Abstract
Epstein-Barr virus (EBV) causes infectious mononucleosis and is associated with cancers in immunocompromised populations. EBV establishes a latent infection and immortalizes and transforms B lymphocytes. Several latent proteins have profound effects on cellular growth, including activation of NF-κB, phosphatidylinositol 3′-OH kinase (PI3K) signaling, and notch signaling. Activation of PI3K can affect the activity of β-catenin, the target of the wnt signaling pathway. Deregulation of β-catenin is associated with a number of malignancies. To determine if β-catenin is regulated by EBV infection, EBV-infected cells were examined for β-catenin levels and localization. β-Catenin was increased in EBV-positive tumor cell lines compared to EBV-negative lines, in EBV-infected Burkitt's lymphoma cell lines, and in EBV-transformed lymphoblastoid cell lines (LCL). In contrast to wnt signaling, EBV consistently induced the accumulation of β-catenin in the cytoplasm but not the nucleus. The β-catenin regulating kinase, glycogen synthase kinase 3β (GSK3β), was shown to be phosphorylated and inactivated in EBV-infected lymphocytes. Inactivated GSK3β was localized to the nucleus of EBV-infected LCL. Neither the cytoplasmic accumulation of β-catenin nor the nuclear inactivation of GSK3β was affected by the inhibition of PI3K signaling. These data indicate that latent infection with EBV has unique effects on β-catenin signaling that are distinct from activation of wnt and independent of its effects on PI3K.
Epstein-Barr Virus (EBV) is the causative agent of lymphomas that develop in immunosuppressed patients, such as posttransplant lymphoma and a significant percentage of AIDS-associated lymphomas (20, 37). EBV is also consistently detected in the endemic form of Burkitt's lymphoma (BL) and found with varying incidence in distinct subtypes of Hodgkin's disease (HD). In developing countries, it is detected in almost all cases of HD. EBV is a major factor in the development of the epithelial cancers nasopharyngeal carcinoma (NPC) and gastric carcinoma.
The expression pattern of EBV latent genes differs depending upon the state of the cell, whether it is resting or activated, as well as immune surveillance. Three distinct patterns of latent EBV gene expression have been identified in tumor samples, and cell lines that mimic these profiles offer attractive model systems for studying the contributions of different EBV proteins to cancer development. During type I latency, characteristic of BL, only EBV nuclear antigen 1 (EBNA1), transcripts from the BamHI-A region of the viral genome, and small nonpolyadenylated RNAs, known as EBERs, are expressed. Latency type II, characteristic of NPC and HD, is characterized by expression of EBNA1, the EBERs, BamHI-A transcripts, and latent membrane proteins 1, 2A, and 2B (LMP1, LMP2A, and LMP2B, respectively). During type III latency, which is characteristic of posttransplant lymphoma, EBNAs 1, 2, 3A, 3B, 3C, and LP, BamHI transcripts, EBERs, LMP1, LMP2A, and LMP2B are expressed.
Several EBV proteins have profound effects on cellular gene expression, growth control, and signaling. The EBNA proteins, EBNA2 and EBNA3A, -B, and -C, and RK-BARF0 impinge on Notch signaling (23). LMP1 interacts with tumor necrosis receptor-associated factors to activate NF-κB and JNK, and LMP2A inhibits B-cell signaling and activates the phosphatidylinositol 3′-OH kinase (PI3K) pathway.
The wnt/wingless signaling cascade is an important pathway that is activated in a number of cancers. β-Catenin, a transcriptional regulatory factor, is a critical component of the wnt signaling pathway (35). Stabilized or free β-catenin can translocate to the nucleus and bind transcription factors, such as the T-cell factor (Tcf) or lymphocyte enhancer factor (Lef), to activate transcription. In Xenopus laevis and Drosophila melanogaster systems, β-catenin-Tcf/Lef complexes regulate a number of important developmental programs and genes. In mammalian cells, β-catenin-Tcf/Lef complexes regulate the expression of a number of proto-oncogenes, including c-myc (15) and cyclin D1 (44, 46), as well as genes important for growth and tumor progression, such as MMP7 (5), PPARδ (14), gastrin (21), connexin 43 (49), and WISP proteins (34). β-Catenin can regulate leukemic cell adhesion, proliferation, and survival (3), and Tcf and Lef have roles in B- and T-cell development (32, 40, 50). However, a targeted β-catenin knockout mouse model suggests that β-catenin is not essential for these processes (4). The specific roles of β-catenin in lymphocyte proliferation and the development of lymphoma are unknown.
β-Catenin and glycogen synthase kinase 3β (GSK3β) are retained in the cytoplasm in a protein complex with adenomatous polyposis coli (APC) and axin (33, 35). Phosphorylation of β-catenin on the amino-terminal serine and threonine residues by GSK3β results in its degradation by ubiquitin-dependent mechanisms. An important step in the regulation of β-catenin is phosphorylation of GSK3β and, in the presence of a wnt signal, GSK3β is phosphorylated and inactivated or sequestered, resulting in the stabilization of β-catenin. Mutations affecting the GSK3β phosphorylation sites on β-catenin or β-catenin binding sites on APC occur in greater than 90% of colon cancers (22). Deregulation of the wnt/wingless pathway is also associated with skin cancer (1), hepatoblastoma (45), ovarian endometrioid adenocarcinoma (55), and medulloblastoma (28, 35).
A recent study from our lab revealed that in epithelial cells EBV LMP2A activates signaling pathways that lead to β-catenin stabilization, nuclear translocation, and β-catenin-dependent transcriptional activation. LMP2A has been shown to activate PI3K signaling, inducing the phosphorylation and activation of Akt, and phosphorylation of Akt targets, forkhead transcription factor family members, and GSK3β (29, 41, 42). While the increased β-catenin in the cytoplasm of LMP2A-expressing cells was independent of PI3K signaling, the nuclear translocation of β-catenin was inhibited by inactivation of PI3K.
