Abstract
Background
Heart failure (HF) is a chronic disease that compromises patients’ quality of life (QoL). Interventions designed to reduce distress and improve disease self-management are needed. We evaluated the efficacy of a telephone-based coping skills training (CST) intervention.
Methods and Results
This randomized clinical trial involved 180 HF outpatients with reduced ejection fraction. Participants ranged in age from 29 to 87 years (mean = 58 years); 27% were women and 47% were non-Caucasian. Participants were randomized to either a CST intervention or heart failure education (HFE), both delivered over 16 weeks. The primary outcomes were: (i) post-intervention effects on QoL and HF disease biomarkers (both with alpha=0.01), and; (ii) a composite measure of time to death or first hospitalization (with alpha=0.03) over a median follow-up period of 3 years. CST resulted in greater improvements in QoL compared to HFE (P<.01), including the Kansas City Cardiomyopathy Questionnaire (KCCQ)(P=.009), depressive symptoms (P=.027), and the six-minute walk test (P=.012). However, it did not differentially improve HF disease biomarkers, or reduce risk of all-cause hospitalizations or death (HR = 0.84 [0.59, 1.12]). Interestingly, exploratory analyses showed that participants randomized to CST experienced a reduction in the composite endpoint of worsening HF hospitalization or death during the 3-year follow-up period (HR = 0.65 [0.44, 0.98], P = .040).
Conclusions
CST improved QoL in patients with HF. Monitoring and improving QoL is emerging as an important aspect of the clinical management of HF that can reduce disease burden and may help improve clinical outcomes in this vulnerable patient population.
Clinical Trial Registration
Keywords: quality of life, depression, biomarker, hospitalization
Introduction
An estimated 5.7 million American adults are living with heart failure (HF), and another 870,000 new cases are diagnosed each year.1 HF is the most costly diagnosis in the Medicare population and is the most common cause for hospitalization in patients over the age of 65 years. Although medical management helps control symptoms and stabilizes patients, HF is nonetheless an inherently unstable condition, with frequent hospitalizations being one manifestation of this instability. The associated annual direct and indirect costs for HF patient care in the United States are estimated to be in excess of $39 billion.1,2
HF is a chronic condition with broad-ranging impact, affecting almost every important aspect of patients’ lives. Consequently, psychological distress is prevalent in patients with HF and quality of life (QoL) is often markedly impaired. Major depressive disorder is a common manifestation of the difficulties in coping with distress experienced by patients with HF.3 Impaired QOL and elevated depressive symptoms are associated with an adverse HF disease trajectory and poor clinical outcomes.4, 5
Traditional behavioral interventions designed to help HF patients remain in stable health and avoid preventable hospital admissions have focused on patient education, diet and medication adherence, and physical activity.6 Overall, the evidence supporting these disease management strategies has been mixed, but some have resulted in prolonged event-free survival, decreased the number of hospital admissions and improved QoL.7, 8 Although the most effective interventions have involved regular home visits by a nurse, telephone-based interventions also have been found to be effective, and because they are more convenient for patients and relatively inexpensive compared to face-to-face encounters, there is a need to further develop and refine these approaches.9
Coping effectively with chronic disease can require significant behavioral change. Coping skills training (CST) is a cognitive-behavioral approach to disease self-management that transforms maladaptive coping styles into more constructive behaviors, facilitates compliance with medical treatment recommendations, and improves psychological well-being.10 CST has been shown to both enhance self-management and improve patient health and QoL in a number of chronic diseases, including diabetes and pulmonary disease.10, 11 However, the CST approach to disease management has not been studied widely in patients with HF.12 Because HF is a chronic disease that requires rigorous medical self-management, health behavior change, and psychological adjustment, a CST intervention focused on both disease self-management and alleviating psychological distress has the potential to be of significant value for HF patients.
The objective of this trial was to evaluate the efficacy of a CST intervention, delivered over the telephone to HF patients, on three outcomes: post-intervention QoL, HF disease biomarkers, and longer term clinical outcomes defined by hospitalization or death. In order to achieve equipoise, a heart failure education (HFE) intervention was selected as the control condition.
Methods
Trial Overview
COPE-HF was a single-site randomized clinical trial in which 180 men and women with HF were randomized to either CST or HFE. Participants were enrolled between September 2009 and January 2014. Details of the study design, assessments and interventions were published previously.13
Participants
The study sample consisted of 180 men and women with documented HF. Patients were recruited from the HF Programs at Duke University Medical Center, the UNC Health Care system, and the Durham VA Medical Center. The protocol was approved by the respective institutional review boards at these centers and written informed consent was provided by each participant. Inclusion criteria were: men or women aged 18 or older; New York Heart Association (NYHA) Class II-III HF of at least 3-months duration; and left ventricular ejection fraction (LVEF) ≤ 40% documented within 6 months of study enrollment. Exclusion criteria were: myocardial infarction or coronary artery revascularization within 3 months of enrollment; HF due to a correctable cause or condition, such as uncorrected primary valvular disease; alcohol or drug abuse within 12 months; illnesses such as malignancies that are associated with a life-expectancy of <12 months; current pregnancy; and inability to provide informed consent.
Stratification and Randomization
Participants were randomized in a 1:1 ratio to either CST or HFE. A conditional randomization procedure (PROC PLAN in SAS 9.2) was used to stratify participants according to etiology of HF (ischemic versus non-ischemic) and age (<60 years versus ≥ 60 years). To maintain allocation concealment, group assignments were placed in sealed envelopes and opened sequentially at the time of randomization.
Outcome Measures
The primary outcomes were: (i) post-intervention effects on QoL and HF disease biomarkers and; (ii) a composite measure of time to either all-cause death or hospitalization. The primary measure of HF disease biomarkers was a global score of B-type Natriuretic Peptide (BNP)14, LVEF15, 24-hour heart rate variability (HRV) indexed by the standard deviation of normal R-R intervals (SDNN)16, flow mediated dilation of the brachial artery (FMD)17, and plasma C-reactive protein (CRP).18 The primary QoL index was a global score of the Kansas City Cardiomyopathy Questionnaire (KCCQ)19, Beck Depression Inventory II (BDI-II)20, Speilberger State-Trait Anxiety Inventory (STAI)21, HF Attitudes About Impairment (AAI) questionnaire22 and the 6-minute walk test (6MWT). Hospitalizations were categorized as “all-cause”‘, “cardiovascular’’ and “HF” (worsening HF) based upon medical records. Death was verified from hospital and EMS records.
