Abstract
Chronic lung allograft dysfunction (CLAD) encompasses a range of pathologies that cause a transplanted lung to not achieve or maintain normal function. CLAD manifests as airflow restriction and/or obstruction and is predominantly a result of chronic rejection. Three distinct phenotypes of chronic rejection are now recognized: bronchiolitis obliterans, neutrophilic reversible allograft dysfunction, and restrictive allograft syndrome. Recent investigations have revealed that each phenotype has a unique pathology and histopathological findings, suggesting that treatment regimens should be tailored to the underlying etiology. CLAD is poorly responsive to treatment once diagnosed, and therefore the prevention of the factors that predispose a patient to develop CLAD is critically important. Small and large animal models have contributed significantly to our understanding of CLAD and more studies are needed to develop treatment regimens that are effective in humans.
Keywords: chronic lung allograft dysfunction, bronchiolitis obliterans, bronchiolitis obliterans syndrome, azithromycin responsive allograft dysfunction, neutrophilic reversible allograft dysfunction, restrictive allograft syndrome
Introduction
The field of lung transplantation has experienced improvements in morbidity and mortality over the last 25 years. When experiences with early cohorts of lung transplant recipients were published in 1990 the one-year survival was reported to be 45%. One-year survival rates have now risen to 83% in recent years for bilateral lung transplants according to the International Society for Heart and Lung Transplantation [1,2]. Despite the improvement in early mortality, the rate of decline after one year has not changed substantially over the last two decades, and the roughly 4,000 patients that undergo lung transplantation annually face a 5-year survival rate of only approximately 50% [2]. The lower long term survival of pulmonary transplant patients compared to recipients of other solid organ transplants can be explained by several factors that are unique to lungs. These include a constant exposure to the external environment (i.e. infection, air pollution), potential for aspiration, susceptibility to ischemia reperfusion injury-mediated graft dysfunction and the organ’s propensity to contain abundant lymphoid tissue [3]; all of these factors acting in concert with allo- and autoimmune responses are thought to contribute to chronic lung allograft dysfunction (CLAD).
CLAD is predominantly a consequence of chronic rejection, resulting in one of three phenotypes: bronchiolitis obliterans (BO), neutrophilic reversible allograft dysfunction (NRAD), and restrictive allograft syndrome (RAS). Each of these phenotypes follows a somewhat predictable course, characterized either by airway obstruction or restriction, and is largely unresponsive to changes in immunosuppression [4]. Recent research has elucidated important physiologic differences between these three phenotypes, suggesting that different therapeutic approaches are warranted for each. In this article, we will examine the recent advances in our understanding of CLAD and review the updates on the diagnosis, classification, and treatments.
Diagnosis and classification
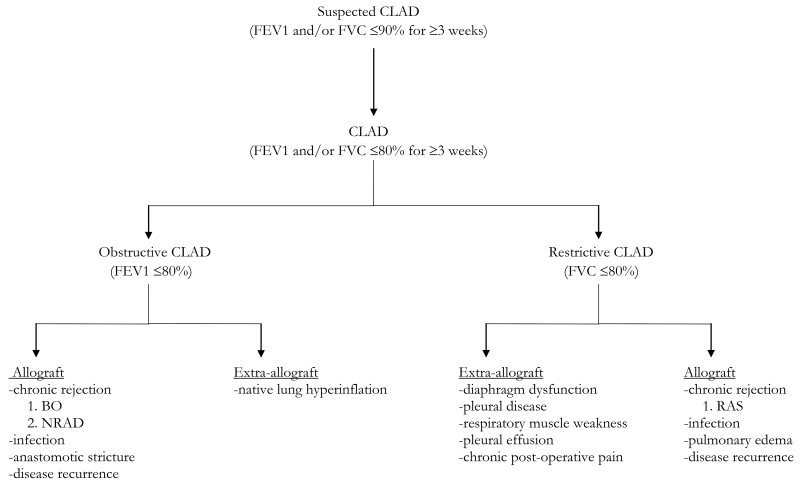
The term CLAD appeared in the transplantation literature relatively recently and encompasses a myriad of pathologies that cause a lung allograft to not achieve or maintain normal function over time (summarized in Table 1). Regardless of the cause, CLAD manifests as chronic airflow obstruction and/or restriction. In 2014, Verleden and colleagues defined CLAD as “FEV1 and/or FVC ≤80% baseline function for ≥3 weeks” [5].
Table 1.
Diagnosis and classification of CLAD based upon recent literature.
