Abstract
The fatty acid elongase [often designated FAE or β-(or 3-) ketoacyl-CoA synthase] is a condensing enzyme and is the first component of the elongation complex involved in synthesis of erucic acid (22:1) in seeds of garden nasturtium (Tropaeolum majus). Using a degenerate primers approach, a cDNA of a putative embryo FAE was obtained showing high homology to known plant elongases. This cDNA contains a 1,512-bp open reading frame that encodes a protein of 504 amino acids. A genomic clone of the nasturtium FAE was isolated and sequence analyses indicated the absence of introns. Northern hybridization showed the expression of this nasturtium FAE gene to be restricted to the embryo. Southern hybridization revealed the nasturtium β-ketoacyl-CoA synthase to be encoded by a small multigene family. To establish the function of the elongase homolog, the cDNA was introduced into two different heterologous chromosomal backgrounds (Arabidopsis and tobacco [Nicotiana tabacum]) under the control of a seed-specific (napin) promoter and the tandem 35S promoter, respectively. Seed-specific expression resulted in up to an 8-fold increase in erucic acid proportions in Arabidopsis seed oil, while constitutive expression in transgenic tobacco tissue resulted in increased proportions of very long chain saturated fatty acids. These results indicate that the nasturtium FAE gene encodes a condensing enzyme involved in the biosynthesis of very long chain fatty acids, utilizing monounsaturated and saturated acyl substrates. Given its strong and unique preference for elongating 20:1-CoA, the utility of the FAE gene product for directing or engineering increased synthesis of erucic acid is discussed.
Very long chain fatty acids (VLCFAs) with 20 carbons or more are widely distributed in nature. In plants, they are mainly found in epicuticular waxes and in the seed oils of a number of plant species, including members of the Brassicaceae, Limnantheceae, Simmondsia, and Tropaeolaceae (Harwood, 1996; Post-Beittenmiller, 1996; Ghanevati and Jaworski, 2001). A strategic goal in oilseed modification is to genetically manipulate high erucic acid germ plasm of the Brassicaceae to increase the content of erucic acid (22:1 Δ13) and other strategic VLCFAs in the seed oil for industrial niche market needs (Puyaubert et al., 2001; Taylor et al., 2001). Erucic acid and its derivatives are feedstocks in manufacturing slip-promoting agents, surfactants, plasticizers, nylon 1313, and surface coatings; more than 1,000 patents have been issued (Sonntag, 1991, 1995; Leonard, 1994). The current market for high erucate oils exceeds $120 million U.S./annum. Worldwide erucic acid demand is predicted to increase from about 40 million pounds (M pds) in 1990 to about 80 M pds by the year 2010. Similarly, demand for the derivative, behenic acid, is predicted to triple to about 102 M pds by 2010 (Sonntag, 1995). In recent years, production has increased to meet market needs, and high erucic acreage in western Canada is currently at a record high (D. Males, Saskatchewan Wheat Pool, personal communication). A Brassica cultivar containing erucic acid levels approaching 80% would significantly reduce the cost of producing erucic acid and its derivatives, and could meet the forecast demand for erucic and behenic acids as renewable, environmentally friendly industrial feedstocks (Leonard, 1994; Taylor et al., 2001).
VLCFAs are synthesized outside the plastid by a membrane-bound fatty acid elongation complex (elongase) using acyl-CoA substrates. The first reaction of elongation involves condensation of malonyl-CoA with a long chain substrate producing a β-ketoacyl-CoA. Subsequent reactions are reduction of β-hydroxyacyl-CoA, dehydration to an enoyl-CoA, followed by a second reduction to form the elongated acyl-CoA (Ghanevati and Jaworski, 2001). The β-ketoacyl-CoA synthase (KCS) catalyzing the condensation reaction plays a key role in determining the chain length of fatty acid products found in seed oils and is the rate-limiting enzyme for seed VLCFA production (Millar and Kunst, 1997; Katavic et al., 2001). The composition of the fatty acyl-CoA pool available for elongation and the presence and size of the neutral lipid sink are additional important factors influencing the types and levels of VLCFAs made in particular cells (Zou et al., 1997; Lardizabal et al., 2000; Han et al., 2001).
Our knowledge of the mechanism of elongation and properties of FAE1 and other elongase condensing enzymes is, in part, limited by their membrane-bound nature—as such they are more difficult to isolate and characterize than soluble condensing enzymes (Ghanevati and Jaworski, 2001; Katavic et al., 2002). The genes encoding FAE1 and its homologs have been cloned from Arabidopsis (James et al., 1995), jojoba (Simondsia chinensis; Lassner et al., 1996), and Brassica napus (Barret et al., 1998).
Ghanevati and Jaworski (2001) have performed site-directed mutagenesis experiments on the Arabidopsis FAE1 to decipher the importance of Cys and His as residues in condensing enzyme catalysis; their results have shown that Cys-223 and four His residues are essential for the enzyme activity.
In this study, we selected garden nasturtium (Tropaeolum majus) as a source of the elongase involved in VLCFA synthesis based on the fact that this plant is capable of producing significant amounts of erucic acid (70%–75% of total fatty acid; Pollard and Stumpf, 1980) and accumulates trierucin as the predominant triacylglycerol (TAG) in its seed oil (Taylor et al., 1992a). Here, we report the isolation of a nasturtium FAE gene and demonstrate the involvement of its encoded protein in the elongation of saturated and especially monounsaturated fatty acids.
RESULTS
Acyl Composition of Nasturtium cv Dwarf Cherry Rose
The acyl composition of the TAG fraction of this cultivar was typical in that it had highly enriched proportions of VLC monounsaturated fatty acids, particularly 22:1 (77.5%) and 20:1 (16.0%) with a trace of 24:1 (1.5%), and a low proportion of total C18 fatty acids (2.5%), primarily oleate (18:1 Δ9; 2.4%). The predominant TAG species were trierucin followed by 22:1/20:1/22:1 (Taylor et al., 1992a, 1993).
Substrate Specificity of Nasturtium Embryo Elongases in Vitro
Although there has been considerable debate regarding the acyl substrate for elongase activity in developing oilseeds (Hlousek-Radojcic et al., 1995), recent studies of developing seeds of B. napus have revealed the presence of two types of elongation activity in vitro: an acyl-CoA-dependent activity and an ATP-dependent activity that apparently utilizes an endogenous acyl primer (Domergue et al., 1999). A 15,000g particulate fraction was isolated from nasturtium embryos collected at mid-development (at 14–17 d after pollination), the stage which exhibited the highest enrichment in acyl-CoA-dependent elongase activity.
