Abstract
Physicochemical similarities between K+ and Na+ result in interactions between their homeostatic mechanisms. The physiological interactions between these two ions was investigated by examining aspects of K+ nutrition in the Arabidopsis salt overly sensitive (sos) mutants, and salt sensitivity in the K+ transport mutants akt1 (Arabidopsis K+ transporter) and skor (shaker-like K+ outward-rectifying channel). The K+-uptake ability (membrane permeability) of the sos mutant root cells measured electrophysiologically was normal in control conditions. Also, growth rates of these mutants in Na+-free media displayed wild-type K+ dependence. However, mild salt stress (50 mm NaCl) strongly inhibited root-cell K+ permeability and growth rate in K+-limiting conditions of sos1 but not wild-type plants. Increasing K+ availability partially rescued the sos1 growth phenotype. Therefore, it appears that in the presence of Na+, the SOS1 Na+-H+ antiporter is necessary for protecting the K+ permeability on which growth depends. The hypothesis that the elevated cytoplasmic Na+ levels predicted to result from loss of SOS1 function impaired the K+ permeability was tested by introducing 10 mm NaCl into the cytoplasm of a patch-clamped wild-type root cell. Complete loss of AKT1 K+ channel activity ensued. AKT1 is apparently a target of salt stress in sos1 plants, resulting in poor growth due to impaired K+ uptake. Complementary studies showed that akt1 seedlings were salt sensitive during early seedling development, but skor seedlings were normal. Thus, the effect of Na+ on K+ transport is probably more important at the uptake stage than at the xylem loading stage.
Soil salinity is one of the most significant abiotic stresses confronting plant agriculture today, and Na+ is one of its major components. Because Na+ is chemically similar to K+, a certain amount of its harmfulness is due to interference at some level with the transport and cytoplasmic functions of K+. Supplying a plant with an abundance of K+ can protect it against the deleterious effects of Na+, and crops varieties bred to resist salinity frequently display a special ability to maintain a high cytosolic K+ to Na+ ratio when challenged with Na+ (Carden et al., 2003; Golldack et al., 2003; Peng et al., 2004). See Yeo (1998) and Maathuis and Amtmann (1999) for detailed reviews of the interrelations of Na+ and K+.
Because neither Na+ nor K+ is altered by metabolism or incorporated into other molecules, cytoplasmic concentration is determined by a combination of influx and efflux transport activities. Much has been learned about the molecules that conduct these fluxes since the first K+ channel genes were cloned from Arabidopsis (Anderson et al., 1992; Sentenac et al., 1992). Now at least 35 genes in the Arabidopsis genome are known or believed to encode molecules that transport K+ (Mäser et al., 2001). These include homologs of animal shaker K+ channels, KCO channels, high-affinity K+ transporter (HKT) cotransporters, and the KUP/HAK/KT family of transporters (Véry and Sentenac, 2003). A reverse genetic approach demonstrated that plant growth depends on the AKT1 (Arabidopsis K+ transporter1) inward-rectifying channel for K+ uptake into roots in certain conditions, but that parallel, non-AKT1 pathways suffice in other conditions (Hirsch et al., 1998; Spalding et al., 1999; Dennison et al., 2001). A combination of electrophysiology and reverse genetics demonstrated that SKOR (shaker-like K+ outward-rectifying channel), a channel expressed in the stele of the root, conducts K+ efflux into the xylem for distribution from root to shoot (Gaymard et al., 1998). The shoots of skor plants contain 50% less K+, but K+ content of the roots is normal (Gaymard et al., 1998).
Uptake of Na+ from the growth medium by roots is, energetically speaking, a downhill process. Thus, passive transporters such as channels may be significant conduits for entry of Na+ into roots. Indeed, electrophysiological studies have shown that Na+-conducting channels exist in the plasma membranes of root cells (Demidchik et al., 2002). However, the genes responsible for these channel activities have not been identified. The HKT1 Na+-K+ symporter is the best-characterized molecule capable of transporting Na+ across the plasma membrane of Arabidopsis root cells (Schachtman and Schroeder, 1994; Rubio et al., 1995; Uozumi et al., 2000). Studies of heterologously expressed HKT1, hkt1 knockout plant lines, and expression analyses indicate that HKT1 conducts Na+ uptake as well as root-to-shoot distribution (Rubio et al., 1999; Uozumi et al., 2000; Mäser et al., 2002a; Garciadeblas et al., 2003).
Regardless of the mechanism by which Na+ enters the cytoplasm, an active mechanism for its removal is necessary for survival in saline conditions (Zhu, 2003). The two strategies Arabidopsis appears to employ are efflux to the apoplast and vacuolar sequestration. In this case, a remarkable amount of molecular information has been obtained. The SOS1 (salt overly sensitive1) gene encodes a plasma membrane Na+-H+ antiporter. Mutations in the gene create a severe salt-sensitive phenotype due to impaired Na+ efflux (Wu et al., 1996; Shi et al., 2000; Qiu et al., 2002). The transcript level of SOS1 in seedlings, very low or undetectable under nonstress growth conditions, appears primarily in root epidermal cells and cells surrounding the vascular tissues in response to Na+ treatment (Shi et al., 2000, 2002). Activity of the SOS1 antiporter is regulated by the SOS2 Ser/Thr protein kinase (Qiu et al., 2002; Quintero et al., 2002), which is in turn regulated by the SOS3 Ca2+-binding protein (Liu and Zhu, 1998; Liu et al., 2000). Loss-of-function sos2 alleles show a salt-sensitive phenotype (Zhu et al., 1998), as do sos3 mutants when faced with the extra demand of Ca2+ deficiency (Liu and Zhu, 1997). Another member of the family of Na+-H+ antiporters to which SOS1 belongs is AtNHX1. This molecule uses the proton gradient existing across the tonoplast to move Na+ into the vacuole (Apse et al., 1999, 2003; Gaxiola et al., 1999; Darley et al., 2000). Overexpression of AtNHX1 in plants creates a remarkable degree of salt tolerance (Apse et al., 1999; Zhang and Blumwald, 2001; Zhang et al., 2001). Thus, Arabidopsis removes Na+ from the cytoplasm by actively transporting it either outward across the plasma membrane or into the vacuole, and in both cases the transport is energized by a difference in proton electrochemical potential.
