Abstract
Vaccines stand as a very powerful means of disease prevention and treatment. Fundamental to the success of vaccination is the efficient delivery of antigenic cargo needed to trigger an effective immune response. In this article, we will review recent advances in delivery technology with a focus on devices designed to optimally maximize responses to antigen cargo. Included with the review is an overview of traditional vaccine applications and how these approaches can benefit by well-designed delivery methods.
Introduction
Vaccines can be divided into two broad groups: live attenuated vaccines and inactivated vaccines. Live attenuated vaccines, which are comprised of weakened forms of disease-causing organisms (pathogens) such as viruses or bacteria, induce immune reactions similar to those resulting from an actual infection [1]. This group of vaccines elicits a strong response and is capable of conferring immunity that can last for decades with a single dose [1]. For example, one vaccination of the smallpox vaccine can maintain substantial immunity to the virus for up to 75 years [2]. Inactivated vaccines, which range from completely inactivated pathogens to the antigen components of those pathogens (including subunit vaccines, toxoid vaccines, carbohydrate vaccines, and conjugate vaccines) induce short-lived protection compared to attenuated vaccines and often require a follow-up booster vaccination to maintain protective immunity [3]. Furthermore, inactivated vaccines typically contain adjuvants, which are additives designed to enhance and shape immune response outcomes [4]. Understanding how to induce protective responses with adjuvants will enable the production of more specific and efficient vaccines, which can confer immunity for longer periods of time [5].
Delivery technology offers advantages in vaccine application by carefully designing the introduction of antigens and adjuvants for a more directed and enhanced immune response. In particular, delivery systems can enhance immunological outcomes by 1) prolonging the deposition of antigens at the site of administration, 2) recruiting sentinel immune cells (termed antigen presenting cells or ACPs) required for immune response initiation, 3) influencing site localization and antigen delivery, and 4) protecting delicate payloads (e.g., nucleic acids) [6,7].
In this review, delivery technology will be evaluated in parallel to traditional vaccines (live attenuated and inactivated whole or component). Emphasis will be placed on how the delivery vector can alter, improve, or accentuate the process of immune response.
Immune Response Cascade and Lessons in Vaccine Design
Upon administration of a live attenuated vaccine, an immune response similar to that of a natural infection is elicited. First, specialized receptors on the surface of dendritic cells (DCs), such as toll-like receptors (TLRs), identify an antigen as a potential threat via pathogen-associated molecular patterns (PAMPs) [1]. The antigen is then internalized by DCs, which differentiate into antigen presenting cells after either destroying or partially degrading the antigen [1]. In a natural infection, DCs may be able to eradicate the pathogen [8]. For an efficient vaccine, however, APCs must activate the adaptive immune system [9] which consists of antibody producing B cells and cytokine/cytolytic molecule producing T cells [1,10] (Figure 1).
