Abstract
Mast cells (MCs) are tissue resident immune cells that are best known for their roles in allergic and inflammatory diseases. In addition to the high affinity IgE receptor (FcεRI), MCs express numerous G protein coupled receptors (GPCRs), which are the most common targets of drug therapy. Neurokinin 1 receptor (NK-1R) is expressed on MCs and contributes to IgE and non-IgE-mediated responses in mice. Although NK-1R antagonists are highly effective in modulating experimental allergic and inflammatory responses in mice they lack efficacy in humans. This article reviews recent findings that demonstrate that while neuropeptides (NPs) activate murine MCs via NK-1R and Mas related G protein coupled receptor B2 (MrgprB2), they activate human MCs via Mas-related G protein coupled receptor X2 (MRGPRX2). Interestingly, conventional NK-1R antagonists have off-target activity against mouse MrgprB2 but not human MRGPRX2. These findings suggest that the failure to translate studies with NK-1R antagonists from in vivo mouse studies to the clinic likely reflects their lack of effect on human MRGPRX2. A unique feature of MRGPRX2 that distinguishes it from other GPCRs is that it is activated by a diverse group of ligands that include; neuropeptides, cysteine proteases, antimicrobial peptides and cationic proteins released from activated eosinophils. Thus, the development of small molecule MRGPRX2-specific antagonists or neutralizing antibodies may provide new targets for the treatment of MC-mediated allergic and inflammatory diseases.
Keywords: Mast cells, NK-1R, MrgprB2, MRGPRX2, anaphylaxis, asthma
Introduction
Mast cells are multifunctional immune cells that are found in all vascularized tissues throughout the body and contribute to vascular homeostasis, innate/adaptive immunity and wound healing [1]. MCs are, however, best known for their roles in allergic and inflammatory diseases such as anaphylaxis, food allergy, rhinitis, itch, urticaria, atopic dermatitis and asthma. In these conditions, activation of MCs via the cross-linking of high affinity IgE receptors (FcεRI) results in the release of preformed and newly synthesized mediators that contribute to signs and symptoms associated with allergic diseases.
Mast cells are generally classified into two types based on the protease content of their secretory granules [2, 3]. In human, most MCs that are found in connective tissues such as the skin contain tryptase, chymase, carboxypeptidase and cathepsin and are known as MCTC. In contrast, majority of MCs that are found in lung and gut express only tryptase and are known as MCT. MCs also differ in their responses to endogenous and exogenous stimuli that promote degranulation. Thus, while both human MC subtypes are activated via the aggregation of FcεRI, only MCTC respond to the neuropeptide substance P (SP) [4, 5]. In mice, connective tissue MCs (CTMCs) resembles MCTC while mucosal MCs (MMCs) resemble MCT [2, 6]. Thus, murine CTMCs are found in the skin and MMC mature in the mucosal tissues such as the lung and gut. In addition, CTMCs are responsive to SP for degranulation but MMCs are not [7, 8].
Substance P activates a variety of cell types including MCs via NK-1R and induces cutaneous vasodilation, plasma extravagation, flare and itch [8–12]. Accordingly, NK-1R antagonists effectively modulate experimental allergic and inflammatory responses in mice but they lack efficacy in clinical trials [13–17]. This article reviews recent findings which indicate that the reason for this difference may reflect differences in GPCR utilization between mouse and human MCs.
Roles of NK-1R on neuropeptide and IgE-mediated responses in mouse MCs in vitro and allergic responses in vivo
It is generally accepted that MMCs such as murine bone marrow-derived cells (BMMCs) do not express NK-1R [8]. However, BMMCs cultured in the presence of interleukin-4 (IL-4) and stem cell factor (SCF), which favor CTMC phenotype, results in the expression neurokinin 1 receptor (NK-1R). Furthermore, SP causes degranulation in these MCs, which is diminished by an NK-1R antagonist or in cells obtained from NK-1R−/− mice [8]. Subcutaneous injection of SP in mice results in MC degranulation, which is followed by increased vascular permeability and marked infiltration of eosinophils and neutrophils [18, 19]. These responses are absent in MC-deficient mice. However, when MC-deficiency is rescued with local engraftment of cultured MCs, SP-induced responses are restored. These findings suggest that activation of NK-1R in murine MCs by SP results in increased vascular permeability and granulocyte recruitment in vivo.
