Abstract
The relationship between expression of a negative regulator of GA signal transduction (RGL2) belonging to the DELLA gene family and repression of Arabidopsis seed germination has been studied (Lee S, Cheng H, King KE, Wang W, He Y, Hussain A, Lo J, Harberd NP, Peng J [2002] Genes and Development 16: 646–658). There is one DELLA gene (LeGAI) present in tomato (Lycopersicon esculentum Mill.), which is expressed in both vegetative and reproductive tissues. During germination of wild-type tomato seed, there was no decline in the expression of LeGAI in either the embryo or the endosperm. Rather, LeGAI transcripts increased in these tissues following imbibition and remained high during and following germination. A similar increase in LeGAI transcripts occurred in the endosperm and embryo of GA-treated gib-1 mutant seed during and following germination. Likewise in soybean (Glycine max) seed, there was no decline in the expression of two DELLA genes in the radicle before or after germination. Upon reexamination of RGL2 in Arabidopsis seeds, a decline in its expression was noted but only after radicle emergence, i.e. after germination had been completed. Taken together, these data are consistent with GA-induced down-regulation of DELLA genes not being a prerequisite for germination of tomato, soybean, and Arabidopsis seeds.
Germination begins when a quiescent seed imbibes water and is completed with the elongation and emergence of the embryo radicle in a turgor-driven process. Viable seeds that do not germinate under favorable conditions are dormant, requiring one or more environmental cues such as light or temperature for this restraint to be overcome (Bewley, 1997a). Two major forms of seed dormancy have been described, namely embryo and coat dormancy. Coat dormancy (sometimes termed coat-enhanced dormancy) occurs when tissues surrounding the embryo physically inhibit protrusion of the radicle, as in seeds of tomato (Lycopersicon esculentum Mill.) and Arabidopsis (Bewley and Black, 1994; Koornneef et al., 2002). Embryos from coat-dormant seeds germinate when removed from their surrounding tissues, while embryo-dormant seeds do not.
Gibberellins (GAs) and abscisic acid (ABA) are growth regulators involved in diverse aspects of plant growth and development, including antagonistic roles in the control of seed germination (Karssen et al., 1989; Bewley, 1997a; Yamaguchi et al., 1998). For instance, ABA establishes dormancy during seed development, also acting to inhibit germination (Karssen et al., 1983), while GA is considered to initiate germination (Peng and Harberd, 2002). These roles for ABA and GA during seed dormancy and germination come primarily from studies of mutants incapable of producing or responding to these plant growth regulators (Koornneef et al., 1982, 1984). For example, ABA biosynthetic mutants in Arabidopsis (aba) have reduced seed dormancy, as do ABA-insensitive mutants (abi; Koornneef et al., 2002). Conversely, intact seeds from mutants unable to produce GA in Arabidopsis (ga1-3) and tomato (gib-1) fail to break coat dormancy and complete germination unless given GA (Koornneef and van der Veen, 1980; Groot and Karssen, 1987).
In seeds, GA is proposed to increase the growth potential of the embryo and to mediate the production of enzymes that hydrolyze the surrounding endosperm in coat-dormant species (Groot and Karssen, 1987; Bradford et al., 2000; Nonogaki et al., 2000). Whether GA responses actually increase embryo growth potential during germination has not been unequivocally demonstrated. The role of GA in the release from coat dormancy is well documented in tomato seed (Bewley, 1997b; Bradford et al., 2000). Herein, the micropylar endosperm partially encloses the radicle tip and must be penetrated by the elongating radicle for germination to be completed. The hemicellulases endo-β-mannanase and β-mannosidase are produced in the presence of GA, degrading the mannan-rich cell walls of the micropylar endosperm and facilitating the protrusion of the radicle through this tissue (Groot and Karssen, 1987; Bewley, 1997b; Mo and Bewley, 2002). In the absence of GA, the embryo radicles of GA-deficient gib-1 embryos neither penetrate the micropylar endosperm nor is there synthesis of cell wall-degrading enzymes (Groot and Karssen, 1987).
Genes encoding protein components of GA signal transduction have been proposed to effect a regulatory role on seed germination (Lee et al., 2002; Peng and Harberd, 2002; Wen and Chang, 2002). For example, DELLA proteins are transcription factors that inhibit GA responses and thus are considered negative regulators of GA signal transduction (Dill et al., 2001; Itoh et al., 2002; Olszewski et al., 2002; Wen and Chang, 2002). Since GA responses are correlated with the completion of seed germination in Arabidopsis, a DELLA protein therefrom, RGL2, has been proposed to act as a repressor of this event (Lee et al., 2002; Peng and Harberd, 2002). The prevailing hypothesis is that transcription of RGL2 is induced following imbibition, and the resultant protein is maintained at a high level in dormant Arabidopsis radicles, where it acts to inhibit GA-induced increases in embryo growth. RGL2 synthesis is then repressed in response to GA produced in the embryo, allowing for the completion of germination (Lee et al., 2002; Peng and Harberd, 2002). In addition to this correlation of RGL2 gene expression with germination, there is genetic evidence that a ga1-3 rgl2 double mutant of Arabidopsis (i.e. one exhibiting no GA synthesis and no GA repressor) is capable of germinating (Lee et al., 2002). Thus, it appears that when RGL2 is not expressed, germination occurs in the absence of GA. Similar observations on Arabidopsis have been reported for other negative regulators of GA signal transduction, including the DELLA protein RGL1 (Wen and Chang, 2002) and the O-linked GlcNAc transferase SPINDLY (Jacobsen et al., 1996); seeds of rgl1 and spindly mutants are capable of germinating in the presence of GA biosynthesis inhibitors that block the germination of wild-type seeds.