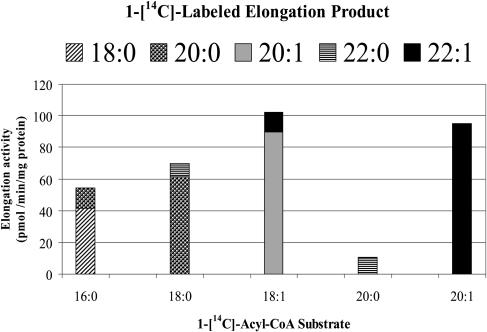
It has been shown that while ATP is necessary for acyl-CoA-dependent elongation in vitro, too high a concentration of ATP strongly inhibited elongase activity (Murphy and Mukherjee, 1989; Domergue et al., 1999). In addition, elongase enzyme activity has been reported to be stimulated by the presence of 10 μm CoA-SH (Barret and Harwood, 1997). In order to optimize reaction conditions, we assessed the roles of these two cofactors. Elongase activity was measured in vitro in the 15,000g particulate fraction from nasturtium embryos under different ATP concentrations (0–5 mm) in the presence of 10 μm CoA-SH with 18 μm [1-14C]18:1-CoA and 200 μm malonyl-CoA. The highest activity was found at cofactor concentrations of 0.75 mm for ATP and 10 μm for CoA-SH (data not shown). These were the reaction conditions used to examine activity with a range of [1-14C]acyl-CoA substrates of different chain length and degree of saturation. Our results indicate that in a developing nasturtium embryo particulate fraction, acyl-CoA-dependent elongases have the capacity to elongate a wide range of saturated (C16–C20) and monounsaturated (C18 and C20) fatty acyl moieties (Fig. 1). Of the [1-14C]-labeled acyl-CoA series (16:0-CoA, 18:0-CoA, 18:1-CoA, 20:0-CoA, 20:1-CoA, 22:1-CoA), tested in vitro, elongase(s) from mid-developing nasturtium embryos exhibited the highest activity with 18:1-CoA and 20:1-CoA (102 and 95 pmol min−1 mg−1 protein, respectively). These elongase activity rates are of the same order of magnitude as that reported for acyl-CoA elongase(s) in a similar particulate fraction from developing rapeseed embryos (Puyaubert et al., 2001). The particulate fraction was also able to elongate, in order of specificity, the saturated substrates 18:0-CoA, 16:0-CoA, and, to a much lesser extent, 20:0-CoA. In general, regardless of the [1-14C]acyl-CoA substrate supplied in vitro, the major labeled fatty acyl product was the C2 extension of its respective precursor (about 80%–90%), with the next respective C4 extension product being present in proportions of about 10% to 20% (Fig. 1). The one critical exception to this trend was the production solely of radiolabeled erucic acid from its respective [1-14C]eicosenoyl-CoA precursor. There was no detectable elongation of [1-14C]-labeled 22:1-CoA to 24:1 (data not shown), even though the latter is found in trace amounts in nasturtium seed oil.
Figure 1.
Substrate specificity of elongase(s) from mid-developing nasturtium embryos. A total of 200 μg of protein from a 15,000g particulate fraction was used in the elongase assay. Reaction conditions were as described in “Materials and Methods.” Results represent the average of three replicates. For each [1-14C]acyl-CoA substrate, the relative proportional distribution of radiolabeled fatty acid elongation product(s) is demarcated.
Isolation of Nasturtium FAE Homolog
Based on sequence homology among plant fatty acid elongase genes, a full-length cDNA clone was amplified by PCR using a degenerate primers approach and the sequence submitted to GenBank (accession no. AY082610). The nucleotide sequence had an open reading frame of 1,512 bp. Subsequently, 3 partial clones of about 0.6 kb, representative of the AY082610 FAE clone, were found among 2,800 expressed sequence tags surveyed (about 0.1% representation) from a nasturtium embryo subtracted cDNA library.
The nasturtium FAE cDNA encodes a polypeptide of 504 amino acids that is most closely related to an FAE2 from roots of maize (Zea mays; 69% amino acid identity; Schreiber et al., 2000; Fig. 2). The nasturtium FAE polypeptide also shared strong identity with FAEs from Limnanthes douglasii (67%; Cahoon et al., 2000; Marillia et al., 2002) and from seeds of jojoba (63%; Lassner et al., 1996). Homology of the nasturtium FAE to two Arabidopsis β-KCSs AraKCS (Todd et al., 1999) and AraCUT1 (Millar et al., 1999) involved in cuticular wax synthesis was on the level of 57% and 53%, respectively. These homologs all exhibit the capability to elongate saturated fatty acids to produce saturated VLCFAs. The FAE1 polypeptides involved in the synthesis of VLCFAs in Arabidopsis (James et al., 1995) and Brassica seeds (Clemens and Kunst, 1997; Katavic et al., 2002) showed approximately 52% to 54% identity with the nasturtium FAE. The nasturtium FAE protein was predicted to have a theoretical pI value of 9.3 using the algorithm of Bjellqvist et al. (1993, 1994) and a molecular mass of 56.8 kD, which are similar to the respective values reported for the B. napus CE7 and CE8 FAE homologs (Barret et al., 1998) as well as those from Brassica rapa (campestris) and Brassica oleracea (Das et al., 2002).
Figure 2.
Comparison of the amino acid sequences of the nasturtium FAE homolog (NasFAE, GenBank accession no. AY0826190) with FAE1 and related β-KCSs from other plant species. The alignment contains the sequences of the maize (ZeaFAE, GenBank accession no. AJ292770), Limnanthes (LimFAE, GenBank accession no. AF247134), jojoba (SimFAE, GenBank accession no. U37088), Arabidopsis (AraFAE, GenBank accession no. U29142), Brassica (BraFAE, GenBank accession no. AF009563), and two Arabidopsis β-KCSs associated with wax synthesis (AraKCS, GenBank accession no. AF053345, and AraCUT, GenBank accession no. AF129511). Conserved Cys and His residues are labeled with diamonds and triangles, respectively. Tyr at residue position 429 in the nasturtium FAE polypeptide is indicated by a star.
Alignment of the amino acid sequence of the nasturtium FAE with other plant condensing enzymes revealed the presence of six conserved Cys residues (Fig. 2). Further sequence analysis showed that one out of the four conserved His residues suggested to be important for Arabidopsis FAE1 activity (Ghanevati and Jaworski, 2001) was substituted with Tyr at residue position 429 in the nasturtium FAE polypeptide.
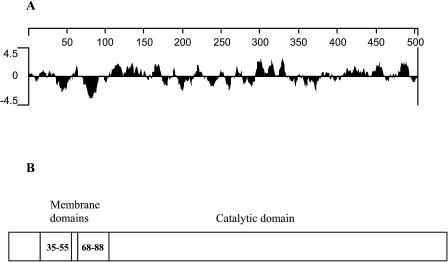
A hydropathy analysis (Kyte-Doolittle) of the amino acid sequence of the nasturtium FAE revealed several hydrophobic domains (Fig. 3A). Protein analyses with the TMAP algorithm (Persson and Argos, 1994) predicted two N-terminal transmembrane domains, the first corresponding to amino acid residues 35 to 55 and the second spanning residues 68 to 88 (Fig. 3B). Like other elongase condensing enzymes, the nasturtium FAE lacks N-terminal signal sequences typically found for plastidial or mitochondrial-targeted plant enzymes (Emanuelsson et al., 2000). It also lacks a KXKXX or KKXX motif (X = any amino acid) often found at the C terminus of proteins retained within endoplasmic reticulum membranes (Jackson et al., 1990). Rather, it is a type IIIa protein, typically present on endoplasmic reticular membranes (Nakai and Kanehisa, 1992; Das et al., 2002).
Figure 3.
Hydropathy analysis of nasturtium FAE. A, Hydropathy plot of FAE indicating the presence of several hydrophobic regions. B, Schematic representation of the putative transmembrane domains of nasturtium FAE amino acid sequence as predicted by TMAP analysis. Numbers shown in the boxes correspond to the residues of each domain in the FAE polypeptide.
An analysis of the nucleotide sequence of the corresponding nasturtium FAE genomic clone (data not shown) revealed the absence of intron sequences. A similar absence of introns was observed in homologs from Arabidopsis FAE1 (Millar and Kunst, 1997), rapeseed CE7 and CE8 (Barret et al., 1998), and high and low erucic lines of B. oleracea, B. rapa, B. napus cv Westar, and B. napus cv Hero (Das et al., 2002; Katavic et al., 2002).