Numerous factors including acute rejection, primary graft dysfunction, respiratory infections and gastroesophageal reflux disease have been shown to predispose lung transplant patients to the development of CLAD [6,7]. The type of chronic rejection is usually determined clinically using spirometry and imaging studies, although bronchoalveolar lavage (BAL) is sometimes used to differentiate between subtypes, such as BO and NRAD [8]. The three predominant types of CLAD (BO, NRAD, and RAS) are discussed below.
Bronchiolitis obliterans
BO presents clinically in a non-specific manner with dyspnea on exertion and a non-productive cough. The symptoms develop insidiously, usually after three months post-transplant. Chest radiographs are typically unchanged from previous films. High resolution CT scan may reveal bronchiectasis (sensitivity 64-80%, specificity 78-90%) and air trapping (sensitivity 80-91%, specificity 80-94%) [9,10]. Pulmonary function testing demonstrates a FEV1 ≤80% of baseline function [5,11].
Transbronchial biopsy has a poor sensitivity for the diagnosis of BO because of small sample sizes and the patchy nature of obliteration of membranous and respiratory bronchioles [12]. A revision of the pathological diagnosis of BO was published in 2007 and now defines BO as “dense eosinophilic hyaline fibrosis in the sub-mucosa of membranous and respiratory bronchioles, resulting in partial or complete luminal occlusion.” Allograft vascular atherosclerosis is often seen on biopsy specimens as well [13]. While neutrophils may be present in the biopsy specimen, the predominant feature is fibrous proliferation; this is in contrast to neutrophilic predominance seen in NRAD (discussed below) [8,14]. Because of the poor sensitivity of transbronchial biopsy, the diagnosis of BO is usually made based on decrements in spirometry, and patients who develop a sustained and otherwise unexplained 20% decrement in FEV1 are given a diagnosis of “bronchiolitis obliterans syndrome” (BOS) and treated in a similar manner [11,12].
A humoral immune response is thought to play an important role in the development of BO. Palmer showed that after lung transplantation some recipients (11%) develop anti-human leukocyte antigen (HLA) antibodies [15]. When human airway epithelial cells are exposed to these anti-HLA antibodies they secrete fibroblast growth factors, induce epithelial cell proliferation, and ultimately cause apoptosis [16]. Additionally, it was recently shown that the presence of anti-HLA antibodies is associated with neutrophilic infiltration of allografts [17]. Patients that develop such antibodies face an increased risk of BOS and decreased survival [15,18-20]. Interestingly, some patients develop pre-transplant antibodies to self-antigens, which was recently found to increase the risk of BOS as well [21]. These studies, among others, provide a link between the humoral immune response and BO and may explain why treating BOS patients with intravenous immunoglobulin and rituximab has yielded some success [22,23].
Neutrophilic reversible allograft dysfunction
In 2003 Gerhardt and colleagues published the results of a pilot study in which they used low-dose azithromycin (AZT) to treat BOS. Five of the six patients in their study demonstrated significant improvement following the addition of AZT to their immunosuppression (mean FEV1 increase of 0.5L) [24]. Since then numerous studies have confirmed the efficacy of AZT, showing that it can reverse and prevent BOS while prolonging the survival of some patients [25-27]. However, not all patients respond to AZT and the understanding of why has revealed another phenotype of CLAD: neutrophilic reversible allograft dysfunction (NRAD).
In 2006, Verleden reported that the difference in AZT responders and non-responders is the presence of pre-treatment neutrophilia and high IL-8 on BAL. Furthermore, there was a correlation between the percentage of neutrophilia and response to treatment [28]. The same group published a follow up study in 2011 showing that AZT responders also had significantly higher levels of inflammatory cytokines (IL-1, IL-8, MCP-1, RANTES), matrix remodeling factors (MMP-8, MMP-9), growth factors (HGF, PDGF), and markers of oxidative stress (MPO) in BAL specimens [8]. These patients also had a significantly higher concentration of bile acid in their BAL specimens, which was previously shown to result in allograft neutrophilia [8,26,29]. Thus, AZT responders, now known to have NRAD, have a form of chronic rejection characterized by active inflammation and neutrophilic infiltration, explaining the efficacy of AZT, which suppresses inflammation both directly and indirectly (antimicrobial action) [30].
NRAD is now accepted as a new phenotype of CLAD, separate from BO [5,8]. Since NRAD has only recently been appreciated, an exact clinical definition is lacking in the literature. Recent work done by Verleden and colleagues suggests that NRAD can be diagnosed when patients have an FEV1 ≤80% of baseline for ≥3 weeks and ≥15% neutrophils on BAL in the absence of infection [31]. Though NRAD responds positively to AZT, multiple studies have demonstrated that NRAD is associated with worse overall survival [32]. Thus, the long-term prognosis of NRAD remains poorly understood.