DELLA proteins from a variety of species, including SLENDER1 (SLN1) in barley (Hordeum vulgare; Fu et al., 2002), SLENDER1 (SLR1) in rice (Oryza sativa; Sasaki et al., 2003), and RGA in Arabidopsis (McGinnis et al., 2003), are posttranslationally regulated by the 26S proteasome. These proteins are nuclear localized until the perception of GA by the cell; then the proteins are phosphorylated and degraded (Itoh et al., 2002; Sasaki et al., 2003). Neither the Arabidopsis DELLA proteins GAI (Fleck and Harberd, 2002) nor RGL1 (Wen and Chang, 2002) are degraded in the presence of GA, suggesting that the proteasome does not regulate all DELLA proteins. Similarly, RGL2 is proposed to be regulated at the level of transcription (Lee et al., 2002).
In this study, we tested the hypothesis that DELLA gene expression regulates seed germination in tomato and soybean (Glycine max). Tomato seeds provide some advantages over those of Arabidopsis because they are larger, with easily separable embryo, endosperm (which can be dissected into physiologically distinct micropylar and lateral regions), and seed coat. There is also a detailed understanding of the physiology of tomato seed dormancy and germination (Bewley, 1997b; Bradford et al., 2000). Data presented here show that only one DELLA gene is present in tomato, making this a valuable species in which to study its role in seed germination. Results obtained demonstrate that the DELLA gene(s) need not be down-regulated prior to the completion of germination in tomato and soybean seeds, the latter of which does not exhibit coat-enhanced dormancy. A reexamination of Arabidopsis seeds also demonstrated that the decrease in RGL2 expression occurs only following the completion of germination.
RESULTS AND DISCUSSION
Tomato Contains a Single DELLA Gene That Is Expressed throughout the Plant
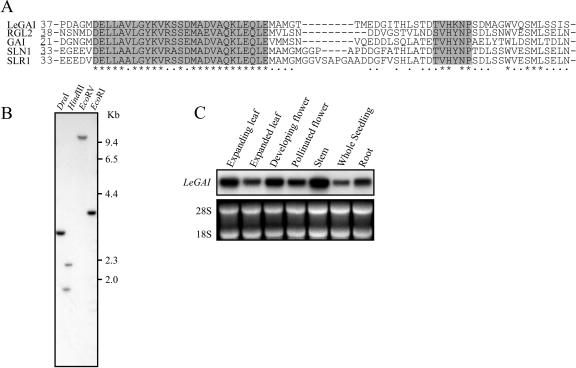
Arabidopsis has five DELLA genes (Dill et al., 2001; Lee et al., 2002), while rice (Itoh et al., 2002) and other species such as barley (Chandler et al., 2002), maize, and wheat (Triticum aestivum; Peng et al., 1999) have only one. All reported DELLA gene sequences lack introns; therefore, we used genomic DNA as template in PCR using degenerate primers to isolate the gene(s) from tomato. Three different sets of primers were designed to conserved domains within known DELLA proteins and used in separate PCRs. All primer pairs yielded overlapping fragments of the same gene, LeGAI, and additional reverse transcription (RT)-PCR with template RNA from various sources also yielded the same LeGAI fragments (data not shown), indicative of this being the only DELLA gene in tomato. The predicted protein sequence of LeGAI contains the conserved -DELLA- and -TVH[K]NP- amino acid domains present exclusively in all other DELLA proteins (Fig. 1A).
Figure 1.
LeGAI encodes a putative DELLA protein. A, Alignment of the deduced amino acid sequences of various DELLA proteins including LeGAI (tomato, GenBank accession no. AY269087), RGL2 (Arabidopsis, NM_111216), GAI (Arabidopsis, NM_101361), SLN1 (barley, AF460219), and SLR1 (rice, AB030956). Identical residues at each position are marked below the alignment with an asterisk (*), similar residues are marked with a dot (·), and the N-terminal-most amino acid shown for each protein is indicated to the left of the sequence. The conserved -DELLA- domain and semi-conserved -TVH[K]NP- domain are shaded. B, DNA gel-blot analysis at low stringency indicates the presence of a single DELLA gene in tomato. Restriction enzymes used to digest tomato DNA are indicated above each lane and DNA molecular mass markers in kb to the right of the autoradiogram. C, RNA gel-blot analysis of LeGAI transcripts showing expression of this gene in various tissues of tomato. Fluorescence attributed to ethidium-bromide-stained 28S and 18S ribosomal RNA is shown below (C) to illustrate there was equal loading of total RNA samples.
To confirm that tomato has a single DELLA gene, DNA gel-blot analysis at low stringency was performed (see “Materials and Methods”) using a probe complementary to LeGAI sequences encoding the conserved DELLA domain. This probe hybridized to a single gene within the tomato genome (Fig. 1B). The LeGAI gene has a known internal HindIII restriction site, and hence two hybridizing bands were obtained with this enzyme, as expected.
Arabidopsis has five DELLA genes, with each exhibiting tissue-specific expression, e.g. RGL2 is exclusively expressed in seeds and flowers (Lee et al., 2002). In contrast, LeGAI expression was present in all tissues of tomato (Fig. 1C). Taken together, these data are consistent with LeGAI being the only DELLA gene in tomato and with its ability to regulate GA responses in all tissues.