Substance P belongs to the tachykinin family of neurokinins and this family has recently been expanded to include hemokinin-1 (HK-1) [20]. Unlike SP, which is released from peripheral nerve endings of the sensory neurons, HK-1 is the only tachykinin peptide that is produced outside the neuronal tissue [20]. Sumpter et al., [21] recently demonstrated that FcεRI activation of murine BMMCs results in enhanced expression of both HK-1 and NK-1R without modifying SP levels. Furthermore, it was found that FcεRI-mediated MC degranulation and TNF/IL-6 production is substantially inhibited in NK-1R−/− BMMCs when compared to wild-type MCs. These findings suggest that HK-1, via its action on NK-1R, acts as an autocrine/paracrine factor for FcεRI-mediated MC mediator release. As described below, HK-1/NK-1R axis has a profound impact on IgE-mediated experimental anaphylaxis and MC-dependent model of chronic atopic airway inflammation, an important feature of asthma [21].
Passive cutaneous anaphylaxis (PCA) is often used as an experimental model to study the mechanism of IgE-mediated type 1 hypersensitivity reactions. In this model, FcεRI-mediated histamine release from dermal MCs leads to increased vascular permeability, resulting in the formation of edema. This is followed by the release of TNF from MCs, which promotes the recruitment of neutrophils at the site of MC activation. Using MC-deficient mice reconstituted with wild-type, Tac1−/− (HK-1) or NK-1R−/− BMMCs, Sumpter et al., [21] showed that HK-1 and NK-1R expressed in murine MCs contribute to both early (edema) and late (neutrophil recruitment) phases of IgE-mediated PCA. In a mouse model of chronic allergic asthma, TNF derived from MCs contribute to Th2/Th17 cytokine production, airway hyperresponsiveness and inflammation [22–24]. Given that FcεRI-mediated TNF production is substantially inhibited in NK-1R−/− BMMCs when compared to wild-type MCs raises the interesting possibility that this GPCR is important for the pathogenesis of allergic asthma. Indeed, absence of Tac1 or NK-1R in MCs leads to substantial decrease in eosinophil and neutrophil recruitment in a MC-dependent model of chronic asthma [21]. These findings suggest that HK-1 and its receptor NK-1R, which are upregulated following FcεRI activation, contribute to both anaphylaxis (Fig. 1A) and chronic allergic asthma in mice (Fig. 1B).
Figure 1. Proposed model for the roles of NK-1R and MrgprB2 on neuropeptide and IgE-mediated responses in mice.
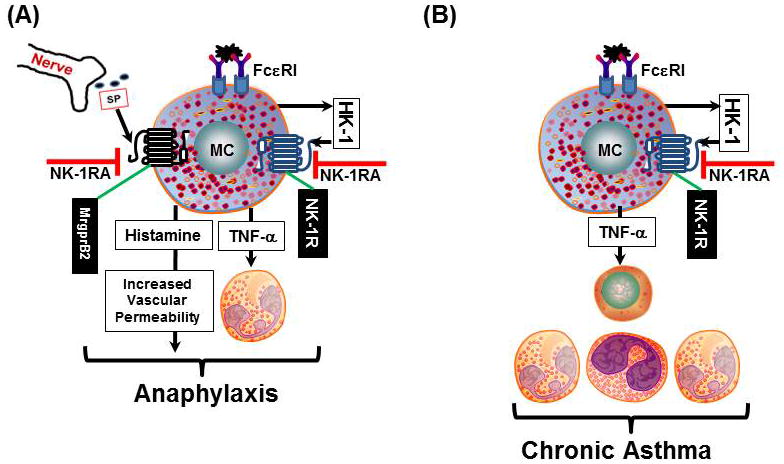
SP released from nerve endings and HK-1 generated from MCs activate NK-1R and MrgprB2 on MCs to induce degranulation and TNF production. These mediators contribute to increased vascular permeability and leukocyte recruitment in (A) anaphylaxis and (B) chronic asthma. It is proposed that conventional NK-1R antagonists modulate these responses in mice by inhibiting both NK-1R and MrgprB2.
Role of MrgprB2 on SP-induced degranulation of murine MCs
In addition to NK-1R, murine CTMCs express Mas-related G protein coupled receptor B2 (MrgprB2) [25]. Generation of a transgenic mouse with td-Tomato reporter under the control of MrgprB2 promoter demonstrated that the expression of this receptor is restricted to CTMC in the skin, gut and trachea [25]. MrgprB2 mutant mice were generated by deleting a 4 base pair in the receptor’s coding region, resulting in a frame shift mutation and early termination of the receptor (shortly after the first transmembrane domain). These mice have no defect in MC number and respond normally to IgE/FcεRI activation. However, peritoneal MCs from these mice show dramatic reduction in degranulation in response to SP in vitro and reduced inflammation in vivo [25, 26]. These findings suggest that IgE and NP-mediated responses in murine CTMCs reflect the activation of two GPCRs; NK-1R and MrgprB2 (Fig 1A).