Tissue-Specific Expression and Copy Number Estimate of Nasturtium FAE
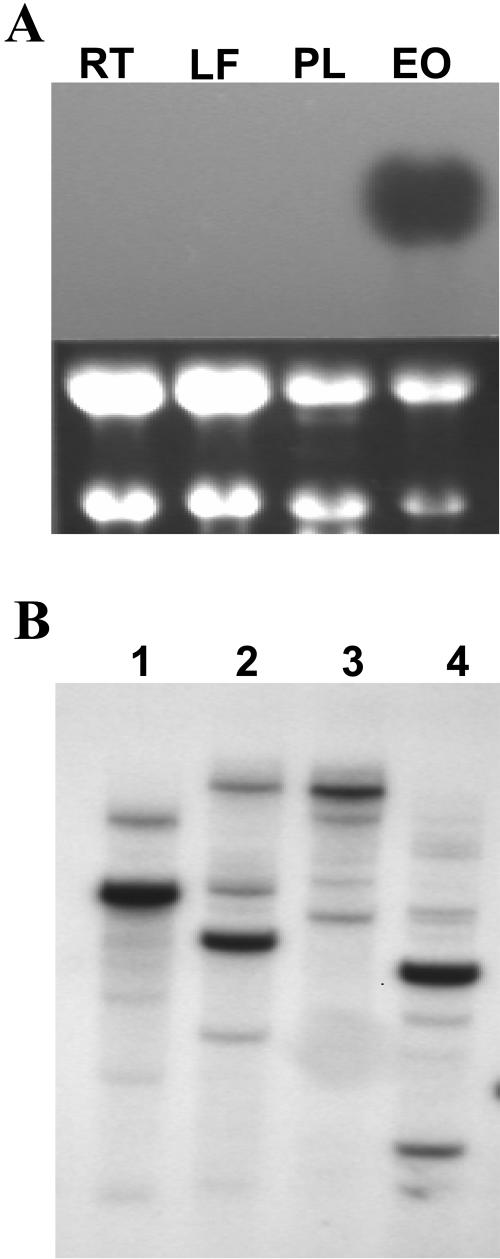
Northern-blot analyses were performed to investigate the expression profile of the FAE gene. Total RNA was isolated from different nasturtium tissues, including roots, leaves, floral petals, and mid-developing embryos. A strong hybridization signal with FAE-specific probe was observed only with RNA isolated from developing embryos (Fig. 4A).
Figure 4.
Northern and Southern analyses of nasturtium FAE. A, Northern analysis of FAE gene expression in nasturtium. Total RNA was isolated from roots (RT), leaves (LF), petals (PL), and embryos (EO). B, Southern-blot analysis of the FAE gene in nasturtium. Genomic DNA was digested with restriction enzymes EcoRI (lane 1), AccI (lane 2), NcoI (lane 3), and HindIII (lane 4).
A Southern-blot hybridization was performed with nasturtium genomic DNA digested with several restriction enzymes, including EcoRI, AccI, NcoI, and HindIII. The FAE gene has no internal EcoRI, AccI, or NcoI sites, while one internal HindIII site exists. Autoradiography revealed the presence of one strongly hybridizing fragment in all cases except with HindIII, for which two strongly hybridizing fragments were evident (Fig. 4B). In addition, a minimum of four weakly hybridizing fragments were detected. After washing under high stringency conditions, the number of hybridizing fragments was unchanged. Thus, we have concluded that nasturtium FAE belongs to a multigenic family consisting of four to six members. A similar multigenic family has been found for a rapeseed FAE1 gene member (Barret et al., 1998).
Heterologous Expression of the Nasturtium FAE in Yeast
To study the function of the protein encoded by the nasturtium FAE, the coding region was linked to the Gal-inducible GAL1 promoter in the expression vector pYES2.1/V5-His-TOPO and transformed into yeast cells: strain Inv Sci1 (Invitrogen) and two mutant strains elo2Δ and elo3Δ (Oh et al., 1997). None of the transgenic yeast strains harboring the nasturtium FAE showed any difference in fatty acid composition in comparison to yeast cells transformed with empty vector (data not shown). To study the elongase activity, microsomal proteins from yeast mutant strains elo2Δ harboring nasturtium FAE were incubated with a range of the [1-14C]-labeled acyl-CoA substrates (16:0-CoA, 18:0-CoA, 18:1-CoA, 20:1-CoA, 22:1-CoA). Nasturtium FAE did not show any elongase activity in vitro with the [1-14C]-labeled acyl-CoA substrates, while a high activity for Arabidopsis FAE1 with 18:1-CoA substrate was found (data not shown). Western-blot analyses performed with anti-His (C-terminal) FAE antibody did not detect any nasturtium FAE/V5-His fusion protein, while in the control, a strong band of about 61 kD corresponding to Arabidopsis FAE1/V5-His was observed (data not shown). The reason for the inability to functionally express the nasturtium FAE in these various yeast strains is not known. As described above, a Limnanthes and a maize FAE showed the highest homology to the nasturtium FAE polypeptide. Therefore, in the current context it is interesting to note that similar difficulties with expression of the Limnanthes and maize FAEs in yeast cells have been experienced by other researchers (E. Cahoon and R. Lessire, respectively, personal communications).
As indicated earlier, a comparison of the predicted amino acid sequence of the nasturtium FAE with other plant condensing enzymes (Fig. 2) showed that one of the four conserved His residues, suggested to be important for Arabidopsis FAE1 activity in yeast cells (Ghanevati and Jaworski, 2001), was substituted with Tyr in the nasturtium FAE polypeptide. To study the importance of His residue for enzyme activity, we used a site-directed mutagenesis approach to replace the Tyr-429 residue with His. This mutated version of nasturtium FAE was expressed in yeast cells. Analyses of fatty acid composition of transformed yeast cells showed that His at position 429 did not restore enzyme activity (data not shown). Therefore, we decided to study the function of nasturtium FAE in plant heterologous chromosomal backgrounds.
Expression of Nasturtium FAE in Tobacco Plants
To establish functional identity, the cDNA for the FAE-related polypeptides was expressed in tobacco (Nicotiana tabacum) plants under the control of the tandem 35S constitutive promoter. In addition, to assess the importance of His residues for enzyme activity, the Tyr at position 429 in the nasturtium FAE was replaced with His and subsequently used to prepare a plant transformation vector under the control of the tandem S35 promoter. Integration of the 35S/FAE/Nos expression cassette into tobacco plants was confirmed by PCR amplification on genomic DNA (data not shown). Fatty acid composition was determined in callus, leaves, and seeds of transgenic tobacco plants.
Expression of the nasturtium FAE homolog in tobacco callus resulted in an increase in proportions of VLCFAs from 3.7% in the wild-type background to as high as 8.6% (a 132% increase) in transgenic lines (Table I). In particular, the increase in proportions of saturated VLCFAs (22:0, 24:0, 26:0) was most pronounced. The fact that the synthesis of the saturated VLCFAs occurs at the expense of 16:0 and 18:0 suggests that the nasturtium FAE is able to elongate C16 and C18 fatty acids. Expression of the mutated version of the nasturtium FAE (SMF) resulted in a slight increase in the VLCFA content in tobacco callus, on average 18.5% in comparison to the wild-type background. Increased proportions of VLCFAs at the expense of LCFAs were observed in leaves of transgenic tobacco plants carrying either the nasturtium FAE or its mutated form (Table II). Comparison of fatty acid composition in tobacco tissues upon expression of the nasturtium FAE and its mutated version revealed that Tyr at position 429 is likely important to achieve full activity of the enzyme. A decreased proportion of 18:3 in leaves of transgenic tobacco lines in comparison to the wild-type (empty vector) background suggests that the metabolic pull of the elongation pathway may be somewhat stronger than that of the competing desaturation pathway.
Table I.