Restrictive allograft syndrome
Historically the diagnosis of BOS included all patients with a FEV1 ≤ 80% of baseline, as all of these patients were thought to have BO. However, recent investigations have revealed that this is not an accurate way to diagnose BO as a newly discovered phenotype of CLAD, termed restrictive allograft syndrome (RAS), meets these diagnostic criteria but has a markedly different pathophysiology. RAS was first proposed as a novel form of CLAD in 2011 by Sato and colleagues of The Toronto Lung Transplantation Program. They showed that 30% of post-bilateral lung transplant patients diagnosed with CLAD had restrictive lung disease that could not be attributed to an extrapulmonary process. In these patients CT scans showed substantial interstitial lung disease with peripheral lung fibrosis and survival rates were significantly worse than patients diagnosed with BOS (median survival 541 vs. 1,421 days, p=0.0003). They diagnosed these patients as having RAS, and proposed clinical criteria of FEV1 <80% and TLC <90% in the absence of extra-allograft pathology for diagnosis [33].
Biopsy specimens from RAS patients reveal “diffuse alveolar damage and extensive fibrosis in the alveolar interstitium, visceral pleura, and interlobular septa, with or without scattered BO lesions” [33]; this is in contrast to the pathology of BO discussed above, which predominantly shows submucosal fibrosis of bronchioles causing luminal occlusion [13]. Recent histopathological studies suggest that the disease process is also characterized by myofibroblast infiltration, predominantly in the subpleural tissues. Diffuse alveolar damage appears early in the disease process and is followed by pleuroparenchymal fibroelastosis [33,34].
Since RAS is a novel form of CLAD there are no animal studies to date and the exact pathophysiology has yet to be elucidated. It has been hypothesized that the mechanism of RAS is similar to IPF, as the two diseases follow a similar clinical course and have similar findings on CT scan [35]. IPF is the result of fibroblast proliferation in response to inflammatory mediators, in particular transforming growth factor beta (TGF-β) [36]. In 2005, years before RAS was recognized as a phenotype of CLAD, Liu treated rat lung transplants with pirfenidone, a drug that has been shown to slow the progression of IPF by inhibiting TGF-β and TNF-α. They observed a decrease in peak airway pressures, collagen content, and TGF-β levels in the treated mice compared to controls [37]. Recently, Vos reported the case of a patient with RAS who was treated with pirfenidone. The patient experienced a slower rate of decline in both FVC and FEV1 and an increase in TLC. A CT scan after treatment with pirfenidone demonstrated an interval decrease in subpleural consolidations and opacities [35]. Though the patient ultimately passed away from RAS, his improvement raises interesting points about the pathology of this newly recognized disease process and the therapeutic potential of pirfenidone.
Medical therapy
Post-transplant acute rejection, pneumonias, gastroesophageal reflux disease and CMV infection have all been shown to predispose patients to developing CLAD; thus, prevention of CLAD is best accomplished with adequate immunosuppression and infection prophylaxis [6]. Maintenance immunosuppression usually consists of a three drug regimen consisting of a glucocorticoid, a calcineurin inhibitor and a nucleotide blocking agent. When CLAD is diagnosed the patient’s calcineurin inhibitor and/or nucleotide blocking agent can be changed to a different drug in the same class; if this fails, substitution with sirolimus or everolimus can be considered [38]. Montelukast is yet another treatment option and has been shown to slow the decline of FEV1 in BOS patients [39]. Furthermore, every effort should be made to prevent gastroesophageal reflux disease in the post-transplant population given its known association with chronic rejection [29].
Pneumonia is the most common infection following lung transplantation, and it is recommended that trimethoprim-sulfamethoxazole be taken prophylactically following transplantation; this antibiotic protects against many of the most common pathogens, including Pneumocystis Jirovecii and Streptococcus pneumonia [40]. In recipients who are at risk for CMV infection, prophylaxis should be given with oral valganciclovir. AZT should be initiated as soon as CLAD is suspected, as supported by the discussion above [24-27]. Most institutions do not routinely place patients on AZT, however, a recent randomized controlled trial done by Ruttens suggests that AZT prophylaxis for CLAD may be indicated. The results of their randomized controlled trial show a significantly lower long-term CLAD prevalence and improved CLAD-free survival, pulmonary function, and functional exercise capacity in the treated group compared to placebo [41]. If these conservative medical measures fail and pulmonary function continues to worsen cytolytic therapy with an agent such as antilymphocyte globulin, antithymocyte globulin, OKT3 monoclonal antibody, or alemtuzumab should be considered. While cytolytic therapy has been shown to slow the rate of decline in lung function, its success has been limited by leukopenia and infectious side effects [42]. Finally, evidence is emerging that supports the use of intravenous immunoglobulin and rituximab in patients that develop anti-HLA antibodies [22,23].