The purpose of this study was to obtain molecular-level information about the mechanism responsible for the well-known interrelationships between Na+ and K+ by studying the mutants that have helped define the current models of K+ acquisition and Na+ tolerance. The results indicate that the SOS Na+-extrusion system protects the AKT1 K+-acquisition system from impairment by Na+.
RESULTS
In Na+-Free Conditions, sos Mutants Are Normal with Respect to K+ Nutrition
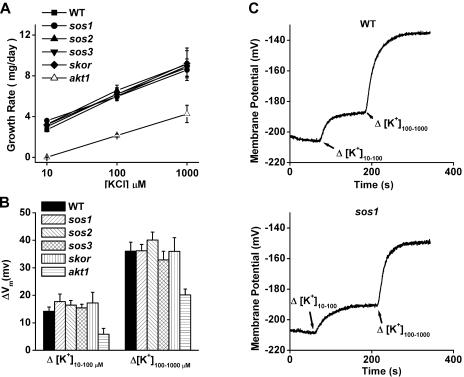
Defective Na+ efflux is clearly an important contributor to the sos mutant phenotype (poor growth in the presence of Na+), but early reports indicated that the sos1 mutation impaired K+ acquisition (Wu et al., 1996), which could contribute to the phenotype by impacting the Na+ to K+ ratio. This possibility was investigated using an approach previously used to demonstrate the key role of AKT1 in K+ uptake. First, the growth rates of sos seedlings were compared to the wild type in conditions where growth rate is limited by K+ availability and not other factors. Previous work established that including millimolar levels of 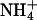 into the growth medium forces Arabidopsis seedlings to rely on the AKT1 channel for K+ uptake by blocking a non-AKT1 mechanism. As shown in Figure 1A, wild-type seedlings on such a growth medium grew at a rate that depended directly on K+ availability. Growth rate increased as K+ concentration was raised in the range from 10 to 1,000 μm. In these K+-limiting (and nominally Na+-free) conditions, each of the three sos mutants grew indistinguishably from the wild type, indicating that their ability to take up K+ was not significantly impaired. Lack of SKOR, a channel not known to play a role in K+ uptake from the medium, also did not affect growth rate in these conditions at this stage of development (days 4–8). By contrast, akt1 grew very slowly as reported previously (Hirsch et al., 1998; Spalding et al., 1999; Dennison et al., 2001). Therefore, in these conditions a mutation that impairs K+ uptake from K+-deficient media produces a phenotype (akt1), but the sos and skor mutants grow normally. Apparently, the sos and skor mutations do not affect K+ uptake to an extent that affects growth when K+ availability is limiting.
into the growth medium forces Arabidopsis seedlings to rely on the AKT1 channel for K+ uptake by blocking a non-AKT1 mechanism. As shown in Figure 1A, wild-type seedlings on such a growth medium grew at a rate that depended directly on K+ availability. Growth rate increased as K+ concentration was raised in the range from 10 to 1,000 μm. In these K+-limiting (and nominally Na+-free) conditions, each of the three sos mutants grew indistinguishably from the wild type, indicating that their ability to take up K+ was not significantly impaired. Lack of SKOR, a channel not known to play a role in K+ uptake from the medium, also did not affect growth rate in these conditions at this stage of development (days 4–8). By contrast, akt1 grew very slowly as reported previously (Hirsch et al., 1998; Spalding et al., 1999; Dennison et al., 2001). Therefore, in these conditions a mutation that impairs K+ uptake from K+-deficient media produces a phenotype (akt1), but the sos and skor mutants grow normally. Apparently, the sos and skor mutations do not affect K+ uptake to an extent that affects growth when K+ availability is limiting.
Figure 1.
Parameters related to K+ nutrition in Na+-sensitive and K+-channel mutants. A, Growth rates of mutant and wild-type seedlings on media designed to be deficient only in K+. Growth rates of all mutants were similar to the wild type, increasing as K+ availability increased, except akt1, which displayed its typical poor growth on K+-limiting conditions. Each value plotted represents the mean of three independent trials ±se, with each trial involving 24 seedlings. B, K+ permeability of the plasma membrane of root cells measured as K+-induced shifts in Vm in the same Na+-sensitive and K+-channel mutants. The sos and skor mutants displayed wild-type K+ permeabilities, whereas the reduced permeability typical of akt1, believed to be the cause of its growth phenotype, was observed. The values shown for each genotype are means ± se of at least five independent measurements on separate seedlings. C, Example recordings of Vm showing wild-type and sos1 individual responses to 10-fold shifts in [K+] of the bathing medium.