Figure 1.
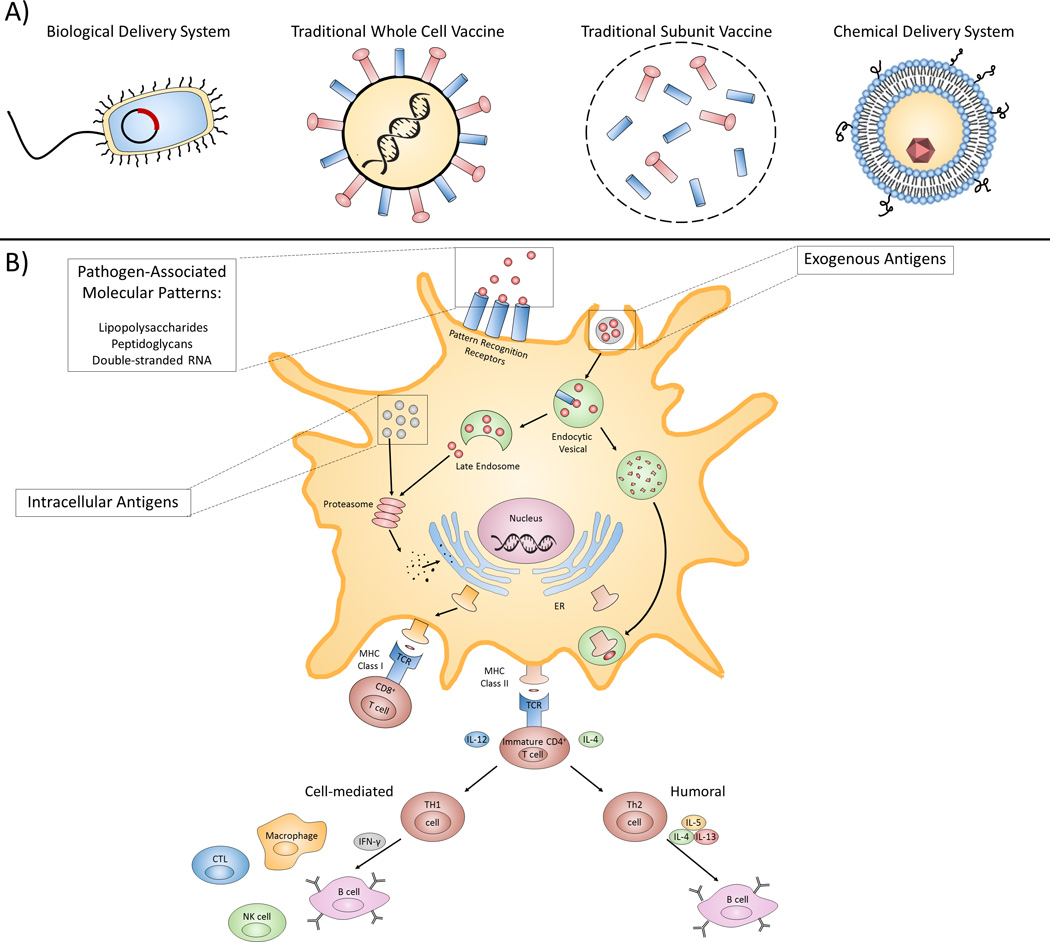
Vaccine types, delivery devices, and immune response outcomes. A) A pictorial representation of different vaccines and delivery devices. Biological delivery systems include avirulent and attenuated recombinant bacterial vectors capable of delivering genetic and protein antigens. Traditional whole cell vaccines, such as the live attenuated vaccine depicted, contain weakened versions of pathogens that do not cause disease but can continue to replicate. Unlike whole cell vaccines, subunit vaccines only contain the most antigenic regions of a pathogen. Liposomes, a type of chemical delivery system, can provide a high degree of multivalent surface antigens. B) Diagram representing the processing and presentation of antigens in dendritic cells (DCs). Pattern recognizing receptors on the surface of DCs identify pathogen associated molecular patterns (PAMPs) which initiate DC activation. Exogenous antigens are internalized by DCs and processed in endocytic vesicles before being loaded onto MHC Class II molecules, forming a peptide-MHC II complex that is presented to immature T cells that can then stimulate either a humoral or cell-mediated (CTL, cytotoxic T cell; NK, natural killer cell) response. Endogenous, as well as exogenous, antigens can also be loaded onto MHC I molecules; the resulting complex then interacts with CD8+ T cells, which have cytotoxic activity.
While a T cell-independent immune response can occur, an effective vaccine must induce a T cell-dependent response. This occurs when T cells interact with the APCs, differentiate into T-helper (Th) cells, such as CD4+ T cells, and begin to secrete cytokines that then affect the behavior of B cells [1,8]. For example, continuously replicating live attenuated vaccines constantly present proteinaceous antigens that are recognized by Th cells. These Th cells trigger a humoral (B cell) response, allowing for the formation of memory B cells that can be reactivated rapidly upon re-infection without further aid of T cells [1,11,12].
Another main component of the adaptive immune system, cytotoxic T cells (CD8+ T cells or killer T cells), secrete cytotoxic factors and cytokines upon interacting with ACPs, which allows them to kill cells that display pathogen-derived proteins [8]. CD8+ T cells are often activated by ACPs displaying antigens derived from foreign or altered nucleic acid content, resulting from cancer aberrations or viral infections, for example, in what is known as a cell-mediated response [13]. It should be noted that a CD8+ response has recently been demonstrated to be indicative of an effective vaccine. For example, one study showed that a CD8+ T-cell response in dengue vaccines was comparable to that of a natural viral infection [14].
