Summary
Cancer stem cells (CSCs) are highly associated with therapy resistance and metastasis. Interplay between CSCs and various immune components is required for tumor survival. However, the response of CSCs to complement surveillance remains unknown. Herein, using stem-like sphere-forming cells prepared from a mammary tumor and a lung adenocarcinoma cell line, we found that CD59 was upregulated to protect CSCs from complement-dependent cytotoxicity. CD59 silencing significantly enhanced complement destruction and completely suppressed tumorigenesis in CSC-xenografted nude mice. Furthermore, we identified that SOX2 upregulates CD59 in epithelial CSCs. In addition, we revealed that SOX2 regulates the transcription of mCd59b, leading to selective mCD59b abundance in murine testis spermatogonial stem cells. Therefore, we demonstrated that CD59 regulation by SOX2 is required for stem cell evasion of complement surveillance. This finding highlights the importance of complement surveillance in eliminating CSCs and may suggest CD59 as a potential target for cancer therapy.
Keywords: CD59, SOX2, complement surveillance, stem cells, complement-dependent cytotoxity, transcription regulation, mCD59a, mCD5b, mouse testis
Highlights
-
•
CD59 upregulation is required for stem cells evading complement surveillance
-
•
SOX2 is responsible for CD59 upregulation in stem cells
-
•
SOX2 regulates mCD59b selective expression in testis spermatogonial stem cells
CD59 and mCD59b in mouse, but not other membrane regulatory proteins, are upregulated by SOX2 in stem cells and are required to evade complement surveillance. CD59 insufficiency may result in near-complete arrest of tumor growth and loss of tumorigenesis of cancer stem cells.
Introduction
Cancer stem cells (CSCs) generally account for a rare subpopulation of cells within tumors; however, some reports showed that up to 25% of cancer cells within certain tumors display the characteristics of CSCs (Kelly et al., 2007, Quintana et al., 2008). CSCs have been defined according to their ability to drive tumor growth in xenografted animals accompanied by self-renewal and differentiation (Clarke et al., 2006). Moreover, CSCs have been reported to be highly associated with therapy resistance, recurrence, and metastasis (Dean et al., 2005, Meacham and Morrison, 2013). During the process of tumor initiation and progression, tumor cells must escape immunologic detection and elimination (Dunn et al., 2002). Given these unique properties of CSCs, these cells may have a stronger capability than differentiated tumor cells of evading various host immune surveillance mechanisms.
The complement system, a main component of innate immunity, circulates to conduct immune surveillance and discriminate invading pathogens and cell debris from healthy host tissues (Morgan et al., 2005, Ricklin et al., 2010). After activation, complement components are cleaved into different fragments with multiple functions: C3a/C5a primes inflammation, C3b/iC3b induces opsono-phagocytosis, and C5b-9(n) (membrane attack complex, MAC) provokes rapid cell death (Dunkelberger and Song, 2010). To protect host cells from bystander complement attack, several membrane complement regulatory proteins (mCRPs) have evolved to restrict complement activation at diverse stages. CD46 acts as a cofactor for the inactivation of cell-bound C4b and C3b by serum factor I, CD55 inactivates C3 and C5 convertases by accelerating the decay of these proteases, and CD59 is the sole mCRP to prevent MAC formation (Zhou et al., 2008). Various endogenous (autologous antibodies, C1q, pentraxins, ficolins, etc.) (Ricklin et al., 2010) and exogenous (therapeutic antibodies, such as rituximab for B lymphoma [Zhou et al., 2008] and cetuximab for certain solid tumors [Hsu et al., 2010]) pattern recognition molecules can substantially activate complement in tumor microenvironment, which is critical in tumor cells, especially CSCs, for eventual survival from complement-mediated elimination (Ricklin et al., 2010).
Numerous studies, including ours, have demonstrated that high expression of mCRPs, mainly CD46, CD55, and CD59, confer tumor cell resistance to antibody-based cancer therapy by preventing complement cascade amplification or MAC formation; therefore, functional inhibition of mCRPs may unleash the resistance (Goswami et al., 2016, Hu et al., 2011, Macor et al., 2015, Wang et al., 2010). Compared with other mCRPs, CD59 has been considered the most effective mCRP to protect tumor cells from complement-mediated lysis (Fishelson, 2003, Zhou et al., 2008). However, there are few reports on CSC evasion of complement-mediated elimination. In addition, normal stem cells may similarly encounter frequent complement attack, which requires high expression of mCRPs. Therefore, mCd59b (Genbank: NM_181858.1) deficiency, but not mCd59a (Genbank: NM_001111060.2) deficiency, could induce male infertility associated with fewer sperm cells (Qin et al., 2003). However, the underlying mechanisms for stem cells escaping complement surveillance remain largely unclear.
In this study, we employed serum-free selection medium to prepare cancer stem-like sphere-forming cells in which CD59, but not CD46 or CD55, was upregulated, conferring resistance to cetuximab-induced complement-dependent cytotoxicity (CDC). CD59 insufficiency in sphere-forming cells completely suppressed tumorigenesis in xenografted nude mice. Furthermore, we illustrated that SOX2 is responsible for CD59 upregulation in CSCs and highly correlates with the selective expression of mCD59b in mouse spermatogonial stem cells.
Results
CD59 Alone Is Upregulated to Confer CSC Resistance to Complement-Mediated Destruction
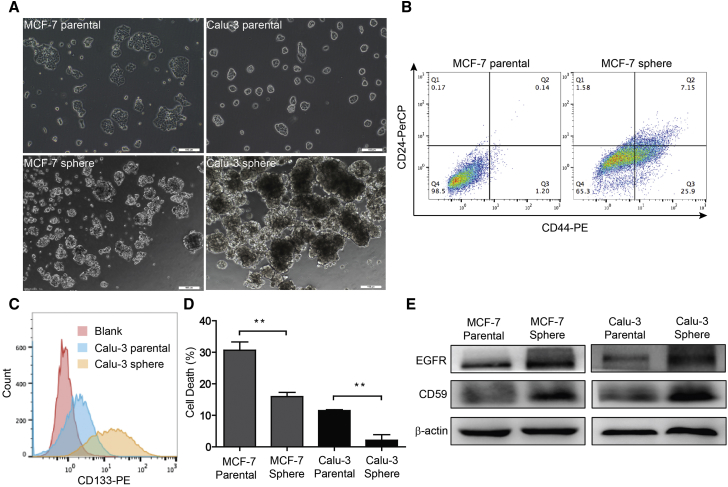
To investigate the response of mCRPs in CSCs, we first prepared stem-like sphere-forming cells from MCF-7 and Calu-3 parental cells. We observed that spheres developed with a diameter larger than 50 μm after 14 days of culture (Figure 1A). Furthermore, we verified the stemness of sphere-forming cells by staining the related biomarkers and by detecting in vivo tumorigenesis abilities. In MCF-7 cells, the subpopulation of stem-like CD44+/CD24− cells was significantly increased from 1.2% in parental cells to 25.9% in sphere-forming cells (Figure 1B). Similarly, in Calu-3 sphere-forming cells, the subpopulation of CD133+ cells was dramatically increased compared with that of the parental cells (Figure 1C). In addition, we implanted 1.0 × 105 Calu-3 sphere and parental cells in each flank of the same nude mouse, and found that sphere-forming cells resulted in much faster tumor growth than parental cells (Figure S1). Therefore, the enriched sphere-forming cells displayed the important characteristics of CSCs.