The relative permeability of a membrane to K+ can be assessed by measuring the change in membrane potential (Vm) caused by 10-fold shifts in the [K+] of the bathing solution. This was previously used to demonstrate the significant effect of the akt1 mutation on the membrane of cells (Hirsch et al., 1998; Spalding et al., 1999; Dennison et al., 2001). The data in Figure 1B show that none of the sos mutations affected the K+ permeability of the membrane as assessed by this method. Neither did the skor mutation. In each case, the magnitude of the change in Vm was indistinguishable from the wild type in the range from 10 μm to 1,000 μm K+. Original recordings of Vm as it undergoes shifts in response to changes in [K+] are shown in Figure 1C to demonstrate that the wild-type and sos1 responses were similar in all respects.
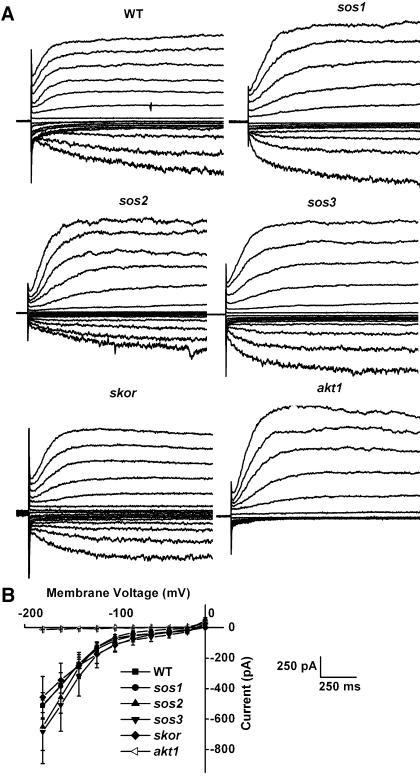
An even more specific and direct determination of how readily K+ can flow across the membrane is achievable with the patch-clamp technique. Voltage clamping in the whole-cell configuration proved to be a very sensitive and accurate method of assessing AKT1 function. Essentially all of the inward K+ currents driven by negative membrane potentials imposed on a wild-type cell were mediated by the AKT1 channel, as evidenced by their absence in the akt1 mutant. The data in Figure 2 show that each sos mutant was normal with respect to AKT1-mediated inward K+ currents. The cells used in these studies were isolated from an apical region of the root that had not yet differentiated into cortical and stelar regions. Perhaps for this reason the skor mutation did not affect the outward currents. The fact that the inward currents are normal in the absence of this shaker-like K+ channel is further evidence that they are exclusively AKT1 mediated. The conclusion to be drawn from the data presented in Figures 1 and 2 is that in Na+-free but K+-limiting conditions, the sos mutants show no indications that K+ uptake or K+ homeostasis is impaired. The membranes of sos mutants are normally permeable to K+, and the mutants grow normally when K+ availability is limiting. Thus, their sensitivity to salt is unlikely to result from a defect in K+ nutrition.
Figure 2.
Patch-clamp recordings of K+ channel currents in cells obtained from the apex of roots of Na+-sensitive and K+-channel mutants. A, Representative recordings of whole-cell currents obtained from cells that were voltage clamped at 20 mV steps between −180 mV and +120 mV. Only the akt1 mutant displayed abnormal inward (negative) currents, which are here plotted downward. B, Steady-state inward K+ current plotted versus the clamped membrane potential. Shown are the average steady-state values ± SEM obtained for five cells of each genotype. The sos and skor mutants were not different than wild type, but akt1 showed its characteristic absence of inward current.
Na+ Impairs the K+ Permeability of sos1 Root Cell Membranes
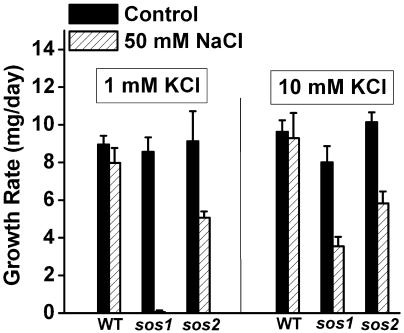
Addition of 50 mm NaCl to a growth medium containing 1 mm KCl did not affect the growth of wild-type plants. This level of Na+ would not be considered stressful to Arabidopsis seedlings. However, sos1 is extremely sensitive to 50 mm Na+, as shown previously and in the growth-rate measurements in Figure 3. While this condition was nearly lethal for sos1 seedlings, sos2 seedlings were able to grow at approximately half the rate as the wild type. Because the medium contained 2.5 mm Ca2+, sos3 mutants did not show a Na+-sensitive phenotype (data not shown). Increasing [K+] in the medium from 1 mm to 10 mm did not increase the growth rate of wild-type plants, but this increase enabled sos1 seedlings to grow and develop at a measurable rate. These results indicate that in the presence of 50 mm Na+, growth of sos1 was limited by K+ availability, an indication of impaired K+ uptake.
Figure 3.
Na+ sensitivity of growth rate in sos1 and sos2 seedlings depends on [K+] of the medium. With 1 mm KCl present in the medium, the growth rate of sos1 seedlings is greatly impaired by the presence of 50 mm NaCl. The growth rate of sos2 seedlings is impaired to a lesser extent, and the wild type is not affected by this salt treatment. Providing 10-fold more K+ (right) increased the growth rate of sos1 seedlings, indicating their growth rate in the presence of 50 mm Na+ is limited by K+ availability. Each value plotted represents the mean of 3 independent trials ±se, with each trial involving 24 seedlings.