With live attenuated vaccines, a potent and long-lasting immune response is typically invoked. However, in the case of inactivated vaccines, adjuvants are often needed to enhance the efficacy of antigens [4]. Each adjuvant can induce different immune responses even with the same antigens, as demonstrated by a recent study on adjuvants for human immunodeficiency virus type-1 (HIV-1) that showed that, while all adjuvants tested in conjunction with HIV-1 gp140 envelope (Env) trimers induced a stronger immune response than the non-adjuvant control, aluminum-based adjuvants (Alhydrogel and Adju-Phos) were less potent than TLR-, Emulsion-, Liposome-, and ISCOM-based adjuvants [15]. Even with these adjuvants, however, the vaccine was still not able to strongly mimic a natural infection, a common issue with modern inactivated vaccines. More research into developing next-generation adjuvants is needed to produce vaccines that can mount the appropriate immune response, increase the generation of memory, and increase the response speed [16].
In a natural infection, pathogen-associated antigens are capable of eliciting both a humoral and cell-mediated immune response by activating two types of T-helper (Th) cells: Th1 and Th2. Th1 cells are pro-inflammatory and induce cell-mediated immunity. Th2 cells cause an anti-inflammatory reaction and invoke a strong antibody response and are therefore associated with the humoral immune system [17]. Antigens associated with parasitic and extracellular bacterial infections, for example, preferentially elicit a strong Th2 response, while those associated with intracellular bacterial infections primarily produce a Th1 response [18].
Most modern vaccines use a humoral immune response to confer protection [16]. However, it has been shown that both the humoral and cell-mediated responses have complementary roles in protection against certain diseases [19], leading to the need to develop adjuvants and antigens that can balance both responses. Currently, there are adjuvants that have been found to produce mixed Th1 and Th2 responses, such as flagellin, a principal component of a bacterium’s flagella [20], but there are few adjuvants that have been designed specifically to do so [21].
Delivery Technology to Enhance Vaccination Effectiveness
Vaccine delivery systems can generally be categorized into biological (e.g. viral or bacterial) and chemical vectors [22]* (Figure 1). An important consideration in adopting delivery technology is effectively using the capabilities and features of the chosen vector to augment, alter, or improve upon traditional vaccine formulations. The following section will focus on certain properties that such vectors can address.
Among biological delivery systems, avirulent recombinant bacterial vectors hold potential in infectious disease and cancer vaccine development. Suitable nonpathogenic options with facile genetic manipulation protocols enable simple production, administration, and engineering for associated vaccination goals; as a byproduct of their bacterial nature (including cell wall composition and macromolecule content), the vectors also serve as potent natural adjuvants [23]. Examples include Salmonella spp. [24–26]*, Mycobacterium bovis [27], Listeria monocytogenes [28–30], Vibrio cholera [31], Lactobacillus spp. [32], Staphylococcus spp. [33], Shigella spp. [34], and E. coli [35].
Due to the ability of the bacterial vector to carry either genetic or protein antigens, delivery can be designed in a way to elicit both strong Th1 and Th2 responses. For example, attenuated L. monocytogenes capable of expressing and secreting the human CD24 protein were used to efficiently enhance both Th1 and Th2 immune responses, which resulted in reduced disease and longer survival rates in mice bearing tumors [36]. The range of natural TLR ligand adjuvants associated with bacterial vectors also offers a way of ensuring or biasing a more comprehensive response [37]*. Such a combination of responses has been shown effective for infectious disease (H1N1 influenza) and additional cancer treatments [38–40]. To this stage, however, few delivery vectors have been designed or utilized to direct a combination of Th1/Th2 responses.
Protein-based delivery formulations also have the potential to augment traditional sub-unit vaccines [41–47]*. As one example of a chemical vector approach, advanced liposomal technology allows simple mixing of liposomes and His-tagged protein antigens through affinity complexation. The end result enables a high degree of surface oriented antigens (theoretically up to 600) and the potential to greatly vary and amplify valency of select target antigens from a pool of potential candidates (an important consideration in a hyper-variable diseases such as pneumococcal infection) [48,49]*. The latter feature is in contrast to vaccine strategies that subject the immune system to a broad but diluted range of antigens (such as proteins, peptides, nucleic acids, carbohydrates, haptens)[50].
While the protein-based nature of such a formulation may bias towards a humoral response, the inclusion of counter-biased adjuvants would offer the potential of a more comprehensive response. In addition, the affinity-based complexation simplicity of such liposomal vectors opens the possibility of a fully synthesized construct featuring the liposome and peptide epitopes, such that no biological recombinant proteins are required, potentially simplifying overall vector production.