Secondary outcomes were the components of the HF disease and QoL global score measures. Health behaviors important for HF self-management were assessed prior to randomization and immediately upon completion of the intervention. Routine daily physical activity was assessed using an accelerometer (Actiwatch®-64, Mini Mitter Co., Inc., Bend, Oregon) worn on the wrist of the non-dominant arm for 24-hours. Medication adherence was assessed using the Medication Event Monitoring System (or MEMS®) bottle cap in–home for 10 days. Dietary sodium intake was estimated from a 24-hour standardized dietary recall with a nutritionist, as well as by 24-hour urinary sodium excretion. In addition, we administered an established questionnaire that was designed to assess self-care in HF patients, defined by a combination of maintenance, symptom perception and management behaviors.23 Post-treatment assessments were performed within 2 weeks after completing the intervention. All outcome assessments were performed by research team members blinded to group assignment.
Interventions
Coping Skills Training (CST)
CST was delivered by a clinical psychologist (B.M.H.), and was comprised of 16 weekly 30-minute individual phone calls. Cognitive behavioral techniques were patterned after previous studies involving pulmonary11, 24 and cardiac25 patients. Motivational interviewing was utilized to enhance adherence to prescribed health behaviors. All CST participants received the same set of topics. For the initial 4 sessions involving health behaviors (diet and salt restriction, daily weighing, physical activity, medication adherence), participants could prioritize the order in which each health behavior was covered. The remaining 12 sessions were presented in the same sequence and including specific coping techniques (relaxation training, cognitive restructuring, visualization, problem solving, and activity pacing). Two optional modules addressing depression and assertiveness training were incorporated into the intervention at the discretion of the interventionist based upon patient needs.
Heart Failure Education (HFE)
The HFE intervention was delivered by a Physician’s Assistant (PA) and also involved 16 weekly individual phone calls of up to 30-minutes duration, providing a control for weekly patient contact, but focused upon the provision and discussion of medical issues important for HF self-management without teaching behavioral skills to improve coping. The interventionist (J.S.) provided information regarding HF health behaviors, including symptom monitoring, importance of daily weighing, adherence to medications, physical activity, and optimal diet.
All study participants continued with their regular medical care, including their routine cardiology visits and management of any episodes of escalating symptoms or disease progression.
Statistical Analysis
All analyses were performed with SAS 9.3 (Cary, NC), and the evaluation of all intervention effects were based on the principle of intention-to-treat, with missing post-treatment data managed using multiple imputation within PROC MI, using 100 imputations. The effects of treatment on the QoL and HF disease severity variables were assessed using two separate general linear models in which a rank-based global score of all QoL and HF disease markers served as the respective outcome variable, as suggested by O’Brien26 for the examination of multiple endpoints.27 This general analytic approach has previously been utilized in multiple cardiac trials and is recommended for examining multiple, related outcome variables within a domain of interest simultaneously.28 Consistent with this ‘gatekeeper’ methodology, if a global score analysis showed evidence of a treatment group difference, we conducted subsequent analyses examining the individual outcomes in a secondary, explanatory step.
Evaluation of treatment effects on clinical outcomes used separate Cox proportional hazards models (PHREG). These analyses addressed all-cause first hospitalization or death as primary and cardiovascular hospitalization or death as supportive. Additional supportive analyses addressed worsening HF hospitalization or death, and death. For these analyses, the time of event was the index date, and participants with no dates were censored at the time of last contact with study staff or documentation in their medical record. All proportional hazards models controlled for age, HF etiology, baseline BNP, LVEF, and number of hospitalizations within the past year. We also examined the impact of treatment on HF hospitalization and death using the Wei-Lin-Weissfeld (WLW) method, which allows deaths after HF hospitalization to be taken into account.29 This approach has been advocated for use among patients with chronic diseases due to the multiple natures of most “hard” clinical events in these populations, which are often clustered within individuals.30
An additional set of proportional hazards analyses were conducted in which changes in QoL served as the predictor of interest. Within this model, time to first HF hospitalization or death, and time to death, using the WLW approach served as the outcome with change in QoL (post-treatment residualized for pre-treatment) as the predictor of interest and baseline QoL, BNP, LVEF, age, etiology of HF, and number of hospitalizations within the past year as control variables. Only events occurring after post-treatment assessments were considered in these models. These exploratory analyses also examined individual QoL indices.
Power Calculation
With respect to the global HF severity and QoL scores, we estimated power assuming an attrition rate of about 20% and multiple imputation for missing data. We identified a standardized effect size of about a half-standard deviation as clinically meaningful for the HF biomarker and QoL global scores.31 For testing a given global index at 0.01, so as to account for multiple comparisons, approximately100 patients in each treatment arm, provides about .90 power to detect a standardized effect size of 0.60.
Power for the Cox regression model was estimated assuming an event rate of 70% in the control group, 42 months for patient accrual, a median follow-up time of 3 years and a minimum follow-up time of 15 months. With approximately 100 patients in each group, we estimated power of about 0.90 to detect a hazard ratio of 0.50 by a two-sided test at 0.05. For testing at 0.03, to account for multiple comparisons, the power is .90 to detect a hazard ratio of 0.47.