Extracorporeal photophoresis and total lymphoid irradiation
Extracorporeal photophoresis (ECP) is an immune modulating therapy that has been used in the United States to treat a variety of immunological disorders since the 1980s. The process involves separating white blood cells from a patient’s whole blood, treating the isolated white blood cells with 8-methoxypsoralen (8-MOP) then ultraviolet radiation in a photoactivation chamber, and finally the white blood cell fraction is reinfused into the patient; this treatment causes crosslinking in DNA and eventually apoptosis of the patient’s leukocytes. Although the exact mechanism of action has not been elucidated, it has been hypothesized that when these apoptotic leukocytes are reinfused into the patient they are phagocytosed by antigen presenting cells, thus expanding regulatory T cells [43]. ECP was first used in the lung transplant population in 1995 to treat acute lung rejection, but a role for ECP in treating chronic rejection has only been recently established [42]. A study published in 2010 by our institution showed a significant reduction in the rate of lung function decline in patients with progressive BOS after ECP [43]. In 2014 a follow-up study was done examining the mechanism of ECP in suppressing chronic lung rejection. The authors found that ECP significantly reduced circulating levels of proinflammatory cytokines, as well as antibodies to lung-associated self antigens and donor-specific antigens; these results have shown that the efficacy of ECP is at least in part due to suppression of the humoral immune response [44].
Total lymphoid irradiation (TLI) is yet another treatment option for chronic rejection refractory to medical therapy. TLI is a method of leukocyte depletion accomplished by radiating the major lymphatic beds of the body, in particular the splenic, paraaortic, pelvic, inguinal and femoral fields. While TLI has been successfully used to slow the progression of cardiac allograft dysfunction, its efficacy in lung transplantation is not as well established [45]. Fischer examined 37 lung recipients with progressive BOS, who underwent TLI. They found that TLI significantly lowered the rate of decline in FEV1. However, 10 patients failed to complete TLI (>8/10 fractions) because of infection (n=2), bone marrow suppression (n=6), or death (n=2) [46]. Since this article was released in 2005 no large studies have been reported studying TLI or comparing ECP to TLI.
Retransplantation
While modern lung retransplantation has a lower risk of death compared to historical outcomes, the survival rate remains significantly worse than primary transplantation despite advances in immunosuppression and postoperative management [47]. Given these poor outcomes and the short supply of donor lungs, only select patients are considered for retransplantation. The recent discoveries on CLAD etiology and classification, particularly regarding RAS, raised the question of the impact that CLAD phenotype has on retransplantation outcomes. Verleden and colleagues addressed this question in a recent multicenter retrospective study. They found that survival was significantly worse for RAS patients than BOS patients following retransplantation. Additionally, RAS patients were more likely to develop recurrent RAS, and CLAD occurred earlier in the postoperative period [48]. Thus, RAS should now be considered a risk factor for a poor outcome along with other risk factors such as pre-operative ventilation, hospitalization, and multiple retransplants [47,48].
Experimental Models of CLAD
Much of what is known about the pathophysiology of CLAD has stemmed from work in animal models of chronic lung rejection. One of the key experimental models to gain mechanistic insight into the development of BO has been the heterotopic tracheal transplant model, where tracheal rings derived from a donor animal are placed underneath the skin or into the abdominal cavity of a recipient [49]. This model has been used in mice, rats and large animals. The main advantage of this approach is that the histological changes associated with graft rejection resemble those observed in BO lesions in human lung transplants. Additional benefits of this model are its technical ease and a high level of reproducibility across laboratories. However, there are important differences between heterotopic tracheal transplantation and lung transplantation in humans that limit the translational value of this model. For example, the lesions in the heterotopic tracheal transplant model involve large rather than small airways, the tracheal segment is not vascularized and the graft is not exposed to the external environment. To address the latter limitation an orthotopic tracheal transplant model was developed, whereby segments of murine donor trachea are interposed into a recipient’s trachea [50]. Rejection of orthotopic tracheal grafts is evidenced by subepithelial fibrosis. This model has been used to establish an association between graft hypoxia as well as viral infections with the development of CLAD [51,52]. In addition, Dutly and colleagues developed a model of tracheal transplantation where the graft is placed into the lung to expose the graft to an intrapulmonary environment [53].