Fatty acid composition of transformed tobacco calli
| Construct | Fatty Acid Composition
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 16:0 | 18:0 | 20:0 | 22:0 | 24:0 | 26:0 | LCFA | VLCFA | |
| % (w/w) of total fatty acids [% increase]a | ||||||||
| RD | 20.38 ± 0.12 | 7.99 ± 0.26 | 1.32 ± 0.03 | 0.59 ± 0.03 | 0.70 ± 0.03 | 0.89 ± 0.16 | 96.28 ± 0.31 | 3.72 ± 0.31 |
| SF | 18.01 ± 0.42 | 5.23 ± 0.41 | 1.58 ± 0.54 [19.7] | 1.32 ± 0.16 [123.9] | 1.93 ± 0.27 [175.7] | 1.31 ± 0.20 [147.2] | 91.37 ± 0.84 | 8.63 ± 0.84 [131.9] |
| SMF | 19.48 ± 0.34 | 7.12 ± 0.19 | 1.30 ± 0.02 | 0.57 ± 0.03 | 0.73 ± 0.04 | 1.01 ± 0.32 | 95.59 ± 0.40 | 4.41 ± 0.40 [18.5] |
Results represent the average (±se) of 10 measurements using independent calli. Constructs: RD, Control (plasmid-only) transgenic calli; SF, 35S: nasturtium FAE transgenic calli; SMF, 35S: mutated nasturtium FAE transgenic calli.
Relative to value for calli from RD, the tobacco control (plasmid-only) calli, set at 100%.
Table II.
Fatty acid composition of transformed tobacco leaves
| Construct | Fatty Acid Composition
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 16:0 | 18:0 | 18:3 | 20:0 | 20:1Δ11 | 22:0 | 24:0 | LCFA | VLCFA | |
| % (w/w) of total fatty acids [% increase]a | |||||||||
| RD | 16.32 ± 0.14 | 3.94 ± 0.11 | 53.30 ± 0.72 | 0.53 ± 0.02 | 1.18 ± 0.00 | 0.27 ± 0.01 | 2.74 ± 0.09 | 93.77 ± 0.29 | 6.23 ± 0.29 |
| SF | 15.83 ± 0.14 | 3.35 ± 0.12 | 47.02 ± 0.66 | 0.91 ± 0.12 [71.7] | 2.34 ± 0.12 [98.3] | 0.42 ± 0.02 [55.6] | 4.14 ± 0.15 [51.1] | 88.64 ± 0.35 | 11.36 ± 0.35 [82.3] |
| SMF | 15.53 ± 0.17 | 4.00 ± 0.12 | 47.25 ± 0.85 | 0.98 ± 0.16 [84.5] | 2.61 ± 0.02 [121.2] | 0.30 ± 0.01 | 3.24 ± 0.08 [18.2] | 90.05 ± 0.28 | 9.95 ± 0.28 [59.7] |
Results represent the average (±se) of 10 measurements using leaf discs from 10 independent transgenic plants. Constructs: RD, Control (plasmid-only) transgenic leaves; SF, 35S: nasturtium FAE transgenic leaves; SMF, 35S: mutated nasturtium FAE transgenic leaves.
Relative to value for leaves from RD, the tobacco control (plasmid-only) plants, set at 100%.
Expression of nasturtium FAE in tobacco seeds resulted in a 50% increase in proportions of VLCFAs from 0.6% in the wild-type background to 0.9% in transgenic plants (data not shown). The relatively low proportions of VLCFAs in tobacco leaves and calli (Tables I and II) may be an indication that (1) in vivo, saturated fatty acids are not present at high concentrations; therefore, even a 50% increase in relative proportions does not result in high levels of VLC saturated fatty acids accumulating in glycerolipids; (2) expression of the nasturtium FAE when under the control of the 35S promoter is relatively weak.
Expression of Nasturtium FAE in Arabidopsis Plants: Constitutive Expression
Since expression of nasturtium FAE did not result in a high accumulation of VLCFA in tobacco seeds, we decided to study the effect of expressing it in Arabidopsis. Using a vacuum-infiltration method, 18 kanamycin-resistant plants were obtained. The fatty acid composition of T2 seeds was determined. A significant increase was observed only in the content of erucic acid (22:1 Δ13). On average, the level of erucic acid increased up to 3.2% (a 50% increase) in transgenic seeds compared to 2.1% in a wild-type background (data not shown). In the best transgenic lines, the content of erucic acid increased up to 4.0% (a 90% increase).
Expression of Nasturtium FAE in Arabidopsis Plants: Seed-Specific Expression
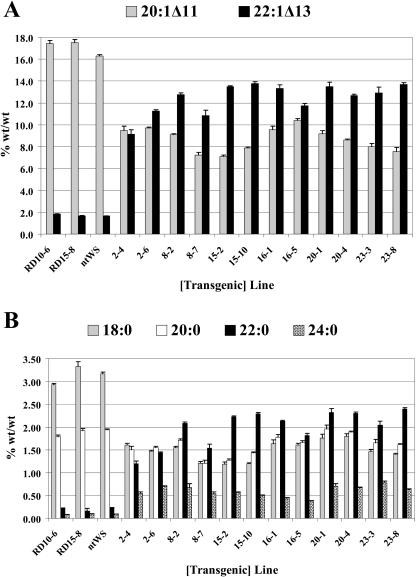
Constitutive tandem 35S-driven FAE expression did not result in a strong increase in VLCFA proportions in tobacco and Arabidopsis seeds. Thus, we decided to use the seed-specific promoter napin to study FAE expression in an Arabidopsis seed background in which total VLCFA proportions in wild-type seed oils are of the order of about 28%. From vacuum-infiltration experiments, 25 kanamycin-resistant T1 plants were selected. The T2 progeny were collected individually from each plant and the fatty acid composition determined. Significant changes in fatty acid composition in comparison to the wild type (empty vector) were found. On average, the proportion of erucic acid (22:1 Δ13) increased from 2.1% in wild type to 9.6% in T2 transgenic seeds at the expense of 20:1 Δ11 (Table III). Homozygous T3 lines were analyzed to examine the range of VLCFA proportional redistribution induced by expression of the nasturtium FAE gene. The 12 best T3 lines are shown in Figure 5, A and B. The erucic acid content was increased by up to 7- to 8-fold in lines 15-2, 15-10, 16-1, 20-1, and 23-8. Small increases in the proportions of 24:1 Δ15 were also observed (Table III). There was also a relatively significant increase in the proportions of the saturated VLCFAs 22:0 and 24:0 at the expense of 18:0 and 20:0. In both the case of the VLC monounsaturated fatty acids (Fig. 5A) and the VLC saturated fatty acids (Fig. 5B), the highest proportional increases in erucic and in [behenic + lignoceric] acids were generally correlated with the concomitant reduction in the proportion of their corresponding elongase primers, eicosenoic and [stearate + arachidic] acids, respectively.
Table III.
Fatty acid composition of transgenic Arabidopsis T2 seed oils
| Construct | Fatty Acid Composition
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 18:0 | 20:1 Δ11 | 22:0 | 22:1 Δ13 | 24:0 | 24:1 Δ15 | LCFA | VLCFA | |
| % (w/w) of total fatty acids {Range} [% increase]a | ||||||||
| RD | 3.72 ± 0.07 {3.35–4.03} | 19.87 ± 0.26 {17.97–20.86} | 0.30 ± 0.01 {0.27–0.34} | 2.12 ± 0.05 {1.88–2.28} | 0.11 ± 0.01 {0.09–0.15} | 0.19 ± 0.01 {0.15–0.24} | 70.15 ± 0.22 {69.35–71.36} | 29.85 ± 0.22 {28.64–30.65} |
| NF | 2.57 ± 0.10 {1.58–3.31} | 12.78 ± 0.42 {8.87–16.85} | 1.57 ± 0.12 {0.66–2.78} [423.3] | 9.63 ± 0.59 {4.43–15.57} [354.2] | 0.46 ± 0.03 {0.24–0.65} [318.2] | 0.46 ± 0.03 {0.29–0.70} [142.1] | 68.77 ± 0.47 {65.53–72.06} | 31.30 ± 0.47 {27.94–34.47} [4.8] |
Results represent the average (±se) of triplicate measurements using 200 seeds from 25 independent Arabidopsis transgenic lines. Constructs: RD, Control (plasmid-only) transgenic seeds; NF, Napin: nasturtium FAE transgenic seeds.