Results
Participant Flow
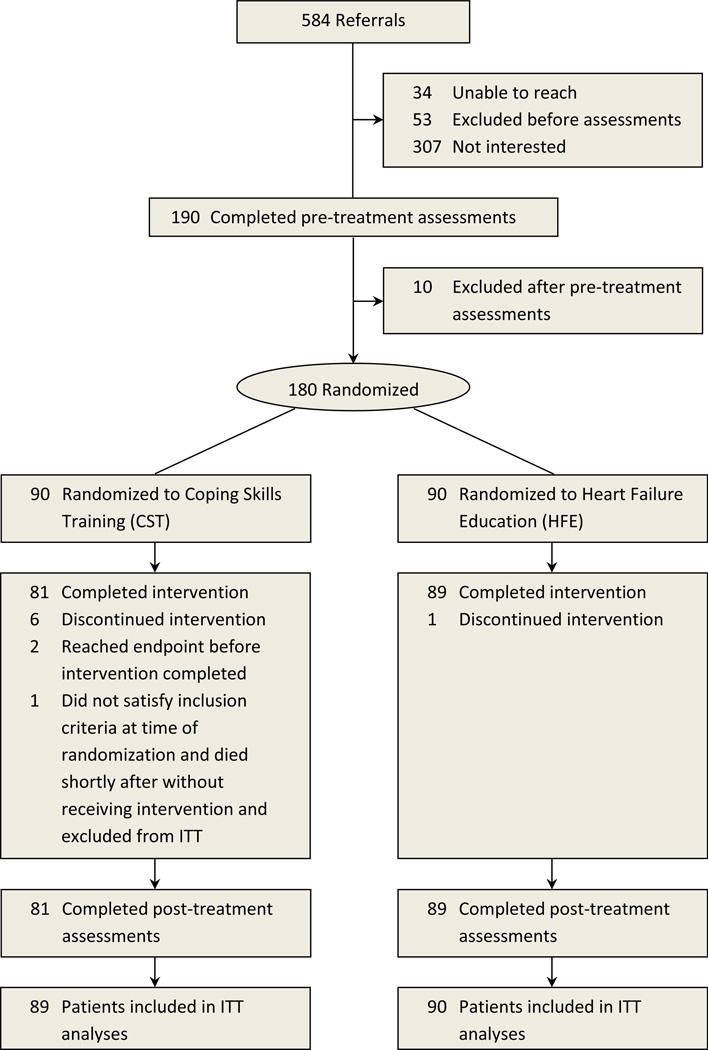
Figure 1 displays the flow of participants during the trial. Of 584 men and women referred for potential participation, 199 were consented, 190 completed pre-treatment assessments, and 180 were randomized. One participant randomized to CST died prior receiving any treatment and was excluded in the analysis. The intent-to-treat (ITT) analyses were therefore based on a study sample of 179 participants.
Figure 1.
CONSORT chart of trial enrollment.
Participant Characteristics
Demographic, clinical, and baseline characteristics are presented in Table 1. Although treatment groups were comparable on most background characteristics, the HFE group tended to have a higher percentage of participants with implantable cardiac defibrillators (ICDs) at baseline, as well as lower LVEF.
Table 1.
Background Characteristics of Study Sample. Values are presented as mean (SD) unless otherwise indicated.
| Variable | CST (n=89) | HFE (n=90) | Cohort (n=179) | P-value |
|---|---|---|---|---|
| Age, years | 57.6 (11.1) | 57.9 (11.9) | 57.7 (11.5) | .90 |
| Female, n (%) | 27 (30%) | 21 (23.3%) | 48 (26.7%) | .29 |
| White, n (%) | 50 (55.6%) | 46 (51.1%) | 96 (53.3%) | .50 |
| Ischemic etiology, n (%) | 35 (38.9%) | 37 (41.1%) | 72 (40%) | .81 |
| Lab Results | ||||
| Serum Creatinine, mg/dL | 1.18 (0.37) | 1.23 (0.42) | 1.2 (0.40) | .44 |
| Hemoglobin, g/dL | 13.5 (1.7) | 13.4 (1.5) | 13.4 (1.6) | .66 |
| Urinary Sodium, mg/24-hr | 3550 (1734) | 3882 (2055) | 3717 (1903) | .25 |
| Dietary Sodium, mg/day | 2993 (1461) | 2806 (1653) | 2899 (1559) | .43 |
| Medications | ||||
| ACEI, n (%) | 64 (72%) | 69 (77%) | 134 (74%) | .47 |
| ARB, n (%) | 21 (23%) | 16 (18%) | 37 (21%) | .34 |
| Aldosterone Antagonists, n (%) | 47 (52%) | 47 (52%) | 94 (52%) | .99 |
| Anticoagulants, n (%) | 26 (29%) | 27 (30%) | 54 (30%) | .91 |
| Aspirin, n (%) | 58 (64%) | 60 (67%) | 118 (65%) | .83 |
| Beta blocker, n (%) | 87 (98%) | 85 (94.4%) | 173 (96%) | .25 |
| Antidepressant, n (%) | 21 (23%) | 33 (37%) | 54 (30%) | .06 |
| Cholesterol drug, n (%) | 62 (69%) | 64 (71%) | 126 (70%) | .83 |
| Diuretic, n (%) | 78 (88%) | 84 (93%) | 163 (91%) | .19 |
| Nitrate, n (%) | 21 (23%) | 25 (28%) | 46 (25%) | .52 |
| Medical History | ||||
| Hypertension, n (%) | 65 (73%) | 67 (74%) | 132 (74%) | .83 |
| Diabetes, n (%) | 41 (46%) | 49 (54%) | 90 (50%) | .26 |
| Hyperlipidemia Dx, n (%) | 57 (64%) | 64 (71%) | 122 (68%) | .31 |
| BiV Pacemaker, n (%) | 32 (37%) | 44 (49%) | 77 (43%) | .08 |
| ICD, n (%) | 63 (71%) | 75 (83%) | 139 (77%) | .05 |
| Quality of Life | ||||
| BDI Score | 14.2 ± 9.5 | 13.5 ± 10.4 | 13.9 ± 9.9 | .64 |
| AAI Score | 47.3 (10.0) | 48.0 (10.7) | 47.6 (10.3) | .66 |
| STAI Score | 28.1 (7.4) | 31.3 (9.9) | 29.7 (8.9) | .03 |
| KCCQ Score | 58.1 (21.5) | 55.6 (21.9) | 56.8 (21.6) | .43 |
| 6-min Walk Distance, meters | 376 (98) | 353 (114) | 365 (107) | .16 |
| Heart Failure Disease Biomarkers | ||||
| BNP, pg/ml | 284.4 (485) | 241.5 (318) | 262.8 (409) | .49 |
| LVEF, % | 31.7 (10.3) | 28.4 (8.7) | 30.0 (9.6) | .02 |
| HRV, SDNN | 88.8 (28.1) | 81.8 (29.2) | 85.7 (28.7) | .19 |
| Flow Mediated Dilation, % | 4.1 (4.1) | 3.9 (4.2) | 4.0 (4.2) | .78 |
| CRP, mg/L | 3.7 (3.4) | 4.0 (3.4) | 3.8 (3.4) | .57 |
ACEI=angiotensin converting enzyme inhibitor; ARB=angiotensin receptor blocker; BiV=biventricular; ICD=implantable cardioverter defibrillator; BDI=Beck Depression Inventory; AAI=Attitudes About Impairment; STAI= State Anxiety; KCCQ=Kansas City Cardiomyopathy Questionnaire; BNP= B-Type Natriuretic Peptide; LVEF=left ventricular ejection fraction; HRV= Heart rate Variability; SDNN=standard deviation of normal to normal heartbeat intervals; CRP=C-reactive protein. Hypertension, diabetes or hyperlipidemia were defined as present if a diagnosis appeared in participants' medical records during the year preceding pre-randomization baseline assessments