Orthotopic lung transplantation in small and large animals most closely mimics the surgical procedure of human lung transplantation. Allan has developed a miniature swine model of orthotopic lung transplantation which develops the spectrum of histopathological changes seen in human chronic lung rejection; this model has the benefit of allowing for surveillance biopsies that is not feasible in smaller animal models [54]. This group has used the miniature swine model to study the role of indirect allorecognition in developing CLAD [55]. Atanasova performed rat orthotopic lung transplants in the Fischer 344 → Lewis strain combination, treated the recipients with cyclosporine postoperatively and instilled lipopolysaccharide into their airways on postoperative day 28; this resulted in pulmonary fibrosis and fibroproliferation of airways resembling human BO [56]. Wilkes and colleagues have used orthotopic lung transplants in mice to elucidate mechanisms that contribute to CLAD. Importantly, their work has demonstrated that IL-17 is a major mediator of BO in mice [57]. A follow-up study in 2013 showed that IL-17 inhibits complement regulatory proteins (CRP) that normally suppress the complement system. They found that the development of BO in mice could be prevented by neutralizing the complement protein C5. In the same study the authors obtained human tissues from lungs explanted during retransplantation for refractory BO and studied the protein expression profiles. They found that the BO specimens had a significantly lower expression of the CRPs, CD46 and CD55 [58]. Oishi treated transplanted mice with the plant derivative halofuginone, which has previously been shown to inhibit Th17 responses. They observed a significant reduction in obliterated airways and parenchymal fibrosis in lung allografts of these mice [59]. The utility of the murine model to study human chronic lung rejection has also been shown by De Vleeschauwer in 2012 [60]. In our own lab, we have shown that immune responses that regulate rejection and tolerance of murine orthotopic lung transplants are generated in the graft independent of secondary lymphoid organs, providing a rationale for the local delivery of immunosuppressants [61,62].
Conclusion
CLAD remains the Achilles heel of lung transplantation. The clinical definition of CLAD has only recently been established, and much remains to be discovered regarding its pathogenesis and treatment. The recognition of three distinct phenotypes of chronic rejection (BO, NRAD, and RAS) represents a significant step forward. Each phenotype has unique histopathological findings and studies suggest that treatment should be tailored accordingly. Given that CLAD is poorly responsive to medical therapy the prevention of predisposing factors, such as gastroesophageal reflux, infection and acute rejection episodes, are of the upmost importance. Small and large animals models have contributed to our understanding of CLAD and hold much potential in further elucidating this disease and its treatment.
Footnotes
Conflict of Interest
Daniel Kreisel reports a patent (“Induction in tolerance of lung allograft transplantation”) issued to Washington University.
Jason Gauthier and Ramsey Hachem declare no conflict of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
•Of importance
••Of major importance
- 1.Grossman RF, Frost A, Zamel N, et al. The Toronto Lung Transplant Group Results of single-lung transplantation for bilateral pulmonary fibrosis. N Engl J Med. 1990;322(11):727–33. doi: 10.1056/NEJM199003153221104. [DOI] [PubMed] [Google Scholar]
- 2.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33(10):1009–24. doi: 10.1016/j.healun.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Moyron-quiroz JE, Rangel-moreno J, Kusser K, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10(9):927–34. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 4.Meyer KC, Raghu G, Verleden GM, et al. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J. 2014;44:1479–503. doi: 10.1183/09031936.00107514. [DOI] [PubMed] [Google Scholar]
- 5••.Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014;33(2):127–33. doi: 10.1016/j.healun.2013.10.022. This manuscript was the first to provide a specific, comprehensive clinical definition of CLAD.
- 6.Kroshus TJ, Kshettry VR, Savik K, John R, Hertz MI, Bolman RM. Risk factors for the development of bronchiolitis obliterans syndrome after lung transplantation. J Thorac Cardiovasc Surg. 1997;114(2):195–202. doi: 10.1016/S0022-5223(97)70144-2. [DOI] [PubMed] [Google Scholar]
- 7.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175(5):507–13. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 8••.Verleden SE, Vos R, Mertens V, et al. Heterogeneity of chronic lung allograft dysfunction: insights from protein expression in broncho alveolar lavage. J Heart Lung Transplant. 2011;30(6):667–73. doi: 10.1016/j.healun.2010.12.008. The realization that patients responsive to AZT have a different pathology than AZT non-responders paved the way for the modern understanding of NRAD.