Figure 1.
CD59 Upregulation Confers Stem-like Sphere-Forming Cell Resistance to Cetuximab-Induced Complement Destruction
(A) The morphological change between parental and sphere-forming cells. Scale bars, 100 μm.
(B and C) The subpopulation of stem cells was remarkably increased in the sphere-forming cells. Stem cell biomarkers: CD44+/CD24− for MCF-7 (B) and CD133+ for Calu-3 (C).
(D) MCF-7 and Calu-3 sphere-forming cells exhibit resistance to cetuximab-induced complement-mediated destruction compared with the parental cells. Data are presented as mean ± SD (n = 3 independent experiments); ∗∗p < 0.01.
(E) The expression levels of EGFR and CD59 were notably increased in the sphere-forming cells.
See also Figure S2.
Next, using a fluorescence-activated cell sorting (FACS) assay, we detected the expression levels of three mCRPs, CD46, CD55, and CD59, in the sphere-forming cell membranes. Compared with the parental cells, sphere-forming cells expressed a much higher level of CD59 in both MCF-7 and Calu-3 cells (Figures S2A and S2B). In contrast, we found that the CD46 levels were significantly reduced in MCF-7 and Calu-3 sphere-forming cells, and the CD55 level was only notably reduced in MCF-7 sphere-forming cells and slightly increased in Calu-3 sphere-forming cells (Figures S2C–S2F). This finding is consistent with previous reports that CD59 is upregulated in colorectal and pancreatic CSCs (Gemei et al., 2013, Zhu et al., 2013). Therefore, CD59 may play a more important role than CD46 and CD55 in protecting CSCs from complement destruction.
To test the resistance to CDC imposed by upregulated CD59, we treated parental and sphere MCF-7 or Calu-3 cells with normal human serum (NHS) and the epidermal growth factor receptor (EGFR)-targeted monoclonal antibody cetuximab, which has been widely used for the treatment of multiple solid tumors (Hsu et al., 2010); we then measured the CDC effect by detecting lactate dehydrogenase (LDH) release. The rate of cell death in sphere-forming cells remarkably decreased compared with that of the parental cells (Figure 1D). To exclude the possibility that the EGFR expression level may decrease and accordingly reduce the CDC effect, we further detected the expression levels of EGFR accompanied by CD59 using an immunoblotting assay. We observed that the EGFR level slightly increased in both MCF-7 and Calu-3 sphere-forming cells compared with that of the parental cells (Figure 1E), which may conversely enhance the cetuximab-mediated CDC effect. Consistent with our other findings, the CD59 level still showed remarkable increases in both sphere-forming cells (Figure 1E). Together, these findings strongly suggest that CD59, but not other mCRPs, was significantly upregulated in sphere-forming CSCs to prevent complement destruction.
CD59 Insufficiency Facilitates Cetuximab-Mediated Complement Damage in CSCs
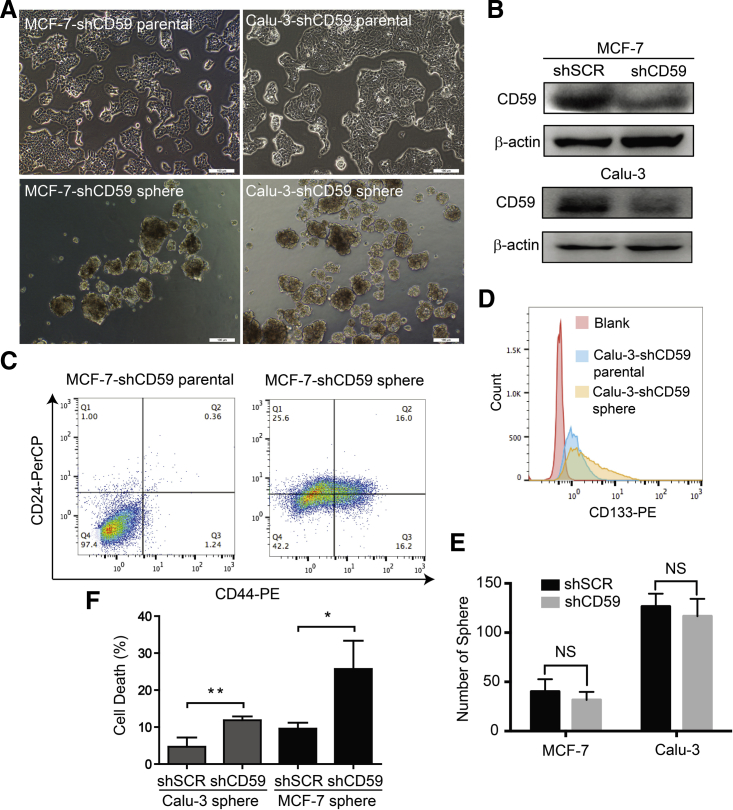
To further validate the function of CD59 in protecting CSCs from complement attack, we first generated stable CD59-insufficient MCF-7 and Calu-3 parental cells by specific small hairpin RNA (shRNA). Then, using the same method previously described, we successfully obtained sphere-forming cells after 14 days of culture (Figure 2A), and CD59 insufficiency was verified by an immunoblotting assay (Figure 2B). Similarly, these sphere-forming cells demonstrated stemness by the significantly increased CD44+/CD24− population in MCF-7-shCD59 sphere-forming cells and CD133+ population in Calu-3-shCD59 sphere-forming cells (Figures 2C and 2D). In addition, we quantitatively compared the sphere formation capacity between CD59-sufficient and CD59-insuficient MCF-7 and Calu-3 cells, which showed no significant change (Figure 2E). Therefore, CD59 insufficiency may not affect sphere formation.
Figure 2.
CD59 Silencing Did Not Affect Sphere Formation but Sensitized Sphere-Forming Cells to Complement-Mediated Destruction
(A) CD59 silencing in MCF-7 and Calu-3 parental cells did not affect sphere formation. Scale bars, 100 μm.
(B) Confirmation of the efficacy of CD59 silencing. shSCR, scrambled shRNA; shCD59, specific shRNA against CD59.
(C and D) The subpopulation of stem cells was still remarkably increased in the CD59-silencing sphere-forming cells. Stem cell biomarkers: CD44+/CD24− for MCF-7 (C) and CD133+ for Calu-3 (D).
(E) Quantitative comparison of the sphere formation capacity between CD59-sufficient and CD59-insufficient cells.
(F) CD59-insufficient MCF-7 and Calu-3 sphere-forming cells were significantly vulnerable to cetuximab-induced complement destruction compared with CD59-sufficient sphere-forming cells. Data are presented as mean ± SD (n = 3 independent experiments), NS, no significance; ∗p < 0.05, ∗∗p < 0.01.
See also Figure S3.