Chemical delivery vectors also include microneedles, a novel vaccine method that aims to replace traditional syringes and targets the network of APCs in the skin layer below the stratum corneum. These systems consist of micron-scale administration devices (to limit injection pain and promote compliance) that are created with appropriate drug formulations and can be divided between four major categories: solid, coated, dissolving, and hollow [51]. Microneedles have been shown to effectively deliver a wide variety of vaccines, including live-attenuated, inactivated, subunit, and DNA formats [52]. For example, a recent study created microneedles composed of dissolvable polyelectrolyte multilayers (using different polymers per layer) encapsulating DNA antigens for HIV, which resulted in the prolonged persistence of antigens in the skin [53].
Due to their unique route of administration, microneedles are capable of heavily impacting the type of elicited immune response. In one case, it was found the microneedle delivery of an M2e-TLR5 ligand fusion protein induced a Th1 biased response which conferred better protection against influenza when compared with the balanced Th1/Th2 response of an intranasal delivery route [54]. This type of class switch could allow for the production of vaccines for difficult pathogens such as HIV as well as improve the effectiveness of existing vaccines.
Summary
Delivery technology offers a means of accentuating or altering the desired immune responses from traditional vaccine formats. A key end goal is to better engineer the vector and corresponding immune response. Such a capability would then offer the potential to optimize vaccination outcomes. In the Table 1, we summarize the delivery technology described in this article, including strengths, weaknesses, and applications. In the future, there may be opportunities to combine these various vector formats as has recently been explored between biological and chemical modalities [35] such that individual advantages of each vector are synergized.
Table 1.
Delivery Technology Summary
| Chemical | Biological | ||
|---|---|---|---|
| Microneedle | Liposome | Bacterial | |
| Advantages |
|
|
|
| Disadvantages |
|
|
|
| Application |
|
|
|
Highlights.
Vaccine potency can be influenced by antigen delivery technology
Chemical vectors covered include microneedle devices and liposomes
Biological vectors covered include attenuated bacterial hosts
A diverse set of properties and tools enable vaccine delivery vector impact
Acknowledgments
The authors recognize support from SUNY STOR and the NIH (AI088485) for funding related to vaccine delivery technology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Recommended Reading
Papers of particular interest, published within the period of review, have been highlighted as:
*of special interest
- 1.Plotkin SA, Orenstein WA, Offit PA. Vaccines. 5th. Philadelphia: Elsevier Saunders; 2008. [Google Scholar]
- 2.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 3.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nature Immunology. 2011;131:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 5.Dane EL, Irvine DJ. Big thinking for adjuvants. Nat Biotech. 2015;33:1146–1148. doi: 10.1038/nbt.3398. [DOI] [PubMed] [Google Scholar]
- 6.Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11:S63–S68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 7.Storni T, Kündig TM, Senti G, Johansen P. Immunity in response to particulate antigen-delivery systems. Advanced Drug Delivery Reviews. 2005;57:333–355. doi: 10.1016/j.addr.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Leo O, Cunningham A, Stern PL. Vaccine immunology. Perspectives in Vaccinology. 2011;1:25–59. [Google Scholar]
- 9.Murphy BR, Whitehead SS. Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol. 2011;29:587–619. doi: 10.1146/annurev-immunol-031210-101315. [DOI] [PubMed] [Google Scholar]