The EBV oncoprotein, LMP1, also activates PI3K signaling (6); however, any role for LMP1 in β-catenin regulation has yet to be established. Interestingly, Kaposi's sarcoma-associated herpesvirus infection latency-associated nuclear antigen regulates the activity of GSK3β by binding and sequestering it in the nucleus, resulting in activation of β-catenin-responsive reporter plasmids (11-13). A comparison of EBV-infected lymphoblastoid cell lines (LCLs) detected elevated β-catenin levels in type III latency compared to type I (43).
In this study, expression of β-catenin was analyzed in a panel of EBV-negative and EBV-positive cell lines, in EBV-negative BL cell lines infected with EBV, and in low-passage LCLs transformed with EBV. In contrast to regulation of β-catenin by LMP2A in epithelial cells (29), increased cytoplasmic β-catenin did not correlate with increased nuclear β-catenin. However, there was a striking increase in the phosphorylation and nuclear localization of the β-catenin regulatory kinase GSK3β that was independent of PI3K activation in EBV-positive cells. These data indicate that in B cells EBV infection induces phosphorylation and nuclear localization of GSK3β without increased nuclear β-catenin. This suggests that β-catenin-dependent gene expression is not activated during EBV infection of B lymphocytes. However, the striking translocation of GSK3β to the nucleus suggests that other targets of GSK3β are likely affected.
MATERIALS AND METHODS
Cell culture.
Cell lines were maintained in RPMI 1640 (Gibco) supplemented with antibiotic-antimycotic mixture and 10% (vol/vol) heat-inactivated fetal bovine serum for most cell lines and 15% fetal bovine serum for LCL 5000 and 5077. Jurkat cells are immature T lymphocytes derived from acute lymphoblastic leukemias. RAJI cells are an EBV-positive mature B-cell BL-derived cell line. DG75 is an EBV-negative BL cell line. HL60 is an EBV-negative promyelocytic leukemia cell line. BJAB is an EBV-negative high-grade B-cell lymphoma. B95-8 is a marmoset LCL transformed with B95-8 virus. CB-B95-8 is an umbilical cord peripheral blood lymphocyte cell line transformed with B95-8 EBV. P3HR1 is a Jijoye-derived BL cell line latently infected with P3HR1 virus containing a deletion of the EBNA2 gene within the viral genome.
BL30 and BL41 are EBV-negative BL cell lines. BL30/B95-8 and BL41/B95-8 are infected with B95-8 virus, while BL30/P3HR1 and BL41/P3HR1 are infected with P3HR1 virus. Peripheral blood lymphocyte (PBL) pools PBL 5000 and PBL 5077 and LCL lines LCL 5000 and LCL 5077 were procured from the University of North Carolina—Chapel Hill Lineberger Comprehensive Cancer Center Tissue Culture Facility. LCL 5000 and 5077 are transformed with EBV strain B95-8.
Cell harvesting and fractionation.
Cell lines were grown in 75-cm2 tissue culture flasks to 3 × 106 cells/ml. Cells treated with PI3K inhibitor were grown in the presence of either LY294002 (50 μM; Calbiochem) or the vehicle control, dimethyl sulfoxide (DMSO; Sigma) for 24 h prior to harvesting. Typically, 5 × 107 cells were harvested by centrifugation and washed with ice-cold phosphate-buffered saline (Gibco). One-fifth or 107 cells were used to make whole-cell lysates by centrifugation and lysis in 100 μl of RIPA buffer (10 mM Tris-HCl [pH 8.0], 140 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 1% deoxycholic acid, and protease and phosphatase inhibitors [Sigma]). The remaining 4 × 107 cells were pelleted by centrifugation, resuspended in 350 μl of buffer A (20 mM HEPES [pH 7.5], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, and protease and phosphatase inhibitors), and incubated on ice for 15 min. Cells were lysed by addition of 10% NP-40 to a final concentration of 1% and vortexed for 1 min. Nuclei were pelleted by centrifugation for 10 min at 1,600 × g at 4°C. The supernatant cytosolic fraction was transferred to a new tube, and the nuclear pellet was washed one time with 400 μl of buffer A and pelleted for 10 min at 1,600 × g at 4°C. Nuclei were solubilized by addition of one pellet volume of NE buffer (20 mM Tris [pH 8.0], 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol, and protease and phosphatase inhibitors), followed by one-fourth pellet volume of 5 M NaCl and one pellet volume of NE buffer and vortexing.
Western blotting.
Cell lysates were clarified by centrifugation and quantitated with a Bio-Rad DC protein assay system (Bio-Rad). Samples were then boiled in SDS sample buffer, and indicated amounts of proteins were separated using 10% acrylamide SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes for Western blotting analysis. Primary antibodies used included actin (I-19), PARP (H-250), and GRP-78 (N-20) (all from Santa Cruz), phospho-GSK3β (Ser9; Cell Signaling Technology), β-catenin (BD Transduction Laboratories), GSK3 (Upstate Biotechnology), CS1-4 (anti-LMP1; Dako), and PE2 (anti-EBNA2 mouse monoclonal) (53). Antibody-bound proteins were detected with horseradish peroxidase-conjugated secondary antibodies (Amersham Pharmacia and Dako) and the Pierce Supersignal West Pico system (Pierce) followed by exposure to film.
Plasmids and RNA probes.
β-Catenin cDNA was synthesized from total RNA isolated from C33A, a human cervical carcinoma cell line, with the BCTRNP3.2 primer (GCCAAGCTTGCCAGTATGATGAGCTTGCTTTC) using SuperScript II RNase H- reverse transcriptase (Invitrogen) according to the manufacturer's directions. cDNA was then amplified with primers BCTRNP3.2 and BCatRNP5 (GCCGAATTCGCGTTTGGCTGAACCATCAC) with platinum Pfx DNA polymerase (Invitrogen) according to the manufacturer's directions. The resulting PCR product was then digested with HindIII and cloned into the HindIII site of pcDNA3 (Invitrogen) to yield plasmid pB-cat RNP containing sequence corresponding to 745 to 1,150 bases of the β-catenin mRNA (accession no. Z19054). Linearized pB-cat RNP was created by digestion with BamHI. Radioactively labeled RNA probes were synthesized from linearized plasmids pB-cat RNP and pTRI-GAPDH mouse (Ambion) with a MAXIscript (Ambion) in vitro transcription kit according to the manufacturer's directions.