Next, we detected the ability of CD59-insufficient sphere-forming cells to avoid cetuximab-mediated CDC. As shown in Figure 2F, CD59 insufficiency resulted in the sphere-forming cells being more vulnerable to the transient cetuximab-mediated complement damage than MCF-7 and Calu-3 cells treated with scrambled shRNA. Therefore, the expression of CD59 is important for CSC survival from complement destruction.
CD59 Level Is a Determinant for the Susceptibility of Parental Cells to Complement Destruction
Considering that the implanted sphere-forming cells could sustain differentiation in in vivo experiments, we first detected the expression levels of CD59 together with CD46 and CD55 in two breast (MCF-7 and SK-BR-3) and two lung (Calu-3 and A549) parental cancer cell lines by FACS assay. As shown in Figure S3A, the CD59 level was gradually increased in the order MCF-7, SK-BR-3, A549, and Calu-3 cells. However, CD46 was expressed at the lowest level in A549 cells compared with those of MCF-7, SK-BR-3, and Calu-3 cells, which express CD46 at a similar level (Figure S3B). Moreover, we observed a notable difference in CD55 expression levels among the four cell lines in the escalating order A549, SK-BR-3, Calu-3, and MCF-7 cells, which was different from CD59 levels (Figure S3C). We also validated the mRNA and protein levels of CD59 among the four cells by qRT-PCR or western blot, respectively (Figures S3D and S3E), and the results were consistent with those in Figure S3A. Furthermore, we used cetuximab together with NHS to induce CDC in these cancer cells. The results of the LDH release assay suggested that the rate of cell death was almost exactly conversely correlated with the expression level of CD59, but not CD46 or CD55 (Figure S3F).
To further validate the role of CD59 in protecting cancer cells from cetuximab-induced CDC, we induced CD59 insufficiency by shCD59 in MCF-7 and Calu-3 cells and then measured cell survival. The results showed that compared with a scrambled control, shCD59 led to higher susceptibility of MCF-7 and Calu-3 cells to the transient cetuximab-mediated CDC (Figure S3G). Therefore, CD59 could more effectively protect cancer cells from cetuximab-induced complement destruction than CD46 and CD55, which is in agreement with the previous conclusion that CD59 is the most relevant mCRP in protecting tumor cells from complement-mediated lysis (Fishelson, 2003).
CD59 Insufficiency Completely Suppressed CSC Tumorigenesis In Vivo
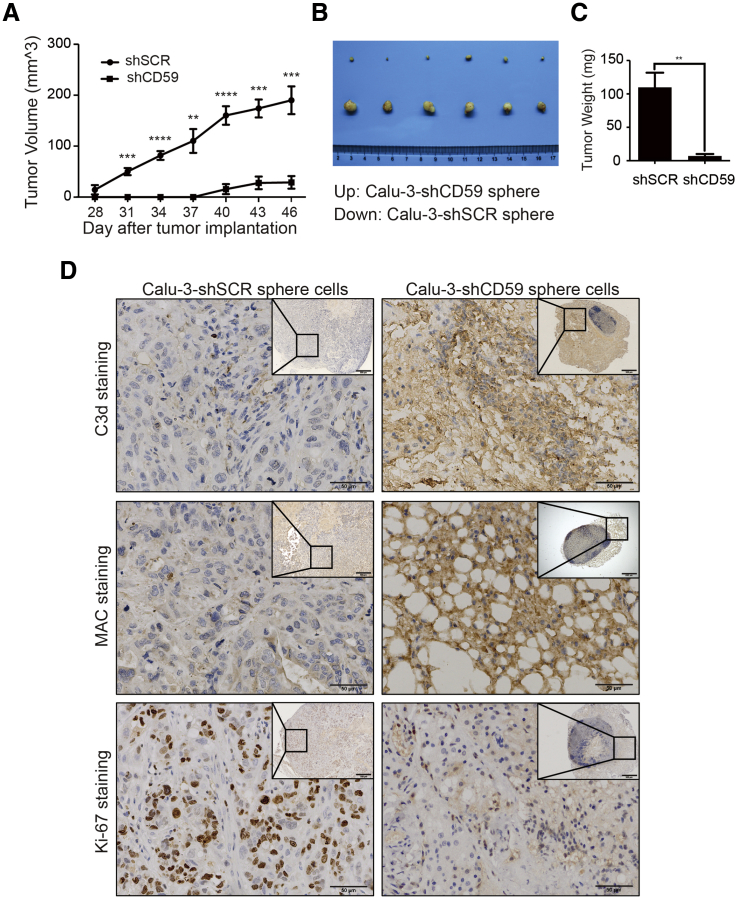
To further confirm whether CD59 insufficiency makes CSCs more vulnerable to complement surveillance in vivo, we subcutaneously implanted CD59-sufficient and CD59-insufficient Calu-3 sphere-forming cells into the respective sides of nude mice axilla. The results showed that CD59 insufficiency nearly completely suppressed tumor growth compared with CD59 sufficiency (Figure 3A). Unlike CD59-sufficient sphere-forming cells, we observed that tumor growth of CD59-insufficient sphere-forming cells lessened on day 46 compared with day 43 (Figure 3A) and considered that combined treatment with the CD59 inhibitor ILYd4 and rituximab on Burkitt's B cell lymphoma xenografted tumors induced a high tumor-free rate (Hu et al., 2011). Therefore, to investigate the status of complement activation and cancer proliferation in tumor tissues, we euthanized mice and collected tumor tissues on day 46. At this endpoint, the tumor size and weight were much smaller in CD59-insufficient tumors than in CD59-sufficient tumors (Figures 3B and 3C). In addition, the CD59-insufficient tumors displayed extensive, strong C3d and MAC staining, whereas positive Ki-67 staining was almost negligible (2.8%) compared with that of CD59-sufficient tumors (65.6%) (Figure 3D). The results suggest extensive complement activation and subsequent MAC-mediated damage, thus almost completely suppressing the tumor growth of CD59-insufficient cells. In addition, we observed that a compartmented nascent lymphoid nodule with abundant lymphocyte accumulation developed and was actively predominant in CD59-insufficient tumor tissue (Figure 3D). Therefore, we suggest that the CD59-insufficient tumors would most likely disappear eventually. This finding strongly suggests that CD59 expression is required for CSC in vivo survival from persistent complement surveillance.
Figure 3.
CD59 Silencing Completely Suppressed Tumor Growth in Calu-3 Sphere-Forming Cells
(A) Tumor growth was completely suppressed from day 43 in nude mice implanted with CD59-insufficient Calu-3 sphere-forming cells. Data are presented as mean ± SEM (n = 6 independent experiments). The significance of tumor growth between CD59-insufficient and CD59-sufficient sphere Calu-3 cells was determined using the Holm-Sidak method in multiple t tests: one per row, with α = 5.000%. Each row was analyzed individually, without assuming a consistent SD. ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
(B and C) The tumor images (B) and tumor weight (C) at the endpoint of day 46. Data are presented as mean ± SD (n = 6 independent experiments); ∗∗p < 0.01.
(D) IHC assay. Complement activation and cancer cell proliferation were determined by C3d and MAC or Ki-67 staining, respectively. Complement was substantially activated, leading to a near-complete cessation of proliferation in CD59-insufficient Calu-3 sphere-forming cells (A). Scale bar, 50 μm.