LeGAI Expression in Tomato Seed Does Not Decrease during or following Germination
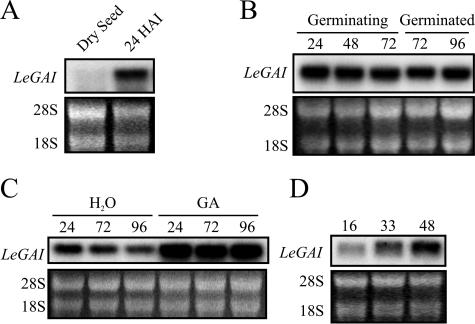
RNA gel-blot analysis revealed that LeGAI transcripts were barely detectable in whole dry wild-type tomato seeds and that they strongly increased by 24 h after imbibition (HAI; Fig. 2A). Tomato seeds were imbibed for various times before their embryos and endosperms were dissected; total RNA was extracted from both tissues and subjected to gel-blot hybridization. LeGAI expression in the embryo following imbibition remained unchanged from 24 to 96 HAI, i.e. during and after the completion of germination (Fig. 2B). Since tomato seed germination was asynchronous, seeds that either had or had not completed germination (i.e. either exhibited or not exhibited radicle protrusion; Bewley, 1997a) were separated at 72 HAI. LeGAI transcript abundance was similar in both germinated and ungerminated seed at this time. Thus, transcriptional down-regulation of LeGAI is not a prerequisite for germination of these seeds.
Figure 2.
LeGAI expression in tomato seeds, embryos dissected from intact seeds, and isolated embryos. A, RNA gel-blot analysis of LeGAI expression in whole wild-type tomato seeds cv Trust, either dry or imbibed for 24 h. B, LeGAI expression in tomato embryos of intact wild-type seeds at various times during (24–72 HAI) and following (72–96 HAI) germination. Nongerminated and germinated seeds were separated at 72 HAI (the time at which approximately 50% had completed germination), and their RNA was analyzed. C, LeGAI expression in embryos of intact seeds of the gib-1 mutant cv Moneymaker during and following germination. GA-imbibed seeds (100 μm GA3) completed germination between 24 and 72 HAI; water-imbibed seeds did not complete germination. D, LeGAI expression during and following germination in embryos isolated from intact gib-1 seeds. Intact seeds were first imbibed on water for 24 h and then embryos excised and placed on water. Isolated embryos completed germination at 33 HAD. Fluorescence attributed to ethidium-bromide-stained 28S and 18S ribosomal RNA is shown below each autoradiogram in A to D to illustrate there was equal loading of total RNA samples. HAI (A–C) or HAD (D) are indicated above each lane.
To further confirm this contrast between changes in the relative abundance of RGL2 transcripts in Arabidopsis and LeGAI transcripts in tomato, seeds of the tomato gib-1 mutant were examined. Seeds of this mutant are unable to synthesize GA and fail to break dormancy and complete germination unless imbibed in GA (Groot and Karssen, 1987). When intact gib-1 seeds were imbibed in water, LeGAI transcripts decreased slightly in the nongerminating embryos up to 96 HAI, whereas in those gib-1 seeds imbibed on GA, which completed germination following 60 HAI, LeGAI transcripts increased and maintained a high level of expression both during and after germination (Fig. 2C). This pattern of DELLA gene transcription in gib-1 tomato seeds also differs from that reported for RGL2 during GA-induced germination of an Arabidopsis mutant (ga1-3) lacking the capacity to synthesize GA, in which transcripts declined sharply (Lee et al., 2002). However, radicle emergence from these Arabidopsis seeds occurred between 24 and 36 h after stimulation to germinate, a time when RGL2 transcripts were still abundant, and the decrease due to GA was only noted at 48 h, when germination was completed and the seedling was well established (Lee et al., 2002).
Germination of Tomato Embryos Occurs Independently of GA
When embryos from gib-1 tomato seeds are removed from their dormancy-imposing surrounding endosperm tissues, they complete germination on water despite their inability to produce GA (Groot and Karssen, 1987). To determine the pattern of LeGAI expression during their germination, intact gib-1 seeds were imbibed for 24 h on water, and then the embryos were dissected from the endosperm and placed on water. LeGAI transcripts were present in isolated gib-1 embryos 16 h after dissection (HAD) and increased in abundance at the commencement of radicle elongation at 33 HAD and in the growing seedling at 48 HAD (Fig. 2D). This again contrasts with the observation in Arabidopsis that a decrease in DELLA gene transcripts is required for germination to be completed (Lee et al., 2002; Peng and Harberd, 2002). It also demonstrates that an increase in LeGAI transcripts during tomato embryo germination occurs independently of a requirement for GA, as does germination itself, whereas the intact seed must be supplied with GA. Similar to embryos of gib-1 tomato, water-imbibed ga1-3 Arabidopsis embryos germinate when their envelope tissues are removed (Debeaujon and Koornneef, 2000).