- 9.Leung AN, Fisher K, Valentine V, et al. Bronchiolitis obliterans after lung transplantation: detection using expiratory HRCT. Chest. 1998;113(2):365–70. doi: 10.1378/chest.113.2.365. [DOI] [PubMed] [Google Scholar]
- 10.Worthy SA, Park CS, Kim JS, Müller NL. Bronchiolitis obliterans after lung transplantation: high-resolution CT findings in 15 patients. AJR Am J Roentgenol. 1997;169(3):673–7. doi: 10.2214/ajr.169.3.9275875. [DOI] [PubMed] [Google Scholar]
- 11.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21(3):297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 12.Kramer MR, Stoehr C, Whang JL, et al. The diagnosis of obliterative bronchiolitis after heart-lung and lung transplantation: low yield of transbronchial lung biopsy. J Heart Lung Transplant. 1993;12(4):675–81. [PubMed] [Google Scholar]
- 13.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26(12):1229–42. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Vanaudenaerde BM, Meyts I, Vos R, et al. A dichotomy in bronchiolitis obliterans syndrome after lung transplantation revealed by azithromycin therapy. Eur Respir J. 2008;32(4):832–43. doi: 10.1183/09031936.00134307. [DOI] [PubMed] [Google Scholar]
- 15.Palmer SM, Davis RD, Hadjiliadis D, et al. Development of an antibody specific to major histocompatibility antigens detectable by flow cytometry after lung transplant is associated with bronchiolitis obliterans syndrome. Transplantation. 2002;74(6):799–804. doi: 10.1097/00007890-200209270-00011. [DOI] [PubMed] [Google Scholar]
- 16.Jaramillo A, Smith CR, Maruyama T, Zhang L, Patterson GA, Mohanakumar T. Anti-HLA class I antibody binding to airway epithelial cells induces production of fibrogenic growth factors and apoptotic cell death: a possible mechanism for bronchiolitis obliterans syndrome. Hum Immunol. 2003;64(5):521–9. doi: 10.1016/s0198-8859(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 17.Denicola MM, Weigt SS, Belperio JA, Reed EF, Ross DJ, Wallace WD. Pathologic findings in lung allografts with anti-HLA antibodies. J Heart Lung Transplant. 2013;32(3):326–32. doi: 10.1016/j.healun.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobo LJ, Aris RM, Schmitz J, Neuringer IP. Donor-specific antibodies are associated with antibody-mediated rejection, acute cellular rejection, bronchiolitis obliterans syndrome, and cystic fibrosis after lung transplantation. J Heart Lung Transplant. 2013;32(1):70–7. doi: 10.1016/j.healun.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Angaswamy N, Saini D, Ramachandran S, et al. Development of antibodies to human leukocyte antigen precedes development of antibodies to major histocompatibility class I-related chain A and are significantly associated with development of chronic rejection after human lung transplantation. Hum Immunol. 2010;71(6):560–5. doi: 10.1016/j.humimm.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bharat A, Narayanan K, Street T, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83(2):150–8. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 21.Bharat A, Saini D, Steward N, et al. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 2010;90(4):1094–101. doi: 10.1016/j.athoracsur.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ius F, Sommer W, Kieneke D, et al. IgM-Enriched Human Intravenous Immunoglobulin-Based Treatment of Patients With Early Donor Specific Anti-HLA Antibodies After Lung Transplantation. Transplantation. 2015 doi: 10.1097/TP.0000000000001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hachem RR, Yusen RD, Meyers BF, et al. Anti-human leukocyte antigen antibodies and preemptive antibody-directed therapy after lung transplantation. J Heart Lung Transplant. 2010;29(9):973–80. doi: 10.1016/j.healun.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerhardt SG, Mcdyer JF, Girgis RE, Conte JV, Yang SC, Orens JB. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: results of a pilot study. Am J Respir Crit Care Med. 2003;168(1):121–5. doi: 10.1164/rccm.200212-1424BC. [DOI] [PubMed] [Google Scholar]
- 25.Vos R, Vanaudenaerde BM, Verleden SE, et al. A randomised controlled trial of azithromycin to prevent chronic rejection after lung transplantation. Eur Respir J. 2011;37(1):164–72. doi: 10.1183/09031936.00068310. [DOI] [PubMed] [Google Scholar]
- 26.Gottlieb J, Szangolies J, Koehnlein T, Golpon H, Simon A, Welte T. Long-term azithromycin for bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2008;85(1):36–41. doi: 10.1097/01.tp.0000295981.84633.bc. [DOI] [PubMed] [Google Scholar]
- 27.Corris PA, Ryan VA, Small T, et al. A randomised controlled trial of azithromycin therapy in bronchiolitis obliterans syndrome (BOS) post lung transplantation. Thorax. 2015;70(5):442–50. doi: 10.1136/thoraxjnl-2014-205998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verleden GM, Vanaudenaerde BM, Dupont LJ, Van raemdonck DE. Azithromycin reduces airway neutrophilia and interleukin-8 in patients with bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2006;174(5):566–70. doi: 10.1164/rccm.200601-071OC. [DOI] [PubMed] [Google Scholar]
- 29.D’ovidio F, Mura M, Tsang M, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005;129(5):1144–52. doi: 10.1016/j.jtcvs.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 30.Vos R, Vanaudenaerde BM, Verleden SE, et al. Anti-inflammatory and immunomodulatory properties of azithromycin involved in treatment and prevention of chronic lung allograft rejection. Transplantation. 2012;94(2):101–9. doi: 10.1097/TP.0b013e31824db9da. [DOI] [PubMed] [Google Scholar]
- 31.Verleden SE, Vandermeulen E, Ruttens D, et al. Neutrophilic reversible allograft dysfunction (NRAD) and restrictive allograft syndrome (RAS) Semin Respir Crit Care Med. 2013;34(3):352–60. doi: 10.1055/s-0033-1348463. [DOI] [PubMed] [Google Scholar]
- 32.Verleden GM, Vos R, Verleden SE, et al. Survival determinants in lung transplant patients with chronic allograft dysfunction. Transplantation. 2011;92(6):703–8. doi: 10.1097/TP.0b013e31822bf790. [DOI] [PubMed] [Google Scholar]
- 33••.Sato M, Waddell TK, Wagnetz U, et al. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant. 2011;30(7):735–42. doi: 10.1016/j.healun.2011.01.712. The discovery of RAS has opened a new door for research in the field of lung transplant immunology. This paper was the first to show that RAS is a form of CLAD distinctly different from BO and NRAD, which has prompted investigation into the underlying etiology of this disease.