- 10.Newell EW, Davis MM. Beyond model antigens: high-dimensional methods for the analysis of antigen-specific T cells. Nature biotechnology. 2014;32:149–157. doi: 10.1038/nbt.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furman D, Davis MM. New approaches to understanding the immune response to vaccination and infection. Vaccine. 2015;33:5271–5281. doi: 10.1016/j.vaccine.2015.06.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis MM. Flexibility for specificity. Science. 2015;347:371–372. doi: 10.1126/science.aaa5082. [DOI] [PubMed] [Google Scholar]
- 13.Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol. 2012;12:479–491. doi: 10.1038/nri3247. [DOI] [PubMed] [Google Scholar]
- 14.Weiskopf D, Angelo MA, Bangs DJ, Sidney J, Paul S, Peters B, de Silva AD, Lindow JC, Diehl SA, Whitehead S, et al. The human CD8+ T cell responses induced by a live attenuated tetravalent dengue vaccine are directed against highly conserved epitopes. J Virol. 2015;89:120–128. doi: 10.1128/JVI.02129-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nkolola JP, Cheung A, Perry JR, Carter D, Reed S, Schuitemaker H, Pau MG, Seaman MS, Chen B, Barouch DH. Comparison of multiple adjuvants on the stability and immunogenicity of a clade C HIV-1 gp140 trimer. Vaccine. 2014;32:2109–2116. doi: 10.1016/j.vaccine.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romagnani S. T-cell subsets (Th1 versus Th2) Ann Allergy Asthma Immunol. 2000;85:9–18. doi: 10.1016/S1081-1206(10)62426-X. quiz 18, 21. [DOI] [PubMed] [Google Scholar]
- 18.Mario M, D'Elios MB, Chiara Della Bella, Amedeo Amedei. T-cell response to bacterial agents. J Infect Dev Ctries. 2011;5 doi: 10.3855/jidc.2019. [DOI] [PubMed] [Google Scholar]
- 19.Mills KHG, Ryan M, Ryan E, Mahon BP. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infection and Immunity. 1998;66:594–602. doi: 10.1128/iai.66.2.594-602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huleatt JW, Jacobs AR, Tang J, Desai P, Kopp EB, Huang Y, Song L, Nakaar V, Powell TJ. Vaccination with recombinant fusion proteins incorporating Toll-like receptor ligands induces rapid cellular and humoral immunity. Vaccine. 2007;25:763–775. doi: 10.1016/j.vaccine.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Korsholm KS, Petersen RV, Agger EM, Andersen P. T-helper 1 and T-helper 2 adjuvants induce distinct differences in the magnitude, quality and kinetics of the early inflammatory response at the site of injection. Immunology. 2010;129:75–86. doi: 10.1111/j.1365-2567.2009.03164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fromen CA, Robbins GR, Shen TW, Kai MP, Ting JPY, DeSimone JM. Controlled analysis of nanoparticle charge on mucosal and systemic antibody responses following pulmonary immunization. Proceedings of the National Academy of Sciences. 2015;112:488–493. doi: 10.1073/pnas.1422923112. This study directly assessed the effects of nanoparticle charge on pulmonary vaccination without affecting other physio/chemical particle characteristics and/or antigen loading by using the Particle Replication in Non-Wetting Templates (PRINT) process.
- 23.Parsa S, Pfeifer B. Engineering bacterial vectors for delivery of genes and proteins to antigen-presenting cells. Molecular Pharmaceutics. 2007;4:4–17. doi: 10.1021/mp0600889. [DOI] [PubMed] [Google Scholar]
- 24.Saxena M, Van TTH, Baird FJ, Coloe PJ, Smooker PM. Pre-existing immunity against vaccine vectors – friend or foe? Microbiology. 2013;159:1–11. doi: 10.1099/mic.0.049601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Curtiss R. Development of Streptococcus pneumoniae vaccines using live vectors. Vaccines. 2014;2:49–88. doi: 10.3390/vaccines2010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang X, Kong W, Wanda S-Y, Xin W, Alamuri P, Curtiss R. Generation of influenza virus from avian cells infected by Salmonella carrying the viral genome. PLoS ONE. 2015;10:e0119041. doi: 10.1371/journal.pone.0119041. This study presents a Salmonella influenza virus vaccine delivery system which is a recombinant attenuated bacterial strain carrying the 8-unit plasmid capable of mediating production of attenuated influenza virus in vivo by delivering its DNA cargo into permissive host cells.