Conventional NK-1R antagonists block MrgprB2-mediated responses in mice but lack efficacy in humans
Although it is currently unknown if HK-1 level is increased following the activation of human MCs, the expression of SP and NK-1R is upregulated in human asthmatic but not in normal individuals [13, 14, 27, 28]. These findings suggest that NK-1R not only participates in experimental MC-mediated allergic responses, it also plays an important role in the human disease. Not surprisingly, a number of NK-1R antagonists have been developed to modulate allergic responses in animal models and for clinical trials in humans [13–16]. These antagonists are highly effective in animal models of allergic asthma and inflammation but lack efficacy in humans. Azimi et al, [17] recently showed that conventional NK-1R antagonists have off-target effect on the mouse MrgprB2. This suggests that the effectiveness of NK-1R antagonists in mice reflect not only their ability to block NK-1R but also their action against MrgprB2 (Fig. 1). The striking failure to translate findings from animal models to the clinic suggests that there are important differences in the mechanisms via which NPs activate murine and human MCs.
Substance P activates human MCTC via Mas-related G protein coupled receptor-X2 (MRGPRX2)
Similar to the situation in murine CTMCs, human skin MCTC and human MC line (LAD2) express NK-1R and respond to SP for degranulation [29, 30]. However, unlike the case with murine CTMCs, NK-1R antagonists have little or no effect on SP-induced response in human MCs [30]. The recent demonstration that MRGPRX2 is the human counterpart of mouse MrgprB2 raises the possibility that SP activates human MCs via this receptor. Tatemoto et al., [31] provided the first demonstration that SP activates human cord blood-derived MCs via MRGPRX2 and this finding was later confirmed in studies with transfected RBL-2H3 cells and primary human skin MCs [1, 30, 32]. Fujisawa et al., [30] showed that skin MCs of patients with severe chronic urticaria express MRGPRX2 at higher level than the healthy subjects. Furthermore, SP causes degranulation and prostaglandin D2 (PGD2) generation in human skin MCs via MRGPRX2. Eosinophils accumulate in chronic urticaria lesions and their granules proteins (major basic protein, MBP and eosinophil peroxidase, EPO) cause MC degranulation via MRGPRX2 [30, 33]. These findings suggest that activation of human MCs by SP via MRGPRX2 serves to initiate the wheal response. Furthermore, recruitment of eosinophils and the subsequent activation of MRGPRX2 by MBP and EPO contribute to the late phase of chronic urticaria (Fig. 2A).
Figure 2. Proposed model for the roles of human MRGPRX2 on chronic urticaria and asthma.
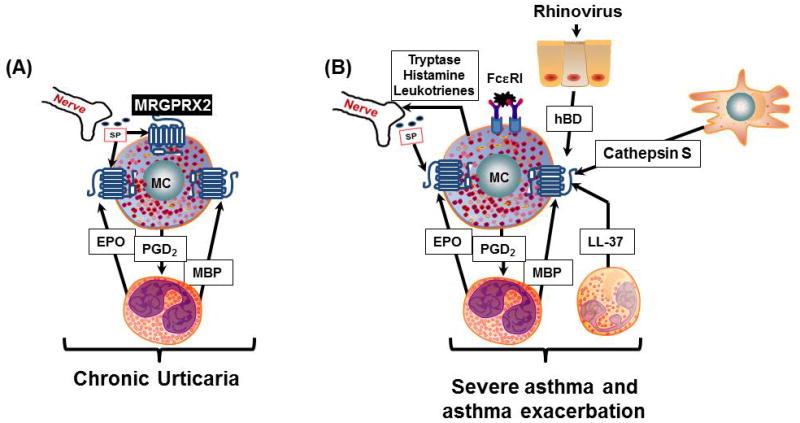
(A): In chronic urticaria, SP released from nerve endings activate skin MCTC via MRGPRX2 to induce degranulation and PGD2 production. The MC-mediated response is further amplified via MRGPRX2-mediated degranulation induced by eosinophil-derived proteins, EPO and MBP. (B): MCTC are recruited to the lung of patients with severe asthma. FcεRI-mediated MC activation leads to the release of mediators (tryptase, histamine and leukotrienes), which stimulate the production of SP from nerve endings. Activation of MRGPRX2 on lung MCTC by SP and EPO/MBP likely contributes to the development of severe asthma. Cathepsin S released from antigen-presenting cells may also contribute to asthma severity by activating lung MCTC via MRGPRX2. It is proposed that human β-defensins (hBD) released from rhinovirus-activated epithelial cells, EPO/MBP from eosinophils and LL-37 from neutrophils induce mediator release from human lung MCTC via MRGPRX2 to cause asthma exacerbation.