Expression of LeGAI Occurs in the Endosperm of Tomato Seed during and following Germination
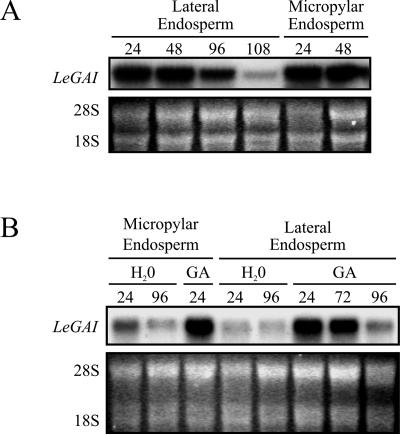
A comparison was made between LeGAI expression in the endosperm and the embryo of tomato seeds. The endosperm surrounds the embryo in tomato, the micropylar endosperm covering the radicle tip and the lateral endosperm being to the outside of the cotyledons. Weakening of the cell walls of the micropylar endosperm by hemicellulases is proposed to be required to permit radicle protrusion (Bradford et al., 2000), and the cell walls of the lateral endosperm are mobilized following germination as an early source of carbohydrates for the growing embryo (Bewley, 1997b).
LeGAI transcripts were barely detectable in the intact mature dry tomato seed (Fig. 2A) that included the endosperm, but, as in the embryo (Fig. 2B), there was an increase in LeGAI expression in the micropylar endosperm during germination and in the lateral endosperm during and following germination (Fig. 3A). The micropylar endosperm was degraded between 48 and 60 HAI, following its penetration by the radicle, but up to this time there was strong expression of the gene. A decline in expression in the lateral endosperm occurred at 96 HAI and later; this could be related to the general decline in its integrity as the cell walls and cellular contents were mobilized. A low abundance of LeGAI transcripts was observed in the micropylar and lateral endosperms of nongerminating gib-1 mutant seeds imbibed in water, up until 96 HAI, but these transcripts increased markedly in both regions in germinating and germinated seeds imbibed in GA as early as 24 HAI before declining in the lateral endosperm as its degradation was being effected at 96 HAI (Fig. 3B). It was not possible to isolate the micropylar endosperm of GA-treated gib-1 seeds after 48 HAI since this tissue had been destroyed following penetration by the germinated embryo. Overall, the patterns of LeGAI expression in wild-type and gib-1 mutant embryos and in the two regions of their endosperms are very similar. The synthesis of various enzymes and proteins associated with weakening of cell walls during and following tomato seed germination is known to be under the control of GA, as shown using gib-1 mutant seed, e.g. both lateral and micropylar forms of endo-β-mannanase (Bradford et al., 2000; Mo and Bewley, 2003), β-mannosidase (Mo and Bewley, 2003), xyloglucan endotransglycoslase, and expansins (Bradford et al., 2000). From this study, it is not possible to draw any direct correlation between enhanced LeGAI expression and an increase in the synthesis of these enzymes, although neither event occurs in the absence of GA.
Figure 3.
LeGAI expression in tomato seed endosperms. A, RNA gel-blot analysis of LeGAI expression in the micropylar and lateral endosperm regions of intact wild-type tomato seeds imbibed in water. Germination was completed between 48 and 96 HAI. Degradation of the micropylar endosperm occurred by 60 HAI, and of the lateral endosperm by 120 HAI. B, LeGAI expression in the micropylar and lateral endosperms of intact tomato seeds of the gib-1 mutant imbibed either in water or in GA (100 μm GA3). GA-imbibed seeds completed germination between 24 and 72 HAI. Degradation of the micropylar endosperm occurred after 24 HAI, and the lateral endosperm by 96 HAI. Fluorescence attributed to ethidium-bromide-stained 28S and 18S ribosomal RNA is shown below each autoradiogram in A and B to illustrate there was equal loading of total RNA samples. HAI are indicated above each lane in A and B.
DELLA Genes Are Not Expressed during Soybean Seed Germination
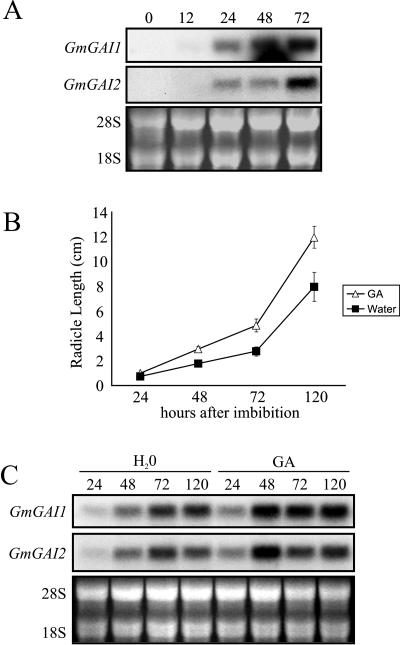
Unlike tomato and Arabidopsis seeds, those of soybean do not exhibit coat-imposed dormancy, nor is GA known to influence soybean germination (Bewley and Black, 1994). The expression of DELLA genes during germination of this seed was therefore examined. Partial cDNA sequences of two genes encoding DELLA proteins (GmGAI1 and GmGAI2) were obtained using a PCR that included soybean genomic DNA and each of the same three primer pairs used to isolate LeGAI. Identical fragments of GmGAI1 and GmGAI2 were also obtained by RT-PCR with RNA isolated from soybean radicles. Both GmGAI1 and GmGAI2 contain the -DELLA- and -TVHYNP- amino acid domains exclusive to DELLA proteins (data not shown).