Treatment Adherence
Treatment adherence during the study intervention was excellent, with 95% of participants completing post-intervention assessments. Average duration of the weekly telephone intervention calls were 10 minutes for HFE and 26 minutes for CST. Among individuals in the CST group, participants were present for an average of 14.5 (SD = 3.5) sessions and 80% of these participants attended 14 or more sessions. Among individuals in the HFE group, participants were present for an average of 15.7 (SD = 1.6) sessions and 90% of individuals participated in all 16 sessions. Post-treatment data were available on all but 9 patients (8 in the CST group and 1 in the HFE group). Among the 9 patients with missing post-treatment assessments, 7 discontinued their randomly assigned intervention prior to its completion (6 in the CST group and 1 in the HFE group). Among the other 2 patients in the CST group with missing post-treatment assessments, 1 died prior to completion of the CST intervention and 1 completed the CST intervention but did not participate in the post-treatment assessment. In relation to the three year follow up of clinical outcomes, there was 0% attrition.
Primary Outcomes
Quality of Life
Examination of post-intervention changes in QoL demonstrated that the CST group exhibited significantly greater improvements in the QoL global score compared with HFE (P <.01) (Table 2). Of the QoL component measures, the CST group exhibited greater improvements the KCCQ and 6MWT distance, and a greater reduction in depressive symptoms measured by the BDI-II compared to HFE. The remaining QoL components (AAI and STAI) showed trends toward greater improvements in the CST group. Follow-up analyses of changes in depressive symptoms also revealed a baseline depression by treatment group interaction (P = .001), such that individuals exhibiting clinically elevated symptoms at baseline (BDI≥ 14; n = 77 [37 in HFE, 40 in CST]) showed reductions in depressive symptoms that were greater among individuals in CST (8.3 [1.4, 5.6]) relative to HFE (3.8 [1.4, 6.5]) (P = .023).
Table 2.
Changes (mean change with confidence intervals) in Measures of Quality of Life (QoL) and Heart Failure Disease Biomarkers
| Variable | Coping Skills Training (n=89) |
Heart Failure Education (n=90) |
P-value |
|---|---|---|---|
| Quality of Life | |||
| Attitudes about Impairment | 3.7 (2.2, 5.3) | 1.8 (0.3, 3.2) | .070 |
| Beck Depression Inventory | −4.5 (−5.8, −3.1) | −2.4 (−3.7, −1.1) | .027 |
| KCCQ Total Score | 8.3 (5.0, 11.5) | 2.3 (−0.8, 5.4) | .009 |
| 6-Min Walk Distance (meters) | 15.8 (4.8, 26.7) | −3.5 (−13.9, 7.0) | .012 |
| State Anxiety | −2.6 (−4.4, −0.8) | −0.2 (−1.9, 1.6) | .057 |
| Heart-Failure Disease Biomarkers | |||
| B-type Natriuretic Peptide (pg/ml) | −23.5 (−63.6, 16.2) | −25.0 (−62.9, 12.8) | .958 |
| Left Ventricular Ejection Fraction (%) |
0.2 (−0.9, 1.4) | −1.3 (−2.4, −0.2) | .066 |
| Heart Rate Variability (SDNN) | 2.0 (−4.7, 8.7) | −3.7 (−11.0, 3.5) | .246 |
| Flow Mediated Dilation (%) | 0.2 (−0.5, 0.9) | 0.0 (−0.7, 0.7) | .733 |
| C-Reactive Protein (mg/L) | −0.3 (−0.9, 0.2) | 0.3 (−0.2, 0.9) | .102 |
Values are adjusted for age, etiology of heart failure (ischemic vs. non-ischemic), and the pre-treatment level of the respective predictor. FMD analyses were also adjusted for baseline arterial diameter. KCCQ=Kansas City Cardiomyopathy Questionnaire; SDNN=standard deviation of normal to normal heartbeat intervals.
HF Biomarkers
Although HF biomarkers showed improvement over time (data not shown), post-treatment comparisons for HF disease biomarkers revealed no significant differences between CST and HFE (P = .114) (Table 2).
