Abstract
Metformin is the mainstay therapy for type 2 diabetes (T2D) and many patients also take salicylate-based drugs [i.e., aspirin (ASA)] for cardioprotection. Metformin and salicylate both increase AMP-activated protein kinase (AMPK) activity but by distinct mechanisms, with metformin altering cellular adenylate charge (increasing AMP) and salicylate interacting directly at the AMPK β1 drug-binding site. AMPK activation by both drugs results in phosphorylation of ACC (acetyl-CoA carboxylase; P-ACC) and inhibition of acetyl-CoA carboxylase (ACC), the rate limiting enzyme controlling fatty acid synthesis (lipogenesis). We find doses of metformin and salicylate used clinically synergistically activate AMPK in vitro and in vivo, resulting in reduced liver lipogenesis, lower liver lipid levels and improved insulin sensitivity in mice. Synergism occurs in cell-free assays and is specific for the AMPK β1 subunit. These effects are also observed in primary human hepatocytes and patients with dysglycaemia exhibit additional improvements in a marker of insulin resistance (proinsulin) when treated with ASA and metformin compared with either drug alone. These data indicate that metformin–salicylate combination therapy may be efficacious for the treatment of non-alcoholic fatty liver disease (NAFLD) and T2D.
Keywords: aspirin, acetyl-CoA carboxylase, salsalate, non-alcoholic fatty liver disease, type 2 diabetes
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is estimated to affect nearly 40 %of all adults and more than 80 %of those with type 2 diabetes (T2D) [1]. Elevated rates of liver fatty acid synthesis (de novo lipogenesis) cause NAFLD in humans [2] and NAFLD may promote the development of T2D by reducing liver insulin sensitivity [3]. Therefore pharmacological strategies aimed at inhibiting liver lipogenesis may be effective to reduce or reverse the prevalence of NAFLD and improve insulin sensitivity.
AMP-activated protein kinase (AMPK) is an αβγ heterotrimer that inhibits a rate limiting step of liver fatty acid synthesis through inhibitory phosphorylation of acetyl-CoA carboxylase 1 and 2 [ACC1 and ACC2; P-ACC); 4,5]. Importantly, mice deficient in the AMPK phosphorylation sites on ACC accumulate enhanced levels of liver fat and develop insulin resistance [6], indicating the importance of this pathway in maintaining metabolic homoeostasis. Metformin and salicylate [the active metabolite of aspirin (ASA) and salsalate] both activate AMPK, albeit by different mechanisms: metformin increases AMPK activity indirectly by inhibiting mitochondrial respiration and increasing AMP levels [7], whereas salicylate-induced activation requires the AMPK β1 subunit [8]. Metformin-and salicylate-based therapeutics (ASA and salsalate) improve liver insulin sensitivity [9–11], effects which may involve the activation of AMPK and subsequent P-ACC [6,8].
Given that metformin and salicylate activate AMPK via distinct mechanisms, we hypothesized that co-treatment using these drugs would synergistically increase liver AMPK activity/P-ACC, reduce lipogenesis and improve insulin sensitivity. In the present study, we provide novel evidence that low, clinically-relevant doses of metformin and salicylate synergistically activate AMPK to inhibit lipogenesis in primary hepatocytes from mice and humans. Synergistic activation of AMPK occurs in cell-free assays and is dependent on the expression of the AMPK β1 isoform. Importantly, combination therapy also improves glucose tolerance, hepatic insulin sensitivity and reduces the accumulation of liver fat in high fat diet (HFD)-fed mice whereas lowering pro-insulin levels (a marker of insulin secretion/sensitivity [12]) in over 8000 human subjects with dysglycaemia over the effects of either drug alone.
MATERIALS AND METHODS
Experimental design
Male C57bl/6 mice were maintained on a 12-h light dark cycle (lights on at 7.00 am) and housed in a pathogen-free facility at 23 °C with bedding enrichment. At 8 weeks of age, mice were either killed to obtain primary hepatocytes or switched from a standard chow diet to a HFD [60 % kcal (1 cal ≡ 4.184 J) from fat, diet D12492 from Research Diets] for 5 weeks. At 13 weeks of age, glucose tolerance tests (GTTs; described in detail below) were performed to confirm a metabolically compromised phenotype (result not shown) and animals were assigned to four groups matched for weight and glucose tolerance. Since we were specifically interested in testing the effects of the low-dose metformin and/or salsalate in obesity, we did not study control chow-fed mice. Importantly, the HFD-fed mice used in the current study had comparable levels of obesity/adiposity, glucose tolerance, insulin resistance and liver triglycerides (TG) compared with those of our recent studies in which both chow and HFD-mice were studied [6,13]. As dictated by design, mice that were not obese (<32 g) after the initial 5 weeks of HFD were excluded from the study and all further analyses. The four groups were then randomly assigned to begin feeding of HFD containing no drug [control], 2.5 g/kg metformin, 1 g/kg salsalate or both 2.5 g/kg metformin and 1 g/kg salsalate (metformin + salsalate) for an additional 5 weeks.
Study approval
All animal procedures were approved by the McMaster University Animal Ethics Research Board (AUP #: 12–12-44). Human results were obtained from data collected during the Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial (NCT00069784, [14]), which was approved a priori by the ethics committee at each site. Written informed consent was obtained from all participants prior to inclusion in the study.