Gene-specific sequences of GmGAI1 and GmGAI2 were used as probes in RNA gel-blot hybridization to examine expression of these genes during and following soybean seed germination. Transcripts of GmGAI1 and GmGAI2 were not present in the dry seed or in the radicle at 12 HAI, before completion of germination, but increased following radicle protrusion 24 HAI and thereafter, i.e. during seedling establishment (Fig. 4A). Since there is no dormancy in soybean seed, GA may not play a role prior to the completion of germination, and hence there is no requirement for the expression of DELLA genes to regulate responses to this hormone. Nevertheless, soybean seeds imbibed in GA displayed enhanced postgermination radicle elongation (Fig. 4B), and coincident with this were GA-mediated increases in GmGAI1 and GmGAI2 gene expression, particularly at 48 HAI during early seedling growth (Fig. 4C). Based on these observations, it is apparent that DELLA gene expression is not a prerequisite for completion of soybean germination but that it does occur during early seedling growth, a GA-responsive phase of development.
Figure 4.
DELLA gene expression and radicle growth in soybean seeds. A, RNA gel-blot analysis with gene-specific probes of GmGAI1 (GenBank accession no. AY269088) and GmGAI2 (AY269089). Germinating (0–12 HAI) and germinated (24–72 HAI) soybean radicles were removed from intact seeds for RNA extraction at the times indicated. Intact dry soybean seeds are represented by 0 HAI. B, Effect of GA (100 μm GA3) on radicle elongation immediately following the completion of germination (24 HAI) and during soybean seedling growth. Error bars represent the se of two replicates of 15 samples each. C, RNA gel-blot analysis of GmGAI gene expression in germinated radicles of soybean seeds removed at the times indicated following imbibition in either water or GA (100 μm GA3). Fluorescence attributed to ethidium-bromide-stained 28S and 18S ribosomal RNA is shown below each autoradiogram in A and C to illustrate there was equal loading of total RNA samples. HAI are indicated above each lane in A and C.
RGL2 Transcripts Remain Abundant during Germination of Arabidopsis Seeds
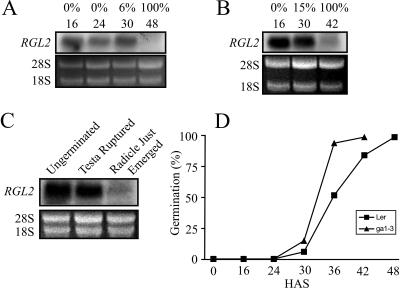
Since the data obtained for tomato and soybean seeds were in marked contrast to those reported for Arabidopsis, seeds of this species were reexamined using a more detailed time scale related to germination than was previously reported (Lee et al., 2002). Germination of Arabidopsis seeds, like those of tomato, is not synchronous. Landsberg wild-type seeds used here took 48 h after stratification (HAS) for the entire population to complete germination (Fig. 5D). When 6% of these wild-type seeds had germinated at 30 HAS, RGL2 transcripts remained as abundant as earlier, during germination, at 16 and 24 HAS (Fig. 5A). The majority of the nongerminated seed population at 30 HAS completed germination within the next 6 h (Fig. 5D). By 48 HAS, all of the seeds had completed germination, and RGL2 transcripts were no longer present (Fig. 5A). Therefore in wild-type Arabidopsis, seed RGL2 transcripts remained abundant immediately prior to radicle elongation but were not present thereafter.
Figure 5.
RGL2 expression during and following the completion of germination in Arabidopsis. RGL2 expression in A, wild-type Arabidopsis seeds at various times HAS, as noted above each lane, along with the percentage of seeds that had completed germination; B, ga1-3 Arabidopsis seeds imbibed in 100 μm GA3 at various times following stratification (HAS). Percentage of the population that had completed germination is shown above the HAS for each time point; C, Arabidopsis seeds separated based on their stage of development: intact ungerminated, testa ruptured signifying incipient radicle emergence, and radicles just emerged from the testa. Fluorescence attributed to ethidium-bromide-stained 28S and 18S ribosomal RNA is shown below each autoradiogram in A to C to illustrate there was equal loading of total RNA samples. D, Germination profiles of wild-type and ga1-3 Arabidopsis seeds. Seeds were imbibed and scored (out of 100) for the completion of germination based upon emergence of their radicles.
Mutant Arabidopsis seeds incapable of producing GA (ga1-3) were also examined over a close time line related to germination. Germination of ga1-3 in the presence of GA3 was faster (Fig. 5D) and more synchronous than wild-type seeds on water, allowing for a further correlation between RGL2 gene expression and the completion of germination. As in the wild-type seed, RGL2 transcripts remained abundant in seed populations about to complete germination at 30 HAS, when 15% of the population had completed germination (Fig. 5B). There was also low but detectable RGL2 expression in germinated seeds at 42 HAS, when all the seeds had completed germination.
Further correlations between RGL2 expression and the completion of germination were conducted with a population of imbibed wild-type Arabidopsis seeds separated on the basis of whether they were either ungerminated and had no sign of testa disruption, ungerminated but their testa had ruptured as a prelude to radicle emergence, or germinated and the radicle had emerged and grown to less than the length of the seed (Fig. 5C). At the time of incipient radicle elongation, when the testa had ruptured, RGL2 transcripts were abundant, as in seeds not showing this change, but when the radicle had just emerged RGL2 expression was low. The decline in RGL2 expression is therefore exclusively postgerminative.