RPA.
Ribonuceotide protection assays (RPA) were performed using a Direct Protect lysate RPA kit (Ambion) according to the manufacturer's directions. Briefly, RPA probes were annealed with RNA from 108 cells per reaction mixture overnight in lysis buffer. The following day samples were digested with RNase and protease and precipitated. Protected probes were resolved on a 5% polyacrylamide gel, dried, and imaged using a PhosphorImager (Molecular Dynamics). Full-length probes for β-catenin and glyceraldehyde phosphate dehydrogenase (GAPDH) (465 and 433 bases, respectively) bound to RNA yielded protected fragments of 406 and 316 bases for the β-catenin and GAPDH probes, respectively.
GST-E-cadherin free β-catenin pull-down assay.
Glutathione S-transferase (GST) pull-down assays were performed as described previously (24). GST or GST-E-cadherin protein was expressed in DH5α Escherichia coli induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside at 37°C. Cells were pelleted, resuspended in ice-cold phosphate-buffered saline, and lysed by sonication in E-cadherin lysis buffer (10 mM sodium phosphate [pH 7.4], 150 mM NaCl, 1% NP-40, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 5 μg of aprotinin/ml) on ice. Bacterial lysates were clarified by centrifugation, and GST or GST-E-cadherin was purified by binding to glutathione-Sepharose beads for 1 h at 4°C. The beads were washed three times and resuspended in E-cadherin lysis buffer. Cells were lysed in E-cadherin lysis buffer containing a protease inhibitor cocktail for 30 min at 4°C. Protein concentrations in lysates were quantified, and indicated quantities of proteins were precleared with GST-bound Sepharose beads for 45 min at 4°C and incubated with Sepharose beads bound to GST-E-cadherin. After 1 h of incubation at 4°C, the beads were washed three times and collected by centrifugation. Bound proteins were eluted in SDS sample buffer and separated via SDS-PAGE and transferred to Immobilon P. Western blotting was carried out using antibodies as described above.
RESULTS
β-Catenin levels in EBV-positive and EBV-negative cell lines.
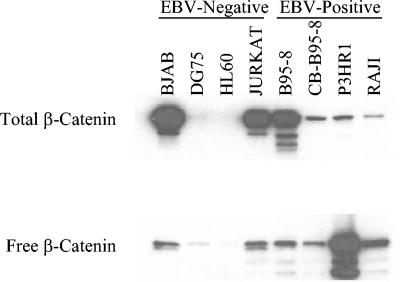
Increased β-catenin levels and activities in lymphocyte cell lines have recently been described in EBV-infected BL cells with type III latency compared with those with type I latency. To determine if EBV consistently affects β-catenin regulation, β-catenin levels were determined in EBV-positive and -negative cell lines. This survey of EBV-positive and -negative lymphocyte cell lines revealed an increase in total and free β-catenin in all EBV-positive cells (Fig. 1). Increased β-catenin was detected in whole-cell lysates in the EBV-negative cell lines BJAB and Jurkat but not in DG75 and HL60 cells. All EBV-positive cell lines contained detectable levels of β-catenin, with B95-8 having the greatest total β-catenin, CB-B95-8 and P3HR1 having intermediate levels, and RAJI cells with low levels. To assess levels of β-catenin that were not bound to axin or E-cadherin complexes, a GST-E-cadherin fusion protein was used to bind free β-catenin. Elution of bound protein and Western blotting revealed that P3HR1 and RAJI, in contrast to findings for total β-catenin levels, had the highest levels of free β-catenin, while B95-8 and CB-B95-8 had less but still easily detectable levels in GST-E-cadherin-bound fractions. All of the EBV-positive cell lines examined had high levels of total and free β-catenin levels, whereas only two of the four EBV-negative cell lines had high levels of total and free β-catenin. Mutations that stabilize and activate β-catenin that occur in the development of a number of malignancies have been described, and similar mutations may have occurred during tumor development or may be selected for during the passage of cell lines.
FIG. 1.
Total and free β-catenin in EBV-negative and -positive cell lines. (Upper) Ten micrograms of total cell lysates from EBV-negative cells, BJAB, DG75, HL60, and Jurkat, and EBV-positive cells, B95-8, cord blood infected with B95-8 virus (CB-B95-8), P3HR1, and RAJI, were analyzed by Western blotting for total β-catenin expression. (Lower) Free β-catenin was determined by incubation of 200 μg of whole-cell lysate with GST-E-cadherin fusion protein. Bound proteins were then eluted and analyzed by Western blotting for β-catenin.
Increased free β-catenin in EBV-infected BL cells.
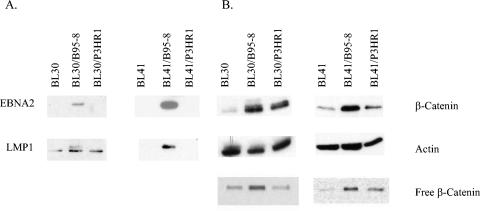
To determine whether β-catenin is increased in cell lines infected with EBV, converted BL cell lines latently infected with EBV were examined for β-catenin levels. The parental cells, BL30 and BL41, are EBV-negative BL cell lines that have each been infected with B95-8 or P3HR1 virus to yield BL30/B95-8, BL30/P3HR1, BL41/B95-8, or BL41/P3HR1. The B95-8-infected cells expressed EBNA2 and most latent proteins, including LMP1 (Fig. 2A). In BL30 and infected BL30 cells in the LMP1 blot, a lower nonspecific band was present in all channels with the LMP1-specific upper band present in the B95-8-infected cells. BL30 and BL41 cells infected with P3HR1 virus, which has been shown to contain a genomic deletion in the EBNA2 gene, do not express EBNA2 or LMP1 but do express EBNA3 (31). In BL30 cells infected with B95-8 and P3HR1 virus, there was an increase in total β-catenin levels (Fig. 2B). A corresponding increase in free β-catenin levels was also detected in the B95-8-infected cells compared to parental BL30 cells. In the BL41 cells, there was an increase in both total and free β-catenin upon B95-8 infection and a slight increase in the P3HR1-infected cell line. These data revealed an increase in β-catenin levels not only in EBV-positive cells compared to EBV-negative cells but also in cells infected with EBV compared to parental cell lines.