Relative to value for seeds from RD, the Arabidopsis control (plasmid-only) plants, set at 100%.
Figure 5.
Fatty acid composition of transgenic Arabidopsis seeds. A, Proportions of 20:1 Δ11 and 22:1 Δ13 in seed oils from nontransformed Arabidopsis ecotype Wassilewskija (ntWS), 2 plasmid-only transgenic control lines (RD10-6 and RD15-8), and the 12 best Arabidopsis T3 homozygous transgenic lines expressing the nasturtium FAE gene under control of the napin promoter. B, Proportions of 18:0, 20:0, 22:0, and 24:0 in seed oils from nontransformed Arabidopsis ecotype Wassilewskija (ntWS), 2 plasmid-only transgenic control lines (RD10-6 and RD15-8), and the 12 best Arabidopsis T3 homozygous transgenic lines expressing the nasturtium FAE gene under control of the napin promoter. The values are the average ± sd of three determinations performed on 200-seed lot.
DISCUSSION
We have isolated a cDNA clone from nasturtium, which exhibits high similarity to the sequences of β-KCSs from various plant species. Our in vitro studies have determined biochemically the range of acyl-CoA specificities of the nasturtium FAE family using experimental conditions that correctly exclude selective conditions fueling the currently heated debate regarding the acyl substrate for elongase activity. Our findings suggest that the FAE proteins in a 15,000g nasturtium particulate fraction have a broad acyl-CoA preference, with the ability to elongate both monounsaturated and saturated C18-CoA and C20-CoA substrates. In like manner, a partially purified jojoba FAE1 showed maximal activity with monounsaturated and saturated C18- and C20-CoAs in vitro (Lassner et al., 1996). However, it is important to note that the particulate elongation activity reported in this study most likely represents the cumulative effect of expression of more than one member of this small gene family. Thus, from this experiment one can only conclude that the capacity to elongate both monounsaturated and saturated acyl moieties is represented in this nasturtium particulate fraction.
While genetic analyses and homology assessments might predict that the isolated nasturtium FAE gene encodes an enzyme that prefers to elongate saturated acyl-CoAs, transgenic experiments in tobacco callus, tobacco leaves, and Arabidopsis seed collectively confirmed that the heterologously expressed nasturtium FAE can elongate monounsaturated and, to a lesser extent, saturated acyl moieties.
Constitutive expression of the nasturtium FAE resulted in a low increase in the content of erucic acid in transgenic Arabidopsis seeds in comparison to seed-specific expression. It is possible that higher VLCFA accumulation in seeds could not be achieved using the 35S promoter. A similar observation was made by Millar and Kunst (1997) in analyzing seed oils from Arabidopsis plants constitutively expressing FAE1. More recently, while trying to overexpress a borage Δ-6 desaturase in evening primrose plants, Mendoza de Gyvest et al. (2004) reported that the 35S promoter was similarly ineffective in seeds.
In comparison, when we introduced the nasturtium FAE (under the control of napin promoter) into Arabidopsis plants, the erucic acid content in seed oils increased by almost an order of magnitude (8-fold) at the concomitant expense of 20:1 Δ11. Indeed eicosenoic acid (20:1 Δ11) proportions fell from about 18% in the plasmid-only control transformants to 7% to 10% in the best T3 transgenics harboring the nasturtium FAE. In a biotechnological context, it is noteworthy that there was no significant increase in the proportion of the next predicted extension product 24:1 Δ15. Thus, the acyl composition of the transgenic Arabidopsis seed oil was reproportioned, such that erucic acid generally surpassed eicosenoic acid as the predominant VLCFA. This suggests a high specificity of the nasturtium elongase for extending 20:1-CoA to yield 22:1 as its primary product. In fact, in a transgenic Arabidopsis background, the nasturtium FAE was much stronger than the previously reported jojoba β-KCS in its ability to increase the level of 22:1: Introducing the jojoba cDNA into Arabidopsis seeds only resulted in an increase in 22:1 proportions from about 2% in the control to 4% in the transgenic jojoba FAE plants (Lassner et al., 1996).
As would be expected, there was some variation in the proportions of 22:1 that accumulated (Fig. 5A), possibly due to positional effects from nasturtium FAE transgene insertion at different sites in the Arabidopsis genome. Similar variations were observed in the expression of a castor fatty acid hydroxylase gene (CFAH12) in the Arabidopsis fad2/fae1 double mutant (Rossak et al., 2001).
The ability of the nasturtium FAE protein to elongate 18:1-CoA and, preferentially, 20:1-CoA is consistent with the observed acyl composition of nasturtium seed oil, which consists primarily of very long chain and, specifically, erucoyl moieties. Thus, the nasturtium FAE homolog described herein may have a larger engineering impact when strongly expressed in a seed-specific manner in high erucic acid Brassicaceae (e.g. B. napus or Brassica carinata), wherein 18:1 Δ9 [and 18:2/18:3] and 20:1 Δ11 represent a potential acyl-CoA elongation substrate pool totaling almost 40% over and above the existing 40% to 45% 22:1 Δ13 content. Given that the pathway for 18:1 elongation has a somewhat stronger metabolic pull over 18:1 desaturation in developing seeds, the resulting erucoyl pool may become quite large. Clearly, the current studies indicate that the nasturtium FAE expression could also be combined with other genetic modifications we have made (e.g. oleoyl-desaturase [FAD 2] silencing) to enhance the VLCFA content of high erucic acid Brassicaceae (Katavic et al., 2001; Taylor et al., 2001; E.-F. Marillia, A. Jadhav, E.M. Giblin, V. Katavic, D.L. Barton, C. Sonntag, V. Babic, and D.C. Taylor, unpublished data) and the proportions of erucic acid, in particular, to provide an industrial feedstock oil of high value and broad applicability.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
All experimental lines propagated in the greenhouse were grown at the Kristjanson Biotechnology Complex greenhouses, Saskatoon, Canada, under natural light conditions supplemented with high-pressure sodium lamps with a 16-h photoperiod (16 h of light and 8 h of darkness) at 22°C and a relative humidity of 25% to 30%. Garden nasturtium (Tropaeolum majus) plants (cv Dwarf Cherry Rose) were grown in the greenhouse, and flowers were hand-pollinated. Seeds at various stages of development were harvested, their seed coats were removed, and embryos were frozen in liquid nitrogen and stored at −80°C. Tobacco (Nicotiana tabacum) plants were grown under sterile conditions on Murashige and Skoog medium (Murashige and Skoog, 1962) as well as under normal greenhouse conditions. Arabidopsis plants were grown in a growth chamber at 22°C with photoperiod of 16 h light (120 μE m−2 s−1) and 8 h dark.
Nasturtium Embryo Protein Preparations and Elongase Assays
A 5,000 to 15,000g particulate fraction enriched in elongase activity was isolated essentially as described previously (Löhden and Frentzen, 1992). Briefly, embryos (2–3 g) were ground in a mortar under liquid nitrogen and then 10 mL of isolation buffer (80 mm HEPES, pH 7.2, containing 2 mm dithiothreitol, 320 mm Suc, and 5% polyvinylpyrrolidone) per g fresh weight was added. The homogenate was filtered through Miracloth and spun for 5 min at 5,000g in a Sorvall refrigerated centrifuge at 5°C, and the supernatant retained and recentrifuged at 15,000g for 25 min. The resulting pellet was resuspended in 80 mm HEPES containing 20% glycerol and 2 mm dithiothreitol. The concentration of protein was determined as described by Bradford (1976). This subcellular fraction was either used directly to determine enzymatic activities or stored at −80°C until used.