Primary cell culture
Mouse primary hepatocytes were obtained from C57bl/6 mice by collagenase digestion, as previously described [6,15]. For human primary hepatocyte experiments, cryopreserved primary human hepatocytes (Triangle Research Labs) were thawed, centrifuged and re-suspended in complete William’s E medium (11 mM glucose) containing 10 % FBS and plated in 24-well collagen coated plates at 0.35 × 106 cells/well and allowed to adhere for 4 h. Donors of cryopreserved human hepatocytes were male [age 46, body mass index (BMI) 23.5] and female (age 59, BMI 29), were non-drug users (including alcohol), non-smokers. All experiments were performed the following morning after hepatocytes were serum starved for 3- (mouse) or 18- (human) h in the William’s E medium (11 mM glucose). For [3H]-acetate fatty acid synthesis, cells were treated with no drug, metformin, salicylate or metformin + salicylate, [3H]-acetate (5 μCi/μl) and sodium acetate (0.5 mM) for 4 h in serum-free William’s E medium (11 mM glucose) as described [6]. Medium was then removed and cells were washed twice with PBS before extraction of total lipids using chloroform–methanol [16]. Drug synergy in fatty acid synthesis experiments was calculated using the Chou–Talalay method and CompuSyn software [17,18]. [14C]-palmitate fatty acid oxidation was performed as described previously [6], with minor modifications including pre-treatment of hepatocytes for 1 h with drugs followed by 4 h treatment with 200 μM palmitate (complexed with 2 % BSA, with radiolabel at 0.5 μCi/ml), 500 μM L-carnitine and drugs in serum-free media before the cells and media were collected for the assay. For Western blots, cells were treated with vehicle or drugs for 2 h. Medium was then removed and cell lysis buffer applied to cells rapidly on ice and plates snap frozen on liquid nitrogen, as described previously [19]. Lysates were stored at − 80 °C for further analyses.
AMPK activity assay
Heterotrimeric human AMPK GST-α1β1γ1 was expressed by transient transfection of COS7 cells cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10 % FBS as described previously [20]. Transfected cells were harvested by washing with ice-cold PBS followed by rapid lysis in situ using 1 ml of lysis buffer [50 mM Tris/HCl, pH 7.4, 150 mM NaCl, 50 mM NaF, 1 mM NaPPi, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 % (v/v) Triton X-100]. Cellular debris was removed by centrifugation, after which AMPK was isolated from the lysates on glutathione Sepharose 4B (GE Life Sciences), extensively washed with lysis buffer and then dephosphorylated with λ-phosphatase (2 mM MnCl2, 2 h, 22 °C) prior to elution in storage buffer 50 mM Tris/HCl (pH 7.4), 150 mM NaCl, 10 % glycerol, 2 mM TCEP [tris(2-carboxyethyl)phosphine], 20 mM reduced glutathione. For AMPK α1β1γ1 and α1β2γ1 expressed in bacteria, all procedures were as described previously [20].
AMPK activity was determined by phosphorylation of the SAMS peptide (HMRSAMSGLHLVKRR) using 100 μM SAMS, 200 μM [γ-32P] ATP, 5 mM MgCl2 and indicated ligands in 25 μl of reaction volume at 30 °C. Reactions were terminated after 10 min by spotting 15 μl on to P81 phosphocellulose paper (Whatman) and washing in 1 % phosphoric acid [21]. Radioactivity was quantified by scintillation counting.
Metabolic studies
GTTs and insulin tolerance tests (ITTs) were performed by intraperitoneal injection of glucose (0.8 g/kg body weight) or human insulin (1 unit insulin/kg body weight) respectively and blood glucose measured by Aviva blood glucose monitor (Roche) from a small tail vein nick at the time points indicated. Mice were fasted for 6 h prior to all tolerance tests. Respiratory exchange ratio (RER) was assessed using a Columbus Laboratory Animal Monitoring System [22]. Whole body adiposity was measured using computed tomography [22]. Fasting (12 h) and fed blood samples were collected by tail vein bleed for serum measurements. Commercially available ELISA kits were used to measure serum salicylate (Neogen) and insulin [Millipore (rodent) and Mercodia (human)]. Serum metformin levels were measured by MS using a PE/Sciex 3000 LC–MS/MS system after solvent extraction. Phenformin was used as an internal standard and metformin was eluted at 3.5 min on the chromatograph.
Hyperinsulinaemic–euglycaemic clamps
Hyperinsulinaemic–euglycaemic clamps were performed as previously described [6]. Briefly, 5 days after cannulating the right jugular vein, mice were fasted for 6 h then infused with D-[3-3H]-glucose for 1 h for assessment of basal glucose disposal. A human insulin infusate containing D-[3-3H]-glucose was then applied and blood glucose monitored and titrated with 50 %dextrose infused at a variable rate to achieve and maintain euglycaemia. Basal and clamped glucose disposal rates (GDRs) and hepatic glucose production (HGP) rates were calculated as previously stated [6]. All tissues were rapidly dissected, snap frozen in liquid nitrogen and stored at − 80 °C for later analyses.
Immunoblotting, histological and biochemical analyses
Tissue or cell lysates were diluted with Western sample buffer and loaded in SDS/PAGE (7.5 % gels) as previously described [6]. After resolution of proteins by molecular mass, proteins were transferred to polyvinylidene difluoride membranes and blocked in 5 %BSA. All primary antibodies were obtained from Cell Signaling Technologies [P-ACC Ser79/212, ACC, β-actin, P-Akt/PKB (protein kinase B) Ser473, P-Akt/PKB Thr308, Akt/PKB] and used at concentrations of 1:1000 except β-actin which was used at 1:5000. For liver histology, all tissues were fixed in formalin (for at least 48 h), paraffin embedded and sectioned for haematoxylin and eosin (H & E) staining. After staining, two sections from each sample were imaged in triplicate. Liver lipid levels were measured by glycerol assay (Sigma) [6].