FIG. 2.
Total and free β-catenin in converted BL cell lines. EBV-negative BL cell lines (BL30 and BL41) and parental cell lines infected with EBV strain B95-8 (BL30/B95-8 and BL41/B95-8) or P3HR1 (BL30/P3HR1 and BL41/P3HR1) were analyzed by Western blotting. Fifty micrograms of total cell lysate was analyzed for latent EBV proteins EBNA2 and LMP1 (A) or β-catenin and actin (B). (B, lower panel) Free β-catenin from 200 and 250 μg of protein from BL30 and BL41 cell lines, respectively, was determined using a GST-E-cadherin pull-down assay followed by Western blotting of bound proteins.
Cytoplasmic localization of β-catenin.
Activation of wnt signaling leads to stabilization and nuclear localization of β-catenin. To determine if there was increased nuclear β-catenin in EBV-infected cell lines, BL30 and BL41 cells as well as EBV-infected counterparts were fractionated into cytoplasmic and nuclear fractions and β-catenin levels were determined by Western blotting (Fig. 3). Although increased total β-catenin was detected in both of the infected BL30 cell lines, the increased β-catenin in the B95-8-infected BL30 cells was in the cytoplasmic fraction, while in the P3HR1-infected BL30 cells a somewhat smaller cytosolic increase was accompanied by an increase in the nuclear fractions (Fig. 3). Similarly, the increased β-catenin in the infected BL41 cell lines was limited to the cytoplasmic fraction for B95-8-infected cells, and β-catenin levels were not significantly increased in the cytoplasmic or nuclear fractions of P3HR1-infected BL41 cells. Actin levels were evaluated to determine equivalent loading, while detection of GRP78, an endoplasmic reticulum protein, indicated nuclear purity (bottom panel, upper band). Although the actin levels of the BL41 compared to the B95-8-infected BL41 cells were somewhat lower, the GRP78 panel confirmed only slight differences in loading between the two lanes and β-catenin levels were consistent with total levels seen in Fig. 2. BL30 and BL41 cell lines infected with B95-8 virus had increased cytoplasmic β-catenin, while P3HR1-infected cells had a smaller nuclear increase. These data indicate that EBV infection of BL cells increases β-catenin with minimal effects on nuclear localization.
FIG. 3.
β-Catenin localization in converted BL cell lines. BL30 (A) and BL41 (B) cell lines were fractionated into nuclear and cytoplasmic fractions, and 50 μg of protein for each fraction was analyzed by Western blotting for β-catenin, actin, and GRP78. Actin was used as a loading control, and GRP78, an endoplasmic reticulum chaperone, was used as an indication of nuclear purity.
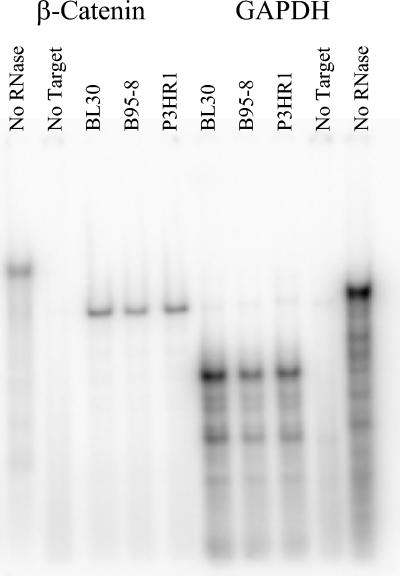
To determine if transcription of β-catenin is altered in EBV-infected cells, RNA was isolated from parental BL30 and well as B95-8- and P3HR1-infected cell lines and was then allowed to anneal to labeled RNA probes for β-catenin and GAPDH messages. RNase protection of bound RNAs and resolution via PAGE revealed that compared with control GAPDH message, the RNA levels of β-catenin in the presence or absence of infection with either of the EBV viruses were unchanged (Fig. 4). These data indicate that the effects of EBV upon β-catenin are posttranscriptional.
FIG. 4.
RNase protection of β-catenin RNA in converted BL cells. RNA from BL30 and infected BL30/B95-8 (B95-8) and BL30/P3HR1 (P3HR1) cells was purified and analyzed by RPA for β-catenin and GAPDH mRNA. Full-length probes for β-catenin and GAPDH (465 and 433 bases, respectively) are shown in the no-RNase lanes. No-target lanes contained probes reacted with RNase in the absence of cellular RNA. Other lanes contained the indicated cellular RNA bound to the probe and reacted with RNase to yield protected fragments of 406 and 316 bases for the β-catenin and GAPDH probes, respectively.
β-Catenin and P-GSK3β in lymphocyte cell lines.
The increased levels of total β-catenin in cell lines suggest that EBV may affect the regulation of β-catenin. However, most of these cell lines were established from BL tumors, have been extensively passaged, and likely have accumulated additional mutations that affect β-catenin regulation. To assess the effect of EBV on β-catenin in recently transformed cell lines, low-passage LCLs were examined for β-catenin levels and localization. Two sets of uninfected PBL before and after infection with EBV strain B95-8 were analyzed by Western blotting for EBNA2 and LMP1, and results indicated that the LCLs expressed the EBV latent proteins LMP1 and EBNA2 (data not shown). Previous studies have shown that β-catenin is not detected in normal resting PBL (48), T cells (3), monocytes (47), or macrophages (27) and is dispensable for hematopoiesis and lymphopoiesis (4). Confirming these previous findings, β-catenin was not detected in the whole-cell lysate for PBL 5000 (Fig. 5). In contrast, the EBV-infected lymphocytes had high levels of β-catenin in the whole-cell lysates (Fig. 5). Analysis of the fractionated LCLs revealed that the increase in β-catenin was completely cytoplasmic, with trace levels of nuclear β-catenin (Fig. 5). Because of limited quantities of PBL material, whole-cell extracts of the LCLs were diluted in serial twofold dilutions to aid with comparisons.
FIG. 5.