- 27.Venkataswamy MM, Ng TW, Kharkwal SS, Carreño LJ, Johnson AJ, Kunnath-Velayudhan S, Liu Z, Bittman R, Jervis PJ, Cox LR, et al. Improving Mycobacterium bovis bacillus Calmette-Guèrin as a vaccine delivery vector for viral antigens by incorporation of glycolipid activators of NKT cells. PLoS ONE. 2014;9:e108383. doi: 10.1371/journal.pone.0108383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seegers JFML. Lactobacilli as live vaccine delivery vectors: progress and prospects. Trends in Biotechnology. 2002;20:508–515. doi: 10.1016/s0167-7799(02)02075-9. [DOI] [PubMed] [Google Scholar]
- 29.Eypper EH, Johnson PV, Purro EI, Hohmann EL. Transcutaneous immunization of healthy volunteers with an attenuated Listeria monocytogenes vaccine strain and cholera toxin adjuvant. Vaccine. 2013;31 doi: 10.1016/j.vaccine.2013.05.028. 10.1016/j.vaccine.2013.1005.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahabi V, Maciag PC, Rivera S, Wallecha A. Live, attenuated strains of Listeria and Salmonella as vaccine vectors in cancer treatment. Bioengineered Bugs. 2010;1:235–239. doi: 10.4161/bbug.1.4.11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luquero FJ, Grout L, Ciglenecki I, Sakoba K, Traore B, Heile M, Diallo AA, Itama C, Page A-L, Quilici M-L, et al. Use of Vibrio cholerae vaccine in an outbreak in Guinea. New England Journal of Medicine. 2014;370:2111–2120. doi: 10.1056/NEJMoa1312680. [DOI] [PubMed] [Google Scholar]
- 32.Bermúdez-Humarán LG, Kharrat P, Chatel J-M, Langella P. Lactococci and lactobacilli as mucosal delivery vectors for therapeutic proteins and DNA vaccines. Microbial Cell Factories. 2011;10:S4–S4. doi: 10.1186/1475-2859-10-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harro JM, Peters BM, O'May GA, Archer N, Kerns P, Prabhakara R, Shirtliff ME. Vaccine development in Staphylococcus aureus : taking the biofilm phenotype into consideration. Fems Immunology and Medical Microbiology. 2010;59:306–323. doi: 10.1111/j.1574-695X.2010.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson RJ, Pasetti MF, Sztein MB, Levine MM, Noriega FR. ΔguaBA attenuated Shigella flexneri 2a strain CVD 1204 as a Shigella vaccine and as a live mucosal delivery system for fragment C of tetanus toxin. Vaccine. 2000;18:2193–2202. doi: 10.1016/s0264-410x(00)00025-6. [DOI] [PubMed] [Google Scholar]
- 35.Jones CH, Ravikrishnan A, Chen M, Reddinger R, Kamal Ahmadi M, Rane S, Hakansson AP, Pfeifer BA. Hybrid biosynthetic gene therapy vector development and dual engineering capacity. Proceedings of the National Academy of Sciences. 2014;111:12360–12365. doi: 10.1073/pnas.1411355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, Hou J, Lin Z, Zhuo H, Chen D, Zhang X, Chen Y, Sun B. Attenuated Listeria monocytogenes as a cancer vaccine vector for the delivery of CD24, a biomarker for hepatic cancer stem cells. Cell Mol Immunol. 2014;11:184–196. doi: 10.1038/cmi.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16:1035–1041. doi: 10.1038/nm.2198. This study proposed an approach to conjugate adjuvant drug-loaded nanoparticles to the surfaces of therapeutic cells, enhancing cell therapy via sustained pseudoautocrine stimulation to donor cells. The strategy is a simple and generalizable way to augment cytoreagents while minimizing the systemic side effects of adjuvant drugs, suggesting therapeutic cells are promising vectors for actively targeted drug delivery.
- 38.Mehta NK, Moynihan KD, Irvine DJ. Engineering new approaches to cancer vaccines. Cancer Immunology Research. 2015;3:836–843. doi: 10.1158/2326-6066.CIR-15-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adib-Conquy M, Scott-Algara D, Cavaillon J-M, Souza-Fonseca-Guimaraes F. TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunol Cell Biol. 2014;92:256–262. doi: 10.1038/icb.2013.99. [DOI] [PubMed] [Google Scholar]
- 40.Pihlgren M, Silva AB, Madani R, Giriens V, Waeckerle-Men Y, Fettelschoss A, Hickman DT, López-Deber MP, Ndao DM, Vukicevic M, et al. TLR4- and TRIF-dependent stimulation of B lymphocytes by peptide liposomes enables T cell–independent isotype switch in mice. Blood. 2012;121:85–94. doi: 10.1182/blood-2012-02-413831. [DOI] [PubMed] [Google Scholar]
- 41.Perrie Y, Crofts F, Devitt A, Griffiths HR, Kastner E, Nadella V. Designing liposomal adjuvants for the next generation of vaccines. Advanced Drug Delivery Reviews. doi: 10.1016/j.addr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 42. García-Vallejo JJ, Ambrosini M, Overbeek A, van Riel WE, Bloem K, Unger WWJ, Chiodo F, Bolscher JG, Nazmi K, Kalay H, et al. Multivalent glycopeptide dendrimers for the targeted delivery of antigens to dendritic cells. Molecular Immunology. 2013;53:387–397. doi: 10.1016/j.molimm.2012.09.012. This study reported design of efficient targeting of peptide antigens with dendritic cell-specific ICAM-3-grabbing non-integrin (DC-SIGN) by characterizing the optimal level of multivalency necessary to achieve the desired internalization, lysosomal delivery, Ag-specific T cell proliferation, and cytokine response, holding potential for the immunotherapy of cancer, autoimmunity, and infectious diseases.