Possible roles of MRGPRX2 in asthma and asthma exacerbation
Mast cells are important effector cells that orchestrate the development of airway hyperresponsiveness and inflammation in asthma via their close interaction with airway smooth muscle cells, T cells and leukocytes [34–36]. Microarray and PCR analyses showed that while high level of MRGPRX2 transcript is expressed in human skin MCs, only low level is present in lung MCs [30, 37]. Mild allergic asthma is generally correlated with an increase in MCT number in both submucosa and smooth muscle but severe asthma is dominated by the presence of MCTC, a MC subtype that expresses MRGPRX2 [38, 39]. MC-derived mediators such as tryptase, histamine and cysteinyl leukotrienes interact with neurons to promote the release of neuropeptides such as SP [40]. The level of SP is elevated in the lung of asthmatics when compared normal lung [27, 28]. Furthermore, SP causes degranulation of MCs obtained from bronchoalveolar lavage indicating a potential for MRGPRX2 in severe asthma [41]. Cathepsin S is a cysteine protease released from antigen-presenting cells and contributes to the pathogenesis of asthma [42–44]. Reddy et al., [45] recently showed that cathepsin S induces Ca2+ mobilization in HeLa cells expressing MRGPRX2. These findings suggest that both cathepsin S and SP contribute to the pathogenesis of severe asthma via the activation of MRGPRX2 in lung MCTC (Fig. 2B).
Respiratory infection by rhinoviruses causes asthma exacerbation in both children and adults. This is associated with MC degranulation and the recruitment of eosinophils and neutrophils to the airways [46–49]. Lung epithelial cells are the principal site of rhinovirus infection in both the upper and lower airways. Interestingly, rhinovirus induces the production of human β-defensins in bronchial epithelial cells [50, 51], which activate human MCs via MRGPRX2 [1, 52, 53]. Neutrophils present in the lung of patients with rhinovirus-induced asthma also secrete the cathelicidin, LL-37, which activates human MCTC via MRGPRX2 [52, 54]. Thus, it is possible that MRGPRX2 expressed in human lung MCTC contributes to rhinovirus-induced asthma exacerbation by responding to ligands generated from epithelial cells (β-defensins) [50, 51], eosinophils (MBP and EPO) [30] and neutrophils (LL-37) (Fig. 2B).
A tripeptide NK-1R antagonist inhibits human MC activation via MRGPRX2
A unique feature of MRGPRX2 that distinguishes it from other GPCRs is that it is activated by multiple cationic ligands and may play important role in a variety of MC-mediated diseases (Fig. 2) [1, 25, 30]. Thus, the development of small molecule MRGPRX2-specific antagonists or neutralizing antibodies may provide potential new therapeutic target for modulating MC-mediated allergic and inflammatory diseases. Azimi et al, [17] showed that a while conventional NK-1R antagonist blocks both NK-1R and MrgprB2, a tripeptide (QWF) NK-1R antagonist also has activity against human MRGPRX2. Thus, QWF inhibits SP-induced responses in HEK-293 cells stably expressing MRGPRX2 and a human MC line (LAD2) naturally expressing the receptor. These findings suggest that while NPs activate murine MCs via NK-1R and MrgprB2 they utilize MRGPRX2 for human MC activation. This difference likely explains why NK-1R antagonists modulate MC-mediated experimental allergic responses in mice but lack efficacy in humans. The tripeptide QWF is, however, unlikely to serve as an MRGPRX2-specific drug in the clinic because it lacks specificity for MRGPRX2 and it is rapidly metabolized in plasma [55]. Despite the potential limitations of QWF, its inhibitory effect on MRGPRX2-mediated MC degranulation provides a strong impetus for the development of specific small molecule MRGPRX2 antagonists or monoclonal antibodies for the treatment of MC-mediated diseases.