β-Catenin and phosphorylated GSK3β levels and localization in LCLs. PBL starting material and LCLs transformed with EBV were analyzed by Western blotting. Nuclear, cytoplasmic, and whole-cell extracts of LCL 5000 and LCL 5077 (25 μg each) and whole-cell extract of PBL 5000 (50 μg) were analyzed for β-catenin, actin, GRP78, P-GSK3β, and total GSK3. Whole-cell extracts were diluted as indicated in serial twofold dilutions.
A critical regulator of β-catenin is GSK3β, which is inactivated by phosphorylation, resulting in β-catenin stabilization. Both of the EBV-infected LCLs had readily detectable phosphorylated P-GSK3β (Fig. 5). Surprisingly, high levels of P-GSK3β were present in the nucleus of both LCL 5000 and 5077. LCL 5000 also had relatively high levels of P-GSK3β in the cytoplasm, whereas LCL 5077 contained very little cytoplasmic P-GSK3β. The detection of the endoplasmic reticulum marker GRP78 only in the cytoplasm confirmed the purity of the nuclear fractions and indicated that the nuclear P-GSK3β did not represent cytoplasmic contamination. These data revealed that EBV has striking effects on β-catenin levels and GSK3β localization, with elevated β-catenin in the cytoplasm but not the nucleus and high levels of inactivated P-GSK3β in the nucleus.
To determine if the phosphorylation of GSK3β by EBV in LCLs was dependent upon PI3K signaling, the LCLs were treated with the PI3K inhibitor LY294002 for 24 h and then fractionated. Western blotting was performed, and the levels of β-catenin and P-GSK3β, as well as of actin, GRP78, and PARP, were determined. Levels of nuclear and cytoplasmic β-catenin and P-GSK3β were unchanged in the presence of LY (Fig. 6). Equal actin levels indicated equal loading between DMSO- and LY-treated samples, and the endoplasmic reticulum marker GRP78 and nuclear marker PARP confirmed the purity of the cytoplasmic and nuclear fractions, respectively. These data revealed that the increased cytoplasmic β-catenin and nuclear P-GSK3β were independent of PI3K and that inactivation of GSK3β by phosphorylation did not result in the accumulation of nuclear β-catenin in this context.
FIG. 6.
β-Catenin and P-GSK3β are unaffected by inhibition of PI3K. LCL 5000 was grown to 3 × 106 cells/ml, separated into equal volumes, and then diluted twofold with fresh medium containing either PI3K inhibitor, 50 μM LY294002 (LY), or DMSO vehicle control and grown for 24 h. Cells were then harvested, fractionated, and analyzed by Western blotting for β-catenin, actin, GRP78, P-GSK3β, and PARP. Quantities of protein used were 100, 40, and 80 μg for whole-cell, nuclear, and cytoplasmic fractions, respectively.
DISCUSSION
In this study, comparisons of EBV-negative versus EBV-positive cell lines, transformed cells infected with EBV versus parental uninfected cells lines, and LCLs transformed with EBV versus PBL starting material revealed that B lymphocytes infected with EBV contain elevated levels of total, free, and cytoplasmic β-catenin. There was also a dramatic translocation of phosphorylated and inactivated GSK3β to the nuclei of LCLs.
These findings confirmed the β-catenin levels reported in a previous study involving Jurkat and HL60 cells (3). Those authors determined that increased β-catenin was important for the growth properties of Jurkat cells and that inhibition of β-catenin led to cell death. In contrast, β-catenin levels and activity were not important for the growth and viability of HL60 cells. The detection of increased cytoplasmic β-catenin in both EBV-converted BL cell lines and LCLs transformed by EBV indicate that latent EBV infection induces the cytoplasmic accumulation of β-catenin.
A recent study described increased β-catenin levels in the presence of EBV type III latency in BL cell lines and reported a five- to sevenfold increase in Tcf/Lef reporter plasmid activation (43). However, EBV-negative cells were not examined, and the cellular localization of β-catenin was not determined. The results presented here indicate that β-catenin can be detected in BL cell lines infected with B95-8 or P3HR1 virus and is not limited to type III latency versus type I latency, as previously described (43). The variability in the effects of EBV in these cell lines may reflect genetic changes accumulated in the BL cell lines. Importantly, these data indicate that in EBV-transformed lymphocytes that have not been established from BL, the increased β-catenin is in the cytoplasm in EBV-infected lymphoid cells, where it would likely have little effect upon gene expression. The previously reported small increases found in reporter assays may reflect trace amounts of β-catenin that move to the nucleus. These data are in stark contrast to those for epithelial cells, where LMP2A induces increased total β-catenin and induces nuclear translocation of β-catenin. In keeping with these observations, nuclear β-catenin has been detected in the epithelial cancer NPC but not in lymphoid malignancy Hodgkin's lymphoma (30). These findings suggest that activation and nuclear translocation of β-catenin is an important aspect of EBV infection of epithelial cells but not in lymphoid cells.
β-Catenin import into and export from the nucleus is a regulated process (8, 16, 38). Multistep activation of β-catenin in peripheral blood mononuclear cells has been suggested by other studies. β-Catenin/Lef-1 complexes are present in the nucleus of normal T cells but are transcriptionally inactive; however, in the transformed T-cell line Jurkat, these complexes are active (36). Chemical treatment of monocytes with the GSK3β inhibitor lithium results in β-catenin stabilization without gene activation; however, stimulation with lipopolysaccharide or zymosan induces β-catenin stabilization and gene activation (47). In epithelial cells, mutations or stimuli that disrupt cellular junctions, of which β-catenin is a component, result in increased cytoplasmic β-catenin without activating gene transcription (19). These data indicate that nuclear translocation and activation of β-catenin require other cellular stimuli in addition to stabilization.