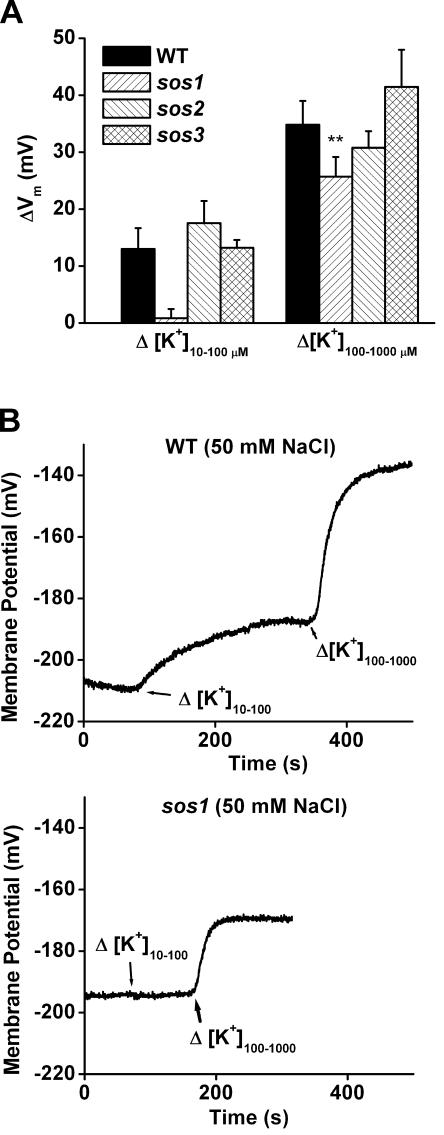
K+-induced shifts in membrane potential were measured in wild-type and sos plants to determine if Na+ treatment impaired K+ permeability of the membrane as evidenced by the growth results. The results in Figure 4, when compared with those in Figure 1B, show that a 10-h pretreatment with 50 mm NaCl did not affect the K+ permeability of wild-type root cells in the 10 to 100 μm range or the 100 to 1,000 μm range. However, the same treatment almost abolished the K+ permeability of sos1 root cells in the lower concentration range and had a smaller though significant effect in the higher concentration range. At least 50% of the K+ permeability measured in these conditions can be attributed to AKT1 channel activity (Spalding et al., 1999). Thus, the plasma membrane of sos1 seedlings treated with 50 mm Na+ in terms of this assay is similar to the membrane of akt1 roots. The 50 mm Na+ pretreatment had no obvious effect on the K+ permeability of sos2 and sos3, consistent with their weaker or nonexistent growth phenotypes in the present conditions. One interpretation is that SOS1 protects the AKT1 channel from Na+ and that higher concentrations or longer pretreatments must be used to create the same effect in the mutants lacking the SOS2 and SOS3 regulatory molecules.
Figure 4.
Na+ impairs the K+ permeability of the plasma membrane of sos1 root cells but not the wild type. A, K+ permeability of the plasma membrane of root cells measured as K+-induced shifts in Vm in seedlings pretreated for 10 h with 50 mm NaCl. The K+ permeability of sos1 root cells measured by shifting [K+] from 10 to 100 μm was severely impaired by the Na+ pretreatment, while other genotypes were not. The response to shifts from 100 to 1,000 μm was also less in sos1 plants relative to wild type (P = 0.01). This effect of Na+ on K+ permeability can explain the growth rate results in Figure 3. The values shown for each genotype are means ± se of at least five independent measurements on separate seedlings. B, Example recordings of Vm showing wild-type and sos1 individual responses to 10-fold shifts in [K+] of the bathing medium.
AKT1 Is a Target of Cytoplasmic Na+
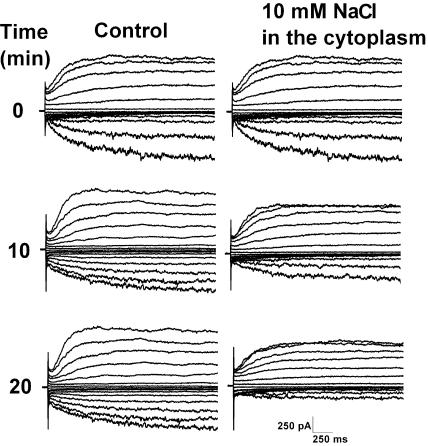
With respect to growth and membrane permeability, sos1 seedlings in the presence of 50 mm Na+ resembled akt1 seedlings, raising the possibility that the AKT1 channel is a target of cytoplasmic Na+. This possibility was tested by introducing 10 mm Na+ into the cytoplasm of a wild-type protoplast via the patch pipette. At various times following attainment of the whole-cell configuration, a set of voltage pulses was applied to drive inward and outward currents. Figure 5 shows that without Na+ in the pipette, the inward and outward currents were fairly constant, unchanging over time. However, when Na+ was present in the patch pipette (and therefore present in the cytoplasm), the inward currents were inhibited. The outward currents were much less affected. Cytoplasmic Na+ specifically impaired AKT1 inward K+ currents. The data in Figures 1 through 5 indicate that sos1 grows poorly when the conditions used here are supplemented with 50 mm Na+ at least in part because excess Na+ in the cytoplasm impairs K+ permeability, at least in part by impairing the AKT1 channel. According to this model, sos1 seedlings presented with Na+ should suffer from reduced K+ and higher Na+ levels, both adversely affecting the Na+ to K+ ratio.
Figure 5.