See also Figure S4.
In addition, it has been reported that pathogen-associated molecular patterns, damage-associated molecular patterns, and surface proteins released from dead cells may activate complement (Ricklin and Lambris, 2013); therefore, we suggest that the extensive complement activation (right panel in Figure 3D) may be amplified by the initial cell death of CD59-insufficient cells. To recapitulate the complement activation in tumor tissues of nude mice implanted by CD59-insufficient cells, we treated MCF-7 parental cells with intermedilysin (ILY), which that can rapidly induce CD59-positive cell death via binding to human CD59 (Hu et al., 2008), following additional NHS or IHS (heat-inactivated human serum) administration. The CD59-deficient MCF-7 cells survived ILY treatment and then were positively stained by antibodies against C3d or MAC after NHS but not IHS challenge (Figures S4A and S4B). Consistent with this, complement activation triggered by NHS and ILY-induced CD59-positive dead cells resulted in higher cell death rate than ILY alone and ILY plus IHS treatment groups (Figure S4C).
SOX2 Regulates CD59 Transcription in CSCs
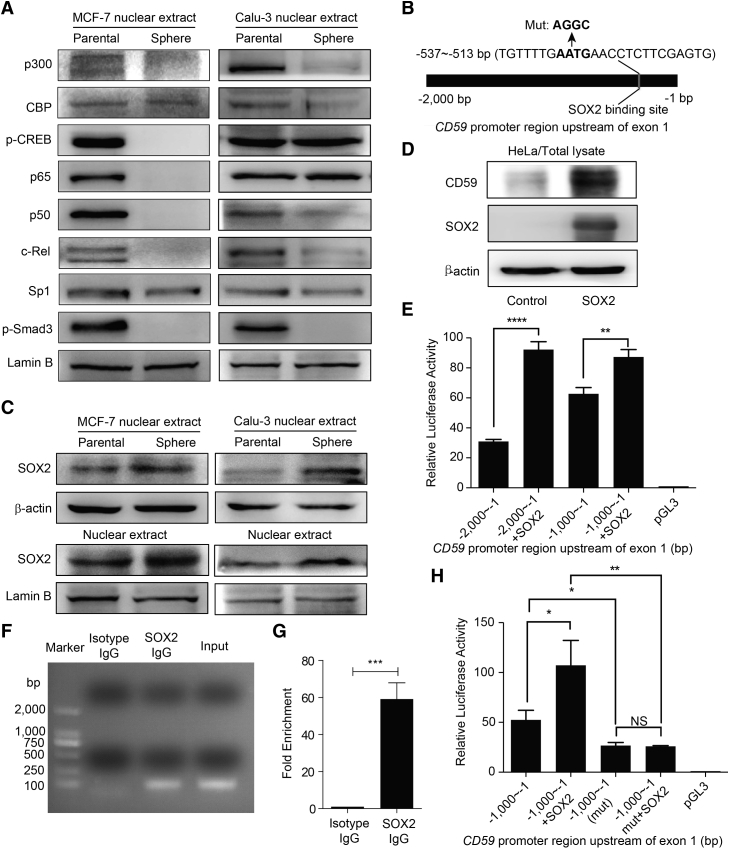
Our previous work illustrated that CD59 constitutive expression is regulated by Sp1, whereas nuclear factor κB (NF-κB) and CREB scaffolded by CBP/p300 proteins are responsible for the inducible expression of CD59 (Genbank: NM_203330.2) in inflammatory conditions (Figure 5C) (Du et al., 2014). Another study showed that Smad3 also regulates CD59 transcription in a certain condition of transforming growth factor β (TGF-β)-induced epithelial-mesenchymal transition (EMT) (Goswami et al., 2016). Therefore, to exclude the effect of the above transcription factors in regulating CD59 transcription in CSCs, we compared their levels in nuclear extracts between the parental and sphere-forming cells. We observed that the levels of CBP/p300, phos-CREB, classic NF-κB subunits p65/p50/c-Rel, Sp1, and phos-Smad3 appear not to change or are even remarkably reduced in the sphere-forming cells (Figure 4A). These results indicate that none of these proteins contribute to CD59 upregulation in CSCs.
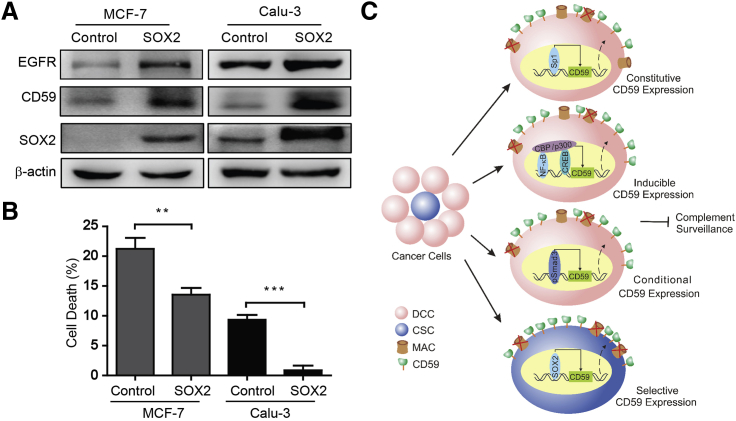
Figure 5.
SOX2 Upregulates CD59 to Protect Cancer Cells from Complement Destruction
(A) Ectopic SOX2 increased the expression levels of EGFR and CD59 in parental cells.
(B) The parental cells with ectopic SOX2 were resistant to cetuximab-induced complement destruction. Data are presented as mean ± SD (n = 3 independent experiments); ∗∗p < 0.01, ∗∗∗p < 0.001.
(C) Schematic for cancer cell evasion of complement surveillance by regulating CD59 transcription. CD59 expression is constitutively regulated by Sp1, inducibly by NF-κB and CREB scaffolds bound to CBP/p300 (Du et al., 2014), conditionally by Smad3 in TGF-β-induced EMT (Goswami et al., 2016), and selectively by SOX2 in CSCs. DCC, differentiated cancer cell; CSC, cancer stem cell; MAC, membrane attack complex. See also Figure S6.
Figure 4.
SOX2 Is a Key Transcription Factor for CD59 Expression in CSCs
(A) The activities or nuclear levels of reported trans-acting factors for CD59 transcription were unchanged or reduced to varying degrees in stem-like sphere-forming cells.
(B) The SOX2 binding site was predicted in the region of −2,000 to −1 bp upstream of CD59 exon1 using MatInspector software. The mutated critical response site of SOX2 is also indicated.
(C) The protein levels of SOX2 in the total lysate and nuclear extract were increased in sphere stem-like cells.
(D) Ectopic SOX2 remarkably increased CD59 expression in HeLa cells.
(E) Dual-luciferase reporter assay in HeLa cells: the promoter activities of the −2,000 to −1 bp and −1,000 to −1 bp regions upstream of CD59 exon1 were significantly increased due to ectopic SOX2.
(F and G) ChIP assay in HeLa cells. A specific antibody against SOX2, but not isotype IgG, could capture the fragment containing the SOX2 response element in the CD59 promoter region, which was amplified by specific primers (Table S2) using PCR (F). The quantitative data are shown (G).