Analyses and modelling of clinical population data
Linear regression analyses were performed on proinsulin levels from patients recruited into the ORIGIN trial [14]. All participants had some degree of glucose elevation that ranged from slightly elevated glucose to clinically important degrees of hyperglycaemia [i.e. median fasting plasma glucose (FPG) = 6.9 mmol/l and median glycated haemoglobin A1c (HbA1c) = 6.4%]. Associations of metformin and/or ASA with proinsulin levels were obtained after adjusting for age, sex, weight, FPG levels, HbA1c and insulin levels.
Statistical analyses
All values are reported as mean ± S.E.M. Data were analysed −using Student’s t tests or one-way ANOVA or repeated measures ANOVA with Tukey’s or Bonferroni post-hoc tests where appropriate. Differences were considered significant when P < 0.05.
RESULTS
Metformin and salicylate synergistically activate AMPK and inhibit lipogenesis in mouse primary hepatocytes
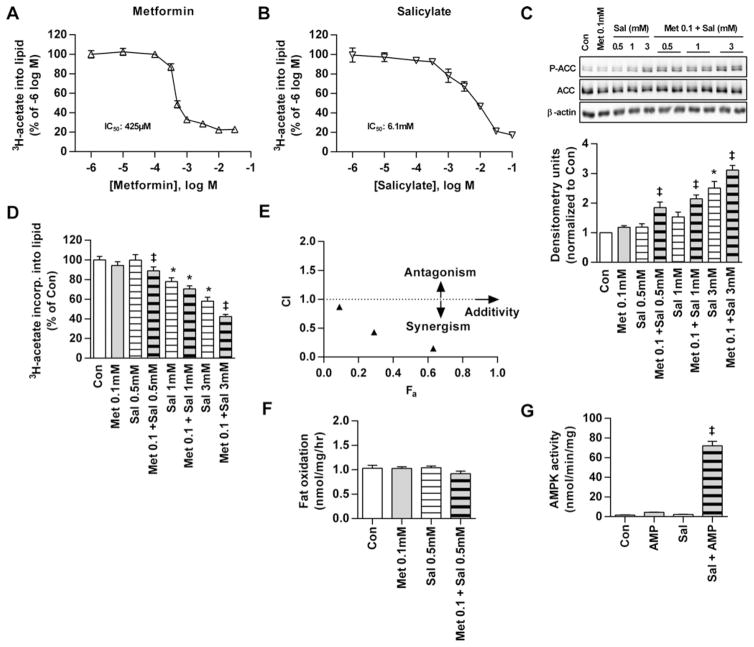
High doses of both metformin (0.5 mM) [6] and salicylate (3–5 mM) [8] regulate fatty acid metabolism in primary mouse hepatocytes through AMPK-mediated P-ACC. P-ACC is considered the best measure of AMPK activation in cells and tissues as it accounts for both allosteric and covalent modification components of AMPK activation [23]. In the present study, we show that metformin (Figure 1A) and salicylate (Figure 1B) both dose-dependently inhibit de novo fatty acid synthesis in mouse primary hepatocytes when administered alone (IC50 values of 425 μM and 6.1 mM respectively). When 0.1 mM metformin (a concentration observed in the portal vein [24]) was combined with 0.5 mM salicylate (a serum concentration achieved following ASA administration [25]), P-ACC was increased ~2-fold despite undetectable changes with individual doses (Figure 1C). Low doses of metformin (0.1 mM) also enhanced P-ACC when combined with salicylate at 1 or 3 mM (Figure 1C) and this effect was conserved when using higher concentrations of metformin (0.25 mM; Supplementary Figure S1A). As expected [6], the inhibitory effects on hepatocyte lipogenesis inversely mirrored the enhanced P-ACC at these combination doses (Figure 1D; Supplementary Figure S1B). The Chou–Talalay mathematical model theorem for drug synergy revealed a synergistic relationship between the two drugs, as indicated by combination Index (CI) values < 1 (Figure 1E; Supplementary Figure S1C).
Figure 1. Metformin and salicylate synergistically activate AMPK and inhibit lipogenesis in primary mouse hepatocytes.
Dose-dependent inhibition of de novo lipogenesis by (A) Met and (B) salicylate in mouse hepatocytes. (C) Ser79/212 P-ACC as a downstream target and marker of AMPK activation in primary mouse hepatocytes treated with no drug (Con), Met, salicylate (Sal) or Met + salicylate. (D) Inverse suppression of lipogenesis in mouse hepatocytes using the drug concentrations in (C). (E) CI compared with fractional effect inhibition (Fa) of lipogenesis in mouse hepatocytes treated with Met + salicylate (concentration ratio of 1:10 Met–salicylate) where CI > 1 indicates an antagonistic, CI = 1 additive or CI < 1 synergistic inhibition. Results represent at least two independent experiments performed in triplicate. (F) Fatty acid oxidation in mouse hepatocytes treated with no drug, low dose Met (0.1 mM), salicylate (0.5 mM) or both. Results from two independent experiments performed in triplicate. (G) Activation of AMPK by salicylate + AMP in vitro using purified, dephosphorylated AMPK α1β1γ1. Results were generated from four independent experiments. All densitometry is a ratio of phosphorylated to total protein. Data are means ± S.E.M. except panel (E), which are −means only. *P < 0.05 compared with Con, ‡ P < 0.05 compared with both respective Met-only and Sal-only doses.