Inhibition of whole-cell K+ currents by intracellular Na+. Wild-type cells displayed relatively constant whole-cell K+ currents over a 20 min time frame when the pipette solution was nominally free of Na+ (left column of curves). When 10 mm Na+ was present in the pipette, the currents were inhibited over this same time period. The inward currents (downward deflections) mediated by the AKT1 channel were inhibited more than the outward currents. Similar results were obtained in three control and three NaCl-treated cells.
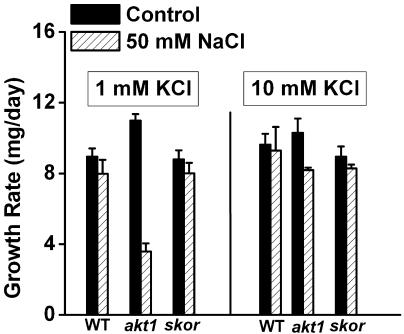
Growth of akt1 Seedlings Is Sensitive to Na+
If maintaining an appropriate Na+ to K+ ratio is a key to withstanding Na+ stress, then akt1 seedlings should be Na+ sensitive due to impaired ability to take up K+. The growth rate data in Figure 6 show this to be the case. Adding 50 mm Na+ to a medium containing 1 mm K+ significantly inhibited growth of akt1 seedlings. The poor growth resulting from the Na+ treatment could be restored to wild-type levels by increasing the [K+] to 10 mm, indicating that the poor growth in the presence of Na+ was related to K+ uptake. The sensitivity to Na+ of akt1 seedlings was not as severe as the sos1 phenotype, perhaps because sos1 plants suffer from reduced ability to remove Na+ and take up K+, whereas akt1 plants suffer only from the latter. The akt1 mutation probably impacts only the K+ side of the Na+ to K+ ratio, whereas the sos1 mutation worsens the ratio by affecting both sides.
Figure 6.
Effect of Na+ on the growth rates of two K+-channel mutants. The growth rate of akt1 seedlings but not skor seedlings was inhibited by 50 mm NaCl when [K+] in the medium was low (1 mm). This poor growth could be rescued by increasing the [K+] to 10 mm. Each value plotted represents the mean of 3 independent trials ±se, with each trial involving 24 seedlings.
The skor mutation did not affect growth on media containing Na+, indicating that loading of K+ into the xylem was either not affected by the mutation in these conditions or that process does not impact the Na+ to K+ ratio to a significant extent.
DISCUSSION
The existence of mutants defective in K+ uptake or distribution (akt1 and skor, respectively), and mutants sensitive to salt (sos1, 2, and 3) make it possible to study the mechanism by which K+ can protect plants against Na+ stress at the molecular level. The physiological results obtained in this study of cells and seedlings indicate that extrusion of Na+ from the cytoplasm by SOS1 protects the K+ permeability of the membrane, and the AKT1 K+ channel in particular, from inhibition by Na+. A logical extension of this conclusion is that AKT1 is a target of salt stress, and its impairment by Na+ at least partly explains why extra K+ mitigates Na+ toxicity.
In the absence of Na+, SOS1 and its regulators appear not to influence the ability of the Arabidopsis root to take up K+. Our tests of K+-channel function, membrane permeability, and growth rate on media specifically designed to reveal deficiencies in K+ uptake found no differences between the three sos mutants and the wild type. This is consistent with a study that reported normal Rb+ uptake in sos2 and sos3 plants (Liu and Zhu, 1997; Zhu et al., 1998) and low expression of the three SOS genes in the absence of Na+ (Liu et al., 2000; Shi et al., 2000). However, other studies employing different techniques have concluded that mutations in the SOS pathway impair K+ uptake in the absence of Na+ to an extent that impacts growth (Wu et al., 1996; Ding and Zhu, 1997; Rus et al., 2001). Thus, there appears to be a direct contradiction between the present and past results with respect to the role of SOS1 in K+ uptake when Na+ is not present or present at nonstressful levels. Several differences in the composition of the media and age of the plant material used in the different studies may account for some of the discrepancy, but other factors such as the orientation of the plate (horizontal versus vertical) and the actual parameter measured (rate of fresh weight increase versus change in root length after transfer) may be of greater significance. In this study, the plates were maintained horizontally, and the rate of fresh weight accumulation was determined. The shoot was not in contact with the medium, so the increase in fresh weight in this arrangement was entirely dependent on the uptake of K+ by the root. This is not necessarily the case when plates are maintained vertically such that the entire surface of the plant is in contact with the K+ supply. When plants are grown on vertical plates, structures other than the root may contribute to K+ uptake. This study quantified the rate at which the mass of the plant increased in a situation where uptake of K+ from the medium by the root was rate limiting. This assay is the most appropriate for the questions addressed by this work. In this assay, a role for the SOS system was not detected.
Another finding presented here that may seem inconsistent with previous results is the different behavior of sos2 and sos3 mutants compared to sos1. If SOS1 activity depends on the SOS2/SOS3 regulatory system as ample evidence indicates (Quintero et al., 2002), then equivalent consequences of mutations in any of the three may be expected. Yet, the impairment of K+ permeability by Na+ in sos1 plants was not observed in sos2 and sos3 roots (Fig. 4A). A probable explanation is that a basal level of SOS1 activity sufficient to maintain K+ permeability of root cells over the time frame covered in this study persists in the absence of the SOS2/SOS3 regulatory system. Indeed, SOS1 expression is still Na+-inducible in sos2 and sos3 backgrounds (Shi et al., 2000). Higher concentrations of Na+ or longer treatment times may produce similar phenotypes in these three sos mutants.