Classical wnt signaling converges on β-catenin bound in a complex that includes APC, axin, and GSK3β. Phosphorylation of GSK3β results in the stabilization of β-catenin and its translocation to the nucleus. In the present study, phosphorylated GSK3β was localized to the nucleus without a corresponding increase in nuclear β-catenin. This finding suggests that the nuclear P-GSK3β may result in increased cytoplasmic β-catenin stability without inducing nuclear translocation of β-catenin. Nuclear GSK3β binds, phosphorylates, and stimulates proapoptotic activity of p53 (51, 52). It is possible that the inactivated GSK3β in the nucleus might bind to p53 and prevent apoptosis. In support of this is the fact that activation of Akt, which phosphorylates and inactivates GSK3β, inhibits p53-mediated apoptosis (26, 39, 56). LMP1 has been shown to specifically inhibit p53-mediated apoptosis, and this may be due to binding of GSK3β to p53 (10). Another pathway regulated by GSK3β is the Notch pathway, which is important for hematopoetic development (25). A reduction in GSK3β levels or activity resulted in a marked reduction in NotchIC levels (9). The presence of inactivated, phosphorylated GSK3β in the nucleus of EBV-infected cells likely affects other critical pathways.
GSK3β is able to phosphorylate and regulate a number of cellular targets other than β-catenin. GSK3β was originally identified for its ability to phosphorylate and inhibit glycogen synthase. Differential regulation of GSK3β by insulin and wnt has been examined (2, 7). Treatment of cells with insulin leads to the phosphorylation and inactivation of GSK3β in a PI3K- and Akt-dependent pathway without increasing β-catenin. In the same cells, treatment with wnt leads to the inactivation of GSK3β and to stabilization and increased β-catenin. The data presented here indicate that EBV regulates GSK3β by yet another mechanism. Phosphorylation of GSK3β in EBV-infected LCLs is independent of PI3K signaling and is distinct from pathways leading to activation and nuclear localization of β-catenin.
In this study neither accumulation of cytoplasmic β-catenin nor nuclear-phosphorylated GSK3β was affected by the inhibition of PI3K, suggesting that β-catenin activation in EBV-infected cells may involve multiple steps. The first step in β-catenin activation would involve cytoplasmic stabilization of the protein in a PI3K-independent pathway. Inhibition of PI3K in the presence of LMP2A resulted in a block in the nuclear translocation of β-catenin but did not block the cytoplasmic stabilization (29). In accordance with those data, inhibition of PI3K also did not block the cytoplasmic accumulation of β-catenin in the present study. LMP2A has been shown to interact with the Nedd4 family of ubiquitin ligases (17, 18, 54). Modulation of the activity of ubiquitin ligases could regulate and stabilize β-catenin in the cytoplasm of EBV-infected cells. Inhibition of deubiquitinating activity in the cell lysates of type III-infected cells resulted in decreased β-catenin (43). This was not unexpected, as the turnover of many cellular proteins is regulated by a balance of ubiquitinating and deubiquitinating enzymes and decreased levels of many proteins by inhibition of deubiquitination would be expected. The first step in β-catenin activation in EBV-infected cells likely occurs via PI3K-independent stabilization of β-catenin by regulation of ubiquitination pathways.
The second step in the activation of β-catenin could occur at the level of nuclear import. In epithelial cells expressing LMP2A, the PI3K pathway was required for the nuclear translocation of β-catenin (29). However, the data presented here indicate that in transformed lymphocytes the increased β-catenin is not nuclear. In contrast, GSK3β is phosphorylated, inactivated, and targeted to the nucleus.
The importance of β-catenin and GSK3β during herpesvirus infection has been identified in several studies. The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen protein has been shown to affect nuclear translocation of GSK3β, and EBV LMP2A activates PI3K, leading to GSK3β phosphorylation. This study reveals that in lymphocytes latent infection with EBV affects the cytoplasmic levels of β-catenin and nuclear translocation and phosphorylation of GSK3β. It will be important to determine how EBV gene expression affects GSK3β phosphorylation and intracellular localization and to identify the targets and pathways affected by the nuclear P-GSK3β and possibly the cytoplasmic β-catenin.
Acknowledgments
We thank Shannon Kenney, Jennifer A. Morrison, Natalie J. Thornburg, and Bernardo A. Mainou for critical reading of the manuscript.
This work was supported by National Institutes of Health grants CA32979 and CA103634 and by the Uehara Memorial Foundation (S.K.).
REFERENCES
- 1.Chan, E. F., U. Gat, J. M. McNiff, and E. Fuchs. 1999. A common human skin tumour is caused by activating mutations in β-catenin. Nat. Genet. 21:410-413. [DOI] [PubMed] [Google Scholar]
- 2.Chen, R. H., W. V. Ding, and F. McCormick. 2000. Wnt signaling to β-catenin involves two interactive components: glycogen synthase kinase-3β inhibition and activation of protein kinase C. J. Biol. Chem. 275:17894-17899. [DOI] [PubMed] [Google Scholar]
- 3.Chung, E. J., S. G. Hwang, P. Nguyen, S. Lee, J. S. Kim, J. W. Kim, P. A. Henkart, D. P. Bottaro, L. Soon, P. Bonvini, S. J. Lee, J. E. Karp, H. J. Oh, J. S. Rubin, and J. B. Trepel. 2002. Regulation of leukemic cell adhesion, proliferation, and survival by β-catenin. Blood 100:982-990. [DOI] [PubMed] [Google Scholar]
- 4.Cobas, M., A. Wilson, B. Ernst, S. J. Mancini, H. R. MacDonald, R. Kemler, and F. Radtke. 2004. β-Catenin is dispensable for hematopoiesis and lymphopoiesis. J. Exp. Med. 199:221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford, H. C., B. M. Fingleton, L. A. Rudolph-Owen, K. J. Goss, B. Rubinfeld, P. Polakis, and L. M. Matrisian. 1999. The metalloproteinase matrilysin is a target of β-catenin transactivation in intestinal tumors. Oncogene 18:2883-2891. [DOI] [PubMed] [Google Scholar]