Conclusions and future direction
Neuropeptides activate murine MCs via NK-1R and MrgprB2 and human MCs via MRGPRX2. Conventional NK-1R antagonists have off target activity against mouse MrgprB2 but not human MRGPRX2. This difference likely explains why conventional NK-1R antagonists modulate experimental allergic responses in mice but lack efficacy in humans [17]. It is noteworthy that MRGPRX2 displays only ~20 and ~50% sequence identity with NK -1R and MrgprB2, respectively. Despite this difference, NK-1R, MrgprB2 and MRGPRX2 are all responsive to SP and the tripeptide NK-1R antagonist (QWF) blocks SP responses to all three receptors [17]. These finding suggest that all three receptors possess specific binding sites for both SP and QWF. Site directed mutagenesis studies of NK-1R demonstrated that amino acid residues 23–25 (NQF) in the receptor’s amino terminus along with H108 (third transmembrane domain), F268 (sixth transmembrane domain), Y287 (7th transmembrane domain) are important for binding substance P [56–58]. Furthermore, histidine at position 197 (H197) in the fifth transmembrane helix of human NK-1R binds specifically to the non-peptide receptor antagonist CP 96345 to block SP action [59]. Thus, identification of SP and QWF binding sites on MRGPRX2 may provide useful information for the development of MRGPRX2-specific antagonists.
Acknowledgments
This work was supported by NIH grants R21-AI108585 and R01-AI124182. I thank Kshitij Gupta, Ph.D. for careful review of this manuscript.
Abbreviations used
- FcεRI
high affinity IgE receptor
- GPCR
G protein coupled receptor
- NK 1R
neurokinin-1 receptor
- MrgprB2
Mas-related G protein coupled receptor-B2
- MRGPRX2
Mas-related G protein coupled receptor-X2
- MC
mast cell
- NP
neuropeptide
- HK-1
hemokinin-1
- SP
Substance P
References
- 1.Subramanian H, Gupta K, Ali H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J Allergy Clin Immunol. 2016;138:700–710. doi: 10.1016/j.jaci.2016.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurish MF, Austen KF. Developmental origin and functional specialization of mast cell subsets. Immunity. 2012;37:25–33. doi: 10.1016/j.immuni.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Irani AM, Bradford TR, Kepley CL, Schechter NM, Schwartz LB. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J Histochem Cytochem. 1989;37:1509–1515. doi: 10.1177/37.10.2674273. [DOI] [PubMed] [Google Scholar]
- 4.Oskeritzian CA, Zhao W, Min HK, Xia HZ, Pozez A, Kiev J, Schwartz LB. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J Allergy Clin Immunol. 2005;115:1162–1168. doi: 10.1016/j.jaci.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukuoka Y, Xia HZ, Sanchez-Munoz LB, Dellinger AL, Escribano L, Schwartz LB. Generation of anaphylatoxins by human beta-tryptase from C3, C4, and C5. J Immunol. 2008;180:6307–6316. doi: 10.4049/jimmunol.180.9.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruitenberg EJ, Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature. 1976;264:258–260. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- 7.Manning BM, Gruba SM, Meyer AF, Haynes CL. Neuropeptide-induced mast cell degranulation and characterization of signaling modulation in response to IgE conditioning. ACS Chem Biol. 2016:3077–3083. doi: 10.1021/acschembio.6b00616. [DOI] [PubMed] [Google Scholar]
- 8.van der Kleij HP, Ma D, Redegeld FA, Kraneveld AD, Nijkamp FP, Bienenstock J. Functional expression of neurokinin 1 receptors on mast cells induced by IL-4 and stem cell factor. J Immunol. 2003;171:2074–2079. doi: 10.4049/jimmunol.171.4.2074. [DOI] [PubMed] [Google Scholar]
- 9.Lotts T, Stander S. Research in practice: substance P antagonism in chronic pruritus. J Dtsch Dermatol Ges. 2014;12:557–559. doi: 10.1111/ddg.12364. [DOI] [PubMed] [Google Scholar]