Follow-up Clinical Events
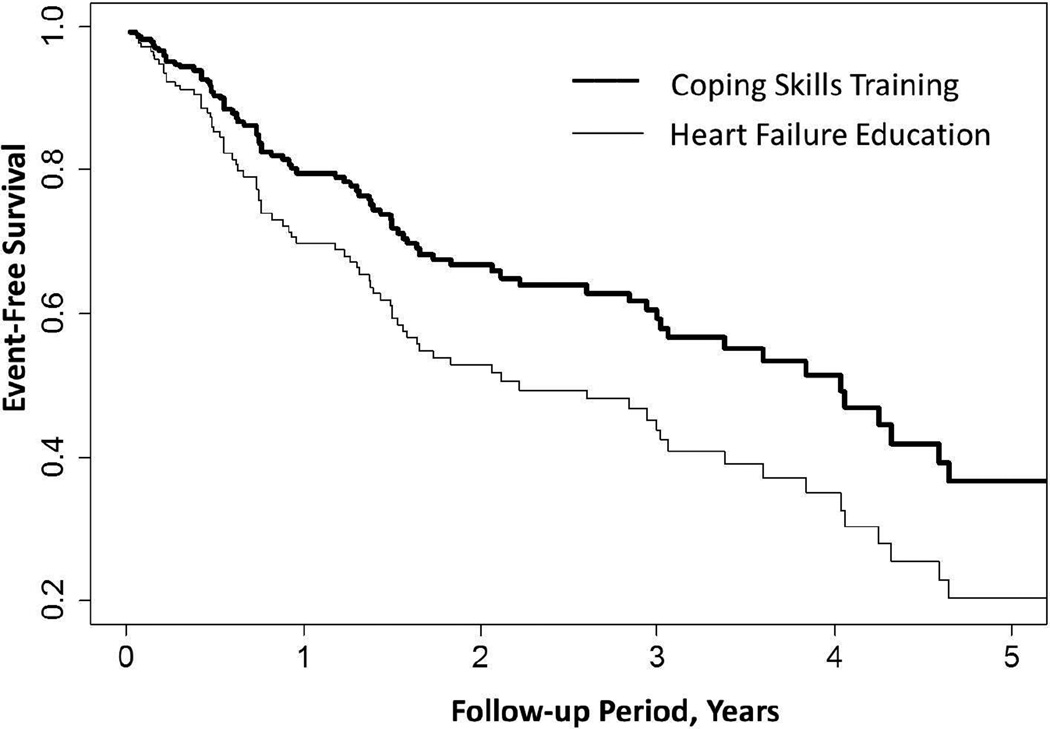
Over a median 3 years of follow-up (range 0.2 to 5.7 years), 127 participants experienced at least one criterion clinical event (all-cause hospitalization or death), 106 experienced at least one cardiac event (MI, stroke, cardiac revascularization, or death), and 80 experienced at least one HF hospitalization (n = 65) or died (n = 15). In this latter set of analyses, 24 participants whose first event was a HF hospitalization subsequently died. As shown in Table 3, compared to HFE, the CST intervention exhibited trends towards reduced risk of first all-cause hospitalization or death (HR = 0.84 [0.59, 1.12], P>.03) and first cardiovascular hospitalization or death (HR = 0.78 [0.53, 1.16], P >.03). CST participants were more clearly less likely to experience a worsening HF hospitalization or die during follow-up (HR = 0.63 [0.40, 1.01], P = .055); this effect appeared to be driven by the CST group tending to have a lower mortality rate (HR = 0.53 [0.27, 1.06], P = .072). Follow-up analyses, integrating both of the latter models using the Wei-Lin-Weissfeld (WLW) method, demonstrated that the CST group was less likely to experience a worsening HF hospitalization or die during follow-up (HR = 0.65 [0.44, 0.98], P = .040), with 3-year worsening HF hospitalization or death rates of 37% for the CST group and 50% for the HFE group (Table 3; Figure 2). Mortality rates in the CST group were nearly 50% less than that observed in HFE, with a 3-year mortality rate of only 8% in the CST group compared to 15% in the HFE group.
Table 3.
Treatment effects on all-cause hospitalization or death and Wei-Lin-Weissfeld integrated model of heart failure hospitalizations or death.
| Variables | All-cause Hospitalization or Death (127 events) |
HF Hospitalization or Death (80 events) |
||
|---|---|---|---|---|
| Model | Hazard Ratio (95% CI) |
P-value | Hazard Ratio (95% CI) |
P-value |
| Age (>60 vs. <60 yr) | 1.07 (0.75, 1.54) | .714 | 1.01 (0.67, 1.53) | .949 |
| Etiology (Ischemic vs. non-Ischemic) | 1.00 (0.69, 1.43) | .986 | 0.77 (0.48, 1.17) | .203 |
| BNP at baseline (1,000 pg/ml) | 2.32 (1.58, 3.40) | <.001 | 3.48 (2.31, 5.12) | <.001 |
| Ejection Fraction at baseline (10%) | 1.00 (0.83, 1.21) | .992 | 0.92 (0.75, 1.13) | .437 |
| Hospitalizations in Past Year (ref=0): 1–2 ≥3 |
0.82 (0.55, 1.22) 1.77 (1.01, 3.07) |
.337 .045 |
0.84 (0.52, 1.34) 1.77 (1.06, 2.97) |
.462 .031 |
| Coping Skills Training | 0.84 (0.59, 1.21) | .352 | 0.65 (0.44, 0.98) | .040 |
Analyses of heart failure and death included 80 first events and 24 subsequent events (i.e. 24 individuals whose first event was a heart failure hospitalization subsequently died).
Figure 2.
Adjusted Cox proportional hazards model comparing CST and HFE from randomization to 3-year follow-up. CST participants exhibited a lower rate of HF hospitalizations and death (HR = 0.65 [0.44, 0.98], P=.040) after adjustment for age, ejection fraction, BNP, and hospitalizations in the year preceding randomization.
Secondary Outcomes
Health Behaviors and HF Self-Management
All participants showed post-intervention improvements in HF self-management scores: HF management (mean increase 8.6 [6.7, 10.5], P = .024), HF maintenance (mean increase 7.6 [6.3, 8.8], P = .036), and HF self-care (mean increase = 10.4 [9.0, 11.7], P < .001), as well as reduced urinary sodium excretion (mean reduction 339 [126, 552], P = .028) and increased physical activity levels (mean increase in arbitrary actigraphy units 18252 [5814, 30690], P = .005). However, there were no significant intervention group differences in these improvements, nor were there treatment group differences in medication adherence.