Previous studies have shown that total Na+ levels are lower in sos1 plants than wild type, an observation that may seem at odds with the present conclusion that extra Na+ in the cytoplasm of sos1 seedlings impairs the K+ permeability of the plasma membrane. However, SOS1 is believed to participate in the loading of Na+ into the xylem for transport to the shoot, where it may be sequestered in vacuoles (Shi et al., 2002). An sos1 plant would be expected to have lower total levels of Na+ due to impairment of this loading function, as well as higher levels in the narrow cytoplasmic compartment of root cells due to impaired efflux to the apoplast. The lower total Na+ measured in whole sos1 plants is probably not inconsistent with higher cytoplasmic concentrations in root cells postulated to explain the present results.
An important issue that this work does not address is the mechanism by which cytoplasmic Na+ impairs AKT1 function. One possibility is a direct effect of the Na+ ion on the AKT1 channel protein, an interaction that reduces the open probability or conductance of the channel. Such a direct effect may be expected to occur faster than the observed time frame of several minutes. However, without knowing how much faster Na+ influx from the pipette is compared to efflux across the membrane of the cell under study, it is difficult to know what time course to expect for a direct effect. An alternative mechanism is that Na+ impairs the activity of a positive regulator of AKT1. A third possibility is that Na+ interferes with the delivery of AKT1 channels to the membrane. It is now clear that the process of channel trafficking to and from the plasma membrane persists in a patch-clamped cell (Hurst et al., 2004). If Na+ impaired the delivery portion of the process more than the endocytotic retrieval portion, the number of active AKT1 channels in the membrane would decrease, as observed. The observed resistance of the outward currents to the same Na+ treatment could be accommodated by this mechanism if the outward-rectifying channels reside in the membrane longer than AKT1.
Another unresolved important issue is the mechanism responsible for Na+ entry. An attractive candidate is the HKT1 Na+-K+ symporter. It is certainly capable of mediating Na+ influx in heterologous systems (Rubio et al., 1995; Uozumi et al., 2000), and mutations in it have been shown to suppress the sos3 phenotype, possibly by impairing Na+ influx (Rus et al., 2001). However, Essah et al. (2003) reported that an hkt1 knockout mutation did not affect Na+ flux into roots. Mäser et al. (2002a) and Berthomieu et al. (2003) concluded that the hkt1 phenotypes and expression pattern of the gene (restricted to the vascular tissue) point to a role for the transporter in the distribution or circulation of Na+ within the plant. Tester and Davenport (2003) and Mäser et al. (2002b) critically address the important issue of how Na+ enters plant cells and discuss the candidate transporters. It is still very much an open question.
No indication of a role for the SKOR channel in K+ nutrition or Na+ resistance was evident in the results obtained here. The best known function of this channel, loading of K+ into the xylem (Gaymard et al., 1998), may not be so critical for seedlings growing in a closed system (taped petri plates). In these nontranspiring conditions and at this early stage of development, distribution of K+ to the hypocotyl and cotyledon may not depend much on loading of K+ into the xylem. Instead, most of the results indicate that the interplay of Na+ and K+ during this phase of seedling establishment occurs at the plasma membranes and in the cytoplasms of cells that interact rather directly with the medium. It is the K+-uptake mechanism, which depends heavily on the AKT1 channel, that is sensitive to Na+ in the absence of SOS1 Na+-efflux activity. The AKT1 channel is a target of cytoplasmic Na+ when influx of this injurious cation is poorly mitigated, as in the sos1 background. This finding and the identification of additional specific targets in the future may facilitate efforts to improve salt tolerance in plants.
MATERIALS AND METHODS
Plant Material and Growth Measurements
Dr. Jian-Kang Zhu (University of California, Riverside, CA) provided seeds of the three sos mutants, which are in the Columbia genetic background. Therefore, Col-0 was the wild-type strain used in all the experiments reported here. Dr. Hervé Sentenac (INRA/CNRS, Montpellier, France) provided skor seeds, which like akt1 are in the Wassilewskija (WS) background. Extensive previous experience has shown no significant differences between Col-0 and WS seedlings in any of the assays employed here (data not shown), so comparisons of responses of akt1 and skor to Col-0 are valid.
The base medium used in the growth studies contained 2.5 mm Ca(NO3)2, 2 mm MgSO4, 0.1 mm NaFeEDTA, 25 μm H3BO3, 2 μm ZnSO4, 2 μM MnSO4, 0.5 μm CuSO4, 0.5 μm Na2MoO4, 0.01 μm CoCl2, 0.5% Suc, and 5 mm MES. The pH of the mixture was adjusted to 5.7 with 1,3 bis[tris(hydroxymethyl) methylamino]propane (BTP). Agarose (0.7%, w/v) was added instead of agar, which contains significant ionic contaminants, and then the mixture was autoclaved for 20 min. To avoid precipitates from forming during autoclaving, NH4H2PO4 was added from a concentrated stock solution afterward to achieve a final concentration of 5 mm 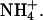 KCl and NaCl were added from concentrated stock solutions to achieve the stated final concentrations.
KCl and NaCl were added from concentrated stock solutions to achieve the stated final concentrations.