- 10.Remrod C, Lonne-Rahm S, Nordlind K. Study of substance P and its receptor neurokinin-1 in psoriasis and their relation to chronic stress and pruritus. Arch Dermatol Res. 2007;299:85–91. doi: 10.1007/s00403-007-0745-x. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. Journal of cellular physiology. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 12.Hossen MA, Inoue T, Shinmei Y, Fujii Y, Watanabe T, Kamei C. Role of substance P on histamine H3 antagonist-induced scratching behavior in mice. J Pharmacol Sci. 2006;100:297–302. doi: 10.1254/jphs.fpj05028x. [DOI] [PubMed] [Google Scholar]
- 13.Hens G, Raap U, Vanoirbeek J, Meyts I, Callebaut I, Verbinnen B, Vanaudenaerde BM, Cadot P, Nemery B, Bullens DM, et al. Selective nasal allergen provocation induces substance P-mediated bronchial hyperresponsiveness. Am J Respir Cell Mol Biol. 2011;44:517–523. doi: 10.1165/rcmb.2009-0425OC. [DOI] [PubMed] [Google Scholar]
- 14.Boot JD, de Haas S, Tarasevych S, Roy C, Wang L, Amin D, Cohen J, Sterk PJ, Miller B, Paccaly A, et al. Effect of an NK1/NK2 receptor antagonist on airway responses and inflammation to allergen in asthma. Am J Respir Crit Care Med. 2007;175:450–457. doi: 10.1164/rccm.200608-1186OC. [DOI] [PubMed] [Google Scholar]
- 15.Rost K, Fleischer F, Nieber K. Neurokinin 1 receptor antagonists--between hope and disappointment. Med Monatsschr Pharm. 2006;29:200–205. [PubMed] [Google Scholar]
- 16.Quartara L, Altamura M, Evangelista S, Maggi CA. Tachykinin receptor antagonists in clinical trials. Expert Opin Investig Drugs. 2009;18:1843–1864. doi: 10.1517/13543780903379530. [DOI] [PubMed] [Google Scholar]
- 17.Azimi E, Reddy VB, Shade KC, Anthony RM, Talbot S, Pereira PJ, Lerner EA. Dual action of neurokinin-1 antagonists on Mas-related GPCRs. JCI Insight. 2016;1:e89362. doi: 10.1172/jci.insight.89362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuda H, Kawakita K, Kiso Y, Nakano T, Kitamura Y. Substance P induces granulocyte infiltration through degranulation of mast cells. J Immunol. 1989;142:927–931. [PubMed] [Google Scholar]
- 19.Yano H, Wershil BK, Arizono N, Galli SJ. Substance P-induced augmentation of cutaneous vascular permeability and granulocyte infiltration in mice is mast cell dependent. J Clin Invest. 1989;84:1276–1286. doi: 10.1172/JCI114295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Lu L, Furlonger C, Wu GE, Paige CJ. Hemokinin is a hematopoietic-specific tachykinin that regulates B lymphopoiesis. Nat Immunol. 2000;1:392–397. doi: 10.1038/80826. [DOI] [PubMed] [Google Scholar]
- 21.Sumpter TL, Ho CH, Pleet AR, Tkacheva OA, Shufesky WJ, Rojas-Canales DM, Morelli AE, Larregina AT. Autocrine hemokinin-1 functions as an endogenous adjuvant for IgE-mediated mast cell inflammatory responses. J Allergy Clin Immunol. 2015;135:1019–1030. e1018. doi: 10.1016/j.jaci.2014.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, Galli SJ. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. J Allergy Clin Immunol. 2007;120:48–55. doi: 10.1016/j.jaci.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 23.Nakae S, Lunderius C, Ho LH, Schafer B, Tsai M, Galli SJ. TNF can contribute to multiple features of ovalbumin-induced allergic inflammation of the airways in mice. J Allergy Clin Immunol. 2007;119:680–686. doi: 10.1016/j.jaci.2006.11.701. [DOI] [PubMed] [Google Scholar]
- 24.Nakae S, Suto H, Berry GJ, Galli SJ. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood. 2007;109:3640–3648. doi: 10.1182/blood-2006-09-046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519:237–241. doi: 10.1038/nature14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaudenzio N, Sibilano R, Marichal T, Starkl P, Reber LL, Cenac N, McNeil BD, Dong X, Hernandez JD, Sagi-Eisenberg R, et al. Different activation signals induce distinct mast cell degranulation strategies. J Clin Invest. 2016;126:3981–3998. doi: 10.1172/JCI85538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nieber K, Baumgarten CR, Rathsack R, Furkert J, Oehme P, Kunkel G. Substance P and β-endorphin-like immunoreactivity in lavage fluids of subjects with and without allergic asthma. J Allergy Clin Immunol. 1992;90:646–652. doi: 10.1016/0091-6749(92)90138-r. [DOI] [PubMed] [Google Scholar]