Association between QOL Improvements and Clinical Outcomes
Post-intervention improvements on our global score measure of QoL were associated with a reduced likelihood of HF hospitalization or death (P = .001). Examination of individual QoL components revealed that greater improvements in the KCCQ (P = .007), 6MWD (P = .012), AII (P = .045) and STAI (P = 0.05) were most strongly associated with reduced clinical events, whereas the BDI-II (P = .088) showed a somewhat weaker association.
Discussion
The COPE-HF randomized clinical trial demonstrated that a coping skills training (CST) intervention resulted in marked and broad-ranging improvements in QoL for patients with HF. Among the most notable improvements were health-related QoL, assessed by the KCCQ, which increased by an average of over 8 points in patients randomized to CST, reflecting a significant improvement in patients’ clinical status.32 Functional capacity assessed by the 6-minute walk test also increased by over 15 meters in the CST group, which is indicative of the favorable clinical changes produced by the CST intervention.32 By comparison, the HF-ACTION trial reported that 3-months of aerobic exercise training also resulted in a similar 15 meter increase in the 6-minute walk test, but a more modest increase in the KCCQ of less than 2 points, compared with usual care.33, 34 Depressive symptoms, assessed by the BDI-II were reduced by over 4 points in participants randomized to CST, and other measures of psychological well-being also showed favorable trends towards improvement compared to the HFE control group. Importantly, post-intervention QoL was associated with improved event-free survival. These findings underscore the perspective that QoL in HF patients should be considered an important treatment target.
Although participants in the CST intervention showed no greater improvement in HF disease biomarkers compared to patients randomized to the education control condition, there was some indication that it lowered the risk of death or hospitalization for worsening HF over an average follow-up period of 3 years. The CST intervention was not associated with reduced all-cause hospitalizations or mortality, but participants in the CST group did show a notable 35% reduction in risk of HF hospitalization or death compared to HFE participants. Importantly, the improved survival benefits found for the CST intervention are compared to a HF education control intervention that itself is also likely to have had a favorable impact on event-free survival, as patient education and the non-specific effects of attention and support have been shown to be beneficial.6 Moreover, the CST intervention involved weekly phone calls for 16 weeks, but its benefits persisted years after its completion, suggesting that the skills and techniques mastered during the intervention were retained over the course of the follow up period.
Because health behaviors that are relevant to HF self-management are important to HF outcomes, we examined the impact of treatment on important HF health behaviors. Our measures of dietary sodium intake indicated that a post-intervention reduction was evident in both CST and HFE groups. Medication adherence was generally high in all participants and did not improve further as a result of the interventions, while self-reported HF self-management behaviors improved in both intervention groups. Therefore, while both treatment groups demonstrated improvements in health behaviors, such improvements do not account for the greater benefits of CST on QoL and reduced likelihood of hospitalization or death due to worsening HF.
Consistent with previous reports,35 over 40% of HF patients in our study sample had clinically significant depressive symptoms (BDI-II≥14). Of this subsample, those randomized to the CST intervention exhibited a marked and clinically-significant 8-point average reduction in BDI II score. These findings are consistent with those of a recent report showing cognitive behavior therapy to be an effective treatment for depression in HF patients with major depressive disorder.12 Unfortunately, evidence regarding the efficacy of antidepressants for treating depressive symptoms and improving outcomes in HF patients has been disappointing.36 We and others have previously shown that elevated depressive symptoms, and depressive symptoms that worsen over time regardless of initial severity, are associated with adverse clinical outcomes in HF, independent of HF disease severity and comorbidity.5
For patients with chronic diseases, patient-centered outcomes and QoL are gaining widespread recognition as being important treatment targets for optimal patient management. QoL is a complex construct consisting of multiple health–related, and disease-specific domains for which there are no universally accepted measures.37–39 Nonetheless, QoL measures have been shown to predict long term clinical outcomes and survival in cancer patients40 and in kidney transplant recipients.41 Several studies of HF patients also have linked QoL, measured by HF disease-specific health-related QoL self-report scales, to survival, but only one recently conducted secondary analysis of data from the COACH study demonstrated that this relationship remained robust after controlling for HF disease severity using HF biomarkers in hospitalized HF patients.4 Our study confirms and extends this finding by further demonstrating that QoL is an important and independent aspect of HF that is related to long-term clinical outcomes in HF outpatients who were on a stable medical regimen. Moreover, our results showed that an intervention designed to reduce psychological distress in HF patients can improve QoL and also help lower the risk of death or hospitalization due to worsening HF.
The present study was relatively small and the follow-up was for an average of only 3 years, limiting the power to detect differences in mortality and hospitalizations between treatment groups. Although our patients came from diverse clinical heart failure programs, they were recruited from only 3 sites, which may limit the generalizability of our findings. A further limitation is that participants in the CST condition had a similar number of phone calls, but sessions were longer compared with HFE. Although the duration of telephone intervention calls was unrelated to any outcome measure within each intervention group, the longer call duration for the CST condition cannot be ruled out as a potential confounding factor. It is of further note that a HF telemonitoring system that had previously demonstrated success in smaller scale trials, failed to scale successfully to a large-scale multi-site randomized controlled trial.42 Therefore, the present findings should be interpreted with caution; a larger scale trial spanning diverse clinical settings may be needed to establish the benefits and cost-effectiveness of coping skills training for HF patients. Nonetheless, the COPE-HF trial findings are encouraging, and are supported by the inclusion of a HFE control group that not only controlled for patient contact and attention, but also provided equipoise in terms of anticipated outcome benefits to participants. It is also noteworthy that while the CST intervention was completed in 16 weekly phone calls, its benefits appeared to persist for years after its completion.
In summary, in a clinical efficacy trial, we found that a CST intervention delivered remotely by telephone showed promising results for improving overall QoL and reducing the risk of worsening HF hospitalizations and mortality in patients with HF. The present findings suggest that CST interventions also may be a feasible approach to reduce the burden on the health care system associated with managing this chronic disease. Our observations also underscore the importance of addressing the distress associated with living with HF, and providing patients with approaches and strategies to improve their ability to cope with their disease and reduce their distress. Remote interventions of this kind may increasingly play an important role for patients with chronic debilitating diseases who may not have access to mental health facilities and may not be receptive to traditional mental health treatments.
