. LuSIV cells: a reporter cell line for the detection and quantitation of a single cycle of HIV and SIV replication. Virology 273:307-315. [DOI] [PubMed] [Google Scholar]
- 18.Weitz-Schmidt, G., K. Welzenbach, V. Brinkmann, T. Kamata, J. Kallen, C. Bruns, S. Cottens, Y. Takada, and U. Hommel. 2001. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat. Med. 7:687-692. [DOI] [PubMed] [Google Scholar]