The 15,000g particulate preparation was used to perform elongation assays as described by Taylor et al. (1992b) with the following modifications. The assay mixture consisted of 80 mm HEPES-NaOH, pH 7.2, containing 0.75 mm ATP, 10 μm CoA-SH, 0.5 mm NADH, 0.5 mm NADPH, 2 mm MgCl2, 200 μm malonyl-CoA, 18 μm [1-14C]acyl-CoA (0.37 GBq mol−1), and nasturtium protein in a final volume of 500 μL. The reaction was started by the addition of 200 μg of protein and incubated in a shaking water bath at 30°C, 100 rpm for 0.5 h. [1-14C]-Radiolabeled acyl-CoAs were synthesized from the corresponding free fatty acids as described (Taylor et al., 1990). Elongase reaction assays were stopped with 3 mL of 100 g/L KOH in methanol. Fatty acid methyl esters (FAMEs) were prepared and quantified by radio-HPLC as described (Taylor et al., 1992b).
Lipid Analyses
The total fatty acid content and acyl composition of tobacco plant lipids and Arabidopsis seed oils were determined by gas chromatography of the FAMEs with 17:0 FAME as an internal standard as described (Zou et al., 1997; Katavic et al., 2001; Taylor et al., 2001).
Isolation of FAE cDNA by a Degenerate Primers Approach
Degenerate primers were designed for amino acid sequences conserved among Arabidopsis KCS1 (GenBank accession no. AF053345), Brassica napus FAE1 (GenBank accession no. AF009563), Limnanthes douglasii FAE (GenBank accession no. AF247134), and jojoba (Simmondsia chinensis) FAE (GenBank accession no. U37088). Single-stranded cDNA template for reverse transcription-PCR was synthesized at 42°C from embryo poly(A) RNA with PowerScript (CLONTECH, Palo Alto, CA). A 50-μL PCR reaction contained single-stranded cDNA derived from 40 ng of poly(A) RNA, 20 pm of each primer: 5′-TCT(A/T)GG(A/T)GG(C/A)ATGGGTTG-3′ [LGGMGC]), and 5′-T(G/A)TA(T/C)GC(C/T)A(A/G)CTC(A/G)TACC)-3′ [WYELAY] and 2.5 units of Taq DNA Polymerase (Amersham Biosciences, Baie d'Urfe, Canada) under standard conditions. An internal part of the elongase sequence was amplified in a thermocycler during 30 cycles of the following program: 94°C for 30 s, 48°C for 30 s, and 72°C for 1 min. The sequence of a 650-bp PCR product was used to design a primer to amplify the 5′ and 3′ ends of the cDNA using the SMART RACE cDNA Amplification kit (CLONTECH). After assembly to determine the full-length sequence of the cDNA, the open reading frame without a stop codon was amplified using the primers 5′-ACCATGTCAGGAACAAAAGC-3′ and 5′-ATTTAATGGAACCTCAACCG-3′, and subsequently cloned into the pYES2.1/V5-His-TOPO expression vector (Invitrogen, Burlington, Canada). Omitting the stop codon allows for the PCR product to be expressed as a fusion to the C-terminal V5 epitope and polyhistidine tag for protein detection and purification.
Expression of Nasturtium FAE in Yeast
The nasturtium FAE in YES2.1/V5-His-TOPO plasmid was transformed into Saccharomyces cerevisiae strain Inv Sci1 (Invitrogen) and two mutant strains elo2Δ and elo3Δ (Oh et al., 1997) using the S.c. EasyComp transformation kit (Invitrogen). Yeast cells transformed with pYES2.1/V5-His-TOPO plasmid only and with Arabidopsis FAE1 were used as controls. The transformants were selected and grown as described previously (Katavic et al., 2002). Fatty acid elongase activity of the microsomal membrane preparation was measured essentially as described by Katavic et al. (2002), with modifications described above. For western-blot analyses, 100 μg of microsomal protein were separated on 15% SDS-PAGE Ready Gel (Bio-Rad) and electrotransferred to PVDF membrane (HybondP; Amersham Biosciences). Recombinant proteins were detected with anti-His (C-terminal) antibody using WesternBreeze kit (Invitrogen) according to manufacturer's protocol.
Site-Directed Mutagenesis of FAE
A site-directed mutagenesis experiment was performed essentially as described (Katavic et al., 2002). The desired mutation (Tyr at position 429 is replaced with His) was introduced into the FAE coding region by PCR using primers 5′-TCGAGGATGTCGCTTCACCGATTTGGAAACAC-3′ and 5′-GTTTCCAAATCGGTGAAGCGACATCCTCGATGG-3′. These primers were complementary to the opposite strands of pYES2.1/V5-His-TOPO containing the nasturtium FAE gene.
cDNA Library Construction
To construct the nasturtium developing cDNA library, immature seeds were collected 17 d after pollination. Total RNA was extracted from embryos as described by Lindstrom and Vodkin (1991), then poly(A) RNA was isolated using Dynabeads Oligo(dT)25 (Dynal Biotech, Lake Success, NY). Copy DNA synthesis was performed on 1 μg of poly(A) RNA using SMART PCR cDNA synthesis kit (CLONTECH) according to manufacturer's protocol. The cDNA population was then subtracted with 12S and 2S seed storage protein cDNA clones using PCR-Select cDNA subtraction kit (CLONTECH). The subtracted embryo cDNA population was cloned and then sequenced as described by Jako et al. (2002).
Sequence Handling
Sequence analyses were performed using Lasergene software (DNAStar, Madison, WI). Sequence similarity searches and other analyses were performed using BLASTN, BLASTX (Altschul et al., 1990), and PSORT (Nakai and Kanehisa, 1992) programs.
Northern Analysis
Total RNA from nasturtium plant material was isolated as described by Lindstrom and Vodkin (1991). A total of 20 μg of RNA was fractionated on a 1.4% formaldehyde-agarose gel, and the gels were then stained with ethidium bromide to ensure that all lanes had been loaded equally (Sambrook et al., 1989). The RNA was subsequently transferred to Hybond N+ membrane (Amersham Biosciences) and hybridized with the 32P-labeled FAE DNA probe, prepared using the Random Primers DNA labeling kit (Gibco-BRL, Cleveland). Membranes were hybridized at 60°C overnight.
Plant Transformation Vectors
The coding regions of the nasturtium FAE (natural and mutated versions named SF and SMF, respectively) were amplified by PCR with primers 5′-taggatccATGTCAGGAACAAAAGC-3′(lowercase shows restriction site for BamHI) and 5′-tagagctcTTAATTTAATGGAACCTCAACC-3′ (lowercase shows restriction site for SacI enzyme) and subsequently cloned as a BamHI and SacI fragment behind the constitutive tandem 35S promoter in binary vector pBI121 (CLONTECH).
The coding region of the nasturtium FAE was cloned behind the seed-specific napin promoter as follows. A BamHI site was introduced in front of the start codon and behind the stop codon of FAE by PCR with primers 5′-taggatccATGTCAGGAACAAAAGC-3′ and 5′-taggatccTTAATTTAATGGAACCTCAACC-3′ (lowercase shows the restriction site for BamHI). The B. napus napin promoter (Josefsson et al., 1987) was cloned in HindIII/XbaI sites of the pUC19 (MBI Fermentas, Burlington, Canada), and the nos terminator (Bevan, 1983) was introduced as an EcoRI/BamHI fragment. The resulting vector was named pDH1. The napin promoter/nos terminator cassette was excised from pDH1 by HindIII/EcoRI digestion and subsequently cloned into the respective sites of pRD400 (CLONTECH), resulting in pVK1. The coding region of FAE was then cloned into the BamHI site of pVK1 behind the napin promoter, and the resulting vector was named NF. Sense orientation of the FAE coding region with respect to promoter was confirmed by restriction analyses with XbaI.