Fatty acid oxidation was unaltered in the presence of low doses of metformin (0.1 mM) or salicylate (0.5 mM) alone or in combination (Figure 1F) and although higher dose salicylate (3 mM) increased fat oxidation, no further increase was observed with high dose metformin (0.5 mM; Supplementary Figure S1D). This is not unexpected since metformin inhibits complex-1 of the respiratory chain [26,27] which is required for the oxidation of fatty acyl-CoA and is consistent with findings from previous studies in hepatocytes [6,28] and skeletal muscle [29].
In cell-free assays, AMP (generated in hepatocytes by metformin [7]) and salicylate increased the activity of purified, non-phosphorylated AMPK α1β1γ1 heterotrimers by greater than 60-fold, but only had minor effects on allosteric activation when administered alone (Figure 1G; Supplementary Figure S1E). Synergy was eliminated in assays using a α1β2γ1 complex, confirming the necessity of the β-subunit isoform for the interaction (Supplementary Figure S1F). These data are the first to indicate that metformin and salicylate synergistically increase AMPK activity/P-ACC through allosteric mechanisms involving AMPK β1 independently of upstream kinases.
Metformin and salicylate synergistically activate AMPK and inhibit lipogenesis in human primary hepatocytes
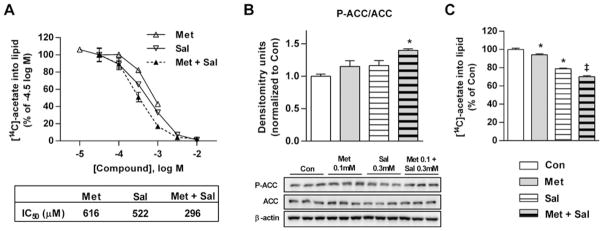
Since AMPK subunit expression profiles differ between mouse and human liver [26], we examined the responses to metformin and salicylate in primary human hepatocytes to assess the translational potential of our findings. Metformin and salicylate each generated dose-dependent inhibition of fatty acid synthesis (IC50 values of 616 and 522 μM respectively; Figure 2A). Importantly, equimolar doses of metformin and salicylate in human hepatocytes further reduced the IC50 for fatty acid synthesis to 296 μM (Figure 2A). As observed in mouse primary hepatocytes, metformin and salicylate also generated parallel synergistic increases in AMPK activation/P-ACC (Figure 2B) and suppression of lipogenesis (Figure 2C).
Figure 2. Metformin and salicylate synergistically activate AMPK and inhibit lipogenesis in primary human hepatocytes.
(A) Dose-dependent inhibition of lipogenesis by Met, salicylate (Sal) or equimolar Met + salicylate (Met + Sal) in human hepatocytes. (B) Ser79/212 P-ACC as a downstream target and marker of AMPK activation in primary human hepatocytes treated with no drug (Con), 0.1 mM Met, 0.3 mM salicylate or Met + salicylate (0.1 and 0.3 mM respectively). (C) Suppression of lipogenesis in human primary hepatocytes treated with the drug conditions in (B). Results represent experiments from two donors performed in triplicate. All densitometry is a ratio of phosphorylated to total protein. Data are means ± S.E.M. *P < 0.05 compared with Con, ‡P < 0.05 compared with both respective Met only and Sal only doses.
Liver lipids are synergistically reduced in high fat diet-fed mice treated with low-dose metformin and salsalate
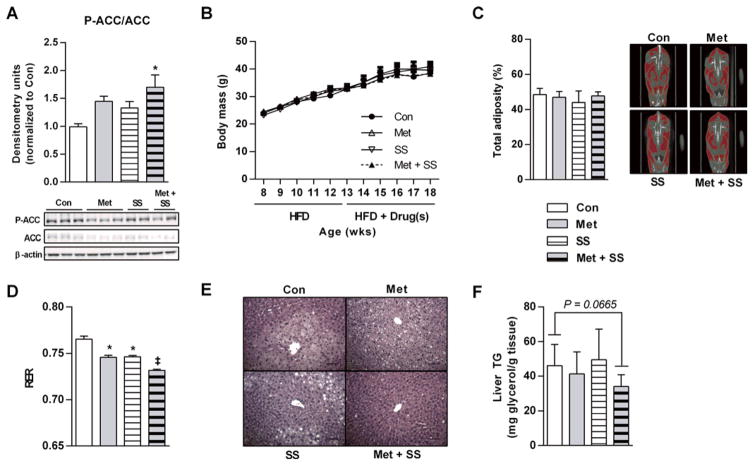
To evaluate whether synergistic effects are also observed in vivo, mice were fed a diet containing 60 % kcal from fat (HFD) for 5 weeks followed by 5 weeks of HFD containing no drug, 2.5 g/kg metformin, 1 g/kg salsalate or 2.5 g/kg metformin plus 1 g/kg salsalate. These doses of metformin and salsalate were chosen based on pilot experiments that generated low systemic venous blood levels of metformin (~8–40 μM; Supplementary Figure S2A) and salicylate (~150–300 μM; Supplementary Figure S2B) and closely mimic those achieved by dosing in humans [i.e. comparable to ~0.5–1.5 g/day metformin [9,30,31], ASA (~1 g/day) or salsalate (~0.75 g/day); 25,32,33]. Consistent with our findings in cultured hepatocytes, liver P-ACC was enhanced in mice receiving metformin–salsalate therapy (Figure 3A). Chronic treatment did not affect weight gain (Figure 3B) or adiposity (Figure 3C), a finding consistent with data from metabolic cages indicating that food intake (Supplementary Figure S2C) and energy expenditure (Supplementary Figure S2D) were unaltered. Despite comparable degrees of adiposity, metformin + salsalate treated mice displayed lower RER values (Figure 3D) (which can indicate reduced rates of whole-body de novo fatty acid synthesis [34]) and a strong tendency for reduced liver lipid (H & E staining; Figure 3E and liver glycerol; Figure 3F).