- 34.Ofek E, Sato M, Saito T, et al. Restrictive allograft syndrome post lung transplantation is characterized by pleuroparenchymal fibroelastosis. Mod Pathol. 2013;26(3):350–6. doi: 10.1038/modpathol.2012.171. [DOI] [PubMed] [Google Scholar]
- 35•.Vos R, Verleden SE, Ruttens D, et al. Pirfenidone: a potential new therapy for restrictive allograft syndrome? Am J Transplant. 2013;13(11):3035–40. doi: 10.1111/ajt.12474. This paper reports the case of a patient with RAS who had marked clinical and radiographic improvement after treatment with pirfenidone, which has previously been successful in treating IPF.
- 36.Sivakumar P, Ntolios P, Jenkins G, Laurent G. Into the matrix: targeting fibroblasts in pulmonary fibrosis. Curr Opin Pulm Med. 2012;18(5):462–9. doi: 10.1097/MCP.0b013e328356800f. [DOI] [PubMed] [Google Scholar]
- 37.Liu H, Drew P, Gaugler AC, Cheng Y, Visner GA. Pirfenidone inhibits lung allograft fibrosis through L-arginine-arginase pathway. Am J Transplant. 2005;5(6):1256–63. doi: 10.1111/j.1600-6143.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- 38.Parada MT, Alba A, Sepúlveda C. Everolimus in lung transplantation in Chile. Transplant Proc. 2010;42(1):328–30. doi: 10.1016/j.transproceed.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Verleden GM, Verleden SE, Vos R, et al. Montelukast for bronchiolitis obliterans syndrome after lung transplantation: a pilot study. Transpl Int. 2011;24(7):651–6. doi: 10.1111/j.1432-2277.2011.01248.x. [DOI] [PubMed] [Google Scholar]
- 40.Martin SI, Fishman JA. Pneumocystis pneumonia in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):272–9. doi: 10.1111/ajt.12119. [DOI] [PubMed] [Google Scholar]
- 41•.Ruttens D, Verleden SE, Vandermeulen E, et al. Prophylactic Azithromycin Therapy After Lung Transplantation: Post hoc Analysis of a Randomized Controlled Trial. Am J Transplant. 2016;16(1):254–61. doi: 10.1111/ajt.13417. This study showed that prophylactic AZT reduces the prevalence of CLAD and improves CLAD-free survival, suggesting that there may be a role for prophylactic AZT in all lung transplant patients.