Supplementary Material
Clinical Perspective.
Heart failure is a chronic, debilitating condition that requires comprehensive medical management by physicians, including pharmacologic and device therapies designed to limit and help reverse its severity. Unhealthy behaviors are often important etiologic factors in the development and progression of heart failure, and adoption of healthy lifestyle practices is therefore an important aspect of successful heart failure management. Findings from the present study confirm and extend growing evidence that implementing strategies designed to enhance patient self-management and improve coping may have beneficial effects on patient-centered outcomes. A novel finding from the study is that coping skills training delivered remotely by telephone improved quality of life more than health behavior education alone. These findings underscore the importance of addressing psychological distress associated with heart failure and providing patients with strategies to improve their ability to cope with their disease. Improving quality of life for heart failure patients also may help reduce the risk of hospitalizations or death due to worsening heart failure. Remote interventions of this kind could play an increasingly important role among heart failure patients, and may help reduce the systemic health care burden associated with managing this complex, chronic disease.
Acknowledgments
We wish to express our appreciation to the members of our Data and Safety Monitoring Board, Mark Appelbaum PhD, Stephanie H. Dunlap, D.O., and Judith McFetridge – Durdle, PhD, RN, and to our expert consultants Ken Resnicow, PhD and Jacqueline Dunbar-Jacob, PhD for their guidance and advice. We also wish to thank our research staff including Michael Ellis, RDMS, RVT, Catherine Wu, MS, Julie Johnson, PA-C, Heidi Scronce, BS, Lauren Williamson, BA, MS, and Monika Grochulski, BS. Thanks also are extended to referring health care providers, including: Michael A. Blazing, MD, Matthew J. Wolf, MD, PhD, Margaret T. Bowers, DNP, RN, Karol S. Harshaw-Ellis, DNP, MSN, Ellen Chrysogelos, ANP at Duke University Medical Center; Jana Glotzer, RN, MSN, C-ANP, Sarah Waters, ANP, at UNC Health Care; and Susan Roberts, ANP, Scott Ward, ANP, at the Durham Veterans Affairs Medical Center.
Sources of Funding: Supported by Grant HL091920 from the National Heart, Lung, and Blood Institute, NIH, Bethesda, MD, and Grant M01-RR-30 from the General Clinical Research Center program, National Center for Research Resources, NIH.
Footnotes
Conflict of Interest: None.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics C and Stroke Statistics S. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Delgado-Passler P, McCaffrey R. The influences of postdischarge management by nurse practitioners on hospital readmission for heart failure. J Am Acad Nurse Pract. 2006;18:154–160. doi: 10.1111/j.1745-7599.2006.00113.x. [DOI] [PubMed] [Google Scholar]
- 3.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 4.Hoekstra T, Jaarsma T, van Veldhuisen DJ, Hillege HL, Sanderman R, Lesman-Leegte I. Quality of life and survival in patients with heart failure. Eur J Heart Fail. 2013;15:94–102. doi: 10.1093/eurjhf/hfs148. [DOI] [PubMed] [Google Scholar]
- 5.Sherwood A, Blumenthal JA, Hinderliter AL, Koch GG, Adams KF, Jr, Dupree CS, Bensimhon DR, Johnson KS, Trivedi R, Bowers M, Christenson RH, O'Connor CM. Worsening depressive symptoms are associated with adverse clinical outcomes in patients with heart failure. J Am Coll Cardiol. 2011;57:418–423. doi: 10.1016/j.jacc.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labrunee M, Pathak A, Loscos M, Coudeyre E, Casillas JM, Gremeaux V. Therapeutic education in cardiovascular diseases: state of the art and perspectives. Ann Phys Rehabil Med. 2012;55:322–341. doi: 10.1016/j.rehab.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Flodgren G, Rachas A, Farmer AJ, Inzitari M, Shepperd S. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2015;9 doi: 10.1002/14651858.CD002098.pub2. CD002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feltner C, Jones CD, Cene CW, Zheng ZJ, Sueta CA, Coker-Schwimmer EJ, Arvanitis M, Lohr KN, Middleton JC, Jonas DE. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160:774–784. doi: 10.7326/M14-0083. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhry SI, Phillips CO, Stewart SS, Riegel B, Mattera JA, Jerant AF, Krumholz HM. Telemonitoring for patients with chronic heart failure: a systematic review. J Cardiac Fail. 2007;13:56–62. doi: 10.1016/j.cardfail.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grey M, Berry D. Coping skills training and problem solving in diabetes. Curr Diabetes Rep. 2004;4:126–131. doi: 10.1007/s11892-004-0068-7. [DOI] [PubMed] [Google Scholar]
- 11.Blumenthal JA, Babyak M, Keefe FJ, Davis RD, Lacaille RA, Carney RM, Freedland KE, Trulock E, Palmer SM. Telephone-based coping skills training for patients awaiting lung transplantation. J Consult Clin Psychol. 2006;74:535–544. doi: 10.1037/0022-006X.74.3.535. [DOI] [PubMed] [Google Scholar]
- 12.Freedland KE, Carney RM, Rich MW, Steinmeyer BC, Rubin EH. Cognitive Behavior Therapy for Depression and Self-Care in Heart Failure Patients: A Randomized Clinical Trial. JAMA Intern Med. 2015;175:1773–1782. doi: 10.1001/jamainternmed.2015.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherwood A, O'Connor CM, Routledge FS, Hinderliter AL, Watkins LL, Babyak MA, Koch GG, Adams KF, Jr, Dupree CS, Chang PP, Hoffman BM, Johnson J, Bowers M, Johnson KS, Blumenthal JA. Coping effectively with heart failure (COPE-HF): design and rationale of a telephone-based coping skills intervention. J Card Fail. 2011;17:201–207. doi: 10.1016/j.cardfail.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troughton RW, Richards AM. BNP for clinical monitoring of heart failure. Heart Fail. Clin. 2006;2:333–343. doi: 10.1016/j.hfc.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Konstam MA, Dracup K, Baker DW, Bottorff MB, Brooks NH, Dacey RA, Dunbar SB, Jackson AB, Jessup M, Johnson JC. Heart failure: evaluation and care of patients with left ventricular systolic dysfunction. J Cardiac Fail. 1995;1:183–187. doi: 10.1016/1071-9164(95)90021-7. [DOI] [PubMed] [Google Scholar]