Figure 3. Metformin–salsalate combination treatment synergistically improves metabolic homoeostasis and lowers liver lipids in HFD-fed mice.
(A) P-ACC Ser79/212 (marker of AMPK activation; P-ACC) in clamped liver samples of C57bl/6 mice fed a 60 % HFD for 5 weeks followed by HFD with no drug (Con), 2.5 g/kg Met, 1 g/kg salsalate (SS) or Met and salsalate (Met + SS) for an additional 5 weeks. Densitometry is the ratio of phosphorylated to total protein. n = 5–8 mice per group. (B) Growth curves for mice on the diets indicated. n = 8 mice per group. (C) Percentage body adiposity by CT scan analysis. n = 8 mice per group. (D) Mean RER over a 24-h light–dark cycle. n = 4 mice per group. (E) Representative H & E staining of hepatic sections (scale bars, 100 μm) illustrate liver lipid deposition and (F) total liver TG assayed as total liver glycerol. n = 8–12 mice per group. Legends are conserved across bar graph panels. All data are means ± S.E.M. *P < 0.05 compared with Con, ‡P < 0.01 compared with both respective Met-only and SS-only doses.
Insulin sensitivity is enhanced in high fat diet-fed mice treated with metformin and salsalsate
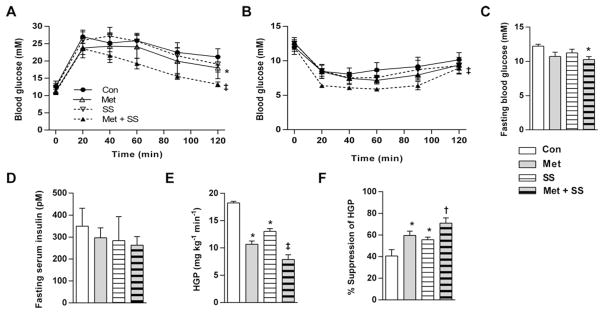
Metformin + salsalate treatment improved glucose (Figure 4A) and insulin tolerance (Figure 4B) and lowered fasting glucose (Figure 4C) compared with control, metformin or salsalate only. Serum insulin also tended to be lowest in mice receiving metformin + salsalate diet (Figure 4D). In hyperinsulinaemic–euglycaemic clamp experiments, all treatments tended to increase glucose infusion rates (GIRs; Supplementary Figures S2E and S2F), however metformin–salsalate combination therapy had the largest effect on liver insulin sensitivity as indicated by reductions in HGP (Figure 4E) and greater suppression of hepatic glucose output (Figure 4F) compared with the effects of metformin and salsalate alone. Serum glucose (Supplementary Figure S2G) and steady-state serum insulin levels (Supplementary Figure S2H) were comparable between groups during the clamp, confirming clamped states and insulin equality respectively. There were no effects of treatments on GDR (Supplementary Figure S2I) or non-esterified fatty acids (NEFA; Supplementary Figure S2J), indicating that peripheral insulin sensitivity (skeletal muscle and adipose tissue) was unaltered. Insulin signalling, as assessed by P-Ser473–Akt–Akt and P-Thr308Akt–Akt was unaltered in clamped livers of mice from any treatment group (Supplementary Figure S2K).
Figure 4. Met and salsalate synergistically improve liver insulin sensitivity in mice.
Intraperitoneal (A) GTT and (B) ITT for C57bl/6 mice fed a 60 % HFD for 5 weeks followed by HFD with no drug (Con), 2.5 g/kg Met, 1 g/kg salsalate (SS) or Met and salsalate (Met + SS) for an additional 5 weeks. (C) Whole blood glucose and (D) serum insulin concentrations following a 12-h fast. (E) HGP and (F) percentage suppression of hepatic glucose output during hyperinsulinaemic–euglycaemic clamps. Legends are conserved across line or bar graph panels. n = 4–5 mice per group for all measures. All data are means ± S.E.M. *P < 0.05 compared with Con, ‡P < 0.01 compared with both respective Met and SS only doses, †P < 0.05 compared with respective SS only.
Metformin and ASA independently reduce proinsulin in humans with dysglycaemia
Human hepatocytes were sensitive to salicylate at concentrations achievable following the intake of ASA (Figure 2A) [25]. Therefore we used data collected as part of the previously completed ORIGIN trial [33] to explore the metabolic effect of metformin and ASA in 8281 people with either prediabetes or diabetes (median FPG = 6.9 mmol/l and median HbA1c = 6.4 %) in whom proinsulin and insulin levels were measured. In large clinical studies (such as ORIGIN), fasting pro-insulin levels are considered a reliable indicator of insulin resistance in individuals with T2D [12]. After adjusting for covariants, metformin (P < 0.0001) and ASA (P = 0.025) were both independently associated with a lower proinsulin level (Table 1) with combination therapy resulting in further reductions (− 0.1801 ± 0.0331, P < 0.0001, n = 3381; Table 1).
Table 1.