(H) Dual-luciferase reporter assay in HeLa cells. Mutation of critical response nucleotides for SOX2 binding abrogated the enhanced promoter activity by ectopic SOX2.
Data are presented as mean ± SD (n = 3, independent repeats in E, H, and technical repeats in G). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. See also Figure S5.
SOX2 is an important transcription factor that is responsible for stemness maintenance in stem cells (Malladi et al., 2016, Takahashi and Yamanaka, 2006). Using MatInspector software (Genomatix Software), we predicted a SOX2 binding site in a region from −537 to −513 bp upstream of CD59 exon 1 (Figure 4B). Therefore, we first evaluated the SOX2 level in the parental and sphere-forming cells. The results showed that SOX2 was notably upregulated in the total lysate and nuclear extract of the sphere-forming cells (Figure 4C). We then overexpressed SOX2, and found that CD59 was accordingly upregulated (Figure 4D). This observation led us to hypothesize that CD59 transcription may be regulated by SOX2 in CSCs.
We next performed a dual-luciferase reporter assay by transfecting pGL3 plasmids containing the sequences 2,000 and 1,000 bp upstream of CD59 exon1 alone or together with a pEGFP-N1-SOX2 plasmid into HeLa cells. The results indicate that ectopic SOX2 significantly enhanced the transcriptional activity (Figure 4E). We further identified whether SOX2 could directly bind to the predicted site by a chromatin immunoprecipitation (ChIP) assay. As shown in Figures 4F and 4G, a specific anti-SOX2 antibody, but not isotype immunoglobulin G (IgG), was able to capture the fragment that could be amplified by the specific primers for the region containing the predicted SOX2 binding site. In addition, we mutated the critical nucleotides for SOX2 activity as indicated in Figure 4B and tested the consequent promoter activity with or without ectopic SOX2. The results showed that the mutation of SOX2 response nucleotides could significantly reduce the promoter activity. Furthermore, even with ectopic SOX2, the transcriptional activity was not increased in the mutant. Mutation of the SOX2 response nucleotides completely abrogated the enhanced promoter activity by ectopic SOX2 (Figure 4H).
To further prove the regulation of CD59 by SOX2, we constructed SOX2-insufficient stable Calu-3 and MCF7 parental cells and then generated the related stem-like sphere-forming cells. The knockdown efficiency of SOX2 was confirmed by qRT-PCR and immunoblotting (Figures S5A and S5C). We then observed that CD59 expression was accordingly downregulated in mRNA and protein levels (Figures S5B and S5C), together with the reduced protein level of EGFR (Figure S5C). Concordantly, the results of FACS further confirmed this finding and demonstrated that the expression levels of CD46 and CD55 were not influenced by SOX2 insufficiency (Figure S5D). Together, these results clearly demonstrated that SOX2 is responsible for CD59 upregulation in CSCs.
Upregulation of CD59 by SOX2 Protects Cancer Cells from Complement Destruction
To further determine whether SOX2 is sufficient to confer cancer cell resistance to complement destruction, we overexpressed SOX2 in MCF-7 and Calu-3 cells. Using an immunoblotting assay, we found that the levels of CD59 and EGFR were increased along with ectopic SOX2 (Figure 5A). Using a FACS assay, the CD59 level in the cell membrane was further verified to be upregulated (Figures S6A and S6B), whereas the membrane levels of CD46 and CD55 did not increase compared with those of the vector control (Figures S6C–S6F).
We conducted a CDC assay to functionally test the effect of upregulated CD59 by ectopic SOX2 in protecting cancer cells from complement destruction. The results demonstrated that SOX2-overexpressing MCF-7 and Calu-3 cells are much more resistant than control cells to cetuximab-mediated complement damage (Figure 5B). These findings demonstrated that SOX2 could transcriptionally upregulate the expression of CD59, but not the expression of CD46 and CD55, in CSCs, thus conferring CSC resistance to complement surveillance (Figure 5C).
SOX2 Is Responsible for mCd59b Selective Expression in Mouse Testis
To elucidate the in vivo function of SOX2 in protecting normal stem cells from complement surveillance by upregulating CD59, we used a mouse model because of accessibility considerations. Mouse Cd59 encodes two duplicate Cd59 isoforms, mCD59a and mCD59b (Powell et al., 1997, Qian et al., 2000). It is controversial whether the distribution of mCD59b is either universal (Qin et al., 2001) or selectively expressed in the testis (Donev et al., 2008). mCd59b deficiency has been reported to induce spontaneous hemolytic anemia and progressive male infertility (Qin et al., 2003). We further demonstrated the abundant expression of mCD59b in mouse testis and revealed that Sp1 regulates mCd59a expression widely, whereas NF-κB and serum response factor (SRF) regulate mCd59b transcription in inflammatory conditions to prevent complement attack (Chen et al., 2015); however, the transcriptional regulation of mCd59b in physiological conditions, which is helpful for explaining the abundant expression of mCD59b in mouse testis, remains unclear. Considering that SOX2 is abundant in stem cells and regulates human CD59 expression in CSCs, we propose that SOX2 may regulate mCd59b transcription.
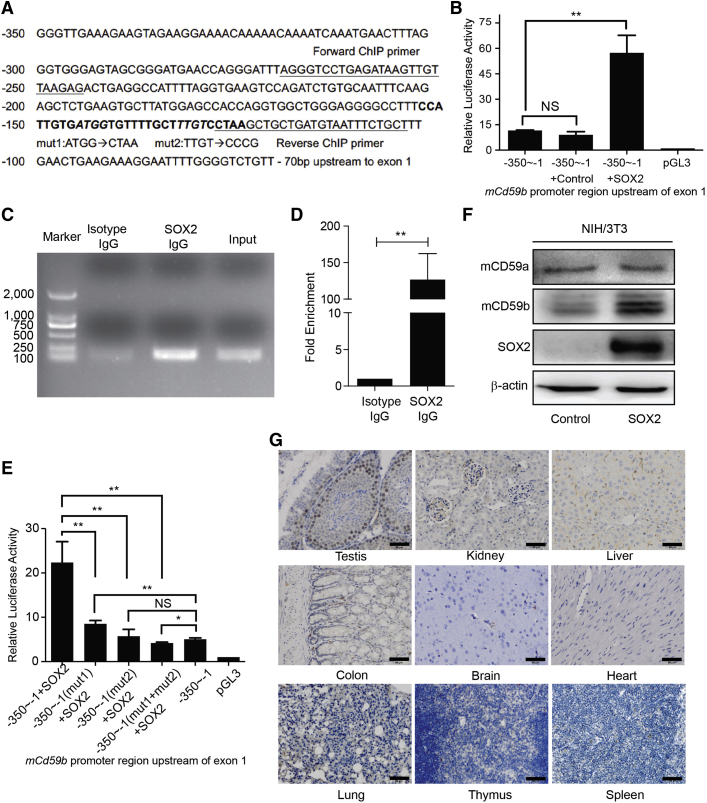
Using MatInspector software, we predicted possible SOX2 response elements in the promoter regions upstream of the mCd59a and mCd59b transcriptional initiation sites. The results indicate two SOX2 binding sites locate at −153 to −124 bp upstream of mCd59b exon 1 (Figure 6A), whereas no SOX2 binding site is located in the mCd59a promoter region.