- 28.Tomaki M, Ichinose M, Miura M, Hirayama Y, Yamauchi H, Nakajima N, Shirato K. Elevated substance P content in induced sputum from patients with asthma and patients with chronic bronchitis. Am J Respir Crit Care Med. 1995;151:613–617. doi: 10.1164/ajrccm.151.3.7533601. [DOI] [PubMed] [Google Scholar]
- 29.Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123:398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, Kuroda K, Nunomura S, Hayama K, Terui T, et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol. 2014;134:622–633. e629. doi: 10.1016/j.jaci.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, Ogasawara H, Edamura K, Takagi H, Iwamura H, et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun. 2006;349:1322–1328. doi: 10.1016/j.bbrc.2006.08.177. [DOI] [PubMed] [Google Scholar]
- 32.Subramanian H, Kashem SW, Collington SJ, Qu H, Lambris JD, Ali H. PMX-53 as a dual CD88 antagonist and an agonist for Mas-related gene 2 (MrgX2) in human mast cells. Mol Pharmacol. 2011;79:1005–1013. doi: 10.1124/mol.111.071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabroe RA, Poon E, Orchard GE, Lane D, Francis DM, Barr RM, Black MM, Black AK, Greaves MW. Cutaneous inflammatory cell infiltrate in chronic idiopathic urticaria: comparison of patients with and without anti-FcεRI or anti-IgE autoantibodies. J Allergy Clin Immunol. 1999;103:484–493. doi: 10.1016/s0091-6749(99)70475-6. [DOI] [PubMed] [Google Scholar]
- 34.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 35.Page S, Ammit AJ, Black JL, Armour CL. Human mast cell and airway smooth muscle cell interactions: implications for asthma. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1313–1323. doi: 10.1152/ajplung.2001.281.6.L1313. [DOI] [PubMed] [Google Scholar]
- 36.Robinson DS. The role of the mast cell in asthma: induction of airway hyperresponsiveness by interaction with smooth muscle? J Allergy Clin Immunol. 2004;114:58–65. doi: 10.1016/j.jaci.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 37.Kajiwara N, Sasaki T, Bradding P, Cruse G, Sagara H, Ohmori K, Saito H, Ra C, Okayama Y. Activation of human mast cells through the platelet-activating factor receptor. J Allergy Clin Immunol. 2010;125:1137–1145. e1136. doi: 10.1016/j.jaci.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 38.Fajt ML, Gelhaus SL, Freeman B, Uvalle CE, Trudeau JB, Holguin F, Wenzel SE. Prostaglandin D2 pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol. 2013;131:1504–1512. doi: 10.1016/j.jaci.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balzar S, Fajt ML, Comhair SA, Erzurum SC, Bleecker E, Busse WW, Castro M, Gaston B, Israel E, Schwartz LB, et al. Mast cell phenotype, location, and activation in severe asthma. Data from the Severe Asthma Research Program. Am J Respir Crit Care Med. 2011;183:299–309. doi: 10.1164/rccm.201002-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Diest SA, Stanisor OI, Boeckxstaens GE, de Jonge WJ, van den Wijngaard RM. Relevance of mast cell-nerve interactions in intestinal nociception. Biochim Biophys Acta. 2012;1822:74–84. doi: 10.1016/j.bbadis.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 41.Heaney LG, Cross LJ, Stanford CF, Ennis M. Substance P induces histamine release from human pulmonary mast cells. Clin Exp Allergy. 1995;25:179–186. doi: 10.1111/j.1365-2222.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- 42.Riese RJ, Mitchell RN, Villadangos JA, Shi GP, Palmer JT, Karp ER, De Sanctis GT, Ploegh HL, Chapman HA. Cathepsin S activity regulates antigen presentation and immunity. J Clin Invest. 1998;101:2351–2363. doi: 10.1172/JCI1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou PP, Zhang WY, Li ZF, Chen YR, Kang XC, Jiang YX. Association between SNPs in the promoter region in cathepsin S and risk of asthma in Chinese Han population. Eur Rev Med Pharmacol Sci. 2016;20:2070–2076. [PubMed] [Google Scholar]
- 44.Deschamps K, Cromlish W, Weicker S, Lamontagne S, Huszar SL, Gauthier JY, Mudgett JS, Guimond A, Romand R, Frossard N, et al. Genetic and pharmacological evaluation of cathepsins in a mouse model of asthma. Am J Respir Cell Mol Biol. 2011;45:81–87. doi: 10.1165/rcmb.2009-0392OC. [DOI] [PubMed] [Google Scholar]