Metformin and ASA independently reduce proinsulin in humans with dysglycaemia
| Variable | Model 1 (n = 8281) | Model 2 (n = 3381) | ||
|---|---|---|---|---|
| Estimate ± S.E.M. | P-value | Estimate ± S.E.M. | P-value | |
| Intercept | 0.3941 ± 0.0741 | <0.0001 | 0.5253 ± 0.1166 | <0.0001 |
| Plasma insulin | 0.4119 ± 0.0104 | <0.0001 | 0.4325 ± 0.0166 | <0.0001 |
| FPG | 0.0518 ± 0.0059 | <0.0001 | 0.0665 ± 0.0088 | <0.0001 |
| HbA1c | 0.1112 ± 0.0125 | <0.0001 | 0.0755 ± 0.0194 | <0.0001 |
| Met | − 0.134 ± 0.023 | <0.0001 | N.D. | N.D. |
| ASA | − 0.049 ± 0.022 | 0.0247 | N.D. | N.D. |
| Met + ASA | N.D. | N.D. | − 0.1801 ± 0.0331 | <0.0001 |
Linear regression analyses of proinsulin levels were adjusted for age, sex, weight, plasma insulin, FPG and HbA1c levels. Analyses of metformin (Met) and ASA were adjusted for ASA and Met respectively. N.D., not determined.
DISCUSSION
Elevated levels of de novo fatty acid synthesis contribute to the aetiology of NAFLD and insulin resistance [2,6] and are observed in insulin resistant [35], diabetic [36] and obese [37] humans under fasting and fed conditions. In addition, recent evidence directly confirms the contribution of de novo lipogenesis to fatty liver disease in humans using NMR spectroscopy [2], highlighting the importance of targeting this process for therapeutic intervention in NAFLD. In agreement with previous reports [6,38–40], we show that metformin and salicylate dose-dependently inhibit de novo lipogenesis in primary hepatocytes from rodents (Figures 1A and 1B). Whereas the concentration of metformin that elicited a 50 % reduction in lipogenesis was comparable between mice and human hepatocytes (Figures 1A and 2A), the IC50 for salicylate was an order of magnitude lower in the human cells (Figure 2A) and comparable to serum levels achieved following ingestion of a regular strength ASA [25], revealing notably greater sensitivity of human primary hepatocytes to salicylate. We also demonstrate that priming of mouse and human hepatocytes with clinical concentrations of metformin that have no effect on fatty acid synthesis on their own, dramatically reduce salicylate-induced inhibition of fatty acid synthesis and activate AMPK in primary hepatocytes and in vitro assays. These data provide the first evidence that metformin and salicylate synergistically activate AMPK and reduce de novo fatty acid synthesis in mouse and human primary hepatocytes at clinically achievable doses. This suggests that low serum levels of salicylate (i.e. closer to those achieved with extra strength ASA [25]) may be appropriate for inhibiting fatty acid synthesis, particularly in NAFLD where hepatic fatty acid synthesis is enhanced. Future studies are required to determine why human hepatocytes have enhanced sensitivity to salicylate-induced inhibition of lipogenesis compared with those of mice.
Consistent with the synergistic effects of metformin and salicylate on liver AMPK activation and de novo lipogenesis, we found that low doses of metformin and salsalate activate AMPK/enhance P-ACC (Figure 3A) and tended to lower TG/NAFLD (Figures 3E and 3F) in livers of mice made obese through feeding of a HFD over the effect of either drug alone. Furthermore, hyperinsulinaemic–euglycaemic clamp experiments revealed that the most profound improvement in insulin sensitivity with combination treatment occurred in the liver, as indicated by synergistic inhibition of HGP (Figure 4F) and elevated percentage suppression of hepatic glucose output (Figure 4G) in metformin + salsalate treated animals over those treated with either drug alone, effects that were also observed in the absence of any notable differences in peripheral GDRs (Figure S2I). These findings are consistent with previous reports indicating that the liver is the primary target of metformin and salsalate [41,42].
We examined the effects of metformin + salsalate treatment in HFD-fed mice since they are considered a clinically relevant model of human obesity and insulin resistance; however, it is known that feeding of a HFD suppresses liver de novo lipogenesis. Therefore, given that metformin–salsalate therapy suppressed de novo lipogenesis and had no effect on fatty acid oxidation, this lower starting lipogenic rate may have explained why metformin–salsalate combination therapy only had modest effects on liver TG and insulin sensitivity. The magnitude of metformin–salsalate effect would be anticipated to be higher in insulin-resistant mouse models that have high rates of liver de novo lipogenesis, such as leptin deficient ob/ob mice or in mice fed a high fructose diet. Future studies investigating this possibility are warranted.
The therapeutic potential offered by combining low doses of metformin and salicylate is highlighted by the fact that the individual doses of these drugs required to fully activate liver AMPK may cause toxicity and/or intolerance [9,43]. Direct pharmaceutical remedies for NAFLD are also lacking, with weight loss being the best therapeutic strategy identified thus far. Whereas metformin consistently reduces liver fat in mice, small studies in humans utilizing the liver biopsy technique have yielded mixed results that suggest the effects of metformin alone may be relatively modest. It may be that larger clinical trials are needed to offset the large sample variance of the liver biopsy technique to clarify these findings. To the best of our knowledge, the role of salicylate-based drugs in regulating liver fat in humans has not been tested. Future clinical trials testing the effect of metformin and salicylate-based drugs on NAFLD using sensitive/direct methods of measuring liver fat and insulin sensitivity (NMR spectroscopy and hyperinsulinaemic–euglycaemic clamps respectively) are warranted.