Figure 6.
SOX2 Is Responsible for mCD59b-Selective Expression in Mouse Testis
(A) The sequence of the region from −350 bp to −70 bp upstream of mCd59b exon1, two SOX2 binding sites (bold), primer sequences, and mutated nucleotides are indicated.
(B) Dual-luciferase reporter assay in NIH/3T3 cells: ectopic SOX2 enhanced the promoter activity.
(C and D) ChIP assay in NIH/3T3 cells. A specific antibody against SOX2, but not isotype IgG, captured the fragment containing the SOX2 response element in the mCd59b promoter region, which was amplified by specific primers (Table S2) using PCR (C). The quantitative data are also shown (D).
(E) Dual-luciferase reporter assay in NIH/3T3 cells. Separate or simultaneous mutation of SOX2 response nucleotides in two SOX2 binding sites abrogated the enhanced promoter activity by ectopic SOX2.
(F) Ectopic SOX2 increased the expression of mCD59b, but not mCD59a, in NIH/3T3 cells.
(G) IHC assay. SOX2 is selectively abundant in testis spermatogonial stem cells, but not in the tested kidney, liver, colon, brain, heart, lung, thymus, and spleen. Scale bars, 50 μm.
Data are presented as mean ± SD (n = 3, independent repeats in B, E and technical repeat in D). NS, no significance; ∗p < 0.05, ∗∗p < 0.01. See also Figure S7.
We then performed dual-luciferase reporter assays by co-transfecting pGL3 plasmid containing −350 to −1 bp region upstream of mCd59b exon1 and SOX2-expressing or empty vector into mouse NIH/3T3 cells. The results showed that ectopic SOX2 could dramatically enhance the promoter activity (Figure 6B). To further identify whether SOX2 directly binds to this mCd59b promoter region, we conducted a ChIP assay in NIH/3T3 cells that were transiently transfected by SOX2-expressing plasmid. We found that the fragment containing the SOX2 binding site, but not isotype IgG, could be remarkably enriched by specific anti-SOX2 antibody (Figures 6C and 6D). Moreover, we mutated the critical response nucleotides in two SOX2 binding sites separately or simultaneously (Figure 6A) and detected the consequent change in promoter activity. The results showed that the enhanced promoter activity almost disappeared in all three mutants with ectopic SOX2 (Figure 6E). Therefore, these results indicated that both SOX2 binding sites were essential for mCd59b transcription. We further functionally identified the role of SOX2 in regulating the expression of mCD59b. The results showed that the expression of mCD59b, but not mCD59a, was significantly increased (Figure 6F). Therefore, we concluded that SOX2 regulates the transcription of mCd59b, but not mCd59a.
Given the selective distribution of mCD59b in the testis (Chen et al., 2015, Donev et al., 2008), we next probed the SOX2 levels in the mouse testis, kidney, liver, colon, brain, lung, thymus, and spleen by immunohistochemistry (IHC) and found that SOX2 was exclusively enriched in testis spermatogonial stem cells compared with the other tested tissues (Figure 6G). To further identify the correlation among SOX2, mCD59a, and mCD59b during testis maturation with age, we collected the testis samples from mice aged 2, 4, 6, and 8 weeks. The result of qRT-PCR showed that compared with those in week 2, the expression levels of mCd59a, mCd59b, and Sox2 (Genbank: NM_011443) increased, respectively, about 1.3-, 60-, and 6-fold in week 4; 2-, 110-, and 9-fold in week 6; and 2.7-, 110-, and 8-fold in week 8 (Figure S7A). We also performed RT-PCR using a pair of primers that can amplify both mCd59a and mCd59b simultaneously (Donev et al., 2008), and visualized the PCR products with 5% PAGE. As shown in Figure S7B, we found that the expression level of mCd59a was slightly increased, while that of mCd59b was dramatically increased with age. The protein levels identified by immunoblotting and IHC were also in agreement with the alteration pattern of mRNAs of mCd59a, mCd59b, and Sox2 with age (Figures S7C and S7D). Moreover, we detected the nuclear levels of the recognized transcription factors of Sp1, SRF, canonical NF-κB, and SOX2 for mCd59a and mCd59b using an immunoblotting assay. Only SOX2 was increased with age in accordance with the alteration of mCD59b but not of mCD59a (Figure S7E). These results therefore indicate the high correlation between mCD59b and SOX2 distribution, which further supports SOX2 regulation of mCd59b transcription in stem cells.
Discussion
CSCs account for a tiny subset of cancer cells; however, as “cancer seeds,” these cells have been considered a major obstacle to curing cancer due to their characteristics of distinctive surface proteins, self-renewal, differentiation, slow-cycling state, and high association with therapy resistance and metastasis (Clarke et al., 2006, Dean et al., 2005, Meacham and Morrison, 2013). Therefore, many efforts have been made to develop small molecules or antibodies that are currently in different clinical phases and that target various signaling pathways in CSCs (Kaiser, 2015). However, it is strongly suggested that CSC-specific therapy should be combined with traditional therapy to quickly eradicate whole tumors (Kaiser, 2015). Therefore, a bispecific target against differentiated and stem cancer cells may hold great potential for cancer therapy. Herein, we demonstrated that CD59 is upregulated by SOX2 in CSCs and that CD59 silencing completely eliminated tumors in a mouse model implanted with stem-like cancer cells.
Accumulating evidence has demonstrated that cancer cells are able to activate the autologous complement system (Cho et al., 2014, Fishelson, 2003, Matsumoto et al., 1997, Niculescu et al., 1992). To evade complement destruction, tumor cells upregulate mCRPs, and CD59 is the most relevant among the three mCRPs (Fishelson, 2003, Macor et al., 2015). Several reports have further shown the close relationship between CD59 expression and CSCs (Gemei et al., 2013, Zhu et al., 2013) and a high level of mCd59b, but not mCd59a, in murine spermatogonial stem cells (Donev et al., 2008). Therefore, CD59 may be such a bispecific target, and CD59-targeted therapy may significantly improve the therapeutic efficacy against cancer by simultaneously eliminating differentiated cells and CSCs, an approach that has already been suggested by several previous reports. We previously found that the human CD59-specific inhibitor ILYd4 combined with rituximab dramatically suppressed tumor growth and achieved a much higher tumor-free rate (50%) than rituximab treatment alone (8.3%) in lymphoma xenografted nude mice (Hu et al., 2011). Furthermore, the combination therapy of two bispecific antibodies against CD20 and CD55 or against CD20 and CD59 completely prevented the development of human/SCID lymphoma (Macor et al., 2015). Moreover, ectopic CD55 and/or CD59 could protect mesenchymal stem cells from complement-mediated lysis (Li and Lin, 2012).