- 6.Dawson, C. W., G. Tramountanis, A. G. Eliopoulos, and L. S. Young. 2003. Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J. Biol. Chem. 278:3694-3704. [DOI] [PubMed] [Google Scholar]
- 7.Ding, V. W., R. H. Chen, and F. McCormick. 2000. Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J. Biol. Chem. 275:32475-32481. [DOI] [PubMed] [Google Scholar]
- 8.Fagotto, F., U. Gluck, and B. M. Gumbiner. 1998. Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of β-catenin. Curr. Biol. 8:181-190. [DOI] [PubMed] [Google Scholar]
- 9.Foltz, D. R., M. C. Santiago, B. E. Berechid, and J. S. Nye. 2002. Glycogen synthase kinase-3β modulates notch signaling and stability. Curr. Biol. 12:1006-1011. [DOI] [PubMed] [Google Scholar]
- 10.Fries, K. L., W. E. Miller, and N. Raab-Traub. 1996. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of the A20 gene. J. Virol. 70:8653-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimuro, M., and S. D. Hayward. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus manipulates the activity of glycogen synthase kinase-3β. J. Virol. 77:8019-8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimuro, M., and S. D. Hayward. 2004. Manipulation of glycogen-synthase kinase-3 activity in KSHV-associated cancers. J. Mol. Med. 82:223-231. [DOI] [PubMed] [Google Scholar]
- 13.Fujimuro, M., F. Y. Wu, C. ApRhys, H. Kajumbula, D. B. Young, G. S. Hayward, and S. D. Hayward. 2003. A novel viral mechanism for dysregulation of β-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat. Med. 9:300-306. [DOI] [PubMed] [Google Scholar]
- 14.He, T. C., T. A. Chan, B. Vogelstein, and K. W. Kinzler. 1999. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell 99:335-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He, T. C., A. B. Sparks, C. Rago, H. Hermeking, L. Zawel, L. T. da Costa, P. J. Morin, B. Vogelstein, and K. W. Kinzler. 1998. Identification of c-MYC as a target of the APC pathway. Science 281:1509-1512. [DOI] [PubMed] [Google Scholar]
- 16.Henderson, B. R., and F. Fagotto. 2002. The ins and outs of APC and β-catenin nuclear transport. EMBO Rep. 3:834-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda, M., A. Ikeda, L. C. Longan, and R. Longnecker. 2000. The Epstein-Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology 268:178-191. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda, M., A. Ikeda, and R. Longnecker. 2001. PY motifs of Epstein-Barr virus LMP2A regulate protein stability and phosphorylation of LMP2A-associated proteins. J. Virol. 75:5711-5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito, K., I. Okamoto, N. Araki, Y. Kawano, M. Nakao, S. Fujiyama, K. Tomita, T. Mimori, and H. Saya. 1999. Calcium influx triggers the sequential proteolysis of extracellular and cytoplasmic domains of E-cadherin, leading to loss of β-catenin from cell-cell contacts. Oncogene 18:7080-7090. [DOI] [PubMed] [Google Scholar]
- 20.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2573. In B. N. Fields, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, S. E. Straus, and D. M. Knipe (ed.), Field's virology, 4th ed., vol. 2. Lippincott Williams & Wilkins Publishers, Philadelphia, Pa. [Google Scholar]
- 21.Koh, T. J., C. J. Bulitta, J. V. Fleming, G. J. Dockray, A. Varro, and T. C. Wang. 2000. Gastrin is a target of the β-catenin/TCF-4 growth-signaling pathway in a model of intestinal polyposis. J. Clin. Investig. 106:533-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korinek, V., N. Barker, P. J. Morin, D. van Wichen, R. de Weger, K. W. Kinzler, B. Vogelstein, and H. Clevers. 1997. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 275:1784-1787. [DOI] [PubMed] [Google Scholar]
- 23.Kusano, S., and N. Raab-Traub. 2001. An Epstein-Barr virus protein interacts with Notch. J. Virol. 75:384-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusano, S., and N. Raab-Traub. 2002. I-mfa domain proteins interact with axin and affect its regulation of the Wnt and c-Jun N-terminal kinase signaling pathways. Mol. Cell. Biol. 22:6393-6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maillard, I., S. H. Adler, and W. S. Pear. 2003. Notch and the immune system. Immunity 19:781-791. [DOI] [PubMed] [Google Scholar]
- 26.Mitsuuchi, Y., S. W. Johnson, M. Selvakumaran, S. J. Williams, T. C. Hamilton, and J. R. Testa. 2000. The phosphatidylinositol 3-kinase/AKT signal transduction pathway plays a critical role in the expression of p21WAF1/CIP1/SDI1 induced by cisplatin and paclitaxel. Cancer Res. 60:5390-5394. [PubMed] [Google Scholar]
- 27.Monick, M. M., A. B. Carter, P. K. Robeff, D. M. Flaherty, M. W. Peterson, and G. W. Hunninghake. 2001. Lipopolysaccharide activates Akt in human alveolar macrophages resulting in nuclear accumulation and transcriptional activity of β-catenin. J. Immunol. 166:4713-4720. [DOI] [PubMed] [Google Scholar]
- 28.Morin, P. J. 1999. β-Catenin signaling and cancer. Bioessays 21:1021-1030. [DOI] [PubMed] [Google Scholar]
- 29.Morrison, J. A., A. J. Klingelhutz, and N. Raab-Traub. 2003. Epstein-Barr virus latent membrane protein 2A activates β-catenin signaling in epithelial cells. J. Virol. 77:12276-12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison, J. A., M. L. Gulley, R. Pathmanathan, and N. Raab-Traub. 2004. Differential signaling pathways are activated in the EBV-associated malignancies nasopharyngeal carcinomas and Hodgkin lymphoma. Cancer Res. 64:5251-5260. [DOI] [PubMed] [Google Scholar]