- 16.Aronson D, Mittleman M, Burger AJ. Measures of heart period variability as preditors of mortality in hospitalized patients with decompensated congestive heart failure. Am J Cardiol. 2004;93:59–63. doi: 10.1016/j.amjcard.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Meyer B, Mortl D, Strecker K, Hulsmann M, Kulemann V, Neunteufl T, Pacher R, Berger R. Flow-mediated vasodilation predicts outcome in patients with chronic heart failure: comparison with B-type natriuretic peptide. J Am Coll Cardiol. 2005;46:1011–1018. doi: 10.1016/j.jacc.2005.04.060. [DOI] [PubMed] [Google Scholar]
- 18.Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, Niebauer J, Hooper J, Volk HD, Coats AJ, Anker SD. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102:3060–3067. doi: 10.1161/01.cir.102.25.3060. [DOI] [PubMed] [Google Scholar]
- 19.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 21.Speilberger CD. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists; 1983. [Google Scholar]
- 22.Turvey CL, Klein DM, Pies CJ, Arndt S. Attitudes about impairment and depression in elders suffering from chronic heart failure. Int J Psychiatr Med. 2003;33:117–132. doi: 10.2190/7DDC-AEHY-T72U-47GU. [DOI] [PubMed] [Google Scholar]
- 23.Riegel B, Carlson B, Moser DK, Sebern M, Hicks FD, Roland V. Psychometric testing of the self-care of heart failure index. J Card Fail. 2004;10:350–360. doi: 10.1016/j.cardfail.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Blumenthal JA, Emery CF, Smith PJ, Keefe FJ, Welty-Wolf K, Mabe S, Martinu T, Johnson JJ, Babyak MA, O'Hayer VF, Diaz PT, Durheim M, Baucom D, Palmer SM. The effects of a telehealth coping skills intervention on outcomes in chronic obstructive pulmonary disease: primary results from the INSPIRE-II study. Psychosom Med. 2014;76:581–592. doi: 10.1097/PSY.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blumenthal JA, Sherwood A, Babyak MA, Watkins LL, Waugh R, Georgiades A, Bacon SL, Hayano J, Coleman RE, Hinderliter A. Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease: a randomized controlled trial. JAMA. 2005;293:1626–1634. doi: 10.1001/jama.293.13.1626. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics. 1984;40:1079–1087. [PubMed] [Google Scholar]
- 27.Sun H, Davison BA, Cotter G, Pencina MJ, Koch GG. Evaluating treatment efficacy by multiple end points in phase II acute heart failure clinical trials: analyzing data using a global method. Circ Heart Fail. 2012;5:742–749. doi: 10.1161/CIRCHEARTFAILURE.112.969154. [DOI] [PubMed] [Google Scholar]
- 28.Felker GM, Maisel AS. A global rank end point for clinical trials in acute heart failure. Circ Heart Fail. 2010;3:643–646. doi: 10.1161/CIRCHEARTFAILURE.109.926030. [DOI] [PubMed] [Google Scholar]
- 29.Wei LJ, Lin DY, Weissfeld L. Regression-Analysis of Multivariate Incomplete Failure Time Data by Modeling Marginal Distributions. J Am Stat Assoc. 1989;84:1065–1073. [Google Scholar]
- 30.Saville BR, Herring AH, Koch GG. A robust method for comparing two treatments in a confirmatory clinical trial via multivariate time-to-event methods that jointly incorporate information from longitudinal and time-to-event data. Stat Med. 2010;29:75–85. doi: 10.1002/sim.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenth RV. Some practical guidelines for effective sample size determination. Am Stat. 2001;55:187–193. [Google Scholar]
- 32.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS Cardiovascular Outcomes Research C. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–715. doi: 10.1016/j.ahj.2004.12.010. [see comment] [DOI] [PubMed] [Google Scholar]
- 33.O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL Investigators H-A. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O'Connor CM, Weinfurt KP. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Havranek EP, Ware MG, Lowes BD. Prevalence of depression in congestive heart failure. Am J Cardiol. 1999;84:348–350. doi: 10.1016/s0002-9149(99)00293-3. [DOI] [PubMed] [Google Scholar]
- 36.O'Connor CM, Jiang W, Kuchibhatla M, Silva SG, Cuffe MS, Callwood DD, Zakhary B, Stough WG, Arias RM, Rivelli SK, Krishnan R. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692–699. doi: 10.1016/j.jacc.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spilker B. Introduction. In: Spilker B, editor. Quality of life assessments in clinical trials. New York: Raven Press; 1990. pp. 3–9. [Google Scholar]
- 38.Leventhal H, Colman S. Quality of life: A process view. Psychol Health. 1997;12:753–767. [Google Scholar]
- 39.Pais-Ribeiro JL. Quality of life is a primary end-point in clinical settings. Clin Nutr. 2004;23:121–130. doi: 10.1016/s0261-5614(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 40.Osoba D. Health-related quality of life and cancer clinical trials. Ther Adv Med Oncol. 2011;3:57–71. doi: 10.1177/1758834010395342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molnar-Varga M, Molnar MZ, Szeifert L, Kovacs AZ, Kelemen A, Becze A, Laszlo G, Szentkiralyi A, Czira ME, Mucsi I, Novak M. Health-related quality of life and clinical outcomes in kidney transplant recipients. Am J Kidney Dis. 2011;58:444–452. doi: 10.1053/j.ajkd.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 42.Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Phillips CO, Hodshon BV, Cooper LS, Krumholz HM. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363:2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.