To measure growth rates, 24 surface-sterilized seeds were sown with equal spacing across square petri plates containing the solidified growth medium. The plates were maintained in darkness at 4°C for 48 h before being placed horizontally in a growth chamber set to deliver 16-h days and 8-h nights at 21°C. After 4 d of growth, the fresh weight of the 24 seedlings was determined to the nearest 0.1 mg. The harvesting/weighing procedure was also performed on a group of seedlings that had grown for 8 d in the same conditions. The difference in mass between the two time points was divided by 4 to obtain the average rate of fresh weight increase (growth rate in mg d−1) for the group of seedlings between 4 and 8 d of growth.
Electrophysiology
To measure Vm, seedlings grown for 4 d on agarose plates containing 100 μm KCl and 1 mm CaCl2 were mounted on an agarose surface in a recording chamber containing a bathing solution that flowed continuously and could be changed by the turn of a stopcock. Membrane potential was measured by inserting a salt-filled, glass microelectrode into a cell within 100 μm of the root apex in intact seedling. The bathing solution consisted of the stated concentrations of KCl and NaCl plus 1 mm CaCl2, and 5 mm MES. The pH was adjusted to 5.7 with BTP. The recording equipment and microelectrode details were described previously (Hirsch et al., 1998). The seedlings used in the experiments that produced the results shown in Figure 4 were grown for 10 h with 50 mm NaCl, 10 μm KCl, and 1 mm CaCl2 before Vm was measured.
Protoplasts for patch-clamping experiments were isolated from root tips harvested from seedlings grown vertically on media containing 100 μm KCl for 5 to 7 d (Yu and Wu, 1999). A micromanipulator-mounted razor was used to excise to most apical 150 μm of the root. Several excised tips were collected and incubated with shaking (approximately 100 rpm) in an enzyme solution containing 15 mg mL−1 Cellulysin (Calbiochem, San Diego), 3 mg mL−1 pectinase (Sigma, St. Louis), and 5 mg mL−1 bovine serum albumin (Sigma) dissolved in the bathing solution described below. After 1 h of incubation, a large excess of bathing solution minus the enzymes was added to the digested tissue, and the whole was poured through a piece of nylon net with a 25-μm pore size. The filtrate was centrifuged at approximately 150g for 5 min, which settled the protoplasts to the bottom of the tube. The supernatant was removed and the protoplasts were resuspended with enzyme-free bathing solution and centrifuged again. The final pellet was maintained on ice for the later usage.
The base of the protoplast isolation solution was the same as the solution used to bath the cells during patch-clamp recording. It consisted of 30 mm KCl, 10 mm CaCl2, 2 mm MgCl2, 5 mm MES, and 250 mm sorbitol. BTP was added to adjust the pH to 5.7. The patch pipette was filled with a solution containing 130 mm K-Glu, 2 mm EGTA, 5 mm HEPES, 4 mm Mg-ATP, and 130 mm sorbitol. The pH was adjusted to 7.0 with BTP. The amplifier, recording equipment, and pipette construction were described previously (Cho and Spalding, 1996).
This work was supported by the National Science Foundation (Career Award no. IBN–9734478 and grant no. IBN–0132803 to E.P.S.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.049213.
References
- Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF (1992) Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 89: 3736–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Overexpression of a vacuolar Na+/H+ antiport confers salt tolerance in Arabidopsis. Science 285: 1256–1258 [DOI] [PubMed] [Google Scholar]
- Apse MP, Sottosanto JB, Blumwald E (2003) Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J 36: 229–239 [DOI] [PubMed] [Google Scholar]
- Berthomieu P, Conejero G, Nublat A, Brackenbury WJ, Lambert C, Savio C, Uozumi N, Oiki S, Yamada K, Cellier F, et al (2003) Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J 22: 2004–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden DE, Walker DJ, Flowers TJ, Miller AJ (2003) Single-cell measurements of the contributions of cytosolic Na+ and K+ to salt tolerance. Plant Physiol 131: 676–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MH, Spalding EP (1996) An anion channel in Arabidopsis hypocotyls activated by blue light. Proc Natl Acad Sci USA 93: 8134–8138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley CP, van Wuytswinkel OC, van der Woude K, Mager WH, de Boer AH (2000) Arabidopsis thaliana and Saccharomyces cerevisiae NHX1 genes encode amiloride sensitive electroneutral Na+/H+ exchangers. Biochem J 351: 241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Davenport RJ, Tester M (2002) Nonselective cation channels in plants. Annu Rev Plant Biol 53: 67–107 [DOI] [PubMed] [Google Scholar]
- Dennison KL, Robertson WR, Lewis BD, Hirsch RE, Sussman MR, Spalding EP (2001) Functions of AKT1 and AKT2 potassium channels determined by studies of single and double mutants of Arabidopsis. Plant Physiol 127: 1012–1019 [PMC free article] [PubMed] [Google Scholar]
- Ding L, Zhu JK (1997) Reduced Na+ uptake in the NaCl-hypersensitive sos1 mutant of Arabidopsis thaliana. Plant Physiol 113: 795–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essah PA, Davenport R, Tester M (2003) Sodium influx and accumulation in Arabidopsis. Plant Physiol 133: 307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciadeblas B, Senn ME, Banuelos MA, Rodriguez-Navarro A (2003) Sodium transport and HKT transporters: the rice model. Plant J 34: 788–801 [DOI] [PubMed] [Google Scholar]
- Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferriere N, Thibaud JB, Sentenac H (1998) Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 94: 647–655 [DOI] [PubMed] [Google Scholar]