- 45.Reddy VB, Sun S, Azimi E, Elmariah SB, Dong X, Lerner EA. Redefining the concept of protease-activated receptors: cathepsin S evokes itch via activation of Mrgprs. Nat Commun. 2015;6:7864. doi: 10.1038/ncomms8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Busse WW, Gern JE, Dick EC. The role of respiratory viruses in asthma. Ciba Found Symp. 1997;206:208–213. doi: 10.1002/9780470515334.ch13. discussion 213–209. [DOI] [PubMed] [Google Scholar]
- 47.Calhoun WJ, Swenson CA, Dick EC, Schwartz LB, Lemanske RF, Jr, Busse WW. Experimental rhinovirus 16 infection potentiates histamine release after antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis. 1991;144:1267–1273. doi: 10.1164/ajrccm/144.6.1267. [DOI] [PubMed] [Google Scholar]
- 48.Zhu J, Message SD, Qiu Y, Mallia P, Kebadze T, Contoli M, Ward CK, Barnathan ES, Mascelli MA, Kon OM, et al. Airway inflammation and illness severity in response to experimental rhinovirus infection in asthma. Chest. 2014;145:1219–1229. doi: 10.1378/chest.13-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rohde G, Message SD, Haas JJ, Kebadze T, Parker H, Laza-Stanca V, Khaitov MR, Kon OM, Stanciu LA, Mallia P, et al. CXC-chemokines and antimicrobial peptides in rhinovirus-induced experimental asthma exacerbations. Clin Exp Allergy. 2014;44:930–939. doi: 10.1111/cea.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duits LA, Nibbering PH, van Strijen E, Vos JB. Mannesse-Lazeroms SP, van Sterkenburg MA, Hiemstra PS: Rhinovirus increases human β-defensin-2 and -3 mRNA expression in cultured bronchial epithelial cells. FEMS Immunol Med Microbiol. 2003;38:59–64. doi: 10.1016/S0928-8244(03)00106-8. [DOI] [PubMed] [Google Scholar]
- 51.Proud D, Sanders SP, Wiehler S. Human rhinovirus infection induces airway epithelial cell production of human β-defensin 2 both in vitro and in vivo. J Immunol. 2004;172:4637–4645. doi: 10.4049/jimmunol.172.7.4637. [DOI] [PubMed] [Google Scholar]
- 52.Subramanian H, Gupta K, Guo Q, Price R, Ali H. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: resistance to receptor phosphorylation, desensitization, and internalization. J Biol Chem. 2011;286:44739–44749. doi: 10.1074/jbc.M111.277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Subramanian H, Gupta K, Lee D, Bayir AK, Ahn H, Ali H. β-Defensins activate human mast cells via Mas-related gene X2. J Immunol. 2013;191:345–352. doi: 10.4049/jimmunol.1300023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta K, Subramanian H, Ali H. Modulation of host defense peptide-mediated human mast cell activation by LPS. Innate Immun. 2016;22:21–30. doi: 10.1177/1753425915610643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hagiwara D, Miyake H, Morimoto H, Murai M, Fujii T, Matsuo M. Studies on neurokinin antagonists. 1. The design of novel tripeptides possessing the glutaminyl-D-tryptophylphenylalanine sequence as substance P antagonists. J Med Chem. 1992;35:2015–2025. doi: 10.1021/jm00089a011. [DOI] [PubMed] [Google Scholar]
- 56.Fong TM, Yu H, Huang RR, Strader CD. The extracellular domain of the neurokinin-1 receptor is required for high-affinity binding of peptides. Biochemistry. 1992;31:11806–11811. doi: 10.1021/bi00162a019. [DOI] [PubMed] [Google Scholar]
- 57.Fong TM, Huang RR, Yu H, Strader CD. Mapping the ligand binding site of the NK-1 receptor. Regul Pept. 1993;46:43–48. doi: 10.1016/0167-0115(93)90010-6. [DOI] [PubMed] [Google Scholar]
- 58.Valentin-Hansen L, Park M, Huber T, Grunbeck A, Naganathan S, Schwartz TW, Sakmar TP. Mapping substance P binding sites on the neurokinin-1 receptor using genetic incorporation of a photoreactive amino acid. J Biol Chem. 2014;289:18045–18054. doi: 10.1074/jbc.M113.527085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fong TM, Cascieri MA, Yu H, Bansal A, Swain C, Strader CD. Amino-aromatic interaction between histidine 197 of the neurokinin-1 receptor and CP 96345. Nature. 1993;362:350–353. doi: 10.1038/362350a0. [DOI] [PubMed] [Google Scholar]