In conclusion, the present study is the first to investigate the potential synergy of two widely used and well-tolerated drugs, metformin and salicylate, on AMPK activity, liver fatty acid synthesis and insulin resistance. The therapeutic potential of the findings are highlighted by our observations showing that metformin combined with salicylate-based therapies (at serum concentrations achieved following the intake of an extra strength ASA) lower fatty acid synthesis in primary human hepatocytes and lower proinsulin levels in a large clinical cohort with dysglycaemia. These data support the use of salicylate-based drugs and metformin in combination to maximize activation of liver AMPK and suppress fatty acid synthesis. As elevated liver fatty acid synthesis is commonly observed in individuals with NAFLD and insulin resistance, combination therapy with salsalate and metformin may be effective for treating these prevalent conditions.
Supplementary Material
Acknowledgments
We thank Yasumichi Hitoshi and Tian Qiang Sun for technical support and R. Rhem and J. Park for computed tomography analysis.
FUNDING
This study was supported by the Canadian Diabetes Association [grant number OG-1-09-2698-65 (to G.R.S.)]; the Canadian Institute for Health Research (CIHR) [grant numbers MOP-11480 (to G.R.S.), and BPF-112934 (to M.D.F.)]; the Canadian Foundation for Innovation [grant number 2008M00051 (to G.R.S.)]; the Canadian Liver Foundation Undergraduate Scholarship; the Australian Research Council [grant number DP130104548 (to B.E.K.)]; the National Health and Medical Research Council [grant number APP1085460 (to B.E.K. and G.R.S.) and grant number APP1049197 (to J.S.O. and J.W.S.)]; the Victorian Government’s Operational Infrastructure Support Program; and Sanofi.
Abbreviations
- ACC
acetyl-CoA carboxylase
- AMPK
AMP-activated protein kinase
- ASA
aspirin
- BMI
body mass index
- CI
combination Index
- Con
control
- FPG
fasting plasma glucose
- GDR
glucose disposal rate
- GTT
glucose tolerance test
- H & E
haematoxylin and eosin
- HbA1c
glycated haemoglobin A1c
- HFD
high fat diet
- HGP
hepatic glucose production
- ITT
insulin tolerance test
- Met
metformin
- NAFLD
non-alcoholic fatty liver disease
- ORIGIN
Outcome Reduction with an Initial Glargine Intervention
- P-ACC
phosphorylation on acetyl-CoA carboxylase at Ser79/212
- PKB
protein kinase B
- RER
respiratory exchange ratio
- Sal
salicylate
- SS
salsalate
- T2D
type 2 diabetes
- TG
triglycerides
Footnotes
AUTHOR CONTRIBUTIONS
Rebecca Ford, Morgan Fullerton, Stephen Pinkosky and Gregory Steinberg designed the study. Rebecca Ford, Emily Day, Morgan Fullerton, Stephen Pinkosky, Adam Bujak., Brennan Smith, Katarina Marcinko, Regje Blümer and Justin Crane performed experiments and data analyses. Hertzel Gerstein analysed human subject data and provided consultation. John Scott, Jonathan Oakhill and Bruce Kemp contributed the AMPK in vitro studies, Rebecca Ford, Stephen Pinkosky, Hertzel Gerstein, Bruce Kemp and Gregory Steinberg wrote the manuscript. All authors contributed to editing the final manuscript draft.
References
- 1.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725. e716. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;123:2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carling D, Clarke PR, Zammit VA, Hardie DG. Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur J Biochem. 1989;186:129–136. doi: 10.1111/j.1432-1033.1989.tb15186.x. [DOI] [PubMed] [Google Scholar]
- 6.Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, O’Neill HM, Ford RJ, Palanivel R, O’Brien M, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19:1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green KA, Mustard KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 10.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 11.Meex RC, Phielix E, Moonen-Kornips E, Schrauwen P, Hesselink MK. Stimulation of human whole-body energy expenditure by salsalate is fueled by higher lipid oxidation under fasting conditions and by higher oxidative glucose disposal under insulin-stimulated conditions. J Clin Endocrinol Metab. 2011;96:1415–1423. doi: 10.1210/jc.2010-1816. [DOI] [PubMed] [Google Scholar]
- 12.Pfutzner A, Kunt T, Hohberg C, Mondok A, Pahler S, Konrad T, Lubben G, Forst T. Fasting intact proinsulin is a highly specific predictor of insulin resistance in type 2 diabetes. Diabetes Care. 2004;27:682–687. doi: 10.2337/diacare.27.3.682. [DOI] [PubMed] [Google Scholar]
- 13.Tsai CY, Lin YS, Yeh TS, Cheong CF, Chang CH, Chen TC, Chen MF. Disrupted hepatic adiponectin signaling impairs liver regeneration of steatotic rats. Chang Gung Med J. 2011;34:248–259. [PubMed] [Google Scholar]