SUMMARY
The data presented here show that a decline in expression of the RGL2 gene in Arabidopsis seed, of the single DELLA gene in tomato seed (LeGAI), and of the two similar genes in soybean (GmGAI1 and GmGAI2) do not need to occur for germination to be completed, i.e. for emergence of the radicle. These data also raise the issue as to whether GA and transduction of a GA signal are essential within the embryo for it to complete germination. For example, embryos isolated from mutant seeds of tomato and Arabidopsis which are incapable of synthesizing GA (gib-1 and ga1-3) are capable of completing germination when isolated, without a requirement for supplied GA (Groot and Karssen, 1987; Debeaujon and Koornneef, 2000). This phenomenon is also observed in GA-deficient genotypes of cereal grains such as rice, which do not exhibit coat dormancy and are capable of completing germination on water (Peng and Harberd, 2002). GA may be present within these mutant seeds, having being imported from the parent plant, which required exogenous hormone to produce viable pollen (Cheng et al., 2004). However, even if there is sufficient GA present in the isolated embryos of the GA-deficient mutants to induce elongation of their radicles, this amount of GA is clearly insufficient to cause them to elongate in the intact seed.
A positive regulator of GA signal transduction has been identified in Arabidopsis and rice, which also supports our premise that GA signaling is not required for the completion of seed germination. The F-box mutants of sleepy1 and gid2 in Arabidopsis and rice, respectively, are unable to degrade their DELLA protein inhibitors and therefore constitutively inhibit GA responses (McGinnis et al., 2003; Sasaki et al., 2003). sleepy1 and gid2 mutant seeds, however, are capable of germinating despite their inability to execute GA responses.
Even though GA signaling involving DELLA genes seems not to be required for the completion of germination, i.e. initiation of radicle elongation, GA action is required for germination of the intact seed of coat-dormant species. In this case, embryos within the seed have to overcome the physical constraint of a surrounding structure, an endosperm or seed coat or both, by inducing their weakening, for which GA is required in several species, including tomato (Bewley, 1997b).
The repressive action of many DELLA proteins can be regulated by the 26S proteasome at the level of protein abundance (Fu et al., 2002; McGinnis et al., 2003; Sasaki et al., 2003). Attempts were made to relate the relative abundance of DELLA proteins to germination using antibodies against a peptide present in LeGAI and against the DELLA proteins SLN1 from barley (Chandler et al., 2002) and GAI from Arabidopsis (Fu et al., 2002). These failed to recognize LeGAI in the tomato seed; therefore, the status of the LeGAI protein during germination remains unknown.
In conclusion, the embryo has the potential to complete germination without a requirement for GA biosynthesis or response. However, in seeds such as those of tomato and Arabidopsis where germination is constrained by surrounding structures, i.e. when dormancy is imposed on the seed, an appropriate amount of GA is required to overcome this. But even when germination does require the intervention of GA, this is not achieved by the suppression of DELLA gene transcription. Why GA should increase the expression of a gene whose product is antagonistic to the action of this growth regulator is an enigma, although there is precedent for this with respect to, for example, the auxin/indole-3-acetic acid family of transcription factors that act to inhibit auxin responses, yet in the presence of auxin their transcript abundance increases (Rouse et al., 1998).
MATERIALS AND METHODS
Plant Material
Tomato (Lycopersicon esculentum cv Trust) seeds were purchased from Stokes Seed (St. Catherines, Canada), and gib-1 mutant tomato seeds were obtained from Dr. Henk Hilhorst (Wageningen University, The Netherlands; Koornneef et al., 1990). Tomato seeds were germinated on a filter paper within a 9-cm diameter petri plate at 25°C in the dark. Soybeans (Glycine max cv OAC Bayfield) were obtained from the Department of Crop Science at the University of Guelph (Guelph, Canada) and germinated on cotton wool within 9-cm diameter petri plates at 25°C in the dark. Wild-type Landsberg and ga1-3 Arabidopsis seeds (Stock no. L53104) were obtained from the Arabidopsis Biological Resource Center (Columbus, OH). Arabidopsis seeds were stratified for 6 d at 4°C, then placed under 18 h light and 6 h dark at 23°C to facilitate germination. GA-deficient plants were sprayed once weekly with 100 μm GA3 (Sigma-Aldrich, St. Louis) up until flowering to restore male fertility. All seeds were stored in a desiccator at 4°C. Germination of GA-deficient ga1-3 and gib-1 seeds was in 9-cm petri plates at 25°C in the dark in the presence of 100 μM GA3.
RNA Isolation and DNA Isolation
Total RNA was extracted from tomato and soybean seeds using the hot borate method of Wan and Wilkins (1994). Total RNA from other tomato plant organs was isolated using Tri Reagent (Sigma-Aldrich) according to the manufacturer's recommendations. Total RNA was isolated from Arabidopsis seeds using the method of Vicient and Delseny (1999). Genomic DNA was isolated from tomato and soybean shoots according to Mo and Bewley (2002).
PCR and Inverse PCR
The tomato DELLA gene (LeGAI) was isolated using PCR and inverse PCR. For PCR, tomato genomic DNA was used as a template since all other DELLA genes isolated from other species lack introns (Peng et al., 1999; Itoh et al., 2002). PCR included 100 ng of genomic DNA and two oligonucleotide primers (GAI-1 Fwd 5′-ATGGCIGAIGTIGCICAIAARYTIGARCA-3′ and GAI-1 Rev 5′-GCIGTRAARTGIGCRAAYTTIARRTAIGGRCA-3′) that corresponded to sequences encoding conserved domains within other DELLA proteins. Resulting PCR products were subcloned into the pGEM T-Easy Vector following the manufacturer's instructions (Promega, Madison, WI) and sequenced using the BigDye Terminator Cycle Sequencing protocol with an ABI 377 DNA Sequencer (Applied Biosystems, Foster City, CA) at the Guelph Molecular Supercenter (Guelph, Canada). Notably, all PCR with tomato genomic DNA or RT-PCR with total RNA isolated from various tissues, along with two additional oligonucleotide primer pairs corresponding to other conserved domains in DELLA proteins, resulted in amplification of sequences that were identical in overlapping regions with those in the tomato DELLA gene isolated with primers GAI-1 Fwd and GAI-1 Rev.