It has been reported that SOX2 controls tumor initiation and CSC functions (Boumahdi et al., 2014); therefore, SOX2 insufficiency in glioblastoma CSCs completely suppresses proliferation and tumorigenicity (Gangemi et al., 2009). We observed that most of the signaling molecules for inducible CD59 expression, including NF-κB, CREB, CBP/p300, and Smad, were strongly inhibited (Figure 4A). This result indicates that SOX2 plays a critical role in regulating CD59 transcription in CSCs. In addition, the normal stem cells of spermatogonia may encounter complement attack, and there is an intact complement system in the female genital tract (Harris et al., 2006); therefore, spermatozoa require the high expression of mCRPs such as CD59. The deficiency of mCd59b resulted in progressive male infertility due to immobile dysmorphic and fewer sperm cells (Qin et al., 2003), indicating the critical role of CD59 in protecting spermatozoa from complement attack. In this study, we further demonstrated that SOX2, which is abundant in mouse testis, is responsible for mCD59b, but not mCD59a, selective expression in mouse testis. Considering the stemness of mouse spermospore and the previous reports that mCd59b deficiency resulted in mouse progressive male infertility due to low sperm count and mobility (Qin et al., 2003) and mCD59b is selectively expressed in mouse testis (Chen et al., 2015, Donev et al., 2008), our finding that SOX2 regulates mCd59b transcription in testis may explain the importance of human CD59 expression in CSCs in protecting them from complement attack. Therefore, we conclude that the loss of tumor-initiating ability and tumorigenicity in CSCs by SOX2 (Genbank: NM_003106) silencing resulted, at least in part, from the consequent CD59 insufficiency.
Recently, a stem-like cancer cell termed latency competent cancer cells, which express SOX2 and Sox9, have been reported to evade natural killer (NK) cell-mediated clearance by attenuating WNT signaling, thereby downregulating ligand expression for NK cell activity (Malladi et al., 2016). Herein, we interestingly observed that SOX2 also regulates EGFR expression, which may confer CSCs a growth signaling for their survival in the tumor microenvironment. Importantly, we further extend the role of SOX2 in protecting CSCs from another innate immune surveillance mechanism. SOX2 regulates CD59 expression in CSCs, and CD59 insufficiency induced a near-complete cessation of proliferation and loss of tumorigenesis in CSCs. This finding highlights the importance of complement surveillance in clearing tumor cells and suggests CD59 as a potential target in cancer therapy.
Experimental Procedures
Sphere Formation Assay
The sphere formation assay was performed as previously described (Rybak et al., 2011, Vlashi et al., 2009). In brief, CD59-sufficient or CD59-insufficient parental cells were dissociated with 0.25% trypsin/EDTA and resuspended with a serum-free medium (DMEM/F12, 3:1 mixture) containing 0.4% BSA and 0.2× B27 lacking vitamin A and supplemented with recombinant EGF (PeproTech) at 10 ng/mL and recombinant basic fibroblast growth factor (PeproTech) at 10 ng/mL. For examination of the sphere-forming capacity of cancer cells, cells were enzymatically dissociated and resuspended at a density of 10,000 cells/mL with the above medium and plated in ultra-low attachment 24-well plates. The medium was exchanged every 7 days, and the spheres were counted and harvested at 14 days. Sphere-forming cells were subcultured with TrypLE Express (Gibco) and resuspended in the above medium at clonal density. The sphere-forming capacity and morphology did not alter even with passage over 15 generations.
CDC Assay
The CDC assay was performed according to our previous report with minor modifications (Hu et al., 2011). In brief, 10,000 cancer cells were plated in 96-well plates for 16 hr and then treated with 200 μg/mL of cetuximab (Merck) and 20% NHS or IHS for 1.5 hr. For evaluation of the CDC effect, released LDH was measured in the supernatant using the Cytotoxicity Detection kit (Roche) based on the manufacturer's instructions. The optical density was measured at 490 nm with a Synergy HT microplate reader (Bio-Tek).
Xenograft Tumors
Six-week-old male BALB/c nude mice were purchased from Vital River. In total, two pairs of cells, 105 Calu-3-shSCR and Calu-3-shCD59 sphere-forming cells, or 105 Calu-3 sphere and parental cells were resuspended in a PBS/Matrigel (Invitrogen) mixture (1:1 volume) and then subcutaneously injected into the left or right sides of the axilla, respectively. The mice were inspected for tumor appearance, and tumor growth was measured every 3 days using a caliper. The tumor volume was determined following a standard formula: length × width2/2. The presence of tumor was confirmed by necropsy, and all the animal experiments were conducted in accordance with experimental protocols approved by the Animal Ethics Committee at Shanghai Medical School, Fudan University.
Statistical Analysis
The data are presented as the mean ± SD unless otherwise specified. The significant differences between two groups were determined using the two-tailed Student's t test for unpaired data, and p < 0.05 was considered statistically significant.
Author Contributions
J.C. and W.H. designed experiments and co-wrote the manuscript. J.C. performed most experiments and analyzed the data. J.C., P.D., L.L., H.G., Q.W., and W.Z. conducted the remaining mouse experiments. X.Z., L.Z., L.G., and N.W. provided technical and material support. W.H. conceptualized and designed the project.
Acknowledgments
This work was supported by grants to W.H. from the National Natural Science Foundation of China (81372258, 81572827), the Major State Basic Research Development Program of China (2013CB910802), and the Program for Professor of Special Appointment (Eastern Scholar, GZ2014002) at Shanghai Institutions of Higher Learning.
Published: December 22, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.11.008.