The final binary vectors (SF, SMF, and NF) were electroporated into Agrobacterium tumefaciens cells strain GV3101 containing helper plasmid pMP90 (Koncz and Schell, 1986). Plasmid integrity was verified by DNA sequencing following its reisolation from A. tumefaciens and transformation into Escherichia coli.
Plant Transformation and Genetic Analysis
Tobacco (cv Xanthi) was transformed using a leaf disc transformation procedure (Horsch et al., 1985). Shoots that rooted in the presence of 50 mg/L kanamycin were considered to be transgenic. Transgenic plants were transferred to soil and grown in the greenhouse.
Arabidopsis (ecotype Wassilewskija) were transformed by vacuum infiltration method (Clough and Bent, 1998). Transgenic plants were selected and analyzed essentially as described by Jako et al. (2001).
Molecular Analysis of Transgenic Plants
DNA was isolated from 2 to 3 g of tobacco or 150 mg of Arabidopsis leaf material using a urea-phenol extraction method (Chen et al., 1992) with the following minor modification: Tissue extraction was performed for 15 min at room temperature, and 400 mm ammonium acetate, pH 5.2, was used for the first two precipitation steps. Stable integration of the napin:FAE:nos cassette into the genome of transgenic plants was checked by PCR amplification on genomic DNA as described by Katavic et al. (2001).
Southern analyses were performed to further confirm and select those transformants containing single or multiple copies of the inserted fragments. Fifteen micrograms of tobacco or 1 μg of Arabidopsis genomic DNA was digested with the restriction enzyme SacI, and the resulting fragments were separated on a 0.9% (w/v) agarose gel, transferred to Hybond N+ nylon membrane (Amersham Biosciences) via an alkali blotting protocol. A 1.5-kb probe containing the coding sequence of FAE was generated by PCR using primers 5′-ATGTCAGGAACAAAAGC-3′ and 5′-TAATTTAATGGAACCTCAACCG-3′ and subsequently radioactively labeled with 32P as described above. Hybridization was performed at 65°C. The filters were washed once in 1× SSPE, 0.1% SDS for 15 min, and 0.1× SSPE, 0.1% SDS for 5 to 10 min at the temperature of hybridization. The blots were exposed to X-OMAT-AR film (Kodak, Rochester, NY).
To estimate the number of FAE isoforms in the nasturtium genome, 15 μg of genomic DNA was digested with restriction enzymes EcoRI, AccI, NcoI, and HindIII. Blotting and hybridization conditions were essentially as above except that filters were washed at low stringency with 1× SSPE, 0.1% SDS for 15 min, autoradiographed and then washed subsequently with 0.1× SSPE, 0.1% SDS, and re-exposed.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number AY082610.
Acknowledgments
We thank Drs. Jitao Zou and Wilf Keller for their critical reviews of this manuscript. We are also grateful to Dr. P. Covello and D. Reed for very helpful discussions during the course of this work, Dr. D. Schwab, B. Panchuk, and Dr. L. Pelcher for primer synthesis and DNA sequencing, and Dr. C. Martin for providing the elo2Δ and elo3Δ mutant yeast strains.
This work was partially supported by Genome Canada Genomics and Human Health Initiative 2 (S.W.) and by the Industrial Research Assistance Program (project no. 418702 awarded to CanAmera Foods; V.K. and D.L.B.). This is National Research Council of Canada Publication Number 46596.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.046839.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410 [DOI] [PubMed] [Google Scholar]
- Barret P, Delourne R, Renard M, Domergue F, Lessire R, Delseny M, Roscoe TJ (1998) A rapeseed FAE1 gene is linked to the E1 locus associated with variation in the content of erucic acid. Theor Appl Genet 96: 177–186 [Google Scholar]
- Barret PB, Harwood JL (1997) Characterization of fatty acid elongase enzymes from germinating pea seeds. Phytochemistry 48: 1295–1304 [DOI] [PubMed] [Google Scholar]
- Bevan M (1983) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12: 8711–8721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjellqvist B, Basse B, Olsen E, Celis JE (1994) Reference points for comparisons of two-dimensional maps of proteins from different human cell types defined in a pH scale where isoelectric points correlate with polypeptide compositions. Electrophoresis 15: 529–539 [DOI] [PubMed] [Google Scholar]
- Bjellqvist B, Hughes GJ, Pasquali CH, Paquet N, Ravier F, Sanchez JC, Frutiger S, Hochstrasser D (1993) The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis 14: 1023–1031 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Cahoon EB, Marillia EF, Stecca KL, Hall SE, Taylor DC, Kinney AJ (2000) Production of fatty acid components of meadowfoam oil in somatic soybean embryos. Plant Physiol 124: 243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Greenblatt IM, Dellaporta SL (1992) Molecular analysis of Ac transposition and DNA replication. Genetics 130: 665–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Kunst L (1997) Isolation of a Brassica napus cDNA (accession no. AF009563) encoding a 3-ketoacyl-CoA synthase, a condensing enzyme involved in the biosynthesis of very long chain fatty acids in seeds (PGR 97-125). Plant Physiol 115: 313–3149380777 [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Das S, Roscoe TJ, Delseny M, Srivastava PS, Lakshmikumaran M (2002) Cloning and molecular characterization of the Fatty Acid Elongase 1 (FAE 1) gene from high and low erucic acid lines of Brassica campestris and Brassica oleracea. Plant Sci 162: 245–250 [Google Scholar]
- Domergue F, Chevalier S, Santarelli X, Cassagne C, Lessire R (1999) Evidence that oleoyl-CoA and ATP-dependent elongations coexist in rapeseed (Brassica napus L.). Eur J Biochem 263: 464–470 [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Ghanevati M, Jaworski JG (2001) Active-site residues of a plant membrane-bound fatty acid elongase β-ketoacyl-CoA synthase, FAE1 KCS. Biochim Biophys Acta 1530: 77–85 [DOI] [PubMed] [Google Scholar]
- Han J, Lühs W, Sonntag K, Zähringer U, Borchardt DS, Wolter FP, Heinz E, Frentzen M (2001) Functional characterization of β-ketoacyl-CoA synthase genes from Brassica napus L. Plant Mol Biol 46: 229–239 [DOI] [PubMed] [Google Scholar]
- Harwood JL (1996) Recent advances in the biosynthesis of plant fatty acids. Biochim Biophys Acta 1301: 7–56 [DOI] [PubMed] [Google Scholar]
- Hlousek-Radojcic A, Imai H, Jaworski J (1995) Oleoyl-CoA is not an immediate substrate for fatty acid elongation in developing seeds of Brassica napus. Plant J 8: 803–809 [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transgenic genes into plants. Science 227: 1129–1231 [Google Scholar]
- Jackson MR, Nilsson T, Petersen PA (1990) Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J 9: 3153–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jako C, Kumar A, Wei Y, Zou J-T, Barton DL, Giblin EM, Covello PS, Taylor DC (2001) Seed-specific over-expression of an Arabidopsis thaliana cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiol 126: 861–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jako C, Coutu C, Roewer I, Reed DW, Pelcher LE, Covello PS (2002) Probing carotenoid biosynthesis in developing seed coats of Bixa oreallana (Bixaceae) through expressed sequence tag analysis. Plant Sci 163: 141–145 [Google Scholar]