To isolate the complete LeGAI gene, inverse PCR was carried out in the following manner. Eighty nanograms of digested tomato genomic were incubated with 20 units of T4 DNA ligase for 3 d at 4°C. Five-microliter aliquots of the genomic DNA ligation reaction were then used as template in an inverse PCR with oligonucleotide primers LeGAI-inv Fwd, 5′-ACCACCAGGTATAGCTCTTAAATCATC-3′ and LeGAI-inv Rev, 5′-CAACTACATCCTCATCTATGGTGACAG-3′ corresponding to sequences in the partial tomato LeGAI gene isolated by regular PCR (see above). The resulting 3-kb PCR product was subcloned into pGEM T-Easy and sequenced. The complete sequence of the LeGAI gene was further confirmed using proofreading DNA polymerase (Invitrogen, Burlington, Canada). Soybean DELLA gene fragments were isolated from both soybean genomic DNA and RT-PCR using radicle RNA as the template and the same primer sets as used for the isolation of LeGAI.
DNA and RNA Gel-Blot Analysis
RNA gel-blot analysis was performed as described by Sambrook et al. (1989). Fifteen micrograms total RNA were electrophoresed on a 1.2% (w/v) agarose gel containing 7% (v/v) formaldehyde, transferred to a positively charged nylon membrane (Hybond-N+; Amersham, Buckinghamshire, UK), and fixed by UV irradiation (Stratalinker; Stratagene, La Jolla, CA). Membranes were prehybridized with Church buffer (0.5 m phosphate buffer, 7% [w/v] SDS, 1 mm EDTA) and 100 μg mL−1 salmon sperm DNA (Sigma-Aldrich) for 4 h. Hybridization was carried out in the same solution but with the addition of the 32P-labeled DNA probe synthesized with the Megaprime DNA labeling system, according to the manufacturer's specifications (Amersham). The LeGAI probe (nucleotides 104–1,108) included the sequence coding for the conserved -DELLA- and -TVHKNP- domains. GmGAI probes consisted of partial gene sequences corresponding to the variable N-terminal region of these proteins, and the Arabidopsis RGL2 probe was the same as that published by Wen and Chang (2002). Following hybridization at 65°C, membranes were washed twice for 15 min each in 2× SSC/0.1% (w/v) SDS and twice in 0.2× SSC/0.1% (w/v) SDS and exposed to x-ray film.
DNA gel-blot analysis was performed essentially as described by Sambrook et al. (1989). Genomic DNA was with either EcoRI, EcoRV, HindIII, or DraI in 100-μL reactions containing 12 μg DNA. Following digestion, DNA was subjected to phenol/chloroform extraction and ethanol precipitation. Ten micrograms were then separated on a 1% (w/v) agarose gel containing 1.5 m NaCl and 0.5 m NaOH. The gel was then neutralized with 0.5 m Tris-HCl, pH 7.0, and the DNA transferred to a positively charged membrane (Amersham). The membrane was fixed in 0.4 m NaOH for 20 min and stored at −20°C until further use. Both low and high stringency DNA gel-blot analyses were performed at hybridization temperatures of 45°C and 65°C, respectively. Washes were performed at the same temperature as hybridization and were the same as for RNA gel-blot analysis.
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial purposes.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AY269087, 111216, 101361, AF460219, AB030956, AY269088, and AY269089.
Acknowledgments
We thank Dr. Camille Steber (Washington State University) for helpful comments relating to the Arabidopsis sleepy1 mutant phenotype and Dr. Henk Hilhorst (Wageningen University, The Netherlands) for generously providing seed of the tomato gib-1 mutant. Thanks also to Dr. Peter Chandler (CSIRO, Canberra, Australia) for the anti-SLN1 antibody and Dr. Nicholas Harberd (John Innes Center, Norwich, UK) for the anti-GAI antibody.
This work was supported by the Natural Sciences and Engineering Research Council of Canada (grant no. 044191 to J.D.B. and grant no. 217291 to R.T.M.). G.W.B. was supported by an NSERC Postgraduate Scholarship.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.034876.