Supplemental Information
References
- Boumahdi S., Driessens G., Lapouge G., Rorive S., Nassar D., Le Mercier M., Delatte B., Caauwe A., Lenglez S., Nkusi E. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- Chen J., Du Y., Ding P., Zhang X., Zhang L., Wang N., Hu W. Mouse Cd59b but not Cd59a is upregulated to protect cells from complement attack in response to inflammatory stimulation. Genes Immun. 2015;16:437–445. doi: 10.1038/gene.2015.29. [DOI] [PubMed] [Google Scholar]
- Cho M.S., Vasquez H.G., Rupaimoole R., Pradeep S., Wu S., Zand B., Han H.D., Rodriguez-Aguayo C., Bottsford-Miller J., Huang J. Autocrine effects of tumor-derived complement. Cell Rep. 2014;6:1085–1095. doi: 10.1016/j.celrep.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M.F., Dick J.E., Dirks P.B., Eaves C.J., Jamieson C.H., Jones D.L., Visvader J., Weissman I.L., Wahl G.M. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Dean M., Fojo T., Bates S. Tumour stem cells and drug resistance. Nat. Rev. Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- Donev R.M., Sivasankar B., Mizuno M., Morgan B.P. The mouse complement regulator CD59b is significantly expressed only in testis and plays roles in sperm acrosome activation and motility. Mol. Immunol. 2008;45:534–542. doi: 10.1016/j.molimm.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Du Y., Teng X., Wang N., Zhang X., Chen J., Ding P., Qiao Q., Wang Q., Zhang L., Yang C. NF-kappaB and enhancer-binding CREB protein scaffolded by CREB-binding protein (CBP)/p300 proteins regulate CD59 protein expression to protect cells from complement attack. J. Biol. Chem. 2014;289:2711–2724. doi: 10.1074/jbc.M113.525501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkelberger J.R., Song W.C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- Fishelson Z. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol. Immunol. 2003;40:109–123. doi: 10.1016/s0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- Gangemi R.M., Griffero F., Marubbi D., Perera M., Capra M.C., Malatesta P., Ravetti G.L., Zona G.L., Daga A., Corte G. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27:40–48. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- Gemei M., Di Noto R., Mirabelli P., Del Vecchio L. Cytometric profiling of CD133+ cells in human colon carcinoma cell lines identifies a common core phenotype and cell type-specific mosaics. Int. J. Biol. Markers. 2013;28:267–273. doi: 10.5301/JBM.5000020. [DOI] [PubMed] [Google Scholar]
- Goswami M.T., Reka A.K., Kurapati H., Kaza V., Chen J., Standiford T.J., Keshamouni V.G. Regulation of complement-dependent cytotoxicity by TGF-beta-induced epithelial-mesenchymal transition. Oncogene. 2016;35:1888–1898. doi: 10.1038/onc.2015.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C.L., Mizuno M., Morgan B.P. Complement and complement regulators in the male reproductive system. Mol. Immunol. 2006;43:57–67. doi: 10.1016/j.molimm.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Hsu Y.F., Ajona D., Corrales L., Lopez-Picazo J.M., Gurpide A., Montuenga L.M., Pio R. Complement activation mediates cetuximab inhibition of non-small cell lung cancer tumor growth in vivo. Mol. Cancer. 2010;9:139. doi: 10.1186/1476-4598-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Ferris S.P., Tweten R.K., Wu G., Radaeva S., Gao B., Bronson R.T., Halperin J.A., Qin X. Rapid conditional targeted ablation of cells expressing human CD59 in transgenic mice by intermedilysin. Nat. Med. 2008;14:98–103. doi: 10.1038/nm1674. [DOI] [PubMed] [Google Scholar]
- Hu W., Ge X., You T., Xu T., Zhang J., Wu G., Peng Z., Chorev M., Aktas B.H., Halperin J.A. Human CD59 inhibitor sensitizes rituximab-resistant lymphoma cells to complement-mediated cytolysis. Cancer Res. 2011;71:2298–2307. doi: 10.1158/0008-5472.CAN-10-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J. The cancer stem cell gamble. Science. 2015;347:226–229. doi: 10.1126/science.347.6219.226. [DOI] [PubMed] [Google Scholar]
- Kelly P.N., Dakic A., Adams J.M., Nutt S.L., Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- Li Y., Lin F. Mesenchymal stem cells are injured by complement after their contact with serum. Blood. 2012;120:3436–3443. doi: 10.1182/blood-2012-03-420612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macor P., Secco E., Mezzaroba N., Zorzet S., Durigutto P., Gaiotto T., De Maso L., Biffi S., Garrovo C., Capolla S. Bispecific antibodies targeting tumor-associated antigens and neutralizing complement regulators increase the efficacy of antibody-based immunotherapy in mice. Leukemia. 2015;29:406–414. doi: 10.1038/leu.2014.185. [DOI] [PubMed] [Google Scholar]
- Malladi S., Macalinao D.G., Jin X., He L., Basnet H., Zou Y., de Stanchina E., Massague J. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. 2016;165:45–60. doi: 10.1016/j.cell.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Takeda J., Inoue N., Hara T., Hatanaka M., Takahashi K., Nagasawa S., Akedo H., Seya T. A novel protein that participates in nonself discrimination of malignant cells by homologous complement. Nat. Med. 1997;3:1266–1270. doi: 10.1038/nm1197-1266. [DOI] [PubMed] [Google Scholar]
- Meacham C.E., Morrison S.J. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B.P., Marchbank K.J., Longhi M.P., Harris C.L., Gallimore A.M. Complement: central to innate immunity and bridging to adaptive responses. Immunol. Lett. 2005;97:171–179. doi: 10.1016/j.imlet.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Niculescu F., Rus H.G., Retegan M., Vlaicu R. Persistent complement activation on tumor cells in breast cancer. Am. J. Pathol. 1992;140:1039–1043. [PMC free article] [PubMed] [Google Scholar]
- Powell M.B., Marchbank K.J., Rushmere N.K., van den Berg C.W., Morgan B.P. Molecular cloning, chromosomal localization, expression, and functional characterization of the mouse analogue of human CD59. J. Immunol. 1997;158:1692–1702. [PubMed] [Google Scholar]
- Qian Y.M., Qin X., Miwa T., Sun X., Halperin J.A., Song W.C. Identification and functional characterization of a new gene encoding the mouse terminal complement inhibitor CD59. J. Immunol. 2000;165:2528–2534. doi: 10.4049/jimmunol.165.5.2528. [DOI] [PubMed] [Google Scholar]
- Qin X., Miwa T., Aktas H., Gao M., Lee C., Qian Y.M., Morton C.C., Shahsafaei A., Song W.C., Halperin J.A. Genomic structure, functional comparison, and tissue distribution of mouse Cd59a and Cd59b. Mamm. Genome. 2001;12:582–589. doi: 10.1007/s00335-001-2060-8. [DOI] [PubMed] [Google Scholar]
- Qin X., Krumrei N., Grubissich L., Dobarro M., Aktas H., Perez G., Halperin J.A. Deficiency of the mouse complement regulatory protein mCd59b results in spontaneous hemolytic anemia with platelet activation and progressive male infertility. Immunity. 2003;18:217–227. doi: 10.1016/s1074-7613(03)00022-0. [DOI] [PubMed] [Google Scholar]
- Quintana E., Shackleton M., Sabel M.S., Fullen D.R., Johnson T.M., Morrison S.J. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D., Lambris J.D. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J. Immunol. 2013;190:3831–3838. doi: 10.4049/jimmunol.1203487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak A.P., He L., Kapoor A., Cutz J.C., Tang D. Characterization of sphere-propagating cells with stem-like properties from DU145 prostate cancer cells. Biochim. Biophys. Acta. 2011;1813:683–694. doi: 10.1016/j.bbamcr.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Vlashi E., Kim K., Lagadec C., Donna L.D., McDonald J.T., Eghbali M., Sayre J.W., Stefani E., McBride W., Pajonk F. In vivo imaging, tracking, and targeting of cancer stem cells. J. Natl. Cancer Inst. 2009;101:350–359. doi: 10.1093/jnci/djn509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Liu Y., Li Z.Y., Fan X., Hemminki A., Lieber A. A recombinant adenovirus type 35 fiber knob protein sensitizes lymphoma cells to rituximab therapy. Blood. 2010;115:592–600. doi: 10.1182/blood-2009-05-222463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Hu W., Qin X. The role of complement in the mechanism of action of rituximab for B-cell lymphoma: implications for therapy. Oncologist. 2008;13:954–966. doi: 10.1634/theoncologist.2008-0089. [DOI] [PubMed] [Google Scholar]
- Zhu J., Nie S., Wu J., Lubman D.M. Target proteomic profiling of frozen pancreatic CD24+ adenocarcinoma tissues by immuno-laser capture microdissection and nano-LC–MS/MS. J. Proteome Res. 2013;12:2791–2804. doi: 10.1021/pr400139c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.