- 31.Murray, R. J., L. S. Young, A. Calender, C. D. Gregory, M. Rowe, G. M. Lenoir, and A. B. Rickinson. 1988. Different patterns of Epstein-Barr virus gene expression and of cytotoxic T-cell recognition in B-cell lines infected with transforming (B95.8) or nontransforming (P3HR1) virus strains. J. Virol. 62:894-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamura, R. M., M. Sigvardsson, J. Galceran, S. Verbeek, H. Clevers, and R. Grosschedl. 1998. Redundant regulation of T cell differentiation and TCRα gene expression by the transcription factors LEF-1 and TCF-1. Immunity 8:11-20. [DOI] [PubMed] [Google Scholar]
- 33.Peifer, M., and P. Polakis. 2000. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science 287:1606-1609. [DOI] [PubMed] [Google Scholar]
- 34.Pennica, D., T. A. Swanson, J. W. Welsh, M. A. Roy, D. A. Lawrence, J. Lee, J. Brush, L. A. Taneyhill, B. Deuel, M. Lew, C. Watanabe, R. L. Cohen, M. F. Melhem, G. G. Finley, P. Quirke, A. D. Goddard, K. J. Hillan, A. L. Gurney, D. Botstein, and A. J. Levine. 1998. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc. Natl. Acad. Sci. USA 95:14717-14722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837-1851. [PubMed] [Google Scholar]
- 36.Prieve, M. G., and M. L. Waterman. 1999. Nuclear localization and formation of β-catenin-lymphoid enhancer factor 1 complexes are not sufficient for activation of gene expression. Mol. Cell. Biol. 19:4503-4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In B. N. Fields, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, S. E. Straus, and D. M. Knipe (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins Publishers, Philadelphia, Pa. [Google Scholar]
- 38.Rosin-Arbesfeld, R., F. Townsley, and M. Bienz. 2000. The APC tumour suppressor has a nuclear export function. Nature 406:1009-1012. [DOI] [PubMed] [Google Scholar]
- 39.Sabbatini, P., and F. McCormick. 1999. Phosphoinositide 3-OH kinase (PI3K) and PKB/Akt delay the onset of p53-mediated, transcriptionally dependent apoptosis. J. Biol. Chem. 274:24263-24269. [DOI] [PubMed] [Google Scholar]
- 40.Schilham, M. W., A. Wilson, P. Moerer, B. J. Benaissa-Trouw, A. Cumano, and H. C. Clevers. 1998. Critical involvement of Tcf-1 in expansion of thymocytes. J. Immunol. 161:3984-3991. [PubMed] [Google Scholar]
- 41.Scholle, F., K. M. Bendt, and N. Raab-Traub. 2000. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J. Virol. 74:10681-10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scholle, F., R. Longnecker, and N. Raab-Traub. 2001. Analysis of the phosphorylation status of Epstein-Barr virus LMP2A in epithelial cells. Virology 291:208-214. [DOI] [PubMed] [Google Scholar]
- 43.Shackelford, J., C. Maier, and J. S. Pagano. 2003. Epstein-Barr virus activates β-catenin in type III latently infected B lymphocyte lines: association with deubiquitinating enzymes. Proc. Natl. Acad. Sci. USA 100:15572-15576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shtutman, M., J. Zhurinsky, I. Simcha, C. Albanese, M. D'Amico, R. Pestell, and A. Ben-Ze'ev. 1999. The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 96:5522-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taniguchi, K., L. R. Roberts, I. N. Aderca, X. Dong, C. Qian, L. M. Murphy, D. M. Nagorney, L. J. Burgart, P. C. Roche, D. I. Smith, J. A. Ross, and W. Liu. 2002. Mutational spectrum of β-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene 21:4863-4871. [DOI] [PubMed] [Google Scholar]
- 46.Tetsu, O., and F. McCormick. 1999. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422-426. [DOI] [PubMed] [Google Scholar]
- 47.Thiele, A., M. Wasner, C. Muller, K. Engeland, and S. Hauschildt. 2001. Regulation and possible function of β-catenin in human monocytes. J. Immunol. 167:6786-6793. [DOI] [PubMed] [Google Scholar]
- 48.Tsutsui, J., M. Moriyama, N. Arima, H. Ohtsubo, H. Tanaka, and M. Ozawa. 1996. Expression of cadherin-catenin complexes in human leukemia cell lines. J. Biochem. (Tokyo) 120:1034-1039. [DOI] [PubMed] [Google Scholar]
- 49.van der Heyden, M. A., M. B. Rook, M. M. Hermans, G. Rijksen, J. Boonstra, L. H. Defize, and O. H. Destree. 1998. Identification of connexin 43 as a functional target for Wnt signalling. J. Cell Sci. 111:1741-1749. [DOI] [PubMed] [Google Scholar]
- 50.Verbeek, S., D. Izon, F. Hofhuis, E. Robanus-Maandag, H. te Riele, M. van de Wetering, M. Oosterwegel, A. Wilson, H. R. MacDonald, and H. Clevers. 1995. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature 374:70-74. [DOI] [PubMed] [Google Scholar]
- 51.Watcharasit, P., G. N. Bijur, L. Song, J. Zhu, X. Chen, and R. S. Jope. 2003. Glycogen synthase kinase-3β (GSK3β) binds to and promotes the actions of p53. J. Biol. Chem. 278:48872-48879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watcharasit, P., G. N. Bijur, J. W. Zmijewski, L. Song, A. Zmijewska, X. Chen, G. V. Johnson, and R. S. Jope. 2002. Direct, activating interaction between glycogen synthase kinase-3β and p53 after DNA damage. Proc. Natl. Acad. Sci. USA 99:7951-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Webster-Cyriaque, J., J. Middeldorp, and N. Raab-Traub. 2000. Hairy leukoplakia: an unusual combination of transforming and permissive Epstein-Barr virus infections. J. Virol. 74:7610-7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winberg, G., L. Matskova, F. Chen, P. Plant, D. Rotin, G. Gish, R. Ingham, I. Ernberg, and T. Pawson. 2000. Latent membrane protein 2A of Epstein-Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases. Mol. Cell. Biol. 20:8526-8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu, R., Y. Zhai, E. R. Fearon, and K. R. Cho. 2001. Diverse mechanisms of β-catenin deregulation in ovarian endometrioid adenocarcinomas. Cancer Res. 61:8247-8255. [PubMed] [Google Scholar]
- 56.Yamaguchi, A., M. Tamatani, H. Matsuzaki, K. Namikawa, H. Kiyama, M. P. Vitek, N. Mitsuda, and M. Tohyama. 2001. Akt activation protects hippocampal neurons from apoptosis by inhibiting transcriptional activity of p53. J. Biol. Chem. 276:5256-5264. [DOI] [PubMed] [Google Scholar]