- 43.Chatin B, Mevel M, Devalliere J, Dallet L, Haudebourg T, Peuziat P, Colombani T, Berchel M, Lambert O, Edelman A, et al. Liposome-based formulation for intracellular delivery of functional proteins. Mol Ther Nucleic Acids. 2015;4:e244. doi: 10.1038/mtna.2015.17. [DOI] [PubMed] [Google Scholar]
- 44.Zhao L, Seth A, Wibowo N, Zhao C-X, Mitter N, Yu C, Middelberg APJ. Nanoparticle vaccines. Vaccine. 2014;32:327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 45.Li W, Joshi MD, Singhania S, Ramsey KH, Murthy AK. Peptide vaccine: progress and challenges. Vaccines. 2014;2:515–536. doi: 10.3390/vaccines2030515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oliveira F, Rowton E, Aslan H, Gomes R, Castrovinci PA, Alvarenga PH, Abdeladhim M, Teixeira C, Meneses C, Kleeman LT, et al. A sand fly salivary protein vaccine shows efficacy against vector-transmitted cutaneous leishmaniasis in nonhuman primates. Science Translational Medicine. 2015;7:290ra290–290ra290. doi: 10.1126/scitranslmed.aaa3043. [DOI] [PubMed] [Google Scholar]
- 47.Trimaille T, Verrier B. Micelle-based adjuvants for subunit vaccine delivery. Vaccines. 2015;3:803–813. doi: 10.3390/vaccines3040803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shao S, Geng J, Ah Yi H, Gogia S, Neelamegham S, Jacobs A, Lovell JF. Functionalization of cobalt porphyrin–phospholipid bilayers with his-tagged ligands and antigens. Nat Chem. 2015;7:438–446. doi: 10.1038/nchem.2236. This study presents the functionalization of cobalt porphyrin–phospholipid bilayers, which represent simple and versatile materials for binding his-tagged proteins and peptides. The approach enables surface orientation of his-tagged polypeptides produced through modern peptide synthesis and genetic engineering, providing a multivalent protein based vaccine delivery platform which can confer targeting or immunogenic properties with a high degree of antigen capacity and variety.
- 49.Botten J, Whitton JL, Barrowman P, Sidney J, Whitmire JK, Alexander J, Kotturi MF, Sette A, Buchmeier MJ. A Multivalent vaccination strategy for the prevention of old world arenavirus infection in humans. Journal of Virology. 2010;84:9947–9956. doi: 10.1128/JVI.00672-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Algar WR, Prasuhn DE, Stewart MH, Jennings TL, Blanco-Canosa JB, Dawson PE, Medintz IL. The controlled display of biomolecules on nanoparticles: a challenge suited to bioorthogonal chemistry. Bioconjugate Chemistry. 2011;22:825–858. doi: 10.1021/bc200065z. [DOI] [PubMed] [Google Scholar]
- 51.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suh H, Shin J, Kim YC. Microneedle patches for vaccine delivery. Clin Exp Vaccine Res. 2014;3:42–49. doi: 10.7774/cevr.2014.3.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeMuth PC, Min Y, Huang B, Kramer JA, Miller AD, Barouch DH, Hammond PT, Irvine DJ. Polymer multilayer tattooing for enhanced DNA vaccination. Nature Materials. 2013;12:367–376. doi: 10.1038/nmat3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang B-Z, Gill HS, He C, Ou C, Wang L, Wang Y-C, Feng H, Zhang H, Prausnitz MR, Compans RW. Microneedle delivery of an M2e-TLR5 ligand fusion protein to skin confers broadly cross-protective influenza immunity. Journal of Controlled Release. 2014;178:1–7. doi: 10.1016/j.jconrel.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