- James DW, Lim E, Keller J, Plooy I, Dooner HK (1995) Directed tagging of the Arabidopsis fatty acid elongation 1 (FAE1) gene with the maize transposon activator. Plant Cell 7: 309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson LG, Lenman M, Ericson ML, Rask L (1987) Structure of a gene encoding the 1.7 S storage protein, napin, from Brassica napus. J Biol Chem 262: 12196–12201 [PubMed] [Google Scholar]
- Katavic V, Friesen W, Barton DL, Gossen KK, Giblin EM, Luciw T, An J, Zou J-T, MacKenzie SL, Keller WA, et al (2001) Improving erucic acid content in rapeseed through biotechnology: What can the Arabidopsis FAE1 and the yeast SLC1-1 genes contribute. Crop Sci 41: 739–747 [Google Scholar]
- Katavic V, Mietkiewska E, Barton DL, Giblin EM, Reed DW, Taylor DC (2002) Restoring enzyme activity in nonfunctional low erucic acid Brassica napus fatty acid elonagase1 by a single amino acid substitution. Eur J Biochem 269: 5625–5631 [DOI] [PubMed] [Google Scholar]
- Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes by a novel type of Agrobacterium binary vector. Mol Gen Genet 204: 383–396 [Google Scholar]
- Lardizabal KD, Metz JG, Sakamoto T, Hutton WC, Pollard MR, Lassner MW (2000) Purification of a jojoba embryo wax synthase, cloning of its cDNA and production of high levels of wax in seeds of transgenic Arabidopsis. Plant Physiol 122: 645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassner MW, Lardizabal K, Metz JG (1996) A jojoba β-ketoacyl-CoA synthase cDNA complements the canola fatty acid elongation mutation in transgenic plants. Plant Cell 8: 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard C (1994) Sources and commercial applications of high erucic vegetable oils. Lipid Technology 4: 79–83 [Google Scholar]
- Lindstrom JT, Vodkin LO (1991) A soybean cell wall protein is affected by seed color genotype. Plant Cell 3: 561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhden I, Frentzen M (1992) Triacylglycerol biosynthesis in developing seeds of Trapaeolum majus L. and Limnanthes douglasii R. Br Planta 188: 215–224 [DOI] [PubMed] [Google Scholar]
- Marillia E-F, Giblin EM, Covello PS, Taylor DC (2002) Expression of meadowfoam Des5 and FAE1 in yeast and in transgenic soybean somatic embryos, and their roles in fatty acid modification. Plant Physiol Biochem 40: 821–828 [Google Scholar]
- Mendoza de Gyvest E, Sparks CA, Sayanova O, Lazzeri P, Napier JA, Jones HD (2004) Genetic manipulation of γ-linolenic acid (GLA) synthesis in a commercial variety of evening primrose (Oenothera sp.). Plant Biotechnol J 2: 351–357 [DOI] [PubMed] [Google Scholar]
- Millar AA, Clemens S, Zachgo S, Giblin EM, Taylor DC, Kunst L (1999) CUT1, an Arabidopsis gene required for cuticular wax biosynthesis and pollen fertility, encodes a very-long-chain fatty acid condensing enzyme. Plant Cell 11: 825–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AA, Kunst L (1997) Very-long-chain fatty acid biosynthesis is controlled through the expression and specificity of the condensing enzyme. Plant J 12: 101–111 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 493–497 [Google Scholar]
- Murphy DJ, Mukherjee KD (1989) Elongases synthesizing very long chain monounsaturated fatty acids in developing oilseeds and their solubilization. Z Naturforsch 44c: 629–634 [Google Scholar]
- Nakai K, Kanehisa M (1992) A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics 14: 897–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh C-S, Toke DA, Mandala S, Martin CE (1997) ELO2 and ELO3, homologues of the Saccharomyces cerevisiae ELO1 gene, function in fatty acid elongation and are required for sphingolipid formation. J Biol Chem 272: 17376–17384 [DOI] [PubMed] [Google Scholar]
- Persson B, Argos P (1994) Prediction of transmembrane segments in proteins utilizing multiple sequence alignments. J Mol Biol 237: 182–192 [DOI] [PubMed] [Google Scholar]
- Pollard MR, Stumpf PK (1980) Long chain (C20 and C22) fatty acid biosynthesis in developing seeds of Tropaeolum majus. Plant Physiol 66: 641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post-Beittenmiller D (1996) Biochemistry and molecular biology of wax production in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 405–430 [DOI] [PubMed] [Google Scholar]
- Puyaubert J, Garbay B, Costagliolo P, Dieryck W, Roscoe TJ, Renard M, Cassagne C, Lessire R (2001) Acyl-CoA elongase expression during seed development in Brassica napus. Biochim Biophys Acta 1533: 141–152 [DOI] [PubMed] [Google Scholar]
- Rossak M, Smith M, Kunst L (2001) Expression of the FAE1 gene and FAE1 promoter activity in developing seeds of Arabidopsis thaliana. Plant Mol Biol 46: 717–725 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schreiber L, Skrabs M, Hartmann K, Becker D, Cassagne C, Lessire R (2000) Biochemical and molecular characterization of corn (Zea mays L.) root elongases. Biochem Soc Trans 28: 647–649 [PubMed] [Google Scholar]
- Sonntag NOV (1991) Erucic, behenic: feedstocks of the 21st century. Inform 5: 449–463 [Google Scholar]
- Sonntag NOV (1995) Industrial utilization of long-chain fatty acids and their derivatives. In DS Kimber, DI McGregor, eds, Brassica Oilseeds. CAB International, Oxon, UK, pp 339–352
- Taylor DC, Barton DL, Rioux KP, Reed DW, Underhill EW, MacKenzie SL, Pomeroy MK, Weber N (1992. b) Biosynthesis of acyl lipids containing very-long chain fatty acids in microspore-derived and zygotic embryos of Brassica napus L. cv. Reston. Plant Physiol 99: 1609–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DC, Katavic V, Zou J-T, MacKenzie SL, Keller WA, An J, Friesen W, Barton DL, Gossen KK, Giblin EM, et al (2001) Field-testing of transgenic rapeseed cv. Hero transformed with a yeast sn-2 acyltransferase results in increased oil content, erucic acid content and seed yield. Mol Breed 8: 317–322 [Google Scholar]
- Taylor DC, Kunst L, MacKenzie SL (1993) Bioassembly of storage lipids in oilseed crops; Target: Trierucin. In J Janick, JE Simon, eds, Progress in New Crops, Wiley, New York, pp 181–191
- Taylor DC, Magus JR, Bhella J, Zou J-T, Mackenzie SL, Giblin EM, Pass EW, Crosby WL (1992. a) Biosynthesis of triacylglycerols in Brassica napus L. cv. Reston; Target: Triercin. In SL MacKenzie, DC Taylor, eds, Seed Oils for the Future. American Oil Chemists' Society, Champaign, IL, pp 77–102
- Taylor DC, Weber N, Hogge LR, Underhill EW (1990) A simple enzymatic method for the preparation of radiolabeled erucoyl-CoA and other long-chain fatty acyl-CoAs and their characterization by mass spectometry. Anal Biochem 184: 311–316 [DOI] [PubMed] [Google Scholar]
- Todd J, Post-Beittenmiller D, Jaworski JG (1999) KCS1 encodes a fatty acid 3-ketoacyl-CoA synthase affecting wax biosynthesis in Arabidopsis thaliana. Plant J 17: 119–130 [DOI] [PubMed] [Google Scholar]
- Zou J-T, Katavic V, Giblin EM, Barton DL, MacKenzie SL, Keller WA, Hu X, Taylor DC (1997) Modification of seed oil content and acyl composition in Brassicaceae by expression of a yeast sn-2 acyltransferase gene. Plant Cell 9: 909–923 [DOI] [PMC free article] [PubMed] [Google Scholar]