- 42.Andreu G, Achkar A, Couetil JP, et al. Extracorporeal photochemotherapy treatment for acute lung rejection episode. J Heart Lung Transplant. 1995;14(4):793–6. [PubMed] [Google Scholar]
- 43.Morrell MR, Despotis GJ, Lublin DM, Patterson GA, Trulock EP, Hachem RR. The efficacy of photopheresis for bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2010;29(4):424–31. doi: 10.1016/j.healun.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 44.Baskaran G, Tiriveedhi V, Ramachandran S, et al. Efficacy of extracorporeal photopheresis in clearance of antibodies to donor-specific and lung-specific antigens in lung transplant recipients. J Heart Lung Transplant. 2014;33(9):950–6. doi: 10.1016/j.healun.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tallaj JA, Pamboukian SV, George JF, et al. Total lymphoid irradiation in heart transplantation: long-term efficacy and survival--an 18-year experience. Transplantation. 2011;92(10):1159–64. doi: 10.1097/TP.0b013e318231e9d3. [DOI] [PubMed] [Google Scholar]
- 46.Fisher AJ, Rutherford RM, Bozzino J, Parry G, Dark JH, Corris PA. The safety and efficacy of total lymphoid irradiation in progressive bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2005;5(3):537–43. doi: 10.1111/j.1600-6143.2004.00709.x. [DOI] [PubMed] [Google Scholar]
- 47.Thomas M, Belli EV, Rawal B, Agnew RC, Landolfo KP. Survival After Lung Retransplantation in the United States in the Current Era (2004 to 2013): Better or Worse? Ann Thorac Surg. 2015;100(2):452–7. doi: 10.1016/j.athoracsur.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 48.Verleden SE, Todd JL, Sato M, et al. Impact of CLAD Phenotype on Survival After Lung Retransplantation: A Multicenter Study. Am J Transplant. 2015;15(8):2223–30. doi: 10.1111/ajt.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hertz MI, Jessurun J, King MB, Savik SK, Murray JJ. Reproduction of the obliterative bronchiolitis lesion after heterotopic transplantation of mouse airways. Am J Pathol. 1993;142(6):1945–51. [PMC free article] [PubMed] [Google Scholar]
- 50.Schrepfer S, Deuse T, Hoyt G, et al. Experimental orthotopic tracheal transplantation: The Stanford technique. Microsurgery. 2007;27(3):187–9. doi: 10.1002/micr.20329. [DOI] [PubMed] [Google Scholar]
- 51.Kuo E, Bharat A, Goers T, et al. Respiratory viral infection in obliterative airway disease after orthotopic tracheal transplantation. Ann Thorac Surg. 2006;82(3):1043–50. doi: 10.1016/j.athoracsur.2006.03.120. [DOI] [PubMed] [Google Scholar]
- 52.Jiang X, Khan MA, Tian W, et al. Adenovirus-mediated HIF-1α gene transfer promotes repair of mouse airway allograft microvasculature and attenuates chronic rejection. J Clin Invest. 2011;121(6):2336–49. doi: 10.1172/JCI46192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dutly AE, Andrade CF, Verkaik R, et al. A novel model for post-transplant obliterative airway disease reveals angiogenesis from the pulmonary circulation. Am J Transplant. 2005;5(2):248–54. doi: 10.1111/j.1600-6143.2004.00680.x. [DOI] [PubMed] [Google Scholar]
- 54.Allan JS, Wain JC, Schwarze ML, et al. Modeling chronic lung allograft rejection in miniature swine. Transplantation. 2002;73(3):447–53. doi: 10.1097/00007890-200202150-00020. [DOI] [PubMed] [Google Scholar]
- 55.Shoji T, Wain JC, Houser SL, et al. Indirect recognition of MHC class I allopeptides accelerates lung allograft rejection in miniature swine. Am J Transplant. 2005;5(7):1626–34. doi: 10.1111/j.1600-6143.2005.00925.x. [DOI] [PubMed] [Google Scholar]
- 56.Atanasova S, Hirschburger M, Jonigk D, et al. A relevant experimental model for human bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2013;32(11):1131–9. doi: 10.1016/j.healun.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 57.Fan L, Benson HL, Vittal R, et al. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am J Transplant. 2011;11(5):911–22. doi: 10.1111/j.1600-6143.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58•.Suzuki H, Lasbury ME, Fan L, et al. Role of complement activation in obliterative bronchiolitis post-lung transplantation. J Immunol. 2013;191(8):4431–9. doi: 10.4049/jimmunol.1202242. Elucidating the role of IL-17 and the complement cascade in the development of BO may lead to new targeted therapies in humans.
- 59.Oishi H, Martinu T, Sato M, et al. Halofuginone treatment reduces interleukin-17A and ameliorates features of chronic lung allograft dysfunction in a mouse orthotopic lung transplant model. J Heart Lung Transplant. 2016;35(4):518–27. doi: 10.1016/j.healun.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 60.De Vleeschauwer S, Jungraithmayr W, Wauters S, et al. Chronic rejection pathology after orthotopic lung transplantation in mice: the development of a murine BOS model and its drawbacks. PLoS ONE. 2012;7(1):e29802. doi: 10.1371/journal.pone.0029802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gelman AE, Li W, Richardson SB, et al. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol. 2009;182(7):3969–73. doi: 10.4049/jimmunol.0803514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li W, Bribriesco AC, Nava RG, et al. Lung transplant acceptance is facilitated by early events in the graft and is associated with lymphoid neogenesis. Mucosal Immunol. 2012;5(5):544–54. doi: 10.1038/mi.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]