- Gaxiola RA, Rao R, Sherman A, Grisafi P, Alper SL, Fink GR (1999) The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc Natl Acad Sci USA 96: 1480–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golldack D, Quigley F, Michalowski CB, Kamasani UR, Bohnert HJ (2003) Salinity stress-tolerant and -sensitive rice (Oryza sativa L.) regulate AKT1-type potassium channel transcripts differently. Plant Mol Biol 51: 71–81 [DOI] [PubMed] [Google Scholar]
- Hirsch RE, Lewis BD, Spalding EP, Sussman MR (1998) A role for the AKT1 potassium channel in plant nutrition. Science 280: 918–921 [DOI] [PubMed] [Google Scholar]
- Hurst AC, Meckel T, Tayefeh S, Thiel G, Homann U (2004) Trafficking of the plant potassium inward rectifier KAT1 in guard cell protoplasts of Vicia faba. Plant J 37: 391–397 [DOI] [PubMed] [Google Scholar]
- Liu JP, Ishitani M, Halfter U, Kim CS, Zhu JK (2000) The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc Natl Acad Sci USA 97: 3730–3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Zhu JK (1997) An Arabidopsis mutant that requires increased calcium for potassium nutrition and salt tolerance. Proc Natl Acad Sci USA 94: 14960–14964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Zhu JK (1998) A calcium sensor homolog required for plant salt tolerance. Science 280: 1943–1945 [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Amtmann A (1999) K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann Bot (Lond) 84: 123–133 [Google Scholar]
- Mäser P, Eckelman B, Vaidyanathan R, Horie T, Fairbairn DJ, Kubo M, Yamagami M, Yamaguchi K, Nishimura M, Uozumi N, et al (2002. a) Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1. FEBS Lett 531: 157–161 [DOI] [PubMed] [Google Scholar]
- Mäser P, Gierth M, Schroeder JI (2002. b) Molecular mechanisms of potassium and sodium uptake in plants. Plant Soil 247: 43–54 [Google Scholar]
- Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJM, Sanders D, et al (2001) Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol 126: 1646–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YH, Zhu YF, Mao YQ, Wang SW, Su WA, Tang ZC (2004) Alkali grass resists salt stress through high [K+] and an endodermis barrier to Na+. J Exp Bot 55: 939–949 [DOI] [PubMed] [Google Scholar]
- Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK (2002) Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci USA 99: 8436–8441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero FJ, Ohta M, Shi H, Zhu JK, Pardo JM (2002) Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc Natl Acad Sci USA 99: 9061–9066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI (1995) Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 270: 1660–1663 [DOI] [PubMed] [Google Scholar]
- Rubio F, Schwarz M, Gassmann W, Schroeder JI (1999) Genetic selection of mutations in the high affinity K+ transporter HKT1 that define functions of a loop site for reduced Na+ permeability and increased Na+ tolerance. J Biol Chem 274: 6839–6847 [DOI] [PubMed] [Google Scholar]
- Rus A, Yokoi S, Sharkhuu A, Reddy M, Lee BH, Matsumoto TK, Koiwa H, Zhu JK, Bressan RA, Hasegawa PM (2001) AtHKT1 is a salt tolerance determinant that controls Na+ entry into plant roots. Proc Natl Acad Sci USA 98: 14150–14155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI (1994) Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature 370: 655–658 [DOI] [PubMed] [Google Scholar]
- Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon JM, Gaymard F, Grignon C (1992) Cloning and expression in yeast of a plant potassium ion transport system. Science 256: 663–665 [DOI] [PubMed] [Google Scholar]
- Shi HZ, Ishitani M, Kim C, Zhu JK (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA 97: 6896–6901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HZ, Quintero FJ, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 14: 465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding EP, Hirsch RE, Lewis DR, Qi Z, Sussman MR, Lewis BD (1999) Potassium uptake supporting plant growth in the absence of AKT1 channel activity. Inhibition by ammonium and stimulation by sodium. J Gen Physiol 113: 909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot (Lond) 91: 503–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozumi N, Kim EJ, Rubio F, Yamaguchi T, Muto S, Tsuboi A, Bakker EP, Nakamura T, Schroeder JI (2000) The Arabidopsis HKT1 gene homolog mediates inward Na+ currents in Xenopus laevis oocytes and Na + uptake in Saccharomyces cerevisiae. Plant Physiol 122: 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry AA, Sentenac H (2003) Molecular mechanisms and regulation of K+ transport in higher plants. Annu Rev Plant Biol 54: 575–603 [DOI] [PubMed] [Google Scholar]
- Wu SJ, Ding L, Zhu JK (1996) SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell 8: 617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo A (1998) Molecular biology of salt tolerance in the context of whole-plant physiology. J Exp Bot 49: 915–929 [Google Scholar]
- Yu CJ, Wu WH (1999) Identification and characterization of inward K+-channels in plasma membranes of Arabidopsis root cortex cells. Sci China Ser C Life Sci 42: 307–315 [DOI] [PubMed] [Google Scholar]
- Zhang HX, Blumwald E (2001) Transgenic salt tolerant tomato plants accumulate salt in the foliage but not in the fruits. Nat Biotechnol 19: 765–768 [DOI] [PubMed] [Google Scholar]
- Zhang HX, Hodson J, Williams JP, Blumwald E (2001) Engineering salt-tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc Natl Acad Sci USA 98: 12832–12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6: 441–445 [DOI] [PubMed] [Google Scholar]
- Zhu JK, Liu JP, Xiong LM (1998) Genetic analysis of salt tolerance in Arabidopsis: evidence for a critical role of potassium nutrition. Plant Cell 10: 1181–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]