- 14.Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 15.Fullerton MD, Hakimuddin F, Bonen A, Bakovic M. The development of a metabolic disease phenotype in CTP:phosphoethanolamine cytidylyltransferase-deficient mice. J Biol Chem. 2009;284:25704–25713. doi: 10.1074/jbc.M109.023846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dzamko N, van Denderen BJ, Hevener AL, Jorgensen SB, Honeyman J, Galic S, Chen ZP, Watt MJ, Campbell DJ, Steinberg GR, Kemp BE. AMPK beta1 deletion reduces appetite, preventing obesity and hepatic insulin resistance. J Biol Chem. 2010;285:115–122. doi: 10.1074/jbc.M109.056762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 18.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 19.Ducommun S, Ford RJ, Bultot L, Deak M, Bertrand L, Kemp BE, Steinberg GR, Sakamoto K. Enhanced activation of cellular AMPK by dual-small molecule treatment: AICAR and A769662. Am J Physiol Endocrinol Metab. 2014;306:E688–696. doi: 10.1152/ajpendo.00672.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott JW, Ling N, Issa SM, Dite TA, O’Brien MT, Chen ZP, Galic S, Langendorf CG, Steinberg GR, Kemp BE, Oakhill JS. Small molecule drug A-769662 and AMP synergistically activate naive AMPK independent of upstream kinase signaling. Chem Biol. 2014;21:619–627. doi: 10.1016/j.chembiol.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Glass DB, Masaracchia RA, Feramisco JR, Kemp BE. Isolation of phosphorylated peptides and proteins on ion exchange papers. Anal Biochem. 1978;87:566–575. doi: 10.1016/0003-2697(78)90707-8. [DOI] [PubMed] [Google Scholar]
- 22.Galic S, Fullerton MD, Schertzer JD, Sikkema S, Marcinko K, Walkley CR, Izon D, Honeyman J, Chen ZP, van Denderen BJ, et al. Hematopoietic AMPK beta1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J Clin Invest. 2011;121:4903–4915. doi: 10.1172/JCI58577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gowans GJ, Hawley SA, Ross FA, Hardie DG. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 2013;18:556–566. doi: 10.1016/j.cmet.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilcock C, Bailey CJ. Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica. 1994;24:49–57. doi: 10.3109/00498259409043220. [DOI] [PubMed] [Google Scholar]
- 25.Ruffin MTt, Krishnan K, Rock CL, Normolle D, Vaerten MA, Peters-Golden M, Crowell J, Kelloff G, Boland CR, Brenner DE. Suppression of human colorectal mucosal prostaglandins: determining the lowest effective aspirin dose. J Natl Cancer Inst. 1997;89:1152–1160. doi: 10.1093/jnci/89.15.1152. [DOI] [PubMed] [Google Scholar]
- 26.Stephenne X, Foretz M, Taleux N, van der Zon GC, Sokal E, Hue L, Viollet B, Guigas B. Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia. 2011;54:3101–3110. doi: 10.1007/s00125-011-2311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 28.Fulgencio JP, Kohl C, Girard J, Pegorier JP. Effect of metformin on fatty acid and glucose metabolism in freshly isolated hepatocytes and on specific gene expression in cultured hepatocytes. Biochem Pharmacol. 2001;62:439–446. doi: 10.1016/s0006-2952(01)00679-7. [DOI] [PubMed] [Google Scholar]
- 29.Collier CA, Bruce CR, Smith AC, Lopaschuk G, Dyck DJ. Metformin counters the insulin-induced suppression of fatty acid oxidation and stimulation of triacylglycerol storage in rodent skeletal muscle. Am J Physiol Endocrinol Metab. 2006;291:E182–E189. doi: 10.1152/ajpendo.00272.2005. [DOI] [PubMed] [Google Scholar]
- 30.Tucker GT, Casey C, Phillips PJ, Connor H, Ward JD, Woods HF. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol. 1981;12:235–246. doi: 10.1111/j.1365-2125.1981.tb01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pentikainen PJ, Neuvonen PJ, Penttila A. Pharmacokinetics of metformin after intravenous and oral administration to man. Eur J Clin Pharmacol. 1979;16:195–202. doi: 10.1007/BF00562061. [DOI] [PubMed] [Google Scholar]
- 32.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–294. doi: 10.2337/dc07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol: Respir Environ Exerc Physiol. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 35.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilke MS, French MA, Goh YK, Ryan EA, Jones PJ, Clandinin MT. Synthesis of specific fatty acids contributes to VLDL-triacylglycerol composition in humans with and without type 2 diabetes. Diabetologia. 2009;52:1628–1637. doi: 10.1007/s00125-009-1405-9. [DOI] [PubMed] [Google Scholar]
- 37.Marques-Lopes I, Ansorena D, Astiasaran I, Forga L, Martinez JA. Postprandial de novo lipogenesis and metabolic changes induced by a high-carbohydrate, low-fat meal in lean and overweight men. Am J Clin Nutr. 2001;73:253–261. doi: 10.1093/ajcn/73.2.253. [DOI] [PubMed] [Google Scholar]
- 38.Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, Brecher P, Ruderman NB, Cohen RA. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem. 2004;279:47898–47905. doi: 10.1074/jbc.M408149200. [DOI] [PubMed] [Google Scholar]
- 39.Beynen AC, Buechler KF, van der Molen AJ, Geelen MJ. Inhibition of hepatic lipogenesis by salicylate. Toxicology. 1982;24:33–43. doi: 10.1016/0300-483x(82)90060-9. [DOI] [PubMed] [Google Scholar]
- 40.Jung TW, Youn BS, Choi HY, Lee SY, Hong HC, Yang SJ, Yoo HJ, Kim BH, Baik SH, Choi KM. Salsalate and adiponectin ameliorate hepatic steatosis by inhibition of the hepatokine fetuin-A. Biochem Pharmacol. 2013;86:960–969. doi: 10.1016/j.bcp.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 41.Natali A, Ferrannini E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia. 2006;49:434–441. doi: 10.1007/s00125-006-0141-7. [DOI] [PubMed] [Google Scholar]
- 42.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldfine AB, Silver R, Aldhahi W, Cai D, Tatro E, Lee J, Shoelson SE. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008;1:36–43. doi: 10.1111/j.1752-8062.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.