References
- Bewley JD (1997. a) Seed germination and dormancy. Plant Cell 9: 1055–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD (1997. b) Breaking down the walls—a role for endo-β–mannanase in release from seed dormancy? Trends Plant Sci 2: 464–469 [Google Scholar]
- Bewley JD, Black M (1994) Seeds: Physiology of Development and Germination, Ed 2. Plenum Press, New York
- Bradford KJ, Chen F, Cooley MB, Dahal P, Downie B, Fukunaga KK, Gee OH, Gurusinghe S, Mella RA, Nonogaki H, et al (2000) Gene expression prior to radicle emergence in imbibed tomato seeds. In M Black, KJ Bradford, J Vazquez-Ramos, eds, Seed Biology: Advances and Applications. CABI Publishing, Wallingford, UK, pp 231–251
- Chandler PM, Marion-Poll A, Ellis M, Gubler F (2002) Mutants at the Slender1 locus of barley cv Himalaya. Molecular and physiological characterization. Plant Physiol 129: 181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Qin L, Lee S, Fu X, Richards DE, Cao D, Luo D, Harberd NP, Peng J (2004) Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 131: 1055–1064 [DOI] [PubMed] [Google Scholar]
- Debeaujon I, Koornneef M (2000) Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol 122: 415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill A, Jung HS, Sun T-P (2001) The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc Natl Acad Sci USA 98: 14162–14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck B, Harberd NP (2002) Evidence that the Arabidopsis nuclear gibberellin signaling protein GAI is not destabilised by gibberellin. Plant J 32: 935–947 [DOI] [PubMed] [Google Scholar]
- Fu X, Richards DE, Ait-Ali T, Hynes LW, Ougham H, Peng J, Harberd NP (2002) Gibberellin-mediated proteasome-dependent degradation of the barley DELLA protein SLN1 repressor. Plant Cell 14: 3191–3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot SPC, Karssen CM (1987) Gibberellins regulate seed germination in tomato by endosperm weakening: a study with gibberellin-deficient mutants. Planta 171: 525–531 [DOI] [PubMed] [Google Scholar]
- Itoh H, Ueguchi-Tanaka M, Sato Y, Ashikari M, Matsuoka M (2002) The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 14: 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen SE, Binkowski KA, Olszewski NE (1996) SPINDLY, a tetratricopeptide repeat protein involved in gibberellin signal transduction in Arabidopsis. Proc Natl Acad Sci USA 93: 9292–9296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karssen CM, Brinkhorst-van der Swan DLC, Breekland AE, Koornneef M (1983) Induction of dormancy during seed development by endogenous abscisic acid: studies on abscisic acid deficient genotypes of Arabidopsis thaliana. Planta 157: 158–165 [DOI] [PubMed] [Google Scholar]
- Karssen CM, Zagorski S, Kepczynski J, Groot SPC (1989) Key role for endogenous gibberellins in the control of seed germination. Ann Bot (Lond) 63: 71–80 [Google Scholar]
- Koornneef M, Bentsink L, Hilhorst H (2002) Seed dormancy and germination. Curr Opin Plant Biol 5: 33–36 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Bosma TDG, Hanhart CJ, Van der Veen JH, Zeevart JAD (1990) The isolation and characterization of gibberellin-deficient mutants in tomato. Theor Appl Genet 80: 852–857 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Jorna ML, Brinkhorst-van der Swan DLC, Karssen CM (1982) The isolation and analysis of abscisic acid (ABA)-deficient mutants by selection of induced revertants in non-germinating gibberellin insensitive lines of Arabidopsis thaliana. Theor Appl Genet 61: 385–393 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Reuling G, Karssen CM (1984) The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Plant Physiol 61: 377–383 [Google Scholar]
- Koornneef M, van der Veen JH (1980) Induction and analysis of gibberellin sensitive mutants from Arabidopsis thaliana. Theor Appl Genet 58: 257–263 [DOI] [PubMed] [Google Scholar]
- Lee S, Cheng H, King KE, Wang W, He Y, Hussain A, Lo J, Harberd NP, Peng J (2002) Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev 16: 646–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis KM, Thomas SG, Soule JD, Strader LC, Zale JM, Sun T-P, Steber CM (2003) The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15: 1120–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo B, Bewley JD (2002) β-Mannosidase (EC 3.2.1.25) activity during and following germination of tomato (Lycopersicon esculentum Mill.) seeds. Purification, cloning and characterization. Planta 215: 141–152 [DOI] [PubMed] [Google Scholar]
- Mo B, Bewley JD (2003) The relationship between β-mannosidase and endo-β-mannanase activities in tomato seeds during and following germination: a comparison of seed populations and individual seeds. J Exp Bot 54: 2503–2510 [DOI] [PubMed] [Google Scholar]
- Nonogaki H, Gee OH, Bradford KJ (2000) A germination specific endo-β-mannanase is expressed in the micropylar endosperm cap of tomato seeds. Plant Physiol 123: 1235–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski N, Sun T-P, Gubler F (2002) Gibberellin signaling: biosynthesis, catabolism, and response pathways. Plant Cell 14: S61–S80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Harberd NP (2002) The role of GA-mediated signaling in the control of seed germination. Curr Opin Plant Biol 5: 376–381 [DOI] [PubMed] [Google Scholar]
- Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F, et al (1999) ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 15: 256–261 [DOI] [PubMed] [Google Scholar]
- Rouse D, Mackay P, Stirnberg P, Estelle M, Leyser O (1998) Changes in auxin response from mutations in an AUX/IAA gene. Science 279: 1371–1373 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Sasaki A, Itoh H, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Jeong DH, An G, Kitano H, Ashikari M, et al (2003) Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299: 1896–1908 [DOI] [PubMed] [Google Scholar]
- Vicient CM, Delseny M (1999) Isolation of total RNA from Arabidopsis seeds. Anal Biochem 268: 412–413 [DOI] [PubMed] [Google Scholar]
- Wan CY, Wilkins TA (1994) A modified hot borate method significantly enhances the yields of high quality RNA from cotton (Gossypium hirsutum L.). Anal Biochem 223: 7–12 [DOI] [PubMed] [Google Scholar]
- Wen CK, Chang C (2002) Arabidopsis RGL1 encodes a negative regulator of gibberellin responses. Plant Cell 14: 87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Smith MW, Brown RGS, Kamiya Y, Sun T-P (1998) Phytochrome regulation and differential expression of gibberellin 3-β-hydroxylase genes in germinating Arabidopsis seeds. Plant Cell 